Abstract
Objective:
To assess the cognitive phenotype of glucocerebrosidase (GBA) mutation carriers with early-onset Parkinson disease (PD).
Methods:
We administered a neuropsychological battery and the University of Pennsylvania Smell Identification Test (UPSIT) to participants in the CORE-PD study who were tested for mutations in PARKIN, LRRK2, and GBA. Participants included 33 GBA mutation carriers and 60 noncarriers of any genetic mutation. Primary analyses were performed on 26 GBA heterozygous mutation carriers without additional mutations and 39 age- and PD duration–matched noncarriers. Five cognitive domains, psychomotor speed, attention, memory, visuospatial function, and executive function, were created from transformed z scores of individual neuropsychological tests. Clinical diagnoses (normal, mild cognitive impairment [MCI], dementia) were assigned blind to genotype based on neuropsychological performance and functional impairment as assessed by the Clinical Dementia Rating (CDR) score. The association between GBA mutation status and neuropsychological performance, CDR, and clinical diagnoses was assessed.
Results:
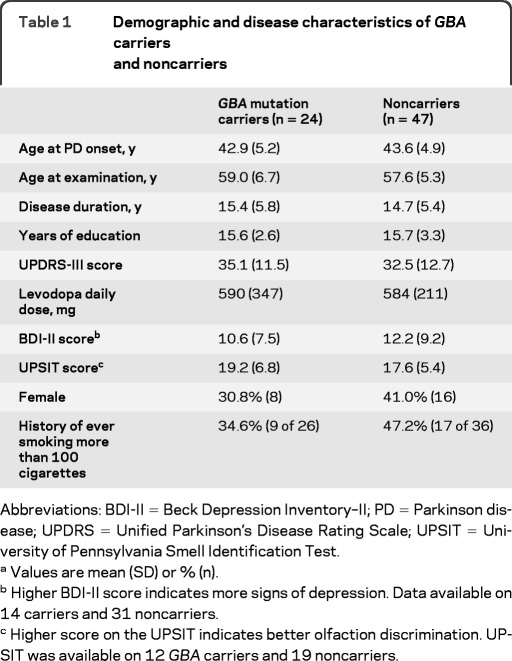
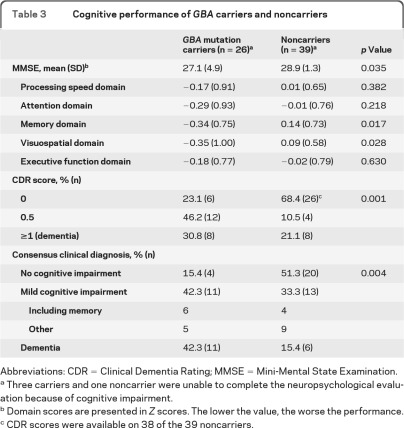
Demographics, UPSIT, and Unified Parkinson's Disease Rating Scale–III performance did not differ between GBA carriers and noncarriers. GBA mutation carriers performed more poorly than noncarriers on the Mini-Mental State Examination (p = 0.035), and on the memory (p = 0.017) and visuospatial (p = 0.028) domains. The most prominent differences were observed in nonverbal memory performance (p < 0.001). Carriers were more likely to receive scores of 0.5 or higher on the CDR (p < 0.001), and a clinical diagnosis of either MCI or dementia (p = 0.004).
Conclusion:
GBA mutation status may be an independent risk factor for cognitive impairment in patients with PD.
Cognitive impairment is one of the most disabling nonmotor complications of Parkinson disease (PD). Older age, longer disease duration, and severity of extrapyramidal signs are the most important risk factors for cognitive impairment in the setting of PD.1–4 Recently, mutations in the glucocerebrosidase (GBA) gene were identified as risk factors for PD,5 affecting up to 6% of all early-onset PD cases in the United States.6 Two independent autopsy studies found that GBA mutations were associated with cortical Lewy bodies, suggesting that Lewy body development may be more extensive in GBA carriers, and might be associated with cognitive impairment.7,8 Among 699 participants in the Consortium on Risk for Early Onset Parkinson's Disease (CORE-PD)9 with age at onset (AAO) <51 years, carriers of GBA mutations (N370S or L444P, n = 37) self-reported cognitive impairment more frequently than noncarriers. While data from the Mini-Mental State Examination (MMSE)10 did not confirm this difference,9 in another study, GBA mutation carriers performed worse than noncarriers on the Montreal Cognitive Assessment (MoCA).11 In addition, a mouse model of Gaucher disease was shown to exhibit memory deficits as well as progressive accumulation of α-synuclein/ubiquitin aggregates in hippocampal neurons.12
In the current study, our primary goal was to examine the neuropsychological profile of GBA carriers compared to noncarriers. To further characterize the GBA phenotype, we assessed extrapyramidal features, olfaction, and depression.
