Abstract
Hepatocyte inducible nitric oxide synthese (iNOS) expression is a tightly controlled pathway that mediates hepatic inflammation and hepatocyte injury in a variety of disease states. We have shown that cyclic adenosine monophosphate (cAMP) regulates cytokine-induced hepatocyte iNOS expression through mechanisms that involve protein kinase B/Akt. We hypothesized that insulin, which activates Akt signaling in hepatocytes, as well as signaling through p38 and MAPK p42/p44, would regulate iNOS expression during inflammation. In primary rat hepatocytes, insulin inhibited cytokine-stimulated nitrite accumulation and iNOS expression in a dose-dependent manner. Inhibition of MAPK p42/p44 with PD98059 had no effect on iNOS activation, whereas SB203580 to block p38 reversed insulin's inhibitory effect. However, insulin did not increase p38 activation and inhibition of p38 signaling with a dominant negative p38 plasmid had no effect on cytokine- or insulin-mediated effects on iNOS. We found that SB203580 blocked insulin-induced Akt activation. Inhibition of Akt signaling with LY294002 or a dominant negative Akt plasmid increased cytokine-stimulated nitrite production and iNOS protein expression and blocked the inhibitory effects of insulin. NF-κB induces iNOS expression and can be regulated by Akt, but insulin had no effect on cytokine-mediated IκBα levels or NF-κB p65 translocation. Our data demonstrate that insulin inhibits cytokine-stimulated hepatocyte iNOS expression and does so through effects on Akt-mediated signaling.
Keywords: nitric oxide, nitric oxide synthase, sepsis, inflammation, liver
hepatic dysfunction was identified decades ago as a component of the multiple organ failure syndrome (10). Liver dysfunction can be manifested in a variety of ways and is associated with increased morbidity and mortality in critically ill patients (13, 18, 25). Nitric oxide synthesis is an important pathway in the host's response to inflammation and mediates hepatic dysfunction and hepatic injury in models of shock and organ failure (19, 28). Proinflammatory cytokines stimulate hepatic nitric oxide production, and we have shown that glucagon and cyclic AMP inhibit hepatocyte inducible nitric oxide synthase (iNOS) expression (14–16). The role of other counterregulatory mediators produced in shock and sepsis on iNOS regulation remain incompletely studied (8). Glucagon and cAMP regulate hepatocyte iNOS expression through intracellular signaling pathways involving JNK and NF-κB (15, 20, 44). Our laboratory and others have demonstrated that cAMP activates protein kinase B/Akt in hepatocytes (9, 27, 43, 45). Akt-mediated signaling changes have profound effects on hepatocyte function and physiology (9, 27, 43), and we have shown that the cAMP-induced changes in iNOS expression are mediated in part through Akt-induced signaling (45).
Insulin is a potent modulator of hepatocyte metabolism and increases hepatocyte gluconeogenesis by regulating hepatocyte gene expression (6, 31, 35). Signaling mechanisms activated by insulin include PI3K, protein kinase B/Akt, and mitogen-activated protein kinases (MAPK) such as p38, p42/p44, and JNK (2, 3, 6, 21, 24, 33). Several of these signaling pathways, such as p38 and MAPK p42/p44, regulate iNOS expression in other tissues and cell types (7, 23, 26). In the liver, the effect of insulin on different signal transduction pathways varies depending on whether primary hepatocytes or hepatic cell lines are studied (2, 3, 6, 21). Although insulin can regulate the expression and activity of several hepatic transcription factors, and therefore the expression of numerous hepatocyte genes (6, 21, 33, 35), insulin's regulation of hepatic function and physiology in sepsis, other than glucose metabolism, has largely been unexplored. Given that insulin activates Akt and the MAPK signaling pathways that have been implicated in iNOS regulation, we hypothesized that insulin would regulate hepatocyte iNOS expression and activity in hepatocytes.
MATERIALS AND METHODS
Reagents.
Williams Medium E, penicillin, streptomycin, L-glutamine, HEPES, human recombinant interleukin 1β (IL-lβ) and murine recombinant interferon (IFN) γ were from Invitrogen Life Science (Carlsbad, CA). Insulin was from Lilly (Indianapolis, IN). Polyclonal antibodies to iNOS were purchased from BD Bioscience (Billerica, MA). LY294002, SB203580, and PD98059 were purchased from Calbiochem (San Diego, CA). Antibodies to Akt, p38, p65, and actin were purchased from Cell Signaling Technology (Danvers, MA), and the antibody to PCNA was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The plasmid expressing the dominant negative Akt was kindly provided by Drs. Burgering and Triest (Utrecht University). The plasmid expressing a dominant negative p38α MAPK (AdDNp38) was purchased from Cell Biolabs (San Diego, CA). All other chemicals were reagent grade and were purchased from Sigma (St. Louis, MO).
