Abstract
Background
There is currently a dearth of knowledge on gender differences in mortality among patients on ART in Africa.
Methods
Using data from the national ART monitoring and evaluation system, a survival analysis of all healthcare workers, teachers, and police/army personnel who accessed ART in Malawi by June, September and December 2006 respectively, was undertaken. Gender differences in survival were analysed using Kaplan-Meier estimates and rate ratios were derived from Poisson regression adjusting for confounding.
Results
4670 ART patients (49.8% female) were followed up for a median of 8.7 months after starting ART. Probability of death was significantly higher for men than women (p<0.001). Controlling for age, WHO clinical stage and occupation, men experienced nearly 2 times the mortality of women RR 1.90 [95% CI: 1.57–2.29]. A higher proportion of men initiated ART in WHO stage 4 (p<0.001).
Conclusion
Among healthcare workers, teachers, police/army personnel, men have higher mortality on ART than women. Possible reasons are unclear but could be biological or because men present for ART at a later clinical stage or have poorer adherence to therapy. Improving early access to ART may reduce mortality, especially among men. A gender difference in adherence to therapy needs further investigation.
Introduction
Since the advent of ART, mortality rates for people living with HIV/AIDS have decreased substantially both in resourcerich1,2 and resource-limited settings3,8 with conflicting reports about gender-related differences in mortality9–16. Where differences have been reported, little is known about the reasons.
Identifying whether men and women benefit equally from ART and ascertaining the reasons underlying any difference could inform strategies designed to address these differences and optimise ART delivery. This is especially important in resource limited settings like Malawi (where ART scale-up is a major focus), and particularly among specific occupational sub-groups of the population who contribute significantly to the socio-economic development of the country and whose skill-base continues to be depleted by HIV/AIDS. Previous studies have considered the general population rather than specific occupational groups.
Previous studies in Malawi focused on the specific occupational groups of health care workers17 teachers18 army19 and police personnel20 separately reporting outcomes in relation to demographic and clinical variables. As the same systems of recording and data collection were used for each study, we decided to combine the databases of the four studies to determine whether there is a gender difference in mortality among adults receiving ART in Malawi, and to explore possible reasons for any difference among healthcare workers, teachers, army and police personnel.
Materials and Methods
Ethics Statement
General measures are provided in all ART facilities to ensure patient confidentiality, consent for HIV testing, and counselling and support for those who receive a positive HIV test result. Data for this study did not include any personal identifiers and were analysed anonymously. The Malawi National Health Science Research Committee provides general oversight and approval for the collection and use of routine programmatic data for monitoring and evaluation, as was the case with this study.
Formal ethical approval was therefore not required from the Malawi Research Committee and as such, no application was made for ethical approval from either Medecins sans Frontieres or The International Union against Tuberculosis and Lung Disease.
Background
Malawi is among the poorest countries in sub-Saharan Africa with an estimated population of about 13 million21. Like other countries in the region, it has experienced a severe HIV epidemic. The national HIV adult prevalence is estimated at about 12% with 810,000 adults living with HIV/AIDS22.
Described previously,23 ART scale-up in Malawi began in June 2004 utilising a national roll-out programme of free ART to all public-sector facilities. ART scale up in the private sector has followed the same process with private treatment offered at a subsidised cost. As of September 2008, there were 170 facilities in the public health sector and 44 sites in the private sector providing ART to over 135,000 patients24.
Patients are eligible for ART if they are HIV-seropositive, understand the implications of starting ART and either present in WHO Clinical Stage 3 or 4 or have a CD4-lymphocyte count of 250/mm3 or less (irrespective of WHO stage)25. Without the equipment to perform CD4-lymphocyte counts, most clinics use clinical staging alone to determine ART eligibility.
Malawi uses a standardised ART approach: use of one generic fixed dose combination of stavudine (d4T), lamivudine (3TC) and nevirapine (NVP). There are two alternative first line regimens for patients with serious side effects, substituting d4T with zivoudine (AZT) or NVP with efavirenz (EFV). Two standard proteinaseinhibitor based second line regimens are available for adults and children with drug failure.
A nationally standardised process of registration, monitoring and reporting of cases and outcomes is used26. Using one master card per patient and one ART register per ART facility23 patient demographic details, occupation, ART start date and WHO stage together with any stage defining conditions at ART initiation, are recorded. After starting ART, patients are followed up at two weeks and then at monthly intervals for clinical assessment and ARV dispensing. Monthly visits are recorded on the master card and patient outcomes are updated quarterly in the ART register. Standardised follow-up outcomes are defined as: alive and on ART (patient alive and on ART at the facility where he/she is registered); dead (patient who has died from any cause while on ART); lost to follow-up (patient on ART who has not attended the ART clinic for 3 months or longer for no known reason and cannot be traced); stopped treatment (patient who is known to be alive and has stopped ART for any reason); transferred out (patient who has transferred-out permanently to another treatment facility). Active follow-up of patients who default is not mandatory due to limited resources but most facilities routinely attempt to trace patients who have failed to attend their scheduled appointment.
