Abstract
The development of the brain tissue damage in ischemic stroke is composed of an immediate component followed by an inflammatory response with secondary tissue damage after reperfusion. Fisetin, a flavonoid, has multiple biological effects, including neuroprotective and antiinflammatory properties. We analyzed the effects of fisetin on infarct size and the inflammatory response in a mouse model of stroke, temporary middle cerebral artery occlusion, and on the activation of immune cells, murine primary and N9 microglial and Raw264.7 macrophage cells and human macrophages, in an in vitro model of inflammatory immune cell activation by lipopolysaccharide (LPS). Fisetin not only protected brain tissue against ischemic reperfusion injury when given before ischemia but also when applied 3 hours after ischemia. Fisetin also prominently inhibited the infiltration of macrophages and dendritic cells into the ischemic hemisphere and suppressed the intracerebral immune cell activation as measured by intracellular tumor necrosis factor α (TNFα) production. Fisetin also inhibited LPS-induced TNFα production and neurotoxicity of macrophages and microglia in vitro by suppressing nuclear factor κB activation and JNK/Jun phosphorylation. Our findings strongly suggest that the fisetin-mediated inhibition of the inflammatory response after stroke is part of the mechanism through which fisetin is neuroprotective in cerebral ischemia.
Keywords: fisetin, flavonoids, macrophages, microglia, stroke
Introduction
Ischemic stroke is a devastating disease representing the second leading cause of death in the Western world and the leading cause of disability in adults (WHO, 2011). Ischemic stroke is believed to evolve in distinct phases (Dirnagl et al, 1999). The initial ischemia leads to apoptosis and necrotic cell death, followed by inflammation triggered and sustained by reperfusion of ischemic brain tissue, which is ultimately replaced by regeneration. We among others have provided evidence in a rodent model of ischemic stroke that poststroke inflammation indeed contributes to secondary tissue damage (Gelderblom et al, 2009).
The flavonoid fisetin (3,3′,4′,7-tetrahydroxyxflavone) has direct intrinsic antioxidant activity and indirect antioxidant effects by increasing levels of glutathione, thus improving survival of neuronal cells upon chemical ischemia in vitro using iodoacetic acid (Maher et al, 2007). Moreover, fisetin has been shown to suppress microglial activation following lipopolysaccharide (LPS) treatment (Zheng et al, 2008). Fisetin not only improves the clinical outcome in a rabbit model of ischemic stroke after 24 hours when given 5 minutes after onset of ischemia (Maher et al, 2007) but has also been reported to reduce infarct size after 24 hours in a permanent middle cerebral artery occlusion (MCAO) model in rats when given 30 minutes after induction of ischemia (Rivera et al, 2004).
Whether fisetin is protective even when given at a clinically relevant time point 3 hours after ischemia and whether the protective effect persists over a longer time course is unknown. Moreover, the mechanisms through which fisetin protects against ischemia have not been analyzed.
We hypothesized that an important aspect of the neuroprotective effect of fisetin in stroke could be due to modulation of the poststroke inflammatory response. Since secondary inflammation is initiated hours after the initial ischemia (Gelderblom et al, 2009), we assumed that delayed treatment with fisetin 3 hours after ischemia should still be similarly protective as pretreatment. To provide evidence that the observed changes in the inflammatory response are indeed due to antiinflammatory effects of fisetin, we also tested whether immune cells respond to fisetin in an in vitro paradigm of immune cell activation by LPS.
