Abstract
Arsenite is critical pharmacologically as a treatment for advanced stage blood cancer. However, environmental exposure to arsenic results in multiple diseases. Previous studies have shown that arsenic decreases expression of CYP3A, a critical drug metabolizing enzyme in human and rat liver. In addition, acute and chronic arsenic exposure in liver stimulates an inflammatory response. Our work has shown that arsenite decreases nuclear levels of RXRα the nuclear receptor that, as a heterodimer partner with PXR, transactivates the CYP3A gene. These results suggest that arsenite decreases transcription of CYP3A by decreasing RXRα. The present report shows that exposure to 5 μM arsenite decreased the activity of a rat CYP3A promoter luciferase reporter in HepG2 cells. The activity of a RARE-luciferase reporter, that is transactivated by the retinoic acid receptor (RAR)/RXRα, was also decreased. Previous studies have shown that arsenic in the concentration range of 2-5 μM affects CYP3A mRNA. When rifampicin-treated primary human hepatocyte cultures were exposed to arsenite concentrations as low as 50 nM, CYP3A mRNA was decreased. Treatment of primary human hepatocytes with the proteasome inhibitor MG132 increased RXRα suggesting the involvement of the proteasome pathway in regulation of RXRα. Finally, arsenic induces a pro-inflammatory response in liver. Surprisingly, we show that in hepatocytes arsenite decreases expression of two inflammatory mediators, TNF and VEGF, an effect that is not predicted from suppression of RXRα activity.
1. Introduction
Arsenic compounds have been used pharmacologically for more than 2400 years [1]. Arsenic has been used as a devitalizing agent prior to teeth fillings, and treatment of skin diseases, stomach ulcers, anemia, emphysema, malaria, and leukemia [1,2]. The toxicological action of arsenic was first recognized when an association between medicinal arsenic compounds and skin cancer was observed in the 1800’s [1]. Multiple liver pathologies have also been linked to arsenic, including chronic inflammation, noncirrhotic portal hypertension, hepatocellular carcinoma, biliary occlusion, and cirrhosis [3-8].
The disease effects of arsenic may involve its action to decrease expression of hepatic cytochrome P450 (CYPs) enzymes that are involved in metabolic inactivation of physiological biomolecules, drugs, and carcinogens [9]. The CYPs mediate the oxidative metabolism of structurally diverse substrates [10]. The CYP3A enzymes are inducible by many natural and xenobiotic substances [11]. The CYP3A’s are involved in the metabolism of more than 50% of drugs in current use [12,13]. In studies using primary rat hepatocyte cultures, arsenite decreases induction of CYPs 3A, 2B and 1A1/2 [9,14]. In primary human hepatocyte cultures, arsenite decreases induction of CYP1A1 and CYP1A2 [15], and decreases both constitutive and induced expression of the human CYP3A enzyme, CYP3A4 [16]. Arsenite also decreases rifampicin (Rif)-induced expression of the rat CYP3A gene, CYP3A23, in primary rat hepatocyte cultures transfected with the human pregnane X receptor (known as SXR in humans or more generally as PXR) [16]. In primary human hepatocyte cultures, arsenite-mediated decreases in CYP3A4 mRNA and protein are associated with decreases in nuclear levels of the retinoid X receptor-α (RXRα) [16]. The RXRα transcription factor regulates CYP3A4 gene transcription as a heterodimer with PXR [16].
Nuclear receptors, such as PXR/RXRα, control transcription of multiple genes [17,18]. In addition to the gene targets related to drug metabolism, there is crosstalk between nuclear receptors and other pathways, including the transcription factor NFκB, which controls expression of inflammatory response genes [19]. Direct association of RXRα and NFκB has been reported [20]. Drugs that activate PXR suppress the immune response [19]. Here, and in previous reports, we show that arsenite reduces PXR/RXRα activity by reducing RXRα. Together these findings suggest that decreases in PXR/RXR by arsenic may stimulate NFκB, resulting in an inflammatory response [19]. Studies of liver following acute [21] and chronic [8] arsenic exposure show increases in inflammatory mediators, including tumor necrosis factor-α (TNF). This response may be mediated by macrophages [8,21]. However, hepatocytes also produce pro-inflammatory mediators, and may contribute to the pro-inflammatory response.
To understand the mechanism of action by arsenic on RXRα and gene expression, the effects of arsenite on primary human hepatocytes and human HepG2 hepatocellular carcinoma cells were examined. Previous studies show that low micromolar concentrations (2-5 μM) affect CYP3A expression [16]. This report includes effects of arsenite at concentrations as low as 50 nM. The effects of arsenic on both a PXR/RXRα-dependent rat CYP3A promoter and a retinoic acid receptor RAR/RXRα-dependent promoter are measured. Arsenic reacts with vicinyl cysteine residues, such as those found in the zinc finger domain of RXRα, and may activate protein degradation. To investigate a possible mechanism of action by arsenic on RXRα, we measure the effects of proteasomal degradation on RXRα levels in primary human hepatocyte cultures and in HepG2 cells. Finally, to determine if arsenite affects immune function in hepatocytes, we test the effects of arsenite on expression of the pro-inflammatory mediators TNF and vascular endothelial growth factor (VEGF) in HepG2 cells. The effects of arsenite to decrease RXRα activity and alter immune mediators may contribute to the toxicity of environmental arsenic and may affect the efficacy of arsenic in cancer treatments.
