Abstract
Activation of the basal forebrain (BF), the primary source of acetylcholine (ACh) in the cortex, broadly increases cortical cerebral blood flow (CBF), a response downstream to ACh release. Although endothelial nitric oxide and cholinoceptive GABA (γ-aminobutyric acid) interneurons have been implicated, little is known about the role of pyramidal cells in this response and their possible interaction with astrocytes. Using c-Fos immunohistochemistry as a marker of neuronal activation and laser-Doppler flowmetry, we measured changes in CBF evoked by BF stimulation following pharmacological blockade of c-Fos-identified excitatory pathways, astroglial metabolism, or vasoactive mediators. Pyramidal cells including those that express cyclooxygenase-2 (COX-2) displayed c-Fos upregulation. Glutamate acting via NMDA, AMPA, and mGlu receptors was involved in the evoked CBF response, NMDA receptors having the highest contribution (∼33%). In contrast, nonselective and selective COX-2 inhibition did not affect the evoked CBF response (+0.4% to 6.9%, ns). The metabolic gliotoxins fluorocitrate and fluoroacetate, the cytochrome P450 epoxygenase inhibitor MS-PPOH and the selective epoxyeicosatrienoic acids (EETs) antagonist 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE) all blocked the evoked CBF response by ∼50%. Together, the data demonstrate that the hyperemic response to BF stimulation is largely mediated by glutamate released from activated pyramidal cells and by vasoactive EETs, likely originating from activated astrocytes.
Keywords: arachidonic acid, GABA, glutamate, neurovascular coupling
Introduction
Stimulus-induced changes in cerebral blood flow (CBF) underlie the basis of functional brain imaging techniques such as positron emission tomography and functional magnetic resonance imaging that use hemodynamic signals to map changes in neuronal activity under physiological and pathological conditions. Although the cellular and molecular bases of this tight coupling are not yet fully elucidated, evidence suggests that the hemodynamic changes are driven by the incoming afferents and their local processing within the activated area (Lauritzen and Gold, 2003). Additional in vitro and in vivo investigations indicate that the coordinated actions of neuronal, glial, and vascular factors are required in mediating the vascular response to a physiological stimulus (Koehler et al, 2009).
Stimulation of the basal forebrain (BF), the primary cholinergic input to the neocortex arising from the substantia innominata-nucleus basalis complex, produces large increases in ipsilateral cortical CBF in the rat concomitant with the local release of acetylcholine (ACh) (for review, see Hamel, 2004). Cholinergic basalocortical afferents have a widerange of targets including excitatory pyramidal cells (Henny and Jones, 2008; Houser et al, 1985), inhibitory interneurons (Cauli et al, 2004), microvessels, and astrocytes (Vaucher and Hamel, 1995). In accordance with these anatomical findings, part of the CBF response elicited by BF stimulation has been attributed to a direct vasodilatory effect of ACh through muscarinic receptor (mAChR)-mediated endothelial, but not neuronal, NO (nitric oxide) release (Zhang et al, 1995). We accordingly found that the hemodynamic response to BF stimulation was virtually eliminated by selective cortical cholinergic deafferentation despite preserved NO synthase containing BF neurons and cortical projections. Further, we showed that the full expression of the CBF response requires GABA-A (γ-aminobutyric acid) receptor activation by specific subsets of cholinoceptive GABA interneurons that contain SOM (somatostatin) and NPY (neuropeptide Y) (Kocharyan et al, 2008). These findings correlated very well with the synchronized bursting induced in these GABA interneurons by mAChR activation (Beierlein et al, 2000), and with the cortical hemodynamic signals induced by γ oscillations—as elicited by activation of BF cholinergic afferents (Cape et al, 2000)—being initiated by the firing of inhibitory interneurons (Niessing et al, 2005).
Pyramidal cells are key players in the neurovascular coupling response to sensory stimulation that is driven by glutamatergic thalamocortical afferents. Pyramidal cells act mainly through the release of cyclooxygenase-2 (COX-2)-derived vasoactive prostanoids (Lecrux et al, 2011; Niwa et al, 2000) and that of glutamate. The latter is thought to induce calcium transients in astrocytes (Zonta et al, 2003), which results in the synthesis and release of potent vasoactive derivatives such as the epoxyeicosatrienoic acids (EETs) (Koehler et al, 2009). Pyramidal cells are also activated following stimulation of cholinergic basalocortical afferents (Kocharyan et al, 2008), but nothing is known regarding their role or that of glutamate or COX-2 products, or about the interplay between pyramidal cells, interneurons, and astrocyte-derived vasoactive messengers in this hyperemic response. In this study, we sought to identify the contribution of these cell types in the CBF response evoked by BF stimulation. Our results demonstrate that glutamate-releasing pyramidal cells and AA (arachidonic acid)-derived cytochrome P450 epoxygenase EETs, but not COX-1 or COX-2 products, mediate the bulk of this neurovascular coupling response.
Materials and methods
Procedures for BF stimulation, CBF measurements, and immunohistochemistry were similar to those detailed previously (Kocharyan et al, 2008; Lecrux et al, 2011). All experiments were approved by the McGill University animal ethics committee and conformed to the Canadian Council on Animal Care.
Animals, Surgical Procedures, and Basal Forebrain Stimulation
The experimental procedure was in two steps: first, chronic implantation of either an electrode or a guide cannula in the BF, and second, 1 week later, electrical or chemical BF stimulation for pharmacological or immunohistochemical studies. Male Sprague-Dawley rats (280 to 300 g; Charles River, Montréal, QC, Canada) were anesthetized with ketamine-xylazine (100/5 mg/kg, intraperitoneally) or isoflurane (2% in a 40%/60% oxygen/air mixture via a face mask) and placed in a stereotaxic frame (model 962; D Kopf Instruments, Tujunga, CA, USA) for chronic implantation of monopolar tungsten electrodes (0.35 mm outer diameter; FHC, Bowdoin, ME, USA) or guide cannulas (Plastics One, Roanoke, VA, USA) in the left substantia innominata (AP: −1.2 mm, lateral: +2.4 mm, ventral: −6.9 mm (electrodes) or −5.3 mm (guide cannulas) from Bregma). Body temperature was maintained at 37°C using a heating pad. One week later, BF electrical stimulation was performed under urethane anesthesia (1.1 g/kg, intraperitoneally) using parameters previously determined as optimal for immunohistochemistry (100 Hz, 80 μA, 0.5 milliseconds, 1 second on/1 second off for 5 minutes) or pharmacological experiments (100 Hz, 50 μA, 0.5 milliseconds, 1 second on/1 second off for 20 seconds) (isolated pulse stimulator; A-M Systems, Sequim, WA, USA) (Kocharyan et al, 2008). During pharmacological studies, a catheter was inserted in the femoral artery for blood pressure measurement (AD Instruments, Colorado Springs, CO, USA) and blood gas analysis (pH, pO2, and pCO2) (Bayer Rapid Lab 348, Siemens Healthcare Diagnostics Inc., Deerfield, IL, USA). All remained within normal range throughout the experiments (see Supplementary Table S1), which lasted at most 2.5 hours. For immunohistochemical studies, we additionally performed chemical BF stimulation by injecting 0.1 μL of sodium glutamate (100 nmol, n=3) or NMDA (N-methyl-D-aspartic acid) (10 nmol, n=3) (over 1 minute; Harvard Apparatus infusion pump, Holliston, MA, USA) using cannulas inserted in the BF, 1.6 mm below the guide cannulas (Kocharyan et al, 2008).
