Abstract
Isolated hepatocytes undergo lipoapoptosis, a feature of hepatic lipotoxicity, on treatment with saturated free fatty acids (FFA) such as palmitate (PA). However, it is unknown if palmitate is directly toxic to hepatocytes or if its toxicity is indirect via the generation of lipid metabolites such as lysophosphatidylcholine (LPC). PA-mediated hepatocyte lipoapoptosis is associated with endoplasmic reticulum (ER) stress, c-Jun NH2-terminal kinase (JNK) activation, and a JNK-dependent upregulation of the potent proapoptotic BH3-only protein PUMA (p53 upregulated modulator of apoptosis). Our aim was to determine which of these mechanisms of lipotoxicity are activated by PA-derived LPC. We employed Huh-7 cells and isolated murine and human primary hepatocytes. Intracellular LPC concentrations increase linearly as a function of the exogenous, extracellular PA, stearate, or LPC concentration. Incubation of Huh-7 cells or primary hepatocytes with LPC induced cell death by apoptosis in a concentration-dependent manner. Substituting LPC for PA resulted in caspase-dependent cell death that was accompanied by activating phosphorylation of JNK with c-Jun phosphorylation and an increase in PUMA expression. LPC also induced ER stress as manifest by eIF2α phosphorylation and CAAT/enhancer binding homologous protein (CHOP) induction. LPC cytotoxicity was attenuated by pharmacological inhibition of JNK or glycogen synthase kinase-3 (GSK-3). Similarly, short-hairpin RNA (shRNA)-targeted knockdown of CHOP protected Huh-7 cells against LPC-induced toxicity. The LPC-induced PUMA upregulation was prevented by JNK inhibition or shRNA-targeted knockdown of CHOP. Finally, genetic deficiency of PUMA rendered murine hepatocytes resistant to LPC-induced apoptosis. We concluded that LPC-induced lipoapoptosis is dependent on mechanisms largely indistinguishable from PA. These data suggest that FFA-mediated cytotoxicity is indirect via the generation of the toxic metabolite, LPC.
Keywords: endoplasmic reticulum stress, c-Jun NH2-terminal kinase, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, palmitate, p53 upregulated modulator of apoptosis
lipotoxicity in the context of the metabolic syndrome is an emerging public health problem in Western societies (3). This syndrome is particularly germane to the liver. Indeed, nonalcoholic fatty liver disease (NAFLD) is highly prevalent and can lead to cirrhosis (1, 13). Lipotoxicity results in cellular demise by apoptosis and is referred to as lipoapoptosis (9, 42), a nefarious process associated with NAFLD severity. Recent data suggest that saturated free fatty acids (FFA) mediate lipoapoptosis (2, 19, 31, 38). However, whether FFA directly or indirectly, by generating toxic metabolites, cause lipoapoptosis is unclear.
In addition to mitochondrial oxidation, esterification into triglycerides, and incorporation into lipoprotein complexes, FFA can also be metabolized to phospholipids (12). For example, saturated FFA can be incorporated into diacylglycerol (DAG), which is derivatized to phosphatidylcholine (PC). Lysophosphatidylcholine (LPC) is a major plasma phospholipid generated from PC by the enzyme phospholipase A2 (PLA2); similarly, PLA2 activity also generates LPC from FFA in cells (15). Moreover, hepatic tissue concentrations of LPC are greater in the liver of NASH patients than in healthy controls (19, 35). A recent study suggested that rats fed a high-fat diet (HFD) displayed elevated serum alanine aminotransferase (ALT) values that correlated with increased PLA2 mRNA expression and LPC levels (21). In this model, metformin treatment reduced HFD-induced levels of LPC and liver injury (21). Thus LPC represents a candidate phospholipid mediator of hepatic lipotoxicity by FFA. Consistent with this concept, metabolism of FFA to LPC was recently reported to be essential for palmitate (PA)-mediated cell death (19). Indeed, previous studies have demonstrated LPC-induced toxicity in various cell types, including endothelial vascular cells, hippocampal progenitor cells, pancreatic islet β-cells, and hepatocytes (4, 8, 19, 20, 30). However, if LPC is the principal mediator of FFA cytotoxicity, LPC should recapitulate the cytotoxic signaling pathways delineated to date for saturated FFA such as PA. The core apoptotic machinery engaged by toxic concentrations of LPC compared with FFA has not yet been reported, a necessary assessment if LPC is to be further implicated as the mediator of hepatic lipotoxicity.
Saturated FFA cause cell death via sustained activation of c-Jun NH2-terminal kinase (JNK) (11, 29). The mechanism of JNK activation is complex and involves, in part, several small GTPase proteins and glycogen synthase kinase (GSK) (6, 17, 22). Activated JNK, via c-Jun, cooperates with endoplasmic reticulum (ER) stress-induced expression of CAAT/enhancer binding homologous protein (CHOP) to upregulate p53-upregulated modulator of apoptosis (PUMA), a potent proapoptotic BH3-only protein member of the Bcl-2 family of proteins. PUMA activates Bax, a multidomain proapoptotic member of the Bcl-2 family (11), which on activation translocates to mitochondria, causing mitochondrial dysfunction and caspase-dependent cell death (40). These are the principal apoptotic mediators implicated so far in hepatic lipotoxicity (23).
In the present study, we examined the mechanism of LPC cytotoxicity to determine whether it activates the apoptotic machinery identified to date for PA-induced lipoapoptosis. Our data indicate that the cytotoxic signaling processes are triggered downstream of PA by the lipid metabolite LPC. These observations further implicate LPC as the lipid mediator of FFA-induced hepatocyte apoptosis.
EXPERIMENTAL PROCEDURES
Cells.
