Abstract
Current medications for drug abuse have had only limited success. Anti-addiction vaccines to elicit antibodies that block the pharmacological effects of drugs have great potential for treating drug abuse. We review the status for two vaccines that are undergoing clinical trials (cocaine and nicotine) and two that are still in pre-clinical development (methamphetamine and heroin). We also outline the challenges and ethical concerns for anti-addiction vaccine development and their use as future therapeutics.
Problems of Drug Abuse and Current Therapies
Drug dependence is a disease state in which physical dependence on a substance leads to compulsive and repetitive use despite negative consequences to the user's health, mental state or social life. Globally, UNODC (United Nations Office on Drugs and Crime ) estimates that between 155 and 250 million people, or 3.5–5.7% of the population aged 15–64, used illicit substances at least once in 2008. Among them 16–38 million became “problem drug users,” which represents 10% to 15% of all people who used drugs. Even though the consequences of the drug abuse are devastating, only 12% to 30% of “problem drug users” had received some type of treatment, which means that 11 to 34 million problem drug users (70% -88%) received no treatment at all (Table 1).1
Table 1.
Illicit drug use at the global level among people aged 15–64 years in 2008
| Drug problem | Number of people (million persons) | Percentage of the population (total 4,396 million persons) |
|---|---|---|
| Used drugs at least once during 2008 | 155 to 250 | 3.5 to 5.7% of the population aged 15–64 |
| “Problem drug users” | 16 to 38 | 10 to 15% of all people who used drugs |
| “Problem drug users” who received no treatment | 11 to 33.5 | 70 to 88% of prob1em drug users received no treatment |
Opioid dependence is considered to be a lifelong, chronic relapsing disorder and substantial therapeutic efforts are needed to keep people drug free. Methadone maintenance therapy (MMT) was introduced in 1960s, and is currently recommended for opioid dependence because its outcomes are far superior to detoxification treatment.2 However, MMT is associated with a number of problems including diversion, overdose, high attrition rates within the first month, and the high cost of the maintenance clinics needed for the daily administration.3 In comparison, buprenorphine has a lower risk of overdose than methadone, limited diversion, fewer toxic medication interactions than methadone, and much greater patient access than methadone treatment.4 However, the reports to date suggest that buprenorphine has not surpassed methadone in its effectiveness for eventual sustained abstinence.2 Both methadone (opioid agonist) and buprenorphine (a partial opioid agonist) are susceptible to diversion, abuse and overdose, and various adverse reactions such as respiratory depression and sudden death.2,4,5 Thus, agonist treatments are not optimal for all patients and most patients will need a transition to some type of antagonist treatment like naltrexone. Naltrexone is a long acting opioid antagonist that does not produce euphoria and is not addicting. It is particularly suitable to prevent a relapse to opioid use after heroin detoxification for those with substantial contingencies that will enhance their compliance with treatment.2,6 However, only weak evidence supports naltrexone's effectiveness in clinical settings despite its theoretically ideal properties.2 Overall, the programs for controlling heroin addiction are costly. For example, the United States spent approximately $21.9 billion dollars on heroin addiction in 1996 alone. High cost has made these opiate maintenance programs unfeasible in much of the world7 and parts of the United States.8 Outcomes are also relatively poor with less than 25% of heroin addicts remaining abstinent after leaving methadone maintenance treatment9 and 60% of heroin addicts lapsing within 3 months following inpatient detoxification treatment.10 Finally, some individuals who are on these programs continue to use illicit drugs, commit crime and engage in behaviors that promote the spread of communicable diseases, such as HIV/AIDS and hepatitis B and C.
Cocaine is the most problematic drug worldwide after the opiates, notably in the Americas. Government surveys indicate that 2.4 million Americans age 12 or older are current users of cocaine, and 18% of them become “problem drug users”.11 There is a clear link between cocaine use and mortality with cocaine involved in close to 40% of all drug deaths in the United States.12 Although promising lines of pharmacotherapy research are examining medications that affect dopaminergic, GABAergic, serotonergic, or glutamatergic systems, there are no pharmacotherapeutic agents currently FDA approved for cocaine addiction.
Methamphetamine use may constitute a threat to health that is similar to abuse of crack cocaine, and its abuse has grown at alarming rates in rural areas in the United States over the past two decades, as well as being widespread in Southeast and East Asia more recently.13,14 Methamphetamine is highly addictive and toxic with abuse leading to high drug accumulation in most body organs and toxicity from potent central and peripheral sympathomimetic effects. 15,16,17 This widespread and long-lasting distribution of methamphetamine parallels its long-lasting behavioral effects, and likely contributes to various medical complications associated with its abuse, underscoring both the need and the challenge in developing an effective therapies.18 Currently, there are no FDA approved medications for treating methamphetamine addiction.
Tobacco addiction, the second-leading cause of death in the world is associated with approximately 5 million deaths each year, or 1 in 10 adult deaths. Currently, there are 1.3 billion smokers worldwide, 19 and with the present smoking trends, tobacco will kill 10 million people each year by 2020.20 To date, three medications are FDA-approved for smoking cessation: nicotine replacement therapy, sustained-release bupropion, and varenicline.21,22 Despite the relative efficacy of these first-line medications, long-term abstinence rates remain disappointingly low. Even through 75% of smokers want to stop smoking, less than 5% of those who make an attempt at quitting are successful.23
In summary, we either lack treatments (cocaine and methamphetamine) or have had limited success with existing therapeutics (heroin and nicotine). These limitations in treatment have opened the opportunity for developing alternatives such as vaccines for drug abuse. Anti-addiction vaccines have distinctly different mechanisms and therapeutic utility from small molecule approaches to treatment. They do not rely on inhibiting drug binding at specific receptors within the brain; rather the antibodies serve as pharmacokinetic antagonists, favorably altering the concentration-time course of drug distribution to multiple organ systems, especially reducing drug concentrations in the brain. Furthermore, vaccine production is less expensive, thus, they have great potential to become available for wider ranges of patients world-wide and for complementing the psychosocial tools needed for a transition to a medication-free and abstinent life.
Vaccines for Drug Abuse
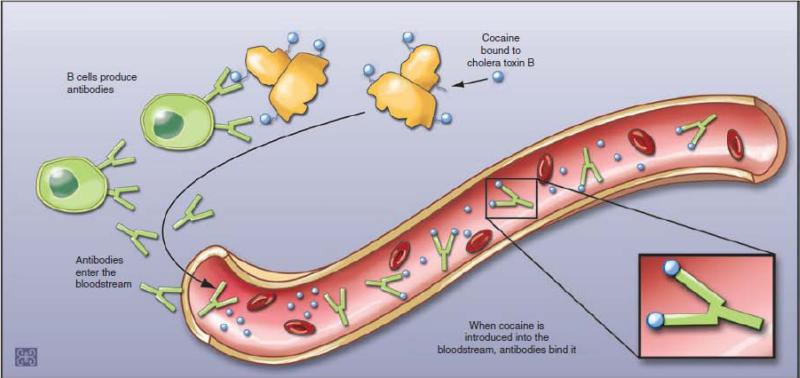
Anti-drug conjugates are typically created by the synthesis of a chemical derivative of the drug (hapten) and attaching that hapten to a highly immunogenic foreign “carrier” protein through a chemical linker. For example, the cocaine vaccine is comprised of succinylnorcocaine molecules covalently linked to a carrier protein derived from the cholera B toxin (rCTB), which is suspended in an aluminum adjuvant. This vaccine can stimulate B-cell to produce antibodies to cocaine as well as rCTB. When cocaine is later introduced into bloodstream, cocaine antibodies can bind to the drug, and form the antibody-drug compound molecules in circulation (Figure. 1). These molecules are too large to cross the blood brain barrier, thereby reducing the rate and quantity of the drug entry into the brain. Denied access to the brain, the drug cannot produce the reinforcement, or “high,” that is a major component of the motivation to continue drug use. Individuals who use a drug without obtaining its reinforcing effects are expected sooner or later to shed the powerful mental associations between the drug and pleasure that underlie craving and relapse (Figure. 2).24,25
Figure 1. Mechanism of action of cocaine vaccine.
The cocaine vaccine is comprised of succinylnorcocaine molecules covalently linked to a carrier protein derived from the cholera B toxin (rCTB), which is suspended in an aluminum adjuvant. This vaccine can stimulate B-cell to produce antibodies to cocaine as well as rCTB. When cocaine later enters the bloodstream, cocaine antibody can bind the drug, and form the antibody-drug compound molecules in circulation that are too large to cross the blood brain barrier.
