Abstract
Limited evidence exists on the relationships between severity of white-matter lesions (WMLs) and cerebral hemodynamics in patients without major cerebral artery disease. To examine changes of cerebral blood flow (CBF), oxygen metabolism, and vascular reserve capacity associated with severity of WML in patients with lacunar stroke, we used a positron emission tomography (PET). Eighteen lacunar patients were divided into two groups according to the severity of WMLs, assessed by Fazekas classification; grades 0 to 1 as mild WML group and grades 2 to 3 as severe WML group. Rapid dual autoradiography was performed with 15O-labeled gas-PET followed by 15O-labeled water-PET with acetazolamide (ACZ) challenge. Compared with the mild WML group, the severe WML group showed lower CBF (20.6±4.4 versus 29.9±8.2 mL/100 g per minute, P=0.008), higher oxygen extraction fraction (OEF) (55.2±7.4 versus 46.7±5.3%, P=0.013), and lower cerebral metabolic rate of oxygen (CMRO2) (1.95±0.41 versus 2.44±0.42 mL/100 g per minute, P=0.025) in the centrum semiovale. There were no significant differences in the ACZ reactivity between the two groups (48.6±22.6% versus 42.5±17.2%, P=0.524). Lacunar patients with severe WMLs exhibited reduced CBF and CMRO2, and increased OEF in the centrum semiovale. The ACZ reactivity was preserved in both patients with severe and mild WMLs in each site of the brain.
Keywords: acetazolamide challenge, centrum semiovale, cerebrovascular reactivity, ischemic stroke, leukoaraiosis
Introduction
White-matter lesions (WMLs), observed as white-mater hyperintensity in T2-weighted magnetic resonance imaging or fluid-attenuated inversion recovery (FLAIR) image, are commonly observed among elderly people (Hachinski et al, 1987). However, they are also associated with hypertension, diabetes, and other vascular risk factors (Murray et al, 2005; Pantoni and Garcia, 1997). Development of WMLs is known to be a cause of cognitive impairment, dementia, and disability (Prins et al, 2005). Recent studies showed that WMLs are not only a stroke risk factor (Streifler et al, 2002) but also a predictor of unfavorable stroke outcome (Koton et al, 2009). Despite accumulating evidence of the clinical significance of WMLs, the pathogenesis of WMLs has not been fully clarified.
Healthy elderly subjects with severe WMLs were reported to have reduced cerebral blood flow (CBF) and preservation of oxygen metabolism (Meguro et al, 1990). Patients with dementia of the Binswanger type have marked decrease of both CBF and oxygen metabolism in the white matter; however, patients without dementia have a lesser decrease in CBF with preservation of almost-normal oxygen metabolism (Yao et al, 1992). These findings indicated that chronic hypoperfusion due to the progression of small artery disease is associated with the development of WMLs. In addition, hemodynamic disturbance induced by internal carotid artery occlusive disease was suggested to contribute to the development of extensive WMLs (Yamauchi et al, 1999).
Limited evidence exists on the relationships between severity of WMLs and hemodynamic disturbance in patients without major cerebral artery occlusive disease. Some studies showed that vascular reactivity was not related to severity of WMLs (Birns et al, 2009; Turc et al, 1994). Other studies reported that vascular reactivity in patients with severe WMLs is impaired (Bakker et al, 1999; Chabriat et al, 2000; Fu et al, 2006; Isaka et al, 1994; Kozera et al, 2010; Mochizuki et al, 1997). These inconsistencies may be due to differences in modalities for evaluation of vascular reserve capacity; i.e., transcranial Doppler ultrasound (Bakker et al, 1999; Birns et al, 2009; Fu et al, 2006; Kozera et al, 2010), perfusion MRI (Chabriat et al, 2000), xenon inhalation computed tomography (Isaka et al, 1994; Mochizuki et al, 1997), and single photon emission computed tomography (Turc et al, 1994). There are also differences in the vasodilatory stimulus used; i.e., CO2 inhalation (Bakker et al, 1999), breath holding, hyperventilation tests (Birns et al, 2009; Kozera et al, 2010), and acetazolamide (ACZ) challenge test (Chabriat et al, 2000; Fu et al, 2006; Isaka et al, 1994; Mochizuki et al, 1997; Turc et al, 1994). Although single photon emission computed tomography study with ACZ challenge can detect stage II hemodynamic failure (Powers, 1991) by positron emission tomography (PET) in patients with major cerebral artery occlusive disease (Hirano et al, 1994), the relationship between ACZ reactivity and oxygen metabolism in patients with WMLs without major artery disease has not been elucidated. We hypothesized that either impairment of vascular reserve capacity or chronic hypoperfusion in the white matter contributes to the development of WMLs without major artery disease.
