Abstract
Cucurbitacins B and D were among the compounds identified as sensitizers of cancer cells to TRAIL-mediated apoptosis in a high-throughput screen. Therefore a series of cucurbitacins was further investigated for TRAIL sensitization and possible mechanisms of action. A total of six cucurbitacins promoted TRAIL-induced apoptosis (B, I, E, C, D, and K) and one (P) was inactive. Sensitization of renal adenocarcinoma cells to TRAIL was apparent after as little as 1–4 h pretreatment and did not require continued presence of cucurbitacin. Active cucurbitacins induced caspase-8 activation only after subsequent TRAIL addition and caspase activation was required for apoptosis suggesting amplified proximal signaling from TRAIL death receptors. Cucurbitacin-sensitized TRAIL-induced cytotoxicity was inhibited by N-acetyl cysteine. Structure–activity relationship analysis in comparison to published studies suggests that TRAIL-sensitizing and general cytotoxic activities of cucurbitacins may be decoupled. Cucurbitacins are reported to be inhibitors of STAT3 activation. However, their TRAIL-sensitizing activity is STAT3-independent. Treatment of renal carcinoma cells with active cucurbitacins produced rapid and dramatic changes in cell morphology and cytoskeletal organization (also prevented by NAC). Therefore, cucurbitacins may be useful as tools for investigating the molecular mechanism(s) of action of TRAIL sensitizers, particularly with regard to temporal aspects of sensitization and modulation of TRAIL signaling by cell morphology, and could form the basis for future therapeutic development in combination with TRAIL death receptor agonists.
Keywords: Cucurbitacins, TRAIL, TRAIL sensitizers, Apoptosis, STAT3, Cell morphology
Introduction
Induction of cancer cell-specific apoptosis via activation of TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) signaling has become an important focus of cancer research [1–5]. Given the relatively common occurrence of TRAIL resistance in many types of cancer [6–9], the search for enhancers of TRAIL-induced cell killing is now widespread (see reviews cited above). This has resulted in the identification of many compounds with a very wide range of putative molecular mechanisms of action [1]. A recent high-throughput screening campaign utilizing the relatively TRAIL-resistant renal cell carcinoma line ACHN resulted in the identification of a number of synergistic TRAIL sensitizers, including cucurbitacins B and D [10]. Cucurbitacins, members of a group of tetracyclic triterpenoids produced by plants of the Cucurbitaceae family, have long been reported to have anticancer effects via several potential mechanisms of action [11]. Cucurbitacins are particularly well-characterized in terms of their ability to modulate JAK2/STAT3 signaling [11–13] and cucurbitacin B has recently been reported to activate generation of reactive oxygen species (ROS) in cells [14]. JAK2/STAT3 signal modulators, including cucurbitacin I [15], sorafenib [16], and tyrphostin AG490 [17, 18] have been reported to enhance TRAIL-induced cell killing in a variety of cancer cells. Similarly, certain other TRAIL sensitizers have been reported to require generation of ROS for activity [5, 19–21]. In order to further investigate the effects of cucurbitacins, a series of seven compounds were evaluated using renal carcinoma cells for their TRAIL sensitizing activity, effects on STAT3 activation, sensitivity to the ROS modulator N-acetyl cysteine (NAC) and effects on cell morphology.
Materials and methods
Chemicals and reagents
Cucurbitacins were obtained from the NCI Developmental Therapeutics Program (DTP) and/or from Chromadex (Irvine, CA—cucurbitacins B, D, E, and I only). 2,3-Bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide (XTT; NSC 601519) was provided by the Drug Synthesis and Chemistry Branch, DTP/NCI (Frederick, MD). Bortezomib was purchased from the National Institutes of Health Pharmacy. Recombinant TRAIL ligand (168 amino acid TNF homologous extracellular domain) was purchased from Peprotech, Inc. (Rocky Hill, NJ). Cell culture media and additives were obtained from Cellgro (Manassas, VA), Hyclone (Logan, UT), Sigma (St. Louis, MO) or Invitrogen (Carlsbad, CA). BCA protein assay kits were obtained from Pierce/Thermo (Rockford, IL). Other chemicals and reagents were obtained from Sigma (St. Louis, MO). Chemical structures were drawn using ChemDraw (CambridgeSoft Corp., Cambridge, MA) using structural information from the PubChem database (http://pubchem.ncbi.nlm.nih.gov/).
Antibodies, western blot reagents
Anti-cleaved caspase-8 (18C8 rabbit monoclonal), and anti-phosphopaxillin (Tyr118) were obtained from Cell Signaling Technology (Danvers, MA). Anti-GAPDH (G8795 mouse monoclonal), conjugated with horse radish peroxidase, was from Sigma. Appropriate secondary antibodies conjugated with horse radish peroxidase were purchased from Thermo Scientific (Rockford, IL).
Cells and cell culture conditions
ACHN and Caki-1 renal ACHN cells (National Cancer Institute, Frederick, MD) were maintained in culture and prepared for assays as described [10].
Cytotoxicity/TRAIL sensitization assays
ACHN cells were trypsinized, transferred to clear 384-well tissue culture treated plates (BD Biosciences; San Jose, CA) at 3,500 cells per well in clear (phenol red-free) medium and allowed to attach overnight. Compounds were diluted into medium (at 10–20× final concentration) and added to plates containing ACHN cells. After 1–4 h incubation, TRAIL (40 ng/ml final concentration) or medium control was added. After 18–20 h, cell numbers were assessed using an XTT assay as described [10]. Cell numbers from the XTT assay were normalized to DMSO-treated controls. For the wash-out experiment, compound-containing medium was removed and the cells were washed (45 μl/well medium) before addition of fresh medium with or without TRAIL. In order to investigate the effect of ROS on sensitization, NAC (10 mM final concentration) was added to cells just before addition of compounds. NAC remained in the plates throughout the experiment. After 4 h with compounds followed by 18–20 h with (or without) TRAIL, cell numbers were estimated using the XTT assay. In this case, medium was removed and the cells washed with fresh medium before XTT addition in order to eliminate interference in the assay due to the NAC reagent. In experiments assessing the effects of the caspase inhibitor ZVAD-FMK and the inactive caspase inhibitor control ZFA-FMK, cell numbers were assessed using the Promega (Madison, WI) MTS assay and normalized to the ZVAD-FMK only or ZFAFMK only as appropriate. In some experiments, cell number was estimated using the CellTiter-Glo Luminescent Cell Viability Assay (Promega) following the manufacturer’s instructions.
