Abstract
The blood–brain barrier (BBB) selectively controls the exchanges between the blood and the brain: it is formed by tight junctions (TJs) between adjacent microvascular endothelial cells. The transmembrane protein claudin-5 is known as a key TJ protein at the BBB, although, the molecular mechanisms by which it regulates TJ tightness are poorly understood. To identify putative claudin-5 partners that contribute to TJ integrity, claudin-5-enriched membrane microdomains were prepared by cell fractionation, using the human brain endothelial cell line hCMEC/D3 and claudin-5 immunoprecipitates were submitted to tandem mass spectrometry. Because a high concentration of mannitol is known to transiently destabilize TJs, this analysis was performed in basal conditions, after mannitol treatment, and after recovery of TJ integrity. We here demonstrate that the G-protein subunit αi2 (Gαi2) interacts with claudin-5 and that association is correlated with TJ integrity in hCMEC/D3 cells; also, a selective expression of Gαi2 is observed in human brain vasculature in situ. Moreover, small interfering RNA-mediated depletion of Gαi2 or claudin-5 in hCMEC/D3 cells similarly increases their paracellular permeability and delays TJ recovery after mannitol treatment. Altogether, our results identify Gαi2 as a novel claudin-5 partner required for TJ integrity in brain endothelial cells.
Keywords: blood–brain barrier, brain endothelial cells, claudin-5, Gαi2, tight junction
Introduction
The blood–brain barrier (BBB) is a selective interface between the blood and the brain, which maintains ionic homeostasis within the brain microenvironment (Abbott et al, 2010). The BBB is present at the level of brain microvascular endothelial cells, sealed by tight junctions (TJs), which constitute the most apical intercellular junctional complex. Tight junctions closely link the plasma membranes of adjacent cells, restricting the paracellular diffusion of macromolecules and polar solutes between brain endothelial cells (Tsukita et al, 2001), as well as between epithelial cells in other physiological barriers, such as the choroid plexus or the intestinal barrier. Tight junctions are constituted by a number of transmembrane and cytosolic proteins assembled in a multiprotein complex. Among the transmembrane proteins involved, the tetraspan proteins occludin (Furuse et al, 1993) and claudins (Tsukita et al, 2001) have a crucial role in TJ integrity; they interact with several cytosolic scaffolding proteins such as zonula occudens (ZO) proteins, MUPP-1, and Cingulin, which link TJs to the actin cytoskeleton and recruit signaling proteins (Tsukita et al, 1999; Wolburg and Lippoldt, 2002).
Claudins are a multigene family of >20 members: brain endothelial cells predominantly express claudin-5 and claudin-3, possibly also claudin-12 (Morita et al, 1999). Importantly, claudin-5-deficient mice present an altered BBB with higher permeability to small molecules (Nitta et al, 2003), whereas exogenous expression of claudin-5 in cultured rat brain endothelial cells strengthens BBB properties (Ohtsuki et al, 2007): both observations point to claudin-5 as a key component of the BBB. It has been established that claudin-5 acts in TJs through a number of protein–protein interactions. Indeed, the second extracellular loop of claudin-5 was shown to mediate claudin/claudin trans-interaction between adjacent MDCK-II cells (Piehl et al, 2010), whereas claudin-5 directly interacts with other TJ components in epithelial or endothelial cells: Occludin (Saitou et al, 1998), ZO proteins (Itoh et al, 1999), MUPP-1 (Poliak et al, 2002), and claudin-3 (Coyne et al, 2003). However, the molecular mechanisms by which claudin-5 regulates BBB permeability are still poorly understood.
The aim of the present study was to identify claudin-5 partners, which contribute to the regulation of TJ integrity at the BBB. We worked with the hCMEC/D3 cell line, a validated in vitro model of human BBB expressing known TJ proteins, presenting a restricted permeability to small molecules (Weksler et al, 2005) and currently widely used by us and others for studying drug transport across the BBB (Dauchy et al, 2009; Poller et al, 2008), cell interaction with brain vasculature (Galan-Moya et al, 2011; Rampon et al, 2008), or pathogen interactions with brain endothelial cells (Coureuil et al, 2009; Zougbede et al, 2011).
We hypothesized that changes in permeability might be associated with an alteration of claudin-5-containing TJ complexes. Using a hypertonic concentration of mannitol—used in clinics to transiently open the BBB for therapeutic purposes (Rapoport, 2000)—we induced a rapid and reversible increase in hCMEC/D3 permeability to small molecules. Claudin-5 was immunoprecipitated from hCMEC/D3 cells that were either left untreated or treated with mannitol followed or not by a recovery period. For each experimental condition, coimmunoprecipitated claudin-5 partners were identified by liquid chromatography coupled with tandem mass spectrometry (nano-LC-MS/MS) analysis. Here, we identify the heterotrimeric G-protein subunit αi2 (Gαi2) that associates with claudin-5 in control conditions, dissociates after mannitol treatment, and reassociates after the recovery period. We show that Gαi2 is expressed by human brain vascular endothelium in situ and by hCMEC/D3 cells at cell–cell junctions. G-protein subunit αi2 as well as claudin-5 downregulation by specific small interfering RNAs (siRNAs) strongly increases endothelial permeability and delays TJ recovery after mannitol treatment. Our results strongly suggest that Gαi2 and claudin-5 are expressed in the same molecular complex that controls TJ integrity in brain endothelial cells.
Materials and methods
Cell Culture Conditions
The human brain microvessel endothelial cell line hCMEC/D3 (Weksler et al, 2005) was cultured on culture inserts (0.4 μm pore size; Corning, Lowell, MA, USA) coated with 150 μg/mL rat tail collagen type I (R&D Systems, Minneapolis, MN, USA). The seeding density was 50,000 cells/cm2. The culture medium contained endothelial basal medium-2 (EBM-2) (Lonza, Walkersville, MD, USA) supplemented with 5% fetal bovine serum ‘Gold,' 10 mM hydroxyethyl-piperazineethane sulfonic acid (HEPES) (PAA Laboratories GmbH, Pasching, Austria), 1% Penicillin–Streptomycin, 1% chemically defined lipid concentrate (Invitrogen Ltd, Paisley, UK), 1.4 μM hydrocortisone, 5 μg/mL ascorbic acid, and 1 ng/mL basic fibroblast growth factor (bFGF) (Sigma-Aldrich, St Louis, MO, USA). Cells were grown for 6 days at 37°C in a humidified incubator in 5% CO2 and the medium was changed 3 days after seeding. When indicated, confluent hCMEC/D3 cells were incubated for 30 minutes at 37°C in a serum-free culture medium supplemented with 1 M D-mannitol (Sigma-Aldrich). For recovery experiments, the mannitol-containing medium was removed after 30 minutes and replaced by standard culture medium for 24 to 48 hours as indicated.
Small Interfering RNA Experiments
Small interfering RNA transfections were performed using various Stealth RNAi duplexes (Invitrogen) against claudin-5 (#HSS186370 and #HSS144294), Gαi2 (#HSS104225 and #HSS178468) or nontargeting siRNAs. Cells were plated onto culture inserts (#3450, Corning) or E-plates for xCELLigence assays (Roche, Basel, Switzerland) in culture medium without antibiotics. One hour after cell seeding, the transfection mix containing Lipofectamine RNAiMAX, 50 nM of siRNAs, and Opti-MEM Reduced Serum Medium (Invitrogen) was added to the culture medium according to the manufacturer's instructions. Cells were incubated for 6 days at 37°C in a CO2 incubator and the culture medium was replaced at day 3 by standard medium (cells on E-plates) or by fresh siRNA-supplemented transfection medium (cells on culture inserts).
Immunoblotting Assays
Confluent hCMEC/D3 cells, untreated or treated with siRNAs, were washed with phosphate-buffered saline (PBS). Cells were incubated on ice for 10 minutes with ice-cold Laemmli lysis buffer. Cell lysates were heated at 100°C for 5 minutes before sodium dodecyl sulfate polyacrylamide gel electrophoresis; proteins were then transferred onto a nitrocellulose membrane (Watman GmbH, Dassel, Germany). After saturation in blocking buffer (Tris-Buffer Saline, 3% nonfat dry milk, 0.05% Tween-20) for 30 minutes, membranes were blotted overnight with antibodies against claudin-5 (Invitrogen), Gαi2 (clone T-19, Santa Cruz Biotechnology, Heidelberg, Germany), VE-cadherin (clone BV6, Santa Cruz Biotechnology) or actin (Sigma-Aldrich) diluted in the blocking buffer. Immunoreactivity was revealed by secondary antibodies coupled to 680 nm fluorophores using the Odyssey LI-COR infrared fluorescent scanner (ScienceTec, Les Ulis, France).