The aim of this study was to examine the changes of CBF, oxygen metabolism, and vascular reserve capacity associated with the severity of WMLs in patients with lacunar stroke.
Materials and methods
Patients
This study was a single-center hospital-based prospective study. The study protocol was governed by the guidelines of national government based on the Helsinki Declaration revised in 1983, and it was approved by the Institutional Research and Ethics Committee of our hospital. All patients gave written informed consent to participate in the study. Patients with lacunar stroke, at least 3 weeks after the onset, were enrolled between April 2009 and April 2010. All patients underwent PET studies with 15O-labeled gas (C15O2, 15O2, C15O) inhalation and 15O-water with ACZ challenge autoradiography as described previously (Kudomi et al, 2005, 2007), as well as MRI studies. Lacunar stroke was defined as a typical clinical syndrome associated with a small infarct, <15 mm in diameter on MRI, restricted to the territory of a perforating artery without adjacent major artery occlusive lesions. Patients with stenosis (>50% in diameter) or occlusion of the internal carotid artery or the trunk of the middle cerebral artery on magnetic resonance angiography or ultrasonography were excluded from the study. The median time interval between the onset of stroke and PET studies was 1,017 days (interquatile range 519 to 1,856).
Baseline clinical characteristics including age, sex, hypertension, diabetes mellitus, dyslipidemia, and current smoking were recorded. Information of risk factors and medical history was collected from a self-reported medical history or inferred from prescribed medication by the primary physicians. Criteria for hypertension, diabetes mellitus, and dyslipidemia were as previously defined (Yokota et al, 2009). Cognitive function was evaluated in all patients by the mini-mental state examination (Folstein et al, 1975) and clinical dementia rating (Hughes et al, 1982). Dementia was defined as clinical dementia rating ≥1, and patients with dementia met the criteria proposed by National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA Alzheimer's Criteria) (Roman et al, 1993).
Magnetic Resonance Imaging
Magnetic resonance imaging was performed on a 1.5-T scanner (Magnetom Vision or Magnetom Sonata; Siemens Medical Systems, Erlangen, Germany). The imaging protocol consisted of a T1-weighted spin-echo, a T2-weighted spin-echo, and FLAIR image. Severity of WMLs was assessed using the FLAIR (repetition time 900 ms, echo time 119 ms, field-of-view 230 × 201 mm2, matrix 256 × 210, 4 mm slice thickness, and 2 mm gap between slices).
Two investigators (CY and TN), who were unaware of all clinical data, graded the degree of severity of WMLs by visual inspection using the Fazekas classification of WMLs as follows: none (grade 0), punctate (grade 1), early confluent (grade 2), and confluent lesions (grade 3) (Fazekas et al, 1987). The patients with grades 0 to 1 were defined as the mild WMLs group and those with grades 2 to 3 were defined as the severe WMLs group. Additionally, WMLs volume was measured manually based on FLAIR imaging (20 slices) using Dr View/LINUX software (AJS, Ver R2.5, Tokyo, Japan).
Positron Emission Tomography Imaging
We used an ECAT47 PET scanner (Siemens Medical Systems), which provided an intrinsic spatial resolution of 4.5 mm full-width at half-maximum at the center of the field-of-view. Data were acquired in 2D mode, and corrected for scatter compensation. A catheter was placed in the brachial artery for continuous monitoring of the arterial blood radioactivity concentration and arterial input function using a scintillator block detector system (BeCON; Molecular Imaging Labo, Suita, Japan) (Kudomi et al, 2003).
Quantitative images of CBF and oxygen extraction fraction (OEF) were obtained from a series of PET scans with 15O-labeled gas (C15O2, 15O2, and C15O) inhalation after a rapid dual autoradiography protocol as reported in a series of publications by Kudomi et al (2005, 2007). Briefly, after a 10-minute transmission scan for the attenuation correction and an 15O-labeled carbon monoxide (C15O) scan for the blood volume assessment, a single dynamic scan was performed for 8 minutes, during which 4,000 MBq of oxygen (15O2) and 5,000 MBq of 15O-labeled carbon dioxide (C15O2) gases were inhaled each >1 minute, sequentially at an interval of 5 minutes. Time to complete the whole dual autoradiography protocol was ∼40 minutes. Cerebral metabolic rate of oxygen (CMRO2) was calculated by multiplying the arterial oxygen content to the product images of OEF times CBF.
Additionally, two sets of PET scans were performed, each followed with 15O-labeled water injection to assess regional CBF images using 15O-water autoradiography (Kanno et al, 1987). The first scan was initiated without any pharmacological or physiological stress (at rest) and the second scan was performed at 10 minutes after an intravenous injection of ACZ titrated to 17 mg/kg. Physiological and laboratory data such as blood pressure, heart rate, and blood gas analysis (Siemens RAPIDLab 1265; Siemens Medical Systems) were obtained during the PET study.