At the end of every quarter, each facility conducts a cohort analysis of patients newly registered during the quarter and of all patients ever registered at the facility. The Ministry of Health's Department of HIV and AIDS and its partners conduct quarterly supervisory and monitoring visits to all public sector ART facilities in the country.
They check the completeness of the ART registers, crosscheck the data recorded on the patient master cards with the data entered in the ART registers and cross-check the cohort analysis23. In the private sector a similar supervisory and monitoring system is conducted.
Data Collection
During three rounds of quarterly supervision visits to all public and private ART facilities between July 2006 and March 2007, individual level patient data were collected for the following occupational cohorts: healthcare workers, teachers and army/police personnel17,18,19,20. The following patient data were transcribed from the ART registers onto structured data collection forms: sex, age, date and WHO clinical stage at ART initiation together with selected stage defining conditions, date and reason for termination of observation at the respective facility (alive on ART at end of observation, transferred to another facility, stopped treatment (‘ART stop’), lost to follow up, death). Observations were censored depending on the time of data collection: healthcare workers (30 June 2006); teachers (30 September 2006); army and police personnel (31 December 2006).
Data Analysis
Data were double entered in Microsoft Access and analysed using STATA version 10. Death was used as the failure event for the analysis of mortality rates. Due to the incomplete ascertainment of deaths and in order to control for potentially differential ascertainment of death by gender, a separate analysis was done combining death, ART stop and loss to follow-up as the failure event (‘ART drop-out’). For the person years denominator, patients were generally considered to come under observation at the time of ART initiation. For 718 patients who had initiated ART before implementation of the standard M&E tools in June 2004, the 1st of June 2004 was used as the observation start date, but their previous exposure time on ART was accurately accounted for in the time at risk. Observations ended at the end of the quarter of data collection or at the time of death, ART stop, transfer-out to another facility or loss to follow-up. Twenty-two cases were excluded because the date of ART initiation or date of termination of observation, were inconsistent or incomplete.
Gender was the main exposure of interest. Age, clinical stage at ART initiation, specific stage defining conditions, occupation and type of ART facility were considered as potential confounders in the association of gender with mortality and ART drop-out. The strength of association between gender and other background variables was measured using the X2 test. Cumulative survival probabilities were estimated using the Kaplan-Meier method and survival between men and women was compared using the log-rank test. Rate ratios were calculated for mortality and ‘ART dropout’. Poisson regression models were used to compare rates between men and women, adjusting for age and WHO clinical stage at ART initiation. P-values were derived from the Wald test and 95% confidence intervals for point estimates are given throughout.
Results
Characteristics of the study population and gender differences in death
Of a total of 4692 patients receiving ART between June 2004 and December 2006, 4670 were included in the analysis. Of these, 2346 (50.2%) were men. Women were on average 3 years younger than men at ART initiation (median age 39 years for men (interquartile range (IQR) 35–44 years) and 36 years for women (IQR 32–42 years).
Men initiated ART in clinically more advanced stages than women. A total of 731 (31.2%) men and 577 (24.8%) women were in WHO clinical stage 4 at ART initiation (p<0.001); 354 (15.1%) men and 394 (17.0%) women were in stage 1 or 2 with a CD4 count below 250 cells/µl (Table 1). Clinical stage at ART initiation was missing for 37 patients (0.8%). More than twice as many men presented with Kaposi's sarcoma at ART initiation (197 men (8.4%) versus 95 women (4.1%), p<0.001), but similar proportions of men and women had pulmonary and extra-pulmonary tuberculosis. (Table 1). Most patients (4413 patients, 94.5%) were from public sector clinics, but the proportion of private sector patients was higher among women (37 men (1.6%) versus 88 women (3.8%), p<0.0001).
Table 1.
