Abstract
Several genetic disorders affecting bone mineralization may manifest during dentin mineralization. Dentin and bone are similar in several aspects, especially pertaining to the composition of the extracellular matrix (ECM) which is secreted by well-differentiated odontoblasts and osteoblasts, respectively. However, unlike bone, dentin is not remodelled and is not involved in the regulation of calcium and phosphate metabolism. In contrast to bone, teeth are accessible tissues with the shedding of deciduous teeth and the extractions of premolars and third molars for orthodontic treatment. The feasibility of obtaining dentin makes this a good model to study biomineralization in physiological and pathological conditions. In this review, we focus on two genetic diseases that disrupt both bone and dentin mineralization. Hypophosphatemic rickets is related to abnormal secretory proteins involved in the ECM organization of both bone and dentin, as well as in the calcium and phosphate metabolism. Osteogenesis imperfecta affects proteins involved in the local organization of the ECM. In addition, dentin examination permits evaluation of the effects of the systemic treatment prescribed to hypophosphatemic patients during growth. In conclusion, dentin constitutes a valuable tool for better understanding of the pathological processes affecting biomineralization.
Keywords: Bone, Tooth, Biomineralization, Osteogenesis imperfecta, Hypophosphatemic rickets
Introduction
Dentin and bone are similar in several aspects, especially pertaining to the composition of the extracellular matrix (ECM), which is secreted by well-differentiated odontoblasts and osteoblasts, respectively. It appears difficult to characterize distinct profiles of the matrix components for either tissue. However, in contrast to bone, dentin is not remodelled and is not involved in the regulation of the calcium and phosphate metabolism. Several genetic disorders disturb both bone and dentin mineralization, including mainly hypophosphatemic rickets, osteogenesis imperfecta, Goldblatt syndrome (MIM 184260) [1], Schimke immunoosseous dysplasia (MIM 242900) [2], brachio-skeleto-genital syndrome [3,4] and osteodysplastic and primordial short stature with severe microdontia, opalescent teeth, and rootless molar [5]. Patients may develop both severe skeletal and dental abnormalities during growth influencing their quality of life. Their dental condition should be part of the diagnosis, treatment and follow-up for each patient.
In this review, we focus on two disorders. The first, hypophosphatemic rickets is related to abnormal secretory proteins involved in the ECM organization of both bone and dentin, as well as in the calcium and phosphatemetabolism. The second, osteogenesis imperfecta affects proteins involved in the local organization of the ECM and is often associated with a dentinogenesis imperfecta [6]. Impairments of bone and dentin mineralization are associated with hypophosphatemic rickets and osteogenesis imperfecta. On the other hand, these diseases do not affect enamel, except for very mild defects subtended by dentin abnormalities, in contrast with amelogenesis imperfecta, which severely affect enamel mineralization but do not affect bone [7].
Tooth constitutes a valuable tool to furthering our understanding of pathological processes induced by these genetic disorders. Tooth is accessible to study biomineralization with the shedding of deciduous teeth and the frequent practice of extraction of premolars and third molars in young adults. Moreover, dentin mineralization improvement in adult permanent teeth reflects the beneficial effect of systemic treatment prescribed during growth in patients with hypophosphatemic rickets.
Comparisons of bone and dentin
Similarities between bone and dentin
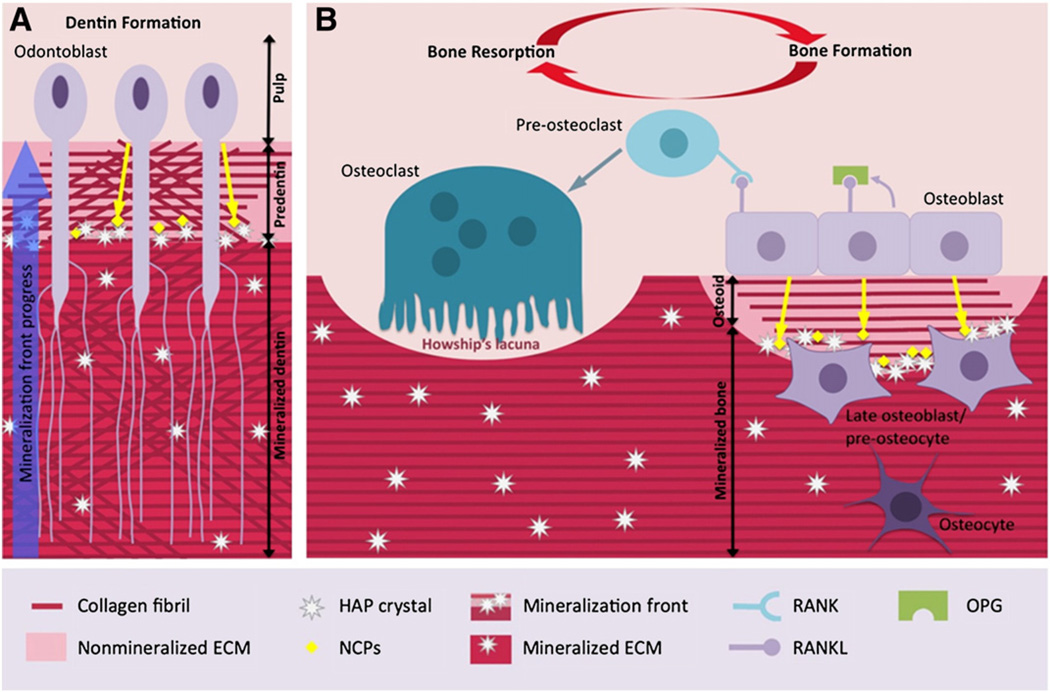
Bone and dentin are mineralized tissues that are similar in their composition and the mechanisms of their formation [8]. They are both composed of an organic matrix rich in type I collagen and a mineral phase consisting of carbonate-substituted hydroxyapatite (HAP) crystals. During osteogenesis and dentinogenesis, mineralization occurs throughout successive steps. Primarily osteoblasts and odontoblasts, the main formative cell types of bone and dentin, secrete a non-mineralized matrix called osteoid and predentin, respectively. Type I collagen, the main protein of predentin and osteoid ECM, is progressively assembled as fibrils to form a well-organized matrix as framework [9]. Calcium and phosphate ions transferred from the vascular network mineralize the matrix predominantly at the mineralization front (Fig. 1).
Fig. 1.
Characteristics of dentin formation and bone remodelling. A: Odontoblasts secrete an ECM composed of type I collagen and NCPs. Within the predentin, type I collagen molecules are assembled as fibrils. Mineralization occurs at the mineralization front by growth and fusion of calcospherites formed by hydroxyapatite (HAP) crystals. This mineralization process is controlled by NCPs and by mineral ion availability. Cell processes remain entrapped within dentin whereas cell bodies remain at the periphery of the pulp. B: Healthy bone is in a dynamic state: the activities of osteoblasts (bone formation) and osteoclasts (bone resorption) are regulated by the OPG/RANK/RANKL axis. RANKL stimulates bone resorption by increasing osteoclast differentiation whereas OPG, a soluble decoy receptor for RANKL, blocks osteoclast formation by inhibiting RANKL binding to RANK.
Bone and dentin ECM contains numerous noncollagenous proteins (NCPs) believed to be responsible for the initiation and control of the mineralization process that transforms predentin and osteoid into dentin and bone, respectively [10]. It has been assumed that some NCPs are associated with specific sites on collagen molecules to promote the nucleation and growth of HAP crystals [11,12]. This hypothesis is strongly supported by studies showing mutations or experimental suppression of NCP genes, especially a phosphoprotein family called SIBLINGs (Small Integrin Binding LIgand N-linked Glycoproteins). SIBLINGs are associated with abnormal phenotypes in the mineralization of bone and/or dentin [13–16]. This family includes dentin sialophosphoprotein (DSPP), dentin matrix protein 1 (DMP1), bone sialoprotein (BSP), matrix extracellular phosphorylated glycoprotein (MEPE) and osteopontin (OPN). In addition to an arginine–glycine–aspartate (RGD) cell adhesion motif, a key feature of these proteins is a highly conserved acidic serine and aspartate-rich motif (ASARM), which is located at the C terminal region of all SIBLINGs, except in OPN where it is located in the mid-region of the protein. The precise role of NCPs in bone and tooth mineralization is still a matter of vigorous debate, as these proteins may function as matrix mineralization nucleators or inhibitors according to the context and the experimental model used [10,17–19].
Distinguishing features of bone and dentin
Although parallels can be drawn between bone and dentin, these tissues display several differences. First, bone is a dynamic tissue, continuously remodelled, whereas dentin shows no turnover. Bone formation occurs through the production of matrix components by osteoblast under the control of local growth factors. In fetal or rapidly forming bone (woven bone), matrix vesicles, produced and released by osteoblasts, are a site of initial mineralization [20,21]. A first nucleus is formed within the vesicle and is used for secondary nucleation and crystal propagation. HAP crystals grow outside the vesicles to form mineralized nodules (calcospherites or mineralization foci) [22,23].