Materials and methods
In Vivo Stroke Model (Middle Cerebral Artery Occlusion)
A total of 90 mice were used in this study. All experiments were approved by and conducted in accordance with the laws and regulations of the regulatory authorities for animal care and use in Hamburg (Behörde fuer Lebensmittelsicherheit und Veterinärwesen—26/07). The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 83-123, revised 1996) and was performed in accordance with the ARRIVE guidelines (http://www.nc3rs.org/ARRIVE). In all, 12-week-old C57Bl/6 wild-type mice (Charles River, Sulzfeld, Germany) were used. The weight of mice ranged between 18 and 24 g. Mice were randomized and the scientists were blinded to group. Sample size calculation was performed (stroke size from pilot experiments, significance level 0.05, power 90%) and resulted in 12 or 8 animals per group to see a difference of 21% or 27% in stroke size, respectively. Temporary MCAO (tMCAO) was performed for 60 minutes as previously described (Gelderblom et al, 2009). Earlier experiments have shown fisetin doses of 30 mg/kg body weight to be effective and doses up to 90 mg/kg body weight (bw) to be safe in rats (Rivera et al, 2004). Animals were injected 20 minutes before or 180 minutes after the onset of ischemia with fisetin (Indofine, Hillsborough, NJ, USA, final concentration high-dose 50 mg/kg body weight, low-dose 25 mg/kg body weight in 15 μL DMSO (dimethyl sulfoxide), diluted immediately before injection in 150 μL 10% cyclodextran; Sigma, St Louis, MO, USA) or placebo (15 μL DMSO in 150 μL 10% cyclodextran; Sigma). Mice were monitored using transcranial temporal laser Doppler and showed a 90% drop in blood flow on placement of the filament (Gelderblom et al, 2009). Every mouse was repeatedly scored on a scale from 0 to 5 (0 no deficit, 1 preferential turning, 2 circling, 3 longitudinal rolling, 4 no movement, 5 death) after reawakening and every day until sacrifice. Only mice with a score greater or equal to one after reawakening were included for stroke size analysis. Clinical scores 1 hour after reawakening were not significantly different between the groups (Supplementary Figure 1). Recovery was defined as the time until mice reached score 1 (mice stopped circling). Mice were killed 3 or 7 days after reperfusion using isoflurane and decapitation. In the pretreatment group, a total of 13 placebo-treated, 12 low-dose fisetin-treated and 8 high-dose fisetin-treated mice were included. In the posttreatment group, 10 placebo-treated and 9 high-dose fisetin-treated mice were included. Mortality was not different between the groups. The brains were harvested, cut into 1 mm standardized slices (Braintree Scientific, Braintree, MA, USA; 1 mm) and vital stained using 2% (w/v) TTC (2,3,5-triphenyl-2-hydroxy-tetrazolium chloride; Sigma) in phosphate buffer. Slices were scanned on a flat bed scanner and infarct volume determined by blinded examiners using NIH ImageJ (rsbweb.nih.gov/ij/) and statistics (analysis of variance, post hoc Tukey–Cramer test, Graph Pad Prism (La Jolla, CA, USA). Fisetin (50 mg/kg bw) did not influence blood pressure after intraperitoneal injection 15 minutes, 1 hour, and 3 hours after intraperitoneal injection compared with placebo (Figure 1F).
Figure 1.
Dose-dependent protection from ischemic stroke by fisetin. (A) Representative vital-stained (2,3,5-triphenyl-2-hydroxy-tetrazolium chloride (TTC)) brain sections of placebo and fisetin (50 mg/kg bw posttreatment) treated mice 3 days after ischemia. (B, C) Fisetin treatment 20 minutes before (A) or 3 hours after onset of ischemia (B). Infarct size as edema corrected infarct volume in μL. Scatter plot with mean. (D, E) Time until animals stopped circling (reached score 1) in days in fisetin pretreatment (D) and fisetin posttreatment (E). Error bars indicate s.d. Significance by one-way analysis of variance (ANOVA) and Bonferroni post hoc test (A, C) or one-sided Student's t-test (B, D). *P<0.05. (F) Systemic blood pressure is not influenced by fisetin. Tail cuff measurements after intraperitoneal injection show no significant changes (two-way ANOVA).
Flow Cytometry
Flow cytometry cell-type analyses were performed as previously described (Gelderblom et al, 2009) (Supplementary Materials and methods). Only brain hemispheres with visible cortical infarcts and mice with scores ≥2 (circling) at 60 minutes after stroke were included in the flow cytometric analysis. For each experiment, three to four hemispheres were pooled. For intracellular cytokine staining, cells were stained with Ly6g (BD Biosciences, San Jose, CA, USA), CD11c (eBioscience, San Diego, CA, USA), CD11b (BD Biosciences), CD45 (eBioscience) for surface staining. Intracellular staining was performed according to the manufacturer's instruction (BD Biosciences), with anti-tumor necrosis factor α (TNFα) monoclonal antibody (BD Bioscience) or the corresponding isotype control (rat IgG1; eBioscience).
In Vitro Assays
Mouse N9 microglial cells or mouse Raw264.7 macrophages were grown in standard medium (Supplementary Materials and methods), treated with LPS (10 ng/mL) alone or following a 30-minute pretreatment with varying doses of fisetin. For analysis of nitric oxide (NO) production, we used the Griess assay (Sigma). Tumor necrosis factor α concentration was measured using a TNFα-kit (R&D Research, Minneapolis, MN, USA) according to the manufacturer's instructions. For western blot antibodies the following were used: phospho-stress-activated protein kinase/c-Jun NH2-terminal kinase (SAPK/JNK), phospho-c-Jun, phospho-IκBα, horseradish peroxidase (HRP)-actin, total SAPK/JNK (Cell Signaling, Danvers, MA, USA); total c-Jun/AP-1 (Santa Cruz, Santa Cruz, CA, USA) and inducible nitric oxide synthase (iNOS) (BD Bioscience), for details see Supplementary Materials and Figures.