Hepatocyte isolation and culture.
Primary hepatocytes were isolated from male Sprague-Dawley rats (200–250 g) using the modified collagenase perfusion technique as previously described (14–16). Hepatocytes were >98% pure and >95% viable as measured by trypan blue exclusion. Hepatocytes were cultured onto collagen-coated 100-mm dishes at 1 × 106 cells/ml (5 ml) or collagen-coated 6-well plates (106 cells/well) in Williams Medium E with L-arginine (0.5 mM), L-glutamine (2 mM), HEPES (15 mM), insulin (1 μM), penicillin, streptomycin, and 10% low endotoxin calf serum (HyClone Laboratories, Logan, UT). After 4 h to allow adherence, the hepatocytes were washed with PBS and cultured overnight in insulin-free media containing 5% calf serum. The cells were then washed with PBS, and the experimental conditions established in insulin-free media except as otherwise described. Experimental conditions were performed in duplicate or triplicate cultures, and experiments were repeated to ensure reproducibility.
Plasmid transfection.
Hepatocytes were plated in 6-well plates and transfected using LipofectAMINE as previously described (44). For experiments investigating p38, recombinant adenovirus expressing the dominant negative p38α MAPK or adenovirus expressing GFP were used. For all other transfection experiments, hepatocytes were transfected with an empty vector (0.2 mg/well pIETLacZ) or the indicated plasmids for 6 h, washed, and allowed to recover for 16 h before experimental conditions were established.
Western blot.
Hepatocytes were washed twice with ice-cold PBS and lysed with 500 μl of lysis buffer (20 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1 mM Na2-EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin, and 1 mM PMSF). They were scraped into Eppendorf tubes, lysed for 30 min at 4° C, centrifuged (15, 000 g for 15 min), and stored at −80°C until use.
Proteins were separated on SDS-PAGE and blot-transferred to nitrocellulose membranes. Nonspecific binding was blocked with TBS-T (50 mM Tris·HCl, ph 7.5, 150 mM NaCl, 0.1% Tween 20) containing 5% nonfat milk for 1 h. Primary antibodies were diluted and incubated with membranes for 1–2 h at room temperature or overnight at 4°C with agitation. After washing three times with TBS-T, secondary antibodies were incubated at 1:10,000 dilution for 1 h. After five additional washes with TBS-T, the bands were visualized with chemiluminescence according to the manufacturer's instructions. The membranes were stripped and reprobed for total unphosphorylated proteins or actin where indicated as loading control. Blots were quantified using Image J software (National Institutes of Health).
MTT viability assay.
Cell viability was assessed by the 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described (32). Briefly, 5 mg/ml of MTT in 70% ethanol was diluted 1:50 with culture media immediately before use. Hepatocytes were cultured overnight in 6-well plates and then stimulated with cytokines and insulin as indicated. After 24 h, the media was aspirated and replaced with the MTT solution. The cells were then incubated for 30 min, the MTT solution was aspirated, and 0.5 ml of DMSO was added. After agitation of plate for ∼5 min, 1/10 vol/vol of 2 M Tris buffer (pH 10.5) was added, the wells were mixed thoroughly, and then a sample was taken to measure absorbance at 570 nm.
NO measurement.
Supernatent NO2− was measured as an index of NO production by the Griess reaction as described (10). Data are presented as means ± SD, and ANOVA was used to determine statistical significance. A P value of <0.05 was used to determine statistical significance.