Dentinogenesis is a continuous process of matrix deposition throughout the life of a tooth. The odontoblast secretes dentin matrix and the cell is displaced to the pulp, leaving its process embedded in the matrix. The cell body is never entrapped in its matrix, unlike osteocytes, except occasionally in pathological situations [24]. There are four types of dentin: (i) the mantle dentin corresponds to the first layer deposited by newly differentiated odontoblasts; (ii) the primary dentin is formed during tooth development (4 μm/day), helping establish the morphology of the tooth germ; (iii) the secondary dentin is physiologically secreted after tooth eruption (0.4 μm/day) and leads to the progressive reduction of the pulp volume (there is no histological difference between primary and secondary dentin); (iv) the tertiary dentin is secreted in response to an injury which serves to protect the underlying pulp [25]. In the instance of moderate injury, surviving odontoblasts secrete so-called ‘reactionary’ dentin. When the damage is significant and the odontoblastic layer is altered, it becomes a reparative dentin. Reparative dentin in the majority of cases is different morphologically from reactionary dentin. For example, it may contain cellular inclusions that resemble osteocytes, forming reparative “osteodentin”.
In contrast to dentin, bone is a repository for stored calcium (about 99% of total body calcium) where it can be quickly mobilized for exchanges with the extracellular space. Bone is richly vascularized and participates in the control of calcium and phosphate metabolism to a large extent (together with the kidney and intestine). Bone cells are continuously renewed by reiterative cycles of proliferation and differentiation on bone surfaces, while odontoblasts are post-mitotic cells. The bone matrix surrounds a network of cells being the terminal differentiated stage of the osteoblasts. These cells, designated as osteocytes, represent up to 95% of the cellular component of adult bone, are marked by the expression of PHEX, sclerostin and DMP1 and have been shown to play various major roles such as the synthesis of new bone matrix, the sensing of pressure or strain, or the trigger in the fracture healing processes [26]. Conversely, bone resorption, a specific bone process, is a result of the action of lysosomal enzymes synthesized by the osteoclast and the acidification of the extracellular compartment of the bone matrix.
Dentin, not remodelling during life, reflects the biomineralization process in both physiological and pathological conditions. Hypophosphatemic rickets and osteogenesis imperfecta illustrate how the tooth can reflect pathological biomineralization.
Familial hypophosphatemic rickets
Familial hypophosphatemic rickets was first described by Albright and collaborators in 1937 as vitamin D resistant rickets [27]. Later, several forms of hypophosphatemic rickets were identified depending on their inheritance (X-linked, autosomal, recessive or dominant) and the gene mutation. The most frequent form is the X-linked form [28]. In 1995 the PEX gene, later renamed PHEX, for Phosphate regulating gene with Homologies to Endopeptidases on the X-chromosome, was identified (MIM 300550) as the principal cause of X-linked (79%) dominant hypophosphatemic rickets (MIM 307800) [29,30]. Later, FGF23 mutations were identified as the cause of autosomal dominant hypophosphatemic rickets [31]; mutations in DMP1 [32] and SLC34A [33,34] cause autosomal recessive hypophosphatemic rickets. Recently, loss-of-function mutations in ENPP1 gene and a translocation involving the Klotho gene were associated with hypophosphatemic rickets [35–37].
The PHEX gene is located on chromosome Xp22.1–22.2 in humans and encodes a 749 amino acid transmembrane endopeptidase. PHEX involved in calcium and phosphate metabolism is expressed by osteoblasts, osteocytes and odontoblasts [38,39]. In vitro studies identified PTHrP107–139 and MEPE and OPN-derived ASARM peptides as PHEX substrates [40–42].
PHEX interacts with MEPE in the bone/dentin ECM protecting this protein from C-terminal cleavage [42,43]. When PHEX is mutated, MEPE is exposed to cleavage in the ECM, liberating a peptide enclosing the ASARM region. This acidic peptide is highly resistant to proteolysis and is a potent inhibitor of the mineralization process [44]. In addition, it is increased in the serum of hypophosphatemic patients and may accumulate in the kidney, inducing hypophosphatemia [43,45,46]. PHEX may also be involved in the cleavage of FGF23 [47] probably via Klotho, neutralizing its hypophosphatemic effect. Nevertheless, the links between PHEX, FGF23, Klotho and MEPE in calcium and phosphate metabolism remain to be clarified.
Two major mice models of the hypophosphatemic rickets, namely Hyp [48] and Gy [49] mice caused by spontaneous deletion of Phex, show that PHEX is necessary for both phosphate homeostasis and bone and dentin mineralization [50,51].
Clinical features
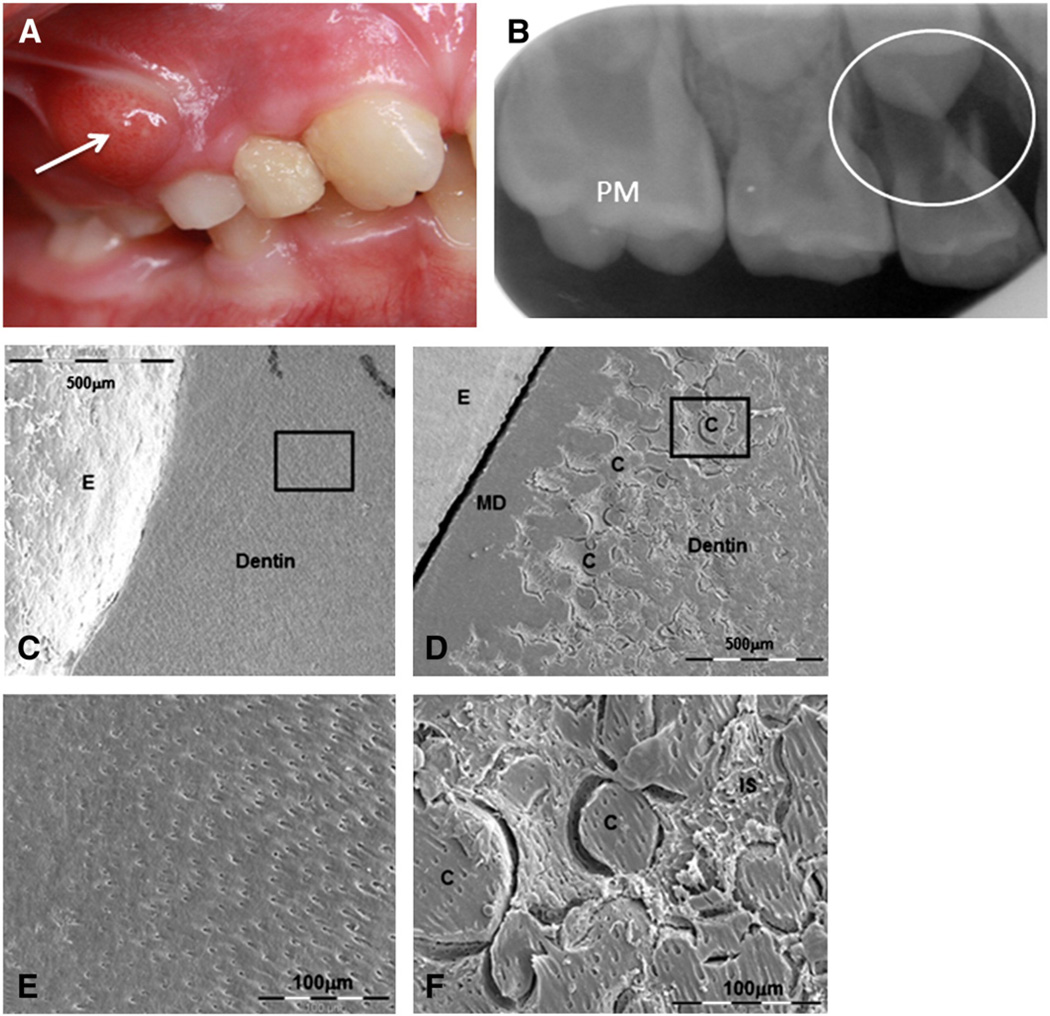
The phenotype of X-linked dominant hypophosphatemic rickets (XLHR), while variable in its severity is characterized by: rickets with leg bowing, short stature, high bone mass, severe dental anomalies, increased alkaline phosphatase activity, hypophosphatemia due to renal tubular phosphate wasting, normal or low normal serum calcium levels with hypocalciuria, normal or low serum levels of 1.25(OH)2 vitamin D, and normal serum levels of parathyroid hormone [52]. The dominant feature in teeth is the occurrence of spontaneous infectious abscesses (on deciduous as well as on permanent teeth), not associated with trauma or decay. DMFT (Decayed, Missing, Filled Tooth; the main index for dental status of children/adults) of patients affected with XLHR is significantly higher than DMFT of healthy age-matched controls [53,54]. On X-rays, hypophosphatemic teeth present an enlarged pulp chamber with prominent horns extending up to the dentin–enamel junction [54]. While some clinical studies report enamel defects [53,54], our histological studies have revealed few enamel abnormalities, with the exception of the occurrence of micro-cracks up to the dentin–enamel junction. In contrast, the dentin was severely disturbed; the entire dentin bulk mineralization was abnormal. We found large nonmineralized interglobular spaces between non-merged calcospherites. Hence, impaired dentin mineralization is the major abnormality of hypophosphatemic teeth. In addition, enamel defects observed may be the consequence of an abnormal underlying dentin [55]. Together with prominent pulp horn and enamel cracks, hypomineralization of hypophosphatemic dentin likely represents a pathway by which bacteria can easily invade the pulp leading to “spontaneous” abscesses of the tooth without any sign of dental decay [55] (Fig. 2).
