Abstract
The objectives of this study were to determine whether neutrophil depletion with anti-neutrophil serum (ANS) or preconditioning with the hydrogen sulfide (H2S) donor NaHS (NaHS-PC) 24 h prior to ischemia-reperfusion (I/R) would prevent postischemic mitochondrial dysfunction in rat intestinal mucosa and, if so, whether calcium-activated, large conductance potassium (BKCa) channels were involved in this protective effect. I/R was induced by 45-min occlusion of the superior mesenteric artery followed by 60-min reperfusion in rats preconditioned with NaHS (NaHS-PC) or a BKCa channel activator (NS-1619-PC) 24 h earlier or treated with ANS. Mitochondrial function was assessed by measuring mitochondrial membrane potential, mitochondrial dehydrogenase function, and cytochrome c release. Mucosal myeloperoxidase (MPO) and TNF-α levels were also determined, as measures of postischemic inflammation. BKCa expression in intestinal mucosa was detected by immunohistochemistry and Western blotting. I/R induced mitochondrial dysfunction and increased tissue MPO and TNF-α levels. Although mitochondrial dysfunction was attenuated by NaHS-PC or NS-1619-PC, the postischemic increases in mucosal MPO and TNF-α levels were not. The protective effect of NaHS-PC or NS-1619-PC on postischemic mitochondrial function was abolished by coincident treatment with BKCa channel inhibitors. ANS prevented the I/R-induced increase in tissue MPO levels and reversed mitochondrial dysfunction. These data indicate that neutrophils play an essential role in I/R-induced mucosal mitochondrial dysfunction. In addition, NaHS-PC prevents postischemic mitochondrial dysfunction (but not inflammation) by a BKCa channel-dependent mechanism.
Keywords: large conductance, calcium-activated potassium channels, mitochondrial membrane potential, mitochondrial respiration, cytochrome c release, anti-neutrophil serum, ileum, myeloperoxidase, TNF-α, rats
preconditioning refers to a phenomenon wherein tissues exposed to mildly noxious stimuli (e.g., ethanol, capsaicin, CGRP, heat, reactive oxygen metabolites, short bouts of ischemia) or a variety of chemical agents [e.g., nitric oxide (NO), hydrogen sulfide (H2S) or carbon monoxide (CO) donors, adenosine, bradykinin, opioids, sildenafil, volatile anesthetics, KATP channel or AMPK activators] exhibit protection from the deleterious effects induced by subsequent exposure to prolonged ischemia and reperfusion (I/R) (2, 9, 10, 13, 14, 17, 18, 20, 33, 38, 57, 58, 60, 66, 67). The protective effects of preconditioning occur over two distinct temporal phases (2, 13, 14, 17, 58). An initial, relatively short-lived phase arises within minutes of exposure to the preconditioning stimulus and then disappears after 1–4 h (acute, early phase, or classical preconditioning). This is followed 12–24 h later by the reappearance of a longer-lived (24–72 h) and often more powerful phase of tolerance to ischemia that is referred to as the second window of protection, late phase, or delayed preconditioning. Interestingly, H2S pretreatment only produces late phase preconditioning (60), a unique finding compared with the large number of preconditioning stimuli studied to date, all of which induce both phases of preconditioning.
H2S, together with NO and CO, belongs to a family of endogenous signaling molecules collectively termed gasotransmitters, which share many similarities (41, 46). As a gasotransmitter, H2S rapidly travels through cell membranes without using specific transporters. The production of H2S occurs through several pathways in mammalian systems, the most prominent of which are two key enzymes in the cysteine biosynthesis pathway, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE). At low micromolar concentrations (less than 100–200 μM), H2S exerts cytoprotective (antinecrotic or antiapoptotic) effects, whereas higher levels of H2S exposure (greater than 250 μM) are cytotoxic (41, 46, 49, 50, 57, 60, 62, 66, 67). Emerging evidence suggests that H2S is a regulator of the N-methyl-d-aspartate (NMDA) receptor and may be central in long-term potentiation of neuronal circuitry (1, 19). In addition, H2S is known to be produced in the vasculature by CSE where it mediates smooth muscle relaxation and subsequent vasodilation and limits baseline leukocyte adhesion (41, 46, 61, 63). Several studies demonstrated that H2S is cardioprotective, reducing myocardial infarct size when administered prior to or during I/R (16, 33, 65). These findings have been extended to the stomach, lungs, liver, and kidneys, where H2S treatment was shown to reduce injury, again when administered prior to or during I/R (8, 15, 43, 59). In the small intestine, we have demonstrated that antecedent treatment with the exogenous H2S donor NaHS (NaHS-PC) induces a preconditioned state wherein postcapillary venules fail to support leukocyte rolling and adhesion on subsequent exposure to I/R 24 h later by a NO- and p38 MAPK-dependent mechanism (60). Liu et al. (26) have shown that H2S administration during ischemia reduces intestinal injury in the rat by a mechanism that may involve increased antioxidant enzyme activity. Elrod and coworkers (6) were the first to demonstrate that H2S during I/R attenuates myocardial injury by preservation of mitochondrial function.
A growing body of evidence indicates that agents that induce the opening of large conductance, calcium-activated potassium channels (BKCa) are cardioprotective in the setting of I/R. For example, Xu et al. (56) first demonstrated that the selective BKCa channel opener NS-1619 leads to a significant reduction in infarct size after global I/R in isolated, perfused rabbit hearts, a cardioprotective effect that was completely prevented by administration of the selective BKCa channel inhibitor paxilline. In addition, the cardioprotective effects of estradiol appear to involve BKCa channel activation in rat ventricular myocytes exposed to simulated ischemia (32). BKCa channels are expressed in most mammalian cells and are activated by elevations in intracellular Ca2+ through the physiological range as well as by membrane depolarization (12, 51, 55). On the basis of these findings, we hypothesized that treatment with a H2S donor (NaHS-PC) 24 h prior to I/R would reduce postischemic intestinal mitochondria dysfunction by a BKCa channel-dependent mechanism. In addition, because neutrophils play an important role in the pathogenesis of I/R-induced mucosal dysfunction (24) and BKCa channel activation appears to play an important role in initiating the effects of NaHS-PC to prevent postischemic leukocyte rolling and adhesion when the bowel is exposed to I/R 24 h later (66), we also sought to determine whether these phagocytic cells contribute to postischemic mucosal mitochondrial dysfunction and, if so, whether there is a mechanistic link between H2S-induced mitochondrial protection and I/R-induced leukocyte infiltration.
METHODS
Animals and Surgery
Male Sprague-Dawley rats (∼250–350 g) were obtained from Hilltop Laboratories (Scottsdale, PA) and were maintained on standard rat chow. Experiments were carried out in accordance with the guidelines set forth by National Institutes of Health and were approved by the University of Missouri Institutional Animal Care and Use Committee. The rats were anesthetized with the mixture of ketamine (90 mg/kg body wt ip) and xylazine (20 mg/kg body wt ip) and intubated to ensure a patent airway. A laparotomy was performed, and the superior mesenteric artery (SMA) was isolated and was occluded with a microvascular clip for 45 min. Intestinal blood flow was reestablished by removal of the clip, and after 60-min reperfusion a 20-cm of ileum was carefully removed, placed on ice, rinsed thoroughly with normal saline, refilled with 10 mM DTT in enterocyte isolation buffer (EIB) (17 mM HEPES, 25 mM NaHCO3 in PBS, pH 7.4), and tied off at both ends. The segment was then gently massaged to remove the mucus. After draining of the luminal contents, the segment was opened longitudinally to expose the intestinal mucosa. The mucosal layer was harvested by gently scraping for subsequent analysis.
Experimental Protocols
The general design of the experimental protocols for each group in the study is described below. All drug dosages were selected from previous reports in the literature.
Group 1: sham.
As a time control for the effects of experimental duration, the SMA was exposed but not subjected to occlusion. Ileal mucosal samples were obtained for assessment of mitochondrial function (n = 6), myeloperoxidase (MPO) content, and TNF-α levels (n = 6).
Group 2: I/R alone.
Rats in this group were treated as described for group 1 except that the SMA was occluded for 45 min followed by reperfusion for 60 min. Ileal mucosal samples were obtained at the end of reperfusion for assessment of mitochondrial function (n = 6), MPO content, and TNF-α levels (n = 6).
Group 3: NaHS + I/R.