METHODS
Participants.
A total of 147 individuals with early-onset PD (33 GBA carriers and 114 noncarriers) who participated in Part II of the CORE-PD study were included. Details of the CORE-PD study have been described.13 In brief, patients with PD, diagnosed by movement disorders specialists, were recruited for Part I of CORE-PD from 16 sites based on AAO of PD <51 years and a score on the MMSE >23. (The inclusion of individuals with MMSE >23 was incorporated to ensure that a reliable history could be obtained from each subject. There was no MMSE exclusion criterion for the follow-up, Part II examination.) A blood sample for DNA was sent to the NINDS Human Genetics Resource Center DNA and Cell Line Repository (http://ccr.coriell.org). Participants were screened for mutations in SNCA, PARKIN, PINK-1, DJ-1, LRRK2, and GBA (N370S and L444P mutations).6 Given the higher frequency of GBA mutations among Ashkenazi Jews, participants who self-reported Ashkenazi Jewish ancestry were further screened for an additional 6 common GBA mutations (V394L, D409G, A456P, R496H, 84GG, and exon 2 IVS2 + 1) by direct sequencing. A total of 147 participants completed the Part II evaluation. The present analysis was restricted to 33 carriers of GBA mutations and 60 individuals who did not have any mutations in the other genes tested.14 The GBA mutation carriers (n = 33) included 7 heterozygous L444P carriers, 16 heterozygous N370S carriers, 1 N370S homozygote, 2 84GG carriers, and 1 R496H carrier. Six individuals were heterozygous for GBA mutations and carried mutations in other PD-related genes (3 with both GBA and PARKIN mutations, 3 with both GBA and LRRK2 G2019S mutations). The analyses presented here focused on the 26 heterozygous GBA mutation carriers who did not have PARKIN or LRRK2 mutations. We also conducted sensitivity analyses including all 33 GBA carriers.
To ensure that the noncarriers were frequency matched to the GBA carriers in age and PD duration at the time of the examination, we included only noncarriers who were 47 years or older with PD duration between 7 and 25 years, similar to GBA carriers. This resulted in the exclusion of 21 noncarriers. The final number of included participants was 72 (33 GBA carriers, 39 noncarriers).
Clinical evaluation.
Clinical evaluation in Part II of CORE-PD included a neurologic examination performed by a research physician, a videotaped assessment of the Unified Parkinson's Disease Rating Scale (UPDRS) evaluated by a movement disorders specialist (E.D.L.), a neuropsychological battery, and a psychiatric evaluation including the Beck Depression Inventory–II (BDI-II).15 The University of Pennsylvania Smell Identification Test (UPSIT, Sensonics, Inc., Haddonfield, NJ)16 was added in 2008 and was available for 31 participants. A Clinical Dementia Rating (CDR)17 score was assigned to each participant by a research physician (L.R.) who administered the neurologic and neuropsychological testing. A consensus panel, including the research physician (L.R.), a neurologist (K.M.), and a neuropsychologist (E.C.), assigned a clinical consensus diagnosis to each participant based on medical history, neurologic examination, neuropsychological performance, and functional impairment. Dementia diagnosis required impairment on neuropsychological evaluation in at least 2 of the following domains: memory, language, executive function, and visuospatial processing, as well as functional impairment as reflected by a CDR score greater than 0. Mild cognitive impairment (MCI) was diagnosed using Petersen's criteria, i.e., impairment in at least 1 neuropsychological domain or low scores in more than 1 domain, with no significant functional impairment.18 None of the researchers was aware of the participants' mutation status at the time of the evaluation or consensus diagnosis.
Standard protocol approvals, registrations, and patient consents.
Institutional review boards at all participating sites approved the protocols and consent procedures. Written informed consent was obtained from all participants in the study.
Neuropsychological evaluation.
Details of the neuropsychological battery have been previously described14 and are summarized in table e-1 on the Neurology® Web site at www.neurology.org. The battery was designed so that it was time-limited (i.e., with an administration time of approximately 30–45 minutes), could be administered in participants' homes, could be administered in English and Spanish, and could be successfully completed by patients with motor impairment. A second MMSE was performed on all participants at the time of the neuropsychological battery but was not included in the domain scores. Five cognitive domains were created based on previous research assessing predictors of cognitive decline in PD (table e-1).14,19–20
In a separate analysis, we further reclassified the battery into 3 domains (table e-2): executive function (including executive function, processing speed, and attention), memory (including memory tests as in table e-1 and California Verbal Learning Test-II recognition errors), and visuospatial domain (unchanged).
Statistical analysis.