Fig. 2.
Dental features of hypophosphatemic rickets. A: Clinical view of a dental abscess on a deciduous molar with no history of trauma or decayB: X-ray of the infected tooth. Enlarged pulp horn can be observed on the permanent molar (PM) and the abscess is circled at the apical zone of the first deciduous molar. Courtesy of Dr Douyère (Hôpital Bretonneau AP-HP, France). Tooth sections observed by Scanning Electron Microscopy (SEM) reveal C: homogenous dentin mineralization for control and D: impaired hypophosphatemic dentin mineralization, except for mantle dentin (MD). Enamel (E) presents a normal aspect in both samples. At higher magnification (black squares), E: regular tubules for control dentin, and F: hypophosphatemic dentin presenting calcospherites (C) between nonmineralized interglobular spaces (IS).
Moreover, we demonstrated ECM protein accumulation in interglobular spaces and identified several peptides derived from type I collagen and NCPs degradation in dentin extracts, particularly a peptide derived from MEPE containing the ASARM region [56]. This peptide, previously identified in the serum of hypophosphatemic patients [46], may explain in part the abnormal dentin mineralization as it is a strong inhibitor of mineralization [44].
In sum, our data show that the tooth is a suitable organ for studying impaired mineralization associated with a genetic disease affecting calcium and phosphate metabolism. By studying hypophosphatemic teeth, we contribute to the knowledge of the underlying mechanisms of the disease, particularly regarding ECM protein degradation and release of pathological peptides.
Systemic treatment
Since the 1970s, a new therapeutic strategy has been introduced for hypophosphatemic children, combining oral phosphate and 1α-hydroxyvitamin D3, a synthetic analogue of 25,OH vitamin D. This treatment has considerably improved growth, bone mineralization and reduced or prevented bone malformation, thus reducing the need for surgical intervention [57]. It also has a clear beneficial impact on dental health, especially for permanent teeth, which mineralize after birth. In 2003, we reported that DMFT of patients treated with phosphate and 1α-hydroxyvitamin D3 since early childhood were comparable to DMFT of healthy age-matched controls [54]. Tooth analysis is a useful approach to the evaluation of the benefits of systemic treatment on mineralization. Indeed, permanent tooth analysis from patients treated since early childhood showed the achievement of an overall healthy mineralized dentin phenotype. A well-mineralized dentin bulk was observed in most treated patients. For some patients, only faint calcospherites were still visible, probably corresponding to a period of lower compliance to treatment (Fig. 3) [55]. Proteomic assays revealed that ECM proteins and particularly MEPE were not degraded in the dentin. In addition, the amount of MEPE-derived ASARM peptide measured was comparable to those of control patients [56,58]. As PHEX is expressed in the tooth [38] it is likely that a deficient expression of the PHEX protein due to a loss of function mutations causes the dental phenotype through the lack of interaction between PHEX and MEPE or PHEX and MEPE-derived ASARM peptides [43]. In addition, the phosphate deficiency resulting from phosphate wasting may impair the phosphorylation of NCPs such as MEPE or OPN and decrease their resistance to cleavage. The deficient 1.25(OH)2 vitamin D production due to the FGF23 inhibition of the 1α hydroxylase may also contribute to the dental anomalies through the control of phosphate transport and MEPE expression. Accordingly, abnormal MEPE cleavage in the dentin of X-linked hypophosphatemic patients does not only result from the lack of direct interaction between the MEPE and PHEX proteins, but also from the phosphate- and vitamin D deficient environment associated with PHEX impairment. As for the rickets and growth, the phosphate load and restoration of normal 1.25(OH)2 vitamin D level compensate for the PHEX gene dysfunction and limits MEPE cleavage allowing efficient dental mineralization [54].
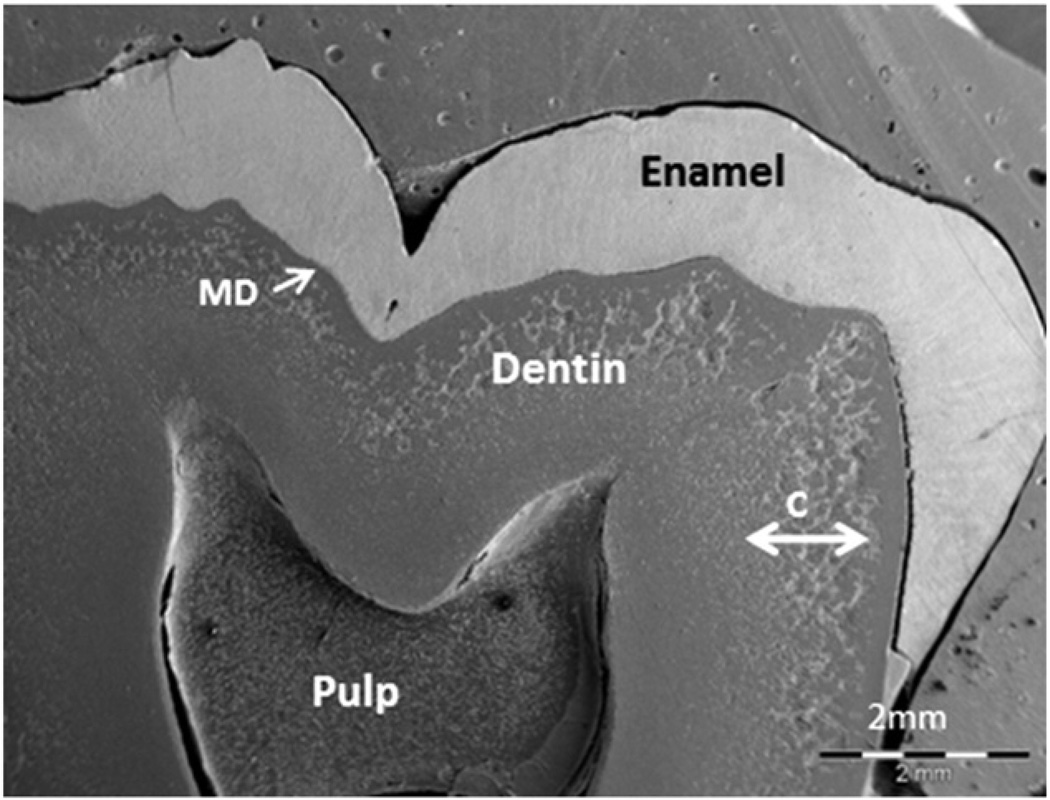
Fig. 3.
Effects of 1–25 (OH)2D and phosphate treatment during childhood on dentin mineralization in permanent teeth. SEM observation of a third molar collected from a hypophosphatemic patient, who received treatment during growth. Dentin structure and ECM organization appear normal in the inner part of dentin, which mineralized correctly during treatment. Calcospherites (C) that did not coalesce as would normally occur are visible in the outer part of dentin, reflecting periods with poor compliance to therapy.
Patients who received a treatment during the growth period and with good compliance to therapy have less dental abnormalities in their permanent teeth, which as a consequence are less prone to spontaneous abscesses. These data highlight the usefulness of studying permanent teeth (extracted for orthodontic reasons) to assess the benefit of the systemic treatment on hypophosphatemic rickets.
Principles of dental management
A dental examination should be carried out twice a year. Intraoral X-rays may be performed to identify periapical lesions, especially for patients whose skeletal analysis suggests an underlying abnormality. Dealing with spontaneous dental abscesses is the major challenge for dental practitioners participating in the care of young hypophosphatemic patients. While systemic treatment improves dentin mineralization in permanent teeth, dentin defects may persist, mainly in deciduous teeth that have impaired mineralization before the beginning of treatment. Therefore, we recommend to protect both deciduous and permanent teeth from bacterial invasion by an early sealing of occlusal surfaces using composite resins [59]. In the past, stainless steel crowns were used to protect the entire crown of the molars. Their use is not recommended currently as their benefit is not as effective as resin sealing, the properties of which have greatly improved over the last decade and which are recommended for children [60]. When abscesses occur, conventional endodontic treatment should be performed on permanent teeth. For primary teeth, extraction might be an option depending on stage of root development. Orthodontic treatment is not contraindicated especially for patients correctly treated with phosphate and 1α-hydroxyvitamin D3 [61]. In our experience, information for both patients and practitioners through the use of pictured documents has greatly improved routine dental care.