Data Analysis
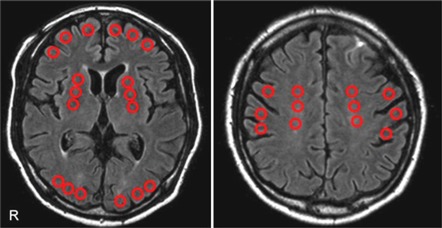
The small circular regions of interest (ROIs) (10 mm in diameter) were placed in the frontal cortex, parietal cortex, occipital cortex, basal ganglia, and centrum semiovale based on automatic registration of MRI to PET by using PVElab (the PVEOut Consortium) (Quarantelli et al, 2004; Svarer et al, 2005). The program is followed by automatic segmentation (running with Statistical Parametric Mapping 5 (SPM5) Software (Institute of Neurology, University College of London, London, UK) and correction of PET counts for fractional volume as determined from the segmentation. The ROIs were manually placed on the FLAIR images and transferred to the CBF images for analysis (Figure 1). We defined the ACZ reactivity as the percentage increase in CBF after ACZ administration relative to baseline CBF. In each subject, the mean measures were obtained by averaging the values for both hemispheres.
Figure 1.
Regions of interest (ROIs) on fluid-attenuated inversion recovery (FLAIR). The small circular ROIs (10 mm in diameter) were placed on the frontal cortex, parietal cortex, occipital cortex, basal ganglia, and the centrum semiovale based on FLAIR image.
Statistical Analysis
Statistical analysis was performed using JMP 7.0 software (SAS Institute, Cary, NC, USA). The statistical significance of intergroup differences was assessed by χ2 tests, unpaired t-tests, and the Mann–Whitney U-test, as appropriate. Logarithmic transformation was performed on WMLs volumes, which was a skewed variable. The relationship between each parameter of PET and log-WML was examined by Pearson's correlation. A value of P<0.05 was considered statistically significant.
Results
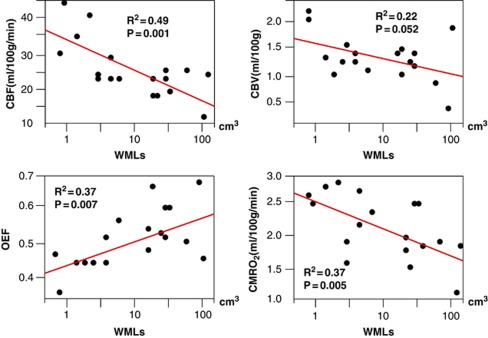
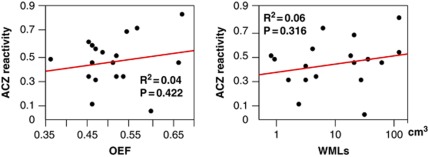
Patients were divided into two groups of severe WMLs (n=9) and mild WMLs (n=9) on the basis of MRI findings. There were no significant differences in age, sex, and vascular risk factors between the two groups (Table 1). Three patients with dementia defined as clinical dementia rating ≥1 were enrolled in the severe WMLs group; however, the rating of mini-mental state examination was not significantly different between the two groups. There were no significant differences in baseline CBF values between the gas-PET and H2O-PET results. Compared with patients in the mild WMLs group, the patients in the severe WMLs group had lower CBF (20.6±4.4 versus 29.9±8.2 mL/100 g per minute, P=0.008), higher OEF (55.2±7.4 versus 46.7±5.3%, P=0.013), and lower CMRO2 (1.95±0.41 versus 2.44±0.42 mL/100 g per minute, P=0.025) in the centrum semiovale, by gas-PET study (Table 2). There were no significant differences in any other parameters of the gas-PET in other ROIs between the two groups. Cerebral blood flow and CMRO2 had a negative correlation with the severity of WMLs, and OEF had a positive correlation with the severity of WMLs (Figure 2). There were no significant differences in ACZ reactivity between the severe and mild WMLs groups in each site of the brain by H2O-PET examination (Table 3). The results of physiological data and blood gas analysis during ACZ challenge were comparable between the two groups (data not shown). The ACZ reactivity was not correlated with the OEF or with the severity of WMLs (P=0.422 and P=0.316, respectively) (Figure 3).
