Abstract
Medical imaging is an invaluable tool for diagnosis, surgical guidance, and assessment of treatment efficacy. The Network for Translational Research (NTR) for Optical Imaging consists of four research groups working to “bridge the gap” between lab discovery and clinical use of fluorescence- and photoacoustic-based imaging devices used with imaging biomarkers. While the groups are using different modalities, all the groups face similar challenges when attempting to validate these systems for FDA approval and, ultimately, clinical use. Validation steps taken, as well as future needs, are described here. The group hopes to provide translational validation guidance for itself, as well as other researchers.
OCIS codes: (170.0110) Imaging systems, (170.3880) Medical and biological imaging
1. Introduction
Accurate diagnosis, surgical treatment, and assessment of response to treatment all depend on the ability to “see” affected tissues and organs. X-ray, CAT (computed axial tomography) scan, MRI (molecular resonance imaging), PET (positron emission tomography), and SPECT (single-photon emission computed tomography) are imaging technologies currently used in clinical practice. With the exception of image-guided invasive biopsy, these imaging modalities are not performed at the same time as treatment. Optical imaging, using fluorescence, as well as photoacoustic modalities, offers the opportunity for both real-time in situ diagnosis and treatment during the same procedure. Even absent therapeutic intervention, optical and photoacoustical imaging may be used as real-time imaging modalities, potentially affording better resolution, enabling better identification of diseased tissue, and expanding the options for obtaining functional and molecular diagnostic information. The Network for Translational Research (NTR) for Optical Imaging is a consortium of researchers at several medical/academic institutions working to move innovative medical imaging technologies from the bench to the clinic. Within the NTR, the Validation/Clinical Studies Core seeks to identify and provide guidance for the validation needs of the participating investigators, and in so doing, establish best practices for other investigators to use in their efforts to validate the combination of medical imaging devices and their companion biomarkers. The need for the NTR and the Validation/Clinical Studies Core has arisen due to a shifting landscape of product development/approval logistics. Previously, physician or bench scientists would hand off inventions to the pharmaceutical industry or device manufacturers for translation into the clinic. Now, industry/academic partnerships may be more advantageous, owing to sustained interactions between inventors, manufacturers, and end-users. This relationship, however, requires new skill sets for academic laboratories in pursuing the validation of their technologies.
Four groups within the NTR are developing different imaging modalities for disease detection and diagnosis, as well as surgery guidance and assessment of therapeutic treatment. Each group focuses on a particular disease as a model pathological state for the development and validation of the imaging system. Three of the four imaging systems include contrast agents along with instrumentation. The following brief descriptions of the modalities will highlight validation efforts and needs, and Table 1 summarizes their needs.
Table 1. NTR Centers, imaging modalities, applications, and validation needs.
| Center | Modality | Clinical Applications | Validation Needs |
|---|---|---|---|
| Washington University at St. Louis | Photoacoustic tomography with and without biomarkers | Sentinel lymph node imaging and guided needle biopsy, early prediction of chemotherapy response, breast cancer screening | In vivo studies in comparison to existing clinical standards |
| The University of Texas Health Science Center—Houston | Near-infrared fluorescence imaging with biomarkers | Lymphedema treatment assessment, non-invasive sentinel and cancerous lymph node identification, surgery guidance | New probes toxicity and batch release, cost/effectiveness |
| Stanford University | Visible/near-infrared fluorescence imaging with biomarkers | Detection of inflammation, premalignancies and cancer, stratifications of tumors for COX-2 expression | Demonstration of clinical efficacy |
| The University of Michigan | Endoscopic multispectral fluorescence imaging with biomarkers | Early detection of colorectal cancer using specific peptides to target flat and depressed lesions | In vivo peptide specificity and toxicity |
2. Optical imaging platforms and validation efforts
2.1. Photoacoustic tomography (PAT)
At Washington University in St. Louis, we are utilizing photoacoustic tomography (PAT), sometimes referred to as optoacoustic tomography, which is defined as cross-sectional or three-dimensional (3D) imaging of a material based on the photoacoustic effect. Therefore, PAT possesses spatial resolution along the depth dimension and at least one of the other two dimensions. In PAT, light is absorbed by biological tissue and converted to transient heating, which is subsequently converted into an ultrasonic wave due to thermoelastic expansion. Detection of the ultrasonic wave yields a tomographic image. Combining rich optical contrast and scalable ultrasonic resolution, PAT is the only imaging modality capable of providing multiscale, high-resolution structural, functional, and molecular imaging of organelles, cells, tissues, and organs in vivo. While functional imaging measures physiological parameters, such as oxygenation and blood flow, molecular imaging senses biomarkers to identify specific cancer cells or detects gene expression products to track gene activation.
PAT involves optical excitation, ultrasonic detection, and image formation. A short-pulsed laser is usually used to efficiently produce ultrasound in biological tissue. The amplitude of the photoacoustic pressure depends on the optical energy deposition, as well as the thermal and mechanical properties of the tissue. Because either unscattered or scattered photons can produce photoacoustic signals, photoacoustic waves can be generated deep within biological tissue. Because the ultrasonic scattering coefficient in tissue is two to three orders of magnitude less than the optical counterpart, high spatial resolution can be achieved by detecting the photoacoustic waves. Consequently, PAT breaks through the optical diffusion limit (~1 mm in the skin) for high-resolution optical-contrast imaging (Fig. 1 ). The image formation is essentially triangulation of the photoacoustic sources according to the time-of-flight signals recorded at multiple locations.