Demographic data, disease characteristics, MMSE, neuropsychological test performance, and UPSIT performance of GBA carriers and noncarriers were compared using χ2, Fisher exact, and Student t tests, as appropriate. Individual neuropsychological test scores for all participants were transformed to create Z scores using means and standard deviations of all participants. Composite scores for each domain were computed by averaging the mean Z scores from the individual tests comprising each domain (see table e-1). Performance on neuropsychological testing was compared between GBA mutation carriers and noncarriers on individual tests and cognitive domains.
CDR scores were categorized as 0 (normal), 0.5, or 1 or higher (dementia) and compared between GBA carriers and noncarriers. The association between clinical diagnosis of cognitive impairment (which was made by consensus meeting based on neuropsychological performance and CDR), either MCI or dementia (dependent variable), and GBA mutation status (independent variable) was assessed in logistic regression models. The association was first assessed in a univariate model, and then in a multivariate model, adjusting for age in years, AAO of PD, gender, UPDRS-III score, motor phenotype (tremor dominant vs postural instability gait difficulty),21 and years of education.
Finally, sensitivity analyses were performed. We repeated the analyses, including all GBA mutation carriers (n = 33), and all noncarriers (n = 60), including those (n = 21) who were excluded for age and disease duration frequency matching.
RESULTS
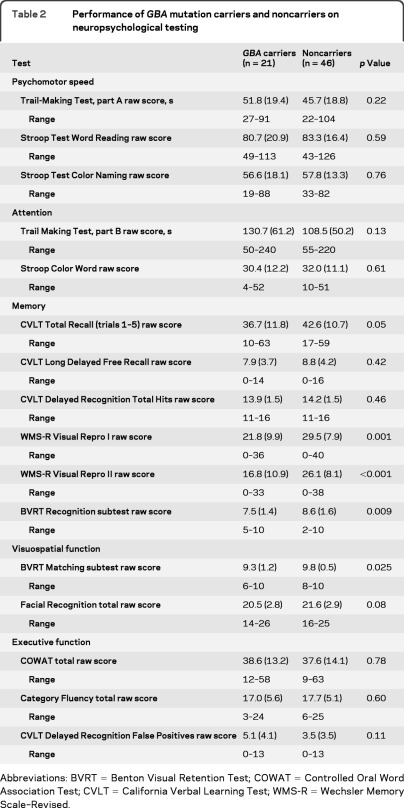
Demographic and disease characteristics of carriers and noncarriers of GBA mutations are presented in table 1. Age (frequency matched), gender, education, AAO, and BDI-II scores did not differ between carriers and noncarriers. UPSIT performance was in the severe microsmia range (score of 25 or less out of 40)16 for 29 of 31 participants with available UPSIT data (table 1). UPSIT performance was not associated with GBA mutation status, neuropsychological performance, or clinical diagnosis of cognitive impairment. Raw scores on individual neuropsychological tests are reported in table 2. Three GBA carriers and 1 noncarrier (whose initial MMSE screen was >23) were too cognitively impaired during the Part II evaluation to complete the full neuropsychological evaluation and therefore domain scores could not be computed. When carriers' performance was compared to noncarriers', carriers scored significantly lower on nonverbal memory tests (Visual Reproduction I, Visual Reproduction II, Benton Visual Retention Test–Recognition; table 2).
Table 1.
Demographic and disease characteristics of GBA carriers and noncarriers
Abbreviations: BDI-II = Beck Depression Inventory–II; PD = Parkinson disease; UPDRS = Unified Parkinson's Disease Rating Scale; UPSIT = University of Pennsylvania Smell Identification Test.
Values are mean (SD) or % (n).
Higher BDI-II score indicates more signs of depression. Data available on 14 carriers and 31 noncarriers.
Higher score on the UPSIT indicates better olfaction discrimination. UPSIT was available on 12 GBA carriers and 19 noncarriers.
Table 2.
Performance of GBA mutation carriers and noncarriers on neuropsychological testing
Abbreviations: BVRT = Benton Visual Retention Test; COWAT = Controlled Oral Word Association Test; CVLT = California Verbal Learning Test; WMS-R = Wechsler Memory Scale–Revised.
MMSE scores, cognitive domain scores, CDR scores, and the clinical consensus diagnoses of cognitive impairment are reported in table 3. GBA carriers' MMSE performance was significantly lower than that of noncarriers. Mean scores in all 5 cognitive domains were lower in the GBA carrier group, reaching significance for the memory (p = 0.017) and visuospatial (p = 0.028) domains. These findings were unchanged when the neuropsychological battery was reclassified into 3 domains (memory, p = 0.015; executive function, p = 0.394; visuospatial, p = 0.028). GBA carriers were more likely to receive higher CDR scores than noncarriers (p < 0.001).
Table 3.
Cognitive performance of GBA carriers and noncarriers
Abbreviations: CDR = Clinical Dementia Rating; MMSE = Mini-Mental State Examination.