Table 1. Baseline characteristics.
| Severe WMLs group (n=9) | Mild WMLs group (n=9) | P | |
|---|---|---|---|
| Age (years) | 76 (73–78) | 74 (70–77) | 0.329 |
| Male | 6 (67) | 8 (89) | 0.577 |
| Current smoker | 7 (78) | 7 (78) | 0.999 |
| Hypertension | 9 (100) | 8 (88) | 0.999 |
| Diabetes mellitus | 3 (33) | 3 (33) | 0.999 |
| Dyslipidemia | 6 (67) | 6 (67) | 0.999 |
| WMLs (cm3) | 33.3 (21.5–90.9) | 3.1 (1.3–4.4) | 0.003 |
| History of stroke | 3 (33) | 2 (22) | 0.999 |
| Time interval between stroke onset and PET study (days) | 953 (445–1,958) | 1,017 (519–1,623) | 0.847 |
| MMSE | 24.0 (20.5–28.5) | 28.0 (24.5–29.5) | 0.140 |
| CDR | 0.5 (0–1) | 0 (0–0.5) | 0.185 |
| Dementia | 3 (33) | 0 (0) | 0.206 |
WMLs, white-matter lesions; PET, positron emission tomography; MMSE, mini-mental state examination; CDR, clinical dementia rating.
Data are number of patients (%), median (interquartile range) for discontinuous variables.
Table 2. Comparison of each parameter of the gas-PET study between patients with severe or mild WMLs in the brain.
| Severe WMLs group (n=9) | Mild WMLs group (n=9) | P | |
|---|---|---|---|
| Frontal cortex | |||
| CBF (mL/100 g per minute) | 35.7±9.0 | 37.8±8.5 | 0.630 |
| CBV (mL/100 g) | 3.0±0.9 | 3.0±0.6 | 0.969 |
| OEF (%) | 54.1±14.7 | 48.3±5.2 | 0.275 |
| CMRO2 (mL/100 g per minute) | 3.24±0.49 | 3.26±0.73 | 0.946 |
| Parietal cortex | |||
| CBF | 40.2±6.9 | 44.1±11.6 | 0.403 |
| CBV | 2.8±0.7 | 3.1±0.5 | 0.284 |
| OEF | 50.6±6.9 | 46.3±4.9 | 0.146 |
| CMRO2 | 3.53±0.35 | 3.62±0.80 | 0.743 |
| Occipital cortex | |||
| CBF | 40.4±8.6 | 47.4±16.1 | 0.266 |
| CBV | 3.5±0.9 | 3.7±1.5 | 0.745 |
| OEF | 55.8±8.8 | 50.4±4.5 | 0.116 |
| CMRO2 | 3.88±0.63 | 4.22±1.16 | 0.442 |
| Basal ganglia | |||
| CBF | 45.1±9.4 | 49.5±13.1 | 0.426 |
| CBV | 2.3±0.7 | 2.5±0.5 | 0.521 |
| OEF | 52.8±7.9 | 50.5±6.3 | 0.505 |
| CMRO2 | 4.14±0.66 | 4.43±0.90 | 0.441 |
| Centrum semiovale | |||
| CBF | 20.6±4.4 | 29.9±8.2 | 0.008 |
| CBV | 1.2±0.4 | 1.4±0.3 | 0.217 |
| OEF | 55.2±7.4 | 46.7±5.3 | 0.013 |
| CMRO2 | 1.95±0.41 | 2.44±0.42 | 0.025 |
CBF, cerebral blood flow; CBV, cerebral blood volume; CMRO2, cerebral metabolic rate of oxygen; OEF, oxygen extraction fraction; PET, positron emission tomography; WMLs, white-matter lesions.
P value by Mann–Whitney U-test.
Figure 2.
Correlation between WML volume and each gas-PET parameter in the centrum semiovale. CBF and CMRO2 had a negative correlation with the severity of WMLs, while OEF was positively correlated with the severity of WMLs. CBF, cerebral blood flow; CBV, cerebral blood volume; OEF, oxygen extraction fraction; CMRO2, cerebral metabolic rate of oxygen; WMLs, white-matter lesions; PET, positron emission tomography.
Table 3. Comparison of CBF between patients with severe or mild WMLs in the brain by H2O-PET.