Caspase-8 activation assays
The caspase-8 assay was performed, as previously described [10]. Briefly, cells were seeded at 7,000 cells/well in white luminescence tissue culture-treated 384-well plates. Alternatively in some experiments, ACHN cells were added to 96-well white luminescence tissue culture plates as previously described [22]. After treatment with cucurbitacins for various periods, TRAIL was added. After different time points, cells were lysed and caspase-8 activity was determined using the Caspase-Glo™ 8 Assay kit from Promega (Madison, WI) according to the manufacturer’s instructions. This assay included MG132 in order to reduce background signal due to proteasome activity in the cells. The correlation of the luminescence assay with caspase-8 activation by western blotting analysis was previously demonstrated for these cells [10, 22].
Western blot
ACHN cells were seeded in 6-well plates at 2.5 × 106 cells/well. After overnight attachment, compounds were added followed by 4 h treatment at which point cells were lysed, proteins separated by SDS-PAGE (4–12% gels, 50 μg protein/well), and transferred to PVDF membranes as described [10]. Lysis buffer was additionally supplemented with Halt Protease and Phosphatase Inhibitor (Pierce/Thermo) for phosphoSTAT3 blots. Western blots for detection of cleaved caspase-8 were prepared and analyzed as described previously [10, 22].
Cell morphology
Changes in cell shape were observed under standard bright field microscopy. For changes in cytoskeleton, cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 followed by fluorescein-phalloidin (Invitrogen) to detect filamentous (F)-actin. Confocal images were collected on a Zeiss LSM 510 confocal microscope (Carl Zeiss MicroImaging GmbH, Jena, Germany).
Data presentation
Unless otherwise noted, values reported represent average ± standard deviation. Statistical significance was estimated by applying the Students t test.
Results
Cucurbitacins sensitize ACHN cells to TRAIL
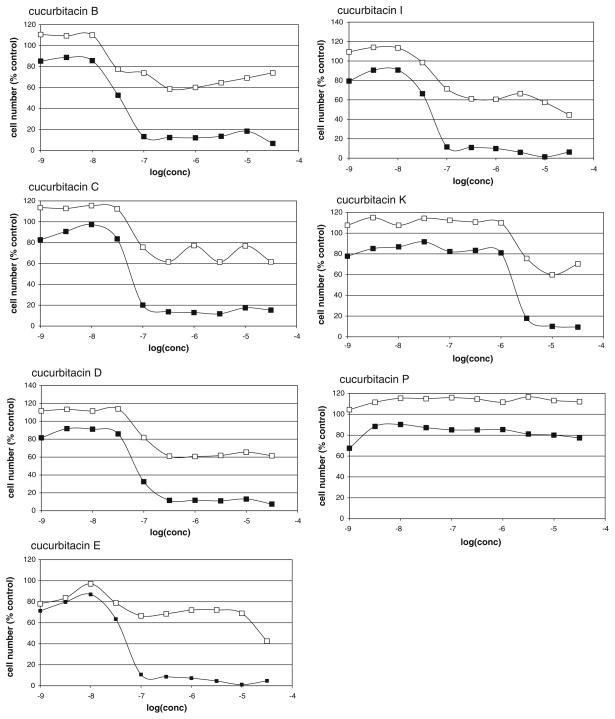
A series of seven cucurbitacins were assessed using the previously described screening assay for the detection of compounds able to sensitize TRAIL-resistant ACHN renal carcinoma cells to TRAIL-induced apoptosis [10]. Results are shown in Fig. 1. Six cucurbitacins showed significant activity when combined with TRAIL, although all six also exhibited some toxicity in the absence of TRAIL. One, cucurbitacin P, had no significant effect on ACHN cells in the presence or absence of TRAIL. Half-maximal growth inhibition values for cucurbitacins in the presence of TRAIL were calculated from the data in Fig. 1 and are listed in Table 1. Cucurbitacin B, the most potent of the cucurbitacins as a TRAIL sensitizer and the cucurbitacin most commonly found in the literature, was also used in several additional experiments. Cucurbitacin also sensitized a number of other human cancer cells to TRAIL-induced apoptosis. A total of 20 cell lines (including ACHN) were tested, including six renal (in addition to ACHN), five breast, four colon, and four melanoma cell lines (see Supplemental Data).
Fig. 1.
Dose-dependent effects of cucurbitacins on ACHN cells. ACHN cells were allowed to attach overnight before treatment for 4 h with variable concentrations of the indicated cucurbitacin followed by addition of TRAIL (40 ng/ml) or culture medium. 20 h after TRAIL addition, viable cells were assessed by an XTT assay. Results were normalized to DMSO controls for each plate (control = 100%). Data points represent the average of 2 days of experiments, duplicate determinations each day. Open symbols represent compound without TRAIL, closed symbols represent compound followed by TRAIL
Table 1.
Structural features and TRAIL sensitizing activities of cucurbitacins
| Feature | Cucurbitacin
|
||||||
|---|---|---|---|---|---|---|---|
| B | C | D | E | I | K | P | |
| C1–C2 | – | – | – | = | = | = | – |
| C2 | –OH | –H | –OH | –OH | –OH | –OH | –OH |
| C3 | =O | –OH | =O | =O | =O | =O | –OH |
| C19 | –H | –OH | –H | –H | –H | –H | –H |
| C23–C24 | = | = | = | = | = | – | – |
| C24 | –H | –H | –H | –H | –H | –OH | –H |
| C25 | –OAc | –OAc | –OH | –OAc | –OH | –OH | –OH |
| IC50 (nM)a | 27.4 | 54.2 | 78.2 | 44.1 | 43.3 | 1970 | Inactive |
IC50 values (average, n = 2) were calculated from the data in Fig. 1 (in the presence of TRAIL)
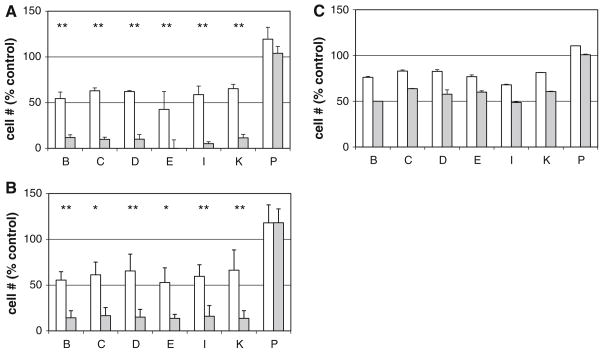
Similar effects were observed when cucurbitacin pretreatment time was reduced from 4 to 1 h. Additionally, a much smaller, but detectable, TRAIL-specific reduction in cell numbers was observed even when cells were only treated with TRAIL for 4 h (rather than 20 h) after 4 h cucurbitacin pretreatment (data not shown). In all of these experiments, the cucurbitacins remained present throughout the TRAIL treatment. In order to assess whether the effects of the cucurbitacins on TRAIL sensitivity required the continued presence of cucurbitacin, a series of washout experiments were performed. After pretreatment of cells for 1 or 4 h with cucurbitacins (1–10 μM—concentrations chosen based on their relative activities as TRAIL sensitizers), the compounds were removed, cells washed with medium, and treated in the presence or absence of TRAIL for 4 or 20 h. Figure 2 shows that after 1 h (A) or 4 h (B) cucurbitacin treatment, the TRAIL sensitization effect persisted even after removal of the compounds (followed by TRAIL for 20 h). In fact, even after only 1 h cucurbitacin followed by as little as 4 h of TRAIL treatment, sensitization could be observed (Fig. 2c).