Fluorescence Microscopy
hCMEC/D3 cells, untreated or treated with siRNAs, were cultured on insert filters. Cells were fixed either with ice-cold ethanol for 10 minutes at −20°C or with 4% paraformaldehyde for 10 minutes at room temperature, respectively, for claudin-5 or Gαi2 staining. Cells were permeabilized with 0.1% Triton X-100 in PBS for 10 minutes at room temperature, incubated in 3% bovine serum albumin in PBS (blocking buffer) for 30 minutes and stained with the indicated primary antibodies, diluted in the blocking buffer overnight at 4°C. After three washes with PBS, cells were incubated for 1 hour with fluorophore-conjugated secondary antibodies (Jackson Immunoresearch Laboratories, West Grove, PA, USA). Labeled preparations washed with PBS were and mounted in Glycergel medium (Dako Inc., Carpinteria, CA, USA). Fluorescence microscopy was performed with a Zeiss Axio Observer Z1 microscope, using a × 40 Oil Objective. Acquisitions were made with the MetaMorph 7 software (Molecular Devices, Sunnyvale, CA, USA).
Permeability Assays
hCMEC/D3 cells were grown on culture inserts for 6 days. Culture medium was removed and replaced by Hank's buffered salt solution (HBSS) transport buffer containing 10 mM HEPES, and 1 mM sodium pyruvate (Invitrogen Ltd, Paisley, UK) in the lower chamber. In the upper chamber, the transport buffer was supplemented with 50 μM Lucifer Yellow (LY) salt, 50 μM fluorescein isothiocyanate (FITC)-Dextran/4 kDa or 20 μM FITC-Dextran/70 kDa (Sigma-Aldrich), as indicated. Incubations were performed in triplicates at 37°C, 95% humidity, and 5% CO2 as previously described (Weksler et al, 2005). Samples were analyzed using a fluorometer (Fusion, Packard Bioscience Company, Meriden, CT, USA). Results are expressed as Permeability coefficients (Pe: 10−3 cm/min) or as the percentage of control permeability (corresponding either to untreated cells or to control siRNA-treated cells, as indicated).
xCELLigence Assays
The xCELLigence system (Roche) is an instrument that measures in real-time electrical impedance across gold microelectrodes integrated in the bottom of culture E-plates. hCMEC/D3 cells were seeded in standard culture medium without antibiotics at density of 15,000 cells/well onto E-plates coated with 150 μg/mL rat tail collagen type I. One hour after cell adhesion, the transfection SiRNA mix was added (as described above) and the cells were maintained in culture for 7 days. As indicated, at t=70 hours of culture, confluent hCMEC/D3 cells were treated with 1 M mannitol in serum-free culture medium for 30 minutes. Then, mannitol solution was removed and standard culture medium was added for a recovery period of 4 days. Impedance measurement was displayed in real time as a Cell Index (an arbitrary unit) by the xCELLigence RTCA Software (Roche, Basel, Switzerland). Results are presented as mean values of cell index±s.d. (n=3) against time (in abscissa), in each condition.
Proteomic Analysis (Sample Preparations and Protein Identification)
Detergent-Free Isolation of Caveolae
hCMEC/D3 cells were grown onto 44 cm2 culture inserts (Corning). Cells were untreated, treated with 1 M mannitol for 30 minutes, or treated with mannitol and left in control medium for a 48-hour recovery period. Detergent-free isolation of caveolae was performed as described previously (Liu et al, 1998) with minor modifications. Briefly, 25 × 106 hCMEC/D3 cells were collected by scraping into 0.7 mL of ice-cold MBS buffer (25 mM morpholine ethane sulfonic acid, 150 mM NaCl, 5 mM EDTA, 500 mM Na2CO3) and protease inhibitors (Roche). Cell lysates were subjected to sonication at 4°C (3 × 20 seconds) and the homogenate was mixed with an equal volume of 80% sucrose in MBS buffer. The resulting 40% sucrose-homogenate was placed at the bottom of ultracentrifuge tubes and overlaid with 6.5 mL of 30% sucrose and 2.5 mL of 5% sucrose in MBS. The sample was centrifuged for 18 hours at 274,000 g, at 4°C using a SW41 rotor (Beckman Instrument, CA, USA). Twelve 1 mL fractions were collected (top to bottom). Both caveolin-1 and claudin-5 were found enriched in fractions 4 to 6 (so-called caveolae-rich fractions). These fractions were centrifuged at 168,500 g for 2 hours and the pellet was solubilized in 20 mM Tris, HCl pH 7.5, 10 mM MgCl2, 150 mM NaCl, 1 mM EDTA and 1% IGEPAL/Nonidet P-40 (immunoprecipitation buffer).
Immunoprecipitation Assays
Caveolae-rich fractions of hCMEC/D3 cells were incubated overnight at 4°C with anti-claudin-5 or anti-Gαi2 monoclonal antibodies in the immunoprecipitation buffer. Immunocomplexes were precipitated with protein G-sepharose beads (GE Healthcare, Waukesha, WI, USA) for 1 hour at 4°C. Beads were washed and proteins were solubilized in Laemmli buffer, boiled at 100°C for 5 minutes; Western blot analysis was performed as indicated above. Before mass spectrometry analysis, samples were prepared as follows: briefly, rabbit polyclonal anti-claudin-5 antibodies were crosslinked to Protein G-sepharose beads. Beads were washed with 0.2 M sodium borate buffer (pH 9.3) and solubilized with 20 mM of DMP (dimethyl pimelimidate) in 0.2 M sodium borate buffer for 1 hour at room temperature. 0.2 M ethanolamine (pH 8.0) was added and beads were washed. Beads were then incubated overnight at 4°C with caveolae-enriched fractions in the immunoprecipitation buffer. Elution was performed with 0.2 M glycine (pH 2.8) and 1 M Tris (pH 8.8) was added for pH neutralization. PPS (3-[3-(1,1-bisalkyloxyethyl) pyridin-1-yl]propane-1 sulfonate) Silent Surfactant (0.2%) in 50 mM ammonium bicarbonate (pH 7.8) and 5 mM of dithiotreitol were added to the immunoprecipitated proteins. The sample was incubated at 50°C for 30 minutes and 15 mM of iodoacetamide was added for 30 minutes at room temperature. Finally, trypsin was added for 4 hours at 37°C followed by 200 mM HCl for 45 minutes at 37°C. Samples were centrifuged at 14,000 r.p.m., for 10 minutes at 4°C and the IGEPAL/NP-40 detergent was completely removed using a strong cation exchanger column (SCX Spin Kit; PROTEA, Biosciences, Inc., Morgantown, WV, USA). After being lyophilized, samples were analyzed by nano-LC-MS/MS.
Mass Spectrometry Analysis and Protein Identification
Protein identification was performed by the University Paris Descartes Proteomics Facility (3P5), using nano-LC-MS/MS as described previously (Tyagi et al, 2009) with minor modifications. Dried peptide eluates were redissolved in 20 μL resuspension solution consisting of 0.1% trifuoroacetic acid (Fluka, Sigma-Aldrich, St Louis, MO, USA) plus 10% acetonitrile (Carlo Erba, Eure, France) and centrifuged at 12,000 g for 5 minutes. A 10-μL portion was injected on an Ultimate 3000 nano-HPLC (Dionex, Olten, Switzerland). Peptides were purified, separated, and deposited on MALDI targets using a Probot automated fraction collector (Dionex). MS spectra generated by a 4800 mass spectrometer (ABSciex, Foster City, CA, USA) were acquired automatically. Raw mass spectra were processed to extract monoisotopic values from peptide isotope clusters. Extracted MS/MS peak lists were subsequently submitted to an in-house mascot (Matrix Science Ltd, London, UK) version 2.2 search engine. The Swiss-prot 55.5 release database was restricted to the human subset of sequences (20,319 sequences). Parent and fragment mass tolerances were set to 20 p.p.m. and 0.3 Da, respectively. Missed trypsin cleavage sites were limited to 1. A filter was applied to the search to reduce false positives and matching redundancies of the same peptide in several hits. All matches above 1% risks of random matching were eliminated (P<0.01). Under these stringent parameters, the minimum protein score was 28. Unless a specific peptide could differentiate them, the best matching protein was selected even when subsets of peptides could match another protein isoform. The probability score calculated by the software was used as a primary criterion for correct identification.