For coculture experiments, primary murine microglia cells were treated with LPS (1 μg/mL; InvivoGen, San Diego, CA, USA) in the presence or absence of fisetin (2.0 μg/mL) for 24 hours. Next, microglia cells were washed and transferred to primary cortical neuronal cultures. Neuronal survival was measured after 24 hours of cocultivation by trypan blue exclusion (for details see Supplementary Materials and methods).
Human Monocyte-Derived Macrophages In Vitro
Human monocyte-derived macrophages were isolated from leukapheresis samples (written permission, anonymous, healthy donors, Eppendorf Blood Bank). Macrophages were stimulated with LPS (1 μg/mL; InvivoGen) in the presence or absence of fisetin. Macrophages were stained for fluorescence activated cell sorting (FACS) with CD80 (BD Bioscience), CD86 (BD Bioscience), and CD11b (Invitrogen, Carlsbad, CA, USA). Tumor necrosis factor α concentrations were measured using human TNFα-kit (BioLegend, San Diego, CA, USA) (for details see Supplementary Materials and methods).
Results
Fisetin Pretreatment and Posttreatment Reduces Infarct Volume 72 Hours After Temporary Middle Cerebral Artery Occlusion
Fisetin has previously been shown in other models to improve stroke severity after 24 hours when given 5 minutes (Maher et al, 2007) or 30 minutes after induction of ischemia (Rivera et al, 2004). To substantiate these findings in a model more prone to poststroke inflammation, we used a reperfusion stroke model, tMCAO mice. When given 20 minutes before onset of 1 hour of ischemia, treatment with 25 mg/kg bw resulted in a trend to a smaller infarct size by 13%, whereas 50 mg/kg bw significantly reduced infarct size by 46% (mean/s.d.: vehicle 56 μL/22 μL, 25 mg/kg fisetin 49 μL/23 μL, 50 mg/kg fisetin 30 μL/23 μL, analysis of variance with Bonferroni post hoc test: vehicle versus 25 mg/kg bw: n.s., vehicle versus 50 mg/kg bw: P<0.05; Figures 1A and 1B). Thus, we tested whether delayed treatment still protects against cerebral ischemia. Even when given 3 hours after onset of ischemia, 50 mg/kg bw reduced infarct size similarly by 35% (mean/s.d. vehicle 42 μL/17 μL, fisetin 27 μL/19 μL, one-sided Student's t-test, P<0.05; Figure 1C). Of note, stroke severity at the time of inclusion did not differ between groups (Supplementary Figure 1) and fisetin did not influence systemic blood pressure (Figure 1F). Either with fisetin pretreatment or posttreatment we found a strong trend toward earlier recovery in fisetin-treated animals compared with vehicle (days until recovery; mean/s.d. pretreatment; Figure 1D: placebo 1.7/1.5; fisetin 25 mg/kg bw 1.1/1.1; fisetin 50 mg/kg bw 1.2/1.5; P>0.05; posttreatment; Figure 1E: placebo 1.2/0.6; 50 mg/kg bw 0.9/0.2; P>0.05).
Fisetin Treatment Reduces Infiltration of the Ischemic Hemisphere With Macrophages, Dendritic Cells, and Lymphocytes
Next, we compared the number and subtype of immune cells in the ischemic hemisphere at day 3 after ischemia in animals pretreated with 50 mg/kg bw fisetin or vehicle using FACS analysis after visualization of subtype-specific surface markers by fluorophore-labeled antibodies (Gelderblom et al, 2009). Fisetin did not change the number of microglia identified as intermediate CD45-positive cells in the ischemic hemisphere (Figure 2A). In contrast, the ischemic hemispheres of fisetin-treated mice contained significantly less highly CD45-positive cells, a group comprised of lymphocytes, macrophages, and dendritic cells (DCs) (cells per ischemic hemisphere; mean±s.d.; vehicle 179.252±23.389; fisetin 93.045±46.963; P=0.045; Figure 2B). For further analysis, highly CD45-positive cells were subdivided into lymphocytes, macrophages, and DCs based on the expression of CD3, CD11b, and CD11c. Since we found similar absolute numbers of microglia in vehicle- and fisetin-treated mice, we used the number of microglia as an internal reference. Using this approach, we observed a significant decrease in percentage of both subtypes of antigen presenting cells, macrophages (percentage of macrophages relative to microglia; mean±s.d.; vehicle 49%±5.2% fisetin 12%±3.3% P=0.037; Figure 2C) and DCs (percentage of DC relative to microglia; mean±s.d.; vehicle 23%±0.7% fisetin 14%±2.3% P=0.038; Figure 2C), as well as lymphocytes (percentage of lymphocytes relative to microglia; mean±s.d.; vehicle 5.7%±0.1% fisetin 3.3%±0.2% P=0.038; Figure 2D) in the ischemic hemisphere of fisetin-treated animals compared with vehicle.