Fig. 2.
Effects of removal of cucurbitacins on enhancement of TRAIL-induced cell killing. ACHN cells were allowed to attach overnight before treatment for 1 or 4 h with 1 μM (cucurbitacins B, C, D, E, and I) or 10 μM (cucurbitacins K and P) followed by removal of drug-containing medium, washing with drug-free medium, and treatment in the presence (gray bars) or absence (open bars) of TRAIL (40 ng/ml). Viable cell numbers were assessed by XTT and normalized to untreated control (control = 100%) for each plate. a 1 h pretreatment followed by overnight incubation ± TRAIL, b 4 h pretreatment followed by overnight incubation ± TRAIL, c 1 h pretreatment followed by 4 h incubation ± TRAIL: n = 4 for (a), n = 3 for (b) (error bars represent standard deviation), and n = 2 for (c) (error bars represent range). *P < 0.05, **P < 0.01, Students t test comparing cucurbitacin alone to cucurbitacin + TRAIL
Sensitization of ACHN cells by cucurbitacins leads to enhanced TRAIL-induced caspase-8 activation
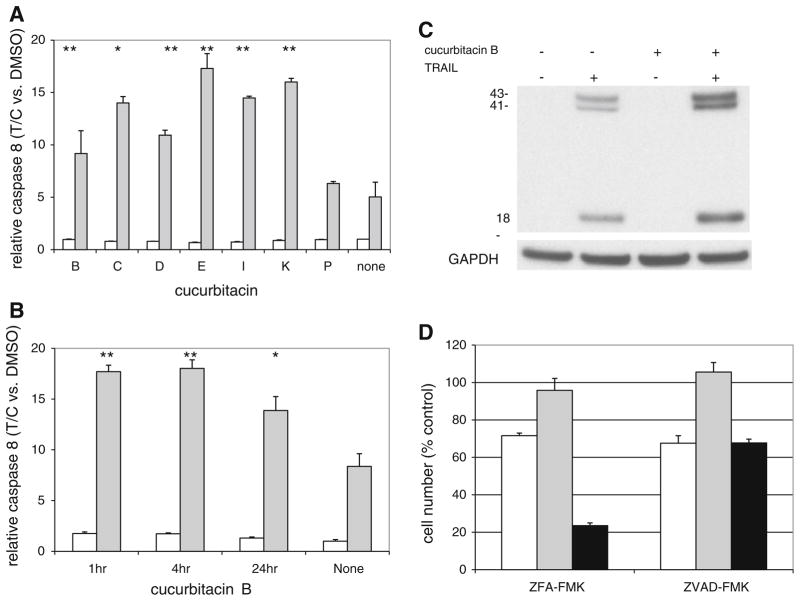
As previously determined for other TRAIL sensitizing compounds, including cucurbitacins B and D [10], the cucurbitacins were assessed for their ability to enhance TRAIL-dependent activation of caspase-8 (Fig. 3a). Cucurbitacins B, C, D, E, I, and K significantly increased caspase-8 activation in the presence of TRAIL. The inactive cucurbitacin P had no effect. None of the cucurbitacins tested increased caspase-8 activity in the absence of TRAIL. In each case (except P), cucurbitacins in the presence of TRAIL increased caspase-8 activity to 2.07–2.75 times the level induced by TRAIL alone (Fig. 3a). Consistent with the sensitization results (Fig. 2a, c), only a 1 h treatment with cucurbitacin B was necessary to observe a subsequent enhancement of TRAIL-induced caspase-8 activation (Fig. 3b). This enhanced TRAIL-induced caspase-8 activation by cucurbitacin B was confirmed by western blot analysis using an antibody specific for activated (i.e., cleaved) caspase-8 (Fig. 3c).
Fig. 3.
Caspase activation in the presence of cucurbitacin B. a ACHN cells were treated for 4 h with DMSO (control) or the indicated cucurbitacin (10 μM) followed by an additional 4 h in the presence (gray bars) or absence (open bars) of TRAIL (40 ng/ml) in the continued presence of cucurbitacin. Caspase-8 activity was determined using the Caspase-Glo™ 8 assay kit. Error bars represent standard deviation (n = 3–5). T/C indicates treated/control ratio of caspase-8 activity, normalized to DMSO control. b ACHN cells were incubated in the presence of cucurbitacin B (1 μM) for 1, 4, or 24 h followed by incubation in the presence (gray bars) or absence (open bars) of TRAIL (1000 ng/ml) for 1 h. Caspase-8 activity was assessed using the Caspase-Glo™ 8 assay kit. T/C indicates treated/control ratio of caspase-8 activity, normalized to DMSO control. Error bars represent standard deviation (n = 3). c ACHN cells were incubated in the presence of cucurbitacin B for 1 h followed by incubation in the presence or absence of TRAIL. After 4 h, cell extracts were assessed by western blot analysis for activated (i.e. cleaved) caspase-8. *P < 0.05, **P < 0.01, Students t test comparing cucurbitacin alone to cucurbitacin + TRAIL. d ACHN cells were preincubated for 2 h with either ZFA-FMK (inactive control) or ZVAD-FMK (pan-caspase inhibitor). This was followed by incubations with cucurbitacin B (100 nM, open bars), TRAIL (40 ng/ml, gray bars) or cucurbitacin B (2–4 h) followed incubation with TRAIL (black bars). Cell numbers were assessed 18–20 h after addition of TRAIL. Data were normalized to ZFA-FMK alone or ZVAD-FMK alone. Error bars represent standard deviation (n = 3). P < 0.01 comparing cucurbitacin B + TRAIL + ZVAD-FMK to cucurbitacin B + TRAIL + ZFA-FMK
TRAIL-induced killing of cucurbitacin-sensitized cells is caspase-dependent
Inclusion of the general caspase inhibitor ZVAD-FMK eliminated the reduction in cell numbers in response to the combination of cucurbitacin B and TRAIL compared to the inactive control inhibitor, ZFA-FMK (Fig. 3d). Interestingly, the reduction in cell number by cucurbitacin B alone was not affected by ZVAD-FMK, suggesting that cucurbitacin B alone may work via a non-apoptotic cell death mechanism or perhaps causes cytostasis rather than cytotoxicity.