2. Materials and Methods
2.1. Chemicals
Sodium meta-arsenite (NaAsO2, 99% purity) (As) was obtained from Sigma (St. Louis, MO). Arsenite is a powerful carcinogen and should be handled with care. Use full personal protection, and the powdered form should be handled with adequate ventilation. N-ethyl-maleimide (NEM), rifampicin, 9-cis-retinoic acid (9cRA), and MG132 were obtained from Sigma. Williams E powder was from GIBCO Laboratories (Grand Island, NY). Polyclonal antibody to human RXRα was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-RXRα was raised against an N-terminal peptide from human RXRα and recognizes a single band at 54 kDa [16]. Alkaline phosphatase-conjugated goat anti-rabbit antibody was purchased from Bio-Rad (Hercules, CA).
2.2. Plasmid constructs
The rat promoter luciferase reporter, CYP3A23-luc, contains 1.4 kb of the rat proximal promoter (−1360 to +82) in the pGL3 parent vector [22,23]. The RARE luciferase reporter (RARE-Luc) contains three copies of the DR5 response element from the mouse RARβ2 promoter. The pCMX-PL2-hSXR expression vector contains the complete protein-coding region of human PXR (steroid and xenobiotic receptor; SXR) [24]. The RXRα-V5-His expression vector was created by PCR amplification from the human RXRα-EBOpLPP plasmid (kind gift of Sutisak Kitareewan and Ethan Dmitrovsky, Dartmouth Medical School), and contains the complete RXRα coding region fused on the C-terminus to a V5-His motif in a pcDNA-3.1 parent vector (Invitrogen, Carlsbad, CA). The renilla luciferase construct, pRL-tk, was from Promega (Madison, WI).
2.3. Cell Culture
Human hepatocytes were prepared from livers not used for whole organ transplant within 24 hr of procurement (Liver Transplant Procurement and Distribution System) (Table 1). Hepatocytes were isolated by a three-step collagenase perfusion technique [25], and plated at a cell density of 2 × 106 cells per well in 6-well plates previously coated with type I collagen. After 2-4 hr, the media was changed to remove unattached cells, and the resulting cultures had a viability of >90%, as determined by trypan blue exclusion. Primary hepatocyte cultures were maintained in Williams E medium supplemented with 10−7 M dexamethasone (DEX), 10−7 M insulin, 100 units/ml penicillin G, 100 μg/ml streptomycin, 1 mM ascorbate, 0.26 mM selenium, 20 mM Hepes, and were kept at 37°C in a humidified incubator with 95% air/5% CO2. Following 24-48 hr in culture, cells were either not treated or were exposed to chemicals, as indicated in the figure legends. Arsenite (made fresh) was dissolved in water. Rif and 9cRA were dissolved in dimethyl sulfoxide (DMSO). In all experiments with inducers, control cultures were treated with DMSO alone. The final concentration of DMSO in culture medium was 1 μl/ml or less. The HepG2 hepatocellular carcinoma cell line was a gift from Thomas Kocarek (Wayne State University, Detroit, Michigan). HepG2 cells were maintained at 5% CO2 in DMEM plus 10% fetal bovine serum (Hyclone, Logan, UT), non-essential amino acids (Mediatech, Herndon, VA), glutamine (GIBCO), and penicillin/streptomycin (GIBCO).
TABLE 1. Clinical Characteristic of Human Hepatocyte Donors.
| Donor (HH #) |
Age (years) |
Sex | Race | Cause of Death |
Drug History |
|---|---|---|---|---|---|
| 1200 | 53 | F | W | CVA | nexium, nebulizer use, zopinex, advair, neurotin, cozar, chlorodiaz, premrin, dopamine |
| 1205 | 45 | M | H | CVA | solumedrol, vecuronium |
| 1209 | 30 | F | W | CVA | dopa-provera, heparin, ancef, gentamycin, ampicillin, propofol, levophed, cefepine, zosyn, fentanyl, morphine |
W, caucasian; H, Hispanic; CVA, cerebrovascular accident.
2.4. Transfection of HepG2 cells
For transfection of HepG2 cells, sufficient cells were plated 24 hr prior to transfection to give 50% confluence on the day of transfection (120 × 105 cells/well, 10 mm well, 48-well plate). For CYP3A23-luc reporter assays, 300 ng of CYP3A23-luc was co-transfected with 30 ng of pRL-tk, and 75 ng of pCMX-PL2-hSXR per well. Due to overlap of firefly and renilla spectra, sufficient renilla pRL-tk was transfected to achieve from 0.1% to 10% of the firefly luciferase signal. For RARE-luc reporter assays, 100 ng of RARE-luc was co-transfected with 50 ng of RXRα-V5-His and 10 ng of pRL-tk per well. Plasmid DNA was complexed with Lipofectamine-2000 (Invitrogen) in OptiMEM media (Invitrogen), as described by the manufacturer. Culture media was removed from cells, and complexed DNA was suspended in fresh culture media and added to cells. Treatment chemicals were added 2 hr after transfection. Cells were lysed 6 hr after treatment (8 hr after transfection). Firefly luciferase activity was normalized to renilla luciferase activity (Firefly/Renilla) for triplicate samples in each treatment condition.
