Abstract
Cholestatic patients often present with clinical features suggestive of adrenal insufficiency. In the bile duct-ligated (BDL) model of cholestasis, the hypothalamic-pituitary-adrenal (HPA) axis is suppressed. The consequences of this suppression on cholangiocyte proliferation are unknown. We evaluated 1) HPA axis activity in various rat models of cholestasis and 2) effects of HPA axis modulation on cholangiocyte proliferation. Expression of regulatory molecules of the HPA axis was determined after BDL, partial BDL, and α-naphthylisothiocyanate (ANIT) intoxication. The HPA axis was suppressed by inhibition of hypothalamic corticotropin-releasing hormone (CRH) expression by central administration of CRH-specific Vivo-morpholinos or by adrenalectomy. After BDL, the HPA axis was reactivated by 1) central administration of CRH, 2) systemic ACTH treatment, or 3) treatment with cortisol or corticosterone for 7 days postsurgery. There was decreased expression of 1) hypothalamic CRH, 2) pituitary ACTH, and 3) key glucocorticoid synthesis enzymes in the adrenal glands. Serum corticosterone and cortisol remained low after BDL (but not partial BDL) compared with sham surgery and after 2 wk of ANIT feeding. Experimental suppression of the HPA axis increased cholangiocyte proliferation, shown by increased cytokeratin-19- and proliferating cell nuclear antigen-positive cholangiocytes. Conversely, restoration of HPA axis activity inhibited BDL-induced cholangiocyte proliferation. Suppression of the HPA axis is an early event following BDL and induces cholangiocyte proliferation. Knowledge of the role of the HPA axis during cholestasis may lead to development of innovative treatment paradigms for chronic liver disease.
Keywords: corticotropin-releasing hormone, glucocorticoids, biliary epithelium, adrenocorticotropic hormone
cholangiocytes are epithelial cells that line the intra- and extrahepatic bile ducts. They are constitutively mitotically dormant but possess marked proliferative capacity (2), which is apparent during experimental conditions, such as cholestasis induced by bile duct ligation (BDL) or α-naphthylisothiocyanate (ANIT) intoxication (1), as well as in human cholangiopathies (2). In humans, cholangiocyte proliferation occurs in extrahepatic biliary obstruction, in the course of chronic cholestatic liver diseases (e.g., primary sclerosing cholangitis, primary biliary cirrhosis, liver allograft rejection, and graft-vs.-host disease) (2), and in many forms of liver injury (e.g., in response to alcohol, toxin, or drugs) (2, 43).
The hypothalamic-pituitary-adrenal (HPA) axis describes a complex set of positive- and negative-feedback influences between the hypothalamus, pituitary gland, and adrenal gland (25). These feedforward and feedback mechanisms work in a neuroendocrine manner to modulate a number of physiological processes, such as immunity (32), digestion (30), and the body's response to stress (30). In addition, the HPA axis has been shown to have an influence on human psychology (7, 53).
The mechanism by which the HPA axis remains in homeostasis depends widely on the release and uptake of several key regulatory molecules. The hypothalamus contains neuroendocrine neurons that secrete corticotropin-releasing hormone (CRH), among other humoral agents. CRH will, in turn, act on the pituitary gland to stimulate the production of ACTH, which is derived from the proteolytic cleavage of proopiomelanocortin (POMC) to release ACTH, as well as other peptide fragments such as β-lipotropin (44), into the circulation. Circulating ACTH then induces the adrenal gland to synthesize and release circulating corticosteroids, such as cortisol and corticosterone. These circulating corticosteroids then modulate the vast array of physiological processes influenced by the HPA axis and are also responsible for initiating a negative-feedback loop to shut down corticosteroid production. There are several mechanisms by which the HPA axis can be suppressed. The hippocampus, which is the major controlling organ of the HPA axis, is the initial location in which the HPA axis can be suppressed. However, every region involved in the HPA axis has a negative-feedback mechanism, all leading to reduced circulating corticosteroid levels (6, 8, 24, 26, 27, 29).
Cholestatic patients exhibit clinical features suggestive of adrenal insufficiency, such as hypovolemia, hypotension, and renal failure (55, 57). Furthermore, patients with congenital hypopituitarism or glucocorticoid deficiency often exhibit cholestatic hepatitis (5, 20, 28). In the BDL rat model of cholestasis, there is a general suppression of HPA axis responsiveness to stress (48), as well as a defective CRH-mediated response (46). However, the implications of HPA axis suppression and the subsequent effects of central CRH administration on cholangiocyte proliferation and function have not been studied. Therefore, the aims of the present study were to elucidate the connection between the HPA axis and the liver, in particular how the HPA axis can affect cholangiocyte proliferation.
MATERIALS AND METHODS
Materials
Unless otherwise indicated, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and were of the highest grade available. Antibodies against proliferating cell nuclear antigen (PCNA), cytochrome P-450 11b1 (Cyp11b1), hydroxysteroid dehydrogenase 3β (HSD-3β), and CRH were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The specific cytokeratin-19 (CK-19) antibody was purchased from Vector Laboratories (Burlingame, CA), and the ACTH-specific antibody was purchased from Abcam (Cambridge, MA). All primers were purchased from SABiosciences (Frederick, MD). The CRH Vivo-morpholino (5′-ACCAGCAGCCGCAGCCGCATGTTTA) and corresponding mismatched control sequence (5′-ACgAcCAGCgGCAGCCcCATcTTTA) were purchased from Gene Tools (Philomath, OR). Recombinant rat ACTH and the enzyme immunoassay (EIA) kits for detecting CRH and ACTH were obtained from Phoenix Pharmaceuticals (Burlingame, CA). Cortisol and corticosterone EIA kits were purchased from Cayman Chemical (Ann Arbor, MI).