TRAIL-induced killing of cucurbitacin-sensitized cells is eliminated by N-acetyl cysteine
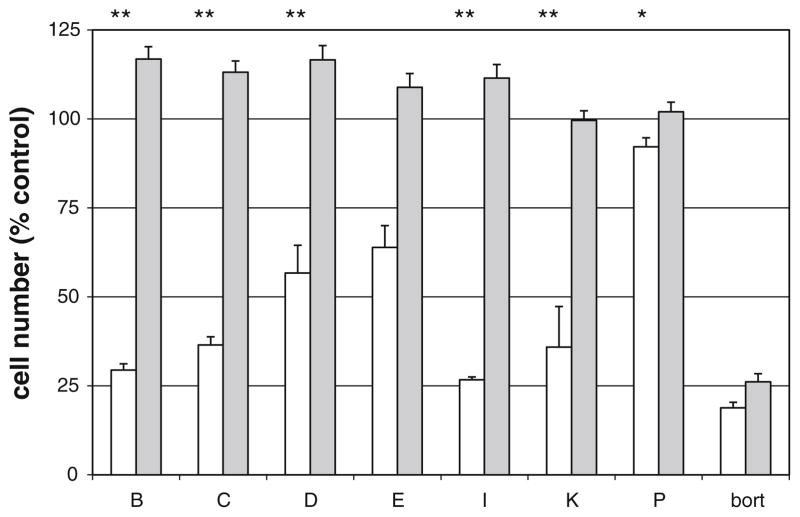
The redox state of a cell can affect STAT3 activity [23] and ROS generation has been reported to specifically mediate some of the effects of cucurbitacins [14]. Therefore, the effects of cucurbitacins on TRAIL-induced cell killing were assessed in the presence and absence of the ROS inhibitor NAC. Addition of NAC at 10 mM (final) eliminated the effects of all of the cucurbitacins (Fig. 4). By contrast, the TRAIL sensitizing effect of the proteasome inhibitor bortezomib was unaffected by NAC (Fig. 4). However, significant generation of ROS following cucurbitacin B treatment (with or without TRAIL) was not detected using MitoSOX or DCF for assessment of mitochondrial and cytosolic ROS respectively (data not shown).
Fig. 4.
Effects of NAC on ACHN cell response to cucurbitacins. ACHN cells were plated at 3,500 cells/well in 384-well plates and allowed to attach overnight. NAC was added to half of the wells at a final concentration of 10 mM followed immediately by the indicated cucurbitacins (or DMSO control). After 4 h, TRAIL (or medium control) was added. After 20 h additional incubation, wells were washed (2 × 100 μl per well), and cell numbers assessed by XTT. Results were normalized to values for cells in the absence of TRAIL or cucurbitacin. Bars represent compounds + TRAIL in the absence (open bars) or presence (gray bars) of NAC. Error bars indicate standard deviation (n = 3). The following concentrations were used: cucurbitacins B, C, D, E, and I: 100 nM; cucurbitacins K and P: 10 μM; bortezomib (bort): 40 nM. *P < 0.05, **P < 0.01, Students t test comparing cucurbitacin + TRAIL with and without NAC
Sensitization of renal carcinoma cells by cucurbitacin differs from bortezomib-induced sensitization
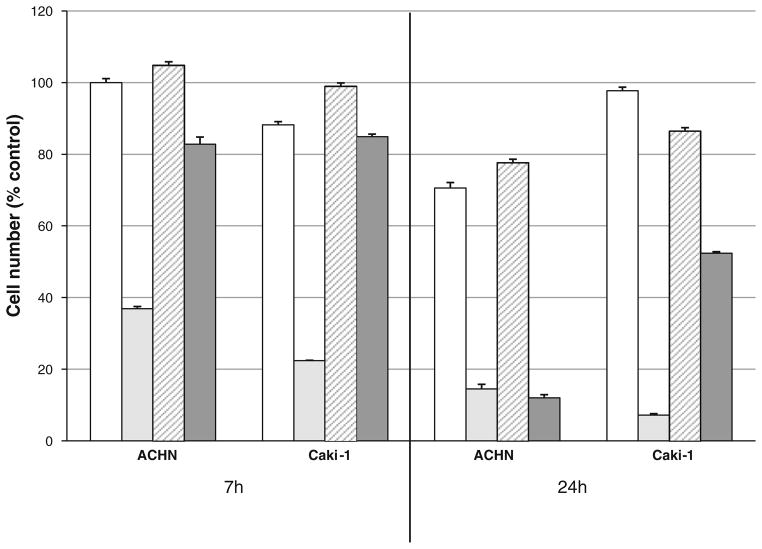
A number of TRAIL-resistant cell lines can be sensitized to TRAIL-induced cell killing by cucurbitacin B, but not by the well-characterized TRAIL sensitizer bortezomib (Supplemental Data). ACHN cells were sensitized by both agents whereas only cucurbitacin B sensitized Caki-1 cells (Fig. 5). In addition, the TRAIL-sensitizing effect of cucurbitacin B was much more rapid than that of bortezomib (Fig. 5). In contrast with bortezomib, cucurbitacin B treatment did not affect the levels of the apoptosis proteins cFLIP, Mcl-1, or XIAP by western blot analysis nor did it affect cell surface levels of TRAIL receptors by FACS analysis (data not shown).
Fig. 5.