RESULTS
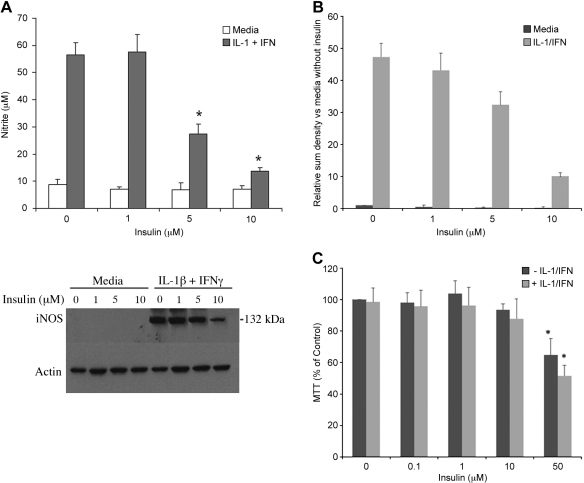
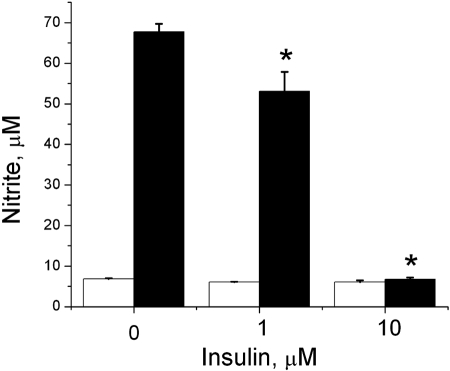
To test the hypothesis that insulin regulates hepatocyte iNOS expression, we cultured hepatocytes with increasing concentrations of insulin in the presence and absence of proinflammatory cytokines to stimulate iNOS. In culture supernatants and cellular proteins collected at 24 h, insulin decreased IL-1β + IFN-stimulated NO2− production and iNOS protein expression in a dose-dependent manner (Fig. 1). Similar findings were evident when hepatocytes were stimulated to produce iNOS with a combination of multiple cytokines (14, 15) (Fig. 2) or IL-1β alone (data not shown). The MTT assay was performed to assess hepatocyte viability and demonstrated no decrease in hepatocyte viability at the insulin concentrations that were effective at inhibiting iNOS expression (Fig. 1C). Decreased viability was seen if the insulin concentration was increased to 50 μM.
Fig. 1.
Insulin decreases cytokine-stimulated inducible nitric oxide synthese (iNOS). Hepatocytes were cultured overnight and then stimulated with cytokines [IL-lβ 200 U/ml; interferon (IFN) 100 U/ml] in the presence of the indicated concentration of insulin. A: samples were collected 24 h later and analyzed for nitrite (top) and iNOS protein (bottom). Data represent one of three similar experiments. *Significant difference vs. media control (P < 0.01; n = 6). B: densitometry analysis. C: hepatocyte viability was measured after 24-h cultures with the indicated concentration of insulin by the 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.
Fig. 2.
Insulin decreases iNOS in response to multiple cytokines. Hepatocytes were stimulated with TNF-α (500 U/ml), IFN (100 U/ml), and IL-1β (200 U/ml) in the presence of the indicated concentration of insulin. Supernatants were collected at 24 h and analyzed for nitrite. The data represent the mean of triplicate cultures from two separate experiments. *Significant difference vs. media control (P < 0.05).
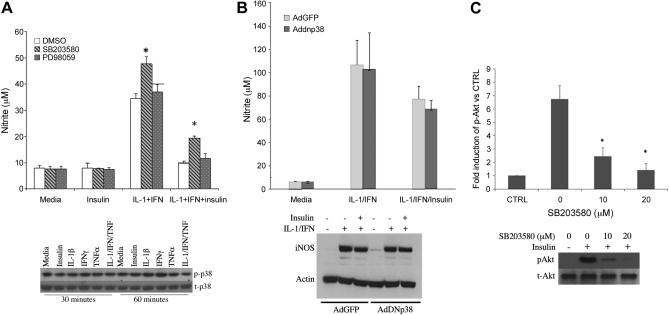
Insulin regulates MAP kinase signaling in hepatic cells (2, 3, 6, 21). To evaluate the role of MAP kinase in mediating the effect of insulin on iNOS activation, we cultured hepatocytes in the presence of SB203580 to inhibit p38 and PD98059 to inhibit MEK/MAPK p42/p44. When measured after 24 h of culture, PD98059 had no effect on the suppression of NO2− production produced by insulin, whereas SB203580 blocked the insulin-induced inhibition of NO (Fig. 3A). PD98059 reliably decreased cytokine-stimulated p42/p44 signaling in our hepatocyte cultures at these concentrations (45). However, when we measured p38 activation by Western blot, insulin did not increase p38 phosphorylation either alone (Fig. 3A) or in the presence of cytokines (not shown). Transfection of hepatocytes with a dominant negative p38 plasmid did not alter NO2− production in response to cytokines or cytokines plus insulin (Fig. 3B). Since several protein kinase inhibitors are not completely specific (4, 5), we evaluated the specificity of SB203580. We found that SB203580 slightly decreased p38 phosphorylation at 20 μM (data not shown) but markedly decreased Akt phosphorylation at 10 μM concentration or greater (Fig. 3C).
Fig. 3.