Chromogenic Immunohistochemistry
Three individuals who died accidentally with no brain involvement, collected in Forensic Medicine, were examined to determine the in vivo expression in human brain of both Gαi2 and claudin-5. Within central nervous system, five areas of interest were selected (frontal and occipital cortex, thalamus, brain stem, and cerebellum), fixed for 1 month in formalin before gross examination, paraffin embedded, and histological prepared (5 μm sections). Neuropathological examinations confirm the absence of pathology in these selected cases. Chromogenic immunohistochemistry was performed on human tissues. After antigen unmasking (97°C, 20 minutes in citrate buffer), monoclonal antibodies against Gαi2 (1:20 dilution) (Santa Cruz) and anti-claudin-5 (1:75 dilution) (Invitrogen) was incubated overnight and revealed with a polymer detection kit (SuperPicTure Zymed, Invitrogen) and the AEC (3 amino-9 ethyl carbazole) as a red chromogen (Vector Lab, Burlingame, CA, USA; http://www.vectorlabs.com). Sections were counterstained with hematoxylin. Negative controls always include normal mouse immunoglobulins at the same dilution, and absence of primary antibody.
Modeling by Ingenuity Pathway Analysis
Nano-LC-MS/MS results were analyzed using ingenuity pathway analysis (Ingenuity Systems, Inc., USA, http://www.ingenuity.com). A network is a graphical representation of the molecular relationships between molecules. We selected only networks scoring ⩾2, with P<0.01 of not being generated by chance. Biological functions were assigned to each network by use of annotations from scientific literature and stored in the IPKB (Ingenuity Pathway Knowledgebase). We used P⩽0.05 to select highly significant biological functions and pathways represented in our proteomic data sets. Molecules are represented as nodes, and the biological relationship between two nodes is represented as an edge (line). All edges are supported by at least one reference from the literature, from a textbook, or from canonical information stored in the IPKB.
Statistical Analysis
Data are presented as the mean values±standard errors (s.d.). Statistical analysis was performed by using Student's t-tests with differences between means considered significant when P<0.05.
Results
Claudin-5 Contributes to the Restriction of Paracellular Permeability in Brain Endothelial Cells
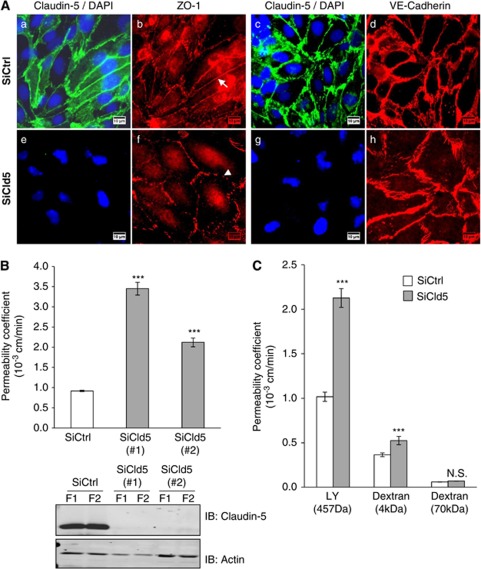
In the first series of experiments, we assessed whether claudin-5 has a predominant role in the barrier feature of the human brain endothelial cell line, hCMEC/D3 which recapitulates in vitro most properties of the human BBB (Poller et al, 2008; Tai et al, 2009; Weksler et al, 2005). For this purpose, we knocked-down claudin-5 expression by RNA interference. Regarding junctional complexes, hCMEC/D3 control cells (transfected with nontargeting siRNA) express claudin-5 and ZO-1 as well as VE-cadherin, markers of TJ and Adherens Junction (AJ) complexes, respectively (Figures 1Aa–1Ad), properly localized at cell–cell junctions. In the absence of claudin-5, hCMEC/D3 cells still form a confluent monolayer and express ZO-1 and VE-cadherin at intercellular contacts (Figures 1Af and 1Ah). However, cells appear bigger and the pattern of ZO-1 expression less continuous in cells treated with claudin-5 siRNA (Figure 1Af, arrowhead) than in control cells (Figure 1Ab, arrow), suggesting a reorganization of junction complexes and/or a loss of junction integrity (Fischer et al, 2002; Romero et al, 2003). Permeability assays using LY, a 457-Da fluorescent marker of paracellular permeability, were performed with claudin-5-depleted hCMEC/D3 cells. Two individual claudin-5 siRNAs (siCld5), which dramatically silenced the expression of claudin-5 (Figure 1B, lower panel), strongly increased the basal LY permeability coefficient of hCMEC/D3 cells: by 3.5- and 2.2-fold, respectively (Figure 1B, upper panel). In three independent experiments, we observed a two- to fourfold increase of LY permeability by siCld5#1 and a 1.5- to 2-fold increase by siCld5#2. Accordingly, siCld5#1 was then used for subsequent assays.
Figure 1.
Claudin-5 knockdown increases paracellular permeability in hCMEC/D3 cells. (A) Immunofluorescence staining of hCMEC/D3 cells, treated by either nontargeting small interfering RNAs (siRNAs) (siCtrl) or siRNAs against claudin-5 (siCld5) at confluence. Labeling with claudin-5 monoclonal antibody and zonula occudens (ZO)-1 polyclonal antibodies (a, b, e, and f) or claudin-5 polyclonal antibodies and VE-cadherin monoclonal antibody (c, d, g, and h). Nuclei in blue were labeled with DAPI. The arrow (b) and arrowhead (c) point to continuous and punctuated ZO-1 staining of cell–cell junctions, respectively. Scale bars represent 10 μm. (B, upper panel) hCMEC/D3 cells treated with siCtrl (white bars) or two individual siCld5 (gray bars) were grown to confluence on Transwell inserts and permeability assays to Lucifer Yellow (LY) were performed: permeability coefficients (Pe) are presented. Results are mean values±s.d. of Pe values of triplicates in one representative experiment (out of three independent experiments). (B, lower panel) Western blot analysis of claudin-5 expression in hCMEC/D3 cells, either treated with siCtrl or with two individual siCld5. Cell extracts were prepared from two duplicate filters (F1/F2), then proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting (IB) was performed with anti-claudin-5 or anti-actin monoclonal antibodies. (C) hCMEC/D3 cells, treated with siCtrl (white bars) or siCld5 #1 (gray bars) were grown to confluence on Transwell inserts. Permeability assays were performed as indicated: to LY (457 Da), 4 kDa FITC-dextran or 70 kDa FITC-dextran; permeability coefficients (Pe) are presented. Results are mean Pe values±s.d. of triplicates in one representative experiment (out of three independent experiments). ***P≤0.005 compared with siCtrl-treated cells, not significant (N.S.).
Because in claudin-5-deficient mice, the BBB permeability to small molecules (compared with larger molecules) was selectively affected (Nitta et al, 2003), permeability assays were further performed with larger molecules such as 4 and 70 kDa FITC-dextrans (Figure 1C). Claudin-5 knockdown increased the permeability coefficient value to LY and 4 kDa FITC-dextrans by 2- and 1.5-fold, respectively. No significant change in the permeability to 70 kDa FITC-dextran was detected, confirming here in vitro that permeability of brain endothelial cells is affected in a size-selective manner by the absence of claudin-5. Taken together, our data further confirmed that the hCMEC/D3 model is highly reminiscent of in vivo BBB and justified its use as a tool to investigate the molecular mechanisms involved in claudin-5 regulation of TJ integrity.