Pharmacological Experiments: Cerebral Blood Flow Measurements by Laser-Doppler Flowmetry
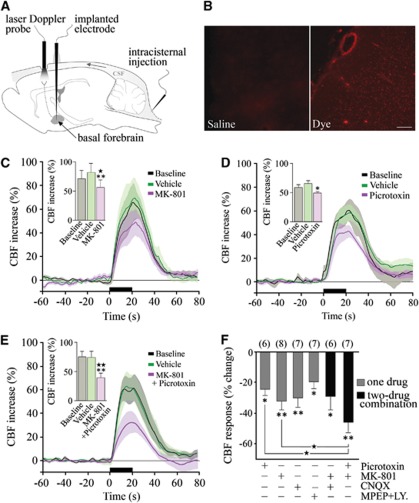
Cerebral blood flow increases induced by BF stimulation were measured by laser-Doppler flowmetry (Transonic Systems, Ithaca, NY, USA) with two needle-shaped probes positioned on the thinned bone overlying the ipsilateral and contralateral frontal cortices (∼4 mm2 anterior from the Bregma). Intracisternal injection (ic) of drugs or vehicles was performed under microscope monitoring using a 30-gauge needle connected by a polyethylene-10 catheter to a Hamilton microsyringe (3 μL over 1 minute; Harvard Apparatus infusion pump) (Figure 1A). After 15 to 20 minutes of CBF stabilization, CBF changes induced by a 20-second BF stimulation were measured at baseline and following vehicle or drug injection. Time courses of drug effects were determined, and the evoked CBF changes were taken at the time of maximal effect. Unless indicated otherwise, these times corresponded to 20 minutes after receptor antagonism or 40 to 50 minutes after enzyme inhibition. Each rat received only one compound or combination of compounds, and the corresponding vehicle. Diffusion of the ic-injected drugs over the cortex was validated on the evoked CBF response to whisker stimulation by the identical reducing effects of picrotoxin after ic injection or cortical superfusion through a closed cranial window (Lecrux et al, 2011). It was further confirmed in the present study in urethane-anesthetized rats injected with saline or 3% Evans blue (n=3/group) and euthanized by decapitation 20 or 60 minutes later. Their brains were cryostat cut (30 μm thick) and cortical diffusion of Evans blue visualized under a light microscope equipped with epifluorescence (Figure 1B).
Figure 1.
Glutamate and GABA (γ-aminobutyric acid) in the cerebral blood flow (CBF) response to basal forebrain (BF) stimulation. (A) Schematic illustration of our experimental set-up. (B) The efficiency of ic injection was confirmed by the distribution of Evans blue throughout the cortical mantle after a single 3 μL Intracisternal (ic) injection of the dye as compared with saline. Scale bar: 500 μm. Significant reductions in ipsilateral CBF responses to BF stimulation were observed after selective antagonism of NMDA (N-methyl-D-aspartic acid) (MK-801) (C) and GABA-A (picrotoxin) (D) receptors, or their combined blockade (E). Average CBF responses at baseline and after vehicle are illustrated. Histograms represent the average peak CBF responses under each condition (★P<0.05; ★★P<0.01 versus baseline; *P<0.05; **P<0.01 versus vehicle, repeated-measures analysis of variance (ANOVA)). (F) Average reductions in peak CBF responses induced by GABA-A and various glutamate receptor antagonists (*P<0.05; **P<0.01, compared with vehicle responses by repeated-measures ANOVA). Combined administration of MK-801 and picrotoxin reduced the evoked CBF response significantly more than either antagonist alone (★P<0.05, one-way ANOVA).
It is well documented that it is impossible to completely block CBF responses induced by increased neuronal activity even with high doses of receptor antagonists or enzyme inhibitors, or through combined blockade of several enzyme activities or receptors (Koehler et al, 2009; Leithner et al, 2010), likely because of overlap and interactions between several effector pathways. Therefore, we used the in vivo pharmacological approach to provide evidence for the implication of specific pathways in the evoked CBF response to BF stimulation rather than a strict quantitative analysis of their respective contribution.
Drug Preparation
Unless otherwise stated, 3 μL of a 10−4 mol/L buffered solution (pH 7.4) of each compound or combination of compounds, or their corresponding vehicle were injected. 6-Cyano-7-nitroquinoxaline-2,3(1H4H)-dione disodium salt (CNQX; vehicle: 0.5 mol/L phosphate-buffered saline (PBS)), MK-801 (vehicle: 0.5 mol/L PBS), and picrotoxin (vehicle: 0.5% ethanol in 0.5 mol/L PBS) were purchased from Tocris Biosciences (Minneapolis, MN, USA). Fluoroacetate sodium (vehicle: 0.5 mol/L PBS), fluorocitrate (vehicle: 0.5 mol/L PBS, 3 × 10−4 mol/L), indomethacin (vehicle: 0.2% ethanol in 0.5 mol/L PBS), MPEP (6-methyl-2-(phenylethynyl)pyridine; vehicle: 0.5 mol/L PBS), LY-367385 (vehicle: 10−3 mol/L NaOH in 0.5 mol/L PBS equilibrated to pH 7.4 with 1 N HCl), and scopolamine (vehicle: 0.5 mol/L PBS, 1 mg/kg, intravenous) were purchased from Sigma-Aldrich Canada Ltd (Oakville, ON, Canada). 14, 15-Epoxyeicosa-5(Z)-enoic acid (14,15-EEZE; vehicle: 0.25% ethanol in 0.5 mol/L PBS), N-(methylsulfonyl)-2-(2-propynyloxy)-benzenehexanamide (MS-PPOH; vehicle: 0.5% ethanol in 0.5 mol/L PBS), N-[2-(cyclohexyloxy)-4-nitrophenyl]-methanesulfonamide (NS-398; vehicle: 1.5% DMSO in 0.5 mol/L PBS) and 5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-(trifluoromethyl)-1H-pyrazole (SC-560; vehicle: 0.5% ethanol in 0.5 mol/L PBS) were obtained from Cayman Chemicals (Ann Arbor, MI, USA).
