Abstract
Objective:
Progressive multifocal leukoencephalopathy (PML) is a severe complication of natalizumab therapy in patients with multiple sclerosis (MS), which is often accompanied by an immune reconstitution inflammatory syndrome (IRIS) after removal of the drug. We describe a patient with MS who presented with simultaneous PML-IRIS 2 months after stopping natalizumab for other reasons.
Case Report and Results:
The patient had widespread PML and severe IRIS. He received corticosteroids and displayed a vigorous JC virus–specific cellular immune response. Elevated myoinositol and lipid/creatine peaks measured in PML lesions by proton magnetic resonance spectroscopy (1H-MRS) corresponded to episodes of contrast enhancement on MRI scans and persisted after the enhancement subsided. He demonstrated steady clinical improvement, but developed marked residual atrophy in areas affected by PML and inflammation, as well as seizures.
Conclusions:
New enhancing white matter lesions, occurring after discontinuation of natalizumab, can be the manifestation of PML-IRIS rather than an MS exacerbation. Elevated myoinositol and lipid/creatine peaks appear to be more sensitive markers of inflammation in PML lesions than contrast enhancement. 1H-MRS may become useful as a biomarker for PML-IRIS by helping clinicians determine the need for corticosteroid administration and anticipate continuing clinical recovery.
Natalizumab has been associated with progressive multifocal leukoencephalopathy (PML). Almost all patients discontinued natalizumab after being diagnosed with PML and developed an immune reconstitution inflammatory syndrome (IRIS).1 We describe a patient with multiple sclerosis (MS) who developed simultaneous PML-IRIS 2 months after interruption of natalizumab.
CASE REPORT
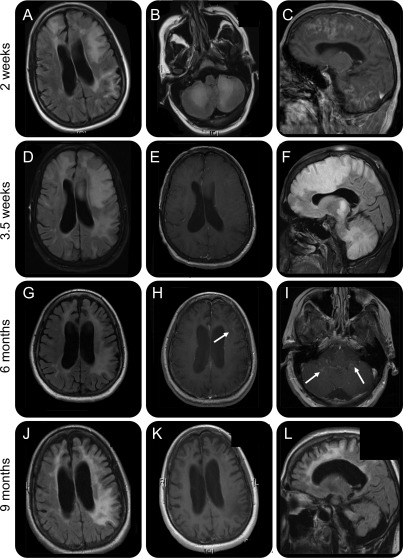
A 23-year-old man with past ethanol abuse and traumatic brain injury 3 years previously was diagnosed with relapsing-remitting MS, after presenting with diplopia, blurred vision, dizziness, and gait ataxia. Imaging showed several enhancing brain lesions and one intramedullary lesion at the C3 level. His CSF examination showed increased cells and proteins, and he had prolonged visual evoked potentials. He was treated with natalizumab monotherapy, which was discontinued after 39 months, because of excessive ethanol consumption. He was subsequently hospitalized with aspiration pneumonia in the setting of ethanol intoxication. Two months after natalizumab interruption, he presented with planning difficulties and ataxia. MRI, 1.5 week after symptom onset (SO), revealed new enhancing lesions in the frontal and left parietal lobes and in the cerebellum. PCR for CSF JC virus (JCV) was positive. He developed a bilateral pyramidal syndrome and aphasia and became bedridden and mute. MRI 2 weeks after SO showed lesion progression in the middle cerebellar peduncles, corticospinal tracts, and left frontal lobe with mass effect (figure, A–C) and a new enhancing lesion in the right temporal lobe. This was consistent with PML-IRIS. PCR for JCV showed 9,900 copies/mL CSF. He received methylprednisone (1 g/day for 5 days) and started mirtazapine (15 mg qhs).
Figure. Overview of MRI evolution.
(A–C) MRI 2 weeks after symptom onset. Axial fluid-attenuated inversion recovery (FLAIR) images demonstrate extensive white matter hyperintense signal in both the frontal and the left parietal lobe (A) and in the bilateral cerebellar white matter (B). (C) Sagittal T1-weighted image after gadolinium injection shows massive enhancement in the frontal lobe and the cerebellum. (D–F) MRI 3.5 weeks after symptom onset. (D) FLAIR image shows extension of the white matter abnormalities with mass effect on the left lateral ventricle and left to right midline shift. (E) T1-weighted image after gadolinium injection shows absence of enhancement. (F) Sagittal FLAIR image shows extensive white matter involvement in the cerebrum and cerebellum with mass effect on the corpus callosum. (G–I) MRI scans 6 months after symptom onset. (G) FLAIR image shows extensive atrophy with enlargement of the sulci and of the lateral ventricles. White matter hyperintense signal persists in both hemispheres. T1-weighted image after gadolinium injection shows a new faint rim of enhancement (H, arrow) and in the cerebellum (I, arrows). (J–L) MRI scans 9 months after symptom onset. (J) FLAIR image shows white matter lesions and atrophy. (K) T1-weighted image after gadolinium injection shows absence of enhancement. (L) Sagittal FLAIR image shows extensive cerebral and cerebellar atrophy.
