Abstract
Chromosome-specific gene regulation is known thus far only as a mechanism to equalize the transcriptional activity of the single male X chromosome with that of the two female X chromosomes. In Drosophila melanogaster, a complex including the five Male-Specific Lethal (MSL) proteins, “paints” the male X chromosome, mediating its hypertranscription. Here, with the molecular cloning of Painting of fourth (Pof), we describe a previously uncharacterized gene encoding a chromosome-specific protein in Drosophila. Unlike the MSL proteins, POF paints an autosome, the fourth chromosome of Drosophila melanogaster. Chromosome translocation analysis shows that the binding depends on an initiation site in the proximal region of chromosome 4 and spreads in cis to involve the entire chromosome. The spreading depends on sequences or structures specific to chromosome 4 and cannot extend to parts of other chromosomes translocated to the fourth. Spreading can also occur in trans to a paired homologue that lacks the initiation region. In the related species Drosophila busckii, POF paints the entire X chromosome exclusively in males, suggesting relationships between the fourth chromosome and the X and between POF complexes and dosage-compensation complexes.
The genome of Drosophila melanogaster is organized into two sex chromosomes (X and Y) and three autosomes (autosomes 2, 3, and 4). The smallest, chromosome 4, is ≈5 megabases (1) and consists of a centromeric, highly condensed region that is underreplicated in polytenic tissues and a banded, polytenized region corresponding to cytological sections 101E–102F. The majority of Drosophila species have a similar microchromosome (2) that is also called the F element (3). In many respects, chromosome 4 is an atypical autosome. Many features indicate that the fourth chromosome of D. melanogaster is largely heterochromatic in nature. The heterochromatin protein HP1 is found associated with much of chromosome 4 (4). Repetitive elements normally confined to heterochromatin are distributed throughout the banded region of chromosome 4 (5). Furthermore, reporter genes carried by transposons inserted at many sites in this chromosome often display a variegated, partially repressed expression typical of heterochromatic position–effect variegation (6, 7).
Several independent lines of evidence have suggested a closer kinship of chromosome 4 with the X chromosome than with the other autosomes (2, 8). First, in contrast to autosomes but similar to the X chromosome, chromosome 4 has “female tendencies,” shifting 2X:3A intersexes toward female development when its dosage is increased and toward male development when the dosage is decreased (9, 10). Second, Triplo-4 causes an increased frequency of X chromosome nondisjunction, suggesting a tendency of chromosome 4 to pair with the X in meiosis (11). Third, the study of the Drosophila busckii karyotype and mutants suggests that D. busckii lacks an independent chromosome 4, that the homologous region is probably located on the proximal part of X, and that the microchromosome of D. melanogaster was in the past a part of the X chromosome (12, 13). Our findings (described below) suggest a possible further similarity between the X and the fourth chromosome; they show that like the X chromosome, the fourth chromosome is associated with chromosome-specific proteins.
In D. melanogaster, two noncoding RNAs, roX1 and roX2, have been shown to be essential components of the X chromosome dosage-compensation system (14). These noncoding RNA components, together with the five Male-Specific Lethal (MSL) proteins, “paint” the dosage-compensated male X chromosome (15). The role played by the roX RNAs in Drosophila has suggested parallels between the fly and the mammalian dosage-compensating systems. In mammalian females, a large noncoding RNA, the product of the Xist gene, is thus far the only known participant in X chromosome inactivation. Xist RNA, like roX1 and 2 RNAs, paints the dosage-compensated X chromosome. Although the way compensation for chromosome dosage is achieved differs between mammals and Drosophila, the basic features of the process display striking similarities. In both systems, spliced polyadenylated, noncoding RNAs become associated with the chromosome to be compensated and paint it by spreading along the chromosome (15, 16). The painting is initiated at nucleation site(s) and spreads from them to involve most of the chromosome.
Here, with the molecular cloning of Painting of fourth (Pof), we describe a previously uncharacterized gene encoding a chromosome-specific protein in Drosophila. In contrast to previously described chromosome-specific proteins, POF paints an autosome, i.e., the fourth chromosome of D. melanogaster. However, examination of the related species D. busckii, in which POF paints the entire X chromosome, suggests a relationship of the fourth chromosome to the X and of POF complexes to dosage-compensation complexes.