Three carriers and one noncarrier were unable to complete the neuropsychological evaluation because of cognitive impairment.
Domain scores are presented in Z scores. The lower the value, the worse the performance.
CDR scores were available on 38 of the 39 noncarriers.
Among participants who received a consensus clinical diagnosis of dementia (11 GBA carriers, 6 noncarriers), GBA mutation carriers did not present a distinctive pattern of impairment in specific cognitive domains. All participants with dementia had both memory and executive impairment. GBA mutation carriers were more likely to receive a clinical consensus diagnosis of MCI or dementia than noncarriers (p = 0.004, table 3). In a univariate logistic model, GBA mutation status was associated with clinical diagnosis of cognitive impairment (either MCI or dementia combined) (odds ratio [OR] = 5.8, 95% confidence interval [CI] 1.7–19.9, p = 0.005). The association persisted when adjusted for age, gender, disease duration, UPDRS-III, motor phenotype, and education (OR = 6.2, 95% CI 1.3–29.0, p = 0.021).
Sensitivity analyses including all GBA carriers (n = 33) and all noncarriers (n = 60) revealed that GBA carriers were more impaired on memory (p = 0.046) and attention (p = 0.007) domains compared to noncarriers, using the 5-domain model. In this larger sample, significant differences between GBA carriers vs noncarriers in CDR scores (p < 0.001) and in the association between GBA mutation status and cognitive impairment (p = 0.001) were similar to those obtained in the primary analyses.
DISCUSSION
While longitudinal follow-up of patients with PD suggests that cognitive impairment occurs in 83% of patients with PD followed up to 20 years,22 studies have shown marked heterogeneity in the profile of cognitive impairment and in the time from onset of motor symptoms to the development of cognitive impairment.23–25Previous studies have shown that genetic risk factors, including variations in microtubule-associated protein tau (MAPT),26 α-synuclein (SNCA),26 and catechol-O-methyl-transferase (COMT)27,28may explain some of the heterogeneity in cognitive performance in PD. We and others have shown that GBA mutations are associated with cortical Lewy body pathology. GBA mutation status was also previously associated with dementia using DSM-IV criteria, after adjusting for covariates, including APOE4 status and Alzheimer disease pathology.7 Our findings of olfactory and cognitive impairment, most notably in nonverbal memory tasks, are all consistent with diffuse cortical neuropathology. The association between GBA mutation status and other nonmotor signs and symptoms of PD (e.g., REM sleep behavior disorder or hallucinations) remains to be investigated.
We may have underestimated differences in cognitive performance. Our study was probably biased toward the null: first, we compared GBA carriers to other affected individuals who by themselves exhibit cognitive impairment (table 2). Second, we included only participants with MMSE >23 on initial evaluation. We cannot conclude whether GBA mutation status is associated with PD dementia only or with dementia with Lewy body as well. Finally, because only selected mutations were genotyped, rather than complete sequencing, we may have inadvertently misclassified other GBA mutation carriers as noncarriers.
While GBA mutation status may explain a portion of the variability in cognitive performance, not all GBA carriers demonstrate cognitive impairment. For example, one of the GBA mutation carriers whose performance on neuropsychological testing was within normal range (table 3) had a motor disease duration of 21 years. Further research is required to understand additional modifiers of cognitive performance, some of which may be protective. Our study was not powered to assess for interactions with MAPT, SNCA, or COMT variants, or to assess for a “dose” effect. It included only a single carrier of 2 GBA mutations, who was excluded from all but the sensitivity analyses. It also was not powered to compare carriers of different GBA mutations, e.g., N370S and R496H vs L444P and 84GG. A previous study has reported that carriers of “severe” GBA mutations (including L444P) may have a higher risk for PD than carriers of “milder” mutations (e.g., N370); however, cognitive impairment was not assessed.29 The neuropsychological battery, which was limited in scope, may have lacked tests that would have identified more specific executive deficits (e.g., Wisconsin Card Sorting Test). Finally, the study design, which includes a single complete neuropsychological evaluation that did not allow us to assess rate of progression, also represents a limitation.
Further research is required to assess whether nonverbal memory may be used as an early marker of cognitive impairment in mutation carriers. GBA mutation carriers may serve as an enriched sample to assess for early cognitive outcomes. Prospective longitudinal studies which include GBA mutation carriers with and without PD will help assess the time and rate of development of cognitive impairment in carriers.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Paul Greene and Linda Winfield for participant referral.
