Abstract
Delayed remote ischemic postconditioning (DRIPost) has been shown to protect the rat brain from ischemic injury. However, extremely short therapeutic time windows hinder its translational use and the mechanism of action remains elusive. Because opening of the mitochondria KATP channel is crucial for cell apoptosis, we hypothesized that the neuroprotective effect of DRIPost may be associated with KATP channels. In the present study, the neuroprotective effects of DRIPost were investigated using adult male Sprague-Dawley rats. Rats were exposed to 90 minutes of middle cerebral artery occlusion followed by 72 hours of reperfusion. Delayed remote ischemic postconditioning was performed with three cycles of bilateral femoral artery occlusion/reperfusion for 5 minutes at 3 or 6 hours after reperfusion. Neurologic deficit scores and infarct volumes were assessed, and cellular apoptosis was monitored by terminal deoxynucleotidyl transferase nick-end labeling. Our results showed that DRIPost applied at 6 hours after reperfusion exerted neuroprotective effects. The KATP opener, diazoxide, protected rat brains from ischemic injury, while the KATP blocker, 5-hydroxydecanote, reversed the neuroprotective effects of DRIPost. These findings indicate that DRIPost reduces focal cerebral ischemic injury and that the neuroprotective effects of DRIPost may be achieved through opening of KATP channels.
Keywords: brain ischemia, KATP, remote ischemic postconditioning, reperfusion injury
Introduction
Stroke is the third leading cause of death in the United States. Approximately 795,000 people experience a stroke and >143,579 people die each year. Of all strokes, 87% are ischemic (Lloyd-Jones et al, 2010). The current lack of clinical treatment for acute stroke necessitates the exploration of novel concepts that may eventually lead to clinical application. One of these concepts is ischemic postconditioning (IPost) (Pignataro et al, 2008a; Zhao et al, 2006), which refers to interference of blood flow by a series of brief, repetitive occlusion and release of cerebral blood vessels after reperfusion. Yang et al (2004) reported that reduction to infarct size by IPost was dependent on opening of KATP channels . However, the extremely short therapeutic time windows may hinder its clinical translation. This specific limitation may prevent its application to those patients in whom reperfusion cannot be immediately and accurately identified.
However, it has been reported that delayed postconditioning conducted 2 days after transient global ischemia attenuates hippocampal injury in gerbils (Burda et al, 2006). In addition, Ren et al (2008) reported that delayed postconditioning reduced ischemic injury after focal ischemia. Recently, a new phenomenon, known as remote IPost (RIPost), was found to induce ischemic tolerance not only within the same piece of tissue, but also in distant tissues as well as in distant organs (Tsubota et al, 2010). Loukogeorgakis et al (2007) revealed that transient limb ischemia induces RIPost in humans by a KATP channel-dependent mechanism. This result suggested that mitochondrial KATP channel activation has a key role in the development of a protective effect during IPost and RIPost. RIPost has greater potential for clinical application than classic IPost as it can be performed in a nonvital organ, avoiding the high risk of IPost in the vital organ. However, all reported IPost procedures have been applied either at the onset of reperfusion or during the ischemic phase. For patients with transient ischemic reperfusion brain injury, pharmacologic and physiologic interventions influence the effective time window of treatment. However, whether delayed remote IPost (DRIPost) attenuates brain injury after cerebral ischemia is unknown. In this study, we tested whether DRIPost, conducted by repetitive occlusion and release of the bilateral femoral arteries, reduces the infarcted area in focal ischemic rats. Accumulating evidence suggests that KATP channel activation is involved in the protective effect of IPost. Thus, we further tested whether DRIPost protects the brain from stroke and the potential protective mechanisms related with KATP channel activation.
Materials and methods
Animals
Male Sprague-Dawley rats weighing 290 to 310 g were provided by the Experimental Animal Centre of the Fourth Military Medical University and housed under diurnal lighting conditions (12 hours darkness/light). All experimental protocols and animal handling procedures were performed in accordance with the National Institutes of Health (NIH, USA) guidelines for the use of experimental animals and the experimental protocols were approved by the Institutional Animal Care and Use Committee of the Fourth Military Medical University. Diazoxide (DIAZ) and 5-hydroxydecanote (5-HD) were purchased from Sigma-Aldrich (St Louis, MO, USA).
Focal Cerebral Ischemia
Sprague-Dawley rats were allowed free access to food and water but were fasted 12 hours before surgery. All animals were anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg in normal saline). The transient middle cerebral artery (MCA) occlusion model was performed as previously described (Xiong et al, 2003). Briefly, the right common carotid artery (CCA) and the right external carotid artery were exposed through a ventral midline neck incision, and were ligated proximally. A 3-0 nylon monofilament suture (Ethicon Inc., Osaka, Japan) with a blunt tip made by burning on a flame was inserted through the arterectomy in the CCA just below the carotid bifurcation, positioned into the internal carotid artery and advanced ∼17 to 18 mm until a mild resistance was felt. Under these conditions, the origin of the MCAs was occluded. Reperfusion was accomplished by withdrawing the suture after 90 minutes of ischemia. The incision sites were infiltrated with 0.25% (v/v) bupivacaine hydrochloride for postoperative analgesia.