Huh-7 cells, a human hepatocellular carcinoma cell line, were maintained in Dulbecco's modified Eagle's medium containing glucose (25 mM) supplemented with 10% fetal bovine serum, 100,000 IU/l penicillin, and 100 mg/l streptomycin. We also employed short-hairpin CHOP (shCHOP) cells derived from Huh-7 cells, which stably express a short-hairpin RNA (shRNA) complementary to CHOP (2). Mouse primary hepatocytes were isolated from C57BL/6 wild-type (Jackson Laboratory, Bar Harbor, ME) and Puma-deficient (Puma−/−) mice by collagenase perfusion and were plated as primary cultures (14). Development and characterization of the Puma−/− mice have been previously reported (25). Mouse primary hepatocytes were maintained in Waymouth's medium supplemented with 10% fetal bovine serum, 100,000 IU/l penicillin, 100 mg/l streptomycin, and 100 nM insulin. Human primary hepatocytes were obtained from the nontumor part of the livers of patients undergoing hepatic resection for clinical indications. The isolation and culture of the human hepatocytes was performed as described previously (27). All animal studies had prior Institutional Animal Care and Use Committee approval; the isolation of human hepatocytes was approved by the Institutional Review Board of Mayo Clinic.
Treatment of hepatocytes with lipids.
One-palmitoyl-sn-glycero-3-phosphocholine (lysophosphatidylcholine; LPC) was obtained from Sigma-Aldrich (L5254; St. Louis, MO). LPC was dissolved in DMSO and DMEM (1:1) at a stock concentration of 25 mM. Cells were cultured in medium containing LPC (0–85 μM). The final concentration of DMSO was ≤0.2% in the medium; the corresponding DMSO concentration was used as a vehicle control. PA (P5585; Sigma-Aldrich) and stearic acid (SA; W303518; Sigma-Aldrich) was dissolved in isopropanol at a stock concentration of 160 mM. Cells were cultured in medium containing fatty acid and 1% BSA to maintain the physiological ratio between bound and unbound FFA (36).
Measurement of LPC concentration.
Cellular-associated LPC was measured by an enzymatic assay as reported by Kishimoto et al. (26). Huh-7 cells (70% confluent) were cultured in six-well plates and treated with PA and SA for 12 h and with LPC for 4 h. Next, cells were washed, resuspended in 50 μl of PBS, and sonicated at 12 kHz for 15 s by employing a XL-2000 Microson (Qsonica, Newton, CT). Protein concentration was measured using the Bradford assay, and each sample was diluted to a final protein concentration of 2 μg/μl in PBS. Each sample (4 μl) was mixed with 240 μl of reaction buffer containing 100 mM Tris·HCl (pH 8.0), 0.01% (wt/vol) Triton X-100, 1 mM calcium chloride, 3 mM N-ethyl-N-(2-hydroxy-3-sulfopropyl)-3-methylaniline, sodium salt, dihydrate (TOOS), 10 kU/l peroxidase, 0.1 kU/l glycerophosphorylcholine phosphodiesterase, and 10 kU/l choline oxidase. After incubation for 5 min at 37°C, 80 μl of lysophospholipase buffer containing 100 mM Tris·HCl, 0.01% Triton X-100, 5 mM 4-aminoantipyrine, and 30 kU/l lysophospholipase were added to each sample. Next, the reaction mix was incubated at 37°C for 5 min, and the absorbance was measured at 570 nm using a synergy HT multimode microplate reader (BioTek Instruments, Winooski, VT). LPC 16:0 and LPC 18:0, standards used for PA- and SA-treated cells, respectively, were diluted in saline and used to generate a standard curve. Saline was used as blank.
Quantitation of apoptosis.
Apoptotic cells were evaluated by both morphological and biochemical approaches as we described previously (2). Briefly, nuclei were stained with 2 μg/ml 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI) for 30 min (Huh-7) or 10 min (primary hepatocytes) at 37°C and analyzed by fluorescence microscopy (Nikon Eclipse TE200; Nikon, Tokyo, Japan). Apoptosis was quantified by assessing the characteristic morphological changes of nuclear chromatin condensation and fragmentation. Apoptosis was expressed as a percentage of total cells counted. For caspase 3/7 activity, a biochemical hallmark of apoptosis, cells were plated in 96-well plates. The assay was performed using the commercially available Apo-One homogeneous caspase 3/7 assay (Promega, Madison, WI) according to the manufacturer's instructions.
Quantitative real-time PCR.
Total cellular RNA was extracted using Trizol reagent (Invitrogen, Camarillo, CA) and was reverse-transcribed into cDNA with Moloney murine leukemia virus reverse transcriptase (Invitrogen) and random primers (Invitrogen) as previously described (5). Quantification of the cDNA template was performed with quantitative real-time PCR (qRT-PCR; LightCycler; Roche Applied Science, Indianapolis, IN) using SYBR green (Molecular Probes, Eugene, OR) as a fluorophore. PCR primers were as follows: human CHOP (NM_004083): forward 5′-ATGGCAGCTGAGTCATTGCCTTTC-3′ and reverse 5′-AGAAGCAGGGTCAAGAGTGGTGAA-3′ (177 bp); human PUMA (NM_001127240), forward 5′-GACGACCTCAACGCACAGTA-3′ and reverse 5′-AGGAGTCCCATGATGAGATTGT-3′ (101 bp). As an internal control, primers for 18S rRNA were used (Ambion, Austin, TX). The target mRNA expression levels were expressed relative to 18S rRNA per each sample as previously described (5).
Immunoblot analysis.
Whole cell lysates were prepared as previously described (5). An equal amount of protein (50–80 μg) was resolved by SDS-PAGE on a 12.5–15% acrylamide gel, transferred to nitrocellulose membranes, and incubated with primary antisera. Membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Bound antibody complexes were visualized using chemiluminescent substrate (ECL; Amersham, Arlington Heights, IL) and exposure to Kodak X-OMAT film (Eastman Kodak, Rochester, NY).