A Global Proteomic Analysis to Identify Claudin-5 Partners
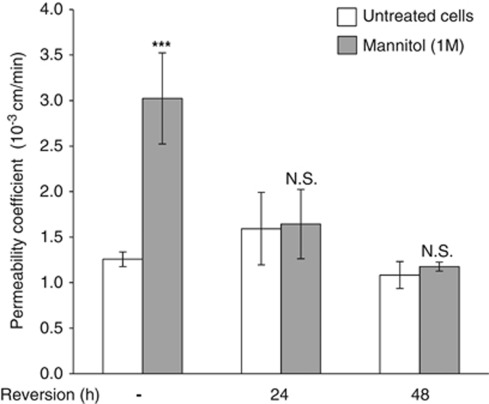
To identify claudin-5 partners that might contribute to BBB formation or maintenance, we hypothesized that such proteins associate with claudin-5 when TJ integrity is maintained and dissociate when TJs are disrupted. To address this issue experimentally, we used a hypertonic concentration of mannitol (1 to 1.4 M) known to transiently open the BBB and induce widening of interendothelial contacts (Rapoport, 2000). hCMEC/D3 cells were treated or not with mannitol (1 M) for 30 minutes, before a 24- or 48-hour recovery period. Permeability assays to LY were performed at 30 minutes, 24, and 48 hours. As shown in Figure 2, mannitol induced a threefold increase of the basal permeability coefficient to LY. As expected, this increase was reversible following a 48-hour recovery period. Similar results were obtained with 1.4 M mannitol (data not shown).
Figure 2.
Hyperosmolar concentration of mannitol induces a transient increase of endothelial permeability to Lucifer Yellow (LY). hCMEC/D3 cells were grown at confluence on Transwell inserts. Cells were treated with 1 M mannitol for 30 minutes, then medium was changed to let the cells recover for up to 48 hours. Permeability to LY was measured directly after mannitol treatment (white bars) and after 24 or 48 hours recovery (gray bars), as indicated. Results are mean Pe values±s.d. from three independent experiments performed in triplicates. ***P<0.005 compared with untreated cells, not significant (N.S.).
hCMEC/D3 cell fractionation on sucrose gradients was performed to obtain caveolae-enriched fractions known to incorporate TJ proteins (Dodelet-Devillers et al, 2009; Lambert et al, 2007). Claudin-5 was enriched, as expected, in these fractions (not shown) and was immunoprecipitated from fractions prepared from untreated hCMEC/D3 cells, cells treated with mannitol (1 M) or treated with mannitol plus a 48-hour recovery period. Coimmunoprecipitated claudin-5 proteins were identified by nano-LC/MS/MS tandem mass spectrometry analysis in each experimental condition. In addition to claudin-5 per se, we could identify 12 proteins coimmunoprecipitated with claudin-5 in control condition (Table 1; Supplementary Figure 1): eight of them (Table 1, asterisks) were integral membrane proteins or membrane-associated proteins known to be localized in caveolae and/or at cell–cell junctions in epithelial or endothelial cells, thus confirming that our approach was efficient to identify junctional proteins. These proteins were caveolin-1, VE-cadherin, p120 catenin, γcatenin, β-actin, Gαi2, and PKCθ isoform. Interestingly, when the same proteomic analysis was performed with cells treated with mannitol and cells treated with mannitol plus 48 hours recovery, only β-actin, Gαi2, and PKCθ could be detected in control as well as recovery conditions but were not found in the mannitol condition. These results, in line with our working hypothesis, pointed to β-actin, Gαi2, and PKCθ as putatively associated with claudin-5 in a functional manner.
Table 1. Claudin-5 partners in hCMEC/D3 cells identified by mass spectrometry.
| Protein name | SWISSPROT access number | Cellular compartment | MW (Da) | Mascot score | Queries |
|---|---|---|---|---|---|
| p120 catenin (*) | O60716 | cyt., pl. mb, nucl. | 108,674 | 1,272 | 22 |
| Guanine nucleotide-binding protein Gi subunit α2 (Gαi2) (*) | P04899 | pl. mb | 40,995 | 728 | 8 |
| Actin (β-actin) (*) | P60709 | cyt. | 42,052 | 282 | 5 |
| HSPA5/BiP (*) | P11021 | cyt. | 72,402 | 200 | 4 |
| Myosin-Ic | O00159 | cyt. | 121,724 | 132 | 4 |
| Stomatin | P27105 | pl. mb. | 31,882 | 174 | 3 |
| Histone H4 | P62805 | nucl. | 11,360 | 140 | 3 |
| VE-cadherin (*) | P33151 | pl. mb. | 87,516 | 122 | 3 |
| HSPA9 | P38646 | cyt. | 73,920 | 121 | 3 |
| Claudin-5 (*) | O00501 | pl. mb. | 23,816 | 154 | 2 |
| Junction plakoglobin, γCatenin (*) | P14923 | pl. mb. | 82,434 | 88 | 2 |
| Caveolin-1 (*) | Q03135 | pl. mb, caveolae | 20,472 | 56 | 1 |
| PKCθ (*) | Q04759 | cyt. | 83,407 | 35 | 1 |
Pl. mb., plasma membrane; cyt., cytosol; nucl., nucleus.
Caveolae fractions were isolated as described in Materials and methods from hCMEC/D3 cells, either untreated or treated with 1 M mannitol for 30 minutes or treated with 1 M mannitol plus a 48-hour recovery period. Claudin-5 was immunoprecipitated with anti-claudin-5 polyclonal antibodies. Coimmunoprecipitated proteins were identified by nano-LC/MS/MS analysis. Each protein was identified in at least three independent experiments. Are indicated: SwissProt access numbers, Mascot scores (scores >35 with P<0.01), queries (number of sequenced peptides identified). Cellular compartments are indicated according to the SwissProt data bank. The table presents data from one out of three independent mass spectrometry analyses. Asterisks (*) indicate proteins known to be localized in caveolae and/or at cell–cell junctions. Proteins in bold (Gαi2, β-actin, and PKCθ) were detected in control and postrecovery conditions, but not detected after 1 M mannitol treatment.
G-Protein Subunit αi2 Physically Interacts with Claudin-5 in a Multimolecular Complex
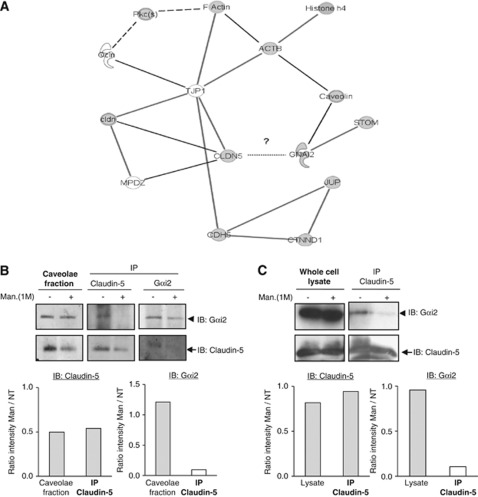
To further examine the links between claudin-5 and its 12 partners identified by our proteomic analysis, a molecular network was created using the Ingenuity software (Ingenuity Systems Inc, Redwood City, CA, USA), which considers only protein–protein interactions validated from the literature (Supplementary Figure 2). Figure 3A represents a part of the network of claudin-5-associated proteins without HSPA5/BiP, HSPA9, and myosin-Ic. Two groups of molecules are identified: a first group, including claudin-5 with its known TJs partners, MUPP-1/MPDZ, other members of the claudin family (cldn) and ZO-1/TJP1 proteins; a second group includes ZO-1/TJP1 partners (VE-cadherin/CDH5, occludin/Ocln, β-actin/ACTB, caveolin, and Gαi2/GNAI2). Gαi2/GNAI2 appears to interact with claudin-5 through caveolin, β-actin and ZO-1, although a direct interaction with claudin-5 cannot be excluded. Among the three proteins (β-actin/ACTB, Gαi2/GNAI2, and PKCθ) we identified above as associated with claudin-5 in control condition, dissociated after mannitol treatment and reassociated after the recovery period, we focused on Gαi2. Indeed, Gαi2 has been suggested to contribute to TJ biogenesis in epithelial cells (Denker et al, 1996; Saha et al, 1998), to directly interact with caveolin-1 (Song et al, 1996) and to be essential for leukocyte extravasation across endothelial cells in vivo (Pero et al, 2007); however, the molecular mechanisms involved are still elusive.
Figure 3.
