Abstract
Background
Esophageal cancer accounts for a considerable proportion of carcinomas of the upper gastrointestinal tract in African Americans. Our aim was to describe the epidemiology of esophageal squamous cell cancer (ESCC) and esophageal adenocarcinoma (EA) among African Americans in the last five decades.
Methods
A total of 601 records of patients with documented esophageal cancer between 1959 and 2007 at Howard University Hospital were reviewed. Demographic characteristics, risk factors, clinical stage and histological findings were reviewed. The change in prevalence of the disease and the interaction between main risk factors with tumor stage of the patients were assessed over the years of this study.
Result
A total of 552 patients (91.8%) had ESCC while 49 patients (8.2%) had EA. The mean age at diagnosis was 60.1 and 60.6 years for ESCC and EA, respectively (P = 0.8). The peak incidence was in the 1980–1989 decade. Out of 136 ESCC patients with TNM staging information, 130 (95.6%) were diagnosed in stage 2 and above. The majority (73%) of the ESCC were in the mid- and upper third of the esophagus and associated with smoking and alcohol exposure. The majority (81%) of the EA were in the mid- and lower third. The most common presenting symptoms were dysphagia (77.7%), and weight loss (31.9%).
Conclusion
ESCC is the predominant esophageal cancer in African Americans and diagnosed in late stages, and its diagnosis in our institution has decreased since 1990. A combination of genetic factors, environmental influences (e.g., those related to diet), and the deleterious changes associated with smoking and alcohol consumption, and differences in tumor histology, are the obvious parameters that should be the focus of future studies, and early diagnosis at an earlier stage should be considered among blacks.
Keywords: African American, Esophageal cancer, Esophageal squamous cell carcinoma, Esophageal adenocarcinoma
Introduction
Esophageal cancer is the sixth most common incident cancer in the world and the third most common cancer of the digestive tract. Its mortality is comparable to leukemia, liver and intrahepatic causes of cancer-related deaths worldwide [1, 2]. In the United States, 5 in 100,000 persons succumb to the disease per year and overall incidence of the disease is highest in men over 50 years of age [3, 4]. Compared with whites, the 5-year survival rate for African American (AA) men and women is lower for every stage of disease for nearly every cancer [5, 6]. Although relatively uncommon, esophageal cancer is usually fatal, with a relative 5-year survival rate of 18% in whites versus 11% in AAs in 1996–2004 in the United States [6]. Moreover, despite long-term decrease in death rates for several other cancers, the rate for esophagus increased especially in men [7, 8].
Histologically, esophageal cancer is classified into esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EA). ESCC accounts for most cases worldwide and about 40% of cases in the USA. Wide differences in the rates of ESCC have provided insight into risk factors associated with the disease. The importance of specific risk factors varies within different geographic regions: for example, the incidence is higher in urban areas (compared to rural areas) of the United States, particularly among AA men. In one report, the incidence among AA men in Washington, DC, was 28.6 per 100,000 [9]. This compares to an overall incidence of approximately 3–4 per 100,000 in other parts of the United States. Moreover, lower socioeconomic status was associated with esophageal ESCC in a large population-based study [10]. In addition, race and lifestyle choices are major factors that strongly determine the occurrence of the disease [10–12]. For instance, Cummings et al. showed that the incidence of esophageal squamous cell carcinoma is higher in AAs rather than whites (5.0 vs. 1.3 cases/100,000/year); however, whites had a higher EA incidence (3.3 vs. 0.8 cases/100,000/year) [8]. Although ESCC is most common among AA men, etiologic factors and demographics in EC in general are, however, not fully determined according to the patients’ race.
The United States SEER Data shows a 30% drop in incidence of ESCC between 1973 and 2002 with declines greatest in black males, although the incidence in this group remains high compared with other groups. Incidence of EA has increased fourfold over the same period with a fivefold increase in males [8, 13–15].
African Americans are more likely to be diagnosed at a later stage of cancer than whites, possibly due to factors such as less awareness about cancer symptoms and reduced access to clinical services which, in turn, may result in lower cure rates and shorter survival times [16, 17]. This is consistent with the high mortality rate from all types of cancers in the US in relation to a lower level of education [3, 6, 9, 10].