| Severe WMLs group (n=9) | Mild WMLs group (n=9) | P | |
|---|---|---|---|
| Frontal cortex | |||
| CBF baseline | 36.1±7.2 | 40.2±7.3 | 0.244 |
| CBF ACZ | 58.5±10.2 | 59.9±10.3 | 0.770 |
| ACZ reactivity (%) | 64.6±28.5 | 49.7±14.9 | 0.183 |
| Parietal cortex | |||
| CBF baseline | 39.7±4.8 | 45.7±10.5 | 0.136 |
| CBF ACZ | 62.0±7.1 | 66.9±14.6 | 0.387 |
| ACZ reactivity (%) | 57.2±17.1 | 47.1±13.5 | 0.181 |
| Occipital cortex | |||
| CBF baseline | 38.1±7.1 | 45.7±11.5 | 0.109 |
| CBF ACZ | 61.7±13.3 | 70.1±17.0 | 0.259 |
| ACZ reactivity (%) | 62.2±21.5 | 54.2±16.6 | 0.392 |
| Basal ganglia | |||
| CBF baseline | 47.1±9.8 | 54.6±11.3 | 0.148 |
| CBF ACZ | 73.7±10.5 | 85.7±24.6 | 0.200 |
| ACZ reactivity (%) | 60.9±31.0 | 55.7±22.9 | 0.694 |
| Centrum semiovale | |||
| CBF baseline | 19.0±4.1 | 29.8±9.2 | 0.005 |
| CBF ACZ | 28.5±5.9 | 41.8±10.9 | 0.005 |
| ACZ reactivity (%) | 48.6±22.6 | 42.5±17.2 | 0.524 |
ACZ, acetazolamide; CBF, cerebral blood flow; PET, positron emission tomography; WMLs, white-matter lesions.
P value by Mann–Whitney U-test.
Figure 3.
The correlation between ACZ reactivity and OEF or WML volume in the centrum semiovale. Neither OEF nor WML volume was correlated with ACZ reactivity. ACZ, acetazolamide; OEF, oxygen extraction fraction; WMLs, white-matter lesions.
Discussion
This study showed reduced CBF, reduced CMRO2, and increased OEF in patients with severe WMLs compared with those with mild WMLs in the centrum semiovale. All patients in this study had lacunar stroke without major cerebral artery disease. The study also showed that ACZ reactivity was not impaired in either the cortex or the white matter of the patients of both groups.
Hatazawa et al (1997) found asymptomatic WMLs subjects exhibited reduction of CBF in the white matter and basal ganglia without decrease in CMRO2. They also observed an increase in OEF in these areas, suggesting a chronic hypoperfusion in these territories. The present study provided additional information of reduction of both CBF and CMRO2 with an increase in OEF in the WML in the patient groups with severe WMLs. Centrum semiovale is located at the border of an area supplied by deep perforating arteries and the terminal branches of the middle cerebral artery. A decrease in CBF with reduction of CMRO2 in the centrum semiovale in the present study should indicate a consequence of a reduced tissue metabolism in this terminal zone.
In the present study, patients with severe WMLs without major artery disease had increased OEF showed by gas-PET; however, their ACZ reactivity by H2O-PET was preserved. The vascular reserve capacity evaluated by ACZ reactivity was preserved in both patients with severe and mild WMLs. Reduction of both CBF and CMRO2 in the white matter was previously shown in patients with the Binswanger type dementia (Yao et al, 1990), being consistent with our results. Postmortem neuropathologic studies have shown decreased neuronal connectivity in the white matter in progressive subcortical vascular encephalopathy of Binswanger type (Yamanouchi et al, 1989, 1990). Functional reduction in cortical neuronal activity due to disruption of connections between the cortex and subcortex, as indicated previously (Pozzilli et al, 1987; Sette et al, 1989), is likely to be associated with a reduction of CMRO2 in the centrum semiovale in the patients with severe WMLs. Furthermore, the cerebral vessels would not dilate during fluctuations in systemic arterial pressure in daily life in these conditions of disruption of connections. Chronic hypoperfusion with a reduction of CMRO2 in accordance with a disconnection between the cortex and subcortex may be the cause of development of WMLs without major artery disease.
To our knowledge, this is the first report of alterations in CBF, CMRO2, and OEF, with preservation of ACZ reactivity in patients with mild or severe WMLs, with careful consideration of possible methodological errors. Indeed, quantitation of physiological parameters using PET is still a challenging issue, particularly in the white-matter area. As shown in earlier studies (Herscovitch and Raichle, 1983; Huang et al, 1987), the absolute values of both CBF and CMRO2 could be biased because the spatial resolution of PET devices is limited compared with the physical size of the brain tissue component, or the partial volume effects. Oxygen extraction fraction is relatively stable and is less affected by partial volume effects. Our observation of increased OEF could not be explained by partial volume effects alone. Scatter is smaller in 2D mode in PET as compared with 3D acquisition. In this study, scatter correction was applied to minimize the contribution of radioactivity from the surrounding tissue components due to scatter. The ROIs were placed carefully with a guide of anatomical MRI to minimize the errors arising from radioactivity counts of surrounding tissues. These factors remain concerns to be dealt with in future investigations.