Materials and Methods
Fly Strains and Crosses.
Established mutants and fly lines used in this study have been described (17). Anssi Saura and the Umeå Drosophila Stock Centre (Umeå University, Sweden) kindly provided stocks. Flies (except for D. busckii) were cultivated in vials with potato-mash–yeast-agar medium; D. busckii was raised in Formula 4-24 Instant medium (Carolina Biological Supply) supplemented with dry yeast.
Transgenic Flies.
The P[yellow+ hsp70:FLAG-Pof] construct expressing the POF complete protein with a FLAG corresponding peptide at the N-terminal region was made as follows. Primer (AACTCGAGCATGGATTACAAGGACGATGACGATAAGATGGATTCAAAACGCGCGGC) that contains an XhoI site followed by a FLAG-epitope tag (Met-DYKDDDK-) (Sigma) fused to the translation start Met of Pof was used in a PCR together with the T7 primer and a Pof cDNA cloned in pBluescript II KS(+) as a template for the pfuTURBO polymerase (Stratagene). The product was cut with XhoI and XbaI and cloned into corresponding sites of the C4Y-hs vector (18). This vector uses the yellow gene as a marker and places the FLAG-Pof sequence under control of the hsp70 promoter. The construct was sequenced to confirm the absence of mutations resulting from errors during PCR. DNA for transformation was prepared by using Maxi Prep cartridges (Qiagen, Chatsworth, CA). Germ-line transformation of the construct was done according to described methods (19), by using the Df(1)w (67c23) strain as host.
Molecular Biology.
In the first screen, the probe was a 1,532-bp fragment of Zip16 cDNA (subsequently renamed Pof) isolated by J.D.C. in a screen for Zeste-interacting-proteins (20). A D. melanogaster (Canton S) embryonic cDNA library (CLONTECH) was screened, and inserts from 10 positive clones were subcloned into pBluescript II KS(+) and sequenced. For Northern blot analysis, poly(A)+ RNA was isolated by using Dynabeads Oligo(dT)25 (Dynal, Great Neck, NY). Adult flies, pupae, and larvae were frozen at −70° and homogenized in 0.1 M Tris⋅HCl, pH 8.0/0.5 M LiCl/10 mM EDTA/1% SDS/5 mM DTT; the instructions of the bead manufacturer were then followed. The samples were separated on a 1.0% formaldehyde-agarose gel and blotted onto a GeneScreenPlus filter (DuPont/NEN) with a VacuGene Vacuum Blotting System. Prehybridization and hybridization were performed with Ultrahyb solution (Ambion, Austin, TX) at 42° C. A Pof cDNA clone (1.6 kb) and an RpS3 cDNA (1.0 kb), 32P-labeled by random priming and purified with an Amersham Pharmacia S-200 h column, were used as probes. The membrane was washed twice for 5 min in 2× SSPE [standard saline phosphate/EDTA (0.15 M NaCl/10 mM phosphate, pH 7.4/1 mM EDTA] at room temperature, three times for 15 min in 2× SSPE/2% (vol/vol) SDS at 65°C, and once in 0.1× SSPE at room temperature for 15 min. In situ hybridization to polytene chromosomes was performed according to described methods (21) by using a digoxigenin-labeled DINE element probe made as described (22). Predictions concerning the Pof gene product were obtained with software obtained at the following electronic sites: http://jura.ebi.ac.uk:8765/gqsrv/submit (genequiz), and http://www.expasy.ch/(pfam and psortii).
Antibodies.
Polyclonal antibodies were raised in hen and rabbit by using a synthetic peptide (TDDQDKEASGGDGSQC) conjugated to KLH (AgriSera, Vännäs, Sweden). The rabbit sera and the purified hen IgY were affinity-purified on an UltraLink Iodoacetyl column as described by the manufacturer (Pierce).
Immunostaining of Polytene Chromosomes.