Immunochemical Staining for Pyramidal Cells Recruited by Basal Forebrain Stimulation
One hour after stimulation of the left BF, rat brains were fixed by intraaortic perfusion (500 mL of 4% paraformaldehyde in 0.1 mol/L phosphate buffer), postfixed by immersion (2 hours, 4°C), cryoprotected, frozen (−45°C in isopentane) and sectioned as free-floating coronal sections (25 μm thick) on a freezing microtome. Activated pyramidal cells were detected by double-immunocytochemistry for c-Fos (rabbit anti-c-Fos, 1:15,000; Oncogene, San Diego, CA, USA) and a ubiquitous pyramidal cell marker (mouse anti-rat brain pyramidal cells (RBPC), 1:2,000; SWANT, Bellinzona, Switzerland) or COX-2 (mouse anti-COX-2, 1:3,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), which is constitutively expressed by a subset of cortical pyramidal cells (Kaufmann et al, 1996). Rat brain pyramidal cell and COX-2 were detected in the first position with an anti-mouse secondary antibody (1:200; Vector Labs, Burlingame, CA, USA) followed by the ABC complex (Vectastain ABC kit; Vector Labs) and 3,3-diaminobenzidine (Vector Labs; brown precipitate), and c-Fos was detected in the second position with a biotinylated secondary antibody and the SG reagent (Vector Labs; blue–gray precipitate) (Kocharyan et al, 2008). Sections were observed under light microscopy and digital pictures taken, calibrated, and edited with MetaMorph 6.1r3 (Universal Imaging, Downington, PA, USA) and Adobe Photoshop 7 (Adobe Systems, San Jose, CA, USA).
Quantitative Analysis
Laser-Doppler flowmetry values were measured as arbitrary units, and CBF changes induced by BF stimulation expressed as percent changes of the peak CBF value compared with 1 minute average baseline. For figure representation, CBF was averaged every 1 second starting 1 minute before until 1 minute after the stimulation, and expressed as percent change compared with the 1-minute average prestimulus baseline. Physiological parameters and changes in peak CBF were compared by repeated-measures ANOVA (analysis of variance) or by one-way ANOVA for three group comparisons followed by post hoc Newman–Keuls comparison tests. C-Fos-positive COX-2 pyramidal cells were counted in double-immunostained sections (2 to 3 sections/rat) directly under the microscope. Counts were performed in layers II to IV and V of the ipsilateral and contralateral frontoparietal cortex at one defined rostro-caudal level (−1.0 to −1.3 mm from the Bregma). Data were expressed as percent of the total number of pyramidal cells immunopositive for COX-2, and compared by Student's t-test between ipsilateral and contralateral sides. All data were expressed as mean±s.e.m., and statistical analyses were performed with GraphPad Prism4 (San Diego, CA, USA). A P<0.05 was considered significant.
Results
Cortical Cerebral Blood Flow Effects of Basal Forebrain Electrical Stimulation
We first confirmed that electrical BF stimulation induced a substantial increase in ipsilateral cortical CBF compared with the contralateral side (65.5%±2.9% versus 21.8%±1.4%, P<0.001). Intracisternal injection of vehicles did not affect the evoked CBF response, and neither BF stimulation, vehicle or drug injections altered physiological parameters (see Supplementary Table S1). Similarly, none of the compounds altered resting CBF (data not shown), except for a small, albeit significant, decrease after COX-1 inhibition (−12% versus vehicle, P<0.05), as previously reported (Niwa et al, 2001).
Immunohistochemical Identification of Activated Pyramidal Cells
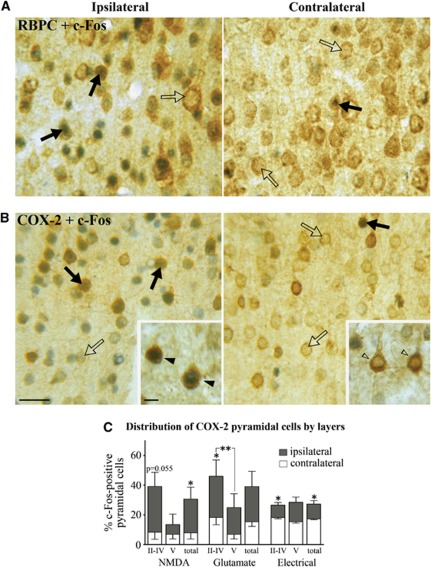
Pyramidal cells being primarily accountable for the increased cortical activity and hemodynamic response to sensory stimuli (Koehler et al, 2009; Lecrux et al, 2011; Norup Nielsen and Lauritzen, 2001; Zonta et al, 2003), we investigated their contribution in the hyperemic response to incoming basalocortical afferents. We used c-Fos as a marker for increased neuronal activity as it is well validated in the neocortex (Staiger et al, 2000) where it was previously found to selectively accumulate in SOM- and NPY-containing GABA interneurons in BF-stimulated rats (Kocharyan et al, 2008). In double-immunostained sections following either chemical or electrical BF stimulation, c-Fos protein levels were significantly upregulated in a large number of pyramidal cells immunopositive for the ubiquitous marker RBPC (Figure 2A, not quantified) and for COX-2, which is coexpressed in a subset of these neurons (Kaufmann et al, 1996). Except for layer V, RBPC immunostaining distributed throughout the different layers of the ipsilateral cortex, whereas COX-2 immunoreactive pyramidal cells were primarily found in layers II/IV (Figures 2B and 2C). A smaller proportion of COX-2 pyramidal cells were activated by electrical compared with chemical stimulation with NMDA or glutamate (Figure 2C). Quantitative analysis showed that NMDA, glutamate, or electrical BF stimulation induced c-Fos in 30.5%±7.9%, 38.9%±10.2%, and 27.1%±2.3% of COX-2 pyramidal cells in the ipsilateral cortex, compared with 8.0%±4.2%, 15.4%±3.1%, and 17.3%±0.5%, respectively, on the contralateral side (Figure 2C). These data indicate activation of various glutamate-releasing pyramidal neurons spanning several layers of the cortex targeted by BF afferents. Together with our previous findings of activated GABA interneurons (Kocharyan et al, 2008), these results demonstrate that both excitatory pyramidal cells and inhibitory interneurons are recruited by BF afferents, consistent with both cell types being targeted by basalocortical afferents (Cauli et al, 2004; Henny and Jones, 2008; Houser et al, 1985).