GLOSSARY
- AAO
age at onset
- BDI-II
Beck Depression Inventory–II
- CDR
Clinical Dementia Rating
- CI
confidence interval
- CORE-PD
Consortium on Risk for Early Onset Parkinson's Disease
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- MoCA
Montreal Cognitive Assessment
- OR
odds ratio
- PD
Parkinson disease
- UPDRS
Unified Parkinson's Disease Rating Scale
- UPSIT
University of Pennsylvania Smell Identification Test
Footnotes
Editorial, page 1372
Supplemental data at www.neurology.org
AUTHOR AFFILIATIONS
From the Department of Neurology (R.N.A., E.C., H.M.-S., M.-X.T., L.R., M.O.R., D.R., E.L., S. Fahn, C.W., L.C., S. Frucht, B.F., R.O., K.M.), Taub Institute for Research on Alzheimer's Disease and the Aging Brain (R.N.A., M.-X.T., B.R., M.V., S.K., E.L., L.N.C., K.M.), Gertrude H. Sergievsky Center (E.L., L.C., R.O., K.M.), Department of Pathology and Cell Biology (L.N.C.), and Center for Human Genetics (L.N.C.), College of Physicians and Surgeons, Department of Epidemiology, Mailman School of Public Health (E.L., R.O.), and Department of Psychiatry (K.M.), Columbia University, New York, NY; Department of Neurological Sciences (C.C.), Rush University Medical Center, Chicago, IL; Parkinson's Disease and Movement Disorders Center (A.C., A.S.), Pennsylvania Hospital, Philadelphia; The Institute for Neurodegenerative Disorders (D.J.), New Haven, CT; Struthers Parkinson's Center (M.N.), Park Nicollet Clinic, Golden Valley, MN; The Alan and Barbara Mirken Department of Neurology (S.B.), Beth Israel Medical Center, New York, NY; Department of Neurology (S.B.), Albert Einstein College of Medicine, Bronx, NY; Dr. John T. MacDonald Foundation (W.K.S.), Department of Human Genetics, Miami Institute for Human Genomics, Miller School of Medicine, University of Miami, Miami, FL; Parkinson's Institute (C.T.), Sunnyvale, CA; Marshfield Clinic (S.M.), Department of Neurology, Marshfield, WI; Data Coordinating Center (H.A.) and Division of Epidemiology (R.O.), New York State Psychiatric Institute, New York; Central DuPage Hospital (M.R.), Neurosciences Institute, Movement Disorders Center, Winfield, IL; Department of Neurology (K.N.), NorthShore University Health System, Evanston, IL; Department of Neurology (K.N.), University of Chicago, Pritzker School of Medicine, Chicago, IL; Butler Hospital (J.H.F.), Providence, RI; Department of Neurology (J.H.F.), Alpert Medical School of Brown University, Providence, RI; Department of Neurology (R.P.), College of Medicine, University of Tennessee Health Science Center, Memphis; Morris K. Udall Parkinson's Disease Research Center of Excellence (L.M.), Department of Psychiatry and Behavioral Sciences (L.M.), and Department of Neurology and Neurological Sciences (L.M.), Johns Hopkins University School of Medicine, Baltimore, MD; Medical College of Wisconsin (B.H.), Milwaukee; New York State Department of Health Wadsworth Center (H.P.), Albany, NY; Parkinson's Disease and Movement Disorders Center (E.M.), Albany Medical Center, Albany, NY; and Department of Neurology (S.F.), Emory University, Atlanta, GA.
AUTHOR CONTRIBUTIONS
Dr. Alcalay: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, statistical analysis, obtaining funding. Dr. Caccappolo: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, statistical analysis. H. Mejia-Santana: study concept or design, study supervision. Dr. Tang: analysis or interpretation of data, statistical analysis. Dr. Rosado: study concept or design, analysis or interpretation of data, acquisition of data, study supervision. Dr. Reilly: drafting/revising the manuscript, study concept or design, acquisition of data. D. Ruiz: study concept or design, acquisition of data. B. Ross: analysis or interpretation of data, acquisition of data. Dr. Verbitsky: analysis or interpretation of data, acquisition of data. S. Kisselev: analysis or interpretation of data, acquisition of data. Dr. Louis: drafting/revising the manuscript, acquisition of data. Dr. Comella: drafting/revising the manuscript, acquisition of data, study supervision. Dr. Colcher: drafting/revising the manuscript, acquisition of data. Dr. Jennings: drafting/revising the manuscript, acquisition of data. Dr. Nance: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data. Dr. Bressman: drafting/revising the manuscript, acquisition of data. Dr. Scott: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data, study supervision. Dr. Tanner: drafting/revising the manuscript, acquisition of data. Dr. Mickel: drafting/revising the manuscript, acquisition of data. Dr. Andrews: analysis or interpretation of data, acquisition of data, study supervision. Dr. Waters: drafting/revising the manuscript, acquisition of data. Dr. Fahn: drafting/revising the manuscript, study concept or design, acquisition of data. Dr. Cote: drafting/revising the manuscript, acquisition of data. Dr. Frucht: analysis or interpretation of data, acquisition of data. Dr. Ford: drafting/revising the manuscript, acquisition of data. Dr. Rezak: drafting/revising the manuscript, acquisition of data. Dr. Novak: drafting/revising the manuscript, acquisition of data, study supervision. Dr. Friedman: drafting/revising the manuscript, acquisition of data. Dr. Pfeiffer: drafting/revising the manuscript, acquisition of data. Dr. Marsh: drafting/revising the manuscript, acquisition of data. Dr. Hiner: drafting/revising the manuscript, study concept or design, acquisition of data, study supervision. Dr. Siderowf: drafting/revising the manuscript, acquisition of data. Dr. Payami: drafting/revising the manuscript, acquisition of data. Dr. Molho: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data. Dr. Factor: drafting/revising the manuscript, acquisition of data. Dr. Ottman: drafting/revising the manuscript, study concept or design, analysis or interpretation of data. Dr. Clark: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision, obtaining funding. Dr. Marder: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, obtaining funding.