Animal Treatment
Male Sprague-Dawley rats (150–175 g body wt) were purchased from Charles River and maintained in a temperature-controlled environment (20–22°C) with a 12:12-h light-dark cycle. Animals had free access to drinking water and standard rat chow. All animal experiments were performed in accordance with the guidelines of the Scott and White Institutional Animal Care and Use Committee, which approved the procotol. Rats underwent surgery to ligate the common bile duct (BDL) or a single lobe (partial BDL), as described previously (18). For the ANIT intoxication studies, rats were fed a diet containing 0.1% ANIT or the control diet (AIN 76, Dyets, Bethlehem, PA), as described elsewhere (34). A description of the experimental groups is outlined in Table 1. On the days indicated postsurgery, tissue and serum were collected for further analysis between 8 and 9 AM to minimize the circadian variations in glucocorticoid levels (11).
Table 1.
Summary of animal treatment groups
| Treatment | Tissue Collection | |
|---|---|---|
| BDL | None | 1, 3, 5, and 7 days |
| Sham BDL | 1, 3, 5, and 7 days | |
| Partial BDL | 7 days | |
| 0.1% ANIT intoxication | 7 and 14 days | |
| ANIT control diet | 7 and 14 days | |
| Adrenalectomy | 7 days | |
| CRH morpholino (4 μg·rat−1·day−1 icv) | 7 days | |
| CRH mismatched morpholino (4 μg·rat−1·day−1 icv) | 7 days | |
| BDL | ACTH (100 μg·kg−1·day−1 ip) | 7 days |
| Sham BDL | ACTH (100 μg·kg−1·day−1 ip) | 7 days |
| BDL | CRH (5 μg·rat−1·day−1 icv) | 7 days |
| Sham BDL | CRH (5 μg·rat−1·day−1 icv) | 7 days |
| BDL | Cortisol (1 mg·kg−1·day−1 ip) | 7 days |
| Sham BDL | Cortisol (1 mg·kg−1·day−1 ip) | 7 days |
| BDL | Corticosterone (1 mg·kg−1·day−1 ip) | 7 days |
| Sham BDL | Corticosterone (1 mg·kg−1·day−1 ip) | 7 days |
Intraperitoneal treatments were administered via implanted osmotic minipump.
BDL, bile duct ligation; ANIT, α-naphthylisothiocyanate; CRH, corticotropin-releasing hormone.
Cholangiocyte Proliferation
Cholangiocyte proliferation was assessed in liver sections from the treatment groups by 1) immunohistochemical staining for CK-19 to assess intrahepatic biliary mass and 2) PCNA immunoreactivity as a marker of proliferative capacity using the method described elsewhere (14, 34). After they were stained, the sections were counterstained with hematoxylin and examined with a microscope (model BX 40, Olympus Optical). Over 100 cholangiocytes were counted in a random, blinded fashion in 3 different fields for each group of animals. Data are expressed as number of CK-19- and PCNA-positive cholangiocytes per portal tract.
Assessment of HPA Axis Activity
The circulating glucocorticoids represent the functional output of the HPA axis (41). Circulating cortisol and corticosterone levels in serum from normal and BDL rats were assessed using commercially available EIA kits according to the manufacturer's instructions.
CRH levels in the hypothalamus.
CRH expression and secretion were assessed at various times after BDL by 1) real-time PCR (9), 2) immunoblotting (10), 3) immunofluorescence (10, 21), and 4) CRH-specific EIA. Briefly, rats underwent BDL surgery, and at various times postsurgery the animals were euthanized and the brain was removed. The hypothalamus was dissected and rapidly snap-frozen in liquid nitrogen. For the real-time PCR analysis, RNA was extracted using the RNeasy Mini kit (Qiagen, Valencia, CA) according to the instructions provided by the vendor and reverse-transcribed using the Reaction Ready First Strand cDNA synthesis kit (SABiosciences-Qiagen, Frederick, MD). These reactions were used as templates for the PCR assays using a SYBR Green PCR Master Mix (SABiosciences-Qiagen) in the real-time thermal cycler (Mx3005P sequence detection system, Agilent) and specific primers against rat CRH (SABiosciences-Qiagen; Ref Seq NM_031019, product size 108 bp, reference position 650 bp). A cycle threshold (ΔΔCT) analysis was performed (9, 35) using the normal tissue as the control sample. Data are expressed as relative mRNA levels (means ± SE, n = 4).
CRH protein expression was assessed in hypothalamic protein lysates using immunoblotting, as described previously (10), and a CRH-specific antibody (C-20, Santa Cruz Biotechnology). The intensity of the bands was determined by scanning video densitometry using a PhosphorImager (Storm 860, Amersham Biosciences, Piscataway, NJ) and ImageQuant TLV 2003.02 software. Furthermore, CRH immunoreactivity was assessed in brain sections by immunofluorescence. Normal or BDL rats were transcardially perfused with 4% paraformalydehyde. The brains were removed and postfixed for a further 24 h in 4% paraformalydehyde and then cryoprotected in 30% sucrose (wt/vol in 1× PBS). Free-floating immunofluorescence staining of CRH was performed in brain sections (20 μm) via the method described previously (10, 21). Negative controls were stained using preimmune serum in the place of the primary antibody. Images were taken on an inverted confocal microscope (model IX71, Olympus).