Role of p38 and MAPK p42/p44 in the regulation of iNOS by insulin. A: hepatocytes were pre-incubated with MAPK p38 inhibitor SB203580 (10 μM) or MAPK p42/p44 inhibitor PD98059 (10 μM) for 30 min and then cultured with IL-1β + IFN and insulin (10 μM) for 24 h. The supernatants were collected for nitrite (top). Bottom: hepatocytes were treated with TNF-α (500 U/ml), IL-1β (200 U/ml), IFN (100 U/ml), or insulin (10 μM) for 30 and 60 min, then analyzed by Western blot for phosphorylated and total p38. *Significant difference vs. DMSO (P < 0.05). B: hepatocytes were infected with AdGFP (light gray) or AdDN-p38 (dark gray) with MOI (10:1) for 24 h. The cells were then treated with IL-1β + IFN with or without insulin (10 μM) for another 24 h. The supernatants were collected for nitrite, and cellular proteins were collected for Western blot. C: hepatocytes were cultured with insulin (10 μM) and SB203580 at the indicated concentration for 30 min. Cellular proteins were then collected for Western blot and quantitated by densitometry.
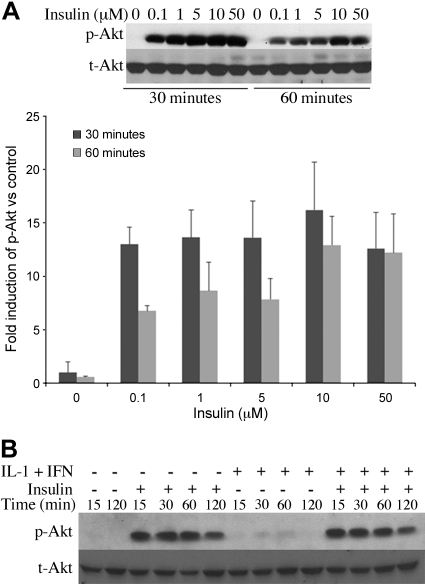
Insulin activates Akt signaling in both hepatic cell lines (6, 35, 41) and primary rat hepatocytes (24). In our cultures, insulin increased Akt phosphorylation in a dose-dependent manner and increased Akt phosphorylation in hepatocytes stimulated with IL-1β + IFN to induce iNOS (Fig. 4). IL-1β + IFN alone induced Akt phosphorylation, consistent with our previous work (45), but not to the magnitude of insulin.
Fig. 4.
Insulin increases Akt phosphorylation. Hepatocytes were cultured overnight in insulin-free media and then stimulated with the indicated concentration of insulin (A) or with insulin (10 μM) with or without IL-1β + IFN for the indicated time points (B). Cellular proteins were isolated and analyzed by Western blot.
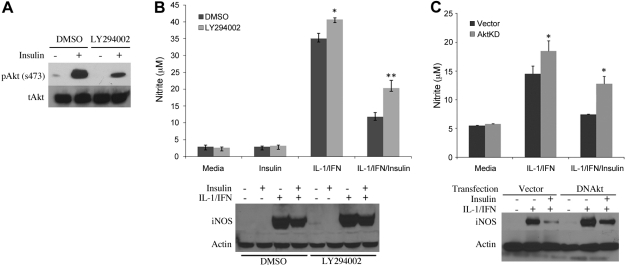
To evaluate the role of Akt in mediating the effect of insulin on iNOS, we blocked Akt signaling by inhibiting upstream PI3K with LY294002. Phosphorylation of Akt by insulin was effectively decreased when hepatocytes were cultured with LY294002 (Fig. 5A). The inhibitory effect of insulin on IL-1β + IFN-induced iNOS activation was partially reversed with LY294002 (Fig. 5B). We also inhibited Akt signaling using a dominant negative Akt plasmid (Akt-KD) that decreases Akt phosphorylation (45). Hepatocytes were transfected with either Akt-KD or a control plasmid, allowed to recover, and then stimulated with IL-1β + IFN to produce NO. Insulin decreased NO production and iNOS protein expression in hepatocytes transfected with the control plasmid. Akt-KD partially prevented the inhibition in NO production and iNOS expression by insulin (Fig. 5C). Akt-KD and LY294002 also increased NO production in IL-1β + IFN-stimulated hepatocytes without insulin, similar to our previous findings (45).
Fig. 5.