Third instar larvae yw; P[yellow+ hsp70:FLAG-Pof] were heat-shocked for 60 min at 37°C and allowed to recover at room temperature for 1 h. Polytene chromosomes were prepared and stained essentially as described (23). Salivary glands were fixed in 2% (vol/vol) formaldehyde for 40 sec followed by 2 min in 50% (vol/vol) acetic acid/1% formaldehyde. Polytene chromosomes were squashed as described (23). The slides were washed 30 min in 1× PBT (phosphate buffered saline with 0.1% Tween-20), transferred to blocking solution (0.1 M maleic acid/0.15 M NaCl/1% Boehringer blocking reagent) and incubated 30 min at room temperature. The slides were then incubated overnight at 4°C with 1:60 diluted M2 anti-FLAG mouse monoclonal primary antibody (Stratagene). The slides were washed twice 10 min in 0.1 M maleic acid/0.15 M NaCl/0.3% Tween 20 and blocked for 30 min. As a secondary antibody, a goat anti-mouse antibody conjugated with Cy3 was used (Zymed), diluted 1:100, and incubated at room temperature for 2 h. The squashes were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; 1 μg/ml) and washed 2 × 10 min before mounting with Vectashield (Vector Laboratories). For staining endogenous POF protein, the same procedure—minus the heat-shock—was used. A polyclonal affinity-purified hen IgY anti-POF was used as the primary antibody (1:100), and a donkey anti-rabbit antibody conjugated with Cy3 (Jackson Laboratories) was used as the secondary antibody (1:300). RNase treatment of salivary glands to test RNA dependence of POF binding was done according to described methods (24). Chromosomes were analyzed with a Zeiss Axiophot microscope equipped with a KAPPA DX3°C charge-coupled device camera. Images were assembled, contrasted, and merged electronically with Adobe photoshop.
Results
Cloning of Pof.
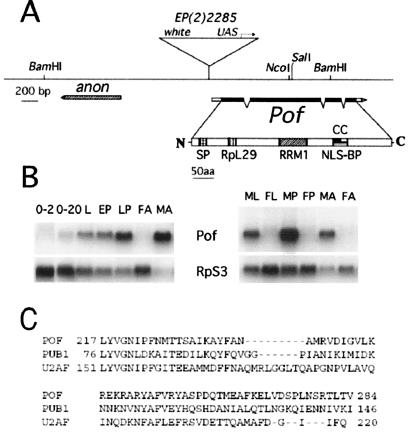
A partial Pof cDNA was originally isolated in a two-hybrid screen for Zeste-interacting proteins (20). This cDNA was then used to isolate additional clones from a cDNA library from D. melanogaster embryos. New cDNA clones (n = 10) were isolated, and their sequence was used to search the Berkeley Drosophila Genome Project database (http://www.fruitfly.org/) for expressed sequence tags and predicted genes (25). The Pof gene is identical to gene CG3691 predicted from the Genome Project with the genomic organization shown in Fig. 1A. Our longest cDNA clone starts 24 bp downstream of the proposed transcription start point of CG3691. The genome sequence and in situ hybridization to polytene chromosomes showed that the Pof gene was located on the right arm of chromosome 2 at 60E (results not shown). Northern blots of poly(A)+ RNA from different stages of development (hybridized with Pof cDNA) revealed that the transcript has little or no maternal component, and that it is weakly expressed in the embryo but becomes progressively stronger during larval and pupal development. In adults, the transcript is predominantly found in males. When RNA from sexed larvae and pupae was used, a small amount of transcript was detectable also in females at these stages but 20- to 30-fold less than in males (Fig. 1B). Line EP(2)2285 has a transposon (EP) element inserted 48-bp upstream of the transcription start point. This EP insertion strongly reduces but does not eliminate expression of Pof and has no detectable phenotypes in males or females.
Figure 1.