Regional cerebral blood flow was monitored using a flexible optical fiber probe attached to the skull over the ipsilateral parietal cortex at one point (1 mm posterior and 5 mm lateral to bregma) by laser Doppler flowmetry (PeriFlux system 5000; Perimed AB, Stockholm, Sweden). Rats in which ipsilateral blood flow was not reduced to <20% of the baseline after placement of the intraluminal filament and whose cerebral blood flow signal was not rapidly restored during reperfusion were excluded from subsequent experiments. Cranial temperature was maintained at 36.8°C to 37.5°C by surface heating and cooling during surgery. In a separate experiment, physiologic parameters (cranial temperature, arterial pH, PaCO2, PaO2, and glucose) were monitored and analyzed in five additional rats. Arterial blood samples were taken 3 minutes before ischemia (baseline), 45 minutes after ischemia, and 30 minutes after reperfusion and DRIPost for gases and plasma glucose measurements.
Animal Recovery and Neurologic Evaluation
Rats were returned to their cages after the suture was withdrawn and were given free access to food and water. The neurologic behavior of rats was scored at 24, 48, and 72 hours after reperfusion by an investigator who was unaware of animal grouping. An 18-point scale of neurologic deficit scores (NDSs) was used for evaluation of neurologic behavior (Garcia et al, 1997). The scale was based on the following six tests: (1) spontaneous activity (0 to 3 points); (2) symmetry in the movement of four limbs (0 to 3 points); (3) forepaw outstretching (0 to 3 points); (4) climbing (1 to 3 points); (5) body proprioception (1 to 3 points); and (6) response to vibrissae touch (1 to 3 points). The six individual test scores were summed up at the end of the evaluation (minimum score, 3; maximum score, 18).
Infarct Volume Measurement
For measurements of infarct volume at 72 hours after cerebral artery reperfusion, rats were killed and the brains were rapidly removed and mildly frozen to keep the morphology intact during slicing. In brief, brains were cut into 2-mm-thick coronal sections in a brain matrix and stained with 2% (w/v) 2,3,5-triphenyltetrazolium chloride (Sigma-Aldrich) for 30 minutes at 37°C followed by overnight immersion in 4% (w/v) paraformaldehyde in phosphate buffer for after fixation. The infarct tissue area remained unstained (white), whereas normal tissue was stained red. Photographs were taken using a digital camera (SONY T9; Sony Corporation, Tokyo, Japan) The infarct area was demarcated and analyzed by Photoshop software (Adobe Photoshop 8.0, Adobe Systems Incorporated, San Jose, CA, USA). To compensate for the effect of brain edema, the corrected infarct volume was calculated as follows: percentage of corrected infarct volume=((contralateral hemisphere area−(ipsilateral hemisphere−measured infarct area))/contralateral hemisphere area) × 100%. After brain extraction, animals observed to have experienced a subarachnoid hemorrhage were excluded from the study.
Experimental Protocol
Experiments were composed of two parts. The first part was designed to prove the neuroprotective effect of DRIPost and explore the optimal method of DRIPost. The second part was designed to investigate the role of KATP channels in the neuroprotective effect of DRIPost in vivo.
Part 1: Neuroprotective Effect of Delayed Remote Ischemic Postconditioning Against Cerebral Ischemia–Reperfusion Injury
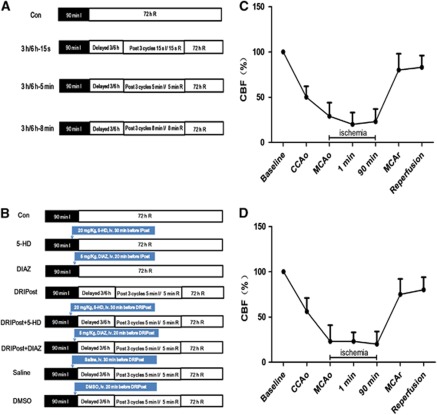
To explore the optimal parameters required to observe the protective effects of DRIPost, our pilot study tested 56 rats with 7 different serials of DRIPost performed at 3 or 6 hours after reperfusion (n=8 for each). The experimental protocols are shown in Figure 1A. Control (Con) group rats were subjected to 90 minutes of ischemia followed by 72 hours of reperfusion. For the DRIPost group, the bilateral femoral arteries were separated below the bilateral groin ligament for later induction of femoral artery occlusion after 90 minutes of ischemia. The occlusion duration varied from 15 seconds, 5 minutes to 8 minutes, and bilateral femoral arteries were released for the same duration and repeated for three cycles.
Figure 1.