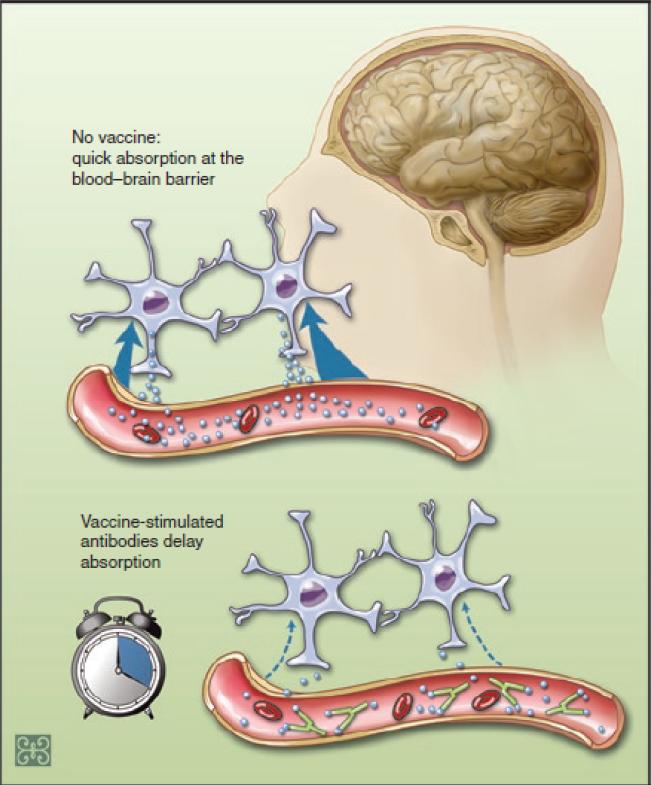
Figure 2. How a cocaine vaccine works.
In the absence of cocaine vaccine, drug readily absorbs at the brain blood barrier and enters brain. This causes reinforcement effects, or “high” of cocaine. Vaccine stimulates antibody production, sequesters drug in the blood circulation. This antibody-drug binding prevents the cocaine from rapidly leaving the blood vessels and entering the brain, reducing the drug's euphoric effects.
The history of addiction vaccines starts nearly 40 years ago with vaccines for opiate addiction.26,27 However, development was halted with the advent of methadone and naltrexone to treat heroin addiction, and the concept lay fallow for two decades after that. As the spread of addiction to stimulants like cocaine and methamphetamine became epidemic, interest in addiction vaccines was rejuvenated, resulting in the development of cocaine conjugate vaccines, as well as vaccines for nicotine. Both of these vaccines have progressed to the point of successful clinical trials. The data from the cocaine and nicotine vaccine trials suggest that many patients may not produce a sufficient antibody response for clinical efficacy, but those patients who attain high levels of antibodies can have excellent abstinence rates.28-30 Extending this technology to other currently abused substances as well as to new generations of “designer drugs” of abuse will be a tremendous opportunity to have innovative pharmacotherapies rapidly available. Indeed, anti-addiction vaccines are in various stages of development and being evaluated for quite a broad array of abused drugs, including cocaine, nicotine, methamphetamine, and heroin.24,30
Cocaine Vaccines
A therapeutic vaccine is particularly critical for cocaine addiction since there is currently no FDA approved pharmacotherapy to assist withdrawal, or to prevent the relapses that so frequently derail addicts’ recovery efforts.31 The current cocaine vaccine's development started with NIDA support in 1994 under Dr. Barbara Fox at Immulogic.32 It was produced by attaching cocaine to the surface of the antigenic carrier protein, cholera toxin B subunit, combined with the FDA approved human adjuvant alum. Both the carrier protein and adjuvant used in this cocaine vaccine are different from what was used in the first animal morphine vaccine, although the proof of principle is the same. Along with other labs, these early studies showed that conjugate cocaine vaccines could elicit strong antibody responses that blocked the pharmacological effects of the cocaine in animal models.
Clinical Trials
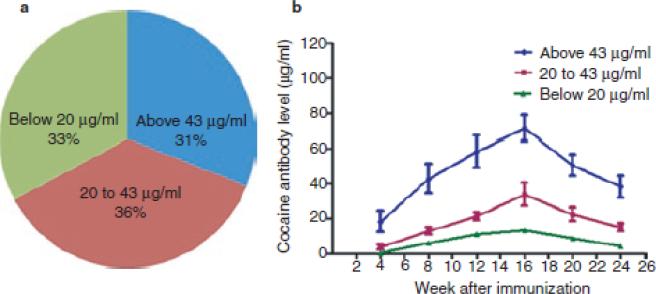
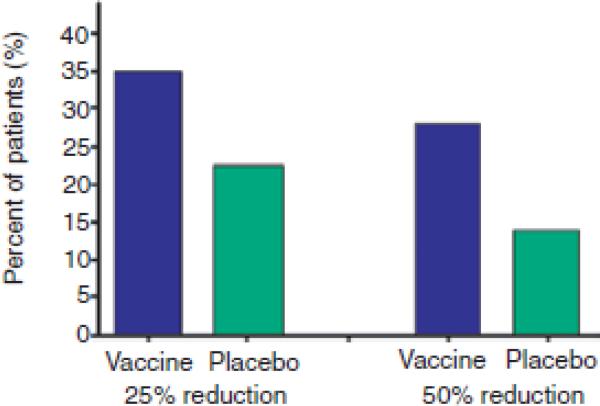
In 1996, our Yale University group was the first to use a cocaine conjugate vaccine in humans, and Phase I and IIa studies with this cocaine vaccine (TA-CD) were completed by 2005.33 The phase IIa studies used human laboratory methods to assess dosing requirements and short term risks from direct administration of cocaine to vaccinated individuals in a controlled laboratory setting. In those studies patients given relatively high vaccine doses for at least four vaccinations attained clinically relevant quantities of cocaine specific antibody, and the cocaine vaccine showed few side effects and no harmful interactions between the cocaine and anti-cocaine antibodies.34,35 From these studies the estimated level of antibodies needed was above 20 μg/ml in order for the TA-CD vaccine to substantially decrease the intoxicating effects of a single smoked cocaine dose.25 A later Phase IIb study (a randomized human outpatient trial to assess clinical efficacy, with vaccinations at week 0, 2, 4, 8, and 12 weeks) showed 67% of the patients attained sufficiently high antibody levels (over 20 μg/ml) after vaccination (Figures 3a), with antibody detectable as early as 4 weeks, and usually reaching peak levels at about 16 weeks (Figures 3b). In 31% of the patients a high antibody level response of 43 μg/ml or higher was attained and remained relatively high during 8 to 24 weeks after the first vaccination (Figure 3b). A higher percentage of vaccinated than placebo patients achieved both a 25% and 50% reduction in cocaine use between weeks 16 through 20 (Figure 4).
Figure 3. Antibody response to cocaine-CTB conjugate vaccine.
Figure 3a (left). Among the patients in our cocaine vaccine study, cocaine antibody levels attained high (over 43 μg/ml), medium (20 to 43 μg/ml) or low (below 20μg/ml) levels at 16 weeks after vaccination in 31%, 36% and 33% of the total patients who participated the phase IIb trial of cocaine vaccine.
Figure 3b (right). Cocaine antibody levels were first detectable at the 4 weeks after the initial vaccination, and reached their peak at 16 weeks, and remained relatively high until 24 weeks after vaccination in patients who attained peak cocaine antibody levels of at least 20 μg/ml.
Figure 4. Reduction of cocaine use in vaccination patients.
A higher percentage of vaccinated than placebo patients achieved both a 25% and 50% reduction in cocaine use between weeks 16 through 20.