Figure 2.
Basal forebrain (BF) stimulation activates cortical pyramidal cells. BF stimulation activated pyramidal cells predominantly in the ipsilateral cortex, as shown by upregulation of c-Fos protein (blue–gray) in the nuclei of RBPC (rat brain pyramidal cell) (A, brown) or cyclooxygenase-2 (COX-2)- (B, brown) immunopositive pyramidal cells (black arrows) compared with nonactivated cells (open arrows). See also insets. (C) Quantitative analysis of c-Fos-positive COX-2 pyramidal cells indicated a greater activation in layers II to IV compared with layer V on the ipsilateral side after NMDA (N-methyl-D-aspartic acid), glutamate, and electrical stimulations (*P<0.05, **P<0.01, paired t-test ipsilateral versus contralateral side). Bars: (A, B) 20 μm, 5 μm in insets.
Glutamate in the Neurovascular Coupling Response to Basal Forebrain Stimulation
We then tested the possible implication of excitatory glutamate-releasing pyramidal cells in the CBF response to BF stimulation. We found significant decreases after selective antagonism of NMDA receptors with MK-801 (−32.2%±5.3%, P<0.01, n=8) (Figures 1C and 1F), AMPA/kainate receptors with CNQX (−30.2%±5.5% at 10 minutes, P<0.01, n=7), and group 1 mGluR1/R5 receptors with LY-367385 and MPEP (−19.6%±4.5% at 30 minutes, P<0.05, n=7) (Figure 1F). No additive effect was observed when MK-801 and CNQX were coadministered (−29.2%±8.4%, P<0.05, n=6) (Figure 1F). Such a finding could be explained by NMDA receptor activation requiring synchronized membrane depolarization provided by AMPA receptors, and consequently glutamatergic synapses lacking AMPA receptors being considered ‘silent' and unable to evoke calcium currents (Levy and Aoki, 2002). Together, these findings show that glutamate neurotransmission, alike its well-defined role in the hyperemic response to sensory stimulation (Lecrux et al, 2011; Norup Nielsen and Lauritzen, 2001; Zonta et al, 2003), drives an important part of the BF-induced CBF response. To interrogate whether combined blockade of glutamate and GABA neurotransmission can alter the BF-induced perfusion response to a greater extent, we coadministered MK-801 and the GABA-A receptor antagonist picrotoxin. We first confirmed the significant decrease in the BF-evoked CBF response following ic administration of picrotoxin (−24.5%±4.1%, P<0.05, n=6) (Figures 1D and 1F) (Kocharyan et al, 2008). Then, we found that the combined administration of MK-801 and picrotoxin had a potent and additive inhibitory effect (−45.9%±6.2%, P<0.01, n=7) that was significantly larger than that induced by each compound alone (P<0.05; Figures 1E and 1F). Overall, these data indicate that both cortical excitatory and inhibitory neurons releasing glutamate and GABA, respectively, contribute to functional hyperemic response to incoming BF afferent input.
Activation of Glutamate- and γ-Aminobutyric Acid-Releasing Cells Is Downstream of mAChR Activation
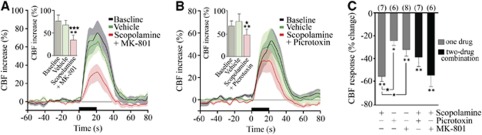
Acetylcholine is the prime instigator of the hyperemic response to BF stimulation (Zhang et al, 1995) and the latter is almost completely abolished (−85%) by cortical cholinergic denervation with the selective cholinotoxin 192-IgG saporin (Kocharyan et al, 2008) and by combined blockade of muscarinic and nicotinic ACh receptors (Biesold et al, 1989). Here, we observed an important role for mAChRs with large decreases in the evoked CBF after scopolamine (−55.5%±4.2% at 40 minutes, P<0.01, n=7) (Figure 3C). Moreover, when scopolamine was coadministered with either MK-801 or picrotoxin (−54.7%±9.5%, P<0.01, n=6 and −38.9%±7.9%, P<0.01, n=7, respectively), no further decrease of the CBF response was observed compared with scopolamine alone (Figures 3A–3C), supporting that GABA and glutamate effects are primarily downstream of mAChR activation. Although not significant, superimposing picrotoxin actually tended to reduce the blocking effect of scopolamine (P=0.051), possibly due to increased spontaneous cortical ACh release under GABA-A receptor blockade (Giorgetti et al, 2000), hence reducing the stimulus-evoked ACh response.
Figure 3.
The reducing effects of NMDA (N-methyl-D-aspartic acid) and picrotoxin on the basal forebrain (BF)-evoked cerebral blood flow (CBF) response are secondary to mAChR (ACh through muscarinic receptor) activation. Average ipsilateral CBF responses at baseline, and following injection of vehicle or combined mAChR (scopolamine) and NMDA (MK-801) (A) or GABA-A (γ-aminobutyric acid) (picrotoxin) (B) receptor antagonists (★P<0.05; ★★★P<0.001 versus baseline, *P<0.05; **P<0.01 versus vehicle, repeated-measures analysis of variance (ANOVA)). (C) Average ipsilateral CBF reductions after vehicle, mAChR blockade alone or combined with that of NMDA or GABA-A receptors. No additive effect was observed compared with scopolamine alone (★P<0.05, one-way ANOVA).
Arachidonic Acid Products in the Basal Forebrain-Evoked Cerebral Blood Flow Response: A Role for Astrocytes?