Inhibition of Akt blocks the effect of insulin on iNOS. A: hepatocytes were cultured with media containing DMSO (control) or LY294002 (10 μM) and then stimulated with insulin (10 μM). Proteins were collected at 60 min for Western blot. B: hepatocytes were treated with DMSO or LY294002 for 60 min and then stimulated with IL-1β + IFN. After 24 h, supernatants were analyzed for nitrite (top) and iNOS protein (bottom). C: hepatocytes were transfected with a control plasmid or a plasmid expressing a dominant negative Akt (Akt KD). After recovering for 16 h, the cells were stimulated to produce iNOS with IL-1β (200 U/ml) plus IFN (100 U/ml) and cultured with insulin (10 μM). Supernatants were collected for nitrite (top) and proteins for Western blot analysis (bottom). Data represent one of three similar experiments. *Significant difference (P < 0.05; n = 6).
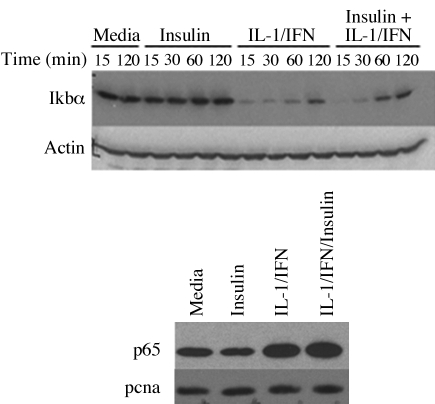
NF-κB is an important regulator of iNOS expression in cytokine-stimulated hepatocytes (15, 20, 38). Hepatocyte NF-κB activation has been shown to be regulated by Akt through Akt-mediated effects on IKKα (17, 29). To evaluate whether the effect of insulin on iNOS was mediated through Akt-induced changes in NF-κB, we stimulated hepatocytes with IL-1β + IFN in the presence and absence of insulin and measured IκBα and nuclear p65 by Western blot. Consistent with our previous work and that of others (20, 38), IL-1β + IFN decreased IκBα, which corresponded to increased levels of p65 in the nucleus (Fig. 6). Insulin had no effect on IκBα levels up to 120 min of culture and did not change nuclear p65, suggesting that insulin did not mediate its effects on iNOS through changes in IκBα or p65 translocation to the nucleus.
Fig. 6.
Effect of insulin on cytokine-induced NF-κB activation. Hepatocytes were stimulated with IL-lβ (200 U/ml) plus IFN (100 U/ml) with or without insulin (10 μM). Top: total proteins were collected at the indicated time points, and Western blot was performed using an antibody against Ikbα. Bottom: nuclear proteins were extracted after 60 min and prepared for Western blot using antibodies against p65 and PCNA (nuclear protein loading control). The blots shown are representative of two similar experiments.
DISCUSSION
The administration of insulin to control glucose levels in critically ill patients has been a topic of considerable interest (30, 37, 40). Hyperglycemia has wide-ranging effects on cellular metabolism and cellular immune function (6, 21, 22, 39). The biological effects of hyperglycemia have been postulated to have important clinical ramifications in many diseases (30, 37, 40). However, understanding the physiological and cellular effects of insulin in sepsis and critically ill patients is equally important.
We have shown that hepatic nitric oxide production is a key mediator in the liver's response to shock, sepsis, and inflammation and that cAMP-elevating agents decrease hepatocyte NO production by mechanisms that involve Akt (14, 15, 19, 45). Insulin is a potent activator of Akt signaling in hepatocytes and also activates several other intracellular signaling pathways (6, 21). We therefore investigated the role of insulin in regulating hepatocyte iNOS expression. Our data demonstrate that exogenous insulin inhibits cytokine-induced hepatocyte NO production and iNOS expression in a dose-dependent manner. This effect of insulin is present whether hepatocytes are stimulated with a single cytokine or multiple proinflammatory cytokines (Figs. 1 and 2). Insulin regulates several signal transduction pathways in hepatic cells, but we could find no role for MAPK p42/p44 or p38 signaling in mediating the effects of insulin on iNOS (Fig. 3). Insulin activates Akt (6, 21, 35, 41), and we demonstrated that insulin increased Akt phosphorylation in hepatocytes stimulated with NO-inducing cytokines. Inhibition of insulin-induced Akt activation with LY294002 or a dominant negative Akt expression plasmid (Akt-KD) partially blocked the inhibitory effect of insulin on iNOS. These results suggest that Akt mediates, in part, the suppressive effect of insulin on hepatocyte iNOS expression. Akt-KD and LY294002 also increased IL-1β + IFN-stimulated NO production in hepatocytes cultured without insulin, suggesting that cytokine-induced Akt signaling may function as an autoregulatory mechanism to control or limit hepatocyte NO production during inflammation.