The antibodies are produced to cocaine as well as cholera toxin b, since the polyclonal antibody response includes the cocaine chemically attached to the cholera toxin. Virtually everyone will produce substantial antibody responses to the cholera protein, but about 33% of patients produce inadequate anti-cocaine antibodies at levels below 20μg/ml (Figure 3a) for unknown reasons. This poor response is being actively investigated with a variety of very interesting leads related to human genetics and the production of immunological tolerance to cocaine. For example, this low level IgG response is associated in some patients with the presence of cocaine binding IgM antibodies which might reflect an immune response to adducts formed by the drug with native proteins in vivo.36 This could represent a T cell independent response to cocaine, which inhibits the desired T cell dependent IgG response to the conjugate vaccine, as has been reported with other haptens.37
The high affinity IgG antibody levels decline substantially over about 3 months after the peak antibody response in the patients who produced sufficient cocaine antibody levels for therapeutic efficacy in the initial response. A booster vaccination is needed to re-stimulate a rise in the antibody levels to the peak. Rather than needing the full series of five vaccinations again, however, a single vaccination may be sufficient to elevate antibody levels back to their therapeutic levels for about 3 months. Thus, for a period of protection lasting two years, patients may need to get about six additional boosters given as one every 3 months. Exposure to cocaine alone will not provoke an increase in antibodies because the cocaine molecule is too small to activate memory B cells by cross linking the antibodies expressed on their surface. However, after boosting with the conjugate vaccine, sufficient quantities of newly produced antibodies will rapidly bind most of a usual dose of cocaine when it enters the bloodstream. This binding prevents the cocaine from rapidly leaving the blood vessels and entering the brain, heart or other organs, reducing the drug's euphoric effects. (Figure 2) Because the antibody–cocaine complex is too large to pass thorough the normal blood brain barrier, the cocaine is then metabolized in the blood and liver to inactive metabolites by one of 3 mechanisms: spontaneous hydrolysis, tissue esterases (especially in the liver), or butyryl cholinesterase (in the bloodstream).38 Fortunately, these metabolites are sufficiently different in structure that they do not bind to the cocaine antibodies, and are simply excreted from the body.
Phase I and II studies with the cocaine vaccine were successful, in part, because of the combination of a good antibody response from conjugation with the cholera toxin carrier and the special advantage of the spontaneous and enzymatic hydrolysis of cocaine into inactive metabolites, as described above. When cocaine is bound by antibodies, the drug is slowly metabolized in the liver or other tissue sites, or excreted unchanged.39
The cocaine vaccine was conceived primarily as an agent for patients who could abstain from cocaine use for a limited period of time but needed help with maintaining that abstinence. This could work by inhibition of a phenomenon occurs across addictions and is called the “priming effect”.40 Briefly, priming involves even a single small dose of cocaine (or any other abused drug) exposure after a period of abstinence. This small exposure markedly intensifies craving, rather than reducing craving, and increases the risk for falling into a binge pattern of abuse and relapse. However, we also found that the vaccine could be useful beyond preventing this priming effect. In the Phase IIb cocaine trials some subjects who continued high level cocaine use did not appear to over-ride the antibody blockade of cocaine effects.34 Therefore, high antibody responders to vaccination may have complete blunting of deliberate attempts to override the vaccine's blockade by using more than an initial one or two drug doses.
The behavioral challenges for any successful vaccination program start with the need to have 2 to 3 months where the patient can be brought to a treatment site for five vaccinations. During these 2 to 3 months, the patients could be vulnerable to relapse if they have already discontinued drug use. While this drug abuse alone does not interfere with the vaccine's ability to induce the required antibody production, it is important that the patient get these vaccinations at appropriate times over the 3 months (e.g., 2, 4, 8 and 12 weeks after the initial vaccination) and continued drug abuse may result in failure to appear for these follow-up visits. Thus, counseling or other treatment efforts will be critical to insure compliance with the schedule of vaccinations; such interventions could vary from residential substance abuse care to outpatient contingency management, in which patients are paid to come for the vaccinations with an escalating pay schedule for each vaccination obtained.
Current Status
The success of the first placebo controlled clinical trial of a cocaine vaccine, as well as the relative ease with which these vaccines can be manufactured, has encouraged a multisite Phase IIb clinical trial of the cocaine vaccine to move forward. This four-month, double-blind, randomized, placebo-controlled, study compares the effect of the cocaine vaccine to placebo in reducing cocaine use in 300 treatment-seeking, cocaine-dependent individuals. Patients receive five vaccinations over a period of twelve weeks and some subjects will likely attain therapeutic antibody levels from the vaccine in weeks 6-8 after the first three vaccinations. Based on the success of this vaccine in the earlier clinical trials, this cocaine vaccine may be one of the first anti-addiction vaccines to be approved by the FDA for human use.
Nicotine Vaccines
Nicotine does not have the advantage of a serum enzyme that breaks it down into an inactive metabolite in the bloodstream. Lacking such a serum enzyme makes a successful nicotine vaccine more difficult if the only goal is to prevent deliberate override of the antibody levels. However, nicotine vaccines may prove as effective as cocaine vaccines because those being vaccinated are typically very motivated to stop smoking and do not have the ambivalence about abstinence that is common among cocaine and other illicit drug users.28 An early nicotine vaccine significantly reduced drug distribution to the brain from single nicotine doses, even after animals were chronically treated with total nicotine daily doses that exceeded the estimated binding capacity of antibody by 33-fold.41 Exceeding the binding capacity reflected that nicotine metabolism continues despite a portion of the drug being bound to antibody, permitting some antibody to be available for binding an additional nicotine dose. This remarkably greater efficacy than binding capacity also is probably due to the rapid on and off binding rates for nicotine as a small molecule that is bound by a single antibody combining site. Nevertheless, having quantitatively high antibody responses to clinical vaccines is critical to success.
Clinical Trials
Because of the substantial market for smoking cessation products, development of a vaccine against nicotine addiction has been of great interest to the pharmaceutical industry. As a result, three pharmaceutical companies have moved nicotine vaccines forward to human studies. (Table 2).42
Table 2.
Current status of anticocaine/antinicotine vaccines in clinical trials
| Vaccine | Target | Status | Characteristics | Company | Country |
|---|---|---|---|---|---|
| TA-CD Cocaine | Cocaine | Phase IIB | Cholera toxin B conjugate | Celtic Pharmaceuticals | United Kingdom |
| NIC002 | Nicotine | Phase II | Virus-like vaccine | Cytos Biotechnology/Novartis | Switzerland |
| TA-NIC | Nicotine | Phase II | A recombinant cholera toxin conjugate | Celtic Pharmaceuticals | United Kingdom |
| NicVax | Nicotine | Phase III | A bacterial exoprotein | Nabi Biopharmaceuticals/GlaxoSmithKline | United States |
1. NIC002
Phase II trials with NIC002 (also known as Nicotine QB or CYT002-NicQB, a Virus like vaccine) from Cytos Biotechnology were conducted in 341 smokers, and the outcome data were available from 239 study subjects who were divided into low, medium and high responders according to their nicotine antibody levels. The top third of the responders had higher abstinence rates at the 6 and 12 month follow ups, however, side-effects occurred in 69.4% of subjects.43 Also the abstinence rates among the low or medium responders were not different from the placebo group. A new Phase II trial in 200 cigarette smokers with a reformulated vaccine with fewer side effects was conducted in 2008 by Novartis. However, this trial also failed to achieve its primary endpoint (continuous abstinence from smoking during weeks 8 to 12 after start of treatment), possibly due to the insufficient antibody titers produced by this vaccine.44
2. TA-NIC
Celtic Pharmaceuticals completed a Phase II trial for TA-NIC (a recombinant cholera toxin) in 2009. This trial involved vaccine doses ranging from 100 to 250 μg to assess the efficacy and safety of the vaccine. While no results were released, in presentations the subjects getting the highest vaccine dose had a 25% abstinence rate from smoking compared to 9% in the placebo group, which was significant.45
3. NicVAX
The most advanced nicotine product is NicVAX (a bacterial exoprotein conjugate vaccine) by Nabi Biopharmaceuticals/GlaxoSmithKline.46 During 2004 to 2006, Nabi conducted five Phase I/II clinical studies involving more than 475 subjects. These trials demonstrated that NicVAX was well-tolerated, highly immunogenic, and gave a dose-dependent increase in antibody concentrations. In addition, these trials showed a correlation between antibody concentration and the ability of subjects to quit smoking and to remain abstinent up to 12 months.47
In 2007, Nabi completed their NicVAX Phase IIb trial, a double-blind, placebo-controlled, dose-ranging study. This trial enrolled a total of 301 heavy smokers who smoked an average of 24 cigarettes per day prior to enrollment. Sixty-one of 201 subjects (30%), who developed the highest level of nicotine antibodies, showed continuous abstinence for 8 weeks between weeks 19–26 at a rate that was almost three times that of the placebo group (16% in this 400 μg group and 6% in the placebo group). Among those 61 who had this high antibody response, but failed to quit, the number of cigarettes smoked per day was reduced from 20 to 10. In the remaining 70% of vaccinated subjects, abstinence rates were no better than placebo. Overall, these trials have not demonstrated nicotine vaccines to be superior to placebo when including all vaccinated subjects, because only a third of those vaccinated subjects developed sufficient levels of antibody to block the effects of nicotine. Another trial using an improved immunization schedule of 400 μg NicVAX given at six applications was completed in 2008. Based on this schedule, 80% of the subjects achieved the target antibody level at 14 weeks after vaccination.47
In 2008 Nabi started a Phase III trial for NicVAX, a double-blind, placebo-controlled trial with 1,000 patients. The primary endpoint of the study was the abstinence rate for 16 weeks ending at 12 months. Abstinence was evaluated by self-reported cigarette consumption and biologically verified by exhaled carbon monoxide. Unfortunately, the results from the first Phase III reported recently failed to meet its primary endpoint, namely the abstinence rate was not significantly greater in those vaccinated compared with the placebo group.47, 48 However, this assessment was done several months after the anti-nicotine antibody levels had fallen well below expected therapeutic levels. Nabi is awaiting the results of a second Phase III trial, but that trial appears to suffer from similar design problems; that is, treatment efficacy is assessed after the active agent responsible for the therapeutic effect (e.g., high antibody levels to nicotine) has been gone for several months.