Socio-demographic and clinical characteristics and outcomes of healthcare workers, teachers, army and police personnel on ART, according to gender
| Males | Females | |||||||
| Variables | ||||||||
| n | % | n | % | n | % | p Value (X2) |
||
| Age starting ART (yrs) | ||||||||
| 19–34 | 1409 | 30.2 | 502 | 21.4 | 907 | 39.0 | <0.001 | |
| 35–44 | 2236 | 47.9 | 1231 | 52.5 | 1005 | 43.2 | ||
| 45+ | 974 | 20.9 | 577 | 24.6 | 397 | 17.1 | ||
| Unknown | 51 | 1.1 | 36 | 1.5 | 15 | 0.7 | ||
| Occupation | ||||||||
| Healthcare worker | 1023 | 21.9 | 363 | 15.5 | 660 | 28.4 | <0.001 | |
| Teacher | 2637 | 56.5 | 1110 | 47.3 | 1527 | 65.7 | ||
| Army personnel | 547 | 11.7 | 526 | 22.4 | 21 | 0.9 | ||
| Police personnel | 463 | 9.9 | 347 | 14.8 | 116 | 5.0 | ||
| WHO clinical Stage | ||||||||
| 1 or 2, CD4 ≥250 | 748 | 16.0 | 354 | 15.1 | 394 | 17.0 | <0.001 | |
| 3 | 2577 | 55.2 | 1238 | 52.8 | 1339 | 57.6 | ||
| 4 | 1308 | 28.0 | 731 | 31.2 | 577 | 24.8 | ||
| Unknown | 37 | 0.8 | 23 | 1.0 | 14 | 0.6 | ||
| Stage defining condition | ||||||||
| Other / unknown | 3338 | 71.5 | 1628 | 69.4 | 1710 | 73.6 | <0.001 | |
| PTB | 845 | 18.1 | 427 | 18.2 | 418 | 18.0 | ||
| Extra-PTB | 194 | 4.2 | 94 | 4.0 | 100 | 4.3 | ||
| Kaposi's sarcoma | 292 | 6.3 | 197 | 8.4 | 95 | 4.1 | ||
| PMTCT | 1 | 0.02 | 0 | 0 | 1 | 0.02 | ||
| ART Facility | ||||||||
| Public | 4413 | 94.5 | 2213 | 94.3 | 2200 | 94.7 | <0.001 | |
| Private | 125 | 2.7 | 37 | 1.6 | 88 | 3.8 | ||
| Unknown | 132 | 2.8 | 96 | 4.1 | 36 | 1.6 | ||
| Time of ART initiation | ||||||||
| Before free ART | 718 | 15.4 | 333 | 14.2 | 385 | 16.6 | 0.03 | |
| After free ART | 3952 | 84.6 | 2013 | 85.8 | 1939 | 83.4 | ||
| Outcome at end of observation | ||||||||
| Alive on ART | 3315 | 70.1 | 1574 | 67.1 | 1741 | 74.9 | <0.001 | |
| Dead | 547 | 11.7 | 341 | 14.5 | 206 | 8.9 | ||
| Lost to follow up | 288 | 6.2 | 147 | 6.3 | 141 | 6.1 | ||
| Stopped ART | 20 | 0.4 | 8 | 0.3 | 12 | 0.5 | ||
| Tranferred out | 500 | 10.7 | 276 | 11.8 | 224 | 9.6 | ||
| Total | 4670 | 100 | 2346 | 50.2 | 2324 | 49.8 |
ART, Antiretroviral Therapy; WHO, World Health Organisation; PTB, Pulmonary Tuberculosis; PMTCT, Prevention of Mother to Child Transmission
A total of 547 patients died, 20 stopped ART and 288 were lost to follow-up in 4,439 person-years of observation (PYO), resulting in a mortality rate of 123.2 per 1000 PYO [95% CI, 113.3–134.0] and an ‘ART drop-out’ rate of 192.6 per 1000 PYO [95% CI, 180.1–206.0]. Mortality was 402.3/ 1000 PYO [95% CI, 356.7–453.6] in month 0–2 after ART initiation, 130.0/ 1000 PYO [95% CI, 135.1–190.5/ 1000 PYO] in month 3–5, 70.2/ 1000 PYO [95% CI, 56.6–87.0/ 1000 PYO] in month 6–11 and 59.8/ 1000 PYO [95% CI, 44.5–80.3/ 1000 PYO] in month 12–17. Table 2 shows that mortality and ART drop-out rates declined in a very similar fashion after ART initiation.
Table 2.
Mortality and ART drop out rates and crude and adjusted rate ratios according to different background variables
| Variables | Mortality | ART drop out | |||||||
| D/PYO | Rate | Rate Ratio [95% CI] |
Adjusted Rate Ratio [95% CI]a |
D/DYO | Rate | Rate ratio [95% CI] |
Adjusted Rate Ratio [95% CI]a |
||
| Sex | |||||||||
| Male | 341/ 2171 | 157.1 | 1.73 [1.45–2.06] | 1.90 [1.57–2.29] | 496/ 2268 | 228.5 | 1.44 [1.26–1.65] | 1.66 [1.43–1.92] | |
| Femaleref | 206/ 2268 | 90.8 | 1 | 1 | 359/ 2171 | 158.3 | 1 | 1 | |
| Age at ART initiation (yrs) | |||||||||
| 19–34 | 162/ 1238 | 130.8 | 1.07 [0.88–1.31] | 1.15 [0.95–1.41] | 262/ 1238 | 211.6 | 1.13 [0.97–1.32] | 1.20 [1.02–1.40] | |
| 35–44 ref | 261/ 2141 | 121.9 | 1 | 1 | 401/ 2141 | 187.3 | 1 | 1 | |
| 45+ | 118/ 986 | 119.6 | 0.98 [0.79–1.22] | 0.97 [0.78–1.21] | 181/ 986 | 183.5 | 0.98 [0.82–1.17] | 0.98 [0.82–1.18] | |
| Unknown | 6/ 73 | 81.9 | 0.67 [0.30–1.51] | 0.61 [0.27–1.38] | 11/ 73 | 150.2 | 0.82 [0.44–1.46] | 0.71 [0.29–1.29] | |
| Occupation | |||||||||