In parallel, circulating (in serum) and tissue (in hypothalamic protein lysates) levels of CRH in normal and BDL rats were assessed using a CRH-specific EIA (Phoenix Pharmaceuticals) following the manufacturer's instructions. Tissue CRH levels are expressed as the ratio of CRH to total protein in the sample, whereas circulating CRH levels are expressed as the amount of CRH per milliliter of serum.
ACTH levels in the pituitary.
ACTH expression and secretion were assessed at various times after BDL by 1) real-time PCR (9), 2) immunoblotting (10), and 3) ACTH-specific EIA. Briefly, rats underwent BDL surgery, and at various times postsurgery the animals were euthanized and the pituitaries were removed and snap-frozen. Primers specific for rat POMC (SABiosciences-Qiagen; Ref Seq NM_13926, product size 160 bp, reference position 673 bp) were used in the real-time PCR analysis as a reflection of ACTH mRNA expression. Immunoblotting for ACTH was performed as outlined above using an antibody directed against amino acids 1–20 of ACTH (clone B427, Abcam). In parallel, circulating (in serum) and tissue (in pituitary protein lysates) levels of ACTH in normal and BDL rats were assessed using an ACTH-specific EIA (Phoenix Pharmaceuticals) following the manufacturer's instructions.
Expression of steroidogenic enzymes in the adrenal glands.
The expression levels of two key enzymes in the synthesis of glucocorticoids, HSD-3β and Cyp11b1, were assessed in the adrenal glands by real-time PCR and immunoblotting. Specifically, rats underwent BDL surgery, and at various times postsurgery the animals were euthanized and the adrenal glands were removed and snap-frozen. Specific primers for HSD-3β (SABiosciences-Qiagen; Ref Seq NM_001007719, product size 96 bp, reference position 1,462 bp) and Cyp11b1 (SABiosciences-Qiagen; Ref Seq NM_012537, product size 84 bp, reference position 1,472 bp) were used in the real-time PCR analysis, as described above. Immunoblotting was also performed using specific antibodies against HSD-3β (Santa Cruz Biotechnology) and Cyp11b1 (Santa Cruz Biotechnology).
RESULTS
HPA Axis Is Suppressed in Experimental Models of Cholestasis
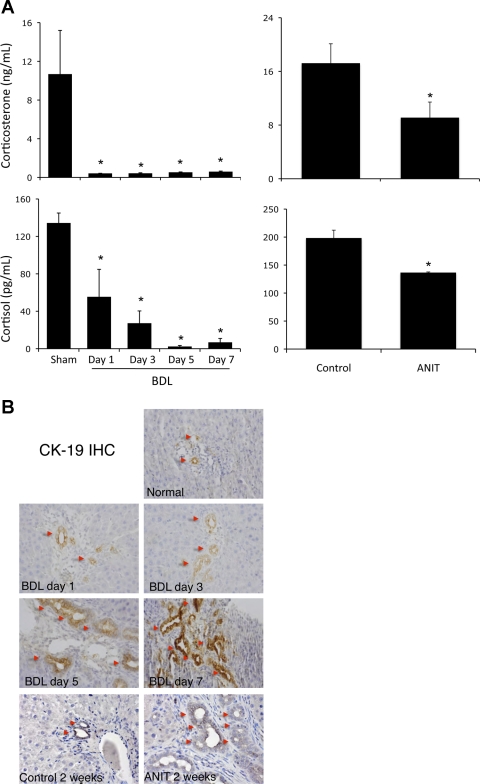
Circulating levels of cortisol and corticosterone were dramatically decreased from 1 to 7 days after BDL surgery compared with sham-operated rats (Fig. 1A) but were not suppressed after partial single-lobe BDL (data not shown). Using another well-characterized model of cholestasis involving the feeding of ANIT to rats, we observed a decrease in circulating levels of cortisol and corticosterone after 2 wk on the ANIT diet (Fig. 1A), but not at 1 wk (data not shown). To put this into a temporal context, we assessed cholangiocyte proliferation in parallel and found a slight increase in biliary mass 1 and 3 days after surgery and a more significant increase 5 and 7 days after surgery (Fig. 1B). In the ANIT model of cholestasis, biliary mass did not change significantly after 1 wk (data not shown) but increased dramatically at 2 wk (Fig. 1B). Because the more dramatic effects on the HPA axis were found using the BDL model of cholestasis, subsequent experiments were performed using this model.
Fig. 1.
Circulating glucocorticoid levels are reduced as an early event after bile duct ligation (BDL) and after 2 wk of α-naphthylisothiocyanate (ANIT) intoxication. A: cortisol and corticosterone in serum from rats after BDL surgery or ANIT feeding. Values are means ± SE; n = 5. *P < 0.05 vs. sham. B: cholangiocyte proliferation at various times after BDL surgery or ANIT feeding assessed by cytokeratin-19 (CK-19) immunohistochemistry (IHC). Original magnification ×20.