Development Considerations
A successful nicotine vaccine could have an important advantage over pharmacological treatments in that they can have a prolonged effect (for 2-3 months after each booster dosing), thereby requiring limited patient cooperation to have adequate adherence, which could reduce relapse rates. Daily administration of a drug is not required; only bimonthly booster shots are needed to maintain an adequate antibody titer. However, there has been inconsistency in the degree of antibody response in large subject groups; some people do not achieve adequate antibody titers. Possible disadvantages of nicotine vaccines include the necessity for injections of the vaccine and the time delay before an effective immune response is achieved. Overall, nicotine vaccines could have significant success relatively soon, since they can be manufactured relatively inexpensively, the total smoking population worldwide is very large, motivation to quit smoking is generally high, and finally patients are quite unlikely to try to deliberately override the antibody capacity, unlike cocaine patients. The low cost of a nicotine vaccine will also facilitate distribution of the vaccine for public health purposes to a wide range of less wealthy populations in both developed and developing nations’ healthcare systems.
Vaccines for Methamphetamine Addiction
By slowing methamphetamine's entry into the brain, antibodies may be effective in reducing the pharmacological effects of this drug on the brain, thereby reducing its behavioral reinforcement, although pharmacokinetic studies have shown that the half life of the drug in circulation is significantly prolonged when bound to the specific antibodies for methamphetamine.49 Both cocaine and methamphetamine have fast uptake (reaching peak brain concentrations within several minutes); however, methamphetamine has slower clearance (hours) from the human brain.50 As a result, a high accumulation of methamphetamine in the brain occurs very quickly and lasts for hours. In vivo pharmacokinetics have shown that high concentrations of anti-methamphetamine monoclonal antibodies can produce a sustainable equilibrium shift of methamphetamine out of the brain and into the blood stream, as measured by substantial reductions in methamphetamine brain concentrations over time accompanied by substantial increases in methamphetamine serum concentrations.51,52 The rate of association and dissociation of the antibody binding to the drug may also influence the rate of drug entry and exit from the brain. These antibody-induced reductions in methamphetamine volume of distribution, clearance from the blood stream, and substantially increased serum protein (predominantly antibody) binding are why antibody treatments are classified as pharmacokinetic antagonists; that is, they favorably change the concentration-time course of methamphetamine in brain and other organ systems.
Even though the vaccines for methamphetamine abuse are still in preclinical development, this is a rapidly evolving field. A number of labs have been working on evaluating the best composition of a vaccine for methamphetamine by considering hapten design, selection of the carrier protein, the chemical positioning of a linker between the target antigen and the carrier protein, and choice of the best adjuvant.53-55 Janda's group has recently reported three methamphetamine conjugates that are able to generate substantial antibody titers (45-108 μg/mL) with moderate affinity (82, 130, and 169 nM).54 The data from our lab have shown that high titer antibodies can be elicited and maintained for 3 months by administration of methamphetamine conjugates in rodents, depending on the conjugate construction and the adjuvants used. Behavioral effects included inhibition of methamphetamine-stimulated locomotor activity in the vaccinated animals (Orson, unpublished). The passive administration of high affinity monoclonal antibodies have been shown to reduce methamphetamine self-administration in rats,56 and to reduce locomotor activity in rats given high dose methamphetamine.57,58 Our data have suggested that methamphetamine binding to antibody is equivalent to a high affinity monoclonal methamphetamine antibody59 in ELISA inhibition assays, when using polyclonal antibodies generated by 6-succinylmethamphetamine conjugated to the carrier protein either keyhole limpet hemocyanin (KLH) or OMPC, the outer membrane protein complex of the bacterium Neisseria meningitidis group B (Orson unpublished data).
Development Considerations
While the published human cocaine/nicotine studies support the feasibility of anti-methamphetamine vaccines, the anti-methamphetamine vaccines that generate efficacious levels of antibodies with sufficient binding affinity to the drug are not yet sufficiently characterized to proceed to clinical studies. The influence of average antibody binding affinity and kinetics from these new vaccines have not been adequately explored with traditional methods, and newer techniques will provide powerful analytical tools to evaluate these binding properties. These new techniques include surface plasmon resonance (which measures real time purified antibody binding kinetics to immobilized targets on a gold foil surface), isothermal titration calorimetry (which determines binding affinity by measuring heats of dilution with sequential addition of the unlabeled drug to purified antibody in solution), and microscale thermopheresis (which measures antibody binding properties in biologically relevant solutions, e.g., containing serum, to fluorescenated targets in capillary tubes during transient heat gradients). The locomotor assay that is commonly used in behavioral testing for screening of cocaine vaccine candidates may have limitations for testing the actual efficacy of vaccines against methamphetamine.53 Other models, for example self administration and conditioned place preference need to be considered for better evaluation of these vaccines against methamphetamine and to assess the degree to which the antibodies can be surmounted.
Vaccines for Opiate Addiction
The history of anti-addiction vaccines starts nearly 40 years ago. The proof of principle for an anti-addiction vaccine was first demonstrated by two studies for morphine vaccines: In 1972, Berkowitz and colleagues 26 published their creation of a morphine vaccine in animals. Using rats, they administered a morphine hapten linked to bovine serum albumin and created anti-morphine antibodies. These antibodies reduced the concentration of free morphine in the plasma of their vaccinated rats. In 1974, Bonese created a similar vaccine in primates, and the vaccinated rhesus monkey primates decreased their self-administration of heroin. 27 Other early studies also showed that morphine conjugates could produce antibodies with specificity to heroin and 6-acetylmorphine, as well as morphine itself.60-62 The binding specificity may differ depending on the hapten used the antibodies, and the antibodies were saturable; so higher drug doses could overcome the binding capacity of the antibodies in circulation.63
In order to produce long lasting morphine/heroin antibodies that block the pharmacological effects the drug, opiate vaccines are currently being revisited.64-67 Anton and Lef65 demonstrated that a morphine-tetanus toxoid vaccine was able to produce antibodies that prevented the acquisition of heroin self-administration in rats. However, this vaccine required four boosts over 60 days, and biweekly boosts over the period of a year in order to keep adequate titers. Janda and his colleagues published their data recently and have found that the polyclonal antibodies produced by a vaccine with a heroin-like hapten linked to KLH had micromolar affinities to 6-acetyl morphine (6-AM), heroin and morphine, but were nonetheless able to prevent the acquisition of heroin self-administration and the antinociceptive effects of heroin in rodents. Conversely, antibodies generated by a morphine-like KLH-vaccine only had adequate affinity for morphine and reduced binding for heroin, but no affinity for 6-acetyl morphine; in addition, the morphine-like vaccine was not effective for prevention of heroin administration acquisition.67
Heroin is a prodrug, rapidly converted to the pharmacologically active opiate 6-acetylmorphine and to morphine by esterases in both the periphery and the central nervous system (CNS).68 About 10% of morphine is converted to morphine-6-glucuronide in the liver within 2 hours after an intravenous injection. A vaccine that produces antibodies which bind to morphine-6-glucuronide is desirable, though likely less important quantitatively, as the penetration of morphine-6-glucuronide into the CNS is slow and it contributes substantially less than the other metabolites to the reinforcing effects of opiates.63 One of the ways to make the morphine conjugate is to link the hapten through the 6 hydroxyl group of morphine to a carrier protein. Earlier study using a morphine-6-succinyl-BSA conjugate produced antisera that bound to heroin, morphine, as well as other active morphine derivatives.61 Using a similar approach, we have evaluated various morphine conjugates for the most effective vaccine design and immunization conditions to elicit high level antibody responses that can bind heroin and its active metabolites. Our recent studies have demonstrated that moderate to high levels of anti-morphine antibodies are elicited by 6-succinylmorphine conjugated to KLH together with either alum or monophosphoryl lipid A (MPL) in rodents 69,70 In agreement with previous studies61, we showed that polyclonal antibodies generated by morphine vaccines were able to bind to morphine and its metabolites (6-acetyl morphine, 3-glucuronide morphine, and 6-glucuronide morphine) with nanomolar affinity.70 In addition, our efficacy studies demonstrated a significant inhibition of morphine induced analgesia69,70 and the reinforcing effects of morphine70 as demonstrated in conditioned place preference in the immunized animals. These behavioral changes in the vaccinated rats were at least in part due to sequestration of the drug in the blood and reduction of the drug levels in the brain by antibodies.70
Development Considerations
A significant challenge to this approach has been the wide range of abused prescription opioids. Although morphine and heroin can both be blocked by antibodies from a single type of vaccine, at least five different vaccine types may be needed to block all the types of synthetic opiates that have now been manufactured as alternatives to morphine due to their widely varying chemical structures. Because prescription opiate abuse has overtaken heroin as the major opiate abuse problem here, a vaccine approach to blocking heroin abuse will be less urgent for clinical use in the USA. However, in the developing world, such as Southeast Asia, the Middle East (Iran) and Mexico, where morphine and heroin are the opiates most commonly abused, an effective vaccine would be life-saving and very cost effective. By building on previous research, designing methodologically sound studies and systematically assessing patient outcomes during the clinical trial and at 6 to 12 month follow-ups, such vaccines can hopefully win FDA approval and then assist in combating this serious public health issue.