In the present study, we investigated characteristics of the esophageal cancer including its two histologic subtypes, its association with the environmental exposure (smoking, alcohol) and the disease trends over the last 50 years.
Materials and Methods
Patients and Data Gathering
Howard University Hospital (HUH) is located in District of Columbia (Washington, DC) and serves the Washington, DC, area, mainly an AA population. We conducted a chart review on all documented cases of esophageal cancer from the cancer registry, pathology, and radiotherapy department of HUH over a period of 48 years (1959–2007). This study was approved by the Howard university institutional review board. The following data were obtained from the pathology reports: age at diagnosis, sex, date of diagnosis, histology, anatomical site of cancer, lifestyle choices (cigarette smoking, alcohol intake), family history of cancer, and presenting symptoms. We limited our study to AA with histologically confirmed squamous cell carcinoma or adenocarcinoma of the esophagus. Other histologies such as lymphoma, etc., and cases without tissue confirmation, were excluded. The cancer staging report was available for 152 patients who were diagnosed after 1980. Cancer staging was reported according to the TNM staging system [18].
Information about smoking and alcohol ingestion and family history of cancer was available for 142 cases from medical records spanning a 22-year time frame between 1985 and 2007. History of tobacco usage was reviewed, and all tobacco users were grouped together as having a “positive” smoking history, i.e. we categorized smoking variable as ever versus never. The type and quantity of alcoholic beverages consumed were not available, so occasional drinkers and non-drinkers were grouped together as “negative” for alcohol drinking history.
Statistical Analysis
Numerical data are expressed as mean ± standard deviation (SD). Student’s t test was used for comparison of means. Categorical variables are compared using the chi-square test. P values less than 0.05 were considered significant. Statistical analysis was performed using the SPSS 16.0 software package (IBM, Somers, NY, USA).
Results
Esophageal Cancer and Its Association with Smoking and Family History
A total of 670 cases of esophageal cancer were identified between 1959 and 2007. After excluding esophageal cancer with histological type other than EA or ESCC and those without tissue confirmation, 601 cases remained [mean age (SD): 60.1 (10.9), age range: 25–98; male/female: 421/180]. Among them, 552 [91.8%; mean age (SD): 60.1 (10.8), age range: 25–98; male/female: 388/164] patients had ESCC and 49 [8.2%; mean age (SD): 60.6 (12.0), age range: 37–84; male/female: 33/16] had EA.
Information regarding tobacco use and alcohol intake was available for 142 EC cases from medical records from 1985 to 2007. A total of 112 (88%) of patients with ESCC had a positive history of tobacco use but just 11 (79%) of EA patients were smokers (P = 0.4). Ninety-eight (77%) and 7 (50%) of ESCC and EA patients, respectively, were drinking alcohol more than occasional usage (P = 0.03). Information regarding family history of cancer was available for 145 patients. Thirty-seven (29%) and 4 (29%) of ESCC and EA patients, respectively, had positive family history of cancer. Out of 37 ESCC with positive family history of cancer, 27 cases had a history of cancer in close relatives. In the EA group, there was no history of cancer in close relatives.
Disease staging was available for those diagnosed after 1980 (ESCC: 136; EA: 16), and is summarized in Table 1. The data showed that most patients were at stage 2 or above at the time of diagnosis while EA cases were at stage 3. There was no association between different stages of ESCC and age, gender, decade of diagnosis, positive family history of cancer, smoking cigarette, and alcohol drinking. Most EA were located in the lower third while most ESSC were located in the upper third of the esophagus. Detailed information regarding anatomic location and esophageal cancer histologic subtypes is reported in Table 1.
Table 1.
