Abstract
RNA-DNA chimeras, in which both DNA and RNA monomers are site-specifically substituted in the same strand, may be prepared only by chemical synthesis. Biochemical studies have revealed a number of surprising and subtle effects resulting from the insertion of either a ribonucleotide into a DNA strand or a deoxyribonucleotide into an RNA strand. The availability of large quantities of these chimeras allows for their crystallization and subsequent x-ray structure determination. We describe a flexible and efficient method for the large-scale preparation of these compounds, their purification, and their crystallization. The methodology is based on a combination of existing DNA phosphoramidite synthons and those recently introduced for the preparation of biochemically active RNA1. We demonstrate that these two different synthons are compatible, produce large quantities of nucleic acid needed for physical studies, and that high resolution diffraction quality crystals may be grown from these chimeras. Of the duplex chimeras synthesized and crystallized, [r(G)d(CGTATACGC)]2, [d(GCGT)r(A)d(TACGC)]2 and [r(GCG)d(TATACCC) + d(GGGTATACGC)] form A-helices and d(CG)r(CG)d(CG)]2 forms a left-handed Z-helix.
Full text
PDF


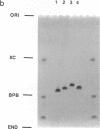
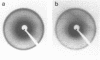
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Egli M., Usman N., Zhang S. G., Rich A. Crystal structure of an Okazaki fragment at 2-A resolution. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):534–538. doi: 10.1073/pnas.89.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Gaffney B. L., Senior M., Riddle R. R., Jones R. A. Methylation of thymine residues during oligonucleotide synthesis. Nucleic Acids Res. 1985 Jan 25;13(2):573–584. doi: 10.1093/nar/13.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrier-Takada C., Lumelsky N., Altman S. Specific interactions in RNA enzyme-substrate complexes. Science. 1989 Dec 22;246(4937):1578–1584. doi: 10.1126/science.2480641. [DOI] [PubMed] [Google Scholar]
- Musier-Forsyth K., Scaringe S., Usman N., Schimmel P. Enzymatic aminoacylation of single-stranded RNA with an RNA cofactor. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):209–213. doi: 10.1073/pnas.88.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musier-Forsyth K., Usman N., Scaringe S., Doudna J., Green R., Schimmel P. Specificity for aminoacylation of an RNA helix: an unpaired, exocyclic amino group in the minor groove. Science. 1991 Aug 16;253(5021):784–786. doi: 10.1126/science.1876835. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Okazaki T. Discontinuous DNA replication. Annu Rev Biochem. 1980;49:421–457. doi: 10.1146/annurev.bi.49.070180.002225. [DOI] [PubMed] [Google Scholar]
- Perreault J. P., Labuda D., Usman N., Yang J. H., Cedergren R. Relationship between 2'-hydroxyls and magnesium binding in the hammerhead RNA domain: a model for ribozyme catalysis. Biochemistry. 1991 Apr 23;30(16):4020–4025. doi: 10.1021/bi00230a029. [DOI] [PubMed] [Google Scholar]
- Pyle A. M., Cech T. R. Ribozyme recognition of RNA by tertiary interactions with specific ribose 2'-OH groups. Nature. 1991 Apr 18;350(6319):628–631. doi: 10.1038/350628a0. [DOI] [PubMed] [Google Scholar]
- Sakata T., Hiroaki H., Oda Y., Tanaka T., Ikehara M., Uesugi S. Studies on the structure and stabilizing factor of the CUUCGG hairpin RNA using chemically synthesized oligonucleotides. Nucleic Acids Res. 1990 Jul 11;18(13):3831–3839. doi: 10.1093/nar/18.13.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng M. K., Liaw Y. C., van der Marel G. A., van Boom J. H., Wang A. H. Effects of the O2' hydroxyl group on Z-DNA conformation: structure of Z-RNA and (araC)-[Z-DNA]. Biochemistry. 1989 Jun 13;28(12):4923–4928. doi: 10.1021/bi00438a001. [DOI] [PubMed] [Google Scholar]
- Urdea M. S., Ku L., Horn T., Gee Y. G., Warner B. D. Base modification and cloning efficiency of oligodeoxyribonucleotides synthesized by the phosphoramidite method: methyl versus cyanoethyl phosphorus protection. Nucleic Acids Symp Ser. 1985;(16):257–260. [PubMed] [Google Scholar]
- Usman N., Cedergren R. Exploiting the chemical synthesis of RNA. Trends Biochem Sci. 1992 Sep;17(9):334–339. doi: 10.1016/0968-0004(92)90306-t. [DOI] [PubMed] [Google Scholar]
- Yang J. H., Perreault J. P., Labuda D., Usman N., Cedergren R. Mixed DNA/RNA polymers are cleaved by the hammerhead ribozyme. Biochemistry. 1990 Dec 25;29(51):11156–11160. doi: 10.1021/bi00503a002. [DOI] [PubMed] [Google Scholar]
- Yang J. H., Usman N., Chartrand P., Cedergren R. Minimum ribonucleotide requirement for catalysis by the RNA hammerhead domain. Biochemistry. 1992 Jun 2;31(21):5005–5009. doi: 10.1021/bi00136a013. [DOI] [PubMed] [Google Scholar]