To determine whether H2S would act as a preconditioning stimulus and prevent postischemic mitochondrial dysfunction, neutrophil sequestration, and increased mucosal TNF-α levels when subjected to I/R, rats in this group were treated with a solution of NaHS (H2S donor, Sigma Chemical, St. Louis, MO; 14 μmol/kg ip) 24 h prior to I/R. Samples were harvested for assessment of mitochondrial function (n = 6), MPO content, and TNF-α levels (n = 6) at the end of the reperfusion period, as described for group 2.
Group 4: paxilline or penitrem A + NaHS + I/R.
To explore the role of BKCa channels as a trigger for the development of NaHS-PC, rats were treated as described for group 3 except that a selective BKCa channel inhibitor, either paxilline (2.5 mg/kg ip) or penitrem A (0.4 μg/kg), was administered 10 min prior to NaHS treatment in separate groups of experiments (n = 6 in each).
Group 5: NS-1619 + I/R.
The aim of this group of experiments was to determine whether preconditioning with the BKCa channel opener, NS-1619 [1-(2′-hydroxy-5′-trifluoromethylphenyl)-5-trifluoromethyl-2(3H) benzimid-axolone], would mimic the effects of NaHS-PC and prevent postischemic mitochondrial dysfunction on subsequent exposure of the small intestine to I/R 24 h later. Rats in this group (n = 6) were treated as described in group 3 except that they received NS-1619 (1.0 mg/kg ip) 24 h prior to I/R in lieu of NaHS.
ANS treatment protocols (groups 6–8).
Male Sprague-Dawley rats (250∼350 g) were divided into three groups.
group 6. ans+i/r (n = 6).
Rats in this group were administered three injections of anti-neutrophil serum (ANS; Inter-Cell Technologies, Jupiter, FL; 1 ml/kg) at 12-h intervals. Three hours after the last injection, the small bowel was subjected to I/R, with ileal samples obtained at the end of the reperfusion period for assessment of mitochondrial function, MPO content, and mucosal TNF-α levels.
group 7. i/r alone (n = 6).
Rats were prepared as described for group 6 above, except that saline was injected in lieu of ANS three times at 12-h intervals.
group 8. sham control (n = 6).
Mice in this group were treated as described for group 7, except that the SMA was not occluded. Ileal samples were obtained at time points comparable to group 6. At the end of each of the experiments described for groups 6–8, blood samples were obtained for evaluation of circulating neutrophil counts.
Circulating Neutrophil Counts
Whole blood was obtained by cardiac puncture at the end of the reperfusion period. To quantify total leukocyte count, blood samples were diluted 1:20 with 1% gentian violet solution, then counted by use of a hemocytometer. To obtain neutrophil differential count, the blood samples were feathered onto a microscope slide followed by Wright Giemsa staining, then counted under the microscope. Circulating neutrophil counts were calculated as the product of total leukocyte count and percentage neutrophils and were expressed as cells per microliter of whole blood.
Measurement of Mitochondrial Membrane Potential of Intestinal Mucosal Cells
Mitochondria membrane potential was determined by using 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide (JC-1) (54). Scraped mucosal cells were resuspended in EIB (with 10 mM DTT and 0.25% BSA) and centrifuged at 500 g for 5 min at 4°C. The pellets were washed with EIB and centrifuged at 500 g for 5 min at 4°C. Following supernatant removal, 0.5 ml pellets were obtained and resuspended with 2 ml EIB. Aliquots of the 2-ml suspension were separated into two centrifuge tubes (1 ml in each tube), and 2 μl of 0.2 mg/ml JC-1 or DMSO (as a control), respectively, were added to the tubes. After 15-min incubation at room temperature in the dark, 200 μl of cell suspension were transferred to an opaque, 96-well plate and analyzed with a SpectraMax M2 plate reader at excitation vs. emission wavelengths for red vs. green fluorescence at 550 and 600 nm vs. 485 and 535 nm, respectively. Results were expressed as the ratio of red to green fluorescence.
Measurement of Mitochondrial Respiration of Intestinal Enterocytes
Mitochondrial respiration of intestinal enterocytes was studied by measuring the mitochondrial dehydrogenase-dependent reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT; Sigma Chemical) to its formazan derivative (MTT-FZ) (52). Scraped mucosal cells were resuspended in EIB (with 10 mM DTT and 0.25% BSA) and centrifuged at 500 g for 5 min at 4°C. The pellets were washed with EIB and centrifuged at 500 g again for 5 min at 4°C. The supernatant was removed and 0.5 ml pellets were taken and resuspended with 2 ml EIB. The cell suspension was then mixed with 2.5 ml MTT solution (2 mg/ml in EIB) and incubated for 15-min at room temperature in the dark. After incubation, the suspension was divided into two 15-ml Corex glass centrifuge tubes (2 ml each) for MTT and DNA assay, respectively. Following centrifugation at 9,000 g for 10 min at 4°C, the supernatant was removed and the pellets were homogenized in 2 ml lysis buffer: 5 mM Tris·HCl, 20 mM EDTA, and 0.1% Triton X-100, pH 8.0 (lysis buffer for DNA analysis did not include Triton X-100). The homogenate was centrifuged again at 9,000 g for 5 min at 4°C, 1 ml of MTT supernatant was solubilized with 2 ml of DMSO, and the absorbance of a 200-μl aliquot of MTT supernatant-DMSO mixture at 540 nm was determined. Total DNA was determined by mixing an aliquot of cell lysate with Hoechst 33258 (bisbenzimide) and measuring fluorescence (360 nm excitation, 460 nm emission). The results were expressed as a ratio of MTT-FZ to DNA concentration (mg/ml).
Enterocyte Protein Extraction and Western Blotting
Mucosal cells were isolated as described above, resuspended in PBS, and centrifuged 5 min at 500 g at 4°C.
BKCa channel expression in ileal enterocytes.
Preliminary Western blot studies showed BKCa channel present in both total membrane and mitochondrial fractions of ileal enterocytes, however, mitochondrial fractions also showed pronounced E-cadherin immunoreactivity, indicating significant contamination of mitochondrial fractions with plasma membrane. Therefore, to resolve the issue of subcellular location of BKCa channel in enterocytes, we performed a more rigorous fractionation procedure using modifications of previously published methods (27, 53).
Distal ileal segments (20 cm) were taken from six rats, thoroughly flushed with ice-cold 0.15 M NaCl, filled with EIB + 10 mM DTT, tied off with suture at both ends, then incubated in a gently shaking water bath in prewarmed saline at 37°C for 10 min. The EIB + DTT was drained out, and segments were then refilled with EIB + DTT + 5 mM EGTA and further incubated as above for 15 min to release gut enterocytes. After incubation, each segment was placed on a glass plate over ice and gently massaged; then the enterocytes were collected by draining the segment into a 50-ml centrifuge tube through a saline-wet gauze sponge. Drained segments were washed once more with EIB + DTT + EGTA; this wash was added to the collection. Harvested enterocytes from all six ileal segments were pooled for further processing. Cells were centrifuged at 500 g for 5 min at 4°C, the supernatant was removed, and the cell pellet was gently resuspended in EIB + DTT + 0.25% BSA. This suspension was centrifuged as above, the supernatant was removed, and the cells were resuspended in EIB as a final wash. All subsequent procedures were carried out on ice in a cold room (4°C).