Figure 2.
Decreased inflammatory cells in postischemic brains of fisetin pretreated mice. (A) Absolute numbers of microglia per hemisphere by flow cytometry 3 days after ischemia are not different between fisetin and vehicle pretreated mice. (B–D) Significant reduction of ischemic brain infiltrating CD45high leukocytes, macrophages, dendritic cell (DC), and lymphocytes relative to numbers of microglia 3 days after stroke (n=3). Error bars indicate s.d. Significance by two-sided Student's t-test. n.s., not significant, *P<0.05, **P<0.05.
Fisetin Modifies the Activation of Infiltrating Macrophages and Resident Microglia in the Ischemic Hemisphere
The activation status of cells infiltrating the brain is more relevant for secondary brain damage than their number. Therefore, we tested whether pretreatment or posttreatment with 50 mg/kg bw fisetin changes the cytokine profile of the brain resident microglia, infiltrating macrophages and DC compared with vehicle using flow cytometry labeling intracellular cytokines of the brain- and spleen-derived cells 3 days after stroke.
Indeed, pretreatment with 50 mg/kg bw fisetin before tMCAO strongly reduced the relative proportion of TNFα producing microglia (3 days after ischemia; vehicle 30.4% fisetin 17.3% Figure 3A) and macrophages (3 days after ischemia; vehicle 24.6% fisetin 2.9% Figure 3B) found in the brain. No change was seen in the brain invading DCs (Supplementary Figure 2A). Similarly, fisetin posttreatment 3 hours after ischemia still resulted in suppression of TNFα production in the brain-derived microglia and macrophages although to a slightly lesser degree (3 days after ischemia; microglia vehicle 36.9% fisetin 22.6% Figure 3A; macrophages vehicle 31.2% fisetin 19.1% Figure 3B). Fisetin treatment did not alter TNFα expression of spleen-derived macrophages after stroke independent of pretreatment or posttreatment with fisetin (pretreatment 3 and 7 days after ischemia; vehicle 4.2%/4.7% fisetin 4.4%/4.5% Figure 4A; posttreatment vehicle 5.5%, fisetin 5.8% Figure 4B) or DCs (3 days after ischemia; vehicle 0.2% fisetin 0.5% Supplementary Figure 2B). Tumor necrosis factor α production in spleen-derived macrophages was not influenced by fisetin alone or by poststroke systemic immunosuppression, since similar percentages were observed in sham-operated mice (Figure 4C).
Figure 3.
Fisetin pretreatment and posttreatment reduces tumor necrosis factor α (TNFα) production in the brain. Flow cytometry of microglia (A) and macrophages (B) isolated from ipsilesional brain hemispheres 3 days and 7 days after stroke following pretreatment or posttreatment with fisetin 50 mg/kg body weight. Staining for intracellular TNFα shows attenuation of TNFα production in fisetin pretreated mice in the brain-derived microglia and macrophages at day 3 and macrophages at day 7 after stroke. The same effect is observed in mice treated with fisetin 3 hours after ischemia. Percentages relative to gated cells. Representative experiments with pooled hemispheres from three mice are shown. TNFα-positive cells are defined by isotype control (not shown).
Figure 4.
Fisetin does not exhibit systemic effects on tumor necrosis factor α (TNFα) production. Flow cytometry of macrophages isolated from spleen following stroke (A, B) or sham-operated mice and pretreatment (A) or posttreatment (B) with fisetin or placebo at day 3 or 7 (fisetin 50 mg/kg body weight). Staining for intracellular TNFα does not show differences in TNFα production in spleen macrophages between fisetin and placebo. Of note, no difference in TNFα production between stroke and sham-operated mice is seen (A–C). Percentages relative to gated cells. TNFα-positive cells are defined by isotype control (not shown). Representative experiments with pooled spleens from three mice are shown.
To examine the stability of these changes to the activation profile of the brain-derived immune cells induced by a single fisetin injection (50 mg/kg bw) before ischemia, we examined mice 7 days after the ischemic insult. Even at that time point, TNFα-positive cerebral macrophages were still reduced by the single dose of fisetin before ischemia (7 days after ischemia; vehicle 33.8% fisetin 13.9% Figure 3B), whereas the fisetin effect on microglial TNFα production had almost disappeared at day 7 (7 days after ischemia; vehicle 31.9% fisetin 26.8% Figure 3A). The abating difference supports the idea that the suppression of TNFα expression is a direct result of fisetin treatment rather than an indirect effect of the smaller infarct size.