Distribution of ESCC and EA by anatomic location (1959–2007) and TNM staging (1980–2007)
| Total no. (%) | ESCC no. (%) | EA no. (%) | P values | |
|---|---|---|---|---|
| Cancer location (n = 601) | <0.001 | |||
| Lower third | 174 (28.9) | 144 (26.6) | 30 (61.2) | |
| Middle third | 202 (28.9) | 192 (34.4) | 10 (20.4) | |
| Upper third | 225 (37.3) | 216 (39.0) | 9 (18.4) | |
| Cancer stage (n = 152) | 0.05 | |||
| 0 | 2 (1.3) | 2 (1.5) | 0 (0) | |
| 1 | 4 (2.6) | 4 (2.9) | 0 (0) | |
| 2 | 51 (33.6) | 50 (36.8) | 1 (6.2) | |
| 3 | 44 (28.9) | 35 (25.7) | 9 (56.2) | |
| 4 | 51 (33.6) | 45 (33.1) | 6 (37.5) |
Esophageal Cancer Pathology and Its Incidence Among African Americans in the Last 50 Years
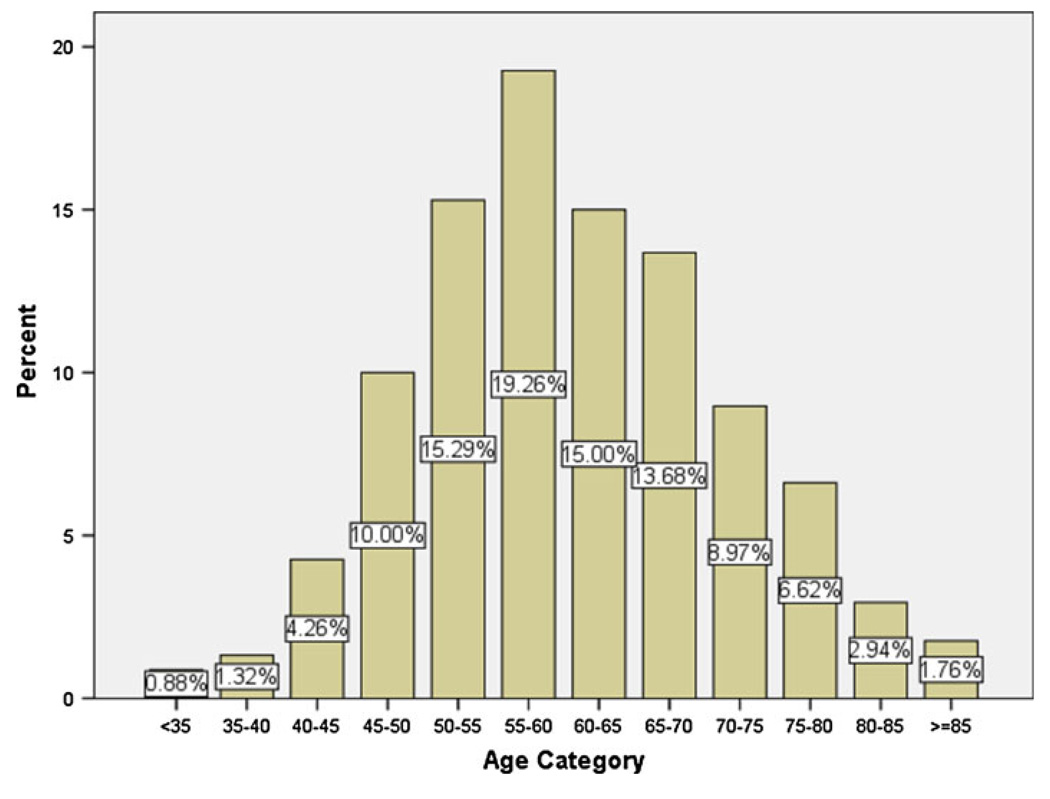
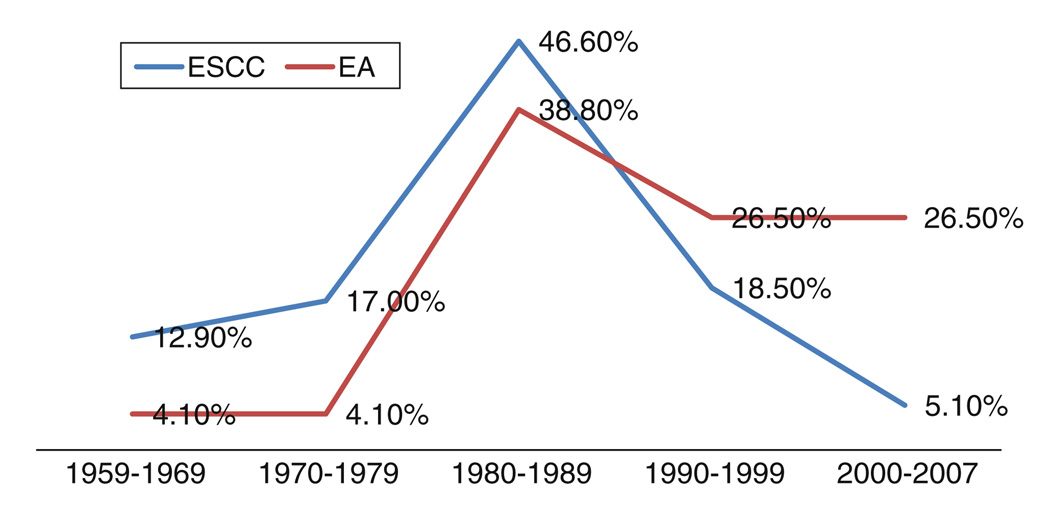
The distribution of EC among different age categories is depicted in Fig. 1. Most of our cases were diagnosed between ages 50 and 70 and the frequency of cancer was the lowest in those less than 35 years old. The proportion of new cases of ESCC to all diagnosed esophageal cancers in our center in the 1960s, 1970s, 1980s, 1990s, and 2000s were 97.3, 97.9, 93.1, 88.7, and 68.3%, respectively. The peak number of cases diagnosed with ESCC was in decade 1980–1989 (46.6%) and since then has markedly declined to 5.1% in 2000–2007 (Fig. 2).
Fig. 1.
The distribution of esophageal cancer frequency (both ESCC and EA) among different age groups, 1959–2007
Fig. 2.
Distribution of ESCC and EA by decade of diagnosis, 1959–2007. The peak number of cases diagnosed with ESCC was in decade 1980–1989 (46.6%) and since then has markedly declined to 5.1% in 2000–2007 (P < 0.001)
Patients (n = 175, 40.3%) with ESCC had poorly differentiated while 36.3% (157), 18.6% (73), and 6.7% (29) were moderately, well and undifferentiated tumors, respectively (Table 2).
Table 2.
Distribution of ESCC and EA by tumor differentiation, 1959–2007
| Differentiation | Esophageal cancer | Total no. (%) | |
|---|---|---|---|
| EA no. (%) | ESCC no. (%) | ||