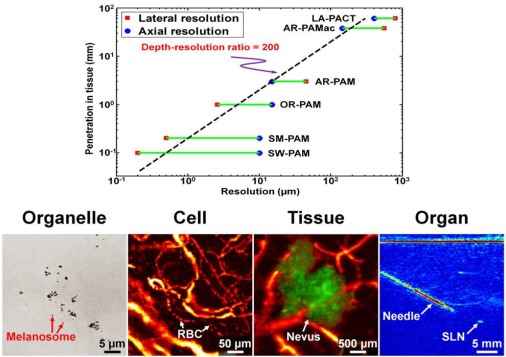
Fig. 1.
(top) Tissue penetration versus resolution for SW-PAM, sub-wavelength photoacoustic microscopy; SM-PAM, sub-micron photoacoustic microscopy; OR-PAM, optical-resolution photoacoustic microscopy; AR-PAM, acoustic-resolution photoacoustic microscopy; AR-PAMac, acoustic-resolution photoacoustic macroscopy; and LA-PACT, linear-array photoacoustic computed tomography; (bottom) in vivo multiscale photoacoustic images at organelle, cell, tissue, and organ scales.
Photoacoustic tomography has two major implementations: photoacoustic microscopy (PAM) and photoacoustic computed tomography (PACT) [1]. Optical-resolution PAM (OR-PAM) relies on tightly focused optical excitation to achieve high lateral resolution within the optical diffusion limit (~1 mm in tissue). This resolution can be refined to a fraction of an optical wavelength or of a micron, as demonstrated in sub-wavelength PAM (SW-PAM) and sub-micron PAM (SM-PAM), respectively. Beyond the limit where optical focusing is not effective, acoustic-resolution PAM (AR-PAM) takes advantage of the much lower acoustic scattering to maintain a high lateral resolution via focused acoustic detection. In contrast to focused-scanning PAM, PACT utilizes an ultrasonic array to simultaneously detect the entire imaging region. An inverse algorithm is then used to reconstruct a high-resolution image. According to the geometry of the array, PACT can be classified into linear-array PACT (LA-PACT) and circular-array PACT (CA-PACT).
PAT provides deep imaging at high resolution, and is capable of multiscale imaging (Fig. 1). To understand the workings of a whole biological system, biological components spanning multiple spatial scales—subcellular organelles (sub-µm scale), through cells (µm), to organs (cm)—must be integrated. Multiscale PAT with the same contrast mechanism—optical absorption—is well positioned for such integration.
PAT is expected to find broad applications in both biology and medicine [2–7]. Preclinical applications include imaging of non-fluorescent pigments (red blood cells & melanin), angiogenesis and anti-angiogenic response, microcirculation physiology and pathology, drug response for screening, brain functions, biomarkers, and gene activities through reporter genes. Clinical applications include melanoma cancer screening, gastrointestinal tract endoscopy, intravascular catheter imaging, neonatal and adult brain imaging, breast cancer detection, prostate cancer detection, guided sentinel lymph node needle/core biopsy for breast cancer staging, early response to chemotherapy, dosimetry in thermal therapy, in vivo label-free histology by photoacoustic imaging of cell nuclei, and blood flow, oxygenation, and tissue metabolism imaging.
Validation of the sensitivity and specificity of PAT is ongoing, and involves comparing in vivo and ex vivo imaging of lymph nodes using the contrast dyes methylene blue and indocyanine green, and placing exogenous animal tissue over test samples to explore imaging depth limits. In the future, the in vivo and ex vivo PAT lymph node imaging will be compared to other imaging techniques and histology analysis to validate results. Other validation plans include GMP (good manufacturing practice) production of dual-modality imaging agents (such as 125I-methylene blue) and SPECT radionuclide-labeled nanoparticles for correlative evaluation of PAT.
2.2. Near-infrared fluorescence (NIRF)
In the University of Texas Health Science Center (UTHSC) Center for Molecular Imaging (CMI), we are developing near-infrared fluorescence technologies. Currently, the only clinically used technologies with sensitivity sufficient for molecular imaging are nuclear imaging techniques. Nuclear imaging techniques have significant drawbacks, however, including radiotracer development at a cyclotron or reactor, radioactivity handling, and lack of any shelf life, causing difficulties with patient scheduling. In addition, nuclear imaging techniques require balancing the half-life of the radiotracer with the pharmacokinetics of the targeting agent. The CMI has pioneered a near-infrared fluorescence imaging technique (Fig. 2 ), and has developed both imaging agents and instrumentation for sensitive detection of a dim fluorophore, indocyanine green (ICG), at femto- to picomolar concentrations in humans [8,9].
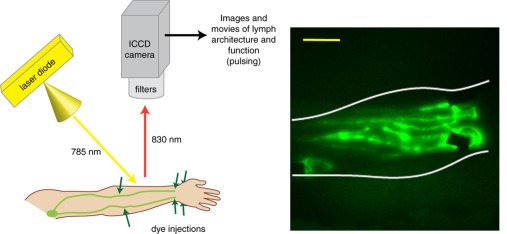
Fig. 2.