2.5. RNA isolation and Invader® RNA analysis
Total RNA was extracted from cells using TRIzol, as described by the manufacturer (Invitrogen). CYP3A4 mRNA was quantified using the CYP3A4 Invader® RNA assay kit, as described by the manufacturer (Third Wave Technologies, Madison, WI) and Noreault et al. [16]. This assay is specific for CYP3A4, and uses a fluorescence resonance energy transfer based signal amplification method that is based on an enzyme-substrate reaction using Cleavase® enzymes. These enzymes cut only the specific complex formed on targeted CYP3A4 mRNA. A standard curve for CYP3A4 mRNA was generated in each assay using purified CYP3A4 mRNA provided by the manufacturer. Expression levels (CYP3A4 mRNA molecules/ng total RNA) were calculated using net signal values from the CYP3A4 standard curve equation, and results are reported as fold induction over control. Each sample was assayed in triplicate using a range of 1-50 ng total RNA per reaction.
2.6 Measuring TNF and VEGF mRNA levels
To measure cytokine mRNA levels in HepG2 cells treated without or with arsenite, total cellular RNA was isolated using the QiaShredder columns and the RNeasy kit (Qiagen). The RNA was quantified using NanoDrop (Thermofisher Scientific). Next, 2 μg of RNA was treated with Turbo-DNA-free (Ambion), and cDNA was created using the Maxima First Strand cDNA kit (Fermentas). RT PCR was performed with RT2 Real Time SYBR master mix (SABiosciences) using standard conditions. Levels of mRNA (Ct) were normalized to the level of β-actin mRNA. All samples were run in triplicate. Melting curves were performed to confirm single PCR products. To verify the size of PCR products, DNA was separated on a 2% NuSieve DNA gels, stained with Syber Gold (Molecular Probes), and imaged using VersaDoc (BioRad). Primer pairs are as follows: TNF: sense – ctcagcctcttctccttcctgat, antisense –- ggttcgagaagatgatctgactg; VEGF: sense – ataaagcattcatcactgtgaaaca, antisense – atgctcagcaagattgtataattcc; β-actin: sense -- attaaggagaagctgtgctacgtc, antisense – atgatggagttgaaggtagtttcg. Each set of primer pairs cross introns.
2.6. Immunoblotting
RXRα was analyzed by immunoblotting as described [16]. Briefly, total protein was quantitatively determined by the method of Lowry [26]. Equal quantities of total protein from samples were separated by electrophoresis in SDS-PAGE (10% acrylamide) and transferred to nitrocellulose electrophoretically at 100V for 1 h. The technique was confirmed by monitoring protein transfer using reversible Ponceau staining of nitrocellulose sheets. The nitrocellulose sheets were blocked overnight at 4°C in phosphate-buffered saline containing 5% non-fat dry milk and 0.3% Tween-20, incubated overnight at 4°C with primary antibody to RXRα, and exposed for 1 hr to an alkaline phosphatase-conjugated goat anti-rabbit secondary antibody. The resulting blots were quantitated by densitometry using Adobe Photoshop, an HP Precision Scanner, and OneDScan software (Scanalytics, Fairfax, VA). In proteasome inhibition studies, whole cell lysates were treated with NEM (5 mM) at the time of lysis.
2.7. Statistical analyses
Treatments within each experiment were performed in duplicate or triplicate, as indicated in the figure legends. In triplicate treatments, results were analyzed either by a Student’s t test, or by ANOVA followed by a Student-Neuman-Kuels multiple comparisons test. A p value of <0.05 was taken to indicate a significant difference. Experiments with HepG2 cells were performed at least twice and representative experiments are shown.
3. Results
3.1. Effects of arsenite on PXR/RXRα and RAR/RXRα promoter activity
To determine if arsenite affected rat CYP3A23 promoter activity after a 6 hr exposure, the CYP3A23-luc reporter and a plasmid expressing human PXR (“hSXR”) were co-transfected into HepG2 hepatocellular carcinoma cells, and the cells were treated with Rif. The activity of the CYP3A23-luc reporter responds to Rif (two-fold increase) when human PXR is ectopically expressed, as is seen for primary rat hepatocytes [16]. CYP3A23-luc reporter activity was decreased by 55% following a 6 hr arsenite (5 μM) exposure (Fig. 1A). Next, the effect of arsenite on transactivation of another RXRα-dependent promoter, RARE-luc, was determined. The RARE-luc reporter contains three copies of the RAR response element that is transcribed by the RAR/RXRα nuclear receptor heterodimer [27]. Although HepG2 cells express some RXRα protein, visible by immunoblot (Fig. 2B), RARE-luc reporter activity was very low without ectopically expressed RXRα (data not shown). Therefore, HepG2 cells were transfected with RARE-luc plus a plasmid expressing RXRα (RXRα-V5His), and treated with the RXRα and RAR ligand, 9cRA. Exposure to arsenite for 6 hr decreased RARE-luc activity by 50% (Fig. 1B). Arsenite did not affect renilla luciferase levels from the pRL-tk loading control (Fig. 1C). These results show that arsenite decreased the promoter activity of both PXR/RXRα-dependent (CYP3A23-luc) and RAR/RXRα-dependent (RARE-luc) reporters.