STUDY FUNDING
This study was funded by NIH NS036630, UL1 RR024156 (K.S.M.), NS050487, NS060113 (L.N.C.), the Parkinson's Disease Foundation (K.S.M., S.F., and L.N.C.), P50 NS039764 (W.K.S.), and NS36960 (H.P.). R.N.A. is a Brookdale Foundation Leadership in Aging Fellow.
DISCLOSURE
Dr. Alcalay receives research support from the NIH (K12 part of UL1 RR024156) and the Brookdale Leadership in Aging Fellowship and received research support from the Michael J. Fox Foundation. Dr. Caccappolo, H. Mejia-Santana, Dr. Tang, Dr. Rosado, Dr. Reilly, D. Ruiz, B. Ross, Dr. Verbitsky, and S. Kisselev report no disclosures. Dr. Louis receives research support from the NIH [NINDS #R01 NS42859 (principal investigator), NINDS #R01 NS39422 (principal investigator), NINDS #R56 NS042859 (principal investigator), NINDS #T32 NS07153-24 (principal investigator), NIA #2P01 AG0027232-16 (principal investigator), and NINDS #R01 NS36630 (coinvestigator)] and the Parkinson's Disease Foundation (principal investigator). Dr. Comella reports no disclosures. Dr. Colcher is on a speaker's bureau for Lundbeck Pharmaceuticals and Teva Pharmaceuticals and has received honoraria from Plan 365, Advanced Health Media, and Healthlogix and Robert Wood Johnson for speaking engagements in 2009. Dr. Jennings reports no disclosures. Dr. Nance has received research funding from Medivation, Santhera, Neurosearch, Juvantia, Schwarcz, Pfizer, Neuraltus, Impax, CHDI, NINDS 5 U10 NS044466-05 (site investigator), NINDS 5 RO1 NS36630 (site investigator), NINDS NS640068-08 (site investigator, Steering Committee), NHGRI/NINDS 501 HG 02449-07 (site investigator), NINDS 1RO1 NS052592-01 (site investigator), NCCAM (site investigator), and 1 RO1 NS060118-01A1 (site investigator); support for Centers of Excellence from National Parkinson Foundation and Huntington Disease Society of America; speaking honoraria from American Academy of Neurology, Huntington Disease Society of America, and Augsburg College; and royalties from Oxford University Press (Juvenile Huntington's Disease, published 2009); and her spouse has served on speaker's bureaus for Genentech and Schering-Plough. Dr. Bressman reports no disclosures. Dr. Scott is a coinventor on patent regarding use of genetic data for assessing risk of developing age-related macular degeneration, licensed to ArcticDx. Dr. Tanner serves on the advisory board of the Michael J. Fox Foundation Scientific Advisory Board and the National Spasmodic Dystonia Association Scientific Advisory Board; has consulted for Impax Pharmaceuticals, Lundbeck Pharmaceuticals, Pacific Health Research Institute (consultant on NIH & Department of Defense funded research), Stanford University (consultant on Muscular Dystrophy Association funded research), and SunHealth Research Institute (consultant on MJFF founded research); and has received research support from the Michael J. Fox Foundation, Brin Foundation, James and Sharron Clark, National Institutes of Health (NIH), Parkinson's Institute and Clinical Center, Parkinson's Disease Foundation, Department of Defense, and Welding Products Manufacturer's Group. Dr. Mickel and Dr. Andrews report no disclosures. Dr. Waters received speaking honorarium from Boehringer and Teva. Dr. Fahn reports receiving support from consulting and advisory board membership with honoraria from Oxford Biomedica (September 2009), Proctor-Goodwin (November 2009), GE Healthcare (November 2009), RJG Foundation (March 2010), IMPAX Pharmaceuticals (May 2010), and Lundbeck (June 2010); he is receiving research support from the Parkinson's Disease Foundation (no salary support); he received a grant from the Smart Family Foundation (no salary support); and received a grant from the US Department of Defense's Telemedicine and Advanced Technology Research Center (TATRC) for the World Parkinson Congress 2010, and a grant from the National Institutes of Health for the World Parkinson Congress 2010. Dr. Fahn received lecture honoraria from Columbia University (July 2009), Sun Pharmaceuticals India (September 2009), World Association of Sleep Medicine (November 2009), American Academy of Neurology (April 2010), and Columbia University (July 2010). Dr. Fahn reports serving as an editor with author honoraria from “Current Neurology and Neurosurgery Report” (annual); and Elsevier for co-author of book Principles and Practice of Movement Disorders. Dr. Cote reports no