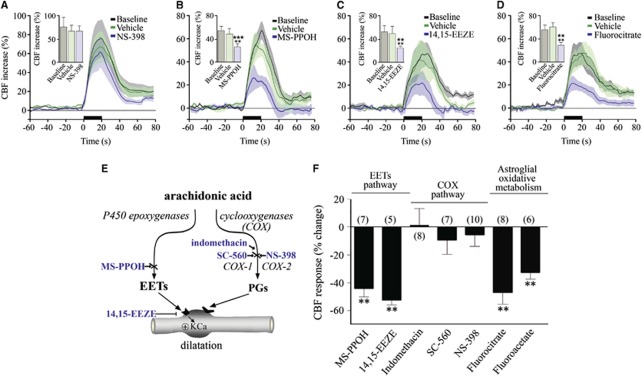
It is believed that glutamate requires intermediary cells and mediators to alter CBF because there is no convincing evidence for dilatory glutamate receptors on brain vessels. In this respect, several AA metabolites display vasodilator properties (Figure 4E), and have been implicated as possible mediators of glutamate-induced CBF changes. The fact that BF stimulation activated COX-2 pyramidal neurons, which have a central role in functional hyperemia to sensory stimulation (Lecrux et al, 2011; Niwa et al, 2000), raised the possibility that COX-2 products may participate in the BF-evoked hemodynamic response. Surprisingly, selective inhibition of COX-2 with NS-398 had no impact on the CBF response to BF stimulation (+6.9%±9.5% at 45 minutes, ns, n=8) (Figures 4A and 4F). Similarly, although COX-1-derived vasoactive metabolites have been implicated in the hemodynamic changes induced by hypercapnia (Niwa et al, 2001), selective inhibition of COX-1 with SC-560 did not affect the evoked CBF response (−9.8%±10.2%, ns, n=7) (Figure 4F). The lack of COX-derived vasoactive products was further confirmed with the nonselective COX inhibitor indomethacin, which was virtually devoid of any effect (+0.4%±12.1%, ns, n=8) (Figure 4F). Together, these findings excluded COX products as mediators of the BF-induced CBF response.
Figure 4.
Astroglial pathways and epoxyeicosatrienoic acids (EETs) contribute to the neurovascular coupling response to basal forebrain (BF) stimulation. (A) Cyclooxygenase-2 (COX-2) inhibition with NS-398 did not alter the cerebral blood flow (CBF) response to BF stimulation, whereas inhibition of EETs synthesis with MS-PPOH (B), EETs receptors with 14,15-EEZE (C) or blockade of astroglial metabolism with fluorocitrate (D) significantly reduced the CBF response (★★P<0.01; ★★★P<0.001 versus baseline, **P<0.01; ***P<0.001 versus vehicle, repeated-measures analysis of variance (ANOVA)). (E) Schematic representation of the synthesis of arachidonic acid (AA) vasoactive products and sites of action ( ) of the inhibitors or antagonists. (F) Average ipsilateral CBF reductions induced by inhibitors of EETs or COX pathways, or astroglial oxidative metabolism compared with their respective vehicles. (**P<0.01, by one-way ANOVA).
) of the inhibitors or antagonists. (F) Average ipsilateral CBF reductions induced by inhibitors of EETs or COX pathways, or astroglial oxidative metabolism compared with their respective vehicles. (**P<0.01, by one-way ANOVA).
Consequently, we investigated whether EETs, AA products synthesized through the cytochrome P450 epoxygenase pathway (Figure 4E) that mediate an important part of the functional hyperemic response to sensory stimulation (Lecrux et al, 2011; Leithner et al, 2010; Peng et al, 2004) could be involved. Using MS-PPOH, a specific substrate inhibitor of P450 epoxygenase and 14,15-EEZE, an EETs receptor antagonist (Koehler et al, 2009), we found respective decreases of 44.2%±6.2% (P<0.01, n=7) and 52.5%±3.6% (after 50 minutes, P<0.01, n=5) (Figures 4B, 4C, and 4F) in the BF-evoked CBF response. The P450 epoxygenase pathway is purportedly selective to astrocytes, which are ideally positioned to regulate synaptic transmission and neurovascular coupling. Their processes contact thousands of synapses, their endfeet are intimately associated with brain microvessels, and a large body of evidence suggests a primary role for astrocytes in the neurovascular coupling response to sensory stimulation (Koehler et al, 2009). To test the implication of astrocytes in the BF-induced perfusion response, we used two metabolic gliotoxins that are preferentially uptaken by astrocytes and act by inhibiting aconitase, a tricarboxylic acid cycle enzyme (Zielke et al, 2007). Both fluorocitrate and fluoroacetate significantly decreased the BF-induced CBF response (47.2%±8.5% at 60 minutes, P<0.01, n=8 and 33.0%±4.9% at 60 minutes, P<0.01, n=6, respectively) (Figures 4D and 4F). These data suggest that metabolically active astrocytes mediate part of the BF-induced CBF increase in the cortex, possibly through the synthesis and release of vasoactive EETs.
Epoxyeicosatrienoic Acids as Intermediaries for Glutamate and γ-Aminobutyric Acid Neurons
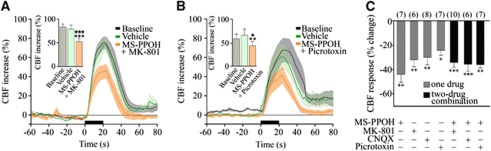
The role of glutamate in neurovascular coupling has been linked, primarily through mGluR5, to the synthesis and release of vasodilatory EETs (Shi et al, 2008), although these receptors would not be required for the initial hemodynamic response (Calcinaghi et al, 2011). Here, we assessed whether the glutamate receptors predominantly involved in the BF-induced increases in CBF also engage the EETs signaling cascade. We coadministered the selective NMDA or AMPA/kainate receptor antagonist with the P450 epoxygenase inhibitor MS-PPOH. Such treatments exerted no additive effect compared with each compound alone (−34.5%±3.5%, P<0.001, n=6 for MK-801+MS-PPOH, and −35.7%±5.8%, P<0.001, n=7 for CNQX+MS-PPOH) (Figures 5A and 5C). Similarly, the combined blockade of GABA-A receptors and EETs synthesis was not more potent than either treatment alone (−36%±2.7%, P<0.01, n=10) (Figures 5B and 5C). Together these results suggest that EETs signaling acts in sequence to NMDA, AMPA/kainate, and GABA-A receptor activation, and that both glutamate and GABA pathways can activate the vasodilatory EETs cascade following BF stimulation.
Figure 5.
Glutamate and GABA (γ-aminobutyric acid) networks modulate the evoked cerebral blood flow (CBF) through epoxyeicosatrienoic acids (EETs). Average CBF responses to basal forebrain (BF) stimulation were reduced by MS-PPOH injection combined to MK-801 (A), picrotoxin (B), or CNQX (C), but these reductions were not additive compared with MS-PPOH alone (C). (★P<0.05; ★★★P<0.001 versus baseline, *P<0.05; **P<0.01 or ***P<0.001 versus vehicle, repeated-measures analysis of variance (ANOVA), followed by Newman–Keuls posttest).
Discussion
The key new findings from our study are (1) activation of glutamate-releasing pyramidal cells, acting though various receptor subtypes, is required for the full expression of the neurovascular coupling response to BF stimulation; (2) COX-2 vasoactive products do not contribute to the CBF response, suggesting that COX-2 pyramidal cells recruited (c-Fos positive) by BF stimulation act through glutamate; (3) NMDA- and GABA-A-receptor-mediated pathways contribute in parallel to the CBF response; (4) EETs are essential in this neurovascular response, possibly acting as intermediaries for both pyramidal cells and GABA interneurons; and (5) metabolically active astrocytes are required, likely to synthesize and release vasoactive EETs.