Figure 1.

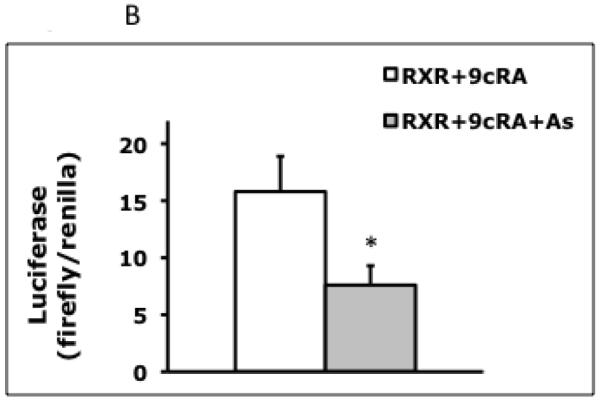

Effects of a 6 hour arsenite exposure on transcription of RXRα-dependent promoters. (A) HepG2 cells were transfected with CYP3A23-luc, hSXR, and pRL-tk. After 2 hr, cells were treated with Rif (5 μM) or Rif plus arsenite (5 μM), and lysed 6 hr after treatment. CYP3A23-luc firefly luciferase values are normalized to renilla luciferase values (Rif -- 160.4 ± 3.0; Rif + As -- 71.9 ± 6.1), and represent the mean ± SD of triplicate samples, *** p<0.001. (B) HepG2 cells were transfected with RARE-luc, RXRα-V5-His, and pRL-tk. After 2 hr, cells were treated with 9cRA (100 nM) or 9cRA plus arsenite (5 μM), and lysed 6 hr after treatment. RARE-luc firefly luciferase values are normalized to renilla luciferase values (9cRA -- 15.8 ± 3.1; 9cRA + As – 7.6 ± 1.7), and represent the mean ± SD of triplicate samples, * p<0.03. (C) Levels of pRL-tk renilla luciferase, used as a transfection control in HepG2 cells, were not significantly decreased by 6 hr treatments with arsenite (5 μM) (no arsenic: 0.464 ±0.033; with arsenic: 0.433 ±0.047, p=0.401; background levels are <0.1 units).
Figure 2.


Effects of proteasomal inhibition on RXRα protein. (A) Primary human hepatocyte cultures were untreated (Ctl) or treated with MG132 (MG) (5 μM) for 6 hr. Whole cells were treated with NEM (5 mM) at the time of lysis, and 20 μg of lysate was immunoblotted for RXRα, as described in Materials and Methods. (B) HepG2 cells were transfected as described in Materials and Methods. After 24 hr, cells were either untreated or treated with MG132 (MG) (5 μM) for 6 hr. Whole cells were treated with NEM (5 mM) at the time of lysis, and 20 μg of lysate was immunoblotted for RXRα, as described in Materials and Methods. Arrows show native RXRα (lower) and V5-His-tagged RXRα (upper), which is ~5 kD larger. Protein markers (85kD and 50kD) are shown on the left. The intermediate band in the “+MG” lane is unidentified.
3.2. Effect of proteasome inhibition on RXRα
Our previous studies in primary human hepatocyte cultures showed that arsenite decreased RXRα protein with no decrease in RXRα mRNA [16]. The RXRα protein is removed by proteasomal activity in some tissues, and may be regulated by proteasomal degradation [17]. A 6 hr treatment with the proteasomal inhibitor MG132 (5 μM) resulted in an increase in RXRα protein in primary human hepatocytes (Fig. 2A). In HepG2 cells, MG132 treatment increased both the native RXRα and a RXRα fusion protein (RXRα-V5-His) (Fig. 2B). These results demonstrate that RXRα is degraded by the proteasomal pathway in both hepatocyte cultures and hepatocellular carcinoma cells.
3.3. Dose response effects of arsenite on CYP3A4 mRNA in primary human hepatocyte cultures
The concentration of arsenite used in the above studies was 5 μM. The effects of lower concentrations of arsenite on CYP3A4 mRNA expression were investigated in control and Rif-induced primary human hepatocyte cultures. Cells were either not treated or treated with Rif for 24 hr to obtain a high level of CYP3A4 mRNA expression. Cultures were then not treated or treated with arsenite for 24 hr. Cultures not treated with Rif showed a concentration-dependent decrease in CYP3A4 mRNA expression, with 100 nM arsenite decreasing CYP3A4 mRNA by 40%, and 1 μM arsenite decreasing CYP3A4 mRNA by 75% (Fig. 3A). Rif (10 μM) caused a 10-fold induction in CYP3A4 mRNA (Fig. 3B), and arsenite co-treatment decreased CYP3A4 mRNA by 50% at 50 nM and by 95% at 1 μM (Fig. 3B). Hence, 24 hr exposures with low nanomolar concentrations of arsenite decrease both basal and induced CYP3A4 mRNA in primary human hepatocyte cultures.