Osteogenesis imperfecta
Osteogenesis imperfecta (OI) is an autosomal dominant disorder of connective tissue that is also known as “brittle bone disease”. COL1A1, COL1A2, CRTAP and LEPRE1 are the genes involved in OI. Based on the common classification, OI patients can be categorized into mild (type I MIM 166240 and MIM 166200), perinatal lethal (type II MIM 166210 and MIM 610854), progressive deforming (type III MIM 259420), and moderately severe (type IV 166220) types [62]; four additional types have been added with distinct features [63,64]. According to the severity in the clinical manifestations, mild forms of OI are usually caused by a premature stop codon or deletion of a single COL1A1 allele on chromosome 17, which reduces the amount of normal type I collagen formation. Severe forms are caused by dominant-negative mutations in COL1A1 or COL1A2 on chromosome 7 that lead to structural defects in the assembled collagen fibril [65]. Type I collagen is the most abundant protein in mammals and the major protein of bone, thus most clinical manifestations of OI impact on osseous structures [66]. Numerous extra-skeletal manifestations may occur, as type I collagen also exists in tendons, ligaments, skin, sclera, teeth, and middle and inner ear [63]. Besides the 90% of collagen mutations, mutations in the CRTAP and LEPRE1 genes responsible for rare, often severe cases of OI (type VIIMIM 610682 and VIIIMIM 610682, respectively) have been reported [67–69]. More recently a novel mutation of SERPINF1 coding for pigment epithelium-derived factor has been identified in type VI OI [70]. Mutations in either gene disrupt the normal folding, assembly, and secretion of collagen molecules. Despite illuminating genetic research, genotype–phenotype correlations are often complex and unpredictable [71].
Many mouse models of OI have been published, and some are available for research on the cellular and molecular defects, or on pharmacological therapy: Mov-13 for OI type I [72], Brittle II for OI type II [73], oim/oim for OI type III [74], Brittle IV for OI type IV [75] and fro/fro for the noncollagenous form [76]. By studying this last model, we, as well as other groups, have clearly demonstrated that sphingomyelinases were involved in bone and dentin mineralization [77–79].
Clinical features
The clinical manifestations of OI include fragile bones, blue sclera, early deafness, dentinogenesis imperfecta (DI), growth deficiency, joint laxity, or any combination of these characteristics. There is extreme phenotypic variation within this population, from the lethal type II OI to the very mild type I OI. The prevalence in infancy is approximately one in 20000 [80], but the real incidence may be higher due to undiagnosed children with mild forms of OI [81].
Paralleling the phenotypic heterogeneity of OI, the orofacial manifestations attributable to OI vary widely, with DI representing the classic oral finding. DI in association with OI is termed DI type I, because there are 2 non-OI associated types of DI recognized (Type II and Type III) [82]. Association of OI and DI is inconsistent; about one half of all OI patients show no obvious clinical signs of DI. The DI phenotype, however, is not clinically evident, but can be detected radiologically [83] or histologically [84]. In contrast, some individuals with OI, while displaying obvious DI, show no detectable bone phenotype [85].
DI is characterized by discoloration of the dentition, severe attrition of the teeth, bulbous crowns and early obliteration of the pulp in both deciduous and permanent dentitions. Pulp obliteration occurs soon after eruption or prior to tooth eruption. Primary dentition is more severely affected than the permanent dentition. This difference may be explained by a faster formation of the primary teeth and a greater expression of collagen during the embryonic developmental stage [83,86]. The enamel, which shows normal structure and normal or infrequently decreased mineral content [87], is often dislodged, exposing the soft dysplastic dentin. Teeth show a greyish-blue to brown-yellow colour with a typical amber translucent appearance [88]. These discolorations are the results of light diffraction through the defective dentin–enamel junction [87].
By X-ray, the features are pathognomonic, with short roots, bulbous shaped crowns constricting at the cervix, pulp obliteration and periapical radiolucencies [82,89]. On histological examination, if the initially formed mantle dentin is normal, the rest of the dentin is characterized by a dysplastic appearance with amorphous areas, irregular dentin tubules, embedded cells, and occasionally interglobular dentin [90,91]. Large spots of nonmineralized matrix and even zones without any dentin tubule are present (Fig. 4) [92].
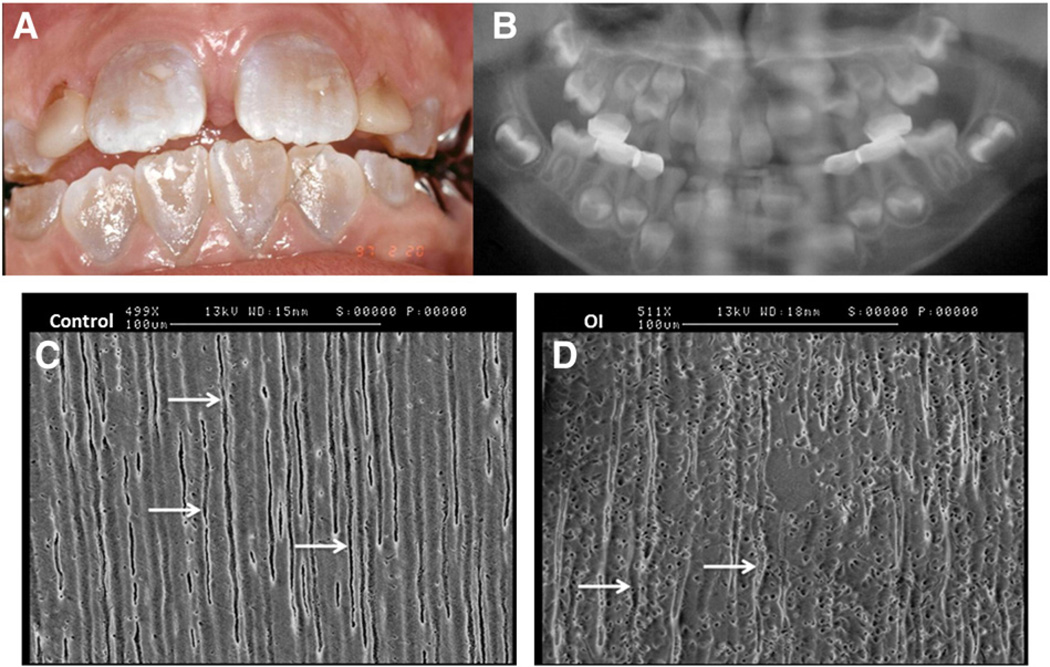
Fig. 4.
Dental features of osteogenesis imperfecta. A: Intrabuccal view of 7 year-old girl with type I osteogenesis imperfecta (OI). The patient also presents a dentition with DI, characterized by amber discoloration. B: Panoramic X-ray of this patient shows typical bulbous crowns and early pulp obliteration in the primarymolars. Courtesy of Dr Vanderzwalm (Hôpital Louis Mourier AP-HP, France). Third molar sections observed by SEMC: control dentin shows regular and aligned dentinal tubules (arrow) D: type I OI dentin shows irregular dentinal tubules (arrow).
Other atypical dental features were reported with OI such as increase rate of heterotopic eruption of first and second molars [84,93]; a high prevalence of permanent tooth agenesis also has been reported (10% to 22%) [84].
OI patients usually present with facial characteristics including a triangular face, protrusive bitemporal bone and prominent frontal bone [94,95], an overhanging occiput [94] and a relatively larger head circumference [96]. Dental malocclusions are marked in many OI subjects and include a high incidence of Class III malocclusions (in 67% to 80% of the patients) [93,97,98], anterior and/or posterior crossbite, and posterior open bite [99]. These effects are attributable to the combination of the skeletal and dentoalveolar abnormalities [98].
Systemic treatment: bisphosphonate therapy
Clinical studies have established that some bisphosphonates (BPs) have beneficial effects on children with OI by reducing the fracture rate, improving the bone density and enhancing growth. However, the overall long-term efficacy and safety for this class of drugs are still debated [100–103]. In adults, an increased incidence of osteonecrosis of the jaw has been associated with the BPs therapy. Several risk factors for Bisphosphonate-Related Osteonecrosis of the Jaw (BRONJ) like dentoalveolar surgery, use of BPs in the context of cancer or high doses of intravenous BPs, have been identified [104]. However, to date, no cases of BRONJ have been reported in the OI children treated with BPs. Two retrospective studies [105,106] and a case-report series [107] investigated children after long-term BP treatment, some of them experiencing dental extraction. None of them showed clinical signs of BRONJ. Considering the lack of prospective clinical studies, preventive measures to reduce the putative risk of BRONJ are recommended [108]. All patients with OI in whom bisphosphonate therapy is planned should be referred for a comprehensive oral screening and therapy session as necessary to identify and eradicate all active and potential sources of infection [109].