After centrifugation, the EIB was removed and the cells were resuspended in 225 mM mannitol, 75 mM sucrose, 30 mM Tris·HCl, pH 7.4, 0.5 mM EGTA, 0.5% BSA and homogenized by use of a Dounce homogenizer (6 strokes with a loose-fitting pestle, and 15 strokes with a tight-fitting pestle). The homogenate was centrifuged at 325 g for 10 min. The pellet was discarded and the supernatant was further centrifuged at 500 g for 5 min. The pellet from this spin was also discarded, and the supernatant was then centrifuged at 9,000 g for 10 min. The supernatant from this spin was saved for subsequent isolation of the total membrane fraction, and the pellet was gently resuspended in 225 mM mannitol, 75 mM sucrose, 30 mM Tris·HCl, pH 7.4, and 0.5% BSA and centrifuged at 10,000 g for 10 min. The supernatant was added to that previously saved, and the pellet was gently washed and centrifuged one last time in 225 mM mannitol, 75 mM sucrose, 30 mM Tris·HCl, pH 7.4 at 10,000 g for 10 min. The supernatant from this final wash was added to those already collected. The crude mitochondrial pellet was resuspended in Laemmli sample buffer (25) without thiol reagent or bromophenol blue, boiled for 5 min, and lysed by sonication for 10 s on ice; this mitochondrial lysate was centrifuged at 10,000 g for 5 min. Supernatant from this spin was saved as the crude mitochondrial fraction. Pooled total membrane fractions were centrifuged at 100,000 g for 60 min in a Beckman MLA-80 rotor, and the membrane pellet was resuspended, boiled, and sonicated as described above for the mitochondrial fraction. These fractions were assayed for protein by use of the Bio-Rad Dc protein assay (Bio-Rad, Hercules, CA). 2-Mercaptoethanol and bromophenol blue were added to 5 and 0.0025%, respectively, and then 40 μg protein from each sample were subjected to SDS-PAGE, followed by electrotransfer onto nitrocellulose membrane. Blots were probed with primary rabbit polyclonal antibodies to BKCa α-subunit (APC-107, Alomone Labs, Jerusalem, Israel) and mitochondrial porin (Sigma, St. Louis, MO), with secondary detection using horseradish peroxidase (HRP)-anti-rabbit IgG (Cell Signaling Technology, Danvers, MA) and mouse monoclonal primary antibody to E-cadherin (BD Biosciences, San Jose, CA) with secondary HRP-anti-mouse IgG (Santa Cruz Biotechnologies, Santa Cruz, CA). Bands were detected by use of the Pierce Super Signal West Pico (Rockford, IL) chemiluminescent detection system.
Cytosolic cytochrome c.
Isolated, washed enterocytes were Dounce homogenized for 30 strokes in 10 volumes of lysis buffer (250 mM sucrose, 20 mM HEPES-KOH, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1.8 mg/ml iodoacetamide, 1 mM PMSF, and 10 μl/ml protease inhibitor cocktail). The homogenate was centrifuged at 1,000 g for 10 min at 4°C. The pellet was discarded and the postnuclear supernatant was centrifuged at 3,000 g for 15 min at 4°C. The initial postmitochondrial supernatant was further centrifuged at 10,000 g for 15 min at 4°C to obtain the final postmitochondrial supernatant, which was kept as the “cytosolic” fraction and stored at −70°C for later analysis. Protein content was assayed as described above.
To quantify cytosolic cytochrome c as a marker of mitochondrial membrane disruption (21), equal amounts of the cytosolic fractions (20 μg) were subjected in a SDS-PAGE and then electroblotting as described above. Blotted membranes were probed with a mouse monoclonal antibody to cytochrome c (Pharmingen/BD Biosciences, San Diego, CA) or β-actin (Sigma), followed by HRP-coupled anti-mouse IgG (Santa Cruz Biotechnologies, Santa Cruz, CA). Blots were developed by chemiluminescent detection, and relative band intensity was quantified by use of Quantity One software (Bio-Rad). Results were expressed as the ratio of cytosolic cytochrome c to β-actin arbitrary densitometric units.
Immunohistochemical Staining of BKCa
Fresh ileal segments (1 cm) were embedded in optimal cutting temperature compound and snap frozen in liquid nitrogen. Frozen sections (10-μm thickness) were cut and fixed in 4% paraformaldehyde for 20 min. They were blocked in 5% BSA + 1% Triton X-100 + PBS for 30 min, then incubated with anti-BKCa (APC-107, 1:50, Alomone Labs) antibody overnight at 4°C. After several washes, secondary antibody HRP-conjugated anti-rabbit (1:100, Cell Signaling) incubation was performed for 2 h at room temperature. Staining was developed with diaminobenzidine for 10 min, and the nuclei were counterstained with hematoxylin. These sections were compared with negative control sections incubated with preabsorbed primary antibody (as described for Western blot measurements) instead of anti-BKCa antibody (APC-107).
Tissue MPO Activity
Mucosal MPO activity was measured in mucosal samples obtained at the end of the reperfusion period, with use of a fluorescence assay kit (Cell Technology, Mountain View, CA). Samples of scraped mucosa were prepared as per kit manufacturers' instructions. MPO activity in the samples was quantified by adding the kit detection reagent and measuring the resulting fluorescence at 530 nm (excitation) and 590 nm (emission). Values were normalized for sample protein content and MPO activity was expressed as milliunits per milligram protein.
TNF-α Assay
Scraped mucosa was homogenized in 1 ml lysis buffer (10 mM Tris·HCl, pH 7.4, 250 mM sucrose, 20 mM HEPES, 1 mM EDTA, 1 mmol PMSF, 10 μl/ml protease inhibitor cocktail) and sonicated for 30 s. The homogenate was then centrifuged at 12,000 g for 20 min at 4°C. Aliquots of the supernatants were stored at −80°C until use. TNF-α levels were measured in duplicate by enzyme-linked immunosorbent assay (Invitrogen KRC3012, Camarillo, CA) according to the manufacturer's instructions. The minimum detectable level of TNF-α is <4 pg/ml. Intestinal mucosal TNF-α levels were expressed as picograms per milligram of protein.
Statistical Analysis
All data were initially analyzed by ANOVA, followed by Newman-Keuls post hoc test. A P value of less than 0.05 was considered to indicate statistical significance.
RESULTS
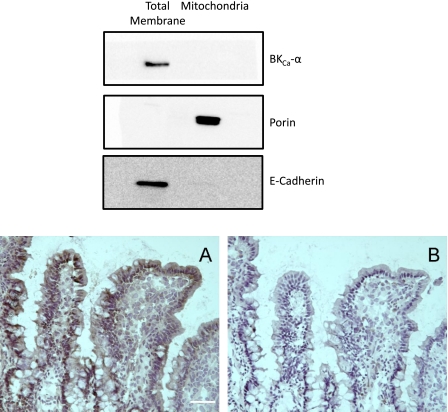
The presence of BKCa in ileal mucosa was demonstrated by immunohistochemistry, with immunoreactivity detected in villus crypts and in enterocytes lining the villus proper (Fig. 1, bottom, A). Subcellular distribution of BKCa α-subunit in ileal enterocytes was examined by Western blot. Total membrane and mitochondrial fractions isolated from freshly isolated enterocytes were probed for expression of the α-subunit of the BKCa channel, along with markers specific for plasma membrane and mitochondria [E-cadherin and VDAC (porin), respectively] (Fig. 1, top). Mitochondrial porin was detected only in the mitochondrial fraction, and although there was some remaining trace contamination of the mitochondrial fraction with E-cadherin, there was no detectable BKCa α-subunit immunoreactivity in the mitochondria, whereas there was robust expression of BKCa α-subunit in the total membrane fraction. To the best of our knowledge, these data provide the first demonstration that BKCa is expressed in small bowel enterocytes.
Fig. 1.
Expression of calcium-activated, large conductance potassium (BKCa) channel in rat intestinal enterocytes. Top: Western blot of total membrane and crude mitochondrial fractions from isolated mouse ileal enterocytes (see methods), each probed for the α-subunit of BKCa channel, mitochondrial porin (VDAC, outer membrane marker for mitochondria), and E-cadherin (plasma membrane protein used as marker for the total membrane fraction). Bottom, A: immunostaining of BKCa α-subunit protein in enterocytes of rat ileal mucosa. Bottom, B: negative controls were performed by using BKCa-α primary antibody that had been preabsorbed by use of a control peptide. Scale bar = 20 μm.