Figure 3.

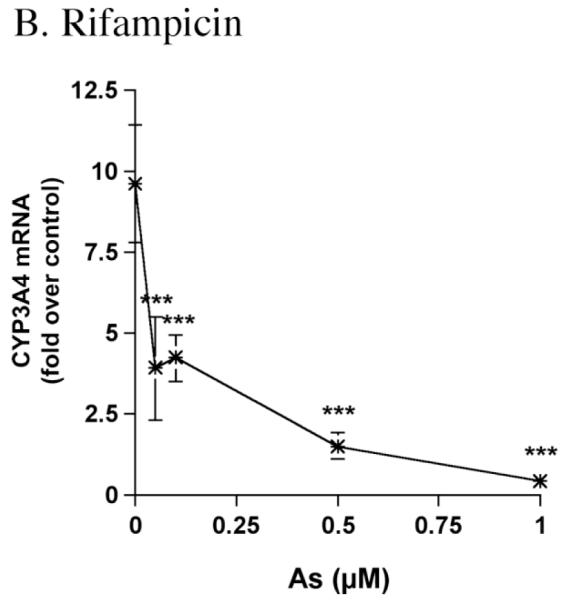
Dose response effects of arsenite on CYP3A4 mRNA in primary human hepatocyte cultures. (A) Hepatocytes were not untreated or treated for 24 hr with 0.5 or 1.0 μM As (HH1200), or 0.1 or 0.5 μM As (HH1205). Total RNA was isolated, and CYP3A4 mRNA was measured using the Invader® RNA Assay, as described in Materials and Methods. Values (molecules CYP3A4 mRNA per ng total RNA) are: HH1200: 0 μM As, 19,410 ± 4730; 0.5 μM As, 5420 ±1960; 1.0 μM As 3500 ±910. Values for HH1205: 0 μM As, 41520 ± 15930; 0.1 μM As, 25130 ±8350; 0.5 μM As 12,770 ±1680. (B) Hepatocytes were treated with 10 μM Rif, and then not further treated or treated for 24 hr with 0.05, 0.1, 0.5 or 1.0 μM As. The results for HH1205 are shown. Values (molecules CYP3A4 mRNA per ng total RNA) for the no arsenite treatment are: 397,060 ±73,210. Values are expressed relative to control levels (“fold over control”), and represent the mean ± SD of triplicate treatments. * p<0.05 vs. Control (“*” is on the Y-axis). ***p<0.001 vs. Control/Rif.
3.4 Effects of arsenite on expression of TNF and VEGF
Arsenite reduces PXR activity by reducing RXRα protein levels in hepatocytes. The PXR/RXRα pathway suppresses the NFκB pathway. By suppressing PXR/RXRα, arsenic may stimulate the immune response, as has been reported in liver [8,21]. Liver-associated macrophages may mediate this effect [8,21]. To determine if hepatocytes are pro-inflammatory, we measured the effects of arsenite on expression of two immune modulators, TNF and VEGF. Cultured HepG2 cells were treated with Rif (5 μM) and 9cRA (100 nM) and then not treated or treated for 6 or 24 hr with arsenite (5 μM). Total RNA was obtained and cDNA prepared for RT PCR. As shown in Fig. 4A, 24 hr arsenite treatments decreased TNF mRNA levels by 70%. Similarly, 24 hr arsenite treatments decreased VEGF modestly (20%), but significantly (Fig. 4B). Shorter (6 hr) arsenite treatments did not affect TNF or VEGF mRNA levels (Fig. 4). Arsenite had no effect (data not shown) on post-transcriptional regulation of VEGF using a VEGF-3′UTR reporter [28] in a macrophage cell line, RAW-264.7, suggesting that the effects of arsenite on VEGF mRNA levels are transcriptional. Additional studies of TNF and VEGF transcription are planned to determine if arsenite acts on immune regulators in hepatocytes via the NFκB pathway.
Figure 4.


Effects of a 6 and 24 hour arsenite exposure on TNF and VEGF expression. Cultured HepG2 cells were plated in triplicate and not treated (“No As”), or treated with 5 μM arsenite for 6 or 24 hr. Total cellular RNA was converted to cDNA for Real-Time PCR. Results are normalized to β-actin mRNA. Values represent the mean ± SD. (A) Tumor necrosis factor-α. * p<0.03 for 0 vs. 24 hr. The 0 hr and 6 hr treatments were not significantly different. Results are representative of two experiments. (B) Vascular endothelial growth factor. * p<0.05 for 0 vs. 24 hr. The 0 hr and 6 hr treatments were not significantly different. Results are representative of three experiments.
4. Discussion
A previous report from this laboratory showed that arsenite decreased CYP3A4 expression in primary human hepatocyte cultures [16]. Decreased CYP3A4 was associated with decreases in protein levels of RXRα, the transcription factor that transactivates the CYP3A4 gene. There was no decrease in PXR, the heterodimer partner of RXRα. Our group has also demonstrated that arsenic decreases the activity of the homologous rat CYP3A enzyme, CYP3A23, in rat hepatocytes [14]. Decreases of CYP3A activity and RXRα protein levels [16] suggest that arsenite decreases CYP3A expression by decreasing transcription by PXR/RXRα [18]. We now demonstrate that arsenite decreases the activity of promoters controlled by PXR/RXRα (Fig. 1A) and RAR/RXRα (Fig. 1B).