Immunocytochemistry for active Bax.
Immunocytochemistry for active Bax was performed as we described previously (2). Briefly, cells plated on coverslips were fixed with 4% paraformaldehyde in PBS and permeabilized with 0.0125% (wt/vol) CHAPS in PBS. The primary antibody was mouse anti-Bax (clone 6A7; Exalpha Biologicals, Watertown, MA), which only recognizes the active conformation of Bax, used at a dilution of 1:500. The secondary antibody was Alexa Fluor 488-conjugated anti-mouse IgG (Molecular Probes), and ProLong Antifade with DAPI (Molecular Probes) was used as mounting medium. Images were acquired by confocal microscopy, employing excitation and emission wavelengths of 488 and 507 nm, respectively. Cells with positive immunoreactivity for the active conformation of Bax were counted and expressed as a percentage of total cells.
Antisera and reagents.
Antisera used were obtained from the following sources. Rabbit anti-phospho-eIF2α (1:1,000; 9721), rabbit anti-eIF2α (1:1,000; 9722), rabbit anti-phospho-JNK (1:1,000; 9251), rabbit anti-JNK (1:1,000; 9252), and rabbit anti-PUMA (rodent specific) (1:500; 74670) were obtained from Cell Signaling Technology (Danvers, MA). Mouse anti-phospho-c-Jun (Ser63) (1:1,000; sc-822), mouse anti-c-Jun (1:1,000; sc-1694), mouse anti-CHOP (1:500; sc-575), and goat anti-β-actin (1:1,000; sc-1616) were obtained from Santa Cruz Biotechnology, and rabbit anti-PUMA (P4743) was obtained from Sigma-Aldrich. The JNK inhibitor SP600125 (420119) and the GSK-3 inhibitor GSK-IX (361550) were obtained from Calbiochem (San Diego, CA). The pan-caspase inhibitor Q-Val-Asp-OPh (QVD-OPh) was obtained from MP Biomedicals (Salon, OH), and TOOS was purchased from Dojindo Molecular Technologies (Rockville, MD). Bromoenol lactone (BEL; B1552), palmityl trifluoromethyl ketone (PACOCF3; P8727), lysophospholipase (EC 3.1.1.5), glycerophosphorylcholine phosphodiesterase (EC 3.1.4.2), peroxidase (EC 1.11.1.7), 4-aminoantipyrine and choline oxidase (EC 1.1.3.17), BSA, Bradford reagent, and other chemicals were all obtained from Sigma-Aldrich.
Statistical analysis.
All data represent at least three independent experiments and are means ± SE. Differences between groups were compared using Student's t-test and one-way analysis of variance with a post hoc Dunnett test; significance was accepted at P values <0.05.
RESULTS AND DISCUSSION
LPC induces hepatocyte apoptosis.
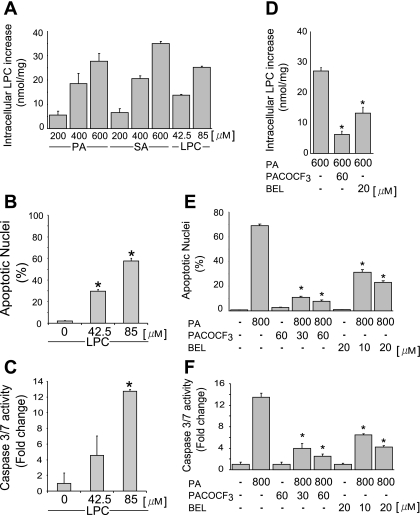
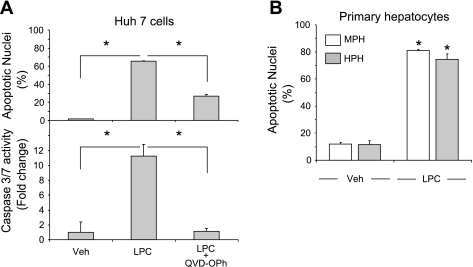
To determine whether LPC is an active lipoapoptosis-inducing metabolite of saturated FFA, we first sought to confirm that saturated FFA augment cellular LPC levels in Huh-7 cells. As anticipated, intracellular LPC levels increased proportionally to extracellular concentration of both PA and SA. An increase of 28 and 35 nmol of LPC/mg protein was identified in cells incubated with 600 μM PA or SA, respectively (Fig. 1A), a concentration within the range of fasting FFA plasma levels observed in human NAFLD (7, 32). Under the cell culture conditions employed for these studies (8.5 × 105 cells/well), this concentration of PA equates to a LPC concentration of 85 μM. Exogenous administration of 85 μM LPC increased intracellular LPC level to the same magnitude observed with 600 μM PA, confirming that LPC is membrane permeant or effectively transported into cells (Fig. 1A). Therefore, we used LPC at or below this concentration and identical cell density culture conditions for the remainder of our studies. Next, we ascertained whether LPC directly induced apoptosis in liver cells. Huh-7 cells were incubated with LPC at 42.5 μM (13.8 nmol of LPC/mg protein) or 85 μM (25.3 nmol of LPC/mg protein). As assessed using both morphological and biochemical criteria, LPC readily induced apoptosis in Huh-7 cells in a concentration-dependent manner (Fig. 1, B and C). LPC is generated from intracellular PC by PLA2 (19, 35). Therefore, we next determined whether pharmacological inhibition of PLA2 with BEL or PACOCF3 (28) inhibits PA-mediated lipoapoptosis. Indeed, concentrations of BEL or PACOCF3 that inhibited intracellular conversion of PA to LPC (Fig. 1D) also reduced PA-mediated lipoapoptosis (Fig. 1, E and F). LPC-induced apoptosis was caspase dependent, because it was blocked by the pan-caspase inhibitor QVD-OPh (Fig. 2A). Primary mouse and human hepatocytes also readily underwent apoptosis on LPC treatment (Fig. 2B). Our results further demonstrate that transformed and primary hepatocytes are susceptible to LPC cytotoxicity at concentrations observed after PA treatment and observed in serum (26) and that the mode of cell death is caspase dependent.