Fisetin Suppresses Activation and Neurotoxicity of Murine Macrophage and Microglial Cell Lines and Human Primary Macrophages in an In Vitro Model of Inflammatory Activation by Lipopolysaccharide
To substantiate the idea that the fisetin-induced reduction of postischemic activation of microglia and infiltrating macrophages demonstrated by suppressed TNFα expression is a direct effect of fisetin on immune cells, we next tested whether fisetin modulates the activation of a murine microglial and a macrophage cell line as well as human primary macrophages on LPS treatment. Fisetin dose dependently reduced TNFα secretion in both murine Raw264.7 macrophage and N9 microglial cells compared with vehicle (mean±s.d.; Raw264.7 macrophages 29%±3.4% P<0.001; N9 microglia 56%±8.8% P<0.001 at 2.9 μg/mL fisetin; Figures 5A and 5B). Fisetin treatment of human monocyte-derived macrophages led to a similar significant, dose-dependent reduction of TNFα secretion on LPS treatment (2.0 μg/mL fisetin 62%±13.4% P<0.05; Figure 5C). In addition, fisetin also reduced NO production and expression of inducible NO synthase as an alternative marker of inflammatory activation in murine immune cell lines (Supplementary Figure 3).
Figure 5.
Fisetin attenuates lipopolysaccharide (LPS)-stimulated tumor necrosis factor α (TNFα) production and LPS-induced neurotoxicity of murine macrophage and microglia as well as human macrophages. Reduced TNFα production in murine macrophage cell lines (A; Raw264.7, n=3), murine microglia cell lines (B; N9, n=3) and human monocyte-derived macrophages (C; n=3) on fisetin treatment. Percentage of TNFα production relative to LPS-stimulated cells. (D) Reduced microglial neurotoxicity after fisetin pretreatment of microglia. Murine primary microglia pretreated with fisetin or dimethyl sulfoxide (DMSO) together with or in the absence of LPS and incubated with primary cortical neurons. Percentage of neuronal death by trypan blue exclusion. Error bars indicate s.d. Significance by one-way analysis of variance (ANOVA) and Bonferroni post hoc test. *P<0.05, **P<0.01, ***P<0.001. LPS=10 ng/mL for (A, B) and 1 μg/mL for (C). Fisetin dose in μg/mL final concentration.
Next, we examined the functional relevance of this activation on neurotoxicity in a murine primary microglia/neuron coculture model. Indeed, we observed that fisetin reduced the neurotoxicity of LPS-stimulated primary microglia (percentage cell death mean±s.d.; DMSO 46%±17% n=13; 2.0 μg/mL fisetin only 47%±11% n=14; LPS+DMSO 69%±12% n=25; 2.0 μg/mL fisetin+LPS 56%±13% n=51; P<0.001; Figure 5D).
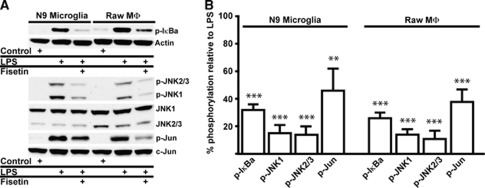
Fisetin Inhibits Lipopolysaccharide-Induced Activation of Proinflammatory Signaling via Nuclear Factor κB and JNK Suppression
To elucidate the mechanism underlying the antiinflammatory actions of fisetin, we looked at the effect of fisetin on several intracellular signal transduction pathways implicated in inflammation including IκB, JNK, and its target c-Jun. Fisetin treatment of LPS-stimulated murine N9 microglial and Raw264.7 macrophage cells significantly reduced both phosphorylated IκB (Figures 6A and 6B) and JNK phosphorylation regardless of JNK isoforms (Figures 6A and 6B). In addition, the phosphorylation of the target of JNK c-Jun was reduced (Figures 6A and 6B) suggesting that fisetin directly inhibits these proinflammatory signaling pathways. The c-Jun signaling pathway has a relevant role in vivo, since immunohistochemically colocalization of c-Jun and phospho-c-Jun with monocytes (CD11+) was observed 3 days after stroke (Supplementary Figure 4).
Figure 6.