G-protein subunit αi2 (Gαi2) interacts with claudin-5 and belongs to a multiprotein complex. (A) A connectivity map of claudin-5, extracted from the whole claudin-5/CLDN5 interactome network (see Supplementary File) established with the Ingenuity software, from molecular interactions validated and published in the literature. Gray nodes represent proteins identified by mass spectrometry in the present study as claudin-5 partners. White nodes represent additional proteins, not identified in the present study: F-actin, zonula occudens (ZO)-1 (TJP1), and occludin (OCLN). Solid lines indicate direct protein–protein interactions; dotted lines indicate indirect interactions. A putative direct interaction between claudin-5 and Gαi2 is suggested by a question mark. GNAI2, Gαi2; CTNND1, p120 catenin; CDH5, VE-cadherin, JUP, γcatenin; ACTB, β-actin; MPDZ, multiple PDZ domain protein (MUPP-1); STOM, stomatin; Pkc(s), PKC protein kinases. (B, C) hCMEC/D3 cells were untreated or treated with 1 M mannitol for 30 minutes. Caveolae fractions (B) were isolated as described in Materials and methods in each condition. Whole cell lysates were solubilized in immunoprecipitation buffer (see Materials and methods). Immunoprecipitations (IP) were performed with mouse monoclonal antibodies directed against claudin-5 or Gαi2 as indicated. Immune complexes were immunoblotted (IB) with anti-claudin-5 or anti-Gαi2 antibodies, as indicated. Results are representative of three independent experiments. Arrows and arrowheads indicate the electrophoretic mobility of claudin-5 and Gαi2, respectively. Immunoblots were scanned and histograms in the lower panels represent the relative intensity of the indicated protein band after mannitol treatment versus without treatment.
To confirm the interaction between endogenous Gαi2 and claudin-5 proteins in hCMEC/D3 cells, coimmunoprecipitation assays were performed using either untreated cells or cells treated with mannitol from caveolae-enriched fractions (Figure 3B) or whole cell lysates (Figure 3C). G-protein subunit αi2 was detected in claudin-5 immunoprecipitates (Figures 3B and 3C); conversely, claudin-5 was detected in Gαi2 immunoprecipitates (Figure 3B). After mannitol treatment, although claudin-5 level was decreased in some experiments for unknown reason (Figure 3B), we observed in all cases that the interaction between claudin-5 and Gαi2 was disturbed. Altogether, these results firmly establish that Gαi2 and claudin-5 constitutively interact with each other, either directly or indirectly through caveolin, β-actin, and ZO-1, which may link the two proteins in a multiprotein complex (Figure 3A).
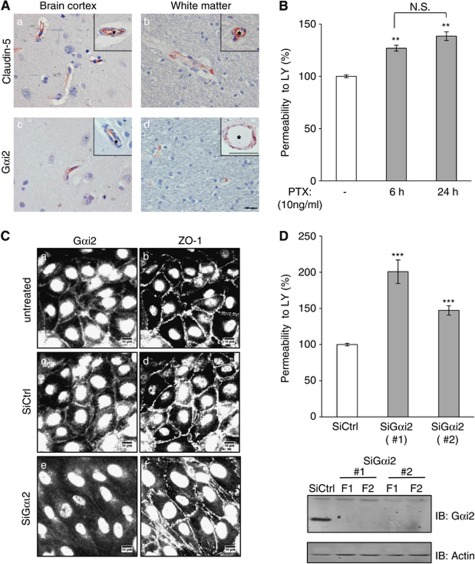
G-Protein Subunit αi2 Is Expressed in Human Brain Microvessels and Contributes to the Integrity of the Cell–Cell Junctions
To get further insight into the physiological relevance of our findings, we investigated whether claudin-5 and Gαi2 might be expressed in the same vascular beds in human brain tissue. For this purpose, immunohistological staining of claudin-5 or Gαi2 was performed on three post-mortem adult human brains and five sections were examined, from brain cortex, thalamus, brain stem, and cerebellum. Gαi2 localization was found to be similar to claudin-5 localization, with a specific labeling of endothelial cells in small capillaries (5 to 8 μm) and larger vessels (25 to 100 μm), in brain cortex (Figures 4Aa and 4Ac) and white matter (Figures 4Ab and 4Ad). Unlike claudin-5, Gαi2 was also detected in neurons (data not shown) as previously described (Khan and Gutierrez, 2004). No claudin-5 or Gαi2 immunostaining was observed in glial cells. These observations reveal that Gαi2 and claudin-5 are expressed by the endothelium of the same vascular beds in human brain. We suggested that Gαi2 may have a role in the integrity of TJs of brain endothelial cells.
Figure 4.
G-protein subunit αi2 (Gαi2) is expressed in human brain endothelium in situ and contributes to the integrity of endothelial junctions. (A) Immunohistochemical detection of claudin-5 and Gαi2 in human central nervous system. Claudin-5 and Gαi2 are detected in the endothelium of blood capillaries in the cortical gray matter and in the white matter. The insets show a small artery (a) and a small venule (b). Asterisks (*) indicate the vessel lumens. The insets show a venula (c) and a small artery (d). The scale bar is 10 μm for all images and 100 μm for the inset in image d. (B) hCMEC/D3 cells were treated for 6 or 24 hours with pertussis toxin (PTX at 10 ng/mL), an inhibitor of G-protein αi GTPase activity. Permeability assays were then performed with Lucifer Yellow (LY). Results are expressed as percentage of control permeability (untreated cells: Pe (LY)=2.86±0.04 × 10−3 cm/min) and are mean values±s.d. of triplicate Pe values in one representative experiment of three independent experiments. (C) Immunostaining of Gαi2 (a, c, and e) and zonula occudens (ZO)-1 (b, d, and f) at the cell membrane of hCMEC/D3 cells, treated with either nontargeting small interfering RNAs (siRNAs) (siCtrl) or siRNAs against Gαi2 (siGαi2). Cells were grown to confluence, fixed, permeabilized, and simultaneously incubated with anti-Gαi2 polyclonal antibodies and anti-ZO-1 monoclonal antibodies. Nuclei staining is not specific. The same fields are shown for ZO-1 and Gαi2 staining. Scale bars represent 10 μm. (D, upper panel) LY permeability assays performed with hCMEC/D3 cells treated with siCtrl (white bars) or two individual siGαi2 (gray bars). Results are expressed as percentage of control permeability (control siRNA-treated cells: Pe (LY)=0.92±0.02 × 10−3 cm/min) and are mean values±s.d. of triplicate Pe values in one representative experiment of three independent experiments. (D, lower panel) Expression level of Gαi2 in hCMEC/D3 cells in the same conditions as above. Whole cell lysates were prepared from 2 duplicate filters (F1, F2) and proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and detected by immunoblotting with anti-Gαi2 or anti-actin antibodies as protein loading controls. ***P⩽0.001, **P⩽0.05 compared with control cells.
Experiments were performed to investigate first whether G-protein αi subunits (hCMEC/D3 cells express the three known subunits: Gαi1, Gαi2, and Gαi3, not shown) are involved in the tightness of cell junctions. hCMEC/D3 cells were treated with PTX (pertussis toxin), a well-known nonselective inhibitor of the GTPase activity of Gαi subunits, for 6 or 24 hours before LY permeability assay. A limited but significant permeability increase was observed (Figure 4B), suggesting that functional Gαi subunits are involved in maintaining the endothelial barrier integrity. This assay, however, could not discriminate between Gαi2 and the two other Gαi subunits (Gαi1 and Gαi3).
Instead, RNA interference approaches were used to investigate the specific role of Gαi2 in the restriction of permeability to LY by hCMEC/D3 cells. First, immunofluorescence analysis confirmed that Gαi2 is expressed at the plasma membrane and at cell–cell junctions in untreated cells or siRNA control-treated cells (Figures 4Ca and 4Cc). We observed that Gαi2 depletion did not prevent cells from getting to confluence, with no visible change in ZO-1 (Figures 4Ce and 4Cf) or VE-cadherin (not shown) accumulation at cell junctions; however, Gαi2-depleted cells appeared more elongated than siRNA control cells. Interestingly, two individual siRNAs that efficiently silenced the expression of Gαi2 (Figure 4D, lower panel) significantly increased the LY permeability coefficient by 1.5- to 2-fold (Figure 4D, upper panel). Altogether, these results clearly demonstrate a functional role of Gαi2 for the maintenance of junction integrity in brain endothelial cells.