Fig. 1.
Lysophosphatidylcholine (LPC) is metabolized from saturated fatty acids by PLA2, and inhibition of PLA2 reduces palmitate (PA)-induced apoptosis in hepatocytes. A: intracellular LPC levels were measured using an enzyme-linked colorimetric assay, as described in experimental procedures. The PA-, stearate (SA)-, and LPC-stimulated increase of LPC above basal values is depicted. Huh-7 cells were treated for 12 h with increasing doses of PA, SA (200, 400, and 600 μM) and LPC (42.5 and 85 μM). B and C: Huh-7 cells were treated for 16 h with LPC at either 42.5 or 85 μM, as indicated. Apoptosis was assessed by morphological criteria after 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI) staining (B). Caspase 3/7 catalytic activity was assessed using a fluorogenic assay and expressed as fold change (C). D: intracellular LPC levels were measured as described in A. Huh-7 cells were treated for 12 h with PA alone or with PA and the PLA2 inhibitor palmityl trifluoromethyl ketone (PACOCF3) or bromoenol lactone (BEL). E and F: Huh-7 cells were treated for 16 h with PA (800 μM) or with PA and the PLA2 inhibitor PACOCF3 (30 or 60 μM) or BEL (10 or 20 μM). Apoptosis was assessed as described in B and E and caspase 3/7 catalytic activity as in C and F, respectively. All data are means ± SE for 3 experiments. *P < 0.01.
Fig. 2.
LPC induces caspase-dependent apoptosis in hepatocytes. A: Huh-7 cells were incubated with LPC (85 μM) in the presence of the pan-caspase inhibitor Q-Val-Asp-OPh (QVD-OPh; 10 μM) for 16 h. Vehicle-treated cells (Veh) were used as controls. Apoptotic nuclei were counted after DAPI staining, and caspase 3/7 catalytic activity was measured using a fluorogenic assay. B: mouse primary hepatocytes (MPH) or human primary hepatocytes (HPH) were treated with LPC for 6 or 3 h, respectively. The concentrations of LPC used were 75 μM for MPH and 85 μM for HPH. Apoptosis was assessed as in A. All data are means ± SE for 3 experiments. *P < 0.01.
LPC treatment induced JNK activation that is GSK-3 dependent.
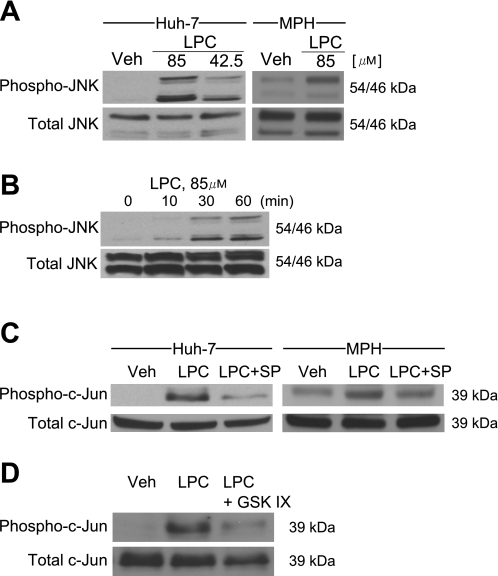
Because JNK activation is a key mediator of hepatocyte lipoapoptosis by PA (2, 19, 29), we assessed whether LPC induces activating phosphorylation of JNK in liver cells. LPC treatment resulted in a significant increase in phospho-JNK levels as assessed by immunoblot analysis in both Huh-7 cells and mouse primary hepatocytes (Fig. 3A). A robust increase in phospho-JNK was observed as early as 30 min following cell treatment with 85 μM LPC (Fig. 3B), and this increase was sustained and persisted at 8 h in Huh-7 cells (Fig. 3A). These results are consistent with other studies demonstrating LPC-induced activation of JNK in pancreatic β-cells or endothelial cells (18, 43). The observed phosphorylation of JNK following LPC treatment is functional, as demonstrated by increased phosphorylation of the transcription factor c-Jun, an endogenous substrate for JNK (Fig. 3C), which was reduced by the pharmacological JNK inhibition (Fig. 3C). Taken together with prior observations (2, 19, 29), these data implicate JNK activation as a downstream effector of hepatocyte lipoapoptosis induced by LPC.
Fig. 3.
LPC induces JNK phosphorylation, and JNK inhibition and glycogen synthase kinase-3 (GSK-3) inhibition reduce c-Jun phosphorylation in hepatocytes. A: immunoblot analysis was performed for phosphorylated (phospho-) and total JNK. Whole cell lysates were prepared from Huh-7 cells incubated with LPC (85 or 42.5 μM) for 8 h or from MPH incubated with LPC (85 μM) for 4 h. B: immunoblot analysis was performed for phosphorylated and total JNK. Whole cell lysates were prepared from Huh-7 cells incubated with LPC (85 μM) for the indicated times. C: immunoblot analysis was performed for phosphorylated and total c-Jun. Whole cell lysates were prepared from Huh-7 cells incubated with LPC at 85 μM in the presence of the JNK inhibitor SP600152 (SP) at 50 μM for 8 h or from MPH incubated with LPC (85 μM) in the presence of SP (50 μM) for 4 h. D: immunoblot analysis was performed for phosphorylated and total c-Jun. Whole cell lysates were prepared from Huh-7 cells incubated with LPC (85 μM) in the presence of the GSK-3 inhibitor GSK-IX (2 μM) for 8 h.