We have shown that limiting excessive NO from iNOS improves hepatic function and reduces mortality after sepsis and hemorrhagic shock (19, 28). Endogenous mediators that promote Akt activation may therefore downregulate hepatocyte iNOS expression in vitro and in vivo (16, 21) and limit iNOS-induced tissue injury in times of stress. Insulin had little effect on p38 in our experiments, and SB203580 had more potent effects on Akt than p38 in these cultures. We cannot exclude the possibility that insulin or SB203580 altered p38 at time points we did not measure or that the SB203580-induced inhibition of Akt was mediated by upstream p38 signaling. Our results suggest, however, that Akt signaling is more important in regulating cytokine-induced hepatocyte iNOS in response to insulin than p38.
Akt signaling can regulate downstream NF-κB activity and MAPK p42/p44 activation (17, 34, 48). Our results, however, demonstrate that insulin did not alter IκBα or p65 nuclear translocation. Inhibition of MAPK p42/p44 with PD98059 had no effect on the insulin-mediated suppression of iNOS. Our results suggest that these signaling pathways do not contribute to insulin's effects on iNOS expression. The identity of the molecular targets of Akt-mediated insulin signaling that inhibit hepatocyte iNOS expression have not been identified. We have previously shown that JNK signaling mediates the cAMP-induced suppression of hepatocyte NO synthesis (44). Akt can regulate JNK in other cell types (1, 11, 12, 46) and activates JNK in hepatocytes (24). It is unknown whether insulin regulates JNK, either directly or through Akt, in hepatocytes stimulated to produce NO during inflammation, and this pathway will require further investigation. We also cannot completely exclude a role for NF-κB in regulating the effect of insulin on iNOS. Although p65 nuclear translocation and IκBα levels were not changed by insulin, NF-κB can potentially mediate the effects of insulin on iNOS through other mechanisms. The p65 subunit of NF-κB can be directly phosphorylated and, once phosphorylated, interacts with the coactivator CBP to interfere with transcription independent of DNA binding (42). The p65 subunit can also bind to other coactivator proteins and regulate transcription (36, 47). Whether insulin regulates other functions of NF-κB in hepatocytes such as competition for coactivators to mediate the expression of iNOS or other genes will require further study.
In conclusion, our data demonstrate that insulin inhibits cytokine-stimulated iNOS expression in primary rat hepatocytes. The suppressive effect of insulin on iNOS was mediated through the upregulation of Akt signaling and was not mediated through effects on MAPK p42/p44 or p38. In addition to regulating glucose levels, insulin may downregulate iNOS expression and limit iNOS-mediated tissue injury and organ dysfunction following shock and inflammation.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Dieases Grant DK-55664 (B. G. Harbrecht).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.G.H. and B.Z. conception and design of research; B.G.H., I.N., and B.Z. analyzed data; B.G.H., I.N., J.W.S., and B.Z. interpreted results of experiments; B.G.H. and B.Z. drafted the manuscript; B.G.H., I.N., J.W.S., and B.Z. edited and revised the manuscript; B.G.H., I.N., and B.Z. approved the final version of the manuscript; I.N. and B.Z. performed experiments; B.Z. prepared figures.