G-Protein Subunit αi2 Is Important for Tight Junctions Reassembly After Mannitol Treatment
To address the role of Gαi2 in TJ formation in brain endothelial cells, the behavior of hCMEC/D3 cells during the recovery period after mannitol treatment was monitored in real time by impedance measurement using the Roche xCELLigence system; values expressed as ‘cell index' are maximal (about 10 arbitrary units, a.u.) with tight monolayers and minimal when cell–cell junction integrity is severely affected (0 to 1 a.u.).
As shown in Figures 5A–5D, hCMEC/D3 cells were depleted in claudin-5 or Gαi2 by siRNAs and compared with cells treated with control siRNA (siCtrl); in addition, codepletion of these proteins was performed by simultaneous treatment with both individual siRNAs and compared with cells treated with control siRNA used at a twofold higher concentration (siCtrl × 2). Experiments were performed for 7 days and the cell index was monitored on-line: hCMEC/D3 cells were cultured until confluence, after 70 hours, they were treated with 1 M mannitol for 30 minutes (asterisk) and medium was changed to remove mannitol for recovery for 4 additional days. Control (siCtrl) hCMEC/D3 cells reached confluence at 56 hours associated with a high cell index plateau (10.4 a.u.). As expected, mannitol treatment induced a dramatic decrease of cell index and after medium change, the impedance gradually increased to reach the plateau value at the end of the experiment (Figure 5A), likely reflecting TJ reassembling during the recovery period. These observations confirmed that the measure of impedance is a good read-out for assessing the integrity of cell–cell junctions. We then used this experimental device to further evaluate the functional consequences of Gαi2 and/or claudin-5 depletion in hCMEC/D3 cells.
Figure 5.
G-protein subunit αi2 (Gαi2) or claudin-5 depletion delays the recovery of cell–cell junction integrity after 1 M mannitol treatment. hCMEC/D3 cells treated with nontargeting small interfering RNAs (siRNAs) (siCtrl) or siRNAs against claudin-5 (siCld5), Gαi2 (siGαi2), or both siCld5+siGαi2 were grown in a 96-well plate of an xCELLigence system (Roche). The impedance measurement (cell index: CI) was monitored in real time and plots were produced using the RTCA Software. At t=70 hours, cells were treated with 1 M mannitol (asterisk). After 30 minutes, the medium was changed for a recovery period (up to 4 days). (A–D) Plots show CI recordings during the whole experiment period (7 days) with asterisk representing time where mannitol was added. Arrowheads (1 to 4) point to times of analysis (as shown in (E)): cell confluence (t=56 hours) and various recovery times (t=84, 112, 164 hours). (E) CI values at the times indicated above (arrowheads 1 to 4 in (A–D)). Results are mean CI values±s.d. (n=3 wells) from one representative experiment out of three independent experiments. (F) Expression level of claudin-5 and Gαi2 proteins in hCMEC/D3 cells either treated with siCtrl, siCld5, siGαi2, siCld5+siGαi2, or siCtrl at a double concentration (2 × ) as control. Whole cell lysates were prepared in each condition and proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and detected by immunoblotting (IB) with anti-claudin-5, anti-Gαi2, or anti-VE-cadherin antibodies. #P<0.005 compared with siCtrl-treated cells; ***P<0.001; not significant (N.S.).
Figure 5E presents impedance values in each experimental condition, with cell index normalization to the value of control cells at confluence. Impedance values are presented when cells get to confluence (t=56 hours) and along the recovery period (t=84, 112, and 164 hours) (Figure 5, arrowheads 1 to 4). Although, claudin-5- and Gαi2-depleted hCMEC/D3 cells, as well as codepleted cells reached confluence, cells silenced for claudin-5 showed at confluence a reduced cell index (70% control), indicating that the absence of claudin-5 affected cell–cell junction integrity (Figure 5E, white bars). By contrast, Gαi2 deletion did not affect the cell index at confluence (although it increased paracellular permeability, as shown in Figure 4D), suggesting that Gαi2 contribution to cell–cell junction integrity was lower than that of claudin-5; alternatively, we cannot exclude that other parameters than junction tightness integrated in the cell index value (such as cell morphology, cell attachment to the matrix) might compensate any change in cell–cell junction integrity, resulting in unchanged cell index. Interestingly, after mannitol treatment of cells depleted in claudin-5 or Gαi2, junction reassembly was significantly delayed: at 42 hours of recovery time (Figure 5E, dark gray bars), cell index was 60% in control cells, but only 17% and 32% in claudin-5- and Gαi2-depleted cells, respectively; codepleted cells reached 40% of the plateau value (Figure 5E, dark gray bars). Consequently, no significant additive effect of the codepletion of claudin-5- and Gαi2 was observed on the delay of cell junction reassembly, suggesting that Gαi2 and claudin-5 might be involved in the same pathway mediating this delay.
Figure 5F presents the expression levels of claudin-5 and Gαi2, as well as VE-cadherin as control, at the end of the experiment, when cell indexes reached 90% to 100% of the initial plateau for each condition (Figure 5E, black bars), indicative of a complete recovery: these data confirm that claudin-5 or Gαi2 depletion was maintained all over the experiments and did not affect the expression of each other, while VE-cadherin expression was not affected by any treatment. Altogether, these data further document that the absence of claudin-5 or Gαi2 clearly affect the integrity of cell–cell junctions in hCMEC/D3 cells by disturbing the process of junction reassembly during recovery after an osmotic shock.
Discussion
To date, TJ architecture in brain endothelial cells remains partly understood in contrast to our extensive knowledge of TJ molecular organization in epithelial cells. Although claudin-5 is known to directly interact with several membrane or membrane-associated proteins in epithelial cells, like occludin (Saitou et al, 1998), ZO proteins (Itoh et al, 1999), MUPP-1 (Poliak et al, 2002), and other members of the claudin superfamily (Coyne et al, 2003), the molecular mechanisms by which claudin-5 regulates BBB permeability are still incompletely understood.
The aim of the present study was to identify putative claudin-5 partners involved in TJ regulation in human brain endothelial cells. In line with an earlier report demonstrating that BBB was loosened in a size-selective manner in claudin-5-deficient mice (Nitta et al, 2003), we observed in vitro, using the hCMEC/D3 human brain endothelial cell line, that knockdown of claudin-5 expression by siRNA interference strongly increased permeability to small molecules (LY, 457 Da), although cell morphology and time to confluence were apparently not affected. Not only did this result confirm that the hCMEC/D3 cell line is an in vitro BBB model, which recapitulates most properties of the BBB in situ, but, more specifically, it also established the feasibility of investigating with this model the molecular mechanisms of regulation of TJ integrity by claudin-5 and claudin-5-associated proteins.
To identify claudin-5 partners that might contribute to the regulation of TJ integrity, we reasoned that these proteins might associate with claudin-5 when TJ integrity is maintained and dissociate when TJs are disrupted. We chose a hypertonic concentration of mannitol used clinically to transiently destabilize brain endothelium TJs for drug delivery to the CNS (Rapoport, 2000), although the molecular mechanisms of mannitol-induced changes in cerebral endothelial cells are poorly understood. An earlier report had described that hyperosmolar concentration of mannitol may not only induce a mechanical shrinkage of the endothelial cells, but also the activation of signaling pathways such as src kinase-dependent phosphorylation of β-catenin and its dissociation from cadherin in cerebral endothelial cells (Farkas et al, 2005). In hCMEC/D3 cells, mannitol (1 M) treatment rapidly induced a marked increase in LY permeability, which, upon medium change, returned to control value after a 24- to 48-hour recovery period, confirming that treatment with mannitol could mimic a transient disruption of TJs tightness. We then took advantage of this observation to identify putative proteins associated with claudin-5 in hCMEC/D3 cells in basal conditions that dissociate after mannitol treatment and reassociate following a 48-hour recovery period. As a first step of our proteomic analysis of claudin-5-associated proteins, hCMEC/D3 cells were fractionated in detergent-free conditions on sucrose gradients and claudin-5 was shown to be enriched in light, caveolin-rich fractions. This observation was in agreement with previous reports, indicating that caveolae are membrane microdomains that incorporate TJ proteins, specific transporters, and adhesion molecules (Dodelet-Devillers et al, 2009). When claudin-5 immunoprecipitation was performed from these fractions, we detected and identified by nano-LC-MS/MS analysis only 12 proteins that constitutively associated with claudin-5. Interestingly, a majority of these proteins are known to be localized to plasma membrane and/or to be associated with junction complexes, such as caveolin-1, VE-cadherin, p120 catenin, Gαi2, and PKCθ, strongly suggesting that our proteomic approach was appropriate to identify membrane proteins with potential involvement in TJs. Surprisingly, ZO proteins, claudin-3, or MUPP-1, previously described as claudin-5 partners by coimmunoprecipitation studies or binding assays with the cytoplasmic tail of claudin-5 (Coyne et al, 2003; Itoh et al, 1999; Poliak et al, 2002), were not detected in our analysis. Interestingly, however, other proteomic studies using claudin-binding bacterial toxin, also failed to detect these proteins (Lohrberg et al, 2009).