Near-infrared fluorescence imaging of human lymphatics utilizes laser illumination of intradermally injected fluorescent dye; (left) fluorophore emissions are captured by an imaging system and ICCD (intensified charge-coupled device) camera, producing real-time images and movies of lymphatic vessel architecture and function, such as the image (right) of ventral forearm lymphatic vessels, yellow scale bar = 5 cm.
In the United States, there are almost a half million surgical procedures per year for nodal staging to determine the number and sites of lymph nodes containing metastatic cancer. Lymph node dissection, coupled with radiation treatment, can result in morbidities that include lymphedema and diminished response to infections. At the UTHSC, we are working to validate the use of our instrumentation to noninvasively detect cancer-positive lymph nodes in breast cancer and melanoma patients in order to develop a non-surgical method for nodal staging. Initial studies show that the instrumentation can be used with a non-specific dye (ICG) to identify draining lymph nodes, compared to currently used (standard-of-care) blue dyes. As one form of validation, ex vivo fluorescent imaging of resected lymph nodes is compared to pathology results to determine specificity. In the process of validating the sensitivity of our NIRF instrumentation in humans, we unexpectedly visualized the “pumping” action of lymphatic vessels draining to lymph nodes and developed the approach for assessing lymphatic disorders, including cancer-related lymphedema. This unexpected discovery was possible owing to the high photon count rate and fast image acquisition (<200 milliseconds). Preclinical studies using dual-labeled imaging agents have demonstrated that the sensitivity of our instrumentation is comparable to nuclear imaging techniques [10].
In addition to our work with ICG, we have conducted safety and toxicity preclinical studies of another near-infrared dye, IRDye800CW [11], and we are assessing other commercially available near-infrared fluorophores. We are also developing antibody- and peptide-based diagnostic agents to tumor cell targets, such as the epithelial cell adhesion molecule (EpCAM), which is expressed in 90% of all cancers [12].
Standard validation so far has included keeping equipment logs for the imaging and support instrumentation, frequent updating of Standard Operating Procedures (SOPs), personnel training records, and the use and validation of batch release specifications, such as optical purity, immunoreactivity, and pyrogenicity. One published validation paper has resulted from these efforts [13], and several more are in preparation. Each time the imaging instruments are used, a SOP for instrument quality assurance is implemented and results are recorded. This process assures that the laser power is correct, the camera is operating within prescribed parameters, and trial information is properly logged by the operator. SciPort is an electronic data capture software developed by Siemens Corporate Research, and is being adapted to our optical imaging needs by the group. After developing features that make the software 21 CFR 11-compliant, the rapid adaptation of good laboratory and clinical practices in optical imaging trials could result.
In addition to agents and instrumentation, we have developed tomographic algorithms for detecting fluorescent agents and have adapted a Siemens PET/CT Inveon scanner with near-infrared technology based upon frequency domain techniques. We are now focusing on translating the algorithms in a stepwise fashion after instrumentation and agents are introduced into a human exploratory study. All of this work will require validation. The technologies that have been translated into the clinic are now being vetted for commercialization across the United States. Under NTR support, we have developed the basic outline of the 510(k) for the instrumentation, as well as a CE (European Commission harmonized standards) mark for its translation in Europe.
Currently, the NIRF technology at the UTHSC is the only technology being used to assess lymphatic dysfunction in patients who are at risk for lymphedema. This technology has been used to demonstrate the efficacy of treatment of lymphedema and wound healing that is currently under a decision for national coverage by Medicare/Medicaid. This is a “validation” effort to assess treatment efficacy [14]. In addition, this technology is used to phenotype subjects for genetic studies to discover the genetic basis for primary and perhaps acquired lymphedema. Validation efforts will have to be adapted to new applications that arise from such discoveries.
2.3. Targeted activation of optical imaging probes
Administration of exogenous molecular probes gives opportunity for specific optical activation of these probes where an enzyme is located, hopefully in diseased tissue. At Stanford University, we focused on COX-2 (cyclooxygenase-2), which is an attractive target for such imaging, because it is not expressed in most normal epithelial cells, but is strongly induced in inflammatory, premalignant, and malignant lesions. It is an important contributor to the resistance of cancer cells to apoptosis and promotes angiogenesis and metastasis [15]. COX-2 inhibitors have demonstrated impressive efficacy in clinical trials for prevention and adjuvant therapy [16,17]. Interestingly, COX-2 inhibitors are only efficacious in adjuvant therapeutic trials in patients who express COX-2 in their tumors [16]. Thus, COX-2-directed imaging agents could find application in early detection, definition of surgical boundaries, and patient stratification for targeted therapies.
The major challenge we faced in developing COX-2-targeted imaging agents was the construction of potent and selective inhibitors that also contained optical imaging moieties. Previous work from our laboratory indicated that amide derivatives of non-selective arylcarboxylic acid COX inhibitors were COX-2-selective, so this was the principal design feature we utilized to construct imaging agents [18]. A wide variety of non-steroidal anti-inflammatory drug cores–e.g., indomethacin, flurbiprofen, ketoprofen, and celecoxib were evaluated. These inhibitors were tethered through a series of alkylenes, piperazines, polyethylene glycols, or phenylenediamines to a diverse range of fluorophores. The fluorophores attached included dansyl, dabsyl, coumarin, fluorescein, rhodamine, Alexa Fluor, Nile Blue, Cy5, Cy7, near-infrared and infrared dyes, as well as lanthanide chelators.