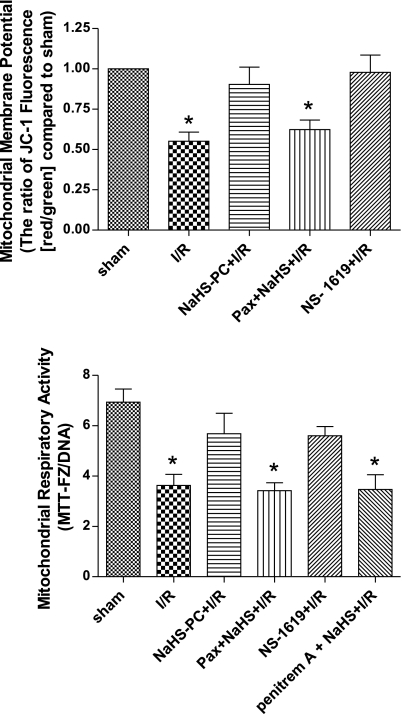
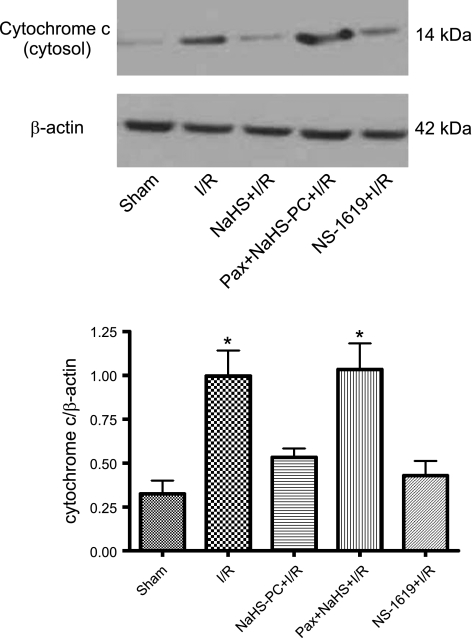
To determine whether I/R induced mucosal mitochondrial dysfunction, we monitored changes in mitochondrial membrane potential (using JC-1, which exists as a green-fluorescent monomer at low membrane potentials but forms red-fluorescent “J-aggregates” at higher potentials), respiratory function (by mitochondrial dehydrogenase-dependent conversion of MTT to its formazan derivative, MTT-FZ), and membrane integrity (by translocation of mitochondrial cytochrome c to the cytosol) in mucosal samples obtained from nonischemic control rats and from animals subjected to intestinal I/R. I/R was associated with a marked decrease in mitochondrial membrane potential (Fig. 2, top) and respiratory activity (Fig. 2, bottom), whereas cytosolic cytochrome c increased (Fig. 3) relative to sham controls. These effects of I/R to induce mitochondrial dysfunction were largely abolished by antecedent treatment with the H2S donor NaHS (NaHS-PC), 24 h prior to I/R (Figs. 2 and 3).
Fig. 2.
Reductions in mitochondrial membrane potential (top) and respiratory activity (bottom) induced by ischemia-reperfusion (I/R) are prevented by preconditioning with the hydrogen sulfide donor NaHS (NaHS-PC+I/R) or the BKCa channel activator NS-1619 (NS-1619+I/R) 24 h earlier. The effects of NaHS-PC to preserve postischemic mitochondrial function are prevented by coincident administration of the BKCa channel inhibitor paxilline (Pax+NaHS-PC+I/R) or penitrem A (penitrem A+NaHS-PC+I/R) with NaHS 24 h prior to I/R. Data are means ± SE for 6 rats per treatment group. *P < 0.05 compared with sham, NaHS-PC+I/R and NS-1619+I/R groups.
Fig. 3.
I/R induced release of cytochrome c into the cytosol of mucosal epithelial cells relative to sham (nonischemic) intestine. This disruption in postischemic mitochondrial membrane integrity was prevented by preconditioning with either the hydrogen sulfide (H2S) donor NaHS (NaHS-PC+I/R) or the BKCa channel activator NS-1619 (NS-1619+I/R) 24 h prior to induction of I/R. Coincident treatment with paxilline, a BKCa channel inhibitor, with NaHS 24 h prior to I/R (Pax+NaHS-PC+I/R) abrogated the protective actions of this H2S donor on postischemic mitochondrial membrane integrity. Top: representative Western blot showing comparison of signals from cytochrome c vs. β-actin. Bottom: densitometric analysis of full data set from 6 rats per treatment group. *P < 0.05 compared with sham, NaHS-PC+I/R, and NS-1619+I/R groups.
Our next series of experiments were directed at determining the role of BKCa channels in initiating or triggering the effects of NaHS-PC to preserve postischemic mitochondrial function in ileal mucosal epithelium. Treating rats with the BKCa channel inhibitor paxilline 10 min prior to administration of NaHS largely abolished the effects elicited by this H2S donor treatment to stabilize postischemic mitochondrial membrane potential (Fig. 2, top), preserve mitochondrial respiratory function (Fig. 2, bottom), and limit cytochrome c release from mitochondria into the cytosol (Fig. 3). Because paxilline exerts other effects in addition to inhibiting BKCa channels (11, 30, 47, 52), we also evaluated the effect of another BKCa channel inhibitor, penitrem A, on mitochondrial respiration in separate groups of rats subjected to sham, I/R, NaHS+I/R, and penitrem A+NaHS+I/R, which demonstrated the same pattern of response as noted in animals treated with paxilline (Fig. 2, bottom). These observations are consistent the hypothesis that NaHS-PC preserves postischemic mitochondrial function in ileal mucosal enterocytes by a BKCa channel-dependent mechanism. This concept is further supported by our observations that preconditioning with the selective BKCa channel activator, NS-1619, in lieu of H2S, was as effective as NaHS-PC in preserving mitochondrial membrane potential, respiratory function, and membrane integrity (Figs. 2 and 3).
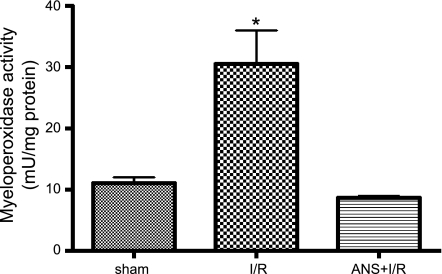
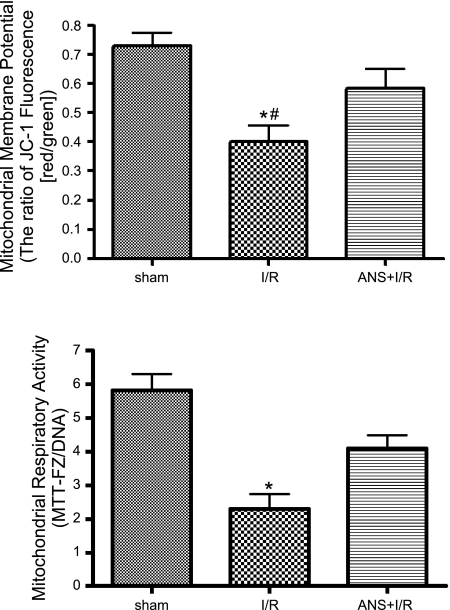
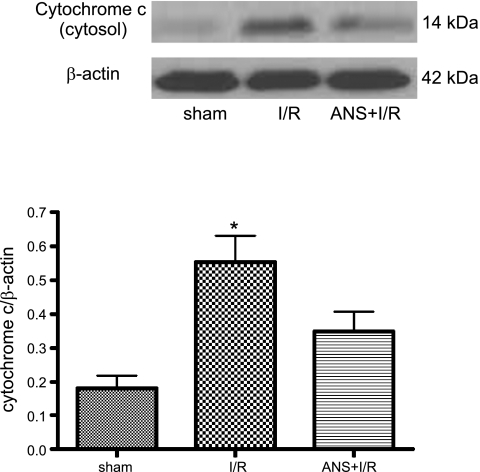
In addition to preserving postischemic mucosal mitochondrial function, other work conducted in our laboratory indicated that NaHS-PC prevented I/R-induced leukocyte rolling along and adhesion to postcapillary venules in the ileal microcirculation by an NO- and p38 MAPK-dependent mechanism (60) that may involve activation of venular BKCa channels (66). Taken together with our present results, these studies suggest that I/R-induced leukocyte infiltration may damage intestinal enterocytes as exemplified by mucosal mitochondrial dysfunction. To determine whether neutrophils contribute to mucosal mitochondrial dysfunction, we evaluated the effect of neutrophil depletion on I/R-induced alterations in mitochondrial membrane potential, respiratory activity, and cytochrome c release. ANS produced a marked reduction in the numbers of circulating neutrophils (Table 1) and prevented the postischemic increase in tissue myeloperoxidase (MPO) activity (Fig. 4). Neutrophil depletion also attenuated I/R-induced reductions in mitochondrial membrane potential (Fig. 5, top) and respiratory activity (Fig. 5, bottom), while limiting the postischemic release of cytochrome c into the cytosol (Fig. 6). These observations indicate that neutrophils play an important role in producing mucosal mitochondrial dysfunction after I/R.
Table 1.
Effect of ANS on number of circulating neutrophils
Data are means ± SE for 6 rats per group. I/R, ischemia-reperfusion; ANS, anti-neutrophil serum.