| Well | 5 (13.9) | 73 (18.6) | 78 (18.4) |
| Moderate | 12 (33.4) | 157 (36.3) | 169 (36.0) |
| Poor | 16 (44.4) | 175 (40.3) | 191 (40.6) |
| Undifferentiated | 3 (8.3) | 29 (6.7) | 32 (6.8) |
| Total | 36 (100) | 434 (100) | 470 (100) |
The symptoms record was available for 467 patients. The most frequent symptom associated with esophageal cancer was dysphagia (76.9%), weight loss (31.8%), abdominal pain (5.5%), chest pain (8.4%), and gastrointestinal bleeding (6.2%). The frequency of symptoms in different types is reported in Table 3.
Table 3.
Frequency of symptoms among all patients and ESCC and EA cases
| Symptom | Total no. (%) |
ESCC no. (%) |
EA no. (%) |
|---|---|---|---|
| Dysphagia | 341 (76.9) | 318 (77.7) | 23 (67.6) |
| Weight loss | 139 (31.8) | 129 (32.0) | 10 (29.4) |
| Abdominal pain | 24 (5.5) | 21 (5.2) | 3 (8.8) |
| Chest pain | 37 (8.4) | 36 (9.0) | 1 (2.9) |
| Gastrointestinal bleeding | 27 (6.2) | 24 (6.0) | 3 (8.8) |
Discussion
The present study supports other studies that ESCC is the most common esophageal cancer in AAs with male sex predominance. However, its frequency decreased from 97.3% in the 1960s to 68.3% in first decade of 2000 at our institution. At the same time, esophageal adenocarcinoma experienced an increase in frequency from 2.7 to 31.7%. Most diagnoses of esophageal cancer at Howard University Hospital were made in 1980s (Fig. 2). About 20% of patients with EC were in the age range of 55–60 years. Most ESCC and EA lesions were found in the upper third (39%) and the lower third (61%) of the esophagus, respectively. Most ESCC cases were in stage 2 while EA patients were in stage 3. More than 40% of EC were reported as poorly differentiated histologically. The family history of cancer, smoking cigarette and alcohol drinking was quite high in both type of cancers, but there was no difference regarding age, sex, family history of cancer and tobacco smoking between these two histologic types of EC except for alcohol drinking, which was more common among ESCC patients (77% vs. 50%; P value 0.03).