There are several issues that need to be addressed, as follows. First, we intended to avoid possible bias in the patient selection, but a relatively small number of subjects could cause selection bias despite our efforts. Second, three patients with dementia were enrolled in the severe WMLs group. Because oxygen metabolism in demented patients was reported to be different from that in non-demented patients (Yao et al, 1992), a reduced CMRO2 with reduced CBF in the severe WMLs group could be attributed to secondary effects arising from decreased cognitive function. Third, we examined the vascular reserve capacity by ACZ challenge. Recently, ACZ-induced vasodilation was reported not to inhibit the visually evoked flow response (Yonai et al, 2010), which indicates that the vasodilatory mechanism during neurovascular coupling may be different from the mechanism of ACZ-induced vasodilation. Acetazolamide at a dose of 17 mg/kg would not cause maximal cerebral vasodilatation. However, there were no significant differences in ACZ reactivity between the two groups, and ACZ reactivity was preserved in all patients in the present study. Fourth, PET imaging in the present study was a single scatter subtraction technique based on the Klein–Nishina formulation which was implemented in the reconstruction software (Watson, 2000). This technique was shown to provide reasonable accuracy in several phantom experiments. It should also be noted that the data were acquired in 2D mode, which has much smaller amount of scatter as compared with recently available 3D mode. Further, the filtered-back projection technique was applied for the image reconstruction. In this procedure, the scatter contribution is likely reduced in the reconstructed images. However, limited spatial resolution of PET devices is a significant source of errors that causes possible contamination of radioactivity counts of cortical grey matter tissue. Exact magnitude of errors in the calculated parameters in the WML cannot be well defined. In addition, PET scanning in the present study has not been applied to age-matched normal subjects. Further systematic study is needed.
In conclusion, we showed that there is reduced CBF and CMRO2, and increased OEF in the centrum semiovale of patients with severe WMLs compared with patients with mild WMLs. The ACZ reactivity was preserved in both patients with severe and mild WMLs. Further studies will be needed to clarify the pathogenesis of WMLs.
The authors declare no conflict of interest.
Footnotes
This study was supported in part by Research Grants for Cardiovascular Diseases (22-4-1) from the Ministry of Health, Labor, and Welfare of Japan; a Grant for Translational Research from the Ministry of Health, Labor, and Welfare of Japan; a Grant for Nano Medicine from the Ministry of Health, Labor, and Welfare of Japan; and a Grant-in-aid for Scientific Research from the Japan Society for the Promotion of Science.
References
- Bakker SL, de Leeuw FE, de Groot JC, Hofman A, Koudstaal PJ, Breteler MM. Cerebral vasomotor reactivity and cerebral white matter lesions in the elderly. Neurology. 1999;52:578–583. doi: 10.1212/wnl.52.3.578. [DOI] [PubMed] [Google Scholar]
- Birns J, Jarosz J, Markus HS, Kalra L. Cerebrovascular reactivity and dynamic autoregulation in ischaemic subcortical white matter disease. J Neurol Neurosurg Psychiatry. 2009;80:1093–1098. doi: 10.1136/jnnp.2009.174607. [DOI] [PubMed] [Google Scholar]
- Chabriat H, Pappata S, Ostergaard L, Clark CA, Pachot-Clouard M, Vahedi K, Jobert A, Le Bihan D, Bousser MG. Cerebral hemodynamics in CADASIL before and after acetazolamide challenge assessed with MRI bolus tracking. Stroke. 2000;31:1904–1912. doi: 10.1161/01.str.31.8.1904. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state'. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fu JH, Lu CZ, Hong Z, Dong Q, Ding D, Wong KS. Relationship between cerebral vasomotor reactivity and white matter lesions in elderly subjects without large artery occlusive disease. J Neuroimaging. 2006;16:120–125. doi: 10.1111/j.1552-6569.2006.00030.x. [DOI] [PubMed] [Google Scholar]