Challenges and Implications
Challenges in Delivering Clinically Effective Anti-Addiction Vaccines
Clinical trials of cocaine and nicotine vaccines have so far reported modest efficacy. The abstinence rates among those vaccinated against nicotine have been no better than placebo overall, but the rates have been superior to placebo in the third of patients who achieved therapeutic antibody levels, demonstrating that the principle of antibody blockade is valid, and that an adequate antibody response is a primary requirement for efficacy. Thus, enhancing the proportion of responders and the magnitude of antibody responses will be critical for achieving broad utility of this approach. Altering the number of doses and/or the size of the dose may improve immunogenicity, however, a requirement for multiple injections may present compliance issues and, in much of the world, significant logistic challenges. These changes could potentially also increase adverse effects so that the safety and efficacy of the revised dosage schedules will need to be established.
Adequate use of adjuvants with a highly immunogenic carrier protein is critical for development of an efficacious conjugate vaccine. The innovative design of the conjugate vaccines using highly immunogenic foreign carrier proteins can dramatically improve the quantity and quality of immune responses. The OMPC carrier has had extraordinary success as the protein conjugate partner for Haemophilus influenza type b polysaccharide vaccine.71 It elicits antipolysaccharide immune responses in young infants and leads to markedly reduced infection rates with this organism. Our preliminary data (unpublished) indicates that OMPC is particularly attractive as a carrier for the anti-addition conjugate vaccines, since it elicits early, high level antibody responses to the drug. KLH vaccines for nicotine72 and cocaine73, 74 have shown excellent results in animals to reduce the free drug level in plasma. Other KLH conjugates are in active clinical evaluation for cancer (bladder, breast myeloma, prostate, ovarian, lymphoma)75 and hypertension.76 It has be demonstrated that the new generation of adjuvants can potentially promote the immune response more robustly and may reduce the number of doses required for the initial immunization series from five to perhaps three administrations.77,78 Currently, there are three licensed adjuvants as listed in the Table 2, and many others are under widespread experimental use or in late stage clinical development. 79 Aluminum salts (alum) have been used as adjuvants with great success since the 1930s and have been particularly effective at promoting protective humoral immunity. The responses to proteins with alum tend to be a mix of Th2 and Th1 cells,80 in humans, and it is used primarily to enhance antibody production.81 Alum is not optimally effective for diseases where cell-mediated immunity is required.82 MF59 (Novartis) is used in Europe with seasonal influenza vaccines in the elderly and pandemic influenza vaccines.83 MF59 is an oil-in-water emulsion based on squalene. It stimulates stronger antibody responses, permits fewer antigen doses, and generates marked memory responses with a mixed Th1-Th2 cell phenotype.84 MPL is now a component of licensed vaccines for HBV and human papilloma virus and has proven to be both safe and effective.85 MPL is recognized specifically by TLR4, and leads to signaling through the TRIF adaptor.86 An efficacious vaccine should have high immunogenicity to elicit and maintain good quality antibodies at sufficient levels throughout a sustained period of several months after the initial vaccination series and then after each booster vaccination. High immunogenicity of the vaccine can also help confer greater antibody specificity to reduce competition for binding to other compounds such as endogenous molecules or inactive metabolites. This improves safety and reduces the likelihood of adverse side effects. Proper use of carrier proteins as well as adjuvants may contribute to development of an efficacious vaccine for drug abuse.
Ethical and Legal Challenges
Apart from the technical and biological development hurdles discussed above, ethical and legal challenges are also barriers to implementation of these innovative treatments. The adoption of new conjugates/adjuvants into licensed vaccines has been slowed by a variety of hypothetical safety concerns. Commercially, vaccines themselves are generally considered high risk with a low profit margin, in large part because of the extended liability that a company can incur with the prolonged legal exposure produced through vaccination. The fundamental stigma of substance abuse, often viewed as a moral failure rather than a brain disease, pervades many aspects of treatment for substance abuse. When addiction is considered a failure of will power or as willful misconduct, then treatment is generally directed toward the behavioral disorder, with little consideration given to direct medical intervention, so that even the addict may not recognize the potential benefit of therapeutic vaccination. The economic correlate of this perception within the pharmaceutical industry is that these patients would provide a poor return on investment for the costs and risks of developing an immunological intervention. This perception has been countered to a significant extent for tobacco smoking, possibly because of its legality and high worldwide prevalence, and as a result, nicotine vaccines have been in clinical trials by three companies.
Vaccines may have potential use as preventive agents in high risk populations; however, certain ethical concerns need to be considered carefully before implementation in that context.87,88 For example, the treatment may be offered as an alternative to imprisonment to persons who have been charged with or convicted of an offence to which their drug dependence has contributed, its main justification being that treating offenders’ drug dependence will reduce their chance of reoffending.89,90 Another example could be vaccinating adolescents against nicotine. If the adolescents then subsequently smoked ten times more cigarettes in order to overcome the nicotine blockade, the result of such high levels of smoking for prolonged periods would be a massive increase of ingested carcinogens, on which the vaccine would have no effect. It may also be feasible to use the vaccines in drug-abusing pregnant women to protect the fetus and mother from drug exposure and its complications. Finally, parents might request that their children be vaccinated to prevent future drug abuse, even though the risks of future drug abuse for them as individuals might not be well defined.88
The ethical and legal challenges have made FDA approval for an anti-addiction vaccine extremely complicated, and the precise requirements for FDA approval are not clear because no such vaccine has yet been approved.89 FDA approval requires demonstration of not only clinical efficacy, but also medical safety. The preventive use of a vaccine in healthy young people would require stronger evidence of safety and efficacy than shorter-term use to reduce relapse in adults who are drug dependent. Obtaining evidence to meet regulatory requirements for such use would likely be even more expensive to accomplish.88,90 As a result, FDA approval concerns are primary issues to those companies who might manufacture, license and sell these vaccines.