Besides the putative BRONJ-risk, administration of BPs during childhood may affect the tooth development. Indeed osteoclastic bone resorption is required for tooth eruption. Consistent with this notion, a human clinical study has shown that BPs delay tooth eruption [110]. Animal studies show inhibition and delay in tooth eruption [111–113] and dental abnormalities [114].
Principles of dental management
According to the American Academy of Pediatric Dentistry guidelines, providing optimal oral health treatment for DI includes: (i) preventing severe attrition associated with enamel loss and rapid wear of the poorly mineralized dentin, (ii) rehabilitating dentition that has undergone severe wear, (iii) optimizing aesthetics, and (iv) preventing caries and periodontal disease [115]. When surgical procedures are needed, particular attention should be paid to the risk of jaw fracture.
If a preventive strategy is preferred, tooth wear requires frequent restorative treatments. Adhesive procedures can be used effectively to treat mild to moderate damage. In more severe cases with significant enamel fracturing and rapid dental wear, the treatment of choice is full coverage restorations in both the primary dentition with stainless steel crowns and the permanent dentition with onlays [116,117]. In primary dentition, stainless steel crowns can be used to stabilize the dentition and avoid the loss of vertical dimension of the face [118].
Bleaching can lighten the colour of DI teeth with some success [119,120]; however, because the discoloration is caused primarily by the underlying yellow-brown dentin, bleaching alone is unlikely to produce a normal appearance in cases of significant discoloration. Different types of veneers can be used to improve the aesthetics and mask the opalescent blue-gray discoloration of the anterior teeth but it is recommended that must be deferred until periodontal maturation is complete i.e. late adolescence.
Conclusion
The tooth is a valuable tool to study the biomineralization process, especially genetic disorders affecting bone as they also frequently involve impaired dentinmineralization. Familial hypophosphatemic rickets and OI are two major examples. Their current treatment has shown a significant improvement of the associated skeletal abnormalities [121] and the effects of hypophosphatemia on teeth [54]. Appropriate dental treatments can improve significantly the quality of life of patients affected by such genetic diseases.
A host of proteins are involved in mineral metabolism and dentin mineralization. Therefore, the study of peptides derived from the bone and dentin ECM such as DMP1,MEPE, OPN or DSP involved in promoting or repressing mineralization is of major interest. These studies may lead to a better understanding of the pathological pathways involving both bone and dentin affecting diseases.
Acknowledgments
This work would not have been possible without Dr Michele Garabédian and her constant involvement with the treatment of patients with genetic diseases affecting bone mineralization. The authors are grateful to Mrs Dominique Septier for teaching them her great knowledge on human tooth histology and Mr Dominique Le Denmat for his imagery skills.
Footnotes
Financial disclosure
None.
Conflict of interest
None.
References
- 1.Bonaventure J, Stanescu R, Stanescu V, Allain JC, Muriel MP, Ginisty D, et al. Type II collagen defect in two sibs with the Goldblatt syndrome, a chondrodysplasia with dentinogenesis imperfecta, and joint laxity. Am J Med Genet. 1992;44:738–753. doi: 10.1002/ajmg.1320440607. [DOI] [PubMed] [Google Scholar]
- 2.da Fonseca MA. Dental findings in the Schimke immuno-osseous dysplasia. Am J Med Genet. 2000;93:158–160. doi: 10.1002/1096-8628(20000717)93:2<158::aid-ajmg14>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 3.Wedgwood DL, Curran JB, Lavelle CL, Trott JR. Cranio-facial and dental anomalies in the Branchio-Skeleto-Genital (BSG) syndrome with suggestions for more appropriate nomenclature. Br J Oral Surg. 1983;21:94–102. doi: 10.1016/0007-117x(83)90053-7. [DOI] [PubMed] [Google Scholar]
- 4.Kantaputra PN. A newly recognized syndrome of skeletal dysplasia with opalescent and rootless teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:303–307. doi: 10.1067/moe.2001.116819. [DOI] [PubMed] [Google Scholar]
- 5.Kantaputra PN. Apparently new osteodysplastic and primordial short stature with severe microdontia, opalescent teeth, and rootless molars in two siblings. Am J Med Genet. 2002;111:420–428. doi: 10.1002/ajmg.10589. [DOI] [PubMed] [Google Scholar]
- 6.Byers PH, Bonadio JF, Cohn DH, Starman BJ, Wenstrup RJ, Willing MC. Osteogenesis imperfecta: the molecular basis of clinical heterogeneity. Ann N Y Acad Sci. 1988;543:117–128. doi: 10.1111/j.1749-6632.1988.tb55324.x. [DOI] [PubMed] [Google Scholar]
- 7.Wright JT. The molecular etiologies and associated phenotypes of amelogenesis imperfecta. Am J Med Genet A. 2006;140:2547–2555. doi: 10.1002/ajmg.a.31358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang B, Sun Y, Maciejewska I, Qin D, Peng T, McIntyre B, et al. Distribution of SIBLING proteins in the organic and inorganic phases of rat dentin and bone. Eur J Oral Sci. 2008;116:104–112. doi: 10.1111/j.1600-0722.2008.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Embery G, Hall R, Waddington R, Septier D, Goldberg M. Proteoglycans in dentinogenesis. Crit Rev Oral Biol Med. 2001;12:331–349. doi: 10.1177/10454411010120040401. [DOI] [PubMed] [Google Scholar]
- 10.Qin C, Baba O, Butler WT. Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med. 2004;15:126–136. doi: 10.1177/154411130401500302. [DOI] [PubMed] [Google Scholar]
- 11.Traub W, Arad T, Weiner S. Origin of mineral crystal growth in collagen fibrils. Matrix. 1992;12:251–255. doi: 10.1016/s0934-8832(11)80076-4. [DOI] [PubMed] [Google Scholar]
- 12.He G, Gajjeraman S, Schultz D, Cookson D, Qin C, Butler WT, et al. Spatially and temporally controlled biomineralization is facilitated by interaction between self-assembled dentin matrix protein 1 and calcium phosphate nuclei in solution. Biochemistry. 2005;44:16140–16148. doi: 10.1021/bi051045l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sreenath T, Thyagarajan T, Hall B, Longenecker G, D'Souza R, Hong S, et al. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem. 2003;278:24874–24880. doi: 10.1074/jbc.M303908200. [DOI] [PubMed] [Google Scholar]
- 14.Sun Y, Lu Y, Chen L, Gao T, D'Souza R, Feng JQ, et al. DMP1 processing is essential to dentin and jaw formation. J Dent Res. 2011;90:619–624. doi: 10.1177/0022034510397839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y, Prasad M, Gao T, Wang X, Zhu Q, D'Souza R, et al. Failure to process dentin matrix protein 1 (DMP1) into fragments leads to its loss of function in osteogenesis. J Biol Chem. 2010;285:31713–31722. doi: 10.1074/jbc.M110.137059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alford AI, Hankenson KD. Matricellular proteins: extracellular modulators of bone development, remodeling, and regeneration. Bone. 2006;38:749–757. doi: 10.1016/j.bone.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Hunter GK, Goldberg HA. Modulation of crystal formation by bone phosphoproteins: role of glutamic acid-rich sequences in the nucleation of hydroxyapatite by bone sialoprotein. Biochem J. 1994;302(Pt 1):175–179. doi: 10.1042/bj3020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher LW, Fedarko NS. Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect Tissue Res. 2003;44(Suppl 1):33–40. [PubMed] [Google Scholar]
- 19.Narayanan K, Ramachandran A, Hao J, He G, Park KW, Cho M, et al. Dual functional roles of dentin matrix protein 1. Implications in biomineralization and gene transcription by activation of intracellular Ca2+ store. J Biol Chem. 2003;278:17500–17508. doi: 10.1074/jbc.M212700200. [DOI] [PubMed] [Google Scholar]
- 20.Boskey AL, Boyan BD, Schwartz Z. Matrix vesicles promote mineralization in a gelatin gel. Calcif Tissue Int. 1997;60:309–315. doi: 10.1007/s002239900234. [DOI] [PubMed] [Google Scholar]