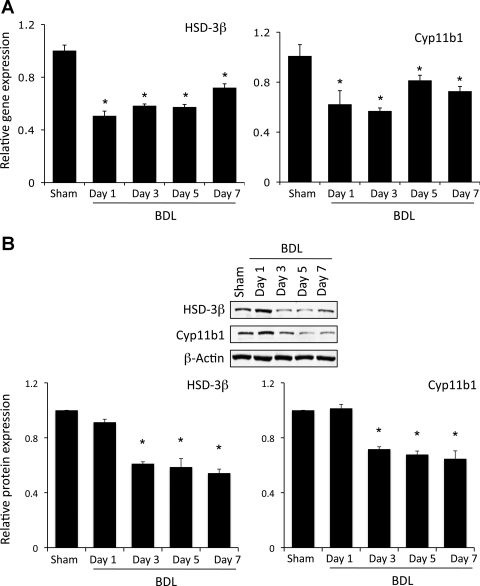
To assess if the reduced circulating glucocorticoid levels are a result of decreased steroidogenesis and to further pinpoint the precise level at which suppression of the HPA axis occurs during cholestasis, expression levels of two of the key regulatory enzymes in the steroidogenic pathway were assessed in the adrenal gland. HSD-3β is an enzyme that is involved in the early stages of conversion of cholesterol to glucocorticoids and is responsible for the enzymatic conversion of pregnenolone and its derivatives to progesterone derivatives (52). The mRNA and protein expression of HSD-3β is suppressed as an early event (1 day) after BDL surgery, an effect that continues up to 7 days (Fig. 2).
Fig. 2.
Key glucocorticoid synthesis enzymes are downregulated in adrenal glands after BDL. Rats underwent BDL surgery, and adrenal glands were dissected at various times postsurgery. Expression of hydroxysteroid dehydrogenase 3β (HSD-3β) and cytochrome P-450 11b1 (Cyp11b1) was assessed by real-time PCR (A) and immunoblotting (B). Values are means ± SE; n = 4. *P < 0.05 vs. sham.
One of the downstream events in steroid biosynthesis is conversion of deoxycorticosterone and 11-deoxycortisol to corticosterone and cortisol, respectively. Both of these reactions are catalyzed by Cyp11b1 (54), the expression of which is also significantly reduced 1–7 days after BDL surgery (Fig. 2). Taken together, our data suggest that the decrease in circulating glucocorticoid levels after BDL is probably due to a decrease in the expression of key regulatory enzymes in the steroidogenesis pathway.
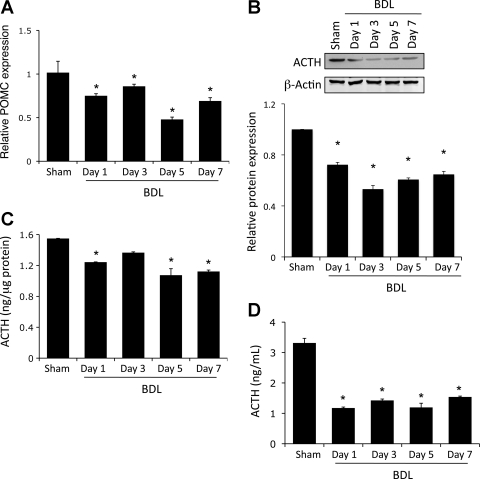
The expression and activity of the steroidogenesis enzymes is regulated by the secretion of ACTH from the anterior pituitary into the bloodstream (40). Therefore, we wished to determine if the reduced expression of the steroidogenesis enzymes is a result of decreased ACTH levels in the pituitary. As mentioned previously, ACTH is derived from the proteolytic cleavage of POMC to release the active ACTH peptide (44). POMC mRNA expression was decreased after BDL (Fig. 3A), suggesting a suppressed expression of the ACTH precursor. In addition, ACTH protein expression and content in the pituitary were decreased significantly after BDL surgery compared with sham-treated animals (Fig. 3, B and C). This suppressed expression also translated to a reduced level of circulating ACTH in the serum of BDL rats from 1 day postsurgery (Fig. 3D).
Fig. 3.
ACTH expression is downregulated in the pituitary after BDL. Rats underwent BDL surgery, and the pituitary was dissected at various times postsurgery. A: expression of the precursor peptide proopiomelanocortin (POMC) assessed by real-time PCR. B: ACTH protein expression assessed by immunoblotting. Values are means ± SE; n = 4. *P < 0.05 vs. sham. C and D: ACTH levels in pituitary lysates and serum, respectively, assessed using commercially available enzyme immunoassay (EIA). Values are means ± SE; n = 5. *P < 0.05 vs. sham.