The mechanisms responsible for JNK activation during lipotoxic stress are complex and likely multifactorial. Activated IRE-1α, an ER resident kinase, can bind the adaptor molecule TNF receptor-associated factor 2 (TRAF2), which further recruits the apoptosis signal-regulating kinase (ASK1); this latter kinase activates the JNK signaling pathway (41). However, a recent report suggests that neither IRE-1α nor ASK-1 are required for saturated FFA-induced JNK phosphorylation (37). On the other hand, we recently reported that PA-induced JNK activation was GSK-3 dependent (22). Therefore, we ascertained whether inhibition of GSK-3α and β-serine/threonine kinases by the pharmacological GSK-3 inhibitor GSK-IX also reduces LPC-induced hepatocyte toxicity. The GSK-3 inhibitor was effective in reducing LPC-induced c-Jun phosphorylation (Fig. 3D). Collectively, these data indicate that, like PA, LPC activates JNK via a GSK-3-sensitive pathway.
LPC-induced JNK activation increased CHOP expression, eIF2α phosphorylation, and PUMA upregulation.
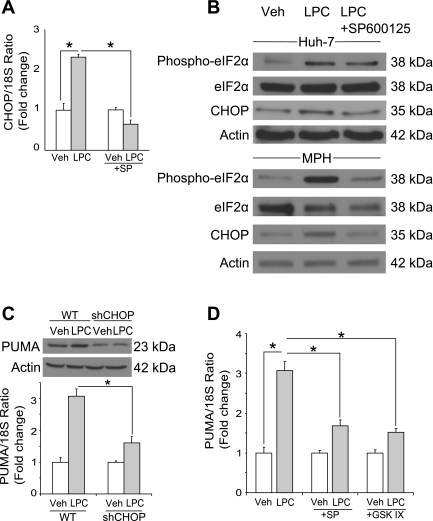
Lipoapoptosis has been linked to induction of ER stress and the subsequent increase in CHOP. Given this information, we initially determined whether LPC induced an ER stress response as indicated by increased CHOP mRNA and protein expression and phosphorylation of eIF2α (22, 34, 39). LPC induced increase of CHOP mRNA and protein level at 8 h after treatment of Huh-7 cells and primary murine hepatocytes (Fig. 4, A and B). LPC also induced phosphorylation of eIF2α in both Huh-7 cells and primary murine hepatocytes (Fig. 4B). Thus LPC, like PA, also induces an ER stress response.
Fig. 4.
LPC induces JNK-dependent CAAT/enhancer binding homologous protein (CHOP) expression and CHOP-mediated p53 upregulated modulator of apoptosis (PUMA) expression. A: total RNA was prepared from Huh-7 cells treated with LPC (85 μM) for 8 h. Vehicle-treated cells were used as controls. CHOP mRNA was quantified by real-time PCR, normalized to 18S rRNA, and expressed as fold change over vehicle. B: whole cell lysates were prepared from Huh-7 cells treated with LPC (85 μM) for 8 h or from MPH treated with LPC (85 μM) for 4 h. Immunoblot analysis was performed for phosphorylated eIF2α, total eIF2α, and CHOP. Actin was used as a loading control. C: cells were treated as described in B. Whole cell lysates were prepared 16 h after treatment, and total RNA was extracted 8 h after treatment. Immunoblot analysis was performed for PUMA. Actin was used as a loading control. PUMA mRNA was quantified by real-time PCR, normalized to 18S rRNA, and expressed as fold change over vehicle. D: total RNA was prepared from Huh-7 cells treated with LPC (85 μM) in the presence of the JNK inhibitor SP (50 μM) or the GSK-3 inhibitor GSK-IX (2 μM) for 8 h. PUMA mRNA was quantified by real-time PCR as described above. All data are means ± SE for 3 experiments. *P < 0.01.
PUMA is a proapoptotic mediator of lipoapoptosis increased in response to CHOP activation. Indeed, LPC treatment was associated with an augmentation of PUMA mRNA and protein levels (Fig. 4C). Interestingly, shRNA-targeted knockdown of CHOP prevented LPC-induced increases in PUMA expression (Fig. 4C), a result similar to the contribution of CHOP in PA-induced toxicity (10). In addition to CHOP, regulation of PUMA transcription is also JNK dependent. Pharmacological inhibition of JNK downregulates CHOP expression induced by the ER stress inducer thapsigargin (33) and prevents LPC-induced increase of CHOP mRNA and protein in our model (Fig. 4, A and B). Also, inhibition of LPC-induced activation of JNK, directly by SP600125 or indirectly by the GSK-3-inhibitor GSK-IX, also prevented LPC-induced upregulation of PUMA mRNA (Fig. 4D).
LPC-induced lipoapoptosis can be blocked by inhibition of JNK, GSK-3, or CHOP.
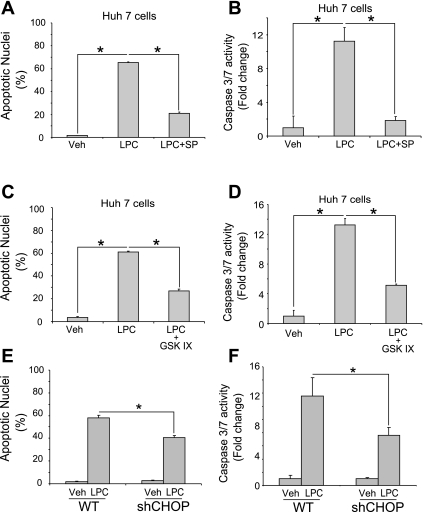
Functionally, activation of JNK by LPC is anticipated to be a key link in the pathway to apoptosis; thus it was important to demonstrate that LPC-induced lipoapoptosis was preventable by inhibition of JNK. Significant numbers of apoptotic nuclei were observed in Huh-7 cells treated with LPC, accompanied by biochemical activation of caspase 3/7 activity. Both indicators of lipoapoptosis were greatly suppressed by cotreatment with the JNK inhibitor SP600125 (Fig. 5, A and B). Consistent with GSK-3-mediated JNK activation, the GSK-3 inhibitor also markedly reduced morphological and biochemical markers of apoptosis in LPC-treated Huh-7 cells (Fig. 5, C and D). The parallel increase of CHOP by lipoapoptosis activation is an additional instigator of death. To determine the contribution of CHOP to LPC-induced lipoapoptosis, parental Huh-7 cells or a stable clone expressing a shRNA targeting CHOP were treated with LPC. Depletion of CHOP partially mitigated cell death in response to LPC (Fig. 5, E and F). These results indicate that both JNK- and CHOP-dependent apoptotic signals are activated by LPC.