Fisetin blocks phosphorylation of JNK, c-Jun, and IκB. (A) Representative western blots of fisetin inhibition of lipopolysaccharide (LPS)-induced phosphorylation of IκB, c-Jun, and JNK isoforms in microglial (N9) and macrophage (Raw264.7) cell lines. Fisetin 2.9 μg/mL (10 μmol). (B) Quantification of the reduction of phosphorylation (in percentage; LPS=100%); Fisetin 2.9 μg/mL (10 μmol). Error bars indicate s.d. Significance by one-way analysis of variance (ANOVA) and Bonferroni post hoc test. **P=0.002, ***P<0.0001; n=3. LPS=10 ng/mL.
Discussion
The flavonoid fisetin has direct glutathione-inducing and antiinflammatory properties (Maher et al, 2007). It also has been shown that fisetin increases ATP levels in an in vitro model of ischemic neuronal cell death, chemical ischemia on treatment with iodoacetic acid (Maher et al, 2007). Thus, fisetin is a promising candidate substance for treatment of ischemic stroke, where oxidative stress, ATP depletion and secondary inflammation are pathophysiologically relevant (recently reviewed in Macrez et al, 2011). In line with these assumptions, fisetin has been shown to be beneficial in a rabbit small clot embolism model and in a rat permanent MCAO model 24 hours after stroke when given up to 30 minutes after onset of ischemia (Maher et al, 2007; Rivera et al, 2004). In contrast, we examined later time points and our animal model of stroke, the occlusion reperfusion model of tMCAO in mice, is a much more potent initiator of poststroke inflammation than permanent ischemia (reviewed in Tuttolomondo et al, 2009). We show that in this model, fisetin is effective when given up to 3 hours after onset of ischemia.
Efficiency of delayed treatment is not only relevant for putative future therapeutic purposes in humans but also indicates that a late stage of secondary infarct growth is modified by fisetin. Thus, we analyzed whether fisetin modifies the inflammatory response after cerebral ischemia. Indeed, fisetin robustly reduced the infiltration of the ischemic hemisphere with macrophages, DCs, and lymphocytes. The reduced postischemic inflammation was not merely due to a difference in chemotaxis as we could demonstrate that fisetin also suppressed the activation of infiltrating macrophages and DCs as well as resident microglia as shown by reduced expression of the proinflammatory cytokine TNFα. However, an additional indirect effect of fisetin on poststroke inflammation via its antiapoptotic effects and consecutive reduction on stroke size cannot be completely excluded. Poststroke inflammation is known to be influenced by infarct size (Hug et al, 2009). Nevertheless, our data indicate that fisetin exhibits direct effects on inflammatory cells, since fisetin suppressed TNFα secretion of murine N9 microglial and macrophage Raw264.7 cells and human primary monocyte-derived macrophages in an in vitro model of inflammatory activation by stimulation with LPS, which is in line with earlier reports (Zheng et al, 2008; Lyu and Park, 2005). In addition, fisetin attenuates LPS-induced neurotoxic effects of microglial cells on primary cortical neurons.
Activation of resident microglia is accepted as a main component of the postischemic periinfarct inflammation and microlia cells are among the main sources of proinflammatory cytokines such as TNFα (Gregersen et al, 2000). Microglial activation is a cause of secondary tissue damage, as is demonstrated by an improved outcome in stroke models through microglia-directed treatment (Liu et al, 2007). Additionally, microglial activation results in the attraction of inflammatory cells from the circulation, which further enhances the inflammatory damage (Yilmaz et al, 2006). Thus, the fisetin-induced reduction of postischemic microglial TNFα expression might explain diminished invasion of macrophages and DCs observed by us on fisetin treatment. Remarkably, the fisetin-induced attenuation of TNFα production in microglia and macrophages in the brain can still be observed when fisetin is applied 3 hours after ischemia. At this time point, inflammatory mechanisms are gaining momentum (Gelderblom et al, 2009), further indicating that antiinflammatory properties of fisetin have a role in this setting.
The mechanisms through which fisetin mediates its antiinflammatory effects can be linked to the suppression of both proinflammatory intracellular pathways, AP-1 and nuclear factor (NF)-κB. These intracellular pathways control the expression of almost all genes encoding proinflammatory cytokines (Dinarello, 2010). The AP-1 pathway uses primarily phosphorylation of c-Jun by JNK for signaling into the nucleus. Nuclear factor-κB on the other hand is associated with the inhibitory protein IκB. Following treatment of cells with proinflammatory stimuli, IκB is phosphorylated and subsequently degraded. The free NF-κB translocates to the nucleus where it can initiate transcription of a diverse array of target genes including cytokines. In our experiments, this dual mode of inhibiting both AP-1 and NF-κB signaling can explain the potent inhibition of LPS-induced TNFα production in mouse and human phagocytes and murine N9 microglial cells. Earlier studies have already reported that fisetin inhibits NF-κB activation on LPS treatment in microglial cells (Zheng et al, 2008). However, whereas fisetin has been shown to reduce phosphorylation of JNK and c-Jun in a prostate cancer cell line (Chien et al, 2010), to our knowledge the efficacy of fisetin to block JNK and c-Jun phosphorylation on inflammatory activation in phagocytes has not been reported before. One can speculate, that this mode of action explains the observed lack of effect on the peripheral immune compartment, since the TNFα expression of spleen-derived macrophages was unaltered by treatment.