- 21.Hoshi K, Ozawa H. Matrix vesicle calcification in bones of adult rats. Calcif Tissue Int. 2000;66:430–434. doi: 10.1007/s002230010087. [DOI] [PubMed] [Google Scholar]
- 22.Anderson HC. Mechanism of mineral formation in bone. Lab Invest. 1989;60:320–330. [PubMed] [Google Scholar]
- 23.McKee MD, Pedraza CE, Kaartinen MT. Osteopontin and wound healing in bone. Cells Tissues Organs. 2011;194:313–319. doi: 10.1159/000324244. [DOI] [PubMed] [Google Scholar]
- 24.Simon S, Smith AJ, Lumley PJ, Berdal A, Smith G, Finney S, et al. Molecular characterization of young and mature odontoblasts. Bone. 2009;45:693–703. doi: 10.1016/j.bone.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg M, Smith AJ. Cells and extracellularmatrices of dentin and pulp: a biological basis for repair and tissue engineering. Crit Rev Oral Biol Med. 2004;15:13–27. doi: 10.1177/154411130401500103. [DOI] [PubMed] [Google Scholar]
- 26.Dallas SL, Bonewald LF. Dynamics of the transition from osteoblast to osteocyte. Ann N Y Acad Sci. 2010;1192:437–443. doi: 10.1111/j.1749-6632.2009.05246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albright F, Butler AM, Bloomberg E. Rickets resistant to vitamin D. Am J Dis Child. 1937;54:529–547. [Google Scholar]
- 28.Winters RW, Graham JB, Williams TF, Mc FV, Burnett CH. A genetic study of familial hypophosphatemia and vitamin D resistant rickets with a review of the literature. Medicine (Baltimore) 1958;37:97–142. doi: 10.1097/00005792-195805000-00001. [DOI] [PubMed] [Google Scholar]
- 29.A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat Genet. 1995;11:130–136. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- 30.Gaucher C, Walrant-Debray O, Nguyen TM, Esterle L, Garabedian M, Jehan F. PHEX analysis in 118 pedigrees reveals new genetic clues in hypophosphatemic rickets. Hum Genet. 2009;125:401–411. doi: 10.1007/s00439-009-0631-z. [DOI] [PubMed] [Google Scholar]
- 31.Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 32.Lorenz-Depiereux B, Bastepe M, Benet-Pages A, Amyere M, Wagenstaller J, Muller-Barth U, et al. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet. 2006;38:1248–1250. doi: 10.1038/ng1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, Abu-Zahra H, et al. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium–phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet. 2006;78:179–192. doi: 10.1086/499409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorenz-Depiereux B, Benet-Pages A, Eckstein G, Tenenbaum-Rakover Y, Wagenstaller J, Tiosano D, et al. Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium–phosphate cotransporter gene SLC34A3. Am J Hum Genet. 2006;78:193–201. doi: 10.1086/499410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy-Litan V, Hershkovitz E, Avizov L, Leventhal N, Bercovich D, Chalifa-Caspi V, et al. Autosomal-recessive hypophosphatemic rickets is associated with an inactivation mutation in the ENPP1 gene. Am J Hum Genet. 2010;86:273–278. doi: 10.1016/j.ajhg.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorenz-Depiereux B, Schnabel D, Tiosano D, Hausler G, Strom TM. Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am J Hum Genet. 2010;86:267–272. doi: 10.1016/j.ajhg.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brownstein CA, Adler F, Nelson-Williams C, Iijima J, Li P, Imura A, et al. A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci U S A. 2008;105:3455–3460. doi: 10.1073/pnas.0712361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruchon AF, Tenenhouse HS, Marcinkiewicz M, Siegfried G, Aubin JE, DesGroseillers L, et al. Developmental expression and tissue distribution of Phex protein: effect of the Hyp mutation and relationship to bone markers. J Bone Miner Res. 2000;15:1440–1450. doi: 10.1359/jbmr.2000.15.8.1440. [DOI] [PubMed] [Google Scholar]
- 39.Thompson DL, Sabbagh Y, Tenenhouse HS, Roche PC, Drezner MK, Salisbury JL, et al. Ontogeny of Phex/PHEX protein expression in mouse embryo and subcellular localization in osteoblasts. J Bone Miner Res. 2002;17:311–320. doi: 10.1359/jbmr.2002.17.2.311. [DOI] [PubMed] [Google Scholar]
- 40.Boileau G, Tenenhouse HS, Desgroseillers L, Crine P. Characterization of PHEX endopeptidase catalytic activity: identification of parathyroid-hormone-related peptide107-139 as a substrate and osteocalcin, PPi and phosphate as inhibitors. Biochem J. 2001;355:707–713. doi: 10.1042/bj3550707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Addison WN, Masica DL, Gray JJ, McKee MD. Phosphorylation-dependent inhibition of mineralization by osteopontin ASARM peptides is regulated by PHEX cleavage. J Bone Miner Res. 2010;25:695–705. doi: 10.1359/jbmr.090832. [DOI] [PubMed] [Google Scholar]
- 42.Addison WN, Nakano Y, Loisel T, Crine P, McKee MD. MEPE–ASARM peptides control extracellular matrix mineralization by binding to hydroxyapatite: an inhibition regulated by PHEX cleavage of ASARM. J Bone Miner Res. 2008;23:1638–1649. doi: 10.1359/jbmr.080601. [DOI] [PubMed] [Google Scholar]
- 43.Martin A, David V, Laurence JS, Schwarz PM, Lafer EM, Hedge AM, et al. Degradation of MEPE, DMP1, and release of SIBLING ASARM-peptides (minhibins): ASARM-peptide(s) are directly responsible for defective mineralization in HYP. Endocrinology. 2008;149:1757–1772. doi: 10.1210/en.2007-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rowe PS, Kumagai Y, Gutierrez G, Garrett IR, Blacher R, Rosen D, et al. MEPE has the properties of an osteoblastic phosphatonin and minhibin. Bone. 2004;34:303–319. doi: 10.1016/j.bone.2003.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu S, Rowe PS, Vierthaler L, Zhou J, Quarles LD. Phosphorylated acidic serine– aspartate-rich MEPE-associated motif peptide from matrix extracellular phosphoglycoprotein inhibits phosphate regulating gene with homologies to endopeptidases on the X-chromosome enzyme activity. J Endocrinol. 2007;192:261–267. doi: 10.1677/joe.1.07059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bresler D, Bruder J, Mohnike K, Fraser WD, Rowe PS. Serum MEPE–ASARM-peptides are elevated in X-linked rickets (HYP): implications for phosphaturia and rickets. J Endocrinol. 2004;183:R1–R9. doi: 10.1677/joe.1.05989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakatani T, Ohnishi M, Razzaque MS. Inactivation of klotho function induces hyperphosphatemia even in presence of high serum fibroblast growth factor 23 levels in a genetically engineered hypophosphatemic (Hyp) mouse model. FASEB J. 2009;23:3702–3711. doi: 10.1096/fj.08-123992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eicher EM, Southard JL, Scriver CR, Glorieux FH. Hypophosphatemia: mouse model for human familial hypophosphatemic (vitamin D-resistant) rickets. Proc Natl Acad Sci U S A. 1976;73:4667–4671. doi: 10.1073/pnas.73.12.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lyon MF, Scriver CR, Baker LR, Tenenhouse HS, Kronick J, Mandla S. The Gy mutation: another cause of X-linked hypophosphatemia in mouse. Proc Natl Acad Sci U S A. 1986;83:4899–4903. doi: 10.1073/pnas.83.13.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu S, Brown TA, Zhou J, Xiao ZS, Awad H, Guilak F, et al. Role of matrix extracellular phosphoglycoprotein in the pathogenesis of X-linked hypophosphatemia. J Am Soc Nephrol. 2005;16:1645–1653. doi: 10.1681/ASN.2004121060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan B, Takaiwa M, Clemens TL, Feng JQ, Kumar R, Rowe PS, et al. Aberrant Phex function in osteoblasts and osteocytes alone underlies murine X-linked hypophosphatemia. J Clin Invest. 2008;118:722–734. doi: 10.1172/JCI32702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.White KE, Larsson TE, Econs MJ. The roles of specific genes implicated as circulating factors involved in normal and disordered phosphate homeostasis: frizzled related protein-4, matrix extracellular phosphoglycoprotein, and fibroblast growth factor 23. Endocr Rev. 2006;27:221–241. doi: 10.1210/er.2005-0019. [DOI] [PubMed] [Google Scholar]