Fig. 5.
Inhibition of JNK and GSK-3 reduces LPC-induced apoptosis, and CHOP mediates LPC-induced apoptosis. A and B: Huh-7 cells were incubated with LPC (85 μM) in the presence of the JNK inhibitor SP (50 μM) for 16 h. Vehicle-treated cells were used as controls. Apoptosis was assessed using morphological criteria after DAPI staining (A). Caspase 3/7 catalytic activity was assessed using a fluorogenic assay and expressed as fold change (B). C and D: Huh-7 cells were incubated with LPC (85 μM) in the presence of the GSK-3 inhibitor GSK-IX (2 μM) for 16 h. Apoptosis was assessed using morphological criteria after DAPI staining (C). Caspase 3/7 catalytic activity was assessed using a fluorogenic assay and expressed as fold change (D). E and F: Huh-7 (wild type; WT) and Huh-7 cells stably expressing shRNA complementary to CHOP (shCHOP) were incubated with LPC (85 μM) for 16 h. Vehicle-treated cells were used as controls. Apoptotic nuclei were counted after DAPI staining, and caspase 3/7 catalytic activity was measured using a fluorogenic assay. All data are means ± SE for 3 experiments. *P < 0.01.
LPC induced JNK- and GSK-dependent activation of Bax.
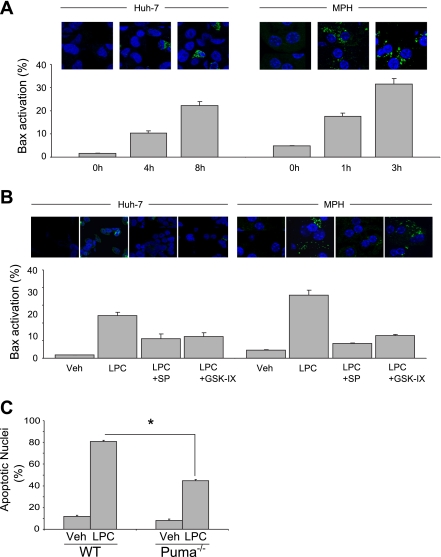
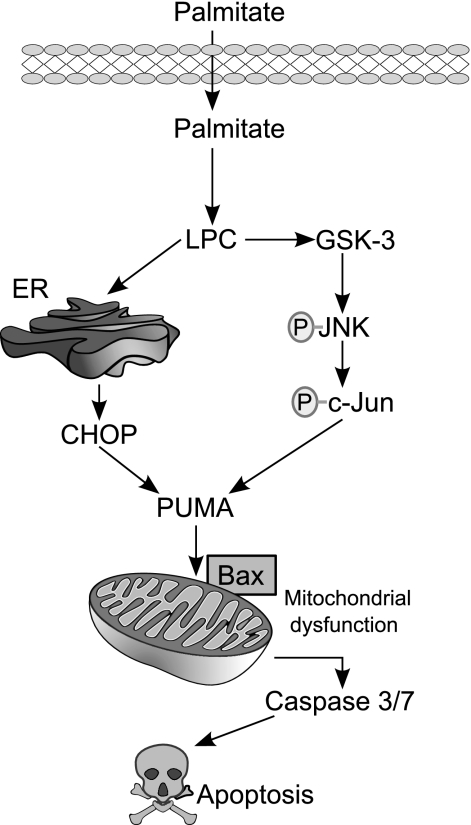
Activation of Bax, and the resultant mitochondrial dysfunction, constitutes a critical mechanism of PA-mediated lipoapoptosis (29). Thus it is significant that PUMA can directly or indirectly activate Bax (16, 24), and we have demonstrated that PA-induced PUMA expression activated Bax in lipoapoptosis (11). On activation, Bax undergoes a conformational change exposing its NH2 terminus, unfolding a highly conserved sequence that is specifically recognized by the monoclonal antibody 6A7. Consistent with its role as a mediator of PA-induced cytotoxicity, LPC treatment induced Bax activation in Huh-7 cells and primary murine hepatocytes in a time-dependent manner (Fig. 6A). Moreover, inhibition of LPC-induced PUMA expression by the JNK inhibitor SP600125 or the GSK-3 inhibitor GSK-IX prevented Bax activation following treatment with LPC (Fig. 6B). Isolated mouse hepatocytes from Puma−/− mice also displayed resistance to LPC-induced apoptosis (Fig. 6C). Together, these data show that LPC cytotoxicity is associated with induced CHOP expression as well as GSK-3-mediated JNK activation. These pathways converge to increase PUMA levels, and, in the context of LPC-induced lipotoxic stress, PUMA promotes Bax activation, resulting in mitochondrial dysfunction and cellular demise (Fig. 7).
Fig. 6.