Although EA in the United States and many Western countries has surpassed ESCC to become the most prevalent form of esophageal cancer [19–22], the frequency of esophageal squamous cell carcinoma is still quite high in our study which is consistent with the review study of Blot et al. [14] and with the SEER database. In this particular review, they found that ESCC was the most common form of EC but that the incidence of EA has been steadily rising by the early 1990s, EA had become the most common form of EC among whites, although SCC still predominated among black patients. We find that the frequency of ESCC has declined since 1990 at Howard University Hospital. This pattern is consistent with Surveillance, Epidemiology, and End Results (SEER) data [4] and several other studies [6–8, 13, 23, 24]. It is possible that a decline in the prevalence of smoking and alcohol drinking especially among men may be responsible for the decline of the incidence of ESCC since 1990 in our study. Other probable explanations could be different health policies over the years. Because the majority of the HUH patient population is AA and the number of EA in this population are so low, comparisons between EA and ESCC by demographic variables, staging and risk factors in this population might be misleading.
Earlier data suggest that AAs typically present with distant disease [3, 6, 7]. The current study shows that more than 95% of ESCC cases, based on staging information, were at stage 2 and above; 45% were at stage 4 at the time of diagnosis. Although the data on the number of cases of EA were too low for comparison (n = 16), 100% pf cases were shown to be above stage 2. Thus, this study sustains the hypothesis that in this particular type of cancer AAs are more likely to be diagnosed at a later stage of the disease process. This result is not consistent with the Cummings study [8] which shows that AAs are not more likely than whites to present with metastatic ESCC but consistent with several other studies [1, 3, 4, 6, 7, 16, 17]. Studies have shown that AAs are less likely to seek medical attention for early stages of disease. Also, their access to medical and/or health facilities is low which may postpone curative treatment if it had been caught at an earlier stage. Unfortunately, even after awareness of the disease, limited access to health care facilities will delay immediate medical assistance. The delay in early detection of ESCC significantly raises the mortality rate, since late detection may result in lower cure rates and shorter life-spans [6, 25].
We observed a difference in the anatomical location of esophageal cancer by histology. Table 1 describes that the ESCC is more common in the upper third of the esophageal followed by the middle and lower third which is consistent with other studies [26, 27], and the same is true for adenocarcinoma which has been shown to be more common in the lower esophagus. These differences in the anatomical locations between ESCC and EA may reflect the different etiological factors behind these two types of esophageal carcinomas.
Information regarding smoking, alcohol intake and family history of cancer was available from medical records at HUH since 1985. The history of tobacco smoking and alcohol abuse was very high in these two types of cancers and alcohol drinking was significantly higher in patients with ESCC. This finding is consistent with another study done to assess the risk factors for developing ESCC, in which they found that low income, moderate to heavy alcohol drinking and tobacco use is associated with almost all cases of ESCC in whites (98%) and blacks (99%) [24, 28]. Although alcohol use in all forms is an important risk factors for ESCC in whites and blacks, the type of alcoholic beverage used does not appear to account for the racial differences in ESCC incidence [29].
The current study is limited by missing detailed information on tobacco and alcohol use. However, it provides interesting results in the light of evidence from EC studies linking tobacco use and alcohol abuse, as in several other studies [11, 12, 27, 28]. Based on our data, about 21% of these patients had a family history of cancer in close relatives of whom 6% were gastric and/or esophageal cancer. Twenty-seven ESCC patients had a history of cancer in close relatives while in the EA group there was no history of cancer in close relatives. The genetic and environmental factors play major role in the development of ESSC and EA. However, Dhillon et al.’s [30] study denied any association between family history of cancer with esophageal cancer and these relationships must be interpreted with caution.
In conclusion, we observed that ESCC with advance stages is the predominant type of esophageal cancer in AAs. However, we have noted a decreasing trend in the diagnosis of esophageal cancer in our institution since 1990.
Acknowledgments
Grant support: CA102681, funded by the National Cancer Institute, NIH, and RCMI, Howard University.