Crosstalk between PXR/RXRα and other transcription factors may explain the many phenotypes of arsenite toxicity [3-8,17]. PXR/RXRα interacts with the inflammatory pathway NFκB and suppresses the inflammatory response [19,20]. Arsenite may increase expression of inflammatory genes by decreasing PXR/RXRα. In animal studies, arsenite stimulates expression of TNF and other inflammatory mediators in liver [8,21]. Although TNF production is thought to be produced by liver-associated macrophages and Kupffer cells, one report has shown that arsenic does not affect TNF in peritoneal macrophages [37]. Hence, the source of inflammatory mediators in liver has not been clearly established. To determine if arsenite affects TNF and VEGF production in hepatocytes, we treated HepG2 cells with clinically relevant levels of arsenite (5 μM). Exposure for 24 hr reduced TNF mRNA by 70% (Fig. 4A). Hepatocytes also produce VEGF, that can act as a cytokine [28,29]. In HepG2 cells, VEGF was modestly reduced following a 24 hr arsenite exposure (Fig. 4B). These results suggest hepatocytes treated with arsenite do not contribute to activation of the immune response. Further studies using chronic exposure to arsenite are needed to determine if hepatocytes assist macrophages in activation of the immune response in liver.
To investigate the mechanism whereby arsenite decreases the RXRα nuclear receptor we evaluated the role of proteasomal degradation in hepatocytes. In non-hepatic tissues, arsenic exposure affects phosphorylation and ubiquitination, and causes a loss of RXRα [17,18,30-33]. Arsenic may initiate proteasomal degradation of RXRα by disrupting protein secondary structure and damaging sulfhydryl-containing residues, such as cysteine [34]. The RXRα protein contains a zinc finger region with multiple vicinyl cysteine residues (C135–C138, C152–C155, C171–C177, C187–C190; human RXRα Gen Bank: NP_002948.1). Both zinc and arsenic bind to vicinyl sulfhydryl groups. Arsenic may displace zinc and react chemically with sulfhydryls to form reactive oxygen species (ROS) [34]. The resulting denatured protein can then initiate ubiquitination and proteasomal degradation [34]. In primary human hepatocyte cultures, we found that proteasomal inhibition increased RXRα protein (Fig. 2A), and additional studies are needed to determine if direct physical and chemical interaction of arsenic with RXRα results in proteasomal degradation.
The studies presented here are relevant to arsenic exposure and environmental health. A recent report, from a large epidemiological study of arsenic exposure (“HEALS” project), measured total blood arsenic in persons exposed to arsenic in well water [35]. Populations with well water arsenic concentrations of 95-560 µg/L (1.3–7.5 µM) have blood arsenic concentrations of 10-64 µg/L (0.14-0.85 µM). To determine the minimal dose of arsenite necessary to decrease CYP3A4 mRNA in primary human hepatocyte cultures, we examined the effects of arsenite concentrations corresponding to very low to moderate exposure levels. Arsenite exposure at 50 nM decreased CYP3A4 mRNA by 50% in Rif-treated primary human hepatocyte cultures (Fig. 3B), suggesting that CYP activity in liver may be affected by low nanomolar arsenic concentrations found in contaminated environments. Further studies are needed to determine if low nanomolar arsenic concentrations are pro-inflammatory. These findings suggest that a large population with sub-micromolar concentrations of arsenic in drinking water may suffer from the toxic effect of arsenic. Our findings also have implications for the current use of arsenic trioxide (ATO) in the micromolar range (1-7 μM) as a chemotherapeutic agent [36]. The mechanism of ATO medicinal action may include effects on RXRα-mediated gene transcription that can affect transcription of multiple genes controlled by RXRα and its heterodimer partners. In addition, the effect of micromolar levels of arsenite on CYP3A4 induction suggests that ATO treatments, and environmental levels of arsenic, may pose a risk for drug-drug interactions.
Highlights.
-
-
We demonstrate that arsenic affects transcription of CYP3A4 in hepatocytes after 6 hours.
-
-
Low nanomolar concentrations of arsenic decrease CYP3A4 levels.
-
-
The proteasome pathway affects RXR-alpha levels in primary human hepatocytes and in the hepatocarcinoma cell line, HepG2.