Effects of cucurbitacin B and bortezomib on ACHN and Caki-1 cells. ACHN or Caki-1 cells were treated with or without cucurbitacin B (200 nM) or bortezomib (20 nM) in the presence of 100 ng/ml TRAIL for 7 or 24 h. Cell numbers were normalized to untreated controls. After treatment with TRAIL alone, cell numbers ranged from 84 to 96% of control. Open bars cucurbitacin B only; light gray cucurbitacin B + TRAIL; cross-hatched bortezomib only; dark gray bortezomib + TRAIL
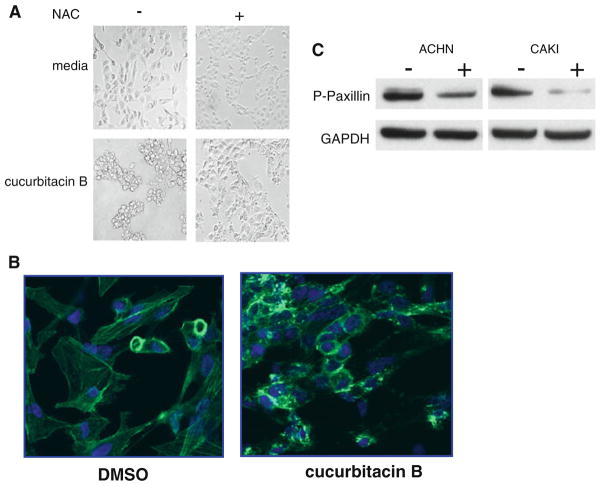
Cucurbitacins rapidly affect cell morphology
Interestingly, sensitizing concentrations of active cucurbitacins always produced dramatic changes in cell shape. These effects were clearly visibly by 1 h after drug exposure and were eliminated by inclusion of NAC (Fig. 6a). By contrast, inactive cucurbitacins, such as cucurbitacin P, did not produce this change in cell morphology. These changes in cell shape were accompanied by significant changes in the cellular distribution of actin. Clumping occurred in cells exposed to cucurbitacin B for 1 h (Fig. 6b). In addition, cucurbitacin B treatment rapidly induced a significant decrease in the levels of phosphopaxillin in both ACHN and Caki-1 cells (Fig. 6c). Unlike cucurbitacins, bortezomib treatment did not result in rapid and dramatic changes in cell shape (data not shown).
Fig. 6.
Effect of cucurbitacin B on cell morphology. a Caki-1 cells were treated with or without cucurbitacin B (200 nM) for 1 h in the presence or absence of 10 mM NAC. b Caki-1 cells were treated for 1 h with 200 nM cucurbitacin B followed by paraformaldehyde fixation and permeabilization with Triton X-100 before visualization of polymerized actin using fluorescein-phalloidin. c ACHN or Caki-1 cells were treated for 1 h with cucurbitacin B (200 nM), solubilized and subjected to western blot analysis using an antibody against phosphopaxillin
Discussion
Cucurbitacins B and D were previously identified in a highthroughput screen for TRAIL synergizing compounds [10]. Cucurbitacin I has been reported to sensitize mouse fibrosarcoma cells, human colorectal carcinoma cells, and human leukemia cells to TRAIL-induced apoptosis [15, 24]. Therefore, additional cucurbitacins were further investigated with regard to their effects on TRAIL-resistant ACHN cells. Based on IC50 values (Fig. 1; Table 1), cucurbitacins B, C, D, E, and I have similar potencies while K is much less potent and P is inactive. The persistent TRAIL-sensitizing effects of cucurbitacins after removal of the compounds (Fig. 2) are consistent with rapid, irreversible effects on TRAIL-induced commitment step(s). It is possible that intracellular cucurbitacin concentrations remain high enough to retain activity even when they are removed from the extracellular medium. Cucurbitacins may participate in a rapid cell surface-reactive event, such as direct interaction with or modification of the TRAIL death receptors themselves, as part of their mechanism of action. The rapid time course also suggests that sensitization does not require changes in gene expression often implicated in sensitization of cells to TRAIL.
The pan-caspase inhibitor ZVAD-FMK blocked sensitization by cucurbitacin B, implying apoptotic cell death via caspase activation. Caspase-8 activation is among the earliest detectable responses of cells to TRAIL-mediated apoptosis [22, 25]. Enhancement of TRAIL-dependent caspase-8 activation by cucurbitacins correlated with cell death from the earliest time points tested. This is consistent with potential threshold effects for initiator caspases and enhanced signaling from the TRAIL death-inducing signaling complex (DISC) [5, 22, 25, 26]. A cell membrane-reactive activity of the cucurbitacins with TRAIL receptors would also be consistent with this observation. Caspase-8 activation and regulation are complicated processes that allow for multiple points of modulation [22, 27, 28]. Cucurbitacins may be useful for probing the molecular mechanisms underlying modulation of caspase activation by TRAIL sensitizers.
Generation of ROS is another mechanism proposed for many TRAIL sensitizers [5, 19–21] and cucurbitacin B as a single agent has been reported to induce ROS generation [14]. Although NAC, an inhibitor of ROS generation, blocked the TRAIL-sensitization effects of cucurbitacins, ROS generation in response to cucurbitacin B (in the presence or absence of TRAIL) was not detected (data not shown). Thus, the inhibitory effects of NAC on cucurbitacins and TRAIL could result from other mechanisms [29], including interference with a possible electrophilic mechanism of cucurbitacin action (via the C23–C24 double bond—Fig. 7). TRAIL sensitizers can be placed in at least two basic categories, those that are inhibited by NAC (including cucurbitacins) and those that are not (for example, bortezomib). The mechanistic bases for this dichotomy are beyond the scope of this report, but may provide important insights into TRAIL signaling pathways.
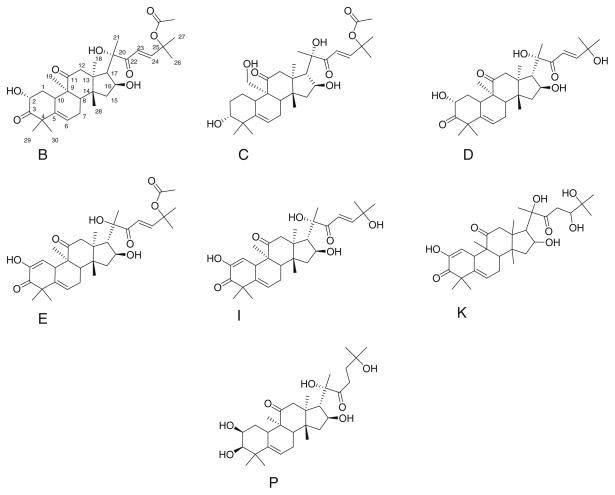
Fig. 7.
Structures of cucurbitacins tested for sensitization of ACHN cells to TRAIL
In addition to the differential effects of NAC, a number of other differences between cucurbitacins and bortezomib were observed. The pattern of sensitization of multiple cell lines by cucurbitacin B (see Supplemental Data) differs from those of other sensitizers including bortezomib (Fig. 5 and Supplemental Data). The TRAIL-sensitizing effect of cucurbitacins was much more rapid than that of bortezomib. Bortezomib also modulates the levels of a number of apoptotic proteins unaffected by cucurbitacin treatment. These differences, along with the effects on cell shape and modulation of activities by NAC, suggest that the molecular mechanisms underlying bortezomib and cucurbitacin B sensitization of cells to TRAIL apoptosis are quite different and distinct.