LPC-induced Bax activation is JNK and GSK-3 dependent, and LPC-induced apoptosis is mediated by PUMA. A: Huh-7 cells or MPH were incubated with LPC (85 μM) for the indicated times. Representative images of 3 independent experiments are depicted. 6A7-immunoreactive cells, indicative of an activating conformational change in Bax, were quantified in 10 random ×40 objective fields for each condition, and the percentages of 6A7-positive vs. total cells are shown in bar graphs. B: Huh-7 cells or MPH were incubated with LPC (85 μM) in the presence of the JNK inhibitor SP (50 μM) or the GSK-3 inhibitor GSK-IX (2 μM) for 8 or 3 h, respectively. Hepatocytes were analyzed for Bax 6A7 immunoreactivity as described in A. C: MPH were isolated from either WT or Puma−/− mice and treated with LPC (75 μM) for 6 h. Vehicle-treated cells were used as controls. All data are means ± SE for 3 experiments. *P < 0.01.
Fig. 7.
Schematic representation of the proposed model for the mechanism of LPC-induced apoptosis. PA-derived LPC induces endoplasmic reticulum (ER) stress and JNK activation, resulting in the induction of the BH3-only protein PUMA. Increased PUMA causes subsequent Bax activation, mitochondrial dysfunction, caspase 3/7 activation, and cellular demise.
SUMMARY
The current study provides new mechanistic insights regarding the mechanisms of LPC cytotoxicity in hepatocytes. The major findings of this study indicate that 1) LPC is increased by incubation with PA in Huh-7 cells; 2) LPC-induced apoptosis is dependent on GSK-3/JNK and caspase activation in hepatocytes; and 3) cytotoxicity by LPC is associated with PUMA upregulation and is PUMA dependent. These current observations further refine in vitro models of FFA-mediated lipoapoptosis. Saturated FFA per se may not be necessary to induce cytotoxic pathways in hepatocytes provided its downstream lipid phosphometabolite LPC is present. Employing the most proximate mediator of this lipid cascade should help identify the most critical and pivotal pathways leading to cell death. We propose that LPC maybe this mediator and, therefore, that future studies on lipotoxicity should encompass the study of this relevant phospholipid. Once the critical LPC-mediated pathways for lipotoxicity have been elucidated, rationally designed therapies for NAFLD can hopefully be developed.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK41876 to G. J. Gores and P30 DK084567 to the Optical Microscopy Core of the Mayo Clinic Center for Cell Signaling in Gastroenterology and the Mayo Foundation.
DISCLOSURES
The authors who have taken part in this study declared that they do not have anything to declare regarding funding from industry or conflict of interest with respect to this manuscript.
AUTHOR CONTRIBUTIONS
Author contributions: K.K., S.C.C., C.D.F., M.E.G., S.F.B., N.W.W., and J.L.M. performed experiments; K.K., S.C.C., C.D.F., M.E.G., S.F.B., N.W.W., and J.L.M. analyzed data; K.K., S.C.C., C.D.F., M.E.G., J.L.M., and G.J.G. interpreted results of experiments; K.K. prepared figures; K.K. drafted manuscript; K.K. and G.J.G. edited and revised manuscript; K.K., S.C.C., C.D.F., M.E.G., N.W.W., J.L.M., and G.J.G. approved final version of manuscript; G.J.G. conception and design of research.
ACKNOWLEDGMENTS
We thank Dr. Harmeet Malhi for reading the manuscript and providing insightful comments and suggestion. We thank Dr. Anuradha Krishnan for isolating the human primary hepatocytes and Courtney N. Riddle for excellent secretarial assistance.
REFERENCES
- 1. Adams LA, Lymp JF, Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 129: 113–121, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Akazawa Y, Cazanave S, Mott JL, Elmi N, Bronk SF, Kohno S, Charlton MR, Gores GJ. Palmitoleate attenuates palmitate-induced Bim and PUMA up-regulation and hepatocyte lipoapoptosis. J Hepatol 52: 586–593, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol 17, Suppl: S186–S190, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Ariyama Y, Tanaka Y, Shimizu H, Shimomura K, Okada S, Saito T, Yamada E, Oyadomari S, Mori M. The role of CHOP messenger RNA expression in the link between oxidative stress and apoptosis. Metabolism 57: 1625–1635, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Barreyro FJ, Kobayashi S, Bronk SF, Werneburg NW, Malhi H, Gores GJ. Transcriptional regulation of Bim by FoxO3A mediates hepatocyte lipoapoptosis. J Biol Chem 282: 27141–27154, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Beier F, Loeser RF. Biology and pathology of Rho GTPase, PI-3 kinase-Akt, and MAP kinase signaling pathways in chondrocytes. J Cell Biochem 110: 573–580, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, Berria R, Ma JZ, Dwivedi S, Havranek R, Fincke C, DeFronzo R, Bannayan GA, Schenker S, Cusi K. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 355: 2297–2307, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Bommiasamy H, Back SH, Fagone P, Lee K, Meshinchi S, Vink E, Sriburi R, Frank M, Jackowski S, Kaufman RJ, Brewer JW. ATF6alpha induces XBP1-independent expansion of the endoplasmic reticulum. J Cell Sci 122: 1626–1636, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brunt EM. Non-alcoholic fatty liver disease: what's new under the microscope? Gut. 2011 doi: 10.1136/gut.2010.218214. [DOI] [PubMed] [Google Scholar]