P < 0.05 compared with sham;
P < 0.001 compared with sham or I/R.
Fig. 4.
Increase in ileal mucosal myeloperoxidase (MPO) activity induced by I/R relative to sham (nonischemic) controls is prevented by neutrophil depletion by use of anti-neutrophil serum (ANS). Data are means ± SE for 6 rats per treatment group. *P < 0.001 compared with sham and ANS+I/R groups.
Fig. 5.
Neutrophil depletion with ANS prevents the reductions in mitochondrial membrane potential (top) and respiratory activity (bottom) induced by I/R relative to sham (nonischemic) controls. Data represents means ± SE for 6 rats per group. *P < 0.01 compared with sham group, #P < 0.05 compared with ANS+I/R group. JC-1, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide; MTT-FZ, formazan derivative of 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide.
Fig. 6.
ANS+I/R treatment prevented the postischemic release of cytochrome c into enterocyte cytosol induced by I/R compared with sham (nonischemic) preparations. Top: representative Western blot from full data set. Bottom: densitometric analysis of aggregate data. Data are means ± SE from 6 rats per group. *P < 0.05 compared with sham and ANS+I/R groups.
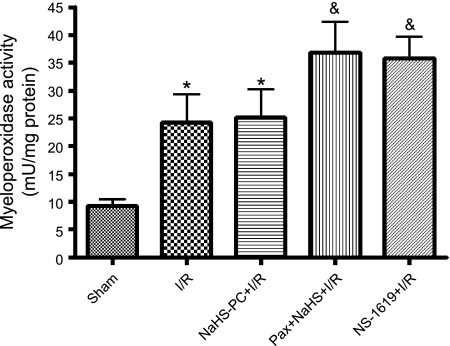
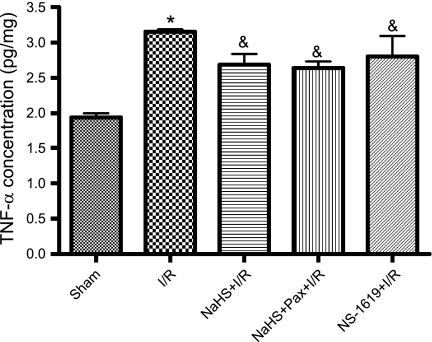
Our results with ANS led us to test whether the effects of preconditioning with NaHS or NS-1619 to preserve mitochondrial function might be related to inhibition of postischemic inflammatory responses. To address this issue, we evaluated the effect of antecedent NaHS or NS-1619 treatment on postischemic tissue neutrophil content and TNF-α levels in a final series of experiments. I/R was associated with a marked infiltration of neutrophils, as indicated by a threefold increase in tissue MPO activity (Fig. 7), and a 1.6-fold increase in mucosal TNF-α levels (Fig. 8), effects that were not prevented by pretreatment with NaHS or NS-1619 24 h earlier. Moreover, treatment with paxilline (Fig. 8) or penitrem A (3.34 ± 0.99 pg/ml TNF-α, n = 3) coincident with NaHS did not exacerbate these inflammatory changes induced by I/R. Thus the ability of NaHS and NS-1619 to preserve mitochondrial function appears to occur even in the face of ongoing inflammation during the period of I/R.
Fig. 7.
I/R induced an increase in intestinal MPO activity relative to sham (nonischemic) controls, an effect that was not attenuated by preconditioning with the H2S donor NaHS (NaHS-PC+I/R) or the BKCa activator NS-1619 (NS-1619+I/R) 24 h prior to I/R. Coincident administration of the BKCa inhibitor paxilline with NaHS 24 h prior to I/R (Pax+NaHS+I/R) was associated with mucosal MPO levels that were significantly elevated relative to sham but were not statistically different from I/R. Data are means ± SE for 6 rats per group. *P < 0.05 compared with sham. &P < 0.001 compared with sham.
Fig. 8.
Ileal mucosal tumor necrosis factor-α (TNF-α) levels were increased following I/R. This postischemic increase in mucosal TNF-α was not affected by preconditioning with the H2S donor NaHS (NaHS-PC+I/R) or the BKCa channel activator NS-1619 (NS-1619+I/R) nor by coincident administration of the BKCa channel inhibitor paxilline with NaHS (Pax+NaHS+I/R) 24 h prior to I/R. Data are means ± SE for 6 rats per group. *P < 0.001 compared with sham, &P < 0.05 compared with sham.
DISCUSSION
Recognition of the fact that deranged mitochondrial function plays a major role in I/R injury has fueled a concerted effort directed at identifying potential therapeutic interventions that might limit this dysfunction. As an example, Wu et al. (54) reported that ischemic preconditioning attenuates I/R-induced mucosal apoptosis by reducing the generation of reactive oxygen species and preserving mitochondrial function, whereas others have reported that inhibitors of the mitochondrial permeability transition pore and mitochondria-targeted antioxidants are cardioprotective in the setting of I/R (3, 37). More recently, Elrod and coworkers (6) demonstrated that delivery of an H2S donor to ischemic hearts at the time of reperfusion attenuated myocardial I/R injury by preservation of mitochondrial function, observations that point to use of H2S donors as a potential new avenue for amelioration of postischemic tissue injury. The results of the present study expand on these findings by demonstrating that administration of the H2S donor NaHS 24 h prior to I/R induced the emergence of a preconditioned state that prevents I/R-induced mitochondrial dysfunction in rat intestinal mucosa. The latter result is consistent with our earlier work demonstrating that NaHS-PC induced the development of an anti-inflammatory phenotype such that postcapillary venules fail to support leukocyte rolling and adhesion in postischemic intestine (60, 66, 67). Another significant new finding of the present study is that NaHS-PC prevented postischemic mitochondrial dysfunction by a BKCa-dependent mechanism. Finally, we demonstrated that neutrophils play an important role in I/R-induced mucosal mitochondrial dysfunction.
With regard to the role of potassium channels in preconditioning, ATP-sensitive potassium channels have received the most attention. However, the results of several recent studies point to the importance of another set of potassium channels, the BKCa channels, as critical triggers for the development of a protected phenotype in response to preconditioning stimuli (40, 47, 56). BKCa channels are expressed on the plasmalemma of cells in a variety of tissues, including endothelial and vascular smooth muscle cells, and are comprised of a pore-forming α-subunit and an auxiliary β-subunit that modulates channel activity and sensitivity to specific agonists (12, 44, 48, 55, 64, 66, 67). This is also true for cardiac myocytes and neurons, where BKCa channels are also expressed in mitochondria (5, 34, 36, 40, 56). Using patch-clamp approaches, Morris et al. (28) were the first to identify channels in the basolateral membrane of rat small intestinal enterocytes that exhibited electrophysiological characteristics consistent with BKCa channel properties. We confirmed the presence of these channels in intestinal mucosa by Western blotting and immunohistochemistry (Fig. 1). Interestingly, Western blotting failed to demonstrate the existence of BKCa channel α subunits in ileal mucosal mitochondrial membranes.
It does not appear that the effects of NaHS-PC to prevent mucosal mitochondrial dysfunction can be attributed to a direct action on neutrophils or mast cells during I/R since recent reports indicate that BKCa channel activity is absent in both human and mouse neutrophils whereas mast cell function is not inhibited by iberiotoxin, a highly selective BKCa channel inhibitor (7, 31). On the other hand, platelets express BKCa channels, which, when activated, hyperpolarize platelet membranes, thereby limiting P-selectin expression and platelet adhesion to endothelial cells under static and flow conditions (22). This raises the possibility that antecedent treatment with NaHS or NS-1619 may modify I/R-induced platelet function via a BKCa channel, which may have implications for our results since platelets participate in promoting postischemic leukocyte sequestration (4, 35). Although we did not evaluate the role of platelets in the development of postischemic mucosal mitochondrial function or whether NaHS exerts protection by an effect on platelets, we feel this explanation is unlikely because the circulating half-life of NaHS is far too short to allow H2S to persist 24 h after NaHS treatment. Thus NaHS-mediated BKCa channel activation during reperfusion 24 h after administration of the H2S donor (the time at which leukocyte infiltration, platelet adhesion, and mast cell degranulation occur in untreated animals) is unlikely, even if each these cell types expressed BKCa. Since platelet-associated P-selectin plays an important role in postischemic leukocyte emigration (but not leukocyte rolling or adhesion) (35), our findings that antecedent NaHS treatment does not attenuate I/R-induced leukocyte accumulation (as assessed by tissue MPO levels, Fig. 7) but does prevent leukocyte rolling and adhesion (60, 66, 67) also support our interpretation that platelets are not a target of NaHS or NS-1619 preconditioning.