Out of these 12 proteins, Gαi2, β-actin, and PKCθ were found to be associated with claudin-5 in basal conditions, to dissociate after mannitol treatment, and to reassociate after recovery, displaying the expected behavior according to our hypothesis. We focused on Gαi2 because several lines of evidence have suggested the involvement of heterotrimeric G-proteins in TJ maintenance and biogenesis in epithelial cells (Denker and Nigam, 1998; Denker et al, 1996). Indeed, MDCK cells expressing Gαi2 or a constitutive active form of Gαi2 developed a high trans-epithelial electrical resistance after calcium switch more rapidly than control cells, suggesting that Gαi2 is important for the formation of TJs in epithelial cells (Saha et al, 1998). In addition, Gαi2 has been described to directly interact with caveolin-1 (Song et al, 1996) and to colocalize with ZO-1 (de Almeida et al, 1994; Denker et al, 1996). In addition, PTX-sensitive heterotrimeric G-proteins were shown to be essential for leukocyte extravasation across endothelial cells in vitro, although the mechanism involved remained elusive (Adamson et al, 2002; Pero et al, 2007).
Here, we present evidence that Gαi2 coimmunoprecipitates with claudin-5 in hCMEC/D3 cells in culture conditions preserving TJ integrity (basal condition and postrecovery after a hyperosmolar shock), strongly suggesting that both proteins interact at TJs in brain endothelial cells. In this regard, we showed that, in adult human brains, Gαi2 and claudin-5 display a similar localization in endothelial cells of small capillaries and larger vessels. We describe Gαi2 as a regulator of barrier integrity in human brain endothelial cells by using RNA interference, which firmly demonstrated that Gαi2 knockdown in hCMEC/D3 cells significantly enhanced their permeability to LY, without affecting cell proliferation or monolayer formation. Because intracellular cAMP is widely thought to decrease brain endothelial permeability (Rubin et al, 1991), we investigated whether Gαi2 depletion might affect the intracellular cAMP level in hCMEC/D3 cells. Interestingly, the depletion of Gαi2 induced a slight increase in cAMP basal level (data not shown), strongly suggesting that the observed increased permeability to LY was independent of the cAMP pathway. This conclusion is in agreement with a previous report, which proposed that PTX-enhanced permeability of brain endothelial cells was mediated via a cAMP-independent pathway (Bruckener et al, 2003).
It is noteworthy that hCMEC/D3 cells also express Gαi1 and Gαi3, the expression of which was not affected by Gαi2 depletion (data not shown), confirming the unique role of Gαi2 in the regulation of permeability in the hCMEC/D3 cell line. Moreover, we showed by impedance measurement that Gαi2 knockdown significantly delayed TJ resealing after a hyperosmotic shock. Interestingly, no additive effect was observed in the absence of both claudin-5 and Gαi2, suggesting that both proteins might be involved in the same pathway. Altogether, these results clearly indicate that Gαi2, similarly to claudin-5, is important for the permeability restriction to small molecules displayed by brain endothelial cells, which reflects the integrity of their TJs.
On the basis of our knowledge of claudin-5 interactome (Figure 3A), we propose that claudin-5 and Gαi2 may control TJ integrity as components of a multiprotein complex, also including caveolin and ZO-1, linked to the actin cytoskeleton; this complex might also include occludin and MUPP-1. Whether the interaction between Gαi2 and claudin-5 is direct or indirect, notably through caveolin, β-actin, and ZO-1 remains to be investigated. It is now well established that TJs and AJs are functionally related and that AJs influence TJ organization. Whereas AJs precede TJs at cell–cell contacts (Gumbiner et al, 1988; Sedar and Forte, 1964), ZO-1 has been implicated in the crosstalk between these two junctional complexes (Ikenouchi et al, 2007). Our observation that claudin-5 or Gαi2 deletion delays junction recovery after a hyperosmotic shock strongly suggests that the claudin-5/Gαi2-containing multiprotein complex may actively contribute to the integrity of cell–cell junctions in human brain endothelial cells through a crosstalk with the VE-cadherin-containing AJ complex.
In conclusion, for the first time to our knowledge, the present study establishes that Gαi2, together with claudin-5, is a key protein controlling TJ integrity in human brain endothelial cells and suggests that both proteins may be present in a multiprotein complex localized at TJs. Further studies will focus on deciphering the functional crosstalk between Gαi2, claudin-5, and their partners for BBB regulation in physiological as well as pathophysiological situations.
Acknowledgments
The authors thank Drs BB Weksler, S Bourdoulous, J Gavard, and C Artus for helpful discussions. The authors are grateful to B Durel (Imaging Facility, Cochin Institute, Paris) and M Andrieu (Immunobiology Facility, Cochin Institute, Paris).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by the European FP7 collaborative grants: the European Stroke Network (ESN, EU FP7, agreement No. 202213) and JUSTBRAIN (Health-F2-2009-241861).
Supplementary Material
References
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Adamson P, Wilbourn B, Etienne-Manneville S, Calder V, Beraud E, Milligan G, Couraud PO, Greenwood J. Lymphocyte trafficking through the blood-brain barrier is dependent on endothelial cell heterotrimeric G-protein signaling. FASEB J. 2002;16:1185–1194. doi: 10.1096/fj.02-0035com. [DOI] [PubMed] [Google Scholar]
- Bruckener KE, el Baya A, Galla HJ, Schmidt MA. Permeabilization in a cerebral endothelial barrier model by pertussis toxin involves the PKC effector pathway and is abolished by elevated levels of cAMP. J Cell Sci. 2003;116:1837–1846. doi: 10.1242/jcs.00378. [DOI] [PubMed] [Google Scholar]
- Coureuil M, Mikaty G, Miller F, Lecuyer H, Bernard C, Bourdoulous S, Dumenil G, Mege RM, Weksler BB, Romero IA, Couraud PO, Nassif X. Meningococcal type IV pili recruit the polarity complex to cross the brain endothelium. Science. 2009;325:83–87. doi: 10.1126/science.1173196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne CB, Gambling TM, Boucher RC, Carson JL, Johnson LG. Role of claudin interactions in airway tight junctional permeability. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1166–L1178. doi: 10.1152/ajplung.00182.2003. [DOI] [PubMed] [Google Scholar]
- Dauchy S, Miller F, Couraud PO, Weaver RJ, Weksler B, Romero IA, Scherrmann JM, De Waziers I, Decleves X. Expression and transcriptional regulation of ABC transporters and cytochromes P450 in hCMEC/D3 human cerebral microvascular endothelial cells. Biochem Pharmacol. 2009;77:897–909. doi: 10.1016/j.bcp.2008.11.001. [DOI] [PubMed] [Google Scholar]
- de Almeida JB, Holtzman EJ, Peters P, Ercolani L, Ausiello DA, Stow JL. Targeting of chimeric G alpha i proteins to specific membrane domains. J Cell Sci. 1994;107 (Pt 3:507–515. doi: 10.1242/jcs.107.3.507. [DOI] [PubMed] [Google Scholar]
- Denker BM, Nigam SK. Molecular structure and assembly of the tight junction. Am J Physiol. 1998;274:F1–F9. doi: 10.1152/ajprenal.1998.274.1.F1. [DOI] [PubMed] [Google Scholar]
- Denker BM, Saha C, Khawaja S, Nigam SK. Involvement of a heterotrimeric G protein alpha subunit in tight junction biogenesis. J Biol Chem. 1996;271:25750–25753. doi: 10.1074/jbc.271.42.25750. [DOI] [PubMed] [Google Scholar]
- Dodelet-Devillers A, Cayrol R, van Horssen J, Haqqani AS, de Vries HE, Engelhardt B, Greenwood J, Prat A. Functions of lipid raft membrane microdomains at the blood-brain barrier. J Mol Med. 2009;87:765–774. doi: 10.1007/s00109-009-0488-6. [DOI] [PubMed] [Google Scholar]