- 10. Cazanave SC, Elmi NA, Akazawa Y, Bronk SF, Mott JL, Gores GJ. CHOP and AP-1 cooperatively mediate PUMA expression during lipoapoptosis. Am J Physiol Gastrointest Liver Physiol 299: G236–G243, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cazanave SC, Mott JL, Elmi NA, Bronk SF, Werneburg NW, Akazawa Y, Kahraman A, Garrison SP, Zambetti GP, Charlton MR, Gores GJ. JNK1-dependent PUMA expression contributes to hepatocyte lipoapoptosis. J Biol Chem 284: 26591–26602, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science 332: 1519–1523, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 44: 865–873, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Faubion WA, Guicciardi ME, Miyoshi H, Bronk SF, Roberts PJ, Svingen PA, Kaufmann SH, Gores GJ. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J Clin Invest 103: 137–145, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide W, Lin X, Watkins SM, Ivanov AR, Hotamisligil GS. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 473: 528–531, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gallenne T, Gautier F, Oliver L, Hervouet E, Noel B, Hickman JA, Geneste O, Cartron PF, Vallette FM, Manon S, Juin P. Bax activation by the BH3-only protein Puma promotes cell dependence on antiapoptotic Bcl-2 family members. J Cell Biol 185: 279–290, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goldsmith ZG, Dhanasekaran DN. G protein regulation of MAPK networks. Oncogene 26: 3122–3142, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Han MS, Lim YM, Quan W, Kim JR, Chung KW, Kang M, Kim S, Park SY, Han JS, Cheon HG, Rhee SD, Park TS, Lee MS. Lysophosphatidylcholine as an effector of fatty acid-induced insulin resistance. J Lipid Res 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Han MS, Park SY, Shinzawa K, Kim S, Chung KW, Lee JH, Kwon CH, Lee KW, Park CK, Chung WJ, Hwang JS, Yan JJ, Song DK, Tsujimoto Y, Lee MS. Lysophosphatidylcholine as a death effector in the lipoapoptosis of hepatocytes. J Lipid Res 49: 84–97, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Hsieh CC, Yen MH, Liu HW, Lau YT. Lysophosphatidylcholine induces apoptotic and non-apoptotic death in vascular smooth muscle cells: in comparison with oxidized LDL. Atherosclerosis 151: 481–491, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Huang Y, Fu JF, Shi HB, Liu LR. Metformin prevents non-alcoholic fatty liver disease in rats: role of phospholipase A2/lysophosphatidylcholine lipoapoptosis pathway in hepatocytes [in Chinese]. Zhonghua Er Ke Za Zhi 49: 139–145, 2011 [PubMed] [Google Scholar]
- 22. Ibrahim SH, Akazawa Y, Cazanave SC, Bronk SF, Elmi NA, Werneburg NW, Billadeau DD, Gores GJ. Glycogen synthase kinase-3 (GSK-3) inhibition attenuates hepatocyte lipoapoptosis. J Hepatol 54: 765–772, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ibrahim SH, Kohli R, Gores GJ. Mechanisms of lipotoxicity in NAFLD and clinical implications. J Pediatr Gastroenterol Nutr 53: 131–140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jabbour AM, Heraud JE, Daunt CP, Kaufmann T, Sandow J, O'Reilly LA, Callus BA, Lopez A, Strasser A, Vaux DL, Ekert PG. Puma indirectly activates Bax to cause apoptosis in the absence of Bid or Bim. Cell Death Differ 16: 555–563, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, MacLean KH, Han J, Chittenden T, Ihle JN, McKinnon PJ, Cleveland JL, Zambetti GP. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 4: 321–328, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Kishimoto T, Soda Y, Matsuyama Y, Mizuno K. An enzymatic assay for lysophosphatidylcholine concentration in human serum and plasma. Clin Biochem 35: 411–416, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Krishnan A, Viker K, Rietema H, Telgenkamp M, Knudsen B, Charlton M. Prolonged engraftment of human hepatocytes in mice transgenic for the deleted form of human hepatocyte growth factor. Hepatol Res 37: 854–862, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Lei X, Barbour SE, Ramanadham S. Group VIA Ca2+-independent phospholipase A2 (iPLA2β) and its role in β-cell programmed cell death. Biochimie 92: 627–637, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem 281: 12093–12101, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Masamune A, Sakai Y, Satoh A, Fujita M, Yoshida M, Shimosegawa T. Lysophosphatidylcholine induces apoptosis in AR42J cells. Pancreas 22: 75–83, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Masuoka HC, Mott J, Bronk SF, Werneburg NW, Akazawa Y, Kaufmann SH, Gores GJ. Mcl-1 degradation during hepatocyte lipoapoptosis. J Biol Chem 284: 30039–30048, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nehra V, Angulo P, Buchman AL, Lindor KD. Nutritional and metabolic considerations in the etiology of nonalcoholic steatohepatitis. Dig Dis Sci 46: 2347–2352, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Oh SH, Lim SC. Endoplasmic reticulum stress-mediated autophagy/apoptosis induced by capsaicin (8-methyl-N-vanillyl-6-nonenamide) and dihydrocapsaicin is regulated by the extent of c-Jun NH2-terminal kinase/extracellular signal-regulated kinase activation in WI38 lung epithelial fibroblast cells. J Pharmacol Exp Ther 329: 112–122, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11: 381–389, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, Contos MJ, Sanyal AJ. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 46: 1081–1090, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Richieri GV, Kleinfeld AM. Unbound free fatty acid levels in human serum. J Lipid Res 36: 229–240, 1995 [PubMed] [Google Scholar]
- 37. Sharma M, Urano F, Jaeschke A. Cdc42 and Rac1 are major contributors to the saturated fatty acid-stimulated JNK pathway in hepatocytes. J Hepatol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Singh R, Wang Y, Xiang Y, Tanaka KE, Gaarde WA, Czaja MJ. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology 49: 87–96, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13: 184–190, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 9: 231–241, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287: 664–666, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology 44: 27–33, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Zhang SD, Peng ZY, Liu S, Pei ZF, Chen F, Yang L. Neferine protects endothelial cells against damages induced by LPC and relationship with asymmetric dimethylarginine [in Chinese]. Zhongguo Zhong Yao Za Zhi 33: 2526–2529, 2008 [PubMed] [Google Scholar]