Our results are consistent with the hypothesis that NaHS-PC attenuates mitochondrial dysfunction by a BKCa channel-dependent mechanism (Figs. 2 and 3). That is, treatment with the BKCa channel inhibitor paxilline coincident with administration of NaHS 24 h prior to I/R abolished the protective effects of antecedent H2S exposure to preserve mitochondrial membrane potential and respiratory activity and prevent cytochrome c release. Although the mechanisms whereby BKCa channels contribute to these beneficial actions of NaHS-PC are unknown, BKCa channel opening allows cytosolic K+ efflux, thereby promoting cell membrane repolarization. This, in turn, reduces Ca2+ entry by closing voltage-dependent Ca2+ channels, which may act to prevent mitochondrial injury induced by [Ca2+]i overload (12, 44, 48, 55, 64). However, since our data support a role for BKCa as a triggering event for entrance into a preconditioned state when H2S is administered, this scenario requires that BKCa channels remain active during the 24 h that elapse between NaHS administration and induction of I/R. This is highly unlikely in view of the highly evanescent nature of H2S, owing to renal excretion and efficient systems that effectively bind (hemoglobin), scavenge (oxidized glutathione, methemoglobin), and metabolize (methylation in the cytosol and oxidation in the mitochondria) this molecule (41, 46). Indeed, even after systemic administration at doses that produce pharmacological effects (as in our study), plasma concentrations only briefly rise above the normal range (46). A more likely explanation is that NaHS-dependent BKCa channel activation initiates a downstream signaling cascade that culminates in increased expression and/or stimulation of effectors such as protein kinase C and heme oxygenase-1 to mediate its protective actions during I/R 24 h later, as has been shown for other forms of preconditioning (2, 9, 10, 13, 14, 17, 18, 33, 38, 57, 58, 67).
It is also unclear how H2S activates BKCa channels, although H2S is known to elicit an oxidative stress (secondary to its effect to inhibit cytochrome oxidase), which may activate BKCa channels (48). Sitdikova and coworkers (39) have suggested that H2S directly increases BKCa channel activity by modulating the redox status of critical sulfhydryl groups located on the cytoplasmic side of the channel protein. Indeed, recent work indicates that H2S signals by sulfhydrating target proteins (29), a process analogous to protein nitrosylation. However, in contrast to nitrosylation, which often inactivates the targeted protein, augmentation of protein activity is more often the case following sulfhydration of cysteine residues. In addition, protein sulfhydration is more prevalent than nitrosylation and may represent a major route for posttranslational modification of protein activity (29). Clearly, much additional work will be required to address these postulates and identify the mechanism for BKCa channel activation as well as the downstream mediators of mitochondrial protection induced by NaHS-PC.
Our conclusions are largely based on the specificity of paxilline and NS-1619 for BKCa channels. However, it is known that these agents produce other effects. For example, paxilline has been shown to inhibit sarco/endoplasmic reticulum Ca2+-ATPase both in vitro and in vivo, albeit at concentrations higher than were achieved in our experiments (47, 52). NS-1619 has also been reported to inhibit complex I of the mitochondrial respiratory chain in tumor cells (30), but again, inhibitor concentrations required to achieve this effect were greater than used in the present study. More troubling to our interpretation are the results of Gáspár et al. (11), who reported that delayed neuronal preconditioning by NS-1619 was not abrogated by BKCa channel inhibition with paxilline. However, we demonstrated that the protective effects of NS-1619 in the postischemic small bowel were inhibited by paxilline in an earlier study (66). Although these results support the specificity of paxilline in our protocol and our interpretation that NaHS-PC may occur by a BKCa channel-dependent mechanism, we also conducted studies using another BKCa channel inhibitor, penitrem A, administered just prior to NaHS treatment. Penitrem A proved to be as effective as paxilline in preventing the protective effects of this H2S donor on postischemic mitochondrial respiration. The latter observation lends additional support to the notion that BKCa channels play a role in NaHS-induced preconditioning.
In light of our earlier work demonstrating that NaHS-PC prevented leukocyte rolling and adhesion induced by I/R 24 h later (60, 66, 67), another objective of the present study was to determine whether neutrophil depletion would limit postischemic mitochondrial dysfunction. ANS administration effectively reduced circulating neutrophil counts, prevented postischemic leukosequestration in the ileum as assessed by tissue MPO levels (Fig. 4), and prevented I/R-induced alterations in mitochondrial membrane potential and respiratory activity as well as cytochrome c release (Figs. 5 and 6). These results indicate that neutrophils play an essential role in the development of postischemic mitochondrial dysfunction in the small bowel. It remains to be determined whether neutrophil-mediated mitochondrial dysfunction contributes to postischemic defects in mucosal lipid absorption and transport, which are also prevented by neutrophil depletion (24).
Coupled with our previous demonstrations that NaHS-PC or NS-1619 preconditioning prevented postischemic leukocyte rolling and adhesion (60, 66, 67), the present observations that these compounds prevent postischemic mitochondrial dysfunction led us to propose that the protective effects of these compounds might be causally linked to prevention of I/R-induced neutrophil infiltration. To address this postulate, we conducted an additional series of experiments to determine whether NaHS-PC or NS-1619 would prevent postischemic leukosequestration and increased ileal levels of the potent chemoattractant TNF-α. Interestingly, neither preconditioning stimulus was effective in preventing the I/R-induced increases in these inflammatory markers (Figs. 7 and 8). These findings were surprising given the observations that acute exposure to H2S exerts powerful anti-inflammatory effects, which include reductions in leukocyte rolling and adhesion and decreased cytokine production (46, 61). However, it is important to remember that the aforementioned responses are direct effects measured during H2S treatment, whereas our work addresses the mechanisms whereby antecedent administration induces the appearance of a preconditioned phenotype 24 h after exposure to H2S. In addition, other reports indicate that H2S contributes to increased leukocyte migration, intestinal MPO levels, and production of cytokines (including TNF-α) in inflammatory conditions (62). Although our latter observations suggest that antecedent treatment with NaHS or NS-1619 preserves mitochondrial function by a mechanism independent of an effect on leukocyte infiltration or expression of TNF-α, it is also possible that the cellular changes associated with mitochondrial protection may limit activation of extravasated neutrophils (Fig. 9). Whatever the explanation, the present data are difficult to reconcile with our earlier work demonstrating that these preconditioning stimuli attenuate I/R-induced leukocyte rolling and adhesion in murine small intestine (60, 66, 67) (Fig. 9). Since the ischemic (45 min) and reperfusion (60 min) times were identical in our rat and mouse models, it is possible that species differences account for the discrepant findings. Indeed, the rat small intestine is more sensitive to ischemia at a given duration, exhibiting irreversible injury at shorter ischemic times compared with murine small bowel (42, 45). This suggests the possibility that NaHS or NS-1619 extends the duration of ischemia that the rat bowel can withstand before injury becomes irreversible. Alternatively, we examined mitochondrial function in ileal mucosa in the present study whereas inflammatory responses were studied in submucosal venules in the jejunal microcirculation in our earlier studies (60, 66, 67). Finally, it is possible that although antecedent NaHS attenuates leukocyte rolling and adhesion (60, 66, 67), preconditioning with this H2S donor may fail to prevent platelet adhesion to leukocytes, a requisite step in postischemic leukocyte emigration (35) (Fig. 9).
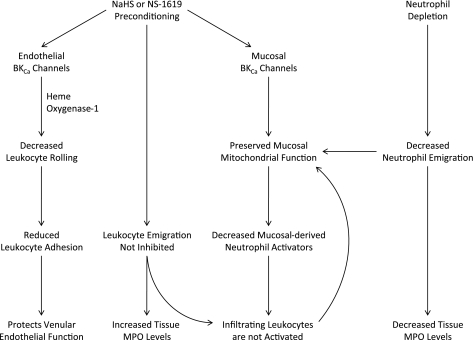
Fig. 9.