Map of the Pof gene. (A) The exon-intron structure of the Pof gene (as deduced from various cDNA clones) is shown below the genomic DNA line (derived from the Berkeley Genome Project). Filled boxes represent coding regions, and open boxes represent untranslated sequences. Motifs in the deduced polypeptide sequence are SP, amino acid sequence used for raising antisera; RpL29, Ribosomal protein L29 signature motif (residues 92–107, predicted by genequiz); RRM1, RNA-binding domain (residues 217–284, predicted by pfam); NLS-BP, a bipartite nuclear localization signal (residues 351–367, predicted by psortii); CC, a region likely to form a coiled coil (residues 353–383, predicted by coils). (B) Developmental Northern blot analysis. RNA samples are from 0- to 2-h and 2- to 20-h embryos (0–2, 2–24), third instar larvae (L), early pupae (EP), late pupae (LP), adult females (FA), adult males (MA), third instar male larvae (ML), third instar female larvae (FL), male pupae (MP), and female pupae (FP). The blot was hybridized with a Pof cDNA probe and then reprobed with RpS3 to control RNA loading. (C) Alignment of POF RRM1 domain to RNA-binding domains of PUB1 (yeast) and 65kDa subunit of splicing factor U2AF (human), accession nos. P32588 and P26368, respectively.
Analysis of the deduced amino acid sequence with conventional software tools predicts that the protein is likely to be nuclear and to contain an RNA-binding domain found in a variety of known RNA-binding proteins. The positions of predicted domains are shown in Fig. 1A; the alignment of the POF RRM1 domain with those of two RNA-binding proteins, PUB1 and U2AF, is shown in Fig. 1C.
Localization of the POF Protein.
To study its nuclear localization, the POF protein was first fused to a FLAG-epitope tag and expressed from the hsp70 promoter in transgenic larvae carrying the P[yellow+ hsp70:FLAG-Pof] construct. After heat shock, staining of polytene chromosomes shows that the anti-FLAG antibody decorates exclusively the fourth chromosome in both male and female larvae in a band pattern approximately complementary to the chromatin bands revealed by DAPI staining (Fig. 2). No staining is seen in nontransgenic flies (results not shown). Overexpression of POF from the hsp70 promoter causes no detectable phenotypes.
Figure 2.
Localization of FLAG-POF during interphase. P[yellow+ hsp70: FLAG-Pof] larvae were heat shocked, and their polytene chromosomes were stained with (A) DAPI and (B) anti-FLAG antibodies. The FLAG-POF protein is found exclusively on chromosome 4. (C) Merged DAPI and FLAG-POF images showing that POF decorates interbands preferentially.
To confirm the binding specificity of the endogenous POF protein, we raised polyclonal antibodies by using a synthetic peptide from the N-terminal part of the protein (Fig. 1A). Antibodies from hen and rabbit were affinity-purified and shown by Western blot analysis to be highly specific for a single polypeptide with the predicted mobility of POF. The protein is found predominantly in males, although it is also detectable at low levels in females (results not shown). The staining of whole salivary glands reveals a nuclear localization with a single staining region in each nucleus that is presumed to correspond to chromosome 4 (Fig. 3 A and B). When wild-type polytene chromosomes are stained with the anti-POF antibody, the protein is found exclusively on chromosome 4 (Fig. 3 C and D). Although the total amount of protein is lower in females, POF protein is clearly detected exclusively on chromosome 4 on polytene chromosomes prepared from female larvae (results not shown). To dissect the nature of this chromosomal specificity further, we used fly strains carrying chromosome 4 translocations. The translocation T(1;4)wm5 reciprocally exchanges the banded region of chromosome 4 (101F–102F) with the tip of the X chromosome (1A–3C). In a T(1;4)wm5 male with karyotype pXd4; p4dX; 4, only the normal copy of the fourth chromosome is stained, and the distal part of the translocated chromosome 4 is not decorated by the antibody (Fig. 4 A–D). These results suggest that a sequence or structure in the proximal region of chromosome 4 is needed for the POF protein to initiate binding, which then spreads to the rest of the chromosome. In some nuclei, the translocated fourth can be found paired with the normal chromosome 4 (Fig. 4 E–H). In these cases, the entire paired region stains, suggesting that the POF complex spreads in trans onto the translocated homologue. In T(3;4)e, the 3L chromosome (from position 79E) is translocated to the tip of chromosome 4 (position 102F1–102F8). Although the chromosome 4 part (p4) of the resulting chromosome is stained, the stain does not spread across the translocation breakpoint into the 3L chromosome (Fig. 4 I–L). These results indicate that the propagation of POF binding depends on sequences or structures specific to chromosome 4. It has been shown that the MSL complex protein MLE depends on RNA for its binding to the male X chromosome. Considering its putative RNA-binding domain, we wondered whether POF binding to chromosome 4 was RNA-dependent, but an RNase treatment had no effect on the chromosome-specific binding of POF (results not shown).