disclosures. Dr. Frucht has received consultation fees from Lundbeck, Jazz Pharmaceuticals, and Merz. Dr. Ford serves on the physician advisory board for Medtronic, Inc. Dr. Rezak is on the speaker bureau of Teva, Medtronic, Novartis, Boehringer Ingelheim, and Galxo. Dr. Novak reports no disclosures. Dr. Friedman has received speaking honorarium from Teva, Boehringer-Ingelheim, and GlaxoSmithKline; received research support from Epivax, Teva, Cephalon, EMD Serono, Acadia, the Michael J. Fox Foundation, and the National Institute of Health; has received consultation fee from Teva, EMD Serono, Acadia, United Biosource, and Clintara; and has received book royalties from Demos Press. Dr. Pfeiffer has received royalties for book editing from CRC Press (Taylor & Francis) and Humana Press; has received speaking honorarium from Boehringer-Ingelheim, Novartis, and Teva; has received consultation honoraria from Solvay, Theravance, Genactis, and Schlesinger Associates; has research support from Novartis, Boehringer-Ingelheim, UCB/Schwarz, and Santhera; received legal consulting fees from Spriggs & Hollingsworth, Davis Graham & Stubbs, and Tucker Ellis & West; and is the journal editor of Parkinsonism and Related Disorders (Elsevier). Dr. Marsh served on the advisory board of the National Parkinson Foundation and the American Parkinson's Disease Association; has received consultation fee from Acadia Pharmaceutical, Ovation Pharmaceutical, Merck Serono, and Boehringer Ingelheim; has received research support from the National Institutes of Health, Forest Research Institute, Eli Lilly, Michael J. Fox Foundation for Parkinson's Research, and the National Parkinson Foundation; and has received book royalties from Taylor and Francis/Informa. Dr. Hiner has received speaking honorarium from Teva Neuroscience. Dr. Siderowf is supported by a Morris K. Udall Parkinson's Disease Research Center of Excellence grant from NINDS (NS-053488), and has been supported by SAP4100027296, a health research grant awarded by the Department of Health of the Commonwealth of Pennsylvania from the Tobacco Master Settlement Agreement under Act 2001-77; has received consulting fees from Teva Neuroscience, Supernus Pharmaceuticals, Schering-Plough, and Merck Serono; and has received speaking honorarium from Teva Neuroscience. Dr. Payami has received funding from the NIH (NS36960). Dr. Molho is supported by the Riley Family Chair in Parkinson's Disease. Dr. Factor reports no disclosures. Dr. Ottman serves on the scientific advisory board for and holds stock options in Trigeminal Solutions, Inc; has received funding for travel from the International League Against Epilepsy, the National Institute for Mental Health, and Coriell Institute for Medical Research; has received speaker honoraria for non-industry sponsored lectures; serves as a consultant to Ortho-McNeil Janssen Scientific Affairs, LLC.; and received research support from the NIH through #R01 NS043472 [PI], #R01 NS036319 [PI], #R01 NS036630 [Co-I], #R03 NS065346 [PI], #RC2 NS070344 [MPI], #R01 NS039422 [Co-I], and #R01 NS053998 [Co-I]. Dr. Clark reports no disclosures. Dr. Marder served on the editorial board of Neurology®; received research support from Amarin Corporation, and NeuroSearch Sweden AB; and receives research support from the NIH [#NS36630 (PI), 1UL1 RR024156-01 (Director PCIR), PO412196-G (Co-I), and PO412196-G (Co-I)], and from the Parkinson Disease Foundation, Huntington's Disease Society of America, and the Parkinson Study Group, and the Michael J. Fox Foundation. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Tison F, Dartigues JF, Auriacombe S, Letenneur L, Boller F, Alperovitch A. Dementia in Parkinson's disease: a population-based study in ambulatory and institutionalized individuals. Neurology 1995;45:705–708 [DOI] [PubMed] [Google Scholar]
- 2. Mayeux R, Chen J, Mirabello E, et al. An estimate of the incidence of dementia in idiopathic Parkinson's disease. Neurology 1990;40:1513–1517 [DOI] [PubMed] [Google Scholar]
- 3. Marder K, Tang MX, Cote L, Stern Y, Mayeux R. The frequency and associated risk factors for dementia in patients with Parkinson's disease. Arch Neurol 1995;52:695–701 [DOI] [PubMed] [Google Scholar]
- 4. Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sorensen P. Risk of dementia in Parkinson's disease: a community-based, prospective study. Neurology 2001;56:730–736 [DOI] [PubMed] [Google Scholar]