Several hundred conjugates were synthesized and evaluated as inhibitors of COX-2 in vitro and in lipopolysaccharide-treated murine macrophages. Indomethacin conjugates to dansyl, dabsyl, coumarin, fluorescein, and rhodamine-derived fluorophores exhibited promising COX-2 inhibition and selectivity both in vitro and in intact cells. The compounds that emerged from our development pathway, the carboxy-X-rhodamine-containing compounds, Fluorocoxib A and Fluorocoxib B (linker = n-butyl), exhibited the best balance of cellular activity and optical properties (λex = 581 nm, λemit = 603 nm) [19].
A major challenge to molecular imaging of cancer is the validation of candidate compounds in intact animals. We devoted at least as much effort to the validation phase as to the discovery phase [19], testing the specificity of the compounds in vitro and in vivo. Fluorocoxibs A and B accumulate in inflamed tissue in COX-2 wild-type mice but not in COX-2 knockout mice. Accumulation in wild-type animals is prevented by pretreatment with selective or nonselective COX-2 inhibitors. Fluorocoxibs A and B selectively accumulate in human tumor xenografts in nude mice if the tumors express COX-2, but not if they do not express COX-2 (Fig. 3 ). Accumulation is slow, requiring up to three hours for maximal accumulation, but then displaying a long lifetime in the tumors—up to 24 hours. Fluorocoxibs A and B exhibit considerable metabolic stability, which appears to be a key feature of their in vivo efficacy. The fluorocoxibs represent the first feasible reagents for clinical detection of tissues containing high levels of COX-2 in settings amenable to fluorescent excitation and analysis by surface measurement or endoscopy (e.g., skin, esophagus, intestine, and bladder). Given the many hurdles that an in vivo imaging agent must overcome, it is perhaps not surprising that our ultimate success rate was approximately one percent, based on the number of candidate compounds synthesized.
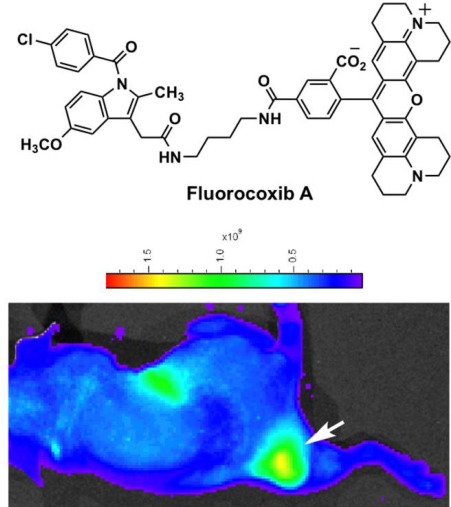
Fig. 3.
Fluorocoxib A, a COX-2-targeting peptide (top); and bioluminescent identification of dorsal mouse tumor (marked with white arrow in bottom image, with minor liver fluorescence) utilizing this COX-2-targeting peptide as the imaging agent.
The next challenge is advancement of the fluorocoxibs into the clinic. Here the challenges are different—e.g., formulation, toxicology, GMP synthesis—and out of the realm of most academic laboratories. This is where programs such as the Network for Translational Research in Imaging are essential to provide the complementary expertise necessary to move molecular imaging agents into human use. Without the NTR, or comparable entities, many compounds will be unable to cross the “Valley-of-Death” from promising candidate to useful agent, and the exciting potential of molecular imaging of cancer will not be realized.
2.4. Targeted detection of colonic dysplasia
The University of Michigan NTR team is developing a multimodality, multispectral imaging platform that uses specific peptides to target overexpressed cell surface receptors in premalignant colonic mucosa (dysplasia) [20]. This integrated imaging strategy (Fig. 4 ) aims to improve methods for early detection and image-guided therapy of colorectal cancer, and is being developed to address an important unmet clinical need for the improved detection of both polypoid and nonpolypoid colonic lesions that may transform into adenocarcinoma [21]. The effectiveness of conventional surveillance colonoscopy has been limited by a significant miss rate (>20%) for polypoid adenomas, as well as nonpolypoid lesions, with flat or depressed morphology, which may represent >25% of all premalignant disease, and may be particularly problematic on the right side of the colon.
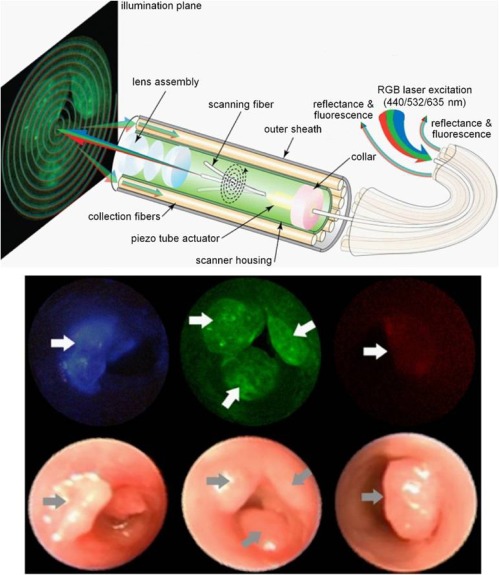
Fig. 4.
Schematic of endoscopic multispectral imaging system (top); and (bottom) fluorescent and white light images of highly specific peptide targeting of colonic dysplasia (arrows) using (left to right) 440, 532, and 635 nm excitation [26].