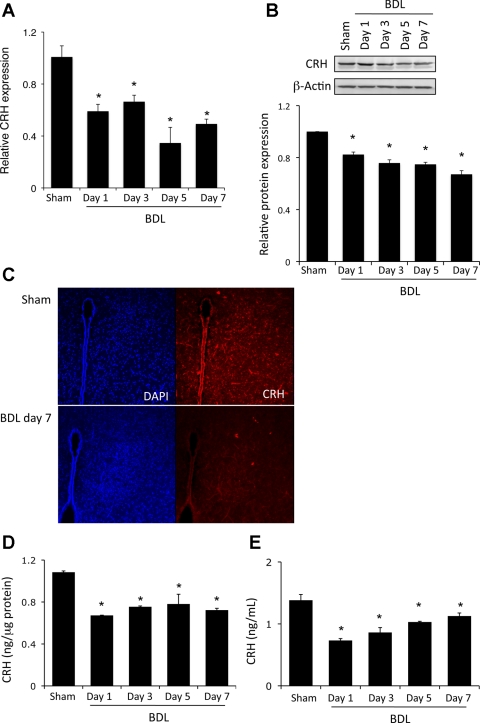
ACTH expression and secretion in the pituitary are under the direct control of CRH released from the hypothalamus (45). Given that we see decreased ACTH expression and release from the pituitary, we also wanted to evaluate if there was a decrease in hypothalamic CRH expression and secretion after BDL. CRH mRNA and protein expression were significantly decreased from 1 to 7 days after BDL surgery (Fig. 4, A and B). Furthermore, CRH was strongly expressed in the paraventricular nucleus of the hypothalamus of sham-operated animals but suppressed after BDL surgery (Fig. 4C). In addition, the CRH content of the hypothalamus decreased significantly 1 day after surgery, an effect that continued up to 7 days (Fig. 4D). Taken together, these data suggest that the hypothalamic CRH expression is suppressed as an early event after BDL surgery. To determine if this also translates to a decrease in CRH secretion, circulating CRH levels were assessed in the serum. Indeed, a decrease in CRH amounts in the serum was observed 1 day after BDL, and this decrease continued over the time course studied (Fig. 4E). Taken together, our data indicate that all regulatory humoral factors regulating the HPA axis are suppressed after BDL, although the precise mechanism by which this occurs is largely unknown.
Fig. 4.
Corticotropin-releasing hormone (CRH) expression is downregulated in the hypothalamus after BDL. Rats underwent BDL surgery, and the hypothalamus was dissected at various times postsurgery. Expression of CRH was assessed by real-time PCR (A) and immunoblotting (B). Values are means ± SE; n = 4. *P < 0.05. C: hypothalamic CRH immunoreactivity assessed by immunofluorescence (red) in whole brain sections 7 days after BDL or sham surgery. Sections were counterstained with 4′,6-diaminido-2-phenylindole (DAPI, blue). Original magnification ×40. D and E: CRH levels in pituitary lysates and serum, respectively, assessed by commercially available EIA. Values are means ± SE; n = 5. *P < 0.05 vs. sham.
Suppression of the HPA Axis Increased Biliary Mass by Inducing Cholangiocyte Proliferation
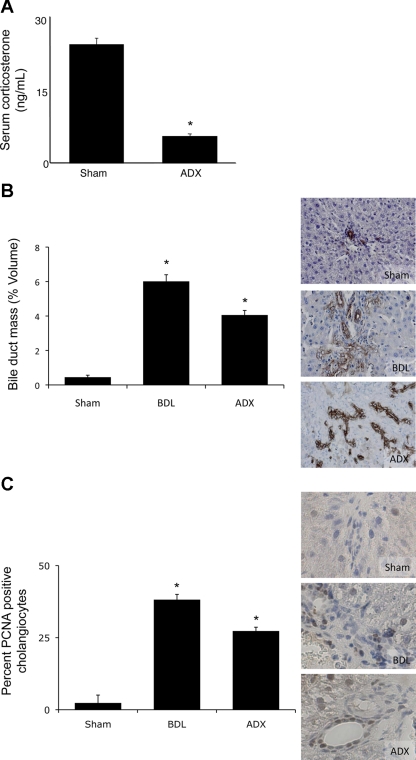
To determine the consequences (if any) of reduced glucocorticoid levels caused by suppression of the HPA axis on cholangiocyte proliferation, we took a two-tiered approach. First, HPA axis activity was suppressed by surgical removal of the adrenal glands. As expected, adrenalectomy significantly reduced the serum levels of corticosterone (Fig. 5A). Suppression of the HPA axis in this manner significantly increased bile duct mass 7 days after surgery, as demonstrated by CK-19 immunoreactivity (Fig. 5B). Furthermore, the percentage of PCNA-positive cholangiocytes increased after BDL and adrenalectomy (Fig. 5C), suggesting that bile duct mass is increased by increasing the proliferative capacity of cholangiocytes.
Fig. 5.
Adrenalectomy (ADX) induces cholangiocyte proliferation. A: circulating corticosterone levels 7 days after ADX or BDL, assessed by commercially available EIA. Values are means ± SE; n = 5. *P < 0.05. B and C: cytokeratin-19 (CK-19)-positive cholangiocytes and proliferating cell nuclear antigen (PCNA)-expressing cholangiocytes in liver sections 7 days after ADX or BDL. Values are means ± SE; n = 7. *P < 0.05 vs. sham.