| Healthcare worker | 118/ 891 | 132.4 | 1.13 [0.91–1.40] | 1.22 [0.99–1.52] | 163/ 891 | 182.9 | 0.90 [0.75–1.07] | 0.96 [0.81–1.15] | |
| Teacher ref | 299/ 2550 | 117.2 | 1 | 1 | 518/2550 | 203.1 | 1 | 1 | |
| Army personnel | 71/ 590 | 120.4 | 1.03 [0.79–1.33] | 0.72 [0.55–0.94] | 84/ 590 | 142.2 | 0.70 [0.56–0.88] | 0.53 [0.42–0.68] | |
| Police personnel | 59/ 407 | 144.9 | 1.24 [0.93–1.63] | 0.94 [0.70–1.25] | 90/ 407 | 221.1 | 1.09 [0.87–1.36] | 0.87 [0.69–1.10] | |
| WHo clinical Stage | |||||||||
| 1 or 2, CD4 ≤250 | 49/ 720 | 68.0 | 0.66 [0.49–0.90] | 0.66 [0.49–0.90] | 86/ 720 | 119.4 | 0.68 [0.54–0.86] | 0.70 [0.55–0.88] | |
| 3 ref | 256/ 2492 | 102.7 | 1 | 1 | 436/ 2492 | 174.9 | 1 | 1 | |
| 4 | 242/ 1187 | 203.8 | 1.98 [1.67–2.37] | 1.91 [1.60–2.28] | 329/ 1187 | 277.2 | 1.58 [1.37–1.83] | 1.56 [1.35–1.80] | |
| Unknown | 0/ 39 | 0.0 | - | - | 4/ 39 | 103.2 | 0.59 [0.22–1.58] | 0.53 [0.20–1.41] | |
| Stage defining condition | |||||||||
| Other / unknown ref | 364/ 3070 | 118.6 | 1 | 577/ 3070 | 188.0 | 1 | |||
| PTB | 88/ 918 | 95.8 | 0.81 [0.64–1.02] | 143/ 918 | 155.8 | 0.83 [0.69–1.0] | |||
| Extra-PTB | 26/ 196 | 132.7 | 1.12 [0.75–1.67] | 36/ 196 | 183.8 | 0.98 [0.70–1.37] | |||
| Kaposi's sarcoma | 69/ 254 | 271.3 | 2.29 [1.7–2.96] | 99/ 254 | 389.3 | 2.07 [1.67–2.56] | |||
| PMTCT | 0/ 0.3 | 0.0 | - | 0/ 0 | 0.0 | - | |||
| ART Facility | |||||||||
| Public ref | 526/ 4205 | 125.1 | 1 | 832/ 4205 | 197.8 | 1 | |||
| Private | 2/ 108 | 18.6 | 0.15 [0.04–0.60] | 3/ 108 | 27.9 | 0.14 [0.05–0.44] | |||
| Unknown | 19/ 126 | 151.2 | 1.21 [0.76–1.91] | 20/ 126 | 159.1 | 0.80 [0.52–1.25] | |||
| Time of ART initiation | |||||||||
| Before free ART | 89/ 1509 | 59.0 | 0.38 [0.30–0.47] | 196/ 1509 | 129.9 | 0.58 [0.49–0.68] | |||
| After free ART ref | 458/ 2929 | 156.4 | 1 | 659/ 2929 | 225.0 | 1 | |||
|
Time since starting ART (months) |
|||||||||
| 0 – 2 ref | 266/ 661 | 402.3 | 1 | 386/ 661 | 583.7 | 1 | |||
| 3 – 5 | 130/ 810 | 160.4 | 0.40 [0.32–0.49] | 196/ 810 | 241.9 | 0.41 [0.35–0.49] | |||
| 6 – 11 | 83/ 1183 | 70.2 | 0.17 [0.14–0.22] | 129/ 1183 | 109.0 | 0.19 [0.15–0.23] | |||
| 12–17 | 44/ 736 | 59.8 | 0.15 [0.11–0.21] | 86/ 736 | 116.9 | 0.20 [0.16–0.25] | |||
Health Organisation; PTB, Pulmonary Tuberculosis; PMTCT, Prevention of Mother to Child Transmission; CI, Confidence interval D/ PYO = deaths or art drop outs per person years of follow up; Rates are per 1000 person years of follow-up ref Reference group for rate ratios;
Adjusted using Poisson regression for age, occupation, clinical stage and time since starting ART; adjusted rate ratios are only shown for the variables found to be associated with the gender difference in mortality
Median follow-up time was 9.0 months for women (range 0.03 – 60.5 months) and 8.4 months for men (range 0.03 – 63.1 months). A total of 341 deaths were recorded in 2171 PYO among men, resulting in a mortality rate of 157.1/ 1000 PYO [95% CI, 141.3–174.7/ 1000 PYO]; 206 deaths were recorded in 2268 PYO among women, resulting in a mortality rate of 90.8/ 1000 PYO [95% CI: 79.3–104.1]. ART drop-out rates were 228.5/ 1000 PYO [95% CI: 209.2–249.5] among men and 158.3/ 1000 PYO [95% CI: 142.8–175.6] among women. The cumulative probability of survival at 3, 6, 12 and 18 months after ART initiation was 90%, 87%, 83% and 80% for men and 94%, 92%, 90% 88% for women. Figure 1 shows the cumulative survival probabilities for men and women, illustrating the significantly lower survival among men (p<0.001). Mortality rate ratios, comparing men with women, were 1.78 [95% CI: 1.39–2.29], 1.37 [95% CI: 0.97–1.94], 1.99 [95% CI: 1.26–3.14] and 1.84 [95% CI; 0.99–3.39] at 0–2 months, 3–5 months, 6–11 months and 12–17 months after ART initiation. Rate ratios for ART drop-out, comparing men with women within the respective intervals, were 1.58 [95% CI: 1.29–1.94], 1.14 [95% CI: 0.86–1.51], 1.50 [95% CI: 1.06–2.13] and 