- 5. Sidransky E, Nalls MA, Aasly JO, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med 2009;361:1651–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alcalay RN, Caccappolo E, Mejia-Santana H, et al. Frequency of known mutations in early-onset Parkinson disease: implication for genetic counseling: the consortium on risk for early onset Parkinson disease study. Arch Neurol 2010;67:1116–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clark LN, Kartsaklis LA, Wolf Gilbert R, et al. Association of glucocerebrosidase mutations with dementia with Lewy bodies. Arch Neurol 2009;66:578–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neumann J, Bras J, Deas E, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson's disease. Brain 2009;132:1783–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alcalay RN, Mejia-Santana H, Tang MX, et al. Self-report of cognitive impairment and mini-mental state examination performance in PRKN, LRRK2, and GBA carriers with early onset Parkinson's disease J Clin Exp Neuropsychol 2010;32:775–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Folstein MF, Folstein SE, McHugh PRP. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 11. Brockmann K, Srulijes K, Hauser AK, et al. GBA-associated PD presents with nonmotor characteristics. Neurology 2011;77:276–280 [DOI] [PubMed] [Google Scholar]
- 12. Sardi SP, Clarke J, Kinnecom C, et al. CNS expression of glucocerebrosidase corrects α-synuclein pathology and memory in a mouse model of Gaucher-related synucleinopathy. Proc Natl Acad Sci USA 2011;108:12101–12106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marder KS, Tang MX, Mejia-Santana H, et al. Predictors of parkin mutations in early-onset Parkinson disease: the consortium on risk for early-onset Parkinson disease study. Arch Neurol 2010;67:731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caccappolo E, Alcalay RN, Mejia-Santana H, et al. Neuropsychological Profile of Parkin Mutation Carriers with and without Parkinson Disease: The CORE-PD Study. J Int Neuropsychol Soc 2011;17:91–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–571 [DOI] [PubMed] [Google Scholar]
- 16. Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav 1984;32:489–502 [DOI] [PubMed] [Google Scholar]
- 17. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414 [DOI] [PubMed] [Google Scholar]
- 18. Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001;58:1985–1992 [DOI] [PubMed] [Google Scholar]
- 19. Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 2005;65:1239–1245 [DOI] [PubMed] [Google Scholar]
- 20. Muslimovic D, Post B, Speelman JD, De Haan RJ, Schmand B. Cognitive decline in Parkinson's disease: a prospective longitudinal study. J Int Neuropsychol Soc 2009;15:426–437 [DOI] [PubMed] [Google Scholar]
- 21. Jankovic J, McDermott M, Carter J, et al. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort: The Parkinson Study Group. Neurology 1990;40:1529–1534 [DOI] [PubMed] [Google Scholar]
- 22. Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord 2008;23:837–44 [DOI] [PubMed] [Google Scholar]
- 23. Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson's disease: progression to dementia. Mov Disord 2006;21:1343–1349 [DOI] [PubMed] [Google Scholar]
- 24. Aarsland D, Bronnick K, Williams-Gray C, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology 2010;75:1062–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maetzler W, Liepelt I, Berg D. Progression of Parkinson's disease in the clinical phase: potential markers. Lancet Neurol 2009;8:1158–1171 [DOI] [PubMed] [Google Scholar]
- 26. Goris A, Williams-Gray CH, Clark GR, et al. Tau and alpha-synuclein in susceptibility to, and dementia in, Parkinson's disease. Ann Neurol 2007;62:145–153 [DOI] [PubMed] [Google Scholar]
- 27. Williams-Gray CH, Hampshire A, Barker RA, Owen AM. Attentional control in Parkinson's disease is dependent on COMT val(158)met genotype. Brain 2008;131:397–408 [DOI] [PubMed] [Google Scholar]
- 28. Foltynie T, Goldberg TE, Lewis SGJ, et al. Planning ability in Parkinson's disease is influenced by the COMT Val(158)Met polymorphism. Mov Disord 2004;19:885–891 [DOI] [PubMed] [Google Scholar]
- 29. Gan-Or Z, Giladi N, Rozovski U, et al. Genotype-phenotype correlations between GBA mutations and Parkinson disease risk and onset. Neurology 2008;70:2277–2283 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.