Glutamate and γ-Aminobutyric Acid in the Perfusion Response to Basal Forebrain Stimulation
The cellular interactions that lead to increased cortical activity following BF stimulation are complex and likely not limited to ACh (Henny and Jones, 2008). However, there is substantial evidence that the neurovascular coupling response to the BF is predominantly driven by ACh: it occurs concurrently with the local release of ACh (Zhang et al, 1995), it is virtually abolished (−85%) by selective cortical cholinergic deafferentation (Kocharyan et al, 2008) and following blockade of mAChR and nicotinic receptors (Biesold et al, 1989). Here, we found that combined blockade of mAChRs and either NMDA or GABA-A receptors had no additive attenuating effect over mAChR blockade alone (∼60%), indicating that activation of these pathways was downstream to mAChR activation and reflected primarily activation of cortical glutamate and GABA neurons targeted by incoming BF afferents.
Accordingly, BF stimulation was accompanied by widespread increased activity (detected by c-Fos) in cortical pyramidal cells, consistent with their innervation by ACh BF terminals (Houser et al, 1985). Although ACh exerts mixed effects on pyramidal cells depending on cortical layers, subdivisions, and receptor subtypes (Gulledge et al, 2009; McCormick and Prince, 1986), strong mAChR-mediated depolarization leading to tonic firing (Carr and Surmeier, 2007) and mAChR-induced persistent spiking (Rahman and Berger, 2011) of pyramidal cells are prominent. Increased firing in pyramidal cells induced by BF stimulation further results from their disinhibition following mAChR activation of electrically coupled SOM interneurons and subsequent silencing of other GABA interneurons, that normally drive pyramidal cell inhibition (for detail, see Kocharyan et al, 2008). Hence, together the combined effects of ACh on pyramidal cells and cortical networks of inhibitory neurons contribute to the overall increased activity in glutamate-releasing pyramidal cells following BF stimulation (Figure 6). This dual mechanism of cortical activation concurs with our findings that combined blockade of NMDA and GABA-A receptors attenuated the BF-evoked CBF response significantly more than blocking either receptor alone.
Figure 6.
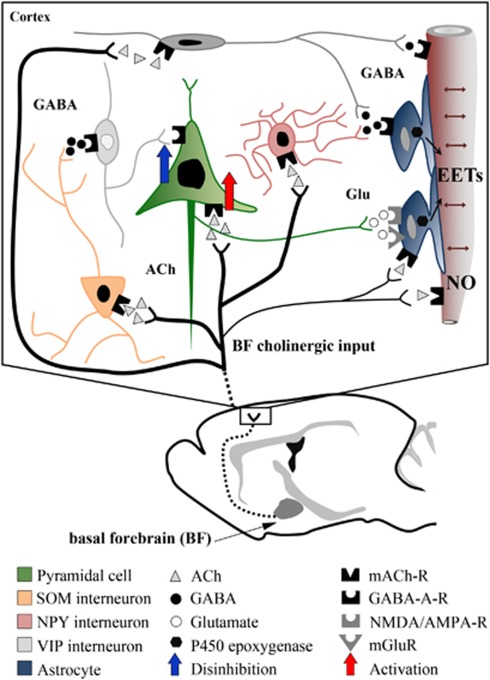
Schematic representation of how glutamate, GABA (γ-aminobutyric acid), and astrocytes may interact in the evoked cerebral blood flow (CBF) response to basal forebrain (BF) stimulation. Our c-Fos studies (black nuclei=activation, white nuclei=no activation or inhibition) indicate that BF cholinergic fibers activate both GABA interneurons—in particular, the somatostatin (SOM) subfamily (Kocharyan et al, 2008)—and glutamate-releasing pyramidal cells. Our pharmacological studies show that glutamate (Glu) and GABA effects are downstream of mAChR (ACh through muscarinic receptor) activation, and that pyramidal cells act through the release of glutamate, and not through cyclooxygenase-2 (COX-2) metabolites. Increased activity in glutamatergic pyramidal cells, either activated directly by acetylcholine (ACh) (red arrow) or indirectly by disinhibition (blue arrow) following activation of GABAergic interneurons, will primarily exert its CBF increasing effect through the epoxyeicosatrienoic acids (EETs), likely released from astrocytes. Direct vascular effects of ACh through mAChR-induced endothelial nitric oxide (NO)-mediated dilation also account for a significant part of the evoked CBF response. Similar, albeit small, dilatory effects of GABA through vascular GABA-A receptors cannot be discarded (Lecrux et al, 2011), as well as possible effects of ACh on astrocytes.
We provided evidence that following its release from activated pyramidal cells, glutamate can act on AMPA, NMDA and, to a lesser extent, group1 mGlu receptors that are expressed by several cortical neurons (Baude et al, 1993) and by astrocytes (Lalo et al, 2006). As mGluRs are normally recruited for selective circuit activation during glutamate spillover (Iserhot et al, 2004), this may explain their relatively modest contribution in the ACh driven-CBF response as compared with that induced by sensory stimulation that is driven by glutamate released by both thalamocortical afferents (Zonta et al, 2003) and local pyramidal cells (Lecrux et al, 2011). The previous (Kocharyan et al, 2008) and current c-Fos data correlated very well with our pharmacological findings, further emphasizing the contribution of excitatory and inhibitory neurons in the neurovascular coupling response to BF afferents, as also reported for thalamocortical and corticocortical afferent pathways (Enager et al, 2009; Lecrux et al, 2011).
Cyclooxygenase Metabolites do not Couple Neural Activity to Cerebral Blood Flow Following Basal Forebrain Stimulation
Nuclear c-Fos was upregulated in COX-2 pyramidal cells, whose products have a substantial role in the functional hyperemic response to incoming glutamatergic afferents following sensory stimulation (Lecrux et al, 2011; Niwa et al, 2000). However, neither COX-2 nor COX-1 products were found to significantly contribute to the BF-induced CBF response. We conclude that COX-2 activation and contribution to functional hyperemia requires high levels of glutamate that cannot be reached following activation of a cholinergic pathway. Evidence supporting this hypothesis includes the failure of mAChR activation to stimulate COX-2 in rat cerebral cortex (Orman et al, 2006), whereas NMDA receptors induce immediate release of neuronal COX-2-derived prostaglandin E2 (Pepicelli et al, 2005). Additionally, light-evoked vasodilatation in the retina, a pathway where neurovascular coupling does not involve glutamate but purinergic transmission, likewise did not operate via COX-2 products (Metea and Newman, 2006).