1.53 [95% CI: 0.99–2.35]. Table 2 shows mortality and ART drop-out rates according to different background variables. Mortality and ART dropout were much higher among patients in stage 4 than stage 3 (RR 1.98 [95% CI: 1.67–2.37] and RR 1.58 [95% CI: 1.37–1.83] respectively). Similarly, Kaposi's sarcoma was associated with over a 2 fold increase in mortality (RR 2.29 [95% CI: 1.7–2.96] and ART drop out (RR 2.07 [95% CI: 1.67–2.56]). Mortality and ART dropout were lower among patients from private sector facilities compared to public sector facilities (RR 0.15 [95% CI: 0.04–0.60] and RR 0.14 [95% CI: 0.05–0.44] respectively) although the private sector was a very small group (n=125). Similarly mortality and ART drop-out were lower among patients who had initiated therapy before the roll-out of free ART (RR 0.38 [95% CI: 0.30–0.47] and RR 0.58 [95% CI: 0.49–0.68] respectively). Mortality and ART drop-out were similar across different age bands and also among the different occupational groups (apart from army personnel who had a lower ART drop-out (RR 0.70 [95% CI: 0.56–0.88]).
Figure 1.
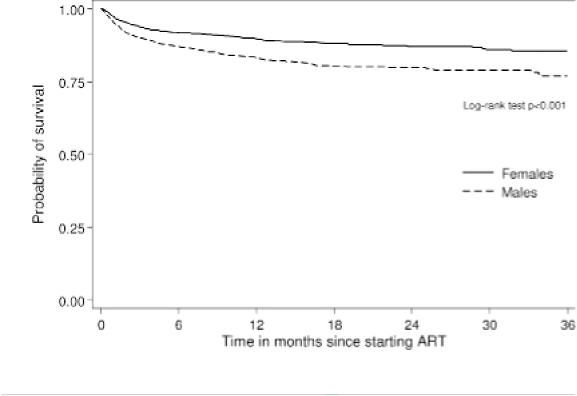
Cumulative survival probabilities by gender
Adjusting for different background characteristics showed that increased mortality among men was partly explained by age, clinical stage and occupation. However, adjusted mortality and ART drop-out rates among men were still approaching nearly two times that of women (RR 1.90 [95% CI: 1.57–2.29] and RR 1.66 [95% CI: 1.43–1.92] respectively) (Table 2). Adjusted mortality rate ratios, comparing men with women, were RR 1.85 [95% CI: 1.42–2.40], RR 1.44 [95% CI: 1.00–2.06], RR 2.11 [95%CI: 1.33–3.34] and RR 1.94 [95% CI: 1.04–3.60] at 0–2 months, 3–5 months, 6–11 months and 12–17 months after ART initiation. There was no statistical evidence tha these rate ratios differed across the different time intervals (p=0.50).
Discussion
Given the conflicting reports about gender differences in mortality of patients on ART in Africa [13–16], this study aimed to determine whether a difference exists and if so possible reasons for it. Whereas previous studies have considered this gender difference among individuals on ART in the general population, this study focussed on individuals on ART from specific occupational groups.
Among healthcare workers, teachers, army and police personnel on ART in Malawi, men were found to experience higher mortality rates and higher ART drop out rates than women after adjusting for age at ART initiation, WHO clinical stage and occupation. By considering these two outcomes separately, we can assume that if all defaulters have actually died then men have nearly 1.7 times higher mortality than women, whereas if all defaulters have silently transferred to another facility and are actually alive on ART elsewhere, then men have 1.9 times higher mortality than women.
There are several possible explanations for higher male mortality. First, increased mortality among male ART patients in our study may have been due or partly due to poorer adherence to therapy compared with women. Increased mortality among male ART patients in Africa has previously been reported, including two reports from Malawi14,15. In one of these reports, the authors suggested that this may be due to men having poorer compliance to therapy, as indicated by their higher loss to follow-up (18.6% versus 13.0% for women)15. However, using loss to follow-up as a measure of adherence to therapy is actually misleading. Loss to follow-up is a measure of clinic retention and does not necessarily reflect adherence to treatment.
For instance, a patient lost to follow-up (i.e. not retained at the clinic) might have transferred to another site and might be taking his/her drugs perfectly regularly.
Another patient presenting perfectly at each appointment might have very poor adherence in terms of taking his/her pills.