Schematic of the design and changes in cerebral blood flow (CBF) in animals for part 1 and part 2 of the experiment. All animals underwent 90 minutes of ischemia (dark bar) and 72 hours of reperfusion (open bar) (n=8 for each). (A) In part 1, rats were subjected to delayed remote ischemic postconditioning (DRIPost) at 3 or 6 hours after reperfusion, and the DRIPost protocol was three cycles of bilateral femoral artery occlusion 15 seconds/5 minutes/8 minutes ischemia (I)/15 seconds/5 minutes/8 minutes reperfusion (R). (B) In part 2, except for delayed 6 hours/5 minutes DRIPost, the KATP channel blocker 5-hydroxydecanote (5-HD) or opener diazoxide (DIAZ), as well as the vehicle physiologic saline and dimethyl sulfoxide (DMSO), was injected through the caudal vein 20 minutes (for 5-HD and saline) or 30 minutes (for DIAZ and DMSO) before DRIPost to investigate the role of KATP channels in DRIPost-induced neuroprotection against focal cerebral ischemia–reperfusion injury. (C, D) Changes in CBF in animals subjected to 90 minutes ischemia followed by 72 hours reperfusion. Right common carotid artery (CCA) occlusion reduced CBF to ∼50% of the baseline, and additional middle cerebral artery (MCA) occlusion further decreased CBF to ∼20%. CCAo, CCA occlusion; MCAo, MCA occlusion; MCAr, MCA release.
Part 2: Effect of KATP Channel Blocking/Opening on Delayed Remote Ischemic Postconditioning-Induced Neuroprotection
In part 2, we further studied the role of KATP channels in the neuroprotective effect of DRIPost. Sixty-four adult male Sprague-Dawley rats were randomly divided into eight groups. The experimental protocols are shown in Figure 1B. Delayed remote ischemic postconditioning (5 minutes ischemia/5 minutes reperfusion in bilateral femoral arteries, three cycles) was induced at 6 hours after reperfusion in the postconditioning groups. This protocol showed a superior outcome in part 1 of the study. To block KATP channels, 20 mg/kg of 5-HD, which was dissolved in normal saline to a concentration of 12 mg/mL, was injected through the caudal vein 30 minutes before DRIPost. To open KATP channels, 5 mg/kg of DIAZ, which was dissolved in dimethyl sulfoxide (DMSO) to a concentration of 3 mg/mL, was administered through the caudal vein 20 minutes before DRIPost. To exclude the effect of administrated drugs on cerebral ischemia–reperfusion injury, two separate control groups were used, which were administered the same dosage of drugs without postconditioning. Vehicle groups (saline group and DMSO group) were also included to preclude any influence.
Terminal Deoxynucleotidyl Transferase Nick-End Labeling and Quantification of Apoptosis
Apoptosis was quantified using a commercially available fluorescent terminal deoxynucleotidyl transferase nick-end labeling (TUNEL) kit, in accordance with the manufacturer protocol (Roche Diagnostics Corporation, Indianapolis, IN, USA). The sections were mounted with 50% (v/v) glycerol for examination under a fluorescence microscope. The total number of TUNEL-positive neurons in the right hemisphere was counted in three different fields for each section in a blind manner by light microscopy at × 400 magnification (BX51; Olympus, Tokyo, Japan), and data from five animals at each stage were averaged.
Statistical Analysis
All data, excepting NDSs, are expressed as the mean±s.d. Physiologic parameters were analyzed by repeated-measures analysis of variance. The NDSs were expressed as median (range). The NDSs among different groups were compared by Kruskal–Wallis test. When Kruskal–Wallis test showed significant difference, the Mann–Whitney U-tests with Bonferroni correction were applied. The infarct volumes and TUNEL-positive neurons were compared among groups by one factor analysis of variance. P<0.05 was considered statistically significant.
Results
Physiologic Parameters and Regional Cerebral Blood Flow
No statistical significance was noted among different time points for any of the physiologic parameters including cranial temperature, blood gas, and glucose concentrations (Table 1). Physiologic parameters remained in the normal range during the experimental period. Monitoring of regional cerebral blood flow ensured successful MCA occlusion (Figures 1C and 1D).
Table 1. Physiological parameters, mean±s.d. (N=6).
| Time point | Temperature (°C) | Pao2 (mm Hg) | Paco2 (mm Hg) | Glucose (mmol/L) | Arterial pH |
|---|---|---|---|---|---|
| Baseline | 37.1±0.2 | 99.8±7.2 | 35.6±4.5 | 5.0±0.2 | 7.38±0.03 |
| Ischemia, 45 minutes | 37.8±0.1 | 95.3±6.5 | 38.5±5.4 | 4.8±0.3 | 7.32±0.02 |
| Reperfusion, 30 minutes | 37.0±0.3 | 90.2±5.4 | 38.6±4.2 | 4.5±0.3 | 7.35±0.12 |
| DRIPost, 10 minutes | 37.2±0.2 | 89.3±6.2 | 39.8±5.7 | 4.2±0.4 | 7.32±0.21 |
DRIPost, delayed remote ischemic postconditioning; PaO2, arterial oxygen tension; PaCO2, arterial carbon dioxide tension.