Acknowledgements
This work was supported by NIH (R01-ES10462; R.C.N., J.F.S.), Merit Review Awards (Department of Veterans Affairs; R.C.N., J.F.S, P.R.S.), the National Center for Research Resources (P20RR1643; R.C.N.), and the Liver Transplant, Procurement, and Distribution System (NIH Contract #N01-DK-9-2310). We thank Sutisak Kitareewan, Ethan Dmitrovsky, for helpful discussions, and Nick Jacobs for discussion and preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Zhu J, Chen Z, Lallemand-Breitenbach V, de The H. How acute promyelocytic leukaemia revived arsenic. Nat Rev Cancer. 2002;2:705–13. doi: 10.1038/nrc887. [DOI] [PubMed] [Google Scholar]
- [2].Wilcox R. Materia Medica and Therapeutics including Pharmacy and Pharmacology. Blakiston’s Son & Co; Philadelphia: 1929. pp. 316–20. [Google Scholar]
- [3].Nevens F, Fevery J, Van Stenbergen W, Sciot R, Desmet V, DeGroote J. Arsenic and non-cirrhotic portal hypertension: a report of eight cases. J Hepatol. 1990;11:80–5. doi: 10.1016/0168-8278(90)90276-w. [DOI] [PubMed] [Google Scholar]
- [4].Centeno J, Mullick F, Martinez L, Page N, Gibb H, Longfellow D, Thompson C, Ladich E. Pathology related to chronic arsenic exposure. Environ Health Perspect. 2002;110:883–6. doi: 10.1289/ehp.02110s5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Farrell G. Drug Induced Liver Disease. Churchill Livingstone; Edinburgh: 1994. [Google Scholar]
- [6].Liu J, Liu Y, Waalkes M. Metallothionein-I/II null mice are more sensitive than controls to chronic arsenic toxicity. The Toxicologist. 1999;48:353. [Google Scholar]
- [7].Neshiwat L, Friedland M, Schorr-Lesnick B, Felman S, Glucksman W, Russo R. Hepatic angiosarcoma. Am J Med. 1992;93:219–22. doi: 10.1016/0002-9343(92)90054-f. [DOI] [PubMed] [Google Scholar]
- [8].Das S, Santra A, Lahiri S, Guha Mazumder DN. Implications of oxidative stress and hepatic cytokine (TNF-alpha and IL-6) response in the pathogenesis of hepatic collagenesis in chronic arsenic toxicity. Toxicol Appl Pharmacol. 2005;204:18–26. doi: 10.1016/j.taap.2004.08.010. [DOI] [PubMed] [Google Scholar]
- [9].Jacobs J, Nichols C, Andrew A, Marek D, Wood S, Sinclair P, et al. Effect of arsenite on induction of CYP1A, CYP2B, and CYP3A in primary cultures of rat hepatocytes. Toxicol Appl Pharmacol. 1999;157:51–9. doi: 10.1006/taap.1999.8659. [DOI] [PubMed] [Google Scholar]
- [10].Vessey D. Metabolism of drugs and toxins by human liver. In: Zakin D, Boyer T, editors. Hepatology: A Textbook of Liver Diseases. W.B. Saunders Co; Philadelphia: 1990. pp. 196–234. [Google Scholar]
- [11].Burk O, Wojnowski L. Cytochrome P450 3A and their regulation. Naunyn Schmiedebergs Arch Pharmacol. 2003;369:105–24. doi: 10.1007/s00210-003-0815-3. [DOI] [PubMed] [Google Scholar]
- [12].Wrighton S, Schuetz E, Thummel K, Shen D, Korzekwa K, Watkins P. The human CYP3A subfamily: practical considerations. Drug Metab Rev. 2000;32:339–61. doi: 10.1081/dmr-100102338. [DOI] [PubMed] [Google Scholar]
- [13].Wrighton S, VandenBranden M, Ring B. The human drug metabolizing cytochromes P450. J Pharmacokinet Biopharm. 1996;24:461–73. doi: 10.1007/BF02353474. [DOI] [PubMed] [Google Scholar]
- [14].Noreault TL, Jacobs JM, Nichols RC, Trask HW, Wrighton SA, Sinclair PR, et al. Arsenite decreases CYP3A23 induction in cultured rat hepatocytes by transcriptional and translational mechanisms. Toxicol Appl Pharmacol. 2005;209:174–82. doi: 10.1016/j.taap.2005.04.008. [DOI] [PubMed] [Google Scholar]
- [15].Vakharia D, Liu N, Pause R, Fasco M, Bessette E, Zhang Q, et al. Effect of metals on polycyclic aromatic hydrocarbon induction of CYP1A1 and CYP1A2 in human hepatocyte cultures. Toxicol Appl Pharmacol. 2001;170:93–103. doi: 10.1006/taap.2000.9087. [DOI] [PubMed] [Google Scholar]
- [16].Noreault TL, Kostrubsky VE, Wood SG, Nichols RC, Strom SC, Trask HW, et al. Arsenite decreases CYP3A4 and RXRα in primary human hepatocytes. Drug Metab Dispos. 2005;33:993–1003. doi: 10.1124/dmd.105.003954. [DOI] [PubMed] [Google Scholar]
- [17].Bastien J, Rochette-Egly C. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene. 2004;328:1–16. doi: 10.1016/j.gene.2003.12.005. [DOI] [PubMed] [Google Scholar]
- [18].Rochette-Egly C. Nuclear receptors: integration of multiple signaling pathways through phosphorylation. Cell Signal. 2003;15:355–66. doi: 10.1016/s0898-6568(02)00115-8. [DOI] [PubMed] [Google Scholar]
- [19].Moreau A, Vilarem MJ, Maurel P, Pascussi JM. Xenoreceptors CAR and PXR activation and consequences on lipid metabolism, glucose homeostasis, and inflammatory response. Mol Pharm. 2008;5:35–41. doi: 10.1021/mp700103m. [DOI] [PubMed] [Google Scholar]