- Hachinski VC, Potter P, Merskey H. Leuko-araiosis. Arch Neurol. 1987;44:21–23. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- Hatazawa J, Shimosegawa E, Satoh T, Toyoshima H, Okudera T. Subcortical hypoperfusion associated with asymptomatic white matter lesions on magnetic resonance imaging. Stroke. 1997;28:1944–1947. doi: 10.1161/01.str.28.10.1944. [DOI] [PubMed] [Google Scholar]
- Herscovitch P, Raichle ME. Effect of tissue heterogeneity on the measurement of cerebral blood flow with the equilibrium C15O2 inhalation technique. J Cereb Blood Flow Metab. 1983;3:407–415. doi: 10.1038/jcbfm.1983.66. [DOI] [PubMed] [Google Scholar]
- Hirano T, Minematsu K, Hasegawa Y, Tanaka Y, Hayashida K, Yamaguchi T. Acetazolamide reactivity on 123I-IMP single photon emission computed tomography in patients with major cerebral artery occlusive disease: correlation with positron emission tomography parameters. J Cereb Blood Flow Metab. 1994;14:763–770. doi: 10.1038/jcbfm.1994.97. [DOI] [PubMed] [Google Scholar]
- Huang SC, Mahoney DK, Phelps ME. Quantitation in positron emission tomography: 8. Effects of nonlinear parameter estimation on functional images. J Comput Assist Tomogr. 1987;11:314–325. [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Isaka Y, Okamoto M, Ashida K, Imaizumi M. Decreased cerebrovascular dilatory capacity in subjects with asymptomatic periventricular hyperintensities. Stroke. 1994;25:375–381. doi: 10.1161/01.str.25.2.375. [DOI] [PubMed] [Google Scholar]
- Kanno I, Iida H, Miura S, Murakami M, Takahashi K, Sasaki H, Inugami A, Shishido F, Uemura K. A system for cerebral blood flow measurement using an H215O autoradiographic method and positron emission tomography. J Cereb Blood Flow Metab. 1987;7:143–153. doi: 10.1038/jcbfm.1987.37. [DOI] [PubMed] [Google Scholar]
- Koton S, Schwammenthal Y, Merzeliak O, Philips T, Tsabari R, Orion D, Dichtiar R, Tanne D. Cerebral leukoaraiosis in patients with stroke or TIA: clinical correlates and 1-year outcome. Eur J Neurol. 2009;16:218–225. doi: 10.1111/j.1468-1331.2008.02389.x. [DOI] [PubMed] [Google Scholar]
- Kozera GM, Dubaniewicz M, Zdrojewski T, Madej-Dmochowska A, Mielczarek M, Wojczal J, Chwojnicki K, Swierblewska E, Schminke U, Wyrzykowski B, Nyka WM. Cerebral vasomotor reactivity and extent of white matter lesions in middle-aged men with arterial hypertension: a pilot study. Am J Hypertens. 2010;23:1198–1203. doi: 10.1038/ajh.2010.152. [DOI] [PubMed] [Google Scholar]
- Kudomi N, Choi E, Yamamoto S, Watabe H, Kim K, Shidahara M, Ogawa M, Teramoto N, Sakamoto E, Iida H. Development of a GSO detector assembly for a continuous blood sampling system. IEEE Trans Nucl Sci. 2003;50:70–73. [Google Scholar]
- Kudomi N, Hayashi T, Teramoto N, Watabe H, Kawachi N, Ohta Y, Kim KM, Iida H. Rapid quantitative measurement of CMRO(2) and CBF by dual administration of (15)O-labeled oxygen and water during a single PET scan-a validation study and error analysis in anesthetized monkeys. J Cereb Blood Flow Metab. 2005;25:1209–1224. doi: 10.1038/sj.jcbfm.9600118. [DOI] [PubMed] [Google Scholar]
- Kudomi N, Watabe H, Hayashi T, Iida H. Separation of input function for rapid measurement of quantitative CMRO2 and CBF in a single PET scan with a dual tracer administration method. Phys Med Biol. 2007;52:1893–1908. doi: 10.1088/0031-9155/52/7/009. [DOI] [PubMed] [Google Scholar]
- Meguro K, Hatazawa J, Yamaguchi T, Itoh M, Matsuzawa T, Ono S, Miyazawa H, Hishinuma T, Yanai K, Sekita Y. Cerebral circulation and oxygen metabolism associated with subclinical periventricular hyperintensity as shown by magnetic resonance imaging. Ann Neurol. 1990;28:378–383. doi: 10.1002/ana.410280313. [DOI] [PubMed] [Google Scholar]
- Mochizuki Y, Oishi M, Takasu T. Cerebral blood flow in single and multiple lacunar infarctions. Stroke. 1997;28:1458–1460. doi: 10.1161/01.str.28.7.1458. [DOI] [PubMed] [Google Scholar]
- Murray AD, Staff RT, Shenkin SD, Deary IJ, Starr JM, Whalley LJ. Brain white matter hyperintensities: relative importance of vascular risk factors in nondemented elderly people. Radiology. 2005;237:251–257. doi: 10.1148/radiol.2371041496. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- Powers WJ. Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol. 1991;29:231–240. doi: 10.1002/ana.410290302. [DOI] [PubMed] [Google Scholar]