Values are expressed as mean±s.d.
Neurologic Deficit Scores
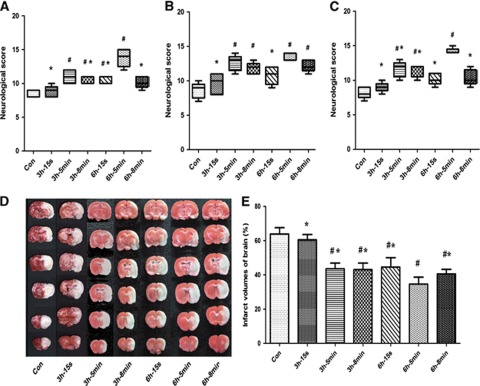
All rats survived until 72 hours after reperfusion. At 24, 48, and 72 hours after reperfusion, the NDS in the 3 h-5 min, 3 h-8 min, and 6 h-5 min groups was significantly higher than that in the Con group (P<0.05). Furthermore, the scores of animals in all groups were significantly lower than that of the 6 h-5 min group at each time point, which highlighted the neuroprotective effect of the 6 h-5 min DRIPost protocol in experiment part 1 (Figures 2A–2C).
Figure 2.
Neurologic scores and infarct volume after 90 minutes of transient middle cerebral artery occlusion in control (CON) and delayed remote limb ischemic postconditioning (DRIPost) groups in part 1 (n=8 for each). DRIPost was performed with bilateral femoral artery occlusion/reperfusion at 3 or 6 hours after reperfusion. Neurologic scores are presented as the median (range); Data for infarct volumes are expressed as the mean±s.d. Neurologic scores were evaluated at 24 hours (A), 48 hours (B), and 72 hours (C) after reperfusion using the Garcia scoring system. (D) Representative 2,3,5-triphenyltetrazolium chloride staining of the cerebral infarct in the rat brain at 72 hours after reperfusion. (E) Statistical analysis of the percentage of infarct volume was determined for each study group. 3 h/6 h-15 s, 5 min and 8 min groups: DRIPost performed at 3 or 6 hours after reperfusion. The occlusion duration varied from 15 seconds, 5 minutes to 8 minutes, and bilateral femoral arteries were released for the same duration and repeated for three cycles. #P<0.05 versus Con group. *P<0.05 versus 6 h-5 min group.
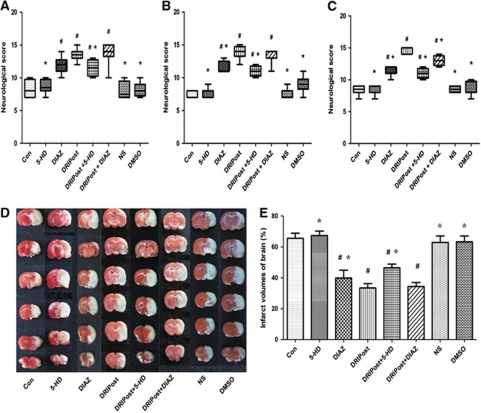
In part 2 of the experiment, at 24, 48, and 72 hours after reperfusion, the NDS in the DIAZ, DRIPost, DRIPost+DIAZ, and DRIPost+5-HD groups was significantly higher than that of the Con group (P<0.05). There was no statistical difference in NDS among the DRIPost and DRIPost+DIAZ groups at 24 and 48 hours after reperfusion (P=0.328 and 0.382, respectively). In contrast, the NDS in the DRIPost+5-HD group was significantly lower than that of the DRIPost group at the three time points (P<0.05; Figures 3A–3C).
Figure 3.
Neurologic scores and infarct volume after 90 minutes of transient middle cerebral artery occlusion in the control (CON), delayed remote limb ischemic postconditioning (DRIPost), 5-HD (KATP blocker, 20 mg/kg, intravenously), DIAZ (KATP opening, 5 mg/kg, intravenously), 0.9% (w/v) saline, dimethyl sulfoxide (DMSO) groups in part 2 (n=8 for each). DRIPost was performed with bilateral femoral artery occlusion/reperfusion at 6 hours after reperfusion. Neurologic scores are presented as the median (range); Date for infarct volumes are expressed as the mean±s.d. Neurologic scores were evaluated at 24 hours (A), 48 hours (B), and 72 hours (C) after reperfusion using the Garcia scoring system. (D) Representative 2,3,5-triphenyltetrazolium chloride staining of the cerebral infarct in the rat brain at 72 hours after reperfusion. (E) Statistical analysis of the percentage of infarct volume was determined for each study group. #P<0.05 versus Con group. *P<0.05 versus DRIPost group. DIAZ, diazoxide; 5-HD, 5-hydroxydecanote; NS, normal saline.