Peptides are well suited for clinical detection of dysplasia because of their rapid binding kinetics and high specificity. In addition, they are small in size, have flexibility for labeling, and incur minimal immunogenicity [22]. Methods of nuclear (scintigraphic) imaging are being developed to localize the anatomic segment of the colon where the premalignant mucosa may be present by radiolabeling the peptides [23]. Then, wide-field fluorescence endoscopy is utilized to perform “red flag” detection of the lesion and to clearly delineate the tumor margins for image-guided therapy [24]. Localization of disease allows for placement of a confocal endomicrosope to validate specific peptide binding to dysplastic colonic crypts with subcellular resolution [25] and for use of an endoscopic ultrasound (EUS) transducer to stage the depth of invasion.
We are partnering with GE Healthcare, Olympus Medical Systems Corp, and Mauna Kea Technologies to standardize and validate the use of this comprehensive, targeted imaging platform. Olympus is modifying a wide-field autofluorescence imaging (AFI) system to deliver narrow-band excitation (440-480 nm) for illuminating the FITC-labeled peptides [27,28]. An investigational new drug (IND) application has been approved by the FDA, and a Phase 1 study has been completed to evaluate the safety and specificity of these molecular probes [29].
Challenges for bringing this targeted imaging strategy to the market include 1) addressing regulatory requirements for safe and effective use of this novel imaging agent with the new instrument, 2) CMS (Centers for Medicare and Medicaid Services) approval for reimbursement of this innovative procedure, and 3) acceptance of this method by the medical community. This targeted approach will improve current methods of colorectal cancer surveillance by identifying lesions that cannot be seen or are missed, increasing the yield for detection of dysplasia, and more accurately defining the margin for tumor resection.
The validation activities are multifaceted and begin with the GMP synthesis and structural elucidation of the fluorescent peptides to be used for imaging studies. The peptide molecular identification and solution concentrations are also confirmed during analytical validation; their safety is established through GLP (good laboratory practices) toxicological studies. Further validation efforts include ex vivo histological evaluation. Eventual confirmation of safety and efficacy from phase 1 in vivo results will be performed by an independent center.
A number of additional validation steps are part of our efforts to bring this technology to the clinic. For example, we use fully validated release assay test procedures running on appropriately qualified analytical instrumentation (UPLC/HPLC/MS [ultraperformance liquid chromatography, high-performance liquid chromatography, mass spectrometry]) at the GMP facility where the affinity peptides are synthesized to confirm the peptide sequence, amino acid analysis, molecular identity, and purity of the drug substance. We also confirm various concentrations of the affinity peptide, both in dosing medium and in biological fluids, during toxicology studies at a GLP facility using fully validated assay test procedures running on appropriately qualified analytical instrumentation (UPLC/HPLC/MS/UV [ultraviolet]). For specificity and sensitivity validation, fluorescence stereomicroscopy of imaging ex vivo data is compared directly to histology results by accurate registration of pathology sections (<1 mm2 resolution) and evaluation by a gastrointestinal pathologist. A final validation step is in the planning stages, and will involve the reverification of the data and results from the current Phase 1 safety and efficacy study by an independent second research center.
3. NTR Validation/Clinical Studies core’s role
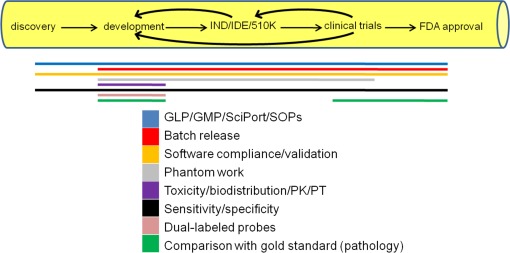
The four NTR centers’ imaging technologies, while different, share similar needs for translational validation. The NTR Validation/Clinical Studies core (VCS) initially tried to accurately and relevantly define these needs. Using a validation self-assessment tool designed for individual investigators, the validation needs and progress were determined for each network partner. In another, broader survey, the VCS core found that all four centers are at slightly different points in the translation process, and use different methods to move along the “translation pipeline,” as represented in Fig. 5 . For example, some NTR researchers are at the 510(k) stage for instrumentation, and at the development stage for markers. Several NTR members are developing technology directly with an industrial partner, or are working independently to produce and test an instrument prototype. One research group utilizes a CRO (contract research organization) for toxicity studies. Several groups have received IND approval, while others have requested help with the IND/IDE (investigational device exemption) process. One of the groups began talking to the FDA before the Office of Combination Products (OCP), which handles device-drug or device-biologic combinations, was established. This NTR group initially completed separate applications for their device and agent. Once OCP was established, the OCP instructed this group to apply through CDER (drugs only). For the off-label use of the imaging agent (indocyanine green), the group will need to apply for an ANDA (abbreviated new drug application), for which no preclinical or clinical data are needed for safety and effectiveness, and the device will use the 501(k) process. Three of the four groups are developing instrumentation and related biomarkers, and thus need guidance through OCP procedures.
Fig. 5.
The translation pipeline and validation needs. Some validation steps are needed throughout the translation process, and some steps are only necessary during portions of the process. Design iterations are designated by arched arrows.