Secondly, increased male mortality may be due to men starting ART at more advanced stages of AIDS than women. We controlled for level of disease progression using WHO clinical stage but it is likely that there was considerabl heterogeneity of immunological stage within the clinical stages. Increased mortality in men may therefore have been due to residual confounding from more advanced disease at the time of treatment initiation. However, one finding in our study suggests that this may not be the case. Given that advanced immunodeficiency is known to be among the most important risk factors for early mortality in patients on ART,3,6,13,15,27,29 one might hypothesise that if men are experiencing a higher mortality than women due to delayed ART initiation, gender differences in early mortality may be much greater than differences in later mortality. In fact, across different time intervals, mortality rate ratios comparing men and women were not statistically different. It must however be highlighted that a comparison of death rates between men and women beyond 6 months in this study is difficult due to relatively few deaths occurring beyond this time and therefore lack of statistical power to make a comparison.
Nevertheless, delayed ART initiation among men is still an issue that probably warrants further examination. Prevention of mother to child transmission (PMTCT) programmes have been proposed as an explanation for why some women present for ART earlier than men. However, only one woman was recorded as starting ART for PMTCT in this current study, and we do not think that this explains these particular findings. Still, it is likely that women generally access health services more frequently than men because of their children (e.g. for child vaccinations, accessing treatment for sick children) and this may partly underpin why they generally access ART earlier than men. Delayed ART initiation among men may also be explained by psychosocial factors such as stigma, pride and fear. A previous study from Malawi, also reporting a delay in ART initiation among men, suggested that this may be due to male dignity and preservation of masculinity, a feature of Malawian culture, supported by anecdotal observation and qualitative study15.
Third, there may be a biological sex difference in the pharmacology and drug response to ART. In this study, all patients starting ART were started on a fixed dose combination of stavudine, lamivudine and nevirapine. In a previous study investigating the pharmacokinetic and pharmacodynamic sex differences of specific antiretroviral drugs including lamivudine, women had 1.6 fold higher plasma concentrations of lamivudine triphosphate than men and achieved a viral load reduction twice as fast as men [30]. Although these data were based on the evaluation of only 33 patients, other studies have also reported that women reach higher plasma concentrations with a number of antiretroviral drugs compared to men31,32. It is therefore possible that the gender difference in mortality found in this study may be related to higher drug levels that are achieved in women.
Finally, there may be other factors involved. We have little information in Malawi or in other resource-poor settings about the comparative rates of cigarette smoking or alcohol consumption between men and women on ART. Yet these may affect ART outcomes. There are suggestions that smoking may contribute to increased morbidity and mortality in HIV-infected populations33 and that alcohol may be strongly associated with non-adherence to ART and worse virological outcomes34,35. The finding in this study, that HIV-infected women on ART tend to live longer than HIVinfected men on ART, must also be considered in light of the fact that women also live longer than men in the general population. In Malawi, life expectancy for men is 49 years versus 51 years for women21. Although small, this intrinsic sex related biological difference may account for at least some of the gender difference seen in mortality.
The study has several strengths. First, the databases were constructed from national surveys using data from a rigorously supervised monitoring and evaluation system and should therefore be nationally representative of all healthcare workers, teachers, police and army personnel on ART in Malawi. Second, by restricting the study to patients in specific occupations, important social factors including healthcare seeking behaviour and socio-economic status should have been controlled for. Third, gender differences in mortality and ART drop out were very similar suggesting that death ascertainment was non differential between men and women, and finally, the defaulter rate in this analysis is lower than the national average, because follow-up ascertainment was likely better in these occupational cohorts than for the total national cohort36.
There were, however, several limitations of the study. CD4 count and viral load measurements were not performed at the start and during the course of ART which may otherwise have offered an explanation for the gender difference in mortality and could have been used to give a clinical indication of adherence to therapy. There was no available data for body mass index, adherence to treatment and changes in ART regimen (the analysis being based on an ‘intention to treat’ analysis) so residual confounding from any of these variables could have meant that the study under- or overestimated the gender difference in mortality. In addition, data on death were noncause specific so some deaths could have been non-HIV related. The study included patients who had transferred out; this could potentially have led to a duplication of observation time which would have led to an underestimation of mortality rates.
However since transfer out rates were very small and very similar for men and women, the effect of this is likely to be small. Finally, our findings can only be generalised to healthcare workers, teachers, army and police personnel in Malawi rather than the general population.
Conclusion and recommendations
Among healthcare workers, teachers, police and army personnel in Malawi, HIVinfected men on ART experience significantly higher mortality than women. This gender difference may be due to men starting ART at a later clinical stage than women (although there is some evidence to doubt this); it may be due to poorer compliance to therapy among men than women; it may be biological or due to other factors such as cigarette smoking and alcohol.
To improve early access one strategy may be to identify patients with advanced clinical disease and implement a system to fast track them onto treatment. It has been suggested that identification of these ‘high risk’ patients could also be facilitated by other disease programmes such as tuberculosis treatment programmes27. With pulmonary tuberculosis (PTB) one of the most common manifestations of WHO stage 3 disease in Malawi (32% of patients in this study who started ART at WHO stage 3 presented with PTB), TB treatment programmes are potentially a key point of access to ART services. In Malawi the national TB programme works in collaboration with the ART programme to identify and refer these patients appropriately. Efforts to strengthen this referral process may facilitate earlier access to treatment. In addition, using qualitative approaches to ascertain why some men delay ART initiation would enable appropriate strategies to be deployed to try and encourage earlier access.