Figure 3.
Localization of wild-type POF. Salivary gland nuclei and polytene chromosomes from wild-type third instar larvae. (A) Nomarski image merged with the DAPI fluorescence image and with the anti-POF immunostaining image. (B) Anti-POF image alone. (C) Polytene chromosomes stained with DAPI and (D) with anti-POF antibody.
Figure 4.
POF localization on translocation chromosomes. T(1;4)wm5 males stained with DAPI (A, C, E, and G) and anti-POF (B, D, F, and H). The distal X chromosome portion translocated to proximal chromosome 4 is indicated by dX, and the proximal X region is labeled pX. The distal 4 portion translocated to X:3C is labeled d4, whereas normal 4 is labeled 4. (A–D) Only the normal 4 is stained when 4 and d4 are not paired. (E–H) Staining spreads in trans when 4 and d4 are paired. (I–L) T(3;4)e chromosomes stained with DAPI (I and K) and anti-POF (J and L). Normal fourth chromosome and proximal chromosome 4 with 3L translocated to the tip are marked 4 and p4, respectively. Translocation breakpoints and cytology are indicated.
Several independent lines of evidence have suggested a closer kinship of chromosome 4 with the X chromosome than with the other autosomes (2, 8). An important support for this kinship comes from the study of D. busckii (12, 13). Krivshenko (12, 13) suggests that in D. busckii, the minichromosome homologue is located at the base of the X chromosome. Therefore, we stained polytene chromosomes from D. busckii with the anti-POF antibody (Fig. 5). The results show that the entire X chromosome of D. busckii is painted by POF protein. However, only male X chromosomes are stained. In contrast to D. melanogaster, no staining is detected in D. busckii females (results not shown). As with the D. melanogaster fourth chromosome, the antibody staining produces a banded pattern apparently complementary to the DAPI-staining chromatin bands. Because the binding of POF to chromosome 4 in D. melanogaster depends on a chromosome 4-specific sequence or structure, we considered the repetitive element DINE-1 as a candidate for this function. DINE-1 is a short repetitive sequence highly enriched in chromosome 4, although it is also found in centric heterochromatin and at the base of other chromosomes (22). When tested by in situ hybridization, the DINE probe does not show any specific hybridization to D. busckii chromosomes (results not shown). This result does not rule out the possible involvement of DINE or other repetitive elements preferentially present on chromosome 4 in the binding of POF to the D. melanogaster chromosome 4.
Figure 5.
POF Localization in D. busckii polytene chromosomes. DAPI (A) and anti-POF (B). The merged image shows that POF staining is more pronounced in interband regions (C).
Discussion
The binding of POF to the fourth chromosome of D. melanogaster is, to our knowledge, a unique characteristic. Thus far, chromosome-specific binding has been described only for the dosage-compensated X chromosome. Although heterochromatin proteins [e.g., HP1 (4)] have been shown to bind extensively to chromosome 4, this binding is accompanied by binding to other heterochromatic regions whereas POF is strictly specific for chromosome 4 and is not detectable in the general centromeric region. In contrast to HP1 [which decorates chromosome 4 bands (26)], POF is found preferentially in interbands rather than in condensed chromatin and is specifically excluded from the base of the chromosome (Fig. 2C). Our study suggests a two-step process for the spreading of POF along the fourth chromosome. Binding initiates at a nucleation site, which, according to the translocation analysis, resides in the proximal region (101E). This initiation is a prerequisite for spreading to the rest of the chromosome. After initiation, POF spreads, but only on fourth chromosome sequences, suggesting that spreading also requires sequences or structures specific to chromosome 4. The spreading in trans to a paired chromosome 4 that lacks its own proximal region indicates that close apposition is sufficient to produce spreading to chromosome 4 sequences. Physical continuity is not required, and the failure to spread to translocated segments of other chromosomes shows that it is not sufficient. The facts that the pairing is not observed in many nuclei, and that the unpaired translocated fourth is not stained, imply either that pairing is determined at some earlier stage and is stably maintained once it occurs, or else that the POF-chromatin complexes on the translocated fourth dissolve very rapidly once pairing is lost.