- Farkas A, Szatmari E, Orbok A, Wilhelm I, Wejksza K, Nagyoszi P, Hutamekalin P, Bauer H, Bauer HC, Traweger A, Krizbai IA. Hyperosmotic mannitol induces Src kinase-dependent phosphorylation of beta-catenin in cerebral endothelial cells. J Neurosci Res. 2005;80:855–861. doi: 10.1002/jnr.20521. [DOI] [PubMed] [Google Scholar]
- Fischer S, Wobben M, Marti HH, Renz D, Schaper W. Hypoxia-induced hyperpermeability in brain microvessel endothelial cells involves VEGF-mediated changes in the expression of zonula occludens-1. Microvasc Res. 2002;63:70–80. doi: 10.1006/mvre.2001.2367. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafushi A, Yonemura S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan-Moya EM, Le Guelte A, Lima Fernandes E, Thirant C, Dwyer J, Bidere N, Couraud PO, Scott MG, Junier MP, Chneiweiss H, Gavard J. Secreted factors from brain endothelial cells maintain glioblastoma stem-like cell expansion through the mTOR pathway. EMBO Rep. 2011;12:470–476. doi: 10.1038/embor.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988;107:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenouchi J, Umeda K, Tsukita S, Furuse M. Requirement of ZO-1 for the formation of belt-like adherens junctions during epithelial cell polarization. J Cell Biol. 2007;176:779–786. doi: 10.1083/jcb.200612080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan ZU, Gutierrez A. Distribution of C-terminal splice variant of G alpha i2 in rat and monkey brain. Neuroscience. 2004;127:833–843. doi: 10.1016/j.neuroscience.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Lambert D, O′Neill CA, Padfield PJ. Methyl-beta-cyclodextrin increases permeability of Caco-2 cell monolayers by displacing specific claudins from cholesterol rich domains associated with tight junctions. Cell Physiol Biochem. 2007;20:495–506. doi: 10.1159/000107533. [DOI] [PubMed] [Google Scholar]
- Liu Y, Casey L, Pike LJ. Compartmentalization of phosphatidylinositol 4,5-bisphosphate in low-density membrane domains in the absence of caveolin. Biochem Biophys Res Commun. 1998;245:684–690. doi: 10.1006/bbrc.1998.8329. [DOI] [PubMed] [Google Scholar]
- Lohrberg D, Krause E, Schumann M, Piontek J, Winkler L, Blasig IE, Haseloff RF. A strategy for enrichment of claudins based on their affinity to Clostridium perfringens enterotoxin. BMC Mol Biol. 2009;10:61. doi: 10.1186/1471-2199-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147:185–194. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5 deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki S, Sato S, Yamaguchi H, Kamoi M, Asashima T, Terasaki T. Exogenous expression of claudin-5 induces barrier properties in cultured rat brain capillary endothelial cells. J Cell Physiol. 2007;210:81–86. doi: 10.1002/jcp.20823. [DOI] [PubMed] [Google Scholar]
- Pero RS, Borchers MT, Spicher K, Ochkur SI, Sikora L, Rao SP, Abdala-Valencia H, O′Neill KR, Shen H, McGarry MP, Lee NA, Cook-Mills JM, Sriramarao P, Simon MI, Birnbaumer L, Lee JJ. Galphai2-mediated signaling events in the endothelium are involved in controlling leukocyte extravasation. Proc Natl Acad Sci USA. 2007;104:4371–4376. doi: 10.1073/pnas.0700185104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piehl C, Piontek J, Cording J, Wolburg H, Blasig IE. Participation of the second extracellular loop of claudin-5 in paracellular tightening against ions, small and large molecules. Cell Mol Life Sci. 2010;67:2131–2140. doi: 10.1007/s00018-010-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S, Matlis S, Ullmer C, Scherer SS, Peles E. Distinct claudins and associated PDZ proteins form different autotypic tight junctions in myelinating Schwann cells. J Cell Biol. 2002;159:361–372. doi: 10.1083/jcb.200207050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poller B, Gutmann H, Krahenbuhl S, Weksler B, Romero I, Couraud PO, Tuffin G, Drewe J, Huwyler J. The human brain endothelial cell line hCMEC/D3 as a human blood-brain barrier model for drug transport studies. J Neurochem. 2008;107:1358–1368. doi: 10.1111/j.1471-4159.2008.05730.x. [DOI] [PubMed] [Google Scholar]
- Rampon C, Weiss N, Deboux C, Chaverot N, Miller F, Buchet D, Tricoire-Leignel H, Cazaubon S, Baron-Van Evercooren A, Couraud PO. Molecular mechanism of systemic delivery of neural precursor cells to the brain: assembly of brain endothelial apical cups and control of transmigration by CD44. Stem Cells. 2008;26:1673–1682. doi: 10.1634/stemcells.2008-0122. [DOI] [PubMed] [Google Scholar]
- Rapoport SI. Osmotic opening of the blood-brain barrier: principles, mechanism, and therapeutic applications. Cell Mol Neurobiol. 2000;20:217–230. doi: 10.1023/A:1007049806660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero IA, Radewicz K, Jubin E, Michel CC, Greenwood J, Couraud PO, Adamson P. Changes in cytoskeletal and tight junctional proteins correlate with decreased permeability induced by dexamethasone in cultured rat brain endothelial cells. Neurosci Lett. 2003;344:112–116. doi: 10.1016/s0304-3940(03)00348-3. [DOI] [PubMed] [Google Scholar]
- Rubin LL, Hall DE, Porter S, Barbu K, Cannon C, Horner HC, Janatpour M, Liaw CW, Manning K, Morales J, et al. A cell culture model of the blood-brain barrier. J Cell Biol. 1991;115:1725–1735. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha C, Nigam SK, Denker BM. Involvement of Galphai2 in the maintenance and biogenesis of epithelial cell tight junctions. J Biol Chem. 1998;273:21629–21633. doi: 10.1074/jbc.273.34.21629. [DOI] [PubMed] [Google Scholar]
- Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, Furuse M, Takano H, Noda T, Tsukita S. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol. 1998;141:397–408. doi: 10.1083/jcb.141.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedar AW, Forte JG. Effects of calcium depletion on the junctional complex between oxyntic cells of gastric glands. J Cell Biol. 1964;22:173–188. doi: 10.1083/jcb.22.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song KS, Li S, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J Biol Chem. 1996;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- Tai LM, Reddy PS, Lopez-Ramirez MA, Davies HA, Male DK, Loughlin AJ, Romero IA. Polarized P-glycoprotein expression by the immortalised human brain endothelial cell line, hCMEC/D3, restricts apical-to-basolateral permeability to rhodamine 123. Brain Res. 2009;1292:14–24. doi: 10.1016/j.brainres.2009.07.039. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M. Structural and signalling molecules come together at tight junctions. Curr Opin Cell Biol. 1999;11:628–633. doi: 10.1016/s0955-0674(99)00016-2. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- Tyagi TK, Ponnan P, Singh P, Bansal S, Batra A, Collin F, Guillonneau F, Jore D, Patkar SA, Saxena RK, Parmar VS, Rastogi RC, Raj HG. Moonlighting protein in Starkeyomyces koorchalomoides: characterization of dihydrolipoamide dehydrogenase as a protein acetyltransferase utilizing acetoxycoumarin as the acetyl group donor. Biochimie. 2009;91:868–875. doi: 10.1016/j.biochi.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P, Male DK, Roux F, Greenwood J, Romero IA, Couraud PO. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- Zougbede S, Miller F, Ravassard P, Rebollo A, Ciceron L, Couraud PO, Mazier D, Moreno A. Metabolic acidosis induced by Plasmodium falciparum intraerythrocytic stages alters blood-brain barrier integrity. J Cereb Blood Flow Metab. 2011;31:514–526. doi: 10.1038/jcbfm.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.