The active cucurbitacins rapidly changed the morphology of both ACHN and Caki-1 cells, possibly via effects on F-actin [30, 31]. In fact, actin distribution was dramatically affected by cucurbitacin B as were levels of phosphopaxillin. Inactive cucurbitacins did not cause changes in cell morphology. In addition, these changes were completely blocked by the presence of NAC in the media. Paxillin phosphorylation is often associated with integrin signaling. Thus it seems likely that cucurbitacin B-induced changes the cell cytoskeleton may play an important role in the amplifying TRAIL apoptosis signaling. This potential role of cell adhesion on TRAIL signaling has recently been reported [32]. The involvement of such cytoskeletal effects on TRAIL signaling is worthy of further investigation. Cucurbitacins may be useful agents for more detailed investigations of the relationships between cell morphology and TRAIL apoptosis signaling.
The differences in structural features among the cucurbitacins assessed in this report (Fig. 7; Table 1—numbering follows Afifi et al. [33]) are subtle. Of particular note, however, is the C23–C24 double bond. Only cucurbitacin K (least potent of the active compounds) and the inactive cucurbitacin P lack this feature. Comparison with two published structure–activity relationship (SAR) analyses of the cytotoxic effects of cucurbitacins [34, 35] suggest both similarities and differences between structural features apparently important for toxicity and for sensitization to TRAIL-induced apoptosis. However neither of the cited studies included all of the cucurbitacins shown in Fig. 7. The cytotoxicity studies identified acetylation of the C25 hydroxyl as being important for increased cytotoxicity. This feature does not correlate with sensitization of ACHN cells to TRAIL. Similarly, the presence of the C1–C2 double bond does not appear to correlate with TRAIL-induced apoptosis, unlike the conclusions drawn by Bartalis and Halaweish [34] regarding cytotoxicity of the compounds alone. The effects of cucurbitacins alone on ACHN cell survival were minimal (Fig. 1), but complete killing curves were not obtained. The report that included cucurbitacin P [35] indicated that it was orders of magnitude less potent than the other cucurbitacins listed (including B, C, D, E, and I) suggesting that cucurbitacin P truly is an outlier (consistent with TRAIL sensitization results). Finally, neither of the cited reports identified the C23–C24 double bond as important in cytotoxicity, yet this correlates quite well with TRAIL sensitization. Future studies may further distinguish molecular features of the cucurbitacins required for TRAIL sensitization requirements in comparison to those that elicit nonspecific cytotoxicity.
Cucurbitacins are also reported to inhibit STAT3 activation and inhibition of STAT3 signaling has been identified as a means of sensitizing cells to TRAIL. However, three lines of evidence suggest that cucurbitacins are not sensitizing ACHN cells to TRAIL via inhibition of STAT3 activation. First, structural features of cucurbitacins identified as important for inhibition of STAT3 activation [36] are not implicated in TRAIL sensitization. This analysis [36] suggested that the presence of a hydroxyl group on C19 results in loss of the ability to block STAT3 activation (cucurbitacin A was used in that study). However, cucurbitacin C, which also has this feature, is a very good TRAIL sensitizer (although A and C differ in other ways). Unfortunately, cucurbitacin A was not available for these studies and Sun et al. [36] did not include cucurbitacin C in their report. There is minimal correlation between structural features required for inhibition of STAT3 activation and those required for sensitization of cells to TRAIL. Second, effects of STAT3 modulation are generally due to changes in STAT3-driven gene expression (e.g. Ref. [37]). However, as little as 1 h pretreatment with cucurbitacins was sufficient to sensitize ACHN cells to TRAIL and by 1–4 h the cells are committed to respond to TRAIL. This rapid sensitization of renal carcinoma cells to cucurbitacins suggests that this response does not rely on transcriptio-driven changes in protein levels. Consistent with this conclusion is the observation that the level of Bcl-xL, transcriptionally regulated by STAT3 [38], was unaffected by cucurbitacin B (see Supplemental Data). Therefore, based on the time courses reported here, the mechanisms of action of cucurbitacin sensitization of renal carcinoma cells to TRAIL are immediate and non-genomic, and may involve a direct interaction of the compound with a readily accessible target that is either on or in the cell. Third, the cucurbitacins tested had minimal effect on constitutively activated STAT3 as measured by western blots of phosphoSTAT3 (see Supplemental Data). Taken together, SAR comparisons (albeit limited), temporal aspects of sensitization, and western blot results for phosphoSTAT3 all suggest that cucurbitacins sensitize ACHN cells to TRAIL by a STAT3-independent mechanism.
Cucurbitacins are not currently clinically useful as single agents due to toxicity and low therapeutic indices [11]. Indeed, normal human renal epithelial cells are also sensitized to TRAIL-mediated apoptosis by cucurbitacin B (data not shown). This would obviously lead to significant off-target effects. Nonetheless, some recent reports have noted in vivo antitumor effects of cucurbitacins alone [39] or when combined with the chemotherapeutic drug gemcitabine in mouse cancer models [40]. Whether there is a therapeutic window for the clinical utilization of cucurbitacins for cancer therapy in humans is unknown. However, cucurbitacins may be useful precursors for clinically useful compounds following appropriate chemical modification (to reduce toxicity) or when used at low concentrations and brief exposures in conjunction with TRAIL. SAR analysis suggests that the TRAIL synergy effects of cucurbitacins are decoupled from their general cytotoxicity. As such, they could form the basis for development of less toxic TRAIL sensitizers.
As noted the rapidity of the response to cucurbitacins would tend to preclude mechanisms requiring changes in gene expression. This conclusion is consistent with the absence of effects of cucurbitacin B on cellular levels of c-FLIP, Mcl-1, and XIAP, or cell surface levels of TRAIL receptors (FACS analysis) (data not shown) as well as Bcl-xL (Supplementary Data). Instead, rapid effects on enzymatic activity and cytoskeletal organization are observed.
The mechanism(s) of cellular resistance to TRAIL and the mechanisms by which TRAIL sensitization can occur (both STAT3-related and STAT3-independent) are not well understood [1]. The temporal aspects of sensitization of cells to TRAIL also remain unclear. The TRAIL-sensitizing action of specific cucurbitacins in renal carcinoma cells requires the activation and cleavage of caspase-8 at very early time points, and suggest an important role for the cytoskeleton in controlling the extent of caspase-8 activation. Sensitization by cucurbitacins is rapid and persistent making them potentially useful reagents for developing increased understanding of the sequence(s) of molecular events that can lead to TRAIL sensitization and subsequent apoptosis. As a result cucurbitacins have potential as valuable tools for investigating early events in sensitization of cells to TRAIL.