Summary diagram illustrating the role of endothelial and mucosal BKCa channels as mediators of the effects of antecedent treatment with NaHS or NS-1619 (NaHS or NS-1619 Preconditioning) to limit I/R-induced leukocyte rolling and adhesion and preserve mucosal mitochondrial function. Neutrophil depletion with ANS prior to the onset of I/R also preserved mucosal mitochondrial function. Since I/R-induced neutrophil infiltration (assessed using tissue MPO activity as a marker) was prevented by ANS treatment, but not by administration of NaHS or NS-1619, our results suggest that infiltrating neutrophils may not be activated to produce collateral injury in the postischemic intestine of animals preconditioned with NaHS or NS-1619. Although the postischemic increase in TNF-α was not attenuated by NaHS or NS-1619 preconditioning, multiple chemotactic agents are released and act in concert to promote neutrophil in the interstitial space after intestinal I/R. It is possible that release of at least some of these neutrophil activators may be abrogated by preconditioning, thereby limiting activation of extravasated leukocytes.
In summary, the present findings indicate that preconditioning with NaHS or NS-1619 24 h prior to I/R limits postischemic mitochondrial dysfunction in intestinal mucosa. Moreover, the effect of NaHS-PC to prevent the postischemic mitochondrial dysfunction appears to be triggered by a BKCa channel-dependent mechanism. Interestingly, our results also indicate that neutrophils play an essential role in the development of postischemic mucosal mitochondrial dysfunction. Since antecedent treatment with NaHS or NS-1619 failed to prevent postischemic leukosequestration or increased TNF-α levels, it appears that these preconditioning stimuli confer mitochondrial protection via a neutrophil-independent mechanism or evoke anti-inflammatory activities that prevent activation of extravasated leukocytes. These observations provide new insight regarding the potential use of BKCa channel activators and NaHS as therapeutic agents to limit I/R injury.
GRANTS
This work was supported by grants from the National Institutes of Health (AA-014945, HL-082186, and HL-095486).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Boehning D, Snyder S. Novel neural modulators. Annu Rev Neurosci 26: 105–131, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Bolli R. Preconditioning: a paradigm shift in the biology of myocardial ischemia. Am J Physiol Heart Circ Physiol 292: H19–H27, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cho J, Won K, Wu D, Soong Y, Liu S, Szeto HH, Hong MK. Potent mitochondria-targeted peptides reduce myocardial infarction in rats. Coron Artery Dis 18: 215–220, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Cooper D, Russell J, Chitman KD, Williams MC, Wolf RE, Granger DN. Leukocyte dependence of platelet adhesion in postcapillary venules. Am J Physiol Heart Circ Physiol 286: H1895–H1900, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Douglas RM, Lai JC, Bian S, Cummins L, Moczydlowski E, Haddad GG. The calcium-sensitive large-conductance potassium channel (BK/MAXI K) is present in the inner mitochondrial membrane of rat brain. Neuroscience 139: 1249–1261, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA 104: 15560–15565, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Essin K, Salanova B, Kettritz R, Sausbier M, Luft FC, Kraus D, Bohn E, Autenrieth IB, Peschel A, Ruth P, Gollasch M. Large-conductance calcium-activated potassium channel activity is absent in human and mouse neutrophils and is not required for innate immunity. Am J Physiol Cell Physiol 293: C45–C54, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Fu Z, Liu X, Geng B, Fang L, Tang C. Hydrogen sulfide protects rat lung from ischemia-reperfusion injury. Life Sci 82: 1196–1202, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Gaskin FS, Kamada K, Yusof M, Korthuis RJ. 5′-AMP-activated protein kinase activation prevents postischemic leukocyte-endothelial cell adhesive interactions. Am J Physiol Heart Circ Physiol 292: H326–H332, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Gaskin FS, Kamada K, Yusof M, Durante W, Gross G, Korthuis RJ. AICAR preconditioning prevents postischemic leukocyte rolling and adhesion: role of KATP channels and heme oxygenase. Microcirculation 16: 167–176, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gáspár T, Katakam P, Snipe JA, Kis B, Domoki F, Bari F, Busija DW. Delayed neuronal preconditioning by NS1619 is independent of calcium activated potassium channels. J Neurochem 105: 1115–1128, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghatta S, Nimmagadda D, Xu X, O'Rourke ST. Calcium-activated potassium channels: structural and functional implications. Pharmacol Ther 110: 103–116, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Gross ER, Gross GJ. Ischemic preconditioning and myocardial infarction: an update and perspective. Drug Discov Today Dis Mech 4: 165–174, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huffmyer J, Raphael J. Physiology and pharmacology of myocardial preconditioning and postconditioning. Semin Cardiothorac Vasc Anesth 13: 5–18, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Jha S, Calvert JW, Duranski MR, Ramachandran A, Lefer DJ. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: role of antioxidant and antiapoptotic signaling. Am J Physiol Heart Circ Physiol 295: H801–H806, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johansen D, Ytrehus K, Baxter GF. Exogenous hydrogen sulfide (H2S) protects against regional myocardial ischemia-reperfusion injury — evidence for a role of KATP channels. Basic Res Cardiol 101: 53–60, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Kamada K, Dayton CB, Yamaguchi T, Korthuis RJ. Antecedent ethanol ingestion prevents postischemic microvascular dysfunction. Pathophysiology 10: 131–137, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Kamada K, Gaskin FS, Yamaguchi T, Carter P, Yoshikawa T, Yusof M, Korthuis RJ. Role of calcitonin gene-related peptide in the postischemic anti-inflammatory effects of antecedent ethanol ingestion. Am J Physiol Heart Circ Physiol 290: H531–H537, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Kimura H. Hydrogen sulfide induces cyclic AMP and modulates the NMDA receptor. Biochem Biophys Res Commun 267: 129–133, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Kitagawa K, Matsumoto M, Tagaya M, Hata R, Ueda H, Niinobe M, Handa N, Fukunaga R, Kimura K, Mikoshiba K. “Ischemic tolerance” phenomenon found in the brain. Brain Res 528: 21–24, 1990 [DOI] [PubMed] [Google Scholar]
- 21. Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev 87: 99–163, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Krotz F, Riexinger T, Buerkle MA, Nithipatikom K, Gloe T, Sohn HY, Campbell WB, Pohl U. Membrane potential-dependent inhibition of platelet adhesion to endothelial cells by epoxysatrienoic acids. Arterioscler Thromb Vasc Biol 24: 595–600, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Kulawiak B, Kudin AP, Szewczyk A, Kunz WS. BK channel openers inhibit ROS production of isolated rat brain mitochondria. Exp Neurol 212: 573–547, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Kurtel H, Fujimoto K, Zimmerman BJ, Granger DN, Tso P. Ischemia-reperfusion-induced mucosal dysfunction: role of neutrophils. Am J Physiol Gastrointest Liver Physiol 261: G490–G496, 1991 [DOI] [PubMed] [Google Scholar]
- 25. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970 [DOI] [PubMed] [Google Scholar]
- 26. Liu H, Bai XB, Shi S, Cao YX. Hydrogen sulfide protects from intestinal ischaemia-reperfusion injury in rats. J Pharm Pharmacol 61: 207–212, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Masola B, Evered DF. Preparation of rat enterocyte mitochondria. Biochem J 218: 441–447, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morris AP, Gallacher D, Lee JA. A large conductance, voltage- and calcium-activated K+ channel in the basolateral membrane of rat enterocytes. FEBS Lett 206: 87–92, 1986 [DOI] [PubMed] [Google Scholar]