Infarct Volume
In experiment part 1, the infarct volume results are shown in Figure 2. All DRIPost protocols, except 3 h-15 s (60.48±3.08%), significantly decreased infarct volumes at 72 hours after reperfusion compared with that of the Con group (63.88±3.66%, P<0.05). Among the protocols implemented in this study, the neuroprotective effect of three cycles of 5 minutes occlusion/reperfusion at 6 hours after reperfusion was superior (34.64±4.00%, P<0.05; Figures 2D and 2E).
In experiment part 2, the infarct volume results are shown in Figure 3. Infarct volume in the DRIPost group (33.45±2.78%) was significantly smaller than that of the Con group (65.58±3.30%) at 72 hours after reperfusion (P=0.000). Administration of DIAZ (DIAZ group and DRIPost+DIAZ group) resulted in a reduction in infarct volume (39.83±5.04% and 34.36±2.56%, respectively) compared with the Con group (P=0.000). Administration of 5-HD (DRIPost+5-HD) eliminated the neuroprotective effect of DRIPost (46.44±2.65% versus 33.45±2.78%, P=0.000). However, there was no significant difference in infarct volume between the DRIPost and DRIPost+DIAZ groups (33.45±2.78% versus 34.36±2.56%, P=0.602; Figures 3D and 3E).
Terminal Deoxynucleotidyl Transferase Nick-End Labeling
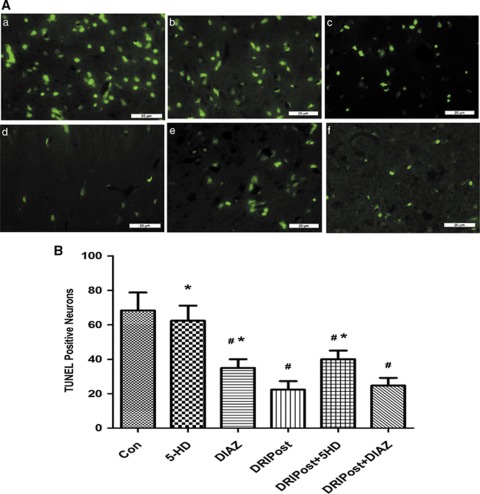
The number of TUNEL-positive neurons in the CA1 region significantly increased at 72 hours after ischemia–reperfusion injury in the Con group (Figure 4Aa). At the same observation time, the number of TUNEL-positive neurons in the CA1 region of the DRIPost group was significantly lower than that in the Con group (P<0.05; Figure 4Ad). Injection of 5-HD, a KATP blocker, before DRIPost, increased the number of TUNEL-positive neurons in the CA1 region after reperfusion (Figure 4Ae). However, there was no significant difference in the number of apoptotic neurons between the DRIPost and DRIPost+DIAZ groups 72 hours after reperfusion (Figure 4Ad and f).
Figure 4.
(A) Representative sections of nuclear DNA fragmentation staining performed by terminal deoxynucleotidyl transferase-mediated nick-end labeling (TUNEL) in the CA1 region of the hippocampus from rats that randomly received 5-HD (20 mg/kg, intravenously) or DIAZ (5 mg/kg, intravenously) after 90 minutes of ischemia and 72 hours of reperfusion (Con). In the presence and absence of 5-HD or DIAZ pretreatment, or with a combination of DRIPost (three cycles of 5 minutes occlusion/reperfusion at 6 hours after reperfusion) and 5-HD or DIAZ. (a) Control (Con); (b) 5-HD; (c) DIAZ; (d) DRIPost; (e) DRIPost+5-HD; (f) DRIPost+DIAZ. (B) Quantitative analysis of the number of TUNEL-positive neurons in the CA1 region of the hippocampus. Data are presented as the mean±s.d. Scale bar, 20 μm. #P<0.05 versus Con group. *P<0.05, versus DRIPost group. DIAZ, diazoxide; 5-HD, 5-hydroxydecanote.
Discussion
This study showed that RIPost with three cycles of 5 minutes limb ischemia/5 minutes reperfusion, which was conducted even 6 hours after focal cerebral ischemia–reperfusion injury, could reduce infarct size and improve neurologic deficits. Ischemia–reperfusion-induced neuronal apoptosis was also markedly attenuated by DRIPost. Activation of KATP channels is critical for endothelial protection by DRIPost because the conditioning stimuli were ineffective when applied in the presence of the nonselective KATP channel blocker 5-HD. Taken together, the present study provides evidence that the neuroprotective effect of DRIPost is associated with the activation of KATP channel opening.