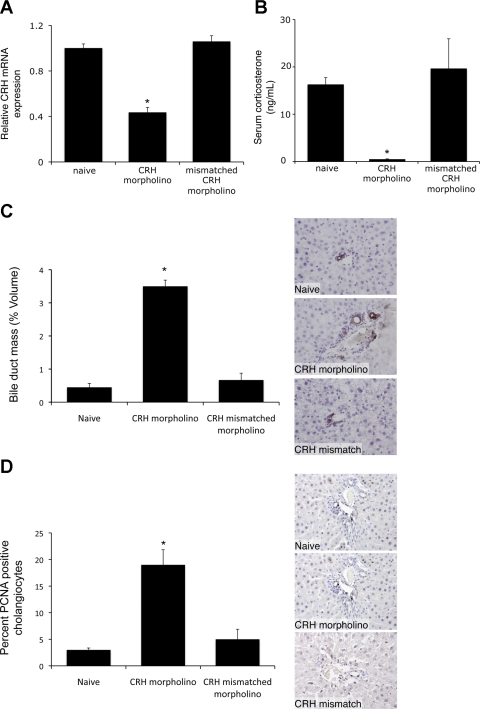
The second approach involved suppression of hypothalamic expression of CRH by the central administration of CRH-specific Vivo-morpholinos for 7 days. Vivo-morpholinos are antisense reagents that contain a novel transporter structure for effective use in vivo, used to block translation or interfere with RNA processing (39). Daily injection (intracerebroventricular) of CRH-specific Vivo-morpholinos decreased CRH expression by ∼60% compared with naive animals. Injection of the mismatched control sequence, having 6 bp changed, had no effect on CRH expression (Fig. 6A). Furthermore, central administration of CRH-specific Vivo-morpholinos significantly decreased the circulating corticosterone levels (Fig. 6B), which suggests that the genetic knockdown of CRH expression in the hypothalamus results in an overall suppression of the HPA axis. In support of the adrenalectomy data described above, suppression of the HPA axis by knockdown of hypothalamic CRH expression also increased biliary mass, which was not observed after intracerebroventricular injections of the mismatched control Vivo-morpholino sequence (Fig. 6C). Furthermore, there was an increased number of PCNA-positive cholangiocytes after central administration of CRH Vivo-morpholinos compared with naive animals or after central administration of the mismatched control sequence (Fig. 6D). Taken together, our data suggest that suppression of the HPA axis has the capacity to induce cholangiocyte proliferation.
Fig. 6.
Knockdown of hypothalamic CRH expression induces cholangiocyte proliferation. Rats were injected with CRH Vivo-morpholino (4 μg·rat−1·day−1 icv) or the mismatched control Vivo-morpholino for 7 days. A: hypothalamic CRH expression assessed by real-time PCR. Values are means ± SE; n = 4. *P < 0.05 vs. naive. B: circulating corticosterone assessed by commercially available EIA. Values are means ± SE; n = 5. *P < 0.05. C and D: CK-19-positive cholangiocytes and PCNA-expressing cholangiocytes in liver sections. Values are means ± SE; n = 7. *P < 0.05 vs. naive.
Restoring HPA Axis Function Attenuates Cholangiocyte Proliferation Observed During Extrahepatic Biliary Obstruction
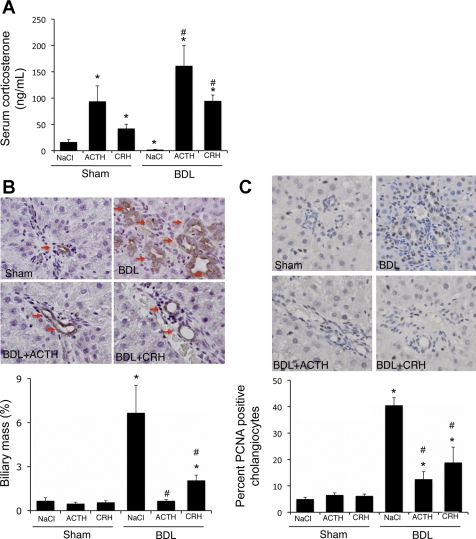
We next evaluated if reactivation of the HPA axis can prevent the cholangiocyte proliferation as a result of biliary obstruction. We again took a parallel approach and administered recombinant ACTH systemically or administered CRH locally into the ventricle (intracerebroventricularly) prior to BDL or sham surgery. Both of these treatment strategies increased corticosterone to levels above those in sham control animals (Fig. 7A). Reactivation of the HPA axis by systemic ACTH or local, central CRH treatment had no effect on biliary mass in sham-operated animals (Fig. 7B). However, both treatment regimens attenuated the increase in biliary mass after BDL surgery (Fig. 7B). Furthermore, there was no observable change in the number of PCNA-positive cholangiocytes after ACTH or CRH treatment in sham-operated animals, whereas there was a marked suppression of PCNA-positive cholangiocytes after CRH and ACTH treatment in BDL animals (Fig. 7C).
Fig. 7.
Restoration of the hypothalamic-pituitary-adrenal (HPA) axis inhibits cholangiocyte proliferation after BDL. Rats underwent BDL or sham surgery and then treated systemically with ACTH (100 μg·kg−1·day−1 ip) or centrally with CRH (5 μg·rat−1·day−1 icv) for 7 days. A: circulating corticosterone levels assessed by commercially available EIA. Values are means ± SE; n = 5. *P < 0.05 vs. sham. #P < 0.05 vs. BDL. B and C: CK-19-positive cholangiocytes and PCNA-expressing cholangiocytes in liver sections. Values are means ± SE; n = 7. *P < 0.05 vs. sham. #P < 0.05 vs. BDL.