Secondly, the issue of adherence to therapy possibly being poorer among men also needs further study. Thirdly, if some of the gender difference in mortality is due to a gender difference in the pharmacology and drug response to ART, this needs to be verified in future studies first before the practical implications of altering drug dosage according to sex are given any due consideration. Fourth, the effects of cigarette smoking and alcohol and other determinants in relation to gender should be investigated. Finally, it is believed that mortality during screening before people are started on ART in Malawi is very high; whether there are differences in survival between men and women at this stage poses yet another question.
| Time in months | 0 | 6 | 12 | 18 | 24 | 30 | 36 |
| Number at risk | |||||||
| Males | 2346 | 1451 | 911 | 581 | 341 | 217 | 121 |
| Females | 2324 | 1414 | 864 | 512 | 288 | 187 | 97 |
| Deaths | |||||||
| Males | - | 255 | 53 | 22 | 5 | 3 | 3 |
| Females | - | 159 | 24 | 13 | 2 | 3 | 1 |
Abbreviations: ART, Antiretroviral therapy
Acknowledgments
The data collected for the healthcare workers, teachers, army and police operations studies were supported through an anonymous donor and linked to routine supervisor visits carried out by the Department of HIV and AIDS in the Malawi Ministry of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authors contributions
Anthony Harries, Erik Schouten and Andreas Jahn were involved in the ART monitoring and evaluation over the period in this study and participated in data collection. Anthony Harries, Erik Schouten, Kelita Kamoto, Sam Phiri and Andreas Jahn designed the study. Katie Taylor-Smith, Hannock Tweya, Anne Ben-Smith and Andreas Jahn analysed and interpreted the data. Katie Taylor-Smith, Hanock Tweya, Anthony Harries and Andreas Jahn wrote the first draft. The second and subsequent drafts were reviewed and critically appraised by all co-authors. All authors have seen and approved the final draft for submission.
References
- 1.Detels R, Munoz A, McFarlane G, et al. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. Multicenter AIDS Cohort Study Investigators. Jama. 1998;280:1497–1503. doi: 10.1001/jama.280.17.1497. [DOI] [PubMed] [Google Scholar]
- 2.CASCADE Collaboration, author. Survival after introduction of HAART in people with known duration of HIV-1 infection. The CASCADE Collaboration. Concerted Action on SeroConversion to AIDS and Death in Europe. Lancet. 2000;355:1158–1159. [PubMed] [Google Scholar]
- 3.Weidle PJ, Malamba S, Mwebaze R, et al. Assessment of a pilot antiretroviral drug therapy programme in Uganda: patients' response, survival, and drug resistance. Lancet. 2002;360:34–40. doi: 10.1016/S0140-6736(02)09330-3. [DOI] [PubMed] [Google Scholar]
- 4.Wester CW, Kim S, Bussmann H, et al. Initial response to highly active antiretroviral therapy in HIV-1C-infected adults in a public sector treatment program in Botswana. J Acquir Immune Defic Syndr. 2005;40:336–343. doi: 10.1097/01.qai.0000159668.80207.5b. [DOI] [PubMed] [Google Scholar]
- 5.Coetzee D, Hildebrand K, Boulle A, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. Aids. 2004;18:887–895. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 6.Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. Jama. 2006;296:782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 7.Lawn SD, Myer L, Harling G, Orrell C, Bekker LG, Wood R. Determinants of mortality and nondeath losses from an antiretroviral treatment service in South Africa: implications for program evaluation. Clin Infect Dis. 2006;43:770–776. doi: 10.1086/507095. [DOI] [PubMed] [Google Scholar]
- 8.Jahn A, Floyd S, Crampin AC, et al. Population-level effect of HIV on adult mortality and early evidence of reversal after introduction of antiretroviral therapy in Malawi. Lancet. 2008;371:1603–1611. doi: 10.1016/S0140-6736(08)60693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez-Hoyos S, del Amo J, Muga R, et al. Effectiveness of highly active antiretroviral therapy in Spanish cohorts of HIV seroconverters: differences by transmission category. Aids. 2003;17:353–359. doi: 10.1097/00002030-200302140-00009. [DOI] [PubMed] [Google Scholar]
- 10.Hall HI, McDavid K, Ling Q, Sloggett A. Determinants of progression to AIDS or death after HIV diagnosis, United States, 1996 to 2001. Ann Epidemiol. 2006;16:824–833. doi: 10.1016/j.annepidem.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Moore AL, Kirk O, Johnson AM, et al. Virologic, immunologic, and clinical response to highly active antiretroviral therapy: the gender issue revisited. J Acquir Immune Defic Syndr. 2003;32:452–461. doi: 10.1097/00126334-200304010-00017. [DOI] [PubMed] [Google Scholar]
- 12.d'Arminio A, Sabin CA, Phillips AN, et al. Cardio and cerebrovascular events in HIV-infected persons. Aids. 2004;18:1811–1817. doi: 10.1097/00002030-200409030-00010. [DOI] [PubMed] [Google Scholar]