The spreading in trans (Fig. 4 E–H) is intriguing and reminiscent of the fact that, in D. melanogaster, complexes containing roX RNA can spread in trans when a transgenic entry site introduced on an autosome is in the heterozygous state (16). The X chromosome of D. melanogaster seems to use ≈35 entry sites to initiate binding of roX RNA complexes. The presence of these entry sites defines the X chromosome as a target for roX complexes. In humans, by contrast, Xist complexes must spread from a single origin Xic to cover most of the human X chromosome (28). A model has been put forward postulating that the presence of abundant LINE elements along the X chromosome may aid in spreading Xist RNA complexes (28). Recently, this model has gained molecular support by the demonstration that LINE elements are enriched 2-fold on the mouse X chromosome (relative to the autosomes), and that a particular variant of this element is associated with the Xic region (29). In our case, a possible candidate to aid in spreading the POF-binding complexes is the DINE-1 element or a specific subset of DINE elements, which might define a site or chromosome structure needed for propagation of POF binding. Our failure to detect DINE sequences on the D. busckii chromosomes might argue against this possibility, or it might mean that D. busckii DINE equivalents are sufficiently diverged from the D. melanogaster DINE sequences to prevent cross-hybridization with the DINE1 probe. Other possible candidates would be any repetitive sequence poorly represented on other euchromatic chromosome arms or present but inaccessible in heterochromatin. Because both the MSL/roX system in Drosophila and the Xist system in mammals rely on a noncoding RNA for function, it is striking that the POF protein includes a putative RNA-binding domain (RRM1). The chromosome-specific binding of POF is not sensitive to RNase treatment; however, binding of MSL1 and MSL2 is also resistant to this treatment (24). The functional relevance of the RNA-binding domain remains to be investigated.
The painting of the entire X chromosome in D. busckii strongly supports the view that the fourth chromosome of D. melanogaster is a part of the X chromosome in that species and that this condition may be ancestral. We suppose that POF may be part of an X chromosome dosage-compensation system in D. busckii and that D. melanogaster has retained or adapted this function to control expression of fourth chromosome genes. This control may include the stimulation of gene expression in a highly heterochromatinized environment. Although some Pof expression can be detected in D. melanogaster females as well as males, the gene is almost male specific. The function of this strong male expression is not clear because the fourth chromosome (the only visible target for POF in salivary glands) is present in two copies in both males and females and not in need of male-specific dosage compensation. It may be that a knowledge of the role POF plays on the D. busckii X chromosome will explain the partial male specificity in D. melanogaster.
Acknowledgments
We thank Astrid Håglund for excellent technical assistance, Anssi Saura for collecting the isofemale D. busckii strain, and Anja Saura for confirming the cytology. This work was supported by grants from the Swedish Natural Science Research Council and the Nilsson–Ehle and Philip Sårenssen Foundations (to J.L.), by European Molecular Biology Organization Short Term Fellowships (to J.L.), and by a grant from the Swiss National Science Foundation (to V.P.).
Abbreviation
- DAPI
4′,6-diamidino-2-phenylindole
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AJ295236).