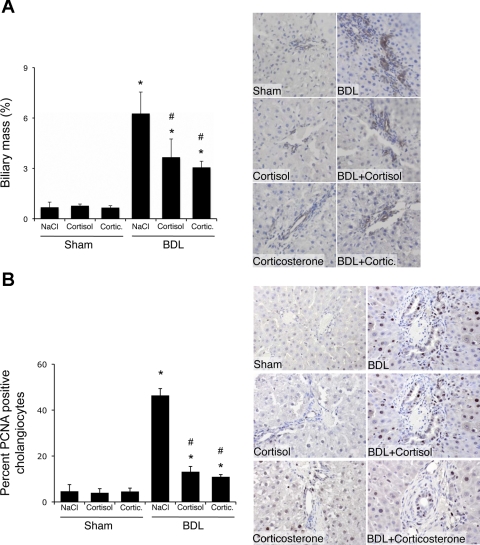
The functional output of HPA axis activation is an increase in glucocorticoid synthesis and release. To test the validity of our hypothesis that restoration of HPA axis activity inhibits cholangiocyte proliferation, we treated rats with systemic cortisol or corticosterone immediately after BDL or sham surgery. Glucocorticoid treatment had no effect on biliary mass in sham-operated animals (Fig. 8A), whereas glucocorticoid administration significantly reduced biliary mass 7 days after BDL surgery (Fig. 8A). In parallel, there was no observable change in the number of PCNA-positive cholangiocytes after treatment with cortisol or corticosterone in sham-operated animals, whereas there was a marked suppression of the number of PCNA-positive cholangiocytes in BDL animals after treatment with either glucocorticoid (Fig. 8B).
Fig. 8.
Systemic treatment of rats with cortisol and corticosterone inhibited cholangiocyte proliferation after BDL. Rats underwent BDL or sham surgery and were treated with cortisol and corticosterone (1 mg·kg−1·day−1) for 7 days postsurgery. A and B: CK-19-positive cholangiocytes and PCNA-expressing cholangiocytes in liver sections. Values are means ± SE; n = 7. *P < 0.05 vs. sham. #P < 0.05 vs. BDL.
DISCUSSION
The major findings of this study relate to the interrelationship between biliary obstruction and the HPA axis. Specifically, the expression and secretion of the key regulatory molecules that drive HPA axis activity and resulting glucocorticoid levels are rapidly suppressed in experimental rodent models of cholestatic liver diseases. Furthermore, our data suggest that suppression of the HPA axis, by a number of surgical and genetic techniques, stimulates cholangiocyte proliferation in a manner similar to that normally observed after BDL. Conversely, reactivation of the HPA axis after BDL attenuated hyperplastic cholangiocyte proliferation. Taken together, our data suggest that modulation of the HPA axis may be a target for the maintenance of biliary mass during cholestatic liver diseases.
In animal models of cholestasis, as well as in human cholangiopathies, cholangiocytes proliferate or are damaged (1, 2, 4). In the BDL rat model, which is widely used for evaluating the mechanisms of cholangiocyte hyperplasia (1), there is an increase in ductal mass (1, 15, 31) and secretin-stimulated cAMP levels (17, 31, 33). In humans, cholangiocyte proliferation occurs in biliary obstruction, in chronic cholestatic liver diseases, and in many forms of liver injury (2, 4, 31). Cholangiopathies share common pathological features, such as the damage of intrahepatic bile ducts and the proliferation of residual ducts (as a mechanism of compensatory repair to maintain biliary homeostasis), but they evolve toward ductopenia, which represents the terminal stage of the disease (2, 4, 31).
The present study demonstrated that the HPA axis is suppressed at every level during the course of cholestasis. CRH expression is decreased in the hypothalamus, which, in turn, has an inhibitory effect on ACTH (POMC) expression in the pituitary, leading to a decreased expression of the key steroidogenic enzymes HSD-3β and Cyp11b1 in the adrenal glands, which results in decreased circulating glucocorticoids. As mentioned above, the link between cholestasis and the HPA axis has been demonstrated clinically, with cholestatic patients often exhibiting clinical features suggestive of adrenal insufficiency, such as hypovolemia, hypotension, and renal failure (55, 57). Furthermore, patients with congenital hypopituitarism or glucocorticoid deficiency often exhibit cholestatic hepatitis (5, 20, 28). In cirrhosis, adrenal insufficiency is associated with increased mortality and hemodynamic impairment (22, 51). Treatment of cirrhotic patients with low doses of hydrocortisone resolves the hemodynamic impairment and is associated with a higher survival rate (13).
The HPA axis is also suppressed in experimental models of cholestasis (37, 46–48). Resection of the bile duct in rats leads to impaired stress-induced CRH release compared with controls, which results in decreased circulating ACTH levels (46, 48). ACTH release from the pituitary as a result of IL-1β treatment is also dampened after bile duct resection (47). Furthermore, McNeilly et al. (37) recently suggested that bile acid buildup as a result of cholestasis may have the ability to inhibit hepatic glucocorticoid clearance, thereby allowing glucocorticoid levels to increase to a level that would inhibit the HPA axis. The data presented here support the concept that the HPA axis is suppressed during cholestasis and that this suppression may have a causal link to other pathological features observed during liver damage. Furthermore, the observation that the HPA axis is not suppressed after only partial BDL in the rat, where serum bile acids are only modestly increased (23), supports a role for perturbed bile acid and/or cholesterol homeostasis in suppression of the HPA axis after complete biliary obstruction. However, instead of affecting hepatic glucocorticoid clearance (37), we hypothesize that the bile acids may enter the brain and suppress the HPA axis directly. Others have shown that the blood-brain barrier becomes leaky in rodent models of cholestasis (12, 56), allowing access of aberrant signaling molecules to the brain. We have preliminary data to suggest that there are increased amounts of bile acids in the brain after BDL and that these bile acids have a dampening effect on the HPA axis (DeMorrow et al., unpublished observations). Whether these bile acids are working through a mechanism involving direct activation of the glucocorticoid receptor [as has been demonstrated previously (38, 49, 50)], thereby activating the negative-feedback loop to shut off the HPA axis, or via direct transcriptional suppression of CRH independent of glucocorticoid receptor activation is under investigation.