We found that the optimal procedure for DRIPost, performed a few hours later, differed from that of rapid RIPost. Currently, there are at least two types of IPost, conventional IPost and RIPost. The conventional IPost refers to a series of brief, repetitive mechanical occlusions/reperfusions at the onset of reperfusion after long-term ischemia (Zhao et al, 2003). As for brain injury, conventional IPost was first reported by Zhao et al (2006) in a rat cerebral ischemia model. This protective concept of IPost has been confirmed by a number of groups using in-vivo global and focal ischemia models (Gao et al, 2008; Pignataro et al, 2008b) and in-vitro ischemic models (Pignataro et al, 2008b). Despite evidence from numerous animal studies, clinical application of in-situ ischemia for postconditioning of the brain is quite an unacceptable concept and impracticable. Recently, RIPost was primarily verified in studies of myocardial injury. Both limb ischemic (Gritsopoulos et al, 2009; Tang et al, 2010) and renal IPost showed potential cardiac protective effects (Liu et al, 2007). A recent study reported that RIPost reduced cerebral infarction by 67% (Ren et al, 2009). In addition, DRIPost initiated as late as 3 hours, but not 6 hours, still robustly reduced brain infarction by 43%. In our study, we found that three cycles of postischemic treatment, either 5 minutes occlusion/5 minutes reperfusion or 8 minutes occlusion/8 minutes reperfusion for each circle, were effective for inducing neuroprotection either applied at 3 hours or 6 hours after reperfusion. Among the effective procedures, 6 h-5 min group showed greater potential in reducing tissue damage after reperfusion. The different results between our group and Zhao's report may mainly reside in the difference of animal model. Taken together, both results suggest that early reperfusion after brain ischemic injury may broadly widen the therapeutic window for DRIPost, thus providing a better chance of neural salvage.
The underlying protective mechanisms of DRIPost are unknown. Nevertheless, our previous research from remote ischemic preconditioning (RIPC) against cerebral and spinal cord ischemia may shed light on our understanding of the protective mechanisms of DRIPost (Dong et al, 2010; Su et al, 2009). Emerging evidence from animal studies suggests that RIPC, IPost, and ischemic preconditioning share common signaling pathways, including triggers (adenosine receptor stimulation) (Kerendi et al, 2005; Steensrud et al, 2010), mediators (protein kinase C activation), and end effectors (opening of mitochondrial KATP channels, activation of prosurvival kinases, and inhibition of mitochondrial permeability transition pore opening; Hausenloy and Yellon, 2009; Meier et al, 2005; Yang et al, 2004). A popular theory has proposed that a substance or humoral factor, such as oxygen free radicals, is carried in the blood stream from the preconditioning organ or tissue to the organ where it manifests its protective effect. After humoral factors arrive to the remote organs, the end effectors (such as KATP channels) appear to be activated and have an important role in the induction of ischemic tolerance by RIPC. The protective effect of KATP has been showed in several tissues, including the intestines, kidneys, liver, and brain (Hai et al, 2005; Tawfik et al, 2009). Opening of KATP channels has been shown to be a prerequisite event for the induction of protection against ischemia–reperfusion injury by RIPC, IPost, and RIPost (Schmidt et al, 2007; Steensrud et al, 2010; Yang et al, 2004). Zhang et al (2010) used an in-vitro model of PC12 cells undergoing oxygen glucose deprivation to reveal that activation of KATP channels elicits protective effects against oxygen glucose deprivation-induced cell apoptosis by caspase-dependent and -independent mitochondrial pathways. In addition, Loukogeorgakis et al (2007) revealed that transient limb ischemia induces RIPost in humans by a KATP channel-dependent mechanism. Thus, we speculate that KATP may also be involved in the neuroprotective effect of DRIPost. In our study, DIAZ, an activator of KATP channels, significantly reduced morphological injury, TUNEL-positive cells, and neurologic deficiency after cerebral ischemia–reperfusion injury. However, the KATP blocker 5-HD reversed the neuroprotective effect of DRIPost. These results indicate that the activation of KATP is involved in the neuroprotective effect of DRIPost.
Several limitations of this study merit comments. First, only six different protocols were designed in this study. The results showed that DRIPost is still effective when applied at 6 hours after reperfusion. Whether the same results can be drawn at even later phases after injury or protocols where other parameters are altered (i.e., changes in the frequency of I/R cycle, application time or duration of occlusion and reperfusion of limb postconditioning) show more potent neuroprotection deserves further elucidation. Our results only exhibited a partial reversal of neuroprotection induced by DRIPost after 5-HD injection, suggesting that opening of KATP channels is not the only cause for the protective effect. In addition, differences could be due to different animal models (heart or brain ischemia–reperfusion injuries) used. Both timing and length of IPost have been confirmed to be important in determining the magnitude of its protective effect. Ren et al (2008) also found that the protective effect of limb RIPC depends on the number and duration of the limb ischemic stimulus. Previous studies have shown that endogenous activation of adenosine receptors, especially the A2A and A3A subtypes, is involved in IPost- and RIPost-mediated cardiac protection (Kerendi et al, 2005; Tsubota et al, 2010). Wang et al (2011) revealed that RIPost performed in one limb alleviated reperfusion injury after focal cerebral ischemia through reactive oxygen species-mediated inhibition of endogenous δ protein kinase C activation signaling cascade in an in-vivo rat model of focal cerebral ischemia. Whether other mediators and effectors such as protein kinase C-ɛ and adenosine A1 receptors that mediate the protective effect of IPost in myocytes could also participate in the induction of ischemic tolerance by DRIPost in the brain remains to be clarified.