Astroglial Cells and Epoxyeicosatrienoic Acids in the Cerebral Blood Flow Response to Basal Forebrain Stimulation
Fluorocitrate and fluoroacetate are preferential inhibitors of astroglial oxidative metabolism in vivo (Zielke et al, 2007), and their administration decreased the evoked CBF response by about 50%, pointing to a role for astrocytes in the BF-induced hyperemic response. Pyramidal cells and interneurons, through respective release of glutamate and GABA, can induce currents in astrocytes (Lalo et al, 2006; Meier et al, 2008), a response linked to the synthesis and release of vasodilatory messengers such as EETs (Koehler et al, 2009). The latter are actually key mediators of the hyperemic response to sensory stimulation (Peng et al, 2004) and of the light-evoked dilatation in the retina (Metea and Newman, 2006). Hence, we tested whether EETs could act as vasoactive messengers in the cholinergic-mediated BF-evoked CBF response. Accordingly, further inspection with blockade of EETs synthesis with MS-PPOH or EETs receptor-mediated effects with 14,15-EEZE demonstrated their major contribution, corresponding to ∼50%, in the evoked hemodynamic response. Moreover, combined blockade of EETs synthesis with either NMDA, AMPA, or GABA-A receptors did not yield to a larger decrease of the evoked CBF response compared with blocking EETs synthesis alone. These findings suggest that not only glutamate-releasing pyramidal cells, but also GABA interneurons may act through EETs to alter CBF, consistent with GABA-A receptors inducing calcium signaling in astrocytes (Lalo et al, 2006; Meier et al, 2008), a key mechanism in the astroglial regulation of brain vessel vasomotricity (Koehler et al, 2009).
Conclusions
Our anatomical and in vivo pharmacological data demonstrate that BF stimulation activates cortical pyramidal cells, including those that constitutionally express COX-2, but that only glutamate is involved in the accompanying CBF response. The results also show that EETs, likely released from astrocytes activated by glutamate and, possibly, GABA from specific subsets of interneurons, mediate an important part of the hemodynamic response. When compared with the CBF response evoked by glutamatergic input elicited by whisker stimulation (Lecrux et al, 2011), our previous (Kocharyan et al, 2008) and current findings on the cholinergic BF afferents demonstrate that distinct cortical interneurons are activated by thalamocortical glutamate (vasoactive intestinal polypeptide and ACh interneurons) and basalocortical ACh (SOM and NPY interneurons) afferents, and that different mediators are involved in the CBF response to whisker (COX-2 products and EETs) and BF (EETs) stimulation, notwithstanding the contribution of NO as a modulator (Lindauer et al, 1999) or a mediator (Zhang et al, 1995) of these respective responses. These findings stress the importance of knowing the circuitry that generates the hemodynamic signals used in neuroimaging techniques. Particularly, BF-derived ACh input to the cerebral cortex is severely reduced in Alzheimer's disease, suggesting that interpretation of perfusion-based brain imaging in such patients should take into consideration the intricate cellular interaction between the various elements of the cortical circuitry.
Acknowledgments
The authors are grateful to Dr Bruno Cauli (ESPCI) for critical reading of the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by a Grant from the Canadian Institute of Health Research (CIHR, MOP-84209, EH), a fellowship from the Heart & Stroke Foundation of Canada/Canadian Stroke Network (CL), and a Jeanne Timmins Costello studentship (CHS).
Supplementary Material
References
- Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci. 2000;3:904–910. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- Biesold D, Inanami O, Sato A, Sato Y. Stimulation of the nucleus basalis of Meynert increases cerebral cortical blood flow in rats. Neurosci Lett. 1989;98:39–44. doi: 10.1016/0304-3940(89)90370-4. [DOI] [PubMed] [Google Scholar]
- Calcinaghi N, Jolivet R, Wyss MT, Ametamey SM, Gasparini F, Buck A, Weber B. Metabotropic glutamate receptor mGluR5 is not involved in the early hemodynamic response. J Cereb Blood Flow Metab. 2011;31:e1–e10. doi: 10.1038/jcbfm.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cape EG, Manns ID, Alonso A, Beaudet A, Jones BE. Neurotensin-induced bursting of cholinergic basal forebrain neurons promotes gamma and theta cortical activity together with waking and paradoxical sleep. J Neurosci. 2000;20:8452–8461. doi: 10.1523/JNEUROSCI.20-22-08452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Surmeier DJ. M1 muscarinic receptor modulation of Kir2 channels enhances temporal summation of excitatory synaptic potentials in prefrontal cortex pyramidal neurons. J Neurophysiol. 2007;97:3432–3438. doi: 10.1152/jn.00828.2006. [DOI] [PubMed] [Google Scholar]
- Cauli B, Tong XK, Rancillac A, Serluca N, Lambolez B, Rossier J, Hamel E. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci. 2004;24:8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enager P, Piilgaard H, Offenhauser N, Kocharyan A, Fernandes P, Hamel E, Lauritzen M. Pathway-specific variations in neurovascular and neurometabolic coupling in rat primary somatosensory cortex. J Cereb Blood Flow Metab. 2009;29:976–986. doi: 10.1038/jcbfm.2009.23. [DOI] [PubMed] [Google Scholar]
- Giorgetti M, Bacciottini L, Giovannini MG, Colivicchi MA, Goldfarb J, Blandina P. Local GABAergic modulation of acetylcholine release from the cortex of freely moving rats. Eur J Neurosci. 2000;12:1941–1948. doi: 10.1046/j.1460-9568.2000.00079.x. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Bucci DJ, Zhang SS, Matsui M, Yeh HH. M1 receptors mediate cholinergic modulation of excitability in neocortical pyramidal neurons. J Neurosci. 2009;29:9888–9902. doi: 10.1523/JNEUROSCI.1366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel E. Cholinergic modulation of the cortical microvascular bed. Prog Brain Res. 2004;145:171–178. doi: 10.1016/S0079-6123(03)45012-7. [DOI] [PubMed] [Google Scholar]
- Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci. 2008;27:654–670. doi: 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR, Crawford GD, Salvaterra PM, Vaughn JE. Immunocytochemical localization of choline acetyltransferase in rat cerebral cortex: a study of cholinergic neurons and synapses. J Comp Neurol. 1985;234:17–34. doi: 10.1002/cne.902340103. [DOI] [PubMed] [Google Scholar]