- 29. Mustafa AK, Gadalla MM, Snyder SH. Signaling by gasotransmitters. Sci Signal 2: re2, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nardi A, Calderone V, Chericoni S, Morelli I. Natural modulators of large-conductance calcium-activated potassium channels. Planta Med 69: 885–892, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Narenjkar J, Marsh SJ, Assem ESK. Inhibition of antigen-induced activation of RBL-2H3 cells by charybdotoxin and cetiedil. Eur J Pharmacol 483: 95–106, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Ohya S, Kuwata Y, Sakamoto K, Muraki K, Imaizumi Y. Cardioprotective effects of estradiol include the activation of large-conductance Ca2+-activated K+ channels in cardiac mitochondria. Am J Physiol Heart Circ Physiol 289: H1635–H1642, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Pan TT, Feng ZN, Lee SW, Moore PK, Bian JS. Endogenous hydrogen sulfide contributes to the cardioprotection by metabolic inhibition preconditioning in the rat ventricular myocytes. J Mol Cell Cardiol 40: 119–130, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Perez-Pinzon MA. Neuroprotective effects of ischemic preconditioning in brain mitochondria following cerebral ischemia. J Bioenerg Biomembr 36: 323–327, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Salter JW, Krieglstein CF, Issekutz AC, Granger DN. Platelets modulate ischemia/reperfusion-induced leukocyte recruitment in the mesenteric circulation. Am J Physiol Gastrointest Liver Physiol 281: G1432–G1439, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Sato T, Saito T, Saegusa N, Nakaya H. Mitochondrial Ca2+-activated K+ channels in cardiac myocytes: a mechanism of the cardioprotective effect and modulation by protein kinase A. Circulation 111: 198–203, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Shanmuganathan S, Hausenloy DJ, Duchen MR, Yellon DM. Mitochondrial permeability transition pore as a target for cardioprotection in the human heart. Am J Physiol Heart Circ Physiol 289: H237–H242, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Shigematsu S, Ishida S, Gute DC, Korthuis RJ. Postischemic anti-inflammatory effects of bradykinin preconditioning. Am J Physiol Heart Circ Physiol 280: H441–H454, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Sitdikova GF, Weiger TM, Hermann A. Hydrogen sulfide increases calcium-activated potassium (BK) channel activity of rat pituitary tumor cells. Pflügers Arch 459: 389–397 [DOI] [PubMed] [Google Scholar]
- 40. Stowe DF, Aldakkak M, Camara AK, Riess ML, Heinen A, Varadarajan SG, Jiang MT. Cardiac mitochondrial preconditioning by Big Ca2+-sensitive K+ channel opening requires superoxide radical generation. Am J Physiol Heart Circ Physiol 290: H434–H440, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Szabó C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov 6: 917–935, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Takeyoshi I, Zhang S, Nomoto M, Zhu Y, Kokudo Y, Suzuki T, Hamada N, Nemoto A, Starzl TE, Todo S. Mucosal damage and recovery of the intestine after prolonged preservation and transplantation in dogs. Transplantation 71: 1–7, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tripatara P, Patel PN, Collino M, Gallicchio M, Kieswich J, Castiglia S, Benetti E, Stewart KN, Brown PA, Yaqoob MM, Fantozzi R, Thiemermann C. Generation of endogenous hydrogen sulfide by cystathionine gamma-lyase limits renal ischemia/reperfusion injury and dysfunction. Lab Invest 88: 1038–1048, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol 8: 321–329, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Walkinshaw M, Downey D, Gottlieb JR, Engrav LH. Ischemic injury to enteric free flaps: an experimental study in the dog. Plast Reconstr Surg 81: 939–945, 1988 [DOI] [PubMed] [Google Scholar]
- 46. Wallace JL. Hydrogen sulfide-releasing anti-inflammatory drugs. Trends Pharmacol Sci 28: 501–505, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Wang X, Yin C, Xi L, Kukreja RC. Opening of Ca2+-activated K+ channels triggers early and delayed preconditioning against I/R injury independent of NOS in mice. Am J Physiol Heart Circ Physiol 287: H2070–H2077, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Wang ZW, Nara M, Wang YX, Kotlikoff MI. Redox regulation of large conductance Ca2+-activated K+ channels in smooth muscle cells. J Gen Physiol 110: 35–44, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Whiteman M, Armstrong J, Chu SH, Jia-Ling S, Wong BS, Cheung NS, Halliwell B, Moore PK. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger'? J Neurochem 90: 765–768, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Whiteman M, Cheung N, Zhu YZ, Chu SH, Siau JL, Wong BS, Armstrong JS, Moore PK. Hydrogen sulphide: a novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem Biophys Res Commun 326: 794–798, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Wickenden AD. K+ channels as therapeutic drug targets. Pharmacol Ther 94: 157–182, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Wiecha J, Munz B, Wu Y, Noll T, Tillmanns H, Waldecker B. Blockade of Ca2+-activated K+ channels inhibits proliferation of human endothelial cells induced by basic fibroblast growth factor. J Vasc Res 35: 363–371, 1998 [DOI] [PubMed] [Google Scholar]
- 53. Wieckowski MR, Giorgi C, Lebiedzinska M, Duszynski J, Pinton P. Isolation of mitochondria-associated membranes and mitochondria from animal tissues and cells. Nat Protoc 4: 1582–1590, 2009 [DOI] [PubMed] [Google Scholar]
- 54. Wu B, Ootani A, Iwakiri R, Fujise T, Tsunada S, Toda S, Fujimoto K. Ischemic preconditioning attenuates ischemia-reperfusion-induced mucosal apoptosis by inhibiting the mitochondria-dependent pathway in rat small intestine. Am J Physiol Gastrointest Liver Physiol 286: G580–G587, 2004 [DOI] [PubMed] [Google Scholar]
- 55. Xia XM, Zeng X, Lingle CJ. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature 418: 880–884, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, O'Rourke B. Cytoprotective role of Ca2+ activated K+ channels in the cardiac inner mitochondrial membrane. Science 298: 1029–1033, 2002 [DOI] [PubMed] [Google Scholar]
- 57. Yamaguchi T, Kamada K, Dayton C, Gaskin FS, Yusof M, Yoshikawa T, Carter P, Korthuis RJ. Role of eNOS-derived NO in postischemic anti-inflammatory effects of antecedent ethanol ingestion in murine small intestine. Am J Physiol Heart Circ Physiol 292: H1435–H1442, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Yamaguchi T, Dayton C, Shigematsu T, Carter P, Yoshikawa T, Gute DC, Korthuis RJ. Preconditioning with ethanol prevents postischemic leukocyte-endothelial cell adhesive interactions. Am J Physiol Heart Circ Physiol 283: H1019–H1030, 2002 [DOI] [PubMed] [Google Scholar]
- 59. Yonezawa D, Sekiguchi F, Miyamoto M, Taniguchi E, Honjo M, Masuko T, Nishikawa H, Kawabata A. A protective role of hydrogen sulfide against oxidative stress in rat gastric mucosal epithelium. Toxicology 241: 11–18, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Yusof M, Kamada K, Kalogeris T, Gaskin FS, Korthuis RJ. Hydrogen sulfide triggers late-phase preconditioning in postischemic small intestine by an NO- and p38 MAPK-dependent mechanism. Am J Physiol Heart Circ Physiol 296: H868–H876, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J 20: 2118–2120, 2006 [DOI] [PubMed] [Google Scholar]
- 62. Zhang H, Zhi L, Moochhala S, Moore PK, Bhatia M. Hydrogen sulfide acts as an upstream inflammatory mediator in cecal ligation and puncture-induced sepsis in mice by upregulating the production of cytokines and chemokines via NF-κB. Am J Physiol Lung Cell Mol Physiol 292: L960–L971, 2007 [DOI] [PubMed] [Google Scholar]
- 63. Zhao W, Wang R. H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol 283: H474–H480, 2002 [DOI] [PubMed] [Google Scholar]
- 64. Zhou XB, Arntz C, Kamm S, Motejlek K, Sausbier U, Wang GX, Ruth P, Korth M. A molecular switch for specific stimulation of the BKCa channel by cGMP and cAMP kinase. J Biol Chem 276: 43239–43245, 2001 [DOI] [PubMed] [Google Scholar]
- 65. Zhu YZ, Wang ZJ, Ho P, Loke YY, Zhu YC, Huang SH, Tan CS, Whiteman M, Lu J, Moore PK. Hydrogen sulfide and its cardioprotective effects in myocardial ischemia in experimental rats. J Appl Physiol 102: 261–268, 2007 [DOI] [PubMed] [Google Scholar]
- 66. Zuidema M, Yang Y, Wang M, Kalogeris T, Liu Y, Meininger CJ, Hill MA, Davis MJ, Korthuis RJ. Antecedent hydrogen sulfide elicits an anti-inflammatory phenotype in postischemic murine small intestine: role of BK channels. Am J Physiol Heart Circ Physiol 299: H1554–H1567, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zuidema MY, Peyton KJ, Fay WP, Durante W, Korthuis RJ. Antecedent hydrogen sulfide elicits an anti-inflammatory phenotype in postischemic murine small intestine: role of heme oxygenase-1. Am J Physiol Heart Circ Physiol 301: H388–H394, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]