In conclusion, DRIPost, even when applied at 6 hours after reperfusion, can induce a potent neuroprotective effect against focal cerebral ischemic reperfusion injury and inhibit apoptotic injury in the ischemic brain. The protective effects may partially be due to opening of KATP channels. Delayed remote ischemic postconditioning may be established as a potential practicable treatment for comparatively late-hour ischemic stroke patients who receive medical care hours after the onset of stroke.
The authors declare no conflict of interest.
Footnotes
This study was supported by the National Natural Science Foundation of China (Beijing, China, grants 30772059, 30972853, and 81128005 to HD), the National Science Foundation for Distinguished Young Scholars (Beijing, China, grant 30725039 to LX), and the Major Program of National Natural Science Foundation of China (Beijing, China, grant 30930091 to LX).
References
- Burda J, Danielisova V, Nemethova M, Gottlieb M, Matiasova M, Domorakova I, Mechirova E, Ferikova M, Salinas M, Burda R. Delayed postconditioning initiates additive mechanism necessary for survival of selectively vulnerable neurons after transient ischemia in rat brain. Cell Mol Neurobiol. 2006;26:1141–1151. doi: 10.1007/s10571-006-9036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HL, Zhang Y, Su BX, Zhu ZH, Gu QH, Sang HF, Xiong L. Limb remote ischemic preconditioning protects the spinal cord from ischemia-reperfusion injury: a newly identified nonneuronal but reactive oxygen species-dependent pathway. Anesthesiology. 2010;112:881–891. doi: 10.1097/ALN.0b013e3181d0486d. [DOI] [PubMed] [Google Scholar]
- Gao X, Zhang H, Takahashi T, Hsieh J, Liao J, Steinberg GK, Zhao H. The Akt signaling pathway contributes to postconditioning's protection against stroke; the protection is associated with the MAPK and PKC pathways. J Neurochem. 2008;105:943–955. doi: 10.1111/j.1471-4159.2008.05218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JH, Liu KF, Ye ZR, Gutierrez JA.1997Incomplete infarct and delayed neuronal death after transient middle cerebral artery occlusion in rats Stroke 282303–2309.2310 [DOI] [PubMed] [Google Scholar]
- Gritsopoulos G, Iliodromitis EK, Zoga A, Farmakis D, Demerouti E, Papalois A, Paraskevaidis IA, Kremastinos DT. Remote postconditioning is more potent than classic postconditioning in reducing the infarct size in anesthetized rabbits. Cardiovasc Drugs Ther. 2009;23:193–198. doi: 10.1007/s10557-009-6168-5. [DOI] [PubMed] [Google Scholar]
- Hai S, Takemura S, Minamiyama Y, Yamasaki K, Yamamoto S, Kodai S, Tanaka S, Hirohashi K, Suehiro S. Mitochondrial K(ATP) channel opener prevents ischemia-reperfusion injury in rat liver. Transplant Proc. 2005;37:428–431. doi: 10.1016/j.transproceed.2004.12.112. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: underlying mechanisms and clinical application. Atherosclerosis. 2009;204:334–341. doi: 10.1016/j.atherosclerosis.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Kerendi F, Kin H, Halkos ME, Jiang R, Zatta AJ, Zhao ZQ, Guyton RA, Vinten-Johansen J. Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res Cardiol. 2005;100:404–412. doi: 10.1007/s00395-005-0539-2. [DOI] [PubMed] [Google Scholar]
- Liu S, Wu XF, Zhang WZ, Sun YX, Cai SL. [Remote postconditioning by brief renal ischemia and reperfusion reduces acute myocardial ischemia and reperfusion induced myocardial apoptosis in rabbits] Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35:757–760. [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- Loukogeorgakis SP, Williams R, Panagiotidou AT, Kolvekar SK, Donald A, Cole TJ, Yellon DM, Deanfield JE, MacAllister RJ. Transient limb ischemia induces remote preconditioning and remote postconditioning in humans by a K(ATP)-channel dependent mechanism. Circulation. 2007;116:1386–1395. doi: 10.1161/CIRCULATIONAHA.106.653782. [DOI] [PubMed] [Google Scholar]