Abbreviations
- AA
African Americans
- EC
Esophageal cancer
- ESCC
Esophageal squamous cell carcinoma
- EA
Esophageal adenocarcinoma
Contributor Information
Hassan Ashktorab, Email: hashktorab@howard.edu, Department of Medicine, Cancer Center, Howard University Cancer Center, Howard University Hospital, Rm#320, 2041 Georgia Ave., Washington, DC 20059, USA.
Zahra Nouri, Department of Medicine, Cancer Center, Howard University Cancer Center, Howard University Hospital, Rm#320, 2041 Georgia Ave., Washington, DC 20059, USA.
Mehdi Nouraie, Department of Medicine, Sickle Cell Disease Center, Howard University, Washington, DC, USA.
Hadi Razjouyan, Department of Medicine, Cancer Center, Howard University Cancer Center, Howard University Hospital, Rm#320, 2041 Georgia Ave., Washington, DC 20059, USA.
Edward E. Lee, Department of Pathology, Howard University, College of Medicine, Washington, DC, USA
Ehsan Dowlati, Department of Medicine, Cancer Center, Howard University Cancer Center, Howard University Hospital, Rm#320, 2041 Georgia Ave., Washington, DC 20059, USA.
El-Waleed El-Seyed, Department of Medicine, Cancer Center, Howard University Cancer Center, Howard University Hospital, Rm#320, 2041 Georgia Ave., Washington, DC 20059, USA.
Adeyinka Laiyemo, Department of Medicine, Cancer Center, Howard University Cancer Center, Howard University Hospital, Rm#320, 2041 Georgia Ave., Washington, DC 20059, USA.
Hassan Brim, Department of Pathology, Howard University, College of Medicine, Washington, DC, USA.
Duane T. Smoot, Department of Medicine, Cancer Center, Howard University Cancer Center, Howard University Hospital, Rm#320, 2041 Georgia Ave., Washington, DC 20059, USA
References
- 1.Jemal A, Center MM, Ward E. The convergence of lung cancer rates between blacks and whites under the age of 40, United States. Cancer Epidemiol Biomarkers Prev. 2009;18:3349–3352. doi: 10.1158/1055-9965.EPI-09-0740. [DOI] [PubMed] [Google Scholar]
- 2.Kamangar F, Chow WH, Abnet CC, Dawsey SM. Environmental causes of esophageal cancer. Gastroenterol Clin North Am. 2009;38:27–57. doi: 10.1016/j.gtc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 4.SEER Cancer Statistics Review. 1975–2005 http://seer.cancer.gov/csr/1975_2005/.
- 5.Jemal A, Center MM, Ward E, Thun MJ. Cancer occurrence. Methods Mol Biol. 2009;471:3–29. doi: 10.1007/978-1-59745-416-2_1. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Ward E, Thun M. Declining death rates reflect progress against cancer. PLoS One. 2010;5:e9584. doi: 10.1371/journal.pone.0009584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings LC, Cooper GS. Descriptive epidemiology of esophageal carcinoma in the Ohio Cancer Registry. Cancer Detect Prev. 2008;32:87–92. doi: 10.1016/j.cdp.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pottern LM, Morris LE, Blot WJ, Ziegler RG, Fraumeni JF., Jr Esophageal cancer among black men in Washington, DC. I. Alcohol, tobacco, and other risk factors. J Natl Cancer Inst. 1981;67:777–783. [PubMed] [Google Scholar]
- 10.Gammon MD, Schoenberg JB, Ahsan H, et al. Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1997;89:1277–1284. doi: 10.1093/jnci/89.17.1277. [DOI] [PubMed] [Google Scholar]
- 11.Wu AH, Wan P, Bernstein L. A multiethnic population-based study of smoking, alcohol and body size and risk of adenocarcinomas of the stomach and esophagus (United States) Cancer Causes Control. 2001;12:721–732. doi: 10.1023/a:1011290704728. [DOI] [PubMed] [Google Scholar]