Conclusions
Current medications for drug abuse have only had limited success for drugs such as cocaine, nicotine, methamphetamine, and heroin. Therapeutic vaccines are very attractive because they sequester the drug in the blood, and have no direct effects on the brain, endocrine system or any other organs. A primary goal for anti-addiction vaccines is to help addicts stay off the abused substance by inhibiting the dramatic reinforcement of drug seeking behavior that can occur from social stresses leading to single or brief drug exposures, especially in the early critical months after withdrawal. Ideally, however, it would be useful to have the capacity to block multiple doses at high concentrations. The results from human studies of the first cocaine vaccine and three nicotine vaccines are promising, and preclinical development of efficacious methamphetamine and opiate vaccines is rapidly progressing. Higher average antibody titers than those yet produced seem likely to be required if vaccines are to become a feasible alternative to the available active compounds. Advances in vaccine conjugate design, carrier protein use, and especially adjuvant optimization should significantly enhance the quantity and quality of the antibodies produced, allowing drug vaccines to become useful clinical tools for the treatment of substance abuse. However, current treatments for opiates in particular may have limited needs for alternatives, since they have a track record of success in maintaining abstinence and preventing complications of abused drugs. Furthermore, concern has been raised regarding the ethical, legal, and regulatory barriers to implementation of anti-addition vaccines.
ACKNOWLEDGEMENTS
Supported by the Department of Veterans Affairs (VA) Merit Review Program and VISN 16 Mental Illness Research, Education and Clinical Center (MIRECC), the VA National Substance Use Disorders Quality Enhancement Research Initiative (QUERI), and the National Institute on Drug Abuse grants K05 DA 0454 (TRK), P50-DA18197 (TRK), 5R01DA030338 (FMO), 1X02DA032939 (TRK, FMO, XYS), R01DA026859 (FMO, XYS).
References
- 1.World drug report 2010. http://www.unodc.org/documents/wdr/WDR_2010/World_Drug_Report_2010_lo-res.pdf.
- 2.Stotts AL, Dodrill CL, Kosten TR. Opioid dependence treatment: options in pharmacotherapy. Expert Opin Pharmacother. 2009;10(11):1727–40. doi: 10.1517/14656560903037168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maxwell S, Shinderman MS. Optimizing long-term response to methadone maintenance treatment: a 152-week follow-up using higher-dose methadone. J Addict Dis. 2002;21(3):1–12. doi: 10.1300/J069v21n03_01. [DOI] [PubMed] [Google Scholar]
- 4.Kahan M, Srivastava A, Ordean A, Cirone S. Buprenorphine: new treatment of opioid addiction in primary care. Can Fam Physician. 2011 Mar;57(3):281–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Kreek MJ, Borg L, Ducat E, Ray B. Pharmacotherapy in the treatment of addiction: methadone. J Addict Dis. 2010;29(2):200–16. doi: 10.1080/10550881003684798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev. 2011;16(2):CD001333. doi: 10.1002/14651858.CD001333.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Tang YL, Zhao D, Zhao C, Cubells JF. Opiate addiction in China: current situation and treatments. Addiction. 2006;101(5):657–65. doi: 10.1111/j.1360-0443.2006.01367.x. [DOI] [PubMed] [Google Scholar]
- 8.Mark TL, Woody GE, Juday T, Kleber HD. The economic costs of heroin addiction in the United States. Drug Alcohol, Depend 1. 2001;61(2):195–206. doi: 10.1016/s0376-8716(00)00162-9. [DOI] [PubMed] [Google Scholar]
- 9.Dekimpe MG, Van de Gucht LM, Hanssens DM, Powers KI. Long run abstinence after narcotics abuse: What are the odds? Management Science. 1998;44:1478–1492. [Google Scholar]
- 10.Gossop M, Stewart D, Browne N, Marsden J. Factors associated with abstinence, lapse or relapse to heroin use after residential treatment: Protective effect of coping responses. Addiction. 2002;97:1259–1267. doi: 10.1046/j.1360-0443.2002.00227.x. [DOI] [PubMed] [Google Scholar]
- 11.National Survey on Drug Use and Health: National Findings . Office of Applied Studies, NSDUH Series H-30, DHHA Publication No. SMA 06-4194. Rockville, MD: 2006. http://www.drugabusestatistics.samhsa.gov/ [Google Scholar]
- 12.Substance Abuse and Mental Health Services Administration (SAMHSA) Office of Applied Studies . Results from the 2007 national survey on drug use and health: National findings. Rockville, MD: [Google Scholar]
- 13.Gonzales R, Mooney L, Rawson RA. The methamphetamine problem in the United States. Annu Rev Public Health. 2010;31:385–398. doi: 10.1146/annurev.publhealth.012809.103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKetin R, Kozel N, Douglas J, Ali R, Vicknasingam B, Lund J, Li JH. The rise of methamphetamine in Southeast and East Asia. Drug Alcohol Rev. 2008;27:220–228. doi: 10.1080/09595230801923710. [DOI] [PubMed] [Google Scholar]
- 15.Darke S, Kaye S, McKetin R, Duflou J. Major physical and psychological harms of methamphetamine use. Drug Alcohol Rev. 2008;27(3):253–262. doi: 10.1080/09595230801923702. [DOI] [PubMed] [Google Scholar]
- 16.Volkow ND, Fowler JS, Wang GJ, Shumay E, Telang F, Thanos PK, Alexoff D, Volkow ND. Distribution and pharmacokinetics of methamphetamine in the human body: clinical implications. PLoS One. 2010;7(12):e15269. doi: 10.1371/journal.pone.0015269. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fowler JS, Kroll C, Ferrieri R, Alexoff D, Logan J, Dewey SL, Schiffer W, Schlyer D, Carter P, King P, Shea C, Xu Y, Muench L, Benveniste H, Vaska P, Volkow ND. PET studies of dmethamphetamine pharmacokinetics in primates: comparison with l-methamphetamine and (–)-cocaine. J Nucl Med. 2007;48:1724–1732. doi: 10.2967/jnumed.107.040279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meijler MM, Matsushita M, Wirsching P, Janda KD. Development of immunopharmacotherapy against drugs of abuse. Curr Drug Discov Technol. 2004;1:77–89. doi: 10.2174/1570163043484851. [DOI] [PubMed] [Google Scholar]
- 19.Esson L, Leeder SR. The millennium development goals and tobacco control: an opportunity for global partnership. World Health Organization (WHO); Geneva: 2004. [Google Scholar]
- 20.World Health Organization Why is tobacco a public health priority? A Tobacco Free Initiative. 2005 http://www.who.int/tobacco/health_priority/en.