Although our data on the mechanism of fisetin effects are highly suggestive, they are based on association and the inhibition of other proinflammatory cascades might as well be involved in the fisetin-mediated protection from cerebral ischemia. Fisetin decreases PGE-2 production, downregulates cyclooxygenase-2, interleukin-1β as well as iNOS expression and NO production on LPS stimulation of BV2 microglial cells and primary microglia (Zheng et al, 2008). We could also show that fisetin is effective in suppressing the LPS-induced iNOS expression and NO production in N9 microglia and Raw264.7 macrophages. Especially, iNOS and cyclooxygenase-2 are additional interesting proinflammatory targets of fisetin as inhibition of cyclooxygenase-2 after experimental stroke in rats results in smaller infarcts and preserved blood–brain barrier (Candelario-Jalil et al, 2007) and the role of iNOS in exacerbating postischemic tissue damage is generally acknowledged (Willmot et al, 2005).
For some of the discussed effects, fisetin has to be available in the central nervous system. Fisetin injected intraperitoneally is quickly glucuronidated and sulfated (Maher, 2009) but biologically active metabolites are detected systemically for >24 hours. Fisetin also crosses the blood–brain barrier and its concentration in the central nervous system peaks 2 hours after injection (Rivera et al, 2004). Off-target effects that can contribute to toxicity are certainly a concern for any potential drug. However, in the case of fisetin, we have no evidence for either short- or long-term toxicity. Mice fed with fisetin at 500 p.p.m. for several months showed no indications of toxicity (Maher et al, 2011).
The concept that antiinflammatory treatments in stroke are beneficial has received more attention over the last few years (reviewed in Iadecola and Anrather, 2011). For example, recent studies have highlighted the beneficial effects on infarct size by inhibition of microglial and macrophage activation using the immunosuppressant FK506 (also known as tacrolimus) or intrathecal steroids (Goericke et al, 2010; Brecht et al, 2009). Similar observations have also been made for other antiinflammatory drugs such as fingolimod (Hasegawa et al, 2010). However, these treatments have the potential drawback of aggravating the systemic immunodepression that is observed after stroke (Klehmet et al, 2009). Unlike these compounds, fisetin seems to have no overt systemic immunosuppressive side effects, as we found no difference in systemic TNFα expression. It rather appears to act specifically under inflammatory conditions like LPS stimulation or stroke.
Taken together, we and others have shown that fisetin has multiple beneficial actions that reduce ischemia-induced brain damage. Ischemic stroke is increasingly recognized as the multiphasic process hypothesized more than a decade ago and is initially composed of necrosis and apoptosis evolving into an immune-mediated process (Dirnagl et al, 1999), leading to further lesion growth. Compounds that modulate more than one of these aspects could provide a very promising approach. Fisetin combines several of these properties without inducing systemic immune suppression. Thus, the experimental evidence supports the idea that fisetin may represent a good starting point for the development of future therapeutic substances for ischemic stroke.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by AHA Grant 075514Y to PM, Landesexzellenzinitiative (LEXI) Hamburg and DFG HO 3312/1-1 to TM.