- Pozzilli C, Itoh M, Matsuzawa T, Fukuda H, Abe Y, Sato T, Takeda S, Ido T. Positron emission tomography in minor ischemic stroke using oxygen-15 steady-state technique. J Cereb Blood Flow Metab. 1987;7:137–142. doi: 10.1038/jcbfm.1987.36. [DOI] [PubMed] [Google Scholar]
- Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Jolles J, Koudstaal PJ, Hofman A, Breteler MM. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain. 2005;128:2034–2041. doi: 10.1093/brain/awh553. [DOI] [PubMed] [Google Scholar]
- Quarantelli M, Berkouk K, Prinster A, Landeau B, Svarer C, Balkay L, Alfano B, Brunetti A, Baron JC, Salvatore M. Integrated software for the analysis of brain PET/SPECT studies with partial-volume-effect correction. J Nucl Med. 2004;45:192–201. [PubMed] [Google Scholar]
- Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A, Moody DM, O'Brien MD, Yamaguchi T, Grafman J, Drayer BP, Bennett DA, Fisher M, Ogata J, Kokmen E, Bermejo F, Wolf PA, Gorelick PB, Bick KL, Pajeau AK, Bell MA, DeCarli C, Culebras A, Korczyn AD, Bogousslavsky J, Hartmann A, Scheinberg P. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- Sette G, Baron JC, Mazoyer B, Levasseur M, Pappata S, Crouzel C. Local brain haemodynamics and oxygen metabolism in cerebrovascular disease. Positron emission tomography. Brain. 1989;112 (Pt 4:931–951. doi: 10.1093/brain/112.4.931. [DOI] [PubMed] [Google Scholar]
- Streifler JY, Eliasziw M, Benavente OR, Alamowitch S, Fox AJ, Hachinski VC, Barnett HJ. Prognostic importance of leukoaraiosis in patients with symptomatic internal carotid artery stenosis. Stroke. 2002;33:1651–1655. doi: 10.1161/01.str.0000018010.38749.08. [DOI] [PubMed] [Google Scholar]
- Svarer C, Madsen K, Hasselbalch SG, Pinborg LH, Haugbol S, Frokjaer VG, Holm S, Paulson OB, Knudsen GM. MR-based automatic delineation of volumes of interest in human brain PET images using probability maps. Neuroimage. 2005;24:969–979. doi: 10.1016/j.neuroimage.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Turc JD, Chollet F, Berry I, Sabatini U, Démonet JF, Ceisis P, Marc-Vergnes JP, Rascol A. Cerebral blood flow, cerebral blood reactivity to acetazolamide, and cerebral blood volume in patients with leukoaraiosis. Cerebrovas Dis. 1994;4:287–293. [Google Scholar]
- Watson CC. New, faster, image-based scatter correction for 3D PET. IEEE Trans Nucl Sci. 2000;47:1587–1594. [Google Scholar]
- Yamanouchi H, Sugiura S, Shimada H. Loss of nerve fibres in the corpus callosum of progressive subcortical vascular encephalopathy. J Neurol. 1990;237:39–41. doi: 10.1007/BF00319666. [DOI] [PubMed] [Google Scholar]
- Yamanouchi H, Sugiura S, Tomonaga M. Decrease in nerve fibres in cerebral white matter in progressive subcortical vascular encephalopathy of Binswanger type. An electron microscopic study. J Neurol. 1989;236:382–387. doi: 10.1007/BF00314894. [DOI] [PubMed] [Google Scholar]
- Yamauchi H, Fukuyama H, Nagahama Y, Shiozaki T, Nishizawa S, Konishi J, Shio H, Kimura J. Brain arteriolosclerosis and hemodynamic disturbance may induce leukoaraiosis. Neurology. 1999;53:1833–1838. doi: 10.1212/wnl.53.8.1833. [DOI] [PubMed] [Google Scholar]
- Yao H, Sadoshima S, Ibayashi S, Kuwabara Y, Ichiya Y, Fujishima M. Leukoaraiosis and dementia in hypertensive patients. Stroke. 1992;23:1673–1677. doi: 10.1161/01.str.23.11.1673. [DOI] [PubMed] [Google Scholar]
- Yao H, Sadoshima S, Kuwabara Y, Ichiya Y, Fujishima M. Cerebral blood flow and oxygen metabolism in patients with vascular dementia of the Binswanger type. Stroke. 1990;21:1694–1699. doi: 10.1161/01.str.21.12.1694. [DOI] [PubMed] [Google Scholar]
- Yokota C, Minematsu K, Ito A, Toyoda K, Nagasawa H, Yamaguchi T. Albuminuria, but not metabolic syndrome, is a significant predictor of stroke recurrence in ischemic stroke. J Neurol Sci. 2009;277:50–53. doi: 10.1016/j.jns.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Yonai Y, Boms N, Molnar S, Rosengarten B, Bornstein NM, Csiba L, Olah L. Acetazolamide-induced vasodilation does not inhibit the visually evoked flow response. J Cereb Blood Flow Metab. 2010;30:516–521. doi: 10.1038/jcbfm.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]