- 21.Aubin HJ, Karila L, Reynaud M. Pharmacotherapy for smoking cessation: present and future. Curr Pharm Des. 2011;17(14):1343–50. doi: 10.2174/138161211796150837. [DOI] [PubMed] [Google Scholar]
- 22.Hays JT, Ebbert JO. Adverse effects and tolerability of medications for the treatment of tobacco use and dependence. Drugs. 2010;24(18):2357–72. doi: 10.2165/11538190-000000000-00000. 70. [DOI] [PubMed] [Google Scholar]
- 23.The Health Consequences of Smoking, Centers for Disease Control and Prevention USA. Surgeon's General Report 2004. [PubMed]
- 24.Orson FM, Kinsey BM, Singh RA, Wu Y, Gardner T, Kosten TR. Substance abuse vaccines. Annals of the New York Academy of Sciences. 2008;1141:257–269. doi: 10.1196/annals.1441.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haney M, Gunderson EW, Jiang H, Collins ED, Foltin RW. Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biol Psychiatry. 2010;67(1):59–65. doi: 10.1016/j.biopsych.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berkowitz B, Spector S. Evidence for active immunity to morphine in mice. Science. 1972;22(67):1290–2. doi: 10.1126/science.178.4067.1290. 178. [DOI] [PubMed] [Google Scholar]
- 27.Bonese KF, Wainer BH, Fitch FW, Rothberg RM, Schuster CR. Changes in heroin self-administration by a rhesus monkey after morphine immunisation. Nature. 1974;20(5485):708–10. doi: 10.1038/252708a0. 252. [DOI] [PubMed] [Google Scholar]
- 28.Maurer P, Bachmann MF. Vaccination against nicotine: an emerging therapy for tobacco dependence. Expert Opin Investig Drugs. 2007;16(11):1775–83. doi: 10.1517/13543784.16.11.1775. [DOI] [PubMed] [Google Scholar]
- 29.Hatsukami DK, Jorenby DE, Gonzales D, Rigotti NA, Glover ED, Oncken CA, Tashkin DP, Reus VI, Akhavain RC, Fahim RE, Kessler PD, Niknian M, Kalnik MW, Rennard SI. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther. 2011;89(3):392–9. doi: 10.1038/clpt.2010.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haney M, Kosten TR. Therapeutic vaccines for substance dependence. Expert Rev Vaccines. 2004;3(1):11–8. doi: 10.1586/14760584.3.1.11. [DOI] [PubMed] [Google Scholar]
- 31.Karila L, Gorelick D, Weinstein A, Noble F, Benyamina A, Coscas S, Blecha L, Lowenstein W, Martinot JL, Reynaud M, Lépine JP. New treatments for cocaine dependence: a focused review. Int J Neuropsychopharmacol. 2008;11(3):425–38. doi: 10.1017/S1461145707008097. [DOI] [PubMed] [Google Scholar]
- 32.Fox BS, Kantak KM, Edwards MA, Black KM, Bollinger BK, Botka AJ, French TL, Thompson TL, Schad VC, Greenstein JL, Gefter ML, Exley MA, Swain PA, Briner TJ. Efficacy of a therapeutic cocaine vaccine in rodent models. Nat Med. 1996;2:1129–1132. doi: 10.1038/nm1096-1129. [DOI] [PubMed] [Google Scholar]
- 33.Kosten TR, Biegel D. Therapeutic vaccines for substance dependence. Expert Rev Vaccines. 2002;1:363–371. doi: 10.1586/14760584.1.3.365. [DOI] [PubMed] [Google Scholar]
- 34.Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, Kosten TR. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66(10):1116–23. doi: 10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martell BA, Mitchell E, Poling J, Gonsai K, Kosten TR. Vaccine pharmacotherapy for the treatment of cocaine dependence. Biol Psychiatry. 2005;58:158–164. doi: 10.1016/j.biopsych.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 36.Deng SX, de Prada P, Landry DW. Anticocaine catalytic antibodies. J Immunol Methods. 2002;1(1-2):299–310. doi: 10.1016/s0022-1759(02)00237-5. 269. [DOI] [PubMed] [Google Scholar]
- 37.Lindroth K, Mastache EF, Roos I, Fernández AG, Fernández C. Understanding thymus-independent antigen-induced reduction of thymus-dependent immune responses. Immunology. 2004;112(3):413–9. doi: 10.1111/j.1365-2567.2004.01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stewart DJ, Inaba T, Lucassen M, Kalow W. Cocaine metabolism: cocaine and norcocaine hydrolysis by liver and serum esterases. Clin. Pharmacol. Ther. 1979;25:464–468. doi: 10.1002/cpt1979254464. [DOI] [PubMed] [Google Scholar]
- 39.Williams RH, Maggiore JA, Shah SM, Erickson TB, Negrusz A. Cocaine and its major metabolites in plasma and urine samples from patients in an urban emergency medicine setting. J Anal Toxicol. 2000;24:478–481. doi: 10.1093/jat/24.7.478. [DOI] [PubMed] [Google Scholar]
- 40.de Wit H. Priming effects with drugs and other reinforcers. Exp & Clin Psychopharm. 1996;4:5–10. [Google Scholar]
- 41.Hieda Y, Keyler DE, Ennifar S, Fattom A, Pentel PR. Vaccination against nicotine during continued nicotine administration in rats: immunogenicity of the vaccine and effects on nicotine distribution to brain. Int J Immunopharmacol. 2000;22(10):809–19. doi: 10.1016/s0192-0561(00)00042-4. [DOI] [PubMed] [Google Scholar]
- 42.Polosa R, Benowitz NL. Treatment of nicotine addiction: present therapeutic options and pipeline developments. Trends Pharmacol Sci. 2011;32(5):281–9. doi: 10.1016/j.tips.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cornuz J, Zwahlen S, Jungi WF, Osterwalder J, Klingler K, van Melle G, Bangala Y, Guessous I, Müller P, Willers J, Maurer P, Bachmann MF, Cerny T. A Vaccine against Nicotine for Smoking Cessation: A Randomized Controlled Trial. PLoS ONE. 2008;3(6):e2547. doi: 10.1371/journal.pone.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cytos Biotechnology . CYT002 Nic Qb: a novel vaccine for nicotine addiction. Cytos Biotechnology; Zurich: 2006. http://www.cytos.com/doc/NicQb_June06_E_fv.pdf. [Google Scholar]
- 45.A Phase 2, Double-Blind, Randomized, Placebo-Controlled, Multicenter, Dose-Ranging Study of 100 or 250 μg of TA-NIC to Assess the Efficacy and Safety of the Vaccine as an Aid to Smoking Cessation. http://www.clinicaltrials.gov/ct2/show/NCT00633321?term=TA-NIC&rank=1.
- 47.Clinical Trials NicVAX® (Nicotine Conjugate Vaccine) http://www.nabi.com/pipeline/clinicaltrials.php.
- 48.News Release, Nabi Biopharmaceuticals Announces Results of First NicVAX(R) Phase III Clinical Trial. Smoking Cessation Immunotherapy Failed to Meet Primary Endpoint. ROCKVILLE, Md: http://phx.corporate-ir.net/phoenix.zhtml?c=100445&p=irolnewsArticle&ID=1586001&highlight= [Google Scholar]
- 49.Orson FM, Kinsey BM, Singh RA, Wu Y, Kosten TR. Vaccines for cocaine abuse. Hum Vaccin. 2009;5(4):194–9. doi: 10.4161/hv.5.4.7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fowler JS, Volkow ND, Logan J, Alexoff D, Telang F, Wang GJ, Wong C, Ma Y, Kriplani A, Pradhan K, Schlyer D, Jayne M, Hubbard B, Carter P, Warner D, King P, Shea C, Xu Y, Muench L, Apelskog K. Fast uptake and long-lasting binding of methamphetamine in the human brain: comparison with cocaine. Neuroimage. 2008;43(4):756–63. doi: 10.1016/j.neuroimage.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laurenzana EM, Byrnes-Blake KA, Milesi-Hallé A, Gentry WB, Williams DK, Owens SM. Use of anti-(+)-methamphetamine monoclonal antibody to significantly alter (+)-methamphetamine and (+)-amphetamine disposition in rats. Drug Metab Dispos. 2003;31(11):1320–6. doi: 10.1124/dmd.31.11.1320. [DOI] [PubMed] [Google Scholar]
- 52.Laurenzana EM, Hendrickson HP, Carpenter D, Peterson EC, Gentry WB, West M, Che Y, Carroll FI, Owens SM. Functional and biological determinants affecting the duration of action and efficacy of anti-(+)-methamphetamine monoclonal antibodies in rats. Vaccine. 2009;23(50):7011–20. doi: 10.1016/j.vaccine.2009.09.072. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Byrnes-Blake KA, Carroll FI, Abraham P, Owens SM. Generation of anti-(+)methamphetamine antibodies is not impeded by (+)methamphetamine administration during active immunization of rats. Int Immunopharmacol. 2001;1(2):329–38. doi: 10.1016/s1567-5769(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 54.Moreno AY, Mayorov AV, Janda KD. Impact of distinct chemical structures for the development of a methamphetamine vaccine. J Am Chem Soc. 2011;4(17):6587–95. doi: 10.1021/ja108807j. 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peterson EC, Gunnell M, Che Y, Goforth RL, Carroll FI, Henry R, Liu H, Owens SM. Using hapten design to discover therapeutic monoclonal antibodies for treating methamphetamine abuse. J Pharmacol Exp Ther. 2007;322(1):30–9. doi: 10.1124/jpet.106.117150. [DOI] [PubMed] [Google Scholar]