- 53.Souza MA, Soares Junior LA, Santos MA, Vaisbich MH. Dental abnormalities and oral health in patients with hypophosphatemic rickets. Clinics (Sao Paulo) 2010;65:1023–1026. doi: 10.1590/S1807-59322010001000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaussain-Miller C, Sinding C, Wolikow M, Lasfargues JJ, Godeau G, Garabedian M. Dental abnormalities in patients with familial hypophosphatemic vitamin D-resistant rickets: prevention by early treatment with 1-hydroxyvitamin D. J Pediatr. 2003;142:324–331. doi: 10.1067/mpd.2003.119. [DOI] [PubMed] [Google Scholar]
- 55.Chaussain-Miller C, Sinding C, Septier D, Wolikow M, Goldberg M, Garabedian M. Dentin structure in familial hypophosphatemic rickets: benefits of vitamin D and phosphate treatment. Oral Dis. 2007;13:482–489. doi: 10.1111/j.1601-0825.2006.01326.x. [DOI] [PubMed] [Google Scholar]
- 56.Boukpessi T, Gaucher C, Leger T, Salmon B, Le Faouder J, Willig C, et al. Abnormal presence of the matrix extracellular phosphoglycoprotein-derived acidic serine- and aspartate-rich motif peptide in human hypophosphatemic dentin. Am J Pathol. 2010;177:803–812. doi: 10.2353/ajpath.2010.091231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glorieux FH, Marie PJ, Pettifor JM, Delvin EE. Bone response to phosphate salts, ergocalciferol, and calcitriol in hypophosphatemic vitamin D-resistant rickets. N Engl J Med. 1980;303:1023–1031. doi: 10.1056/NEJM198010303031802. [DOI] [PubMed] [Google Scholar]
- 58.Boukpessi T, Septier D, Bagga S, Garabedian M, Goldberg M, Chaussain-Miller C. Dentin alteration of deciduous teeth in human hypophosphatemic rickets. Calcif Tissue Int. 2006;79:294–300. doi: 10.1007/s00223-006-0182-4. [DOI] [PubMed] [Google Scholar]
- 59.Douyere D, Joseph C, Gaucher C, Chaussain C, Courson F. Familial hypophosphatemic vitamin D-resistant rickets—prevention of spontaneous dental abscesses on primary teeth: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:525–530. doi: 10.1016/j.tripleo.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 60.Ahovuo-Saloranta A, Hiiri A, Nordblad A, Makela M, Worthington HV. Pit and fissure sealants for preventing dental decay in the permanent teeth of children and adolescents. Cochrane Database Syst Rev. 2008:CD001830. doi: 10.1002/14651858.CD001830.pub3. [DOI] [PubMed] [Google Scholar]
- 61.Kawakami M, Takano-Yamamoto T. Orthodontic treatment of a patient with hypophosphatemic vitamin D-resistant rickets. ASDC J Dent Child. 1997;64:395–399. [PubMed] [Google Scholar]
- 62.Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979;16:101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004;363:1377–1385. doi: 10.1016/S0140-6736(04)16051-0. [DOI] [PubMed] [Google Scholar]
- 64.Cabral WA, Chang W, Barnes AM, Weis M, Scott MA, Leikin S, et al. Prolyl 3- hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nat Genet. 2007;39:359–365. doi: 10.1038/ng1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gajko-Galicka A. Mutations in type I collagen genes resulting in osteogenesis imperfecta in humans. Acta Biochim Pol. 2002;49:433–441. [PubMed] [Google Scholar]
- 66.Millington-Ward S, McMahon HP, Farrar GJ. Emerging therapeutic approaches for osteogenesis imperfecta. Trends Mol Med. 2005;11:299–305. doi: 10.1016/j.molmed.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 67.Baldridge D, Schwarze U, Morello R, Lennington J, Bertin TK, Pace JM, et al. CRTAP and LEPRE1 mutations in recessive osteogenesis imperfecta. Hum Mutat. 2008;29:1435–1442. doi: 10.1002/humu.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marini JC, Cabral WA, Barnes AM. Null mutations in LEPRE1 and CRTAP cause severe recessive osteogenesis imperfecta. Cell Tissue Res. 2010;339:59–70. doi: 10.1007/s00441-009-0872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Willaert A, Malfait F, Symoens S, Gevaert K, Kayserili H, Megarbane A, et al. Recessive osteogenesis imperfecta caused by LEPRE1 mutations: clinical documentation and identification of the splice form responsible for prolyl 3-hydroxylation. J Med Genet. 2009;46:233–241. doi: 10.1136/jmg.2008.062729. [DOI] [PubMed] [Google Scholar]
- 70.Becker J, Semler O, Gilissen C, Li Y, Bolz HJ, Giunta C, et al. Exome sequencing identifies truncating mutations in human SERPINF1 in autosomal-recessive osteogenesis imperfecta. Am J Hum Genet. 2011;88:362–371. doi: 10.1016/j.ajhg.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roughley PJ, Rauch F, Glorieux FH. Osteogenesis imperfecta—clinical and molecular diversity. Eur Cell Mater. 2003;5:41–47. doi: 10.22203/ecm.v005a04. discussion 7. [DOI] [PubMed] [Google Scholar]
- 72.Schnieke A, Harbers K, Jaenisch R. Embryonic lethal mutation in mice induced by retrovirus insertion into the alpha 1(I) collagen gene. Nature. 1983;304:315–320. doi: 10.1038/304315a0. [DOI] [PubMed] [Google Scholar]
- 73.Stacey A, Bateman J, Choi T, Mascara T, Cole W, Jaenisch R. Perinatal lethal osteogenesis imperfecta in transgenic mice bearing an engineered mutant pro-alpha 1(I) collagen gene. Nature. 1988;332:131–136. doi: 10.1038/332131a0. [DOI] [PubMed] [Google Scholar]
- 74.Chipman SD, Sweet HO, McBride DJ, Jr, Davisson MT, Marks SC, Jr, Shuldiner AR, et al. Defective pro alpha 2(I) collagen synthesis in a recessive mutation in mice: a model of human osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1993;90:1701–1705. doi: 10.1073/pnas.90.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Forlino A, Porter FD, Lee EJ, Westphal H, Marini JC. Use of the Cre/lox recombination system to develop a non-lethal knock-in murine model for osteogenesis imperfecta with an alpha1(I) G349C substitution. Variability in phenotype in BrtlIV mice. J Biol Chem. 1999;274:37923–37931. doi: 10.1074/jbc.274.53.37923. [DOI] [PubMed] [Google Scholar]
- 76.Aubin I, Adams CP, Opsahl S, Septier D, Bishop CE, Auge N, et al. A deletion in the gene encoding sphingomyelin phosphodiesterase 3 (Smpd3) results in osteogenesis and dentinogenesis imperfecta in the mouse. Nat Genet. 2005;37:803–805. doi: 10.1038/ng1603. [DOI] [PubMed] [Google Scholar]
- 77.Opsahl S, Septier D, Aubin I, Guenet JL, Sreenath T, Kulkarni A, et al. Is the lingual forming part of the incisor a structural entity? Evidences from the fragilitas ossium (fro/fro) mouse mutation and the TGFbeta1 overexpressing transgenic strain. Arch Oral Biol. 2005;50:279–286. doi: 10.1016/j.archoralbio.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 78.Goldberg M, Opsahl S, Aubin I, Septier D, Chaussain-Miller C, Boskey A, et al. Sphingomyelin degradation is a key factor in dentin and bone mineralization: lessons from the fro/fro mouse. The chemistry and histochemistry of dentin lipids. J Dent Res. 2008;87:9–13. doi: 10.1177/154405910808700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khavandgar Z, Poirier C, Clarke CJ, Li J, Wang N, McKee MD, et al. A cellautonomous requirement for neutral sphingomyelinase 2 in bone mineralization. J Cell Biol. 2011;194:277–289. doi: 10.1083/jcb.201102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith R. Osteogenesis imperfecta, non-accidental injury, and temporary brittle bone disease. Arch Dis Child. 1995;72:169–171. doi: 10.1136/adc.72.2.169. discussion 71-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Engelbert RH, Pruijs HE, Beemer FA, Helders PJ. Osteogenesis imperfecta in childhood: treatment strategies. Arch Phys Med Rehabil. 1998;79:1590–1594. doi: 10.1016/s0003-9993(98)90426-9. [DOI] [PubMed] [Google Scholar]
- 82.Shields ED, Bixler D, el-Kafrawy AM. A proposed classification for heritable human dentine defects with a description of a new entity. Arch Oral Biol. 1973;18:543–553. doi: 10.1016/0003-9969(73)90075-7. [DOI] [PubMed] [Google Scholar]
- 83.Lund AM, Jensen BL, Nielsen LA, Skovby F. Dental manifestations of osteogenesis imperfecta and abnormalities of collagen I metabolism. J Craniofac Genet Dev Biol. 1998;18:30–37. [PubMed] [Google Scholar]
- 84.Malmgren B, Norgren S. Dental aberrations in children and adolescents with osteogenesis imperfecta. Acta Odontol Scand. 2002;60:65–71. doi: 10.1080/000163502753509446. [DOI] [PubMed] [Google Scholar]