- [20].Gu X, Ke S, Liu D, Sheng T, Thomas PE, Rabson AB, Gallo MA, Xie W, Tian Y. Role of NF-kappaB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J Biol Chem. 2006;281:17882–89. doi: 10.1074/jbc.M601302200. [DOI] [PubMed] [Google Scholar]
- [21].Liu J, Kadiiska MB, Liu Y, Lu T, Qu W, Waalkes MP. Stress-related gene expression in mice treated with inorganic arsenicals. Toxicol Sci. 2001;61:314–20. doi: 10.1093/toxsci/61.2.314. [DOI] [PubMed] [Google Scholar]
- [22].Burger H, Schuetz J, Schuetz E, Guzelian P. Paradoxical transcriptional activation of rat liver cytochrome P-450 3A1 by dexamethasone and the antiglucocorticoid pregnenolone 16 alpha-carbonitrile: analysis by transient transfection into primary monolayer cultures of adult rat hepatocytes. Proc Natl Acad Sci. 1992;89:2145–49. doi: 10.1073/pnas.89.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Xie W, Barwick J, Downes M, Blumberg B, Simon C, Nelson M, et al. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature. 2000;406:435–39. doi: 10.1038/35019116. [DOI] [PubMed] [Google Scholar]
- [24].Blumberg B, Sabbagh W, Juguilon H, Bolado J, van Meter C, Ong E, et al. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12:3195–205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Strom S, Pisarov L, Dorko K, Thompson M, Schuetz J, Schuetz E. Use of human hepatocytes to study P450 gene induction. Methods Enzymol. 1996;272:388–401. doi: 10.1016/s0076-6879(96)72044-x. [DOI] [PubMed] [Google Scholar]
- [26].Lowry O, Rosebrough N, Farr A, Randall R. Protein measurement with the Folin-Phenol reagents. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- [27].Lin R, Evans R. Acquisition of oncogenic potential by RAR chimeras in acute promyelocytic leukemia through formation of homodimers. Mol Cell. 2000;5:821–30. doi: 10.1016/s1097-2765(00)80322-6. [DOI] [PubMed] [Google Scholar]
- [28].Du M, Roy KM, Zhong L, Shen Z, Meyers HE, Nichols RC. VEGF gene expression is regulated post-transcriptionally in macrophages. FEBS J. 2006;273:732–45. doi: 10.1111/j.1742-4658.2006.05106.x. [DOI] [PubMed] [Google Scholar]
- [29].Hollingshead HE, Killins RL, Borland MG, Girroir EE, Billin AN, Willson TM, Sharma AK, Amin S, Gonzalez FJ, Peters JM. Peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) ligands do not potentiate growth of human cancer cell lines. Carcinogenesis. 2007;28:2641–49. doi: 10.1093/carcin/bgm183. [DOI] [PubMed] [Google Scholar]
- [30].Tarrade A, Bastien J, Bruck N, Bauer A, Gianni M, Rochette-Egly C. Retinoic acid and arsenic trioxide cooperate for apoptosis through phosphorylated RXR alpha. Oncogene. 2005;24:2277–88. doi: 10.1038/sj.onc.1208402. [DOI] [PubMed] [Google Scholar]
- [31].Kopf E, Plassat JL, Vivat V, de The H, Chambon P, Rochette-Egly C. Dimerization with retinoid X receptors and phosphorylation modulate the retinoic acid-induced degradation of retinoic acid receptors alpha and gamma through the ubiquitin-proteasome pathway. J Biol Chem. 2000;275:33280–8. doi: 10.1074/jbc.M002840200. [DOI] [PubMed] [Google Scholar]
- [32].Ye X, Liu S, Wu Q, Lin X, Zhang B, Wu J. Degradation of retinoid X receptor α by TPA through proteasome pathway in gastric cancer cells. World J Gastroenterol. 2003;9:1915–19. doi: 10.3748/wjg.v9.i9.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Boudjelal M, Wang Z, Voorhees JJ, Fisher GJ. Ubiquitin/proteasome pathway regulates levels of retinoic acid receptor gamma and retinoid X receptor alpha in human keratinocytes. Cancer Res. 2000;60:2247–52. [PubMed] [Google Scholar]
- [34].Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ. Arsenic exposure and toxicology: a historical perspective. Toxicol Sci. 2011;123:305–32. doi: 10.1093/toxsci/kfr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hall M, Chen Y, Ahsan H, Slavkovich V, van Geen A, Parvez F, et al. Blood arsenic as a biomarker of arsenic exposure: Results from a prospective study. Toxicology. 2006;225:225–33. doi: 10.1016/j.tox.2006.06.010. [DOI] [PubMed] [Google Scholar]
- [36].Shen Z, Chen G, Ni J, Li X, Xiong S, Qiu Q. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–60. [PubMed] [Google Scholar]
- [37].Arkusz J, Stańczyk M, Lewińiska D, Stepnik M. Modulation of murine peritoneal macrophage function by chronic exposure to arsenate in drinking water. Immunopharmacol Immunotoxicol. 2005;27:315–30. doi: 10.1081/iph-200067947. [DOI] [PubMed] [Google Scholar]