REFERENCES
- 1. Aikin R, Maysinger D, Rosenberg L. Cross-talk between phosphatidylinositol 3-kinase/AKT and c-Jun NH2-terminal kinase mediates survival of isolated human islets. Endocrinology 145: 4522–4531, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Allister EM, Borradaile NM, Edwards JY, Huff MW. Inhibition of microsomal triglyceride transfer protein expression and apolipoprotein B100 secretion by the citrus flavonoid naringenin and by insulin involves activation of the mitogen-activated protein kinase pathway in hepatocytes. Diabetes 54: 1676–1683, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Au WS, Kung HF, Lin MC. Regulation of microsomal triglyceride transfer protein gene by insulin in HepG2 cells: roles of MAPKerk and MAPKp38. Diabetes 52: 1073–1080, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J 371: 199–204, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bain J, Plater L, Elliott M, Shapiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J 408: 297–315, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barthel A, Schmoll D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am J Physiol Endocrinol Metab 285: E685–E692, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Chio CC, Chang YH, Hsu YW, Chi KH, Lin WW. PKA-dependent activation of PKC, p38 MAPK, and IKK in macrophage: implication in the induction of inducible nitric oxide synthase and interleukin-6 by dibutyryl cAMP. Cell Signal 16: 565–575, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Collins JL, Vodovotz Y, Yoneyama T, Hatakeyama K, Green AM, Billiar TR. Catecholamines decrease nitric oxide production by cytokine-stimulated hepatocytes. Surgery 130: 256–264, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Cullen KA, McCool J, Anwer MS, Webster CRL. Activation of cAMP-guanine exchange factor confers PKA-independent protection from hepatocyte apoptosis. Am J Physiol Gastrointest Liver Physiol 287: G334–G343, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Eiseman B, Beart R, Norton L. Multiple organ failure. Surg Gynecol Obstet 144: 323–326, 1977 [PubMed] [Google Scholar]
- 11. Figueroa C, Tarras S, Taylor J, Vojtek AB. Akt2 negatively regulates assembly of the POSH-MLK-JNK signaling complex. J Biol Chem 278: 47922–47927, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Funakoshi-Tago M, Tago K, Sonoda Y, Tominaga SI, Kasahara T. TRAF6 and C-SRC induce synergistic AP-1 activation via PI3-kinase-AKT-JNK pathway. Eur J Biochem 270: 1257–1268, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Harbrecht BG, Zenati MS, Doyle HR, McMichael J, Townsend RN, Clancy KD, Peitzman AB. Hepatic dysfunction increases length of stay and risk of death after injury. J Trauma 53: 517–523, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Harbrecht BG, Wirant EM, Kim YM, Billiar TR. Glucagon inhibits hepatocyte nitric oxide synthesis. Arch Surg 131: 1266–1272, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Harbrecht BG, Taylor BS, Xu Z, Ramalakshmi S, Ganster RW, Geller DA. cAMP inhibits inducible nitric oxide synthase expression and NF-κB binding activity in cultured rat hepatocytes. J Surg Res 99: 258–264, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Harbrecht BG, Perpetua M, Fulmer M, Zhang B. Glucagon regulates hepatic inducible nitric oxide synthesis in vivo. Shock 22: 157–162, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Hatano E, Brenner DA. Akt protects mouse hepatocytes from TNF-α and Fas-mediated apoptosis through NF-κB activation. Am J Physiol Gastrointest Liver Physiol 282: G1357–G1368, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Hebert PC, Drummond AJ, Singer J, Bernard GR, Russell JA. A simple multiple system organ failure scoring system predicts mortality of patients who have sepsis syndrome. Chest 104: 230–235, 1993 [DOI] [PubMed] [Google Scholar]
- 19. Hierholzer C, Harbrecht BG, Menezes J, Kane J, MacMicking J, Nathan CF, Peitzman AB, Billiar TR, Tweardy DJ. Essential role of induced nitric oxide in the initiation of the inflammatory response following hemorrhagic shock. J Exp Med 187: 917–928, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hong G, Zhang B, Harbrecht BG. Cyclic AMP inhibits IL-1β plus IFNγ-induced NF-κB translocation in hepatocytes by a PKA-independent mechanism. J Surg Res 159: 565–571, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keeton AB, Amsler MO, Venable DY, Messina JL. Insulin signal transduction pathways and insulin-induced gene expression. J Biol Chem 277: 48565–48573, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Khoury W, Klausner JM, Ben-Abraham R, Szold O. Glucose control by insulin for critically ill surgical patients. J Trauma 57: 1132–1138, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Kim SH, Kim J, Sharma RP. Inhibition of p38 and ERK MAP kinases blocks endotoxin-induced nitric oxide production and differentially modulates cytokine expression. Pharmacol Res 49: 433–439, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Kim SK, Woodcroft KJ, Khodadadeh SS, Novak RF. Insulin signaling regulates γ-glutamylcysteine ligase catalytic subunit expression in primary cultured rat hepatocytes. J Pharm Exp Ther 311: 99–108, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Kortgen A, Paxian M, Werth M, Recknagel P, Rauchfub F, Lupp A, Krenn CG, Muller D, Claus RA, Reinhart K, Settmacher U, Bauer M. Prospective assessment of hepatic function and mechanisms of dysfunction in the critically ill. Shock 32: 358–365, 2009 [DOI] [PubMed] [Google Scholar]