It is important to distinguish “verification” from “validation” in the context of technological product development. Verification is a quality control process that evaluates if an engineering system satisfies its original design specification. For example, the NIRF systems pictured in Fig. 2 are verified using a checksheet/SOP prior to each use in clinical trials. Laser output, software performance, and image quality are confirmed and documented. Validation is a quality assurance process to establish that an imaging system fulfills the needs of end users–for example, the system actually images and distinguishes cancerous tissue.
In the context of translation, validation exists on several levels, and these levels can be difficult to conceptualize. At the most basic level for meeting GLP/GMP/GCP (good clinical practices) compliance, routine recordkeeping and use of standard operating procedures (SOPs) are essential. A higher level of validation necessitates proving imaging agent purity, sterility, immunogenicity, pyrogenicity, stability, or imaging device sterility, pyrogenicity, and reliability. The highest level of validation addresses clinical effectiveness of imaging agents or devices for diagnosing and treating disease, and may include cost analysis. The Validation/Clinical Studies (VCS) Core has identified specific validation guidelines for each of the levels.
The VCS Core recognized a number of validation hurdles. For example, despite several publications that have addressed translation of optical imaging devices [30–32], very few published validation protocols exist for batch release criteria for imaging devices and molecular imaging agents. Also, many researchers do not have CRO skill sets and institutional regulatory guidance. As a response to these needs, the VCS core contributed a section to an optical imaging-specific handbook [33], which is a consolidation of appropriate FDA guidances and other sources that are most relevant to validation efforts within the NTR network. The information in the handbook is tailored to three of the four NTR research groups, which are developing combinatorial imaging agents and devices, but most of the handbook is applicable to those researchers developing devices without imaging agents. The handbook lists useful links and references that support pre-IND/IDE submissions, IND/IDE submissions, and guidances and regulations relevant to the NTR, such as a guidance for combination products. The VCS Core’s section of the handbook specifically addresses validation topics, starting with an abbreviated glossary of terms needed for mid-level validations, and a general procedural outline for designing mid-level and high-level validation efforts.
The VCS Core has attempted to establish concrete validation objectives and outcomes for NTR. At the ground-floor level, involving SOPs and other regulatory paperwork, validation can be accomplished using a software tool (SciPort) [34] for electronic data entry and analysis. Software such as SciPort or other electronic data capture (EDC) systems can and should be used for bench and pre-clinical data collection and analysis, as well as SOP access, and computers used with bench equipment such as spectrophotometers and plate readers should employ 21 CFR Part 11-compliant software to document data entry, assure instrument performance, and deter data falsification. One paper produced by the UTHSC group has addressed a mid-level validation issue [13], and could be used as a guide by other groups. Another paper [35] describes the development of a phantom validation approach. The highest validation level, addressing effectiveness of imaging agents or devices for diagnosing and treating disease, remains to be fully developed by the core. Several papers produced by the UT group have assessed outside treatments using a UT medical device [14,36], and have compared optical to nuclear imaging using dual-labeling approaches [10]. While dual-labeled markers cannot yield perfect comparisons, simultaneous fluorescent and radioactive targeting that corresponds to radioactive targeting alone can give important information on the usefulness and validity of new fluorescent imaging techniques. Other methods need similar attention at this level. For instance, a pathology/histology study could help provide guidelines for the investigators. Additionally, cost effectiveness may be addressed, either by the investigator, by NTR, or a collaborative effort, perhaps involving CMS.
4. Future ambitions of the NTR Validation/Clinical Studies core and the NTR centers
Future goals of the VCS core include several publications that could aid not only NTR members, but also other groups and researchers striving to bridge the gap between lab bench discoveries and clinical use. A publication describing the real or hypothetical validation or quality control for pathology use in optical imaging could set a standard for specificity and sensitivity measurement in optical imaging. For instance, a pathology/histology study could help provide guidelines for the investigators, noting the need to engage pathologists throughout the project timeline [37]. A publication describing a real or hypothetical cost/benefit analysis for an optical imaging application would address a new, but most relevant, issue concerning CMS approval and reimbursement. Products that do not merit such approval have little chance of market success, and therefore, translational investment support.
Failure of innovative medical devices and agents to “make it” to the marketplace represents wasted private and tax dollars, deepens researchers’ frustrations, and discourages potential investors from supplying much-needed development capital. Development of tools to aid researchers in moving innovations from concepts to lab tests to clinical applications and marketable products is vital to continuing the momentum of current medical research. Improved medical care, healthier societies, and increased tax dollar revenues are all benefits of smooth translation of technologies. Imaging lies at the heart of medical diagnosis and treatment, and rapid translation of new optical imaging modalities is of vital importance. The NTR Validation/Clinical Studies Core will continue to develop tools for researchers within and out of the NTR network to efficiently translate their cutting-edge technologies.
Acknowledgment
We would like to acknowledge funding from the National Institutes of Health (NIH) U54 CA136429.