- Iserhot C, Gebhardt C, Schmitz D, Heinemann U. Glutamate transporters and metabotropic receptors regulate excitatory neurotransmission in the medial entorhinal cortex of the rat. Brain Res. 2004;1027:151–160. doi: 10.1016/j.brainres.2004.08.052. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci USA. 1996;93:2317–2321. doi: 10.1073/pnas.93.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocharyan A, Fernandes P, Tong XK, Vaucher E, Hamel E. Specific subtypes of cortical GABA interneurons contribute to the neurovascular coupling response to basal forebrain stimulation. J Cereb Blood Flow Metab. 2008;28:221–231. doi: 10.1038/sj.jcbfm.9600558. [DOI] [PubMed] [Google Scholar]
- Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 2009;32:160–169. doi: 10.1016/j.tins.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Kirchhoff F, North RA, Verkhratsky A. NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J Neurosci. 2006;26:2673–2683. doi: 10.1523/JNEUROSCI.4689-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen M, Gold L. Brain function and neurophysiological correlates of signals used in functional neuroimaging. J Neurosci. 2003;23:3972–3980. doi: 10.1523/JNEUROSCI.23-10-03972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrux C, Toussay X, Kocharyan A, Fernandes P, Neupane S, Levesque M, Plaisier F, Shmuel A, Cauli B, Hamel E. Pyramidal neurons are ‘neurogenic hubs' in the neurovascular coupling response to whisker stimulation. J Neurosci. 2011;31:9836–9847. doi: 10.1523/JNEUROSCI.4943-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leithner C, Royl G, Offenhauser N, Fuchtemeier M, Kohl-Bareis M, Villringer A, Dirnagl U, Lindauer U. Pharmacological uncoupling of activation induced increases in CBF and CMRO2. J Cereb Blood Flow Metab. 2010;30:311–322. doi: 10.1038/jcbfm.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy RB, Aoki C. Alpha7 nicotinic acetylcholine receptors occur at postsynaptic densities of AMPA receptor-positive and -negative excitatory synapses in rat sensory cortex. J Neurosci. 2002;22:5001–5015. doi: 10.1523/JNEUROSCI.22-12-05001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindauer U, Megow D, Matsuda H, Dirnagl U. Nitric oxide: a modulator, but not a mediator, of neurovascular coupling in rat somatosensory cortex. Am J Physiol. 1999;277:H799–H811. doi: 10.1152/ajpheart.1999.277.2.H799. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Mechanisms of action of acetylcholine in the guinea-pig cerebral cortex in vitro. J Physiol. 1986;375:169–194. doi: 10.1113/jphysiol.1986.sp016112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier SD, Kafitz KW, Rose CR. Developmental profile and mechanisms of GABA-induced calcium signaling in hippocampal astrocytes. Glia. 2008;56:1127–1137. doi: 10.1002/glia.20684. [DOI] [PubMed] [Google Scholar]
- Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci. 2006;26:2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- Niwa K, Araki E, Morham SG, Ross ME, Iadecola C. Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. J Neurosci. 2000;20:763–770. doi: 10.1523/JNEUROSCI.20-02-00763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa K, Haensel C, Ross ME, Iadecola C. Cyclooxygenase-1 participates in selected vasodilator responses of the cerebral circulation. Circ Res. 2001;88:600–608. doi: 10.1161/01.res.88.6.600. [DOI] [PubMed] [Google Scholar]
- Norup Nielsen A, Lauritzen M. Coupling and uncoupling of activity-dependent increases of neuronal activity and blood flow in rat somatosensory cortex. J Physiol. 2001;533:773–785. doi: 10.1111/j.1469-7793.2001.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orman B, Reina S, Sterin-Borda L, Borda E. Signaling pathways leading to prostaglandin E(2) production by rat cerebral frontal cortex. Prostaglandins Leukot Essent Fatty Acids. 2006;74:255–262. doi: 10.1016/j.plefa.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Peng X, Zhang C, Alkayed NJ, Harder DR, Koehler RC. Dependency of cortical functional hyperemia to forepaw stimulation on epoxygenase and nitric oxide synthase activities in rats. J Cereb Blood Flow Metab. 2004;24:509–517. doi: 10.1097/00004647-200405000-00004. [DOI] [PubMed] [Google Scholar]
- Pepicelli O, Fedele E, Berardi M, Raiteri M, Levi G, Greco A, Ajmone-Cat MA, Minghetti L. Cyclo-oxygenase-1 and -2 differently contribute to prostaglandin E2 synthesis and lipid peroxidation after in vivo activation of N-methyl-D-aspartate receptors in rat hippocampus. J Neurochem. 2005;93:1561–1567. doi: 10.1111/j.1471-4159.2005.03150.x. [DOI] [PubMed] [Google Scholar]
- Rahman J, Berger T. Persistent activity in layer 5 pyramidal neurons following cholinergic activation of mouse primary cortices. Eur J Neurosci. 2011;34:22–30. doi: 10.1111/j.1460-9568.2011.07736.x. [DOI] [PubMed] [Google Scholar]
- Shi Y, Liu X, Gebremedhin D, Falck JR, Harder DR, Koehler RC. Interaction of mechanisms involving epoxyeicosatrienoic acids, adenosine receptors, and metabotropic glutamate receptors in neurovascular coupling in rat whisker barrel cortex. J Cereb Blood Flow Metab. 2008;28:111–125. doi: 10.1038/sj.jcbfm.9600511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger JF, Bisler S, Schleicher A, Gass P, Stehle JH, Zilles K. Exploration of a novel environment leads to the expression of inducible transcription factors in barrel-related columns. Neuroscience. 2000;99:7–16. doi: 10.1016/s0306-4522(00)00166-4. [DOI] [PubMed] [Google Scholar]
- Vaucher E, Hamel E. Cholinergic basal forebrain neurons project to cortical microvessels in the rat: electron microscopic study with anterogradely transported Phaseolus vulgaris leucoagglutinin and choline acetyltransferase immunocytochemistry. J Neurosci. 1995;15:7427–7441. doi: 10.1523/JNEUROSCI.15-11-07427.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Xu S, Iadecola C. Role of nitric oxide and acetylcholine in neocortical hyperemia elicited by basal forebrain stimulation: evidence for an involvement of endothelial nitric oxide. Neuroscience. 1995;69:1195–1204. doi: 10.1016/0306-4522(95)00302-y. [DOI] [PubMed] [Google Scholar]
- Zielke HR, Zielke CL, Baab PJ, Tildon JT. Effect of fluorocitrate on cerebral oxidation of lactate and glucose in freely moving rats. J Neurochem. 2007;101:9–16. doi: 10.1111/j.1471-4159.2006.04335.x. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.