MRI, 3.5 weeks after SO, showed decreased enhancement, but continuous progression of the lesions and mass effect (figure, D–F). He had one focal motor seizure, treated with levetiracetam, and received mefloquine (250 mg once a day for 3 days), followed by mefloquine (250 mg weekly for 24 weeks). MRI 1 week later showed enhancement, and he was given a prednisone taper, starting at 90 mg/day for 1 month.
Three months after SO, he answered in two-word phrases and could take a few steps. MRI showed a decrease in the size of the PML lesions and absence of mass effect but also atrophy and a faint rim of enhancement in the left frontal lobe.
Three months later, he could walk unassisted and care for most of his daily activities. He still had expressive aphasia, planning difficulties, left-sided neglect, and a bilateral cerebellar syndrome. MRI 6 months after SO showed further atrophy at the site of the PML lesions. There was limited residual contrast enhancement in the left frontal lobe, but new enhancement in the cerebellar lesions, indicating ongoing IRIS (figure, G–I).
He continued to improve, and MRI performed 3 months later showed resolution of enhancement and marked atrophy (figure, J–L). One year after onset, he spoke in short sentences and had a residual cerebellar syndrome and a minimal right-sided pyramidal syndrome. No new lesions were seen.
We used proton magnetic resonance spectroscopy (1H -MRS) 3, 6, 9, and 15 months after SO to measure the concentration of N-acetyl-l-aspartate (NAA), a neuronal marker, choline (Cho), a component of cell membranes, myoinositol (mI), a glial marker associated with inflammation, and LIP1 and LIP2, markers of anaerobic metabolism, within PML lesions. Creatine (Cr) is a measure of basal metabolism, and all values are expressed as ratios. There was a decline in the NAA/Cr ratio until 9 months after SO, consistent with neuronal and axonal damage, and an increase at the last time point in the left frontal and cerebellar lesions. The Cho/Cr ratio showed a continuous decline consistent with decreased turnover in cell membranes. mI/Cr, LIP1/Cr, and LIP2/Cr ratios increased in both lesions between 3 and 6 months after SO, concomitant with new enhancement in the cerebellum. When enhancement subsided 9 months after SO, the LIP2/Cr ratio remained elevated in both lesions, whereas the mI/Cr ratio remained elevated in the frontal lesion and the LIP1/Cr ratio continued to rise in the cerebellar lesion. Fifteen months after onset, the mI/Cr ratio decreased in the frontal lesion but increased again in the cerebellar lesion, whereas the lipid/Cr ratios decreased in both.
JCV-specific T-cell responses were detected at the same time points in his blood. Although the CD4+ T-lymphocyte response remained low, the CD8+ T-lymphocyte response became robust over time.
DISCUSSION
This case illustrates that PML can develop even after discontinuation of natalizumab and occur simultaneously with IRIS. Simultaneous PML-IRIS had been mainly restricted to patients with AIDS starting HIV treatment.2 Because natalizumab should be fully cleared from the circulation after approximately 2 months (5 half-lives),3 it is likely that PML started to develop in our patient while the medication was still active. However, it only became clinically apparent when the effect of the medication wore off, allowing the return of lymphocytes back into the brain parenchyma, causing IRIS. This represents a challenge, because neurologic dysfunction and white matter lesions occurring after natalizumab interruption may be caused by MS exacerbations.4 In addition, our patient was hospitalized with ethanol intoxication before the development of frank cerebellar symptoms, and the cognitive dysfunction caused by PML may have been compounded by ethanol.
Interestingly, this patient did not experience a relapse of MS over a 20-month period after PML onset. Indeed, 21% of patients with MS experience a relapse of MS during natalizumab interruption.3 Although his PML lesions seemed extensive, he regained partial neurologic function and some of the abnormal MRI signal subsided after corticosteroid treatment, indicating that impairment was in part caused by reversible inflammation rather than permanent demyelination. Another explanation for his remarkable improvement could be remyelination, as seen in MS. However, this has not been previously described in the setting of PML, and the absence of histologic material precludes any definite conclusion.
There are no biomarkers to define IRIS,2,5 and estimating PML outcome remains difficult. Survivors of PML are more likely to mount a JCV-specific cellular immune response than progressors, but this does not differentiate between patients with PML with or without IRIS.6 This patient displayed a persistent T-cell response against JCV, which did not decrease once IRIS subsided and persisted regardless of corticosteroid use.
Lesions of survivors of PML have higher mI/Cr ratio peaks than those of progressors.7 Of note, mI/Cr, LIP1/Cr, and LIP2/Cr ratios seemed more sensitive than contrast enhancement as surrogate markers of inflammation in our patient.8 Despite improvement, he developed marked brain atrophy, indicating the extent of demyelination caused by PML. 1H- MRS may be an important marker in PML-IRIS, which could be used to monitor ongoing inflammation, help determine the need for corticosteroid administration, and anticipate continuing recovery when enhancement has already subsided.