- 26. LaPointe MC, Isenovic E. Interleukin 1β regulation of inducible nitric oxide synthase and cyclooxygenase-2 involves the p42/44 and p38 MAPK signaling pathways in cardiac myocytes. Hypertension 33: 276–282, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Li J, Yang S, Billiar TR. Cyclic nucleotides suppress tumor necrosis factor α-mediated apoptosis by inhibiting caspase activation and cytochrome c release in primary hepatocytes via a mechanism independent of Akt activation. J Biol Chem 275: 13026–13034, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Menezes J, Hierholzer C, Watkins SC, Lyons V, Peitzman AB, Billiar TR, Tweardy DJ, Harbrecht BG. A novel nitric oxide scavenger decreases liver injury and improves survival after hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol 277: G144–G151, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Nakanishi H, Kaibori M, Teshima S, Yoshida H, Kwon AH, Kamiyama Y, Nishizawa M, Ito S, Okumura T. Pirfenidone inhibits the induction of iNOS stimulated by interleukin-1β at a step of NF-κB DNA binding in hepatocytes. J Hepatol 41: 730–736, 2004 [DOI] [PubMed] [Google Scholar]
- 30. NICE-SUGAR Study Investigators Intensive versus conventional glucose control in critically ill patients. N Engl J Med 360: 1283–1297, 2009 [DOI] [PubMed] [Google Scholar]
- 31. O'Riordain MG, Ross JA, Fearon KCH, Mainjay J, Farouk M, Garden OJ, Carter DC. Insulin and counterregulatory hormones influence acute-phase protein production in human hepatocytes. Am J Physiol Endocrinol Metab 269: E323–E330, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Plumb JA, Milroy R, Kaye SB. Effects of pH dependence of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Res 49: 4435–4440, 1989 [PubMed] [Google Scholar]
- 33. Puigsever P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegleman BM. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1α interaction. Nature 423: 550–555, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Reusch HP, Zimmermann S, Schaefer M, Paul M, Moelling K. Regulation of Raf by Akt controls growth and differentiation in vascular smooth muscle cells. J Biol Chem 276: 33630–33637, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Schmoll D, Walker KS, Alessi DR, Grempler R, Burchell A, Guo S, Walther R, Unterman TG. Regulation of glucose-6-phosphatase gene expression by protein kinase Bα and the forkhead transcription factor FKHR. J Biol Chem 275: 36324–36333, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Sheppard KA, Phelps KM, Williams AJ, Thanos D, Glass CK, Rosenfeld MG, Gerritsen ME, Collins T. Nuclear integration of glucocorticoid receptor and nuclear factor-κB signaling by CREB-binding protein and steroid receptor coactivator-1. J Biol Chem 273: 29291–29294, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Soylemz Wiener R, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA 300: 933–944, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Taylor BS, de Vera ME, Ganster RW, Wang Q, Shapiro RA, Morris SM, Jr, Billiar TR, Geller DA. Multiple NF-κB enhancer elements regulate cytokine induction of the human inducible nitric oxide synthase gene. J Biol Chem 273: 15148–15156, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Turina M, Christ-Crain M, Polk HC., Jr Diabetes and hyperglycemia: strict glycemic control. Crit Care Med 34: S291–S300, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med 345: 1359–1367, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Villafuerte BC, Phillips LS, Rane MJ, Zhao W. Insulin-response element-binding protein1: a novel Akt substrate involved in transcriptional action of insulin. J Biol Chem 279: 26650–36659, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Waltner-Law M, Daniels MC, Sutherland C, Granner DK. NF-κB inhibits glucocorticoid and cAMP-mediated expression of the phosphoenolpyruvate carboxykinase gene. J Biol Chem 275: 31847–31856, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Webster CRL, Usechak P, Anwer MS. cAMP inhibits bile acid-induced apoptosis by blocking caspase activation and cytochrome c release. Am J Physiol Gastrointest Liver Physiol 283: G727–G738, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Zhang B, Perpetua M, Fulmer M, Harbrecht BG. JNK signaling is involved in the effects of cyclic AMP on IL-1β plus IFN-induced inducible nitric oxide synthase expression in hepatocytes. Cell Signaling 16: 837–847, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Zhang B, Li S, Harbrecht BG. Akt-mediated signaling is induced by cytokines and cyclic adenosine monophosphate and suppresses hepatocyte inducible nitric oxide synthase expression independent of MAPK p44/p42. Biochim Biophys Acta 1813: 73–79, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang QG, Wang XT, Han D, Yin XH, Zhang GY, Xu TL. Akt inhibits MLK3/JNK3 signaling by inactivating Rac1: a protective mechanism against ischemic brain injury. J Neurochem 98: 1886–1898, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Zhong H, Voll RE, Ghosh S. Phosphorylation of NF-κB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell 1: 661–667, 1998 [DOI] [PubMed] [Google Scholar]
- 48. Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (Protein kinase B). Science 286: 1741–1744, 1999 [DOI] [PubMed] [Google Scholar]