- 12.Vioque J, Barber X, Bolumar F, et al. Esophageal cancer risk by type of alcohol drinking and smoking: a case–control study in Spain. BMC Cancer. 2008;8:221. doi: 10.1186/1471-2407-8-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184–1187. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blot WJ, McLaughlin JK. The changing epidemiology of esophageal cancer. Semin Oncol. 1999;26:2–8. [PubMed] [Google Scholar]
- 15.Younes M, Henson DE, Ertan A, Miller CC. Incidence and survival trends of esophageal carcinoma in the United States: racial and gender differences by histological type. Scand J Gastroenterol. 2002;37:1359–1365. doi: 10.1080/003655202762671215. [DOI] [PubMed] [Google Scholar]
- 16.Mesihovic R, Vanis N, Gribajcevic M. Pretherapeutic staging of the esophageal cancer using endoscopic ultrasound. Med Arh. 2006;60:110–114. [PubMed] [Google Scholar]
- 17.Brown LM, Hoover R, Silverman D, et al. Excess incidence of squamous cell esophageal cancer among US Black men: role of social class and other risk factors. Am J Epidemiol. 2001;153:114–122. doi: 10.1093/aje/153.2.114. [DOI] [PubMed] [Google Scholar]
- 18.Frederick L, Greene CMB, Haller DG, Morrow M. AJCC Cancer Staging Manual. (6th ed.) 2002 [Google Scholar]
- 19.Balaji NS, DeMeester SR, Wickramasinghe KS, Hagen JA, Peters JH, DeMeester TR. Etiology of intestinal metaplasia at the gastroesophageal junction. Surg Endosc. 2003;17:43–48. doi: 10.1007/s00464-002-8944-1. [DOI] [PubMed] [Google Scholar]
- 20.Derakhshan MH, Malekzadeh R, Watabe H, et al. Combination of gastric atrophy, reflux symptoms and histological subtype indicates two distinct aetiologies of gastric cardia cancer. Gut. 2008;57:298–305. doi: 10.1136/gut.2007.137364. [DOI] [PubMed] [Google Scholar]
- 21.Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85:1457–1459. doi: 10.1046/j.1365-2168.1998.00940.x. [DOI] [PubMed] [Google Scholar]
- 22.Demeester SR. Epidemiology and biology of esophageal cancer. Gastrointest Cancer Res. 2009;3:S2–S5. [PMC free article] [PubMed] [Google Scholar]
- 23.Kort EJ, Sevensma E, Fitzgerald TL. Trends in esophageal cancer and body mass index by race and gender in the state of Michigan. BMC Gastroenterol. 2009;9:47. doi: 10.1186/1471-230X-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am. 2002;11:235–256. doi: 10.1016/s1055-3207(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 25.Ghafoor A, Jemal A, Cokkinides V, et al. Cancer statistics for African Americans. CA Cancer J Clin. 2002;52:326–341. doi: 10.3322/canjclin.52.6.326. [DOI] [PubMed] [Google Scholar]
- 26.Chalasani N, Wo JM, Waring JP. Racial differences in the histology, location, and risk factors of esophageal cancer. J Clin Gastroenterol. 1998;26:11–13. doi: 10.1097/00004836-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Fan YJ, Song X, Li JL, et al. Esophageal and gastric cardia cancers on 4238 Chinese patients residing in municipal and rural regions: a histopathological comparison during 24-year period. World J Surg. 2008;32:1980–1988. doi: 10.1007/s00268-008-9674-x. [DOI] [PubMed] [Google Scholar]
- 28.Freedman J, Lagergren J, Bergstrom R, Naslund E, Nyren O. Cholecystectomy, peptic ulcer disease and the risk of adenocarcinoma of the oesophagus and gastric cardia. Br J Surg. 2000;87:1087–1093. doi: 10.1046/j.1365-2168.2000.01459.x. [DOI] [PubMed] [Google Scholar]
- 29.Brown LM, Hoover R, Gridley G, et al. Drinking practices and risk of squamous-cell esophageal cancer among Black and White men in the United States. Cancer Causes Control. 1997;8:605–609. doi: 10.1023/a:1018446430228. [DOI] [PubMed] [Google Scholar]
- 30.Dhillon PK, Farrow DC, Vaughan TL, et al. Family history of cancer and risk of esophageal and gastric cancers in the United States. Int J Cancer. 2001;93:148–152. doi: 10.1002/ijc.1294. [DOI] [PubMed] [Google Scholar]