Supplementary Material
Acknowledgments
Thanks to Heidi Bokesh and Kirk Gustafson for performing and interpreting chemical analysis to confirm identities of the compounds obtained from the DTP repository. Thanks also to Anna Maciag for assistance with ROS assays and to Tommy Turbyville for help with actin staining and confocal microscopy. This project has been funded in whole or in part with Federal funds from the national Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government. This research was supported (in part) by the Intramural Research Program of NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10495-011-0652-7) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Curtis J. Henrich, Email: Curtis.Henrich2@nih.gov, Molecular Targets Laboratory, NCI-Frederick, Frederick, MD, USA, Basic Research Program, SAIC-Frederick, Inc., NCI-Frederick, Building 538, Room 141, Frederick, MD 21702, USA
Cheryl L. Thomas, Molecular Targets Laboratory, NCI-Frederick, Frederick, MD, USA
Alan D. Brooks, Basic Research Program, SAIC-Frederick, Inc., NCI-Frederick, Building 538, Room 141, Frederick, MD 21702, USA, Laboratory for Experimental Immunology and Cancer Inflammation Program, NCI-Frederick, Frederick, MD, USA
Nancy Lynn Booth, Molecular Targets Laboratory, NCI-Frederick, Frederick, MD, USA.
Evan M. Lowery, Laboratory for Experimental Immunology and Cancer Inflammation Program, NCI-Frederick, Frederick, MD, USA
Richard J. Pompei, Laboratory for Experimental Immunology and Cancer Inflammation Program, NCI-Frederick, Frederick, MD, USA
James B. McMahon, Molecular Targets Laboratory, NCI-Frederick, Frederick, MD, USA
Thomas J. Sayers, Basic Research Program, SAIC-Frederick, Inc., NCI-Frederick, Building 538, Room 141, Frederick, MD 21702, USA, Laboratory for Experimental Immunology and Cancer Inflammation Program, NCI-Frederick, Frederick, MD, USA
References
- 1.Pavet V, Portal MM, Moulin JC, Herbrecht R, Gronemeyer H. Towards novel paradigms for cancer therapy. Oncogene. 2010;30:1–20. doi: 10.1038/onc.2010.460. [DOI] [PubMed] [Google Scholar]
- 2.Holoch PA, Griffith TS. TNF-related apoptosis-inducing ligand (TRAIL): a new path to anti-cancer therapies. Eur J Pharmacol. 2009;625:63–72. doi: 10.1016/j.ejphar.2009.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahmood Z, Shukla Y. Death receptors: targets for cancer therapy. Exp Cell Res. 2010;316:887–899. doi: 10.1016/j.yexcr.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalvez F, Ashkenazi A. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene. 2010;29:4752–4765. doi: 10.1038/onc.2010.221. [DOI] [PubMed] [Google Scholar]
- 5.Sayers TJ. Targeting the extrinsic apoptosis signaling pathway for cancer therapy. Cancer Immunol Immunother. 2011;60:1173–1180. doi: 10.1007/s00262-011-1008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivanov VN, Bhoumik A, Ronai Z. Death receptors and melanoma resistance to apoptosis. Oncogene. 2003;22:3152–3161. doi: 10.1038/sj.onc.1206456. [DOI] [PubMed] [Google Scholar]
- 7.Rahman M, Pumphrey JG, Lipkowitz S. The TRAIL to targeted therapy of breast cancer. Adv Cancer Res. 2009;103:43–73. doi: 10.1016/S0065-230X(09)03003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Testa U. TRAIL/TRAIL-R in hematologic malignancies. J Cell Biochem. 2010;110:21–34. doi: 10.1002/jcb.22549. [DOI] [PubMed] [Google Scholar]
- 9.Stegehuis JH, de Witt LH, de Vries EG, Groen HJ, de Jong S, Kruyt FA. TRAIL receptor targeting therapies for non-small cell lung cancer: current status and perspectives. Drug Resist Updates. 2010;13:2–15. doi: 10.1016/j.drup.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Booth NL, Sayers TJ, Brooks AD, Thomas CL, Jacobsen K, Goncharova EI, McMahon JB, Henrich CJ. A cell-based high-throughput screen to identify synergistic TRAIL sensitizers. Cancer Immunol Immunother. 2009;58:1229–1244. doi: 10.1007/s00262-008-0637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee DH, Iwanski GB, Thoennissen NH. Cucurbitacin: ancient compound shedding new light on cancer treatment. Scientific World Journal. 2010;5:413–418. doi: 10.1100/tsw.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng J, Grande F, Neamati N. Small molecule inhibitors of Stat3 signaling pathway. Curr Cancer Drug Targets. 2007;7:91–107. doi: 10.2174/156800907780006922. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, Sung B, Ichikawa H. Targeting signal-transducer- and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann NY Acad Sci. 2006;1091:151–169. doi: 10.1196/annals.1378.063. [DOI] [PubMed] [Google Scholar]
- 14.Yasuda S, Yogosawa S, Izutani Y, Nakamura Y, Watanabe H, Sakai T. Cucurbitacin B induces G2 arrest and apoptosis via a reactive oxygen species-dependent mechanism in human colon adenocarcinoma SW480 cells. Mol Nutr Food Res. 2010;54:559–565. doi: 10.1002/mnfr.200900165. [DOI] [PubMed] [Google Scholar]
- 15.Finnberg N, Klein-Szanto AJ, El-Deiry WS. TRAIL-R deficiency in mice promotes susceptibility to chronic inflammation and tumorigenesis. J Clin Invest. 2008;118:111–123. doi: 10.1172/JCI29900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang S, Sinicrope FA. Sorafenib inhibits STAT3 activation to enhance TRAIL-mediated apoptosis in human pancreatic cancer cells. Mol Cancer Ther. 2010;9:742–750. doi: 10.1158/1535-7163.MCT-09-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanuti P, Bertagnolo V, Pierdomenico L, Bascelli A, Santavenere E, Alinari L, Capitani S, Miscia S, Marchisio M. Enhancement of TRAIL cytotoxicity by AG-490 in human ALL cells is characterized by downregulation of cIAP-1 and cIAP-2 through inhibition of JAK2/STAT3. Cell Res. 2009;19:1079–1089. doi: 10.1038/cr.2009.80. [DOI] [PubMed] [Google Scholar]