To our knowledge, this is the first study that demonstrates a causal link between the suppressed HPA axis and cholangiocyte proliferation observed after BDL. Suppression of the HPA axis by knockout of hypothalamic CRH expression or surgical removal of the adrenal glands induced cholangiocyte proliferation and increased biliary mass in vivo. Furthermore, reactivation of the HPA axis after BDL by central administration of CRH, systemic treatment with ACTH, or low levels of cortisol or corticosterone prevented the biliary outgrowth seen after BDL. Conversely, treatment of rats with high doses of the synthetic glucocorticoid dexamethasone (up to 2.7 mg·rat−1·day−1) increases bicarbonate secretion from cholangiocytes (3). We previously showed that the effects of a compound on ductal secretion often parallel its effects on cholangiocyte proliferation (14, 18, 19, 33, 36). The glucocorticoid treatments were ∼10-fold less in the present study than in the above-mentioned study, with considerably less potency than dexamethasone, and were designed to restore the HPA axis and circulating glucocorticoid levels to physiological levels. The difference in concentrations and the potency of the glucocorticoid used in these studies may account for the difference in effects.
Treatment of cholestatic liver diseases with glucocorticoids (e.g., dexamethasone) has had varied success in alleviating symptoms (16, 42). Any beneficial effects of glucocorticoids in the management of these diseases seem to be outweighed by the increased adverse effects and complications (16, 42). Therefore, alternative strategies aimed at modulating the HPA axis without producing the nonspecific side effects are necessary. We believe that elucidation of the mechanism by which chronic cholestasis results in a suppressed HPA axis may provide a more specific target for successful management of the balance between biliary hyperplasia and ductopenia, depending on the stage of the disease.
In conclusion, we have provided further evidence for suppression of the HPA axis in an experimental model of cholestasis. However, we have extended our current knowledge by demonstrating a causal link between suppression of the HPA axis and induction of cholangiocyte proliferation after BDL. Further knowledge of the role of the HPA axis during cholestasis and the mechanism by which the HPA axis is suppressed during cholestasis may lead to development of innovative treatment paradigms for chronic liver disease.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-082435 and K01-DK-078532 (to S. DeMorrow), Health and Labour Sciences Research Grants for Research on Measures for Intractable Diseases from the Ministry of Health, Labour, and Welfare of Japan, and Grant-in-Aid for Scientific Research C (21590822) from the Japan Society for the Promotion of Science (to Y. Ueno).
This material is the result of work supported with resources and the use of facilities at the Central Texas Veterans Health Care System.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.Q. and S.D. are responsible for conception and design of the research; M.Q., Y.U., H.Y.P., L.H., G.F., C.G., H.F., D.H., and M.M. performed the experiments; M.Q., Y.U., H.Y.P., L.H., G.F., C.G., H.F., D.H., M.M., and S.D. analyzed the data; M.Q., Y.U., and S.D. interpreted the results of the experiments; M.Q. and S.D. prepared the figures; M.Q. and S.D. drafted the manuscript; M.Q., Y.U., H.Y.P., L.H., G.F., C.G., H.F., D.H., M.M., and S.D. approved the final version of the manuscript; Y.U., H.Y.P., L.H., G.F., M.M., and S.D. edited and revised the manuscript.
REFERENCES
- 1. Alpini G, Lenzi R, Sarkozi L, Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest 81: 569–578, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alpini G, Prall R, LaRusso NF. The pathobiology of biliary epithelia. Liver Biol Pathobiol 4E: 421–435, 2001 [Google Scholar]
- 3. Alvaro D, Gigliozzi A, Marucci L, Alpini G, Barbaro B, Monterubbianesi R, Minetola L, Mancino MG, Medina JF, Attili AF, Benedetti A. Corticosteroids modulate the secretory processes of the rat intrahepatic biliary epithelium. Gastroenterology 122: 1058–1069, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Alvaro D, Mancino MG, Glaser S, Gaudio E, Marzioni M, Francis H, Alpini G. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology 132: 415–431, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Binder G, Martin DD, Kanther I, Schwarze CP, Ranke MB. The course of neonatal cholestasis in congenital combined pituitary hormone deficiency. J Pediatr Endocrinol Metab 20: 695–702, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Carsia RV, Malamed S. Glucocorticoid control of steroidogenesis in isolated rat adrenocortical cells. Biochim Biophys Acta 763: 83–89, 1983 [DOI] [PubMed] [Google Scholar]
- 7. Christensen MV, Kessing LV. The hypothalamo-pituitary-adrenal axis in major affective disorder: a review. Nord J Psychiatry 55: 359–363, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Dayanithi G, Antoni FA. Rapid as well as delayed inhibitory effects of glucocorticoid hormones on pituitary adrenocorticotropic hormone release are mediated by type II glucocorticoid receptors and require newly synthesized messenger ribonucleic acid as well as protein. Endocrinology 125: 308–313, 1989 [DOI] [PubMed] [Google Scholar]
- 9. DeMorrow