- 13.Calmy A, Pinoges L, Szumilin E, Zachariah R, Ford N, Ferradini L. Generic fixed-dose combination antiretroviral treatment in resource-poor settings: multicentric observational cohort. Aids. 2006;20:1163–1169. doi: 10.1097/01.aids.0000226957.79847.d6. [DOI] [PubMed] [Google Scholar]
- 14.Ferradini L, Jeannin A, Pinoges L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335–1342. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 15.Chen SC, Yu JK, Harries AD, et al. Increased mortality of male adults with AIDS related to poor compliance to antiretroviral therapy in Malawi. Trop Med Int Health. 2008;13:513–519. doi: 10.1111/j.1365-3156.2008.02029.x. [DOI] [PubMed] [Google Scholar]
- 16.Etard JF, Ndiaye I, Thierry-Mieg M, et al. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. Aids. 2006;20:1181–1189. doi: 10.1097/01.aids.0000226959.87471.01. [DOI] [PubMed] [Google Scholar]
- 17.Makombe SD, Jahn A, Tweya H, et al. A national survey of the impact of rapid scale-up of antiretroviral therapy on health-care workers in Malawi: effects on human resources and survival. Bull World Health Organ. 2007;85:851–857. doi: 10.2471/BLT.07.041434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makombe SD, Jahn A, Tweya H, et al. A national survey of teachers on antiretroviral therapy in Malawi: access, retention in therapy and survival. PLoS ONE. 2007;2:e620. doi: 10.1371/journal.pone.0000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banda AC, Makombe SD, Jahn A, et al. Antiretroviral therapy in the Malawi defence force: access, treatment outcomes and impact on mortality. PLoS ONE. 2008;3:e1445. doi: 10.1371/journal.pone.0001445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makombe S, Jahn A, Tweya H, et al. The impact of national antiretroviral treatment programme on public services in Malawi: access and survival on therapy among the police force. Malawi Medical Journal. 2008;20:23–27. doi: 10.4314/mmj.v20i1.10951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization, author. WHO Statistical Information System. 2008. http://www.who.int/countries/mwi/en/
- 22.National AIDS Commission, author. HIV and syphilis serosurvey and national HIV prevalence and AIDS estimates. Lilongwe: Malawi Ministry of Health; 2007. [Google Scholar]
- 23.Libamba E, Makombe S, Harries AD, et al. Scaling up antiretroviral therapy in Africa: learning from tuberculosis control programmes - the case of Malawi. Int J Tuberc Lung Dis. 2005;9:1062–1071. [PubMed] [Google Scholar]
- 24.Malawi Ministry of Health, author. Antiretroviral therapy in the public and private sectors in Malawi: results up to 30th September, 2008. Lilongwe: HIV Unit; 2008. [Google Scholar]
- 25.Malawi Ministry of Health, author. Treatment of AIDS: Guidelines for the use of antiretroviral therapy in Malawi. Second Edition. Lilongwe: HIV Unit; 2006. [Google Scholar]
- 26.Libamba E, Makombe S, Mhango E, et al. Supervision, monitoring and evaluation of nationwide scale-up of antiretroviral therapy in Malawi. Bull World Health Organ. 2006;84:320–326. doi: 10.2471/blt.05.023952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. Aids. 2005;19:2141–2148. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 28.Zachariah R, Fitzgerald M, Massaquoi M, et al. Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. Aids. 2006;20:2355–2360. doi: 10.1097/QAD.0b013e32801086b0. [DOI] [PubMed] [Google Scholar]
- 29.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 30.Anderson PL, Kakuda TN, Kawle S, Fletcher CV. Antiviral dynamics and sex differences of zidovudine and lamivudine triphosphate concentrations in HIV-infected individuals. Aids. 2003;17:2159–2168. doi: 10.1097/00002030-200310170-00003. [DOI] [PubMed] [Google Scholar]
- 31.Cressey TR, Lallemant M. Pharmacogenetics of antiretroviral drugs for the treatment of HIV-infected patients: an update. Infect Genet Evol. 2007;7:333–342. doi: 10.1016/j.meegid.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Umeh OC, Currier JS. Sex differences in pharmacokinetics and toxicity of antiretroviral therapy. Expert Opin Drug Metab Toxicol. 2006;2:273–283. doi: 10.1517/17425255.2.2.273. [DOI] [PubMed] [Google Scholar]
- 33.Marshall MM, McCormack MC, Kirk GD. Effect of cigarette smoking on HIV acquisition, progression, and mortality. AIDS Education and Prevention. 2009;21:28–39. doi: 10.1521/aeap.2009.21.3_supp.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: review and metaanalysis. J Acquir Immune Defic Syndr. 2009 Aug 7; doi: 10.1097/QAI.0b013e3181b18b6e. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miguez-Burbano MJ, Lewis JE, Fishman J, Asthana D, Malow RM. The influence of different types of alcoholic beverages on disrupting highly active antiretroviral treatment (HAART) outcome. Alcohol. 2009;44:366–371. doi: 10.1093/alcalc/agp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malawi Ministry of Health, author. ART in the public and private sectors in Malawi: results up to 31st December, 2007. Lilongwe: HIV Unit; 2008. [Google Scholar]