- 56.McMillan DE, Hardwick WC, Li M, Gunnell MG, Carroll FI, Abraham P, Owens SM. Effects of murine-derived anti-methamphetamine monoclonal antibodies on (+)-methamphetamine self-administration in the rat. J Pharmacol Exp Ther. 2004;309(3):1248–55. doi: 10.1124/jpet.103.061762. [DOI] [PubMed] [Google Scholar]
- 57.Byrnes-Blake KA, Laurenzana EM, Landes RD, Gentry WB, Owens SM. Monoclonal IgG affinity and treatment time alters antagonism of (+)-methamphetamine effects in rats. Eur J Pharmacol. 2005;3(1-3):86–94. doi: 10.1016/j.ejphar.2005.08.016. 521. [DOI] [PubMed] [Google Scholar]
- 58.Gentry WB, Laurenzana EM, Williams DK, West JR, Berg RJ, Terlea T, Owens SM. Safety and efficiency of an anti-(+)-methamphetamine monoclonal antibody in the protection against cardiovascular and central nervous system effects of (+)-methamphetamine in rats. Int Immunopharmacol. 2006;6:968–77. doi: 10.1016/j.intimp.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 59.Daniels JR, Wessinger WD, Hardwick WC, Li M, Gunnell MG, Hall CJ, Owens SM, McMillan DE. Effects of anti-phencyclidine and anti-(+)-methamphetamine monoclonal antibodies alone and in combination on the discrimination of phencyclidine and (+)-methamphetamine by pigeons. Psychopharmacology (Berl) 2006;185(1):36–44. doi: 10.1007/s00213-005-0299-6. [DOI] [PubMed] [Google Scholar]
- 60.Van Vunakis H, Wasserman E, Levine L. Specificities of antibodies to morphine. J Pharmacol Exp Ther. 1972;180(2):514–21. [PubMed] [Google Scholar]
- 61.Wainer BH, Fitch FW, Fried J, Rothberg RM. A measurement of the specificities of antibodies to morphine-6-succinyl-BSA by competitive inhibition of 14 C-morphine binding. J Immunol. 1973;110(3):667–73. [PubMed] [Google Scholar]
- 62.Koida M, Takahashi M, Muraoka S, Kaneto H. Antibodies to BSA conjugates of morphine derivatives: strict dependency of the immunological specificity on the hapten structure. Jpn J Pharmacol. 1974;24(1):165–7. doi: 10.1254/jjp.24.165. [DOI] [PubMed] [Google Scholar]
- 63.Rook EJ, Huitema AD, van den Brink W, van Ree JM, Beijnen JH. Pharmacokinetics and pharmacodynamics of high doses of pharmaceutically prepared heroin, by intravenous or by inhalation route in opioid-dependent patients. Basic Clin Pharmacol Toxicol. 2006;98:86–96. doi: 10.1111/j.1742-7843.2006.pto_233.x. [DOI] [PubMed] [Google Scholar]
- 64.Akbarzadeh A, Mehraby M, Zarbakhsh M, Farzaneh H. Design and synthesis of a morphine-6-succinyl-bovine serum albumin hapten for vaccine development. Biotechnol Appl Biochem. 1999;30:139–46. [PubMed] [Google Scholar]
- 65.Anton B, Leff P. A novel bivalent morphine/heroin vaccine that prevents relapse to heroin addiction in rodents. Vaccine. 2006;12(16):3232–40. doi: 10.1016/j.vaccine.2006.01.047. 24. [DOI] [PubMed] [Google Scholar]
- 66.Ma LX, Zhou Q, Zheng HB, Li SB. Preparation and characterization of anti-morphine vaccine antibody. Chinese Cellular and Molecular Immunology. 2006;22(3):368–70. [PubMed] [Google Scholar]
- 67.Stowe GN, Vendruscolo LF, Edwards S, Schlosburg JE, Misra KK, Schulteis G, Mayorov AV, Zakhari JS, Koob GF, Janda KD. A Vaccine Strategy that Induces Protective Immunity against Heroin. J Med Chem. 2011;28(14):5195–204. doi: 10.1021/jm200461m. 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Inturrisi CE, Schultz M, Shin S, Umans JG, Angel L, Simon EJ. Evidence from opiate binding studies that heroin acts through its metabolites. Life Sci. 1983;33(Suppl 1):773–776. doi: 10.1016/0024-3205(83)90616-1. [DOI] [PubMed] [Google Scholar]
- 69.Shen XY, Lopez BM, Kinsey BM, Singh RA, Wu Y, Mao B, Orson FM. Morphine Vaccination and Inhibition of Morphine Induced Analgesia in Mice. Abstract for World Vaccine Congress. 2011:341. [Google Scholar]
- 70.Shen XY, Kosten TA, Lopez BM, O'Malley PW, Wu Y, Kinsey BM, Orson FM. Morphine Vaccination and its Inhibition of Morphine Induced CPP and Analgesia in Rats. Abstract for College on Problems of Drug Dependence Conference. 2011:81. [Google Scholar]
- 71.Donnelly JJ, Deck RR, Liu MA. Immunogenicity of a Haemophilus influenzae polysaccharide-Neisseriae meningitidis outer membrane protein vaccine. J. Immunol. 1990;143:3071. [PubMed] [Google Scholar]
- 72.Hieda Y, Keyler DE, Vandevoort JT, Kane JK, Ross CA, Raphael DE, Niedbalas RS, Pentel PR. Active immunization alters the plasma nicotine concentration in rats. J Pharmacol Exp Ther. 1997;283(3):1076–81. [PubMed] [Google Scholar]
- 73.Ettinger RH, Ettinger WF, Harless WE. Active immunization with cocaine-protein conjugate attenuates cocaine effects. Pharmacol Biochem Behav. 1997;58(1):215–20. doi: 10.1016/s0091-3057(97)00005-1. [DOI] [PubMed] [Google Scholar]
- 74.Bagasra O, Forman LJ, Howeedy A, Whittle P. A potential vaccine for cocaine abuse prophylaxis. Immunopharmacology. 1992;23(3):173–9. doi: 10.1016/0162-3109(92)90023-6. [DOI] [PubMed] [Google Scholar]
- 75.Clinical trials.gov http://clinicaltrials.gov/ct2/results?term=keyhole+limpet+hemocyanin+
- 76.Brown MJ. Success and failure of vaccines against renin-angiotensin system components. Nat Rev Cardiol. 2009;6(10):639–47. doi: 10.1038/nrcardio.2009.156. [DOI] [PubMed] [Google Scholar]
- 77.Banzhoff A, Pellegrini M, Del Giudice G, Fragapane E, Groth N, Podda A. MF59-adjuvanted vaccines for seasonal and pandemic influenza prophylaxis. Influenza Other Respi Viruses. 2008;2(6):243–9. doi: 10.1111/j.1750-2659.2008.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schwarz TF. Clinical update of the AS04-adjuvanted human papillomavirus-16/18 cervical cancer vaccine, Cervarix. Adv Ther. 2009;26(11):983–98. doi: 10.1007/s12325-009-0079-5. [DOI] [PubMed] [Google Scholar]
- 79.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;29(4):492–503. doi: 10.1016/j.immuni.2010.10.002. 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, Larocque D, Van Mechelen M, Garçon N. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol. 2009;5(10):6186–97. doi: 10.4049/jimmunol.0901474. 183. [DOI] [PubMed] [Google Scholar]
- 81.Gavin K, Hoebe B, Duong T, Ota C, Beutler Martin B., Nemazee D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schijns VE, Lavelle EC. Trends in vaccine adjuvants. Expert Rev Vaccines. 2011;10(4):539–50. doi: 10.1586/erv.11.21. [DOI] [PubMed] [Google Scholar]
- 83.Mbow ML, Gregorio E. De, Valiante NM, Rappuoli R. New adjuvants for human vaccines. Curr. Opin. Immunol. 2010;22:411–416. doi: 10.1016/j.coi.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 84.Ott G, Barchfeld GL, Chernoff D, Radhakrishnan R, van Hoogevest P, Van Nest G. MF59. Design and evaluation of a safe and potent adjuvant for human vaccines. Pharm. Biotechnol. 1995;6:277–296. doi: 10.1007/978-1-4615-1823-5_10. [DOI] [PubMed] [Google Scholar]
- 85.Casella CR, Mitchell TC. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci. 2008;65(20):3231–40. doi: 10.1007/s00018-008-8228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;15(5831):1628–32. doi: 10.1126/science.1138963. 316. [DOI] [PubMed] [Google Scholar]
- 87.Hall W, Carter L. Ethical implications of using a cocaine vaccine to treat and prevent cocaine dependence. J Med Ethics. 2004;30:337–340. doi: 10.1136/jme.2003.004739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hall W, Gartner C. Ethical and policy issues in using vaccines to treat and prevent cocaine and nicotine dependence. Curr Opin Psychiatry. 2011;24(3):191–6. doi: 10.1097/YCO.0b013e328345922b. [DOI] [PubMed] [Google Scholar]
- 89.Harwood HJ, Myers TG. New Treatments for Addiction: Behavioral, Ethical, Legal, and Social Questions. National Academies Press (US) 2004 [PubMed] [Google Scholar]
- 90.Spooner C, Hall W, Mattick RP. An overview of diversion strategies for drug related offenders. Drug Alcohol Rev. 2001;20:281–294. [Google Scholar]