References and links
- 1.Wang L. V., Hu S., “Photoacoustic tomography: in vivo imaging from organelles to organs,” Science (to be published). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim C. H., Erpelding T. N., Jankovic L., Wang L. V., “Performance benchmarks of an array-based hand-held photoacoustic probe adapted from a clinical ultrasound system for non-invasive sentinel lymph node imaging,” Philos. Transact. A Math. Phys. Eng. Sci. 369(1955), 4644–4650 (2011). 10.1098/rsta.2010.0353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim C. H., Erpelding T. N., Maslov K., Jankovic L., Akers W. J., Song L., Achilefu S., Margenthaler J. A., Pashley M. D., Wang L. V., “Handheld array-based photoacoustic probe for guiding needle biopsy of sentinel lymph nodes,” J. Biomed. Opt. 15(4), 046010 (2010). 10.1117/1.3469829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim C. H., Erpelding T. N., Jankovic L., Pashley M. D., Wang L. V., “Deeply penetrating in vivo photoacoustic imaging using a clinical ultrasound array system,” Biomed. Opt. Express 1(1), 278–284 (2010). 10.1364/BOE.1.000278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L. V., “Multiscale photoacoustic microscopy and computed tomography,” Nat. Photonics 3(9), 503–509 (2009). 10.1038/nphoton.2009.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L. V., “Prospects of photoacoustic tomography,” Med. Phys. 35(12), 5758–5767 (2008). 10.1118/1.3013698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X., Pang Y., Ku G., Xie X., Stoica G., Wang L. V., “Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain,” Nat. Biotechnol. 21(7), 803–806 (2003). 10.1038/nbt839 [DOI] [PubMed] [Google Scholar]
- 8.Rasmussen J. C., Tan I.-C., Marshall M. V., Fife C. E., Sevick-Muraca E. M., “Lymphatic imaging in humans with near-infrared fluorescence,” Curr. Opin. Biotechnol. 20(1), 74–82 (2009). 10.1016/j.copbio.2009.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasmussen J. C., Tan I.-C., Marshall M. V., Adams K. E., Kwon S., Fife C. E., Maus E. A., Smith L. A., Covington K. R., Sevick-Muraca E. M., “Human lymphatic architecture and dynamic transport imaged using near-infrared fluorescence,” Transl Oncol 3(6), 362–372 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sampath L., Kwon S., Hall M. A., Price R. E., Sevick-Muraca E. M., “Detection of cancer metastases with a dual-labeled near-infrared/positron emission tomography imaging agent,” Transl Oncol 3(5), 307–317 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall M. V., Draney D., Sevick-Muraca E. M., Olive D. M., “Single-dose intravenous toxicity study of IRDye 800CW in Sprague-Dawley rats,” Mol. Imaging Biol. 12(6), 583–594 (2010). 10.1007/s11307-010-0317-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall M. A., Kwon S., Robinson H., Lachance P. A., Azhdarinia A., Ranganathan R., Price R. E., Chan W., Sevick-Muraca E. M., “Imaging prostate cancer lymph node metastases with a multimodality contrast agent,” Prostate 72(2), 129–146 (2012). 10.1002/pros.21413 [DOI] [PubMed] [Google Scholar]
- 13.Aldrich M. B., Wang X., Hart A., Kwon S., Sampath L., Marshall M. V., Sevick-Muraca E. M., “Assessment of free dye in solutions of dual-labeled antibody conjugates for in vivo molecular imaging,” Mol. Imaging Biol. 13(1), 32–42 (2011). 10.1007/s11307-010-0328-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams K. E., Rasmussen J. C., Darne C., Tan I. C., Aldrich M. B., Marshall M. V., Fife C. E., Maus E. A., Smith L. A., Guilloid R., Hoy S., Sevick-Muraca E. M., “Direct evidence of lymphatic function improvement after advanced pneumatic compression device treatment of lymphedema,” Biomed. Opt. Express 1(1), 114–125 (2010). 10.1364/BOE.1.000114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dannenberg A. J., Lippman S. M., Mann J. R., Subbaramaiah K., DuBois R. N., “Cyclooxygenase-2 and epidermal growth factor receptor: pharmacologic targets for chemoprevention,” J. Clin. Oncol. 23(2), 254–266 (2005). 10.1200/JCO.2005.09.112 [DOI] [PubMed] [Google Scholar]
- 16.Bertagnolli M. M., Eagle C. J., Zauber A. G., Redston M., Solomon S. D., Kim K., Tang J., Rosenstein R. B., Wittes J., Corle D., Hess T. M., Woloj G. M., Boisserie F., Anderson W. F., Viner J. L., Bagheri D., Burn J., Chung D. C., Dewar T., Foley T. R., Hoffman N., Macrae F., Pruitt R. E., Saltzman J. R., Salzberg B., Sylwestrowicz T., Gordon G. B., Hawk E. T., APC Study Investigators , “Celecoxib for the prevention of sporadic colorectal adenomas,” N. Engl. J. Med. 355(9), 873–874 (2006). 10.1056/NEJMoa061355 [DOI] [PubMed] [Google Scholar]
- 17.Edelman M. J., Watson D., Wang X., Morrison C., Kratzke R. A., Jewell S., Hodgson L., Mauer A. M., Gajra A., Masters G. A., Bedor M., Vokes E. E., Green M. J., “Eicosanoid modulation in advanced lung cancer: cyclooxygenase-2 expression is a positive predictive factor for celecoxib + chemotherapy--Cancer and Leukemia Group B Trial 30203,” J. Clin. Oncol. 26(6), 848–855 (2008). 10.1200/JCO.2007.13.8081 [DOI] [PubMed] [Google Scholar]