References
- 1.Locke J, McDermid H. Chromosoma. 1993;102:718–723. doi: 10.1007/BF00650898. [DOI] [PubMed] [Google Scholar]
- 2.Ashburner M. Drosophila: A Laboratory Handbook. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 3.Muller H J. In: The New Systematics. Huxley J, editor. Oxford: Clarendon; 1940. pp. 185–268. [Google Scholar]
- 4.Eissenberg J C, Morris G D, Reuter G, Hartnett T. Genetics. 1992;131:345–352. doi: 10.1093/genetics/131.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miklos G L G, Yamamoto M T, Davies J, Pirrotta V. Proc Natl Acad Sci USA. 1988;85:2051–2055. doi: 10.1073/pnas.85.7.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallrath L, Guntur V, Rosman L, Elgin S. Chromosoma. 1996;104:519–527. doi: 10.1007/BF00352116. [DOI] [PubMed] [Google Scholar]
- 7.Wallrath L, Elgin S. Genes Dev. 1995;9:1263–1277. doi: 10.1101/gad.9.10.1263. [DOI] [PubMed] [Google Scholar]
- 8.Hochman B. In: The Genetics and Biology of Drosophila. Ashburner M, Novitski E, editors. San Diego: Academic; 1976. pp. 903–928. [Google Scholar]
- 9.Bridges C B. Am Nat. 1925;59:127–137. [Google Scholar]
- 10.Fung S-T C, Gowen J W. Genetics. 1960;45:988–989. [Google Scholar]
- 11.Sandler L, Novitski E. Genetics. 1956;41:189–193. doi: 10.1093/genetics/41.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krivshenko J D. Proc Natl Acad Sci USA. 1955;41:1071–1079. doi: 10.1073/pnas.41.12.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krivshenko J D. Genetics. 1959;44:1027–1040. doi: 10.1093/genetics/44.6.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franke A, Baker B S. Mol Cell. 1999;4:117–122. doi: 10.1016/s1097-2765(00)80193-8. [DOI] [PubMed] [Google Scholar]
- 15.Stuckenholz C, Kageyama Y, Kuroda M I. Trends Genet. 1999;15:454–458. doi: 10.1016/s0168-9525(99)01855-7. [DOI] [PubMed] [Google Scholar]
- 16.Kelley R, Meller V, Gordadze P, Roman G, Davis R, Kuroda M I. Cell. 1999;98:513–522. doi: 10.1016/s0092-8674(00)81979-0. [DOI] [PubMed] [Google Scholar]
- 17.Lindsley D L, Zimm G G. The Genome of Drosophila Melanogaster. New York: Academic; 1992. [Google Scholar]
- 18.Poux S, McCabe D, Pirrotta V. Development (Cambridge, UK) 2000;128:75–85. doi: 10.1242/dev.128.1.75. [DOI] [PubMed] [Google Scholar]
- 19.Spradling A C. In: Drosophila, a Practical Approach. Roberts D B, editor. Oxford: IRL; 1986. pp. 175–197. [Google Scholar]
- 20.Chen J D. Dissertation. Houston: Baylor College of Medicine; 1992. [Google Scholar]
- 21.Heino T. Chromosoma. 1994;103:4–15. doi: 10.1007/BF00364721. [DOI] [PubMed] [Google Scholar]
- 22.Locke J, Howard L T, Aippersbach N, Podemski L, Hodgetts R B. Chromosoma. 1999;108:356–366. doi: 10.1007/s004120050387. [DOI] [PubMed] [Google Scholar]
- 23.White R A H. In: Drosophila, a Practical Approach. Roberts D B, editor. Oxford: IRL; 1998. pp. 215–240. [Google Scholar]
- 24.Richter L, Bone J R, Kuroda M I. Genes Cells. 1996;1:325–336. doi: 10.1046/j.1365-2443.1996.26027.x. [DOI] [PubMed] [Google Scholar]
- 25.FlyBase Consortium. Nucleic Acids Res. 1999;27:85–88. doi: 10.1093/nar/26.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.James T C, Eissenberg J C, Craig C, Dietrich V, Hobson A, Elgin S C. Eur J Cell Biol. 1989;50:170–180. [PubMed] [Google Scholar]
- 27.Lee J T, Jaenisch R. Curr Opin Genet Dev. 1997;7:274–280. doi: 10.1016/s0959-437x(97)80138-4. [DOI] [PubMed] [Google Scholar]
- 28.Lyon M F. Cytogenet Cell Genet. 1998;80:133–137. doi: 10.1159/000014969. [DOI] [PubMed] [Google Scholar]
- 29.Bailey J A, Carrel L, Chakravarti A, Eichler E E. Proc Natl Acad Sci USA. 2000;97:6634–6639. doi: 10.1073/pnas.97.12.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]