- 18.Kusuba M, Nakao K, Goto T, Nishimura D, Kawashimo H, Shibata H, Motoyoshi Y, Taura N, Ichikawa T, Hamasaki K, Eguchi K. Abrogation of constitutive STAT3 activity sensitizes human hepatoma cells to TRAIL-mediated apoptosis. J Hepatol. 2007;47:546–555. doi: 10.1016/j.jhep.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Kannappan R, Ravindran J, Prasad S, Sung B, Yadav VR, Reuter S, Chaturvedi MM, Aggarwal BB. Gamma-tocotrienol promotes TRAIL-induced apoptosis through reactive oxygen species/extracellular signal-regulated kinase/p53-mediated upregulation of death receptors. Mol Cancer Ther. 2010;9:2196–2207. doi: 10.1158/1535-7163.MCT-10-0277. [DOI] [PubMed] [Google Scholar]
- 20.Lee TJ, Um HJ, Min do S, Park JW, Choi KS, Kwon TK. Withaferin A sensitizes TRAIL-induced apoptosis through reactive oxygen species-mediated up-regulation of death receptor 5 and down-regulation of c-FLIP. Free Radic Biol Med. 2009;46:1639–1649. doi: 10.1016/j.freeradbiomed.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Sung B, Ravindran J, Prasad S, Pandey MK, Aggarwal BB. Gossypol induces death receptor-5 through activation of the ROS-ERK-CHOP pathway and sensitizes colon cancer cells to TRAIL. J Biol Chem. 2010;285:35418–35427. doi: 10.1074/jbc.M110.172767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Brooks AD, Jacobsen KM, Li W, Shanker A, Sayers TJ. Bortezomib sensitizes human renal cell carcinomas to TRAIL apoptosis through increased activation of caspase-8 in the death-inducing signaling complex. Mol Cancer Res. 2010;8:729–738. doi: 10.1158/1541-7786.MCR-10-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L, Cheung S-h, Evans EL, Shaw PE. Modulation of gene expression and tumor cell growth by redox modification of STAT3. Cancer Res. 2010;70:8222–8232. doi: 10.1158/0008-5472.CAN-10-0894. [DOI] [PubMed] [Google Scholar]
- 24.Ishidorj G, Johnston JB, Gibson SB. Inhibition of constitutive activation of STAT3 by cucurbitacin-I (JSI-124) sensitized human B-leukemia cells to apoptosis. Mol Cancer Ther. 2010;9:3302–3314. doi: 10.1158/1535-7163.MCT-10-0550. [DOI] [PubMed] [Google Scholar]
- 25.Ganten TM, Koschny R, Haas TL, Sykora J, Li-Weber M, Herzer K, Walczak H. Proteasome inhibition sensitizes hepatocellular carcinoma cells, but not human hepatocytes, to TRAIL. Hepatology. 2005;42:588–597. doi: 10.1002/hep.20807. [DOI] [PubMed] [Google Scholar]
- 26.Hellwig CT, Kohler BF, Lehtivarjo A-K, Dussmann H, Courtney MJ, Prehn JHM, Rehm M. Real time analysis of tumor necrosis factor-related apoptosis-inducing ligand/cycloheximide-induced caspase activities during apoptosis initiation. J Biol Chem. 2008;283:21676–21685. doi: 10.1074/jbc.M802889200. [DOI] [PubMed] [Google Scholar]
- 27.Oberst A, Pop C, Tremblay AG, Blais V, Denault J-B, Salvesen GS, Green DR. Inducible dimerization and inducible cleavage reveal a requirement for both processes in caspase-8 activation. J Biol Chem. 2010;285:16632–16642. doi: 10.1074/jbc.M109.095083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pennarun B, Meijer A, de Vries EGE, Kleibeuker JH, Kruyt F, de Jong S. Playing the DISC: turning on TRAIL death receptor-mediated apoptosis in cancer. Biochim Biophys Acta. 2010;1805:123–140. doi: 10.1016/j.bbcan.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci. 2003;60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin D, Wakimoto N, Xing H, Lu D, Huynh T, Wang X, Black KL, Koeffler HP. Cucurbitacin B markedly inhibits growth and rapidly affects the cytoskeleton in glioblastoma multiforme. Int J Cancer. 2008;123:1364–1375. doi: 10.1002/ijc.23648. [DOI] [PubMed] [Google Scholar]
- 31.Haritunians T, Gueller S, Zhang L, Badr R, Yin D, Xing H, Fung MC, Koeffler HP. Cucurbitacin B induces differentiation, cell cycle arrest, and actin cytoskeletal alterations in myeloid leukemia cells. Leuk Res. 2008;32:1366–1373. doi: 10.1016/j.leukres.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 32.Phipps LE, Hino S, Muschel RJ. Targeting cell spreading: a method of sensitizing metastatic tumor cells to TRAIL-induced apoptosis. Mol Cancer Res. 2011;9:249–258. doi: 10.1158/1541-7786.MCR-11-0021. [DOI] [PubMed] [Google Scholar]
- 33.Afifi MS, Ross SA, ElSohly MA, Naeem ZE, Halaweish FT. Cucurbitacins of Cucumis prophetarum. J Chem Ecol. 1999;25:847–859. [Google Scholar]
- 34.Bartalis J, Halaweish FT. Relationship between cucurbitacins reversed-phase high-performance liquid chromatography hydrophobicity index and basal cytotoxicity on HepG2 cells. J Chromatogr B. 2005;818:159–166. doi: 10.1016/j.jchromb.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 35.van Dang G, Rode BM, Stuppner H. Quantitative electronic structure–activity relationship (QESAR) of natural cytotoxic compounds: maytansinoids, quassinoids and cucurbitacins. Eur J Pharm Sci. 1994;2:331–350. [Google Scholar]
- 36.Sun J, Baskovich MA, Jove R, Livingston SK, Coppola D, Sebti SM. Cucurbitacin Q: a selective STAT3 activation inhibitor with potent antitumor activity. Oncogene. 2005;24:3236–3245. doi: 10.1038/sj.onc.1208470. [DOI] [PubMed] [Google Scholar]
- 37.Gamero AM, Young HA, Wiltrout RH. Inactivation of Stat3 in tumor cells: releasing a brake on immune responses against cancer? Cancer Cell. 2004;5:111–112. doi: 10.1016/s1535-6108(04)00028-5. [DOI] [PubMed] [Google Scholar]
- 38.Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, Koca C, Day S, Sung B. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann NY Acad Sci. 2009;1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boykin C, Zhang G, Chen YH, Zhang RW, Fan XE, Yang WM, Lu Q. Cucurbitacin IIa: a novel class of anti-cancer drug inducing non-reversible actin aggregation and inhibiting survivin independent of JAK2/STAT3 phosphorylation. Br J Cancer. 2011;104:781–789. doi: 10.1038/bjc.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwanski GB, Lee DH, En-Gal S, Doan NB, Castor B, Vogt M, Toh M, Bokemeyer C, Said JW, Thoennissen NH, Koeffler HP. Cucurbitacin B, a novel in vivo potentiator of gemcitabine with low toxicity in the treatment of pancreatic cancer. Br J Pharmacol. 2010;160:998–1007. doi: 10.1111/j.1476-5381.2010.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.