ACKNOWLEDGMENT
The authors thank the patient and his family for giving us permission to publish his case.
GLOSSARY
- Cho
choline
- Cr
creatine
- 1H-MRS
proton magnetic resonance spectroscopy
- IRIS
immune reconstitution inflammatory syndrome
- JCV
JC virus
- mI
myoinositol
- MS
multiple sclerosis
- NAA
N-acetyl-l-aspartate
- PML
progressive multifocal leukoencephalopathy
- SO
symptom onset
AUTHOR CONTRIBUTIONS
Dr. Gheuens was involved in patient care, imaging and immunologic studies, writing the first draft of the manuscript, and preparing the figures. Dr. Smith was involved in patient care and revising the manuscript. Dr. Wang was involved in performing the imaging and collecting the MRS data and revising the manuscript. Dr. Alsop was involved in performing the imaging and collecting the MRS data and revising the manuscript. Dr. Lenkinski was involved in performing the imaging and collecting the MRS data and revising the manuscript. Dr. Koralnik was involved in patient care, imaging and immunologic studies, and supervising, editing and revising the manuscript, and preparing the figures.
DISCLOSURE
Dr. Gheuens is funded by NIH grant T32 AI07387-21 and is a fellow of the Clinical Investigator Training Program: Beth Israel Deaconess Medical Center–Harvard/MIT Health Sciences and Technology, in collaboration with Pfizer Inc. and Merck & Co. Dr. Smith has received consulting/advisory fees and speaking honoraria but not grant support or standing committee income from all of the manufacturers of FDA-approved products for multiple sclerosis treatment. Dr. Wang is supported in part by NIH grant R01 NS047029. Dr. Alsop is an inventor on several patents related to perfusion MRI (US Patent Nos. 7,545,142, 7,369,888, 6,980,845, 6,717,405), for which he has received royalties from GE Healthcare and Siemens Medical; receives research support from GE Healthcare and is also supported by grants from the NIH, CA115745, EB004582, MH80729, MH077073, DC008796, NS047029, CA101942, AG031720, AG028076, DK084463, and the Congressionally Directed Military Research Program of the Department of Defense, SC090251; and serves as Associate Editor of Magnetic Resonance in Medicine. Dr. Lenkinski is supported in part by NIH grant R01 NS047029 and receives research support from GE Healthcare. Dr. Koralnik is funded by NIH grants R56 NS 041198, R01 NS 047029 and K24 NS 060950; has received a research grant from Biogen Idec and the National Multiple Sclerosis Society; served on scientific advisory boards for Hoffmann La Roche, GlaxoSmithKline and Merck Serono; received consulting fees from Bristol Myers Squibb, Ono Pharmaceuticals, Merck Serono, Hoffmann La Roche, GlaxoSmithKline, Perseid Therapeutics, Vertex Pharmaceutical, Johnson & Johnson; and is an editorial board member for the Journal of NeuroVirology and receives royalties from UpToDate for topics on the management of HIV and CNS mass lesions and on PML. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Vermersch P, Kappos L, Gold R, et al. Clinical outcomes of natalizumab-associated progressive multifocal leukoencephalopathy. Neurology 2011; 76: 1697–1704 [DOI] [PubMed] [Google Scholar]
- 2. Johnson T, Nath A. Neurological complications of immune reconstitution in HIV-infected populations. Ann NY Acad Sci 2010; 1184: 106–120 [DOI] [PubMed] [Google Scholar]
- 3. O'Connor PW, Goodman A, Kappos L, et al. Disease activity return during natalizumab treatment interruption in patients with multiple sclerosis. Neurology 2011; 76: 1858–1865 [DOI] [PubMed] [Google Scholar]
- 4. Miravalle A, Jensen R, Kinkel RP. Immune reconstitution inflammatory syndrome in patients with multiple sclerosis following cessation of natalizumab therapy. Arch Neurol 2011; 68: 186–191 [DOI] [PubMed] [Google Scholar]
- 5. Bonham S, Meya DB, Bohjanen PR, Boulware DR. Biomarkers of HIV immune reconstitution inflammatory syndrome. Biomark Med 2008; 2: 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gheuens S, Bord E, Kesari S, et al. Role of CD4+ and CD8+ T-cell responses against JC virus in the outcome of patients with progressive multifocal leukoencephalopathy (PML) and PML with immune reconstitution inflammatory syndrome. J Virol 2011; 85: 7256–7263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Katz-Brull R, Lenkinski RE, Du Pasquier RA, Koralnik IJ. Elevation of myoinositol is associated with disease containment in progressive multifocal leukoencephalopathy. Neurology 2004; 63: 897–900 [DOI] [PubMed] [Google Scholar]
- 8. Cuvinciuc V, Martin-Blondel G, Marchou B, Bonneville F. Proton MR spectroscopy of progressive multifocal leukoencephalopathy-immune reconstitution inflammatory syndrome. AJNR Am J Neuroradiol 2010; 31: E69–E70; author reply E71 [DOI] [PMC free article] [PubMed] [Google Scholar]