- 18.Kalgutkar A. S., Crews B. C., Rowlinson S. W., Marnett A. B., Kozak K. R., Remmel R. P., Marnett L. J., “Biochemically based design of cyclooxygenase-2 (COX-2) inhibitors: facile conversion of nonsteroidal antiinflammatory drugs to potent and highly selective COX-2 inhibitors,” Proc. Natl. Acad. Sci. U.S.A. 97(2), 925–930 (2000). 10.1073/pnas.97.2.925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uddin M. J., Crews B. C., Blobaum A. L., Kingsley P. J., Gorden D. L., McIntyre J. O., Matrisian L. M., Subbaramaiah K., Dannenberg A. J., Piston D. W., Marnett L. J., “Selective visualization of cyclooxygenase-2 in inflammation and cancer by targeted fluorescent imaging agents,” Cancer Res. 70(9), 3618–3627 (2010). 10.1158/0008-5472.CAN-09-2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsiung P.-L., Hardy J., Friedland S., Soetikno R., Du C. B., Wu A. P., Sahbaie P., Crawford J. M., Lowe A. W., Contag C. H., Wang T. D., “Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy,” Nat. Med. 14(4), 454–458 (2008). 10.1038/nm1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.T. D. Wang, “Targeted imaging of flat and depressed colonic neoplasms,” in Non-Polypoid (Flat and Depressed) Colorectal Neoplasms. Gastrointestinal Endoscopy Clinics of North America, R. M. Soetikno and T. Kaltenbach, eds. (Elsevier Saunders, 2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goetz M., Wang T. D., “Molecular imaging in gastrointestinal endoscopy,” Gastroenterology 138(3), 828–833.e1 (2010). 10.1053/j.gastro.2010.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joshi B. P., Wang T. D., “Exogenous molecular probes for targeted imaging in cancer: focus on multi-modal imaging,” Cancers 2(2), 1251–1288 (2010). 10.3390/cancers2021251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller S. J., Joshi B. P., Feng Y., Gaustad A., Fearon E. R., Wang T. D., “In vivo fluorescence-based endoscopic detection of colon dysplasia in the mouse using a novel peptide probe,” PLoS ONE 6(3), e17384 (2011). 10.1371/journal.pone.0017384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elahi S. F., Miller S. J., Joshi B., Wang T. D., “Targeted imaging of colorectal dysplasia in living mice with fluorescence microendoscopy,” Biomed. Opt. Express 2(4), 981–986 (2011). 10.1364/BOE.2.000981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller S. J., Lee C. M., Joshi B. P., Gaustad A., Seibel E. J., Wang T. D., “Targeted detection of murine colonic dysplasia in vivo with flexible multi-spectral scanning fiber endoscopy,” J. Biomed. Opt. (to be published). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsiung P. L., Wang T. D., “In vivo biomarkers for targeting colorectal neoplasms,” Cancer Biomark. 4(6), 329–340 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.M. Li and T. D. Wang, “Targeted endoscopic imaging,” in Enhanced Endoscopic Imaging. Gastrointestinal Endoscopy Clinics of North America, K. Wang and G. Elta, eds. (Elsevier Saunders, 2009). [Google Scholar]
- 29.T. D. Wang, University of Michigan, 109 Zina Pitcher Place, Ann Arbor, MI 48109, USA, is preparing a manuscript to be called “Phase 1 study of molecular probes to colonic dysplasia.”
- 30.S. S. Mehta, Commercializing Successful Biomedical Technologies (Cambridge University Press, 2008). [Google Scholar]
- 31.S. L. Gibbs-Strauss, M. Rosenberg, B. L. Clough, S. L. Troyan, and J. V. Frangioni, “First-in-human clinical trials of imaging devices: an example from optical imaging,” in Annual International Conference of the IEEE Engineering in Medicine and Biology Society,2009. EMBC 2009 (IEEE, 2009), pp. 2001–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gioux S., Choi H. S., Frangioni J. V., “Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation,” Mol. Imaging 9(5), 237–255 (2010). [PMC free article] [PubMed] [Google Scholar]
- 33.Network for Translational Research, Handbook for NTR Investigators (Nov. 9, 2010), http://ntroi.wustl.edu/wiki/ntroi/images/d/d7/Handbook_for_NTR_Investigators_110910_for_upload.pdf
- 34.Wang F., Hussels P., Liu P., “Securely and flexibly sharing a biomedical data management system,” Proc. SPIE 7264(726402), 1–8 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu B., Tan I. C., Rasmussen J. C., Sevick-Muraca E. M., “Validating the sensitivity and performance of near-infrared fluorescence imaging and tomography devices using a novel solid phantom and measurement approach,” Technol. Cancer Res. Treat. 11(1), 95–104 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Tan I. C., Maus E. A., Rasmussen J. C., Marshall M. V., Adams K. E., Fife C. E., Smith L. A., Chan W., Sevick-Muraca E. M., “Assessment of lymphatic contractile function after manual lymphatic drainage using near-infrared fluorescence imaging,” Arch. Phys. Med. Rehabil. 92(5), 756–764.e1 (2011). 10.1016/j.apmr.2010.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wells W. A., Barker P. E., MacAulay C., Novelli M., Levenson R. M., Crawford J. M., “Validation of novel optical imaging technologies: the pathologists’ view,” J. Biomed. Opt. 12(5), 051801 (2007). 10.1117/1.2795569 [DOI] [PubMed] [Google Scholar]