- 85.Pallos D, Hart PS, Cortelli JR, Vian S, Wright JT, Korkko J, et al. Novel COL1A1 mutation (G559C) [correction of G599C] associated with mild osteogenesis imperfecta and dentinogenesis imperfecta. Arch Oral Biol. 2001;46:459–470. doi: 10.1016/s0003-9969(00)00130-8. [DOI] [PubMed] [Google Scholar]
- 86.Luder HU, van Waes H, Raghunath M, Steinmann B. Mild dental findings associated with severe osteogenesis imperfecta due to a point mutation in the alpha 2(I) collagen gene demonstrate different expression of the genetic defect in bone and teeth. J Craniofac Genet Dev Biol. 1996;16:156–163. [PubMed] [Google Scholar]
- 87.Lindau BM, Dietz W, Hoyer I, Lundgren T, Storhaug K, Noren JG. Morphology of dental enamel and dentine–enamel junction in osteogenesis imperfecta. Int J Paediatr Dent. 1999;9:13–21. doi: 10.1046/j.1365-263x.1999.00101.x. [DOI] [PubMed] [Google Scholar]
- 88.Majorana A, Bardellini E, Brunelli PC, Lacaita M, Cazzolla AP, Favia G. Dentinogenesis imperfecta in children with osteogenesis imperfecta: a clinical and ultrastructural study. Int J Paediatr Dent. 2010;20:112–118. doi: 10.1111/j.1365-263X.2010.01033.x. [DOI] [PubMed] [Google Scholar]
- 89.Witkop CJ., Jr Hereditary defects of dentin. Dent Clin North Am. 1975;19:25–45. [PubMed] [Google Scholar]
- 90.Hall RK, Maniere MC, Palamara J, Hemmerle J. Odontoblast dysfunction in osteogenesis imperfecta: an LM, SEM, and ultrastructural study. Connect Tissue Res. 2002;43:401–405. doi: 10.1080/03008200290001005. [DOI] [PubMed] [Google Scholar]
- 91.Lygidakis NA, Smith R, Oulis CJ. Scanning electron microscopy of teeth in osteogenesis imperfecta type I. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:567–572. doi: 10.1016/s1079-2104(96)80048-5. [DOI] [PubMed] [Google Scholar]
- 92.Malmgren B, Lindskog S. Assessment of dysplastic dentin in osteogenesis imperfecta and dentinogenesis imperfecta. Acta Odontol Scand. 2003;61:72–80. doi: 10.1080/00016350310001398. [DOI] [PubMed] [Google Scholar]
- 93.Schwartz S, Tsipouras P. Oral findings in osteogenesis imperfecta. Oral Surg Oral Med Oral Pathol. 1984;57:161–167. doi: 10.1016/0030-4220(84)90206-8. [DOI] [PubMed] [Google Scholar]
- 94.Freedus MS, Schaaf NG, Ziter WD. Orthognathic surgery in osteogenesis imperfecta. J Oral Surg. 1976;34:830–834. [PubMed] [Google Scholar]
- 95.Libman RH. Anesthetic considerations for the patient with osteogenesis imperfecta. Clin Orthop Relat Res. 1981:123–125. [PubMed] [Google Scholar]
- 96.Lund AM, Muller J, Skovby F. Anthropometry of patients with osteogenesis imperfecta. Arch Dis Child. 1999;80:524–528. doi: 10.1136/adc.80.6.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stenvik A, Larheim TA, Storhaug K. Incisor and jaw relationship in 27 persons with osteogenesis imperfecta. Scand J Dent Res. 1985;93:56–60. doi: 10.1111/j.1600-0722.1985.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 98.O'Connell AC, Marini JC. Evaluation of oral problems in an osteogenesis imperfecta population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:189–196. doi: 10.1016/s1079-2104(99)70272-6. [DOI] [PubMed] [Google Scholar]
- 99.Isshiki Y. Morphological studies on osteogenesis imperfecta, especially in teeth, dental arch and facial cranium. Bull Tokyo Dent Coll. 1966;7:31–49. [PubMed] [Google Scholar]
- 100.Ward LM, Rauch F, Whyte MP, D'Astous J, Gates PE, Grogan D, et al. Alendronate for the treatment of pediatric osteogenesis imperfecta: a randomized placebo-controlled study. J Clin Endocrinol Metab. 2011;96:355–364. doi: 10.1210/jc.2010-0636. [DOI] [PubMed] [Google Scholar]
- 101.Ward KA, Adams JE, Freemont TJ, Mughal MZ. Can bisphosphonate treatment be stopped in a growing child with skeletal fragility? Osteoporos Int. 2007;18:1137–1140. doi: 10.1007/s00198-007-0330-3. [DOI] [PubMed] [Google Scholar]
- 102.Bachrach LK, Ward LM. Clinical review 1: bisphosphonate use in childhood osteoporosis. J Clin Endocrinol Metab. 2009;94:400–409. doi: 10.1210/jc.2008-1531. [DOI] [PubMed] [Google Scholar]
- 103.Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998;339:947–952. doi: 10.1056/NEJM199810013391402. [DOI] [PubMed] [Google Scholar]
- 104.Urade M. Bisphosphonates and osteonecrosis of the jaws. Clin Calcium. 2007;17:241–248. [PubMed] [Google Scholar]
- 105.Malmgren B, Astrom E, Soderhall S. No osteonecrosis in jaws of young patients with osteogenesis imperfecta treated with bisphosphonates. J Oral Pathol Med. 2008;37:196–200. doi: 10.1111/j.1600-0714.2007.00607.x. [DOI] [PubMed] [Google Scholar]
- 106.Chahine C, Cheung MS, Head TW, Schwartz S, Glorieux FH, Rauch F. Tooth extraction socket healing in pediatric patients treated with intravenous pamidronate. J Pediatr. 2008;153:719–720. doi: 10.1016/j.jpeds.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 107.Schwartz S, Joseph C, Iera D, Vu DD. Bisphosphonates, osteonecrosis, osteogenesis imperfecta and dental extractions: a case series. J Can Dent Assoc. 2008;74:537–542. [PubMed] [Google Scholar]
- 108.Migliorati CA, Schubert MM, Peterson DE. Bisphosphonate osteonecrosis (BON): unanswered questions and research possibilities. Rev Recent Clin Trials. 2009;4:99–109. doi: 10.2174/157488709788185978. [DOI] [PubMed] [Google Scholar]
- 109.Huber MA. Osteogenesis imperfecta. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:314–320. doi: 10.1016/j.tripleo.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 110.Kamoun-Goldrat A, Ginisty D, Le Merrer M. Effects of bisphosphonates on tooth eruption in children with osteogenesis imperfecta. Eur J Oral Sci. 2008;116:195–198. doi: 10.1111/j.1600-0722.2008.00529.x. [DOI] [PubMed] [Google Scholar]
- 111.Hodgson B. More about bisphosphonates. J Am Dent Assoc. 2009;140:829–830. doi: 10.14219/jada.archive.2009.0262. discussion 30. [DOI] [PubMed] [Google Scholar]
- 112.Bradaschia-Correa V, Massa LF, Arana-Chavez VE. Effects of alendronate on tooth eruption and molar root formation in young growing rats. Cell Tissue Res. 2007;330:475–485. doi: 10.1007/s00441-007-0499-y. [DOI] [PubMed] [Google Scholar]
- 113.Grier RLt, Wise GE. Inhibition of tooth eruption in the rat by a bisphosphonate. J Dent Res. 1998;77:8–15. doi: 10.1177/00220345980770011201. [DOI] [PubMed] [Google Scholar]
- 114.Hiraga T, Ninomiya T, Hosoya A, Nakamura H. Administration of the bisphosphonate zoledronic acid during tooth development inhibits tooth eruption and formation and induces dental abnormalities in rats. Calcif Tissue Int. 2010;86:502–510. doi: 10.1007/s00223-010-9366-z. [DOI] [PubMed] [Google Scholar]
- 115.Guideline on oral heath care/dental management of heritable dental development anomalies. Pediatr Dent. 2008;30:196–201. [PubMed] [Google Scholar]
- 116.Bouvier D, Leheis B, Duprez JP, Bittar E, Coudert JL. Dentinogenesis imperfecta: long-term rehabilitation in a child. J Dent Child (Chic) 2008;75:192–196. [PubMed] [Google Scholar]
- 117.Bouvier D, Duprez JP, Morrier JJ, Bois D. Strategies for rehabilitation in the treatment of dentinogenesis imperfecta in a child: a clinical report. J Prosthet Dent. 1996;75:238–241. doi: 10.1016/s0022-3913(96)90478-3. [DOI] [PubMed] [Google Scholar]
- 118.Sapir S, Shapira J. Dentinogenesis imperfecta: an early treatment strategy. Pediatr Dent. 2001;23:232–237. [PubMed] [Google Scholar]
- 119.Bidra AS, Uribe F. Successful bleaching of teeth with dentinogenesis imperfecta discoloration: a case report. J Esthet Restor Dent. 2011;23:3–10. doi: 10.1111/j.1708-8240.2010.00379.x. [DOI] [PubMed] [Google Scholar]
- 120.Croll TP, Sasa IS. Carbamide peroxide bleaching of teeth with dentinogenesis imperfecta discoloration: report of a case. Quintessence Int. 1995;26:683–686. [PubMed] [Google Scholar]
- 121.Jehan F, Gaucher C, Nguyen TM, Walrant-Debray O, Lahlou N, Sinding C, et al. Vitamin D receptor genotype in hypophosphatemic rickets as a predictor of growth and response to treatment. J Clin Endocrinol Metab. 2008;93:4672–4682. doi: 10.1210/jc.2007-2553. [DOI] [PubMed] [Google Scholar]