Supplementary Material
References
- Brecht S, Waetzig V, Hidding U, Hanisch UK, Walther M, Herdegen T, Neiss WF. FK506 protects against various immune responses and secondary degeneration following cerebral ischemia. Anat Rec (Hoboken) 2009;292:1993–2001. doi: 10.1002/ar.20994. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Gonzalez-Falcon A, Garcia-Cabrera M, Leon OS, Fiebich BL. Post-ischaemic treatment with the cyclooxygenase-2 inhibitor nimesulide reduces blood-brain barrier disruption and leukocyte infiltration following transient focal cerebral ischaemia in rats. J Neurochem. 2007;100:1108–1120. doi: 10.1111/j.1471-4159.2006.04280.x. [DOI] [PubMed] [Google Scholar]
- Chien CS, Shen KH, Huang JS, Ko SC, Shih YW. Antimetastatic potential of fisetin involves inactivation of the PI3K/Akt and JNK signaling pathways with downregulation of MMP-2/9 expressions in prostate cancer PC-3 cells. Mol Cell Biochem. 2010;333:169–180. doi: 10.1007/s11010-009-0217-z. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Anti-inflammatory agents: present and future. Cell. 2010;140:935–950. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E, Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- Goericke SL, Engelhorn T, Forsting M, Speck U, Maderwald S, Ladd ME, Doerfler A. Intrathecal corticoids in permanent focal cerebral ischemia in rats. Part i: a new therapeutic approach in the acute phase. J Cereb Blood Flow Metab. 2010;30:801–807. doi: 10.1038/jcbfm.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen R, Lambertsen K, Finsen B. Microglia and macrophages are the major source of tumor necrosis factor in permanent middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2000;20:53–65. doi: 10.1097/00004647-200001000-00009. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Suzuki H, Sozen T, Rolland W, Zhang JH. Activation of sphingosine 1-phosphate receptor-1 by fty720 is neuroprotective after ischemic stroke in rats. Stroke. 2010;41:368–374. doi: 10.1161/STROKEAHA.109.568899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug A, Dalpke A, Wieczorek N, Giese T, Lorenz A, Auffarth G, Liesz A, Veltkamp R. Infarct volume is a major determiner of post-stroke immune cell function and susceptibility to infection. Stroke. 2009;40:3226–3232. doi: 10.1161/STROKEAHA.109.557967. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:196–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klehmet J, Harms H, Richter M, Prass K, Volk HD, Dirnagl U, Meisel A, Meisel C. Stroke-induced immunodepression and post-stroke infections: lessons from the preventive antibacterial therapy in stroke trial. Neuroscience. 2009;158:1184–1193. doi: 10.1016/j.neuroscience.2008.07.044. [DOI] [PubMed] [Google Scholar]
- Liu M, Eguchi N, Yamasaki Y, Urade Y, Hattori N, Urabe T. Focal cerebral ischemia/reperfusion injury in mice induces hematopoietic prostaglandin d synthase in microglia and macrophages. Neuroscience. 2007;145:520–529. doi: 10.1016/j.neuroscience.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Lyu SY, Park WB. Production of cytokine and NO by RAW 264.7 macrophages and PBMC in vitro incubation with flavonoids. Arch Pharm Res. 2005;28:573–581. doi: 10.1007/BF02977761. [DOI] [PubMed] [Google Scholar]
- Macrez R, Ali C, Toutirais O, Le Mauff B, Defer G, Dirnagl U, Vivien D.2011Stroke and the immune system: from pathophysiology to new therapeutic strategies Lancet Neurol 10471–480.Review [DOI] [PubMed] [Google Scholar]
- Maher P. Modulation of multiple pathways involved in the maintenance of neuronal function during aging by fisetin. Genes Nutr. 2009;4:297–307. doi: 10.1007/s12263-009-0142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P, Dargusch R, Bodai L, Gerard PE, Purcell JM, Marsh JL. ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington's disease. Hum Mol Genet. 2011;20:261–270. doi: 10.1093/hmg/ddq460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P, Salgado KF, Zivin JA, Lapchak PA. A novel approach to screening for new neuroprotective compounds for the treatment of stroke. Brain Res. 2007;1173:117–125. doi: 10.1016/j.brainres.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera F, Urbanavicius J, Gervaz E, Dajas F. Some aspects of the in vivo neuroprotective capacity of flavonoids: bioavailability and structure-activity relationship. Neurotox Res. 2004;6:543–553. doi: 10.1007/BF03033450. [DOI] [PubMed] [Google Scholar]
- Tuttolomondo A, Di Sciacca R, Di Raimondo D, Renda C, Pinto A, Licata G. Inflammation as a therapeutic target in acute ischemic stroke treatment. Curr Top Med Chem. 2009;9:1240–1260. doi: 10.2174/156802609789869619. [DOI] [PubMed] [Google Scholar]
- World Health Organization The top 10 causes of death. WHO media centre. Factsheet N°310. Updated June 2011 2011.
- Willmot M, Gibson C, Gray L, Murphy S, Bath P. Nitric oxide synthase inhibitors in experimental ischemic stroke and their effects on infarct size and cerebral blood flow: a systematic review. Free Radic Biol Med. 2005;39:412–425. doi: 10.1016/j.freeradbiomed.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- Zheng LT, Ock J, Kwon B-M, Suk K. Suppressive effects of flavonoid fisetin on lipopolysaccharide-induced microglial activation and neurotoxicity. Int Immunopharmacol. 2008;8:484–494. doi: 10.1016/j.intimp.2007.12.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.