- Meier JM, Urban P, Goy JJ. Postconditioning inhibits mitochondrial permeability transition. Future Cardiol. 2005;1:457–460. doi: 10.2217/14796678.1.4.457. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Meller R, Inoue K, Ordonez AN, Ashley MD, Xiong Z, Gala R, Simon RP. In vivo and in vitro characterization of a novel neuroprotective strategy for stroke: ischemic postconditioning. J Cereb Blood Flow Metab. 2008a;28:232–241. doi: 10.1038/sj.jcbfm.9600559. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Meller R, Inoue K, Ordonez AN, Ashley MD, Xiong Z, Gala R, Simon RP. In vivo and in vitro characterization of a novel neuroprotective strategy for stroke: ischemic postconditioning. J Cereb Blood Flow Metab. 2008b;28:232–241. doi: 10.1038/sj.jcbfm.9600559. [DOI] [PubMed] [Google Scholar]
- Ren C, Gao X, Niu G, Yan Z, Chen X, Zhao H. Delayed postconditioning protects against focal ischemic brain injury in rats. PLoS One. 2008;3:e3851. doi: 10.1371/journal.pone.0003851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C, Yan Z, Wei D, Gao X, Chen X, Zhao H. Limb remote ischemic postconditioning protects against focal ischemia in rats. Brain Res. 2009;1288:88–94. doi: 10.1016/j.brainres.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MR, Smerup M, Konstantinov IE, Shimizu M, Li J, Cheung M, White PA, Kristiansen SB, Sorensen K, Dzavik V, Redington AN, Kharbanda RK. Intermittent peripheral tissue ischemia during coronary ischemia reduces myocardial infarction through a KATP-dependent mechanism: first demonstration of remote ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2007;292:H1883–H1890. doi: 10.1152/ajpheart.00617.2006. [DOI] [PubMed] [Google Scholar]
- Steensrud T, Li J, Dai X, Manlhiot C, Kharbanda RK, Tropak M, Redington A. Pretreatment with the nitric oxide donor SNAP or nerve transection blocks humoral preconditioning by remote limb ischemia or intra-arterial adenosine. Am J Physiol Heart Circ Physiol. 2010;299:H1598–H1603. doi: 10.1152/ajpheart.00396.2010. [DOI] [PubMed] [Google Scholar]
- Su B, Dong H, Ma R, Zhang X, Ding Q, Xiong L. Cannabinoid 1 receptor mediation of spinal cord ischemic tolerance induced by limb remote ischemia preconditioning in rats. J Thorac Cardiovasc Surg. 2009;138:1409–1416. doi: 10.1016/j.jtcvs.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Tang Y, Mennander A, Oksala N, Atalay M, Syvala H, Lagerstedt A, Kuuslahti M, Nieminen R, Anas N, Moilanen E, Tarkka M. Postconditioning and remote postconditioning of ischemic rat cardiac grafts. Eur Surg Res. 2010;45:1–8. doi: 10.1159/000315479. [DOI] [PubMed] [Google Scholar]
- Tawfik MK, Abo-Elmatty DM, Ahmed AA. The role of ATP-sensitive potassium channel blockers in ischemia-reperfusion-induced renal injury versus their effects on cardiac ischemia reperfusion in rats. Eur Rev Med Pharmacol Sci. 2009;13:81–93. [PubMed] [Google Scholar]
- Tsubota H, Marui A, Esaki J, Bir SC, Ikeda T, Sakata R. Remote postconditioning may attenuate ischaemia-reperfusion injury in the murine hindlimb through adenosine receptor activation. Eur J Vasc Endovasc Surg. 2010;40:804–809. doi: 10.1016/j.ejvs.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang X, Ding Q, Hu B, Xie Y, Li X, Yang Q, Xiong L. Limb remote postconditioning alleviates cerebral reperfusion injury through reactive oxygen species-mediated inhibition of delta protein kinase C in rats. Anesth Analg. 2011;113:1180–1187. doi: 10.1213/ANE.0b013e31822b885f. [DOI] [PubMed] [Google Scholar]
- Xiong L, Zheng Y, Wu M, Hou L, Zhu Z, Zhang X, Lu Z. Preconditioning with isoflurane produces dose-dependent neuroprotection via activation of adenosine triphosphate-regulated potassium channels after focal cerebral ischemia in rats. Anesth Analg. 2003;96:233–237. doi: 10.1097/00000539-200301000-00047. [DOI] [PubMed] [Google Scholar]
- Yang XM, Proctor JB, Cui L, Krieg T, Downey JM, Cohen MV. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol. 2004;44:1103–1110. doi: 10.1016/j.jacc.2004.05.060. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhao D, Wang Z, Zheng D. Diazoxide preconditioning alleviates caspase-dependent and caspase-independent apoptosis induced by anoxia-reoxygenation of PC12 cells. J Biochem. 2010;148:413–421. doi: 10.1093/jb/mvq074. [DOI] [PubMed] [Google Scholar]
- Zhao H, Sapolsky RM, Steinberg GK. Interrupting reperfusion as a stroke therapy: ischemic postconditioning reduces infarct size after focal ischemia in rats. J Cereb Blood Flow Metab. 2006;26:1114–1121. doi: 10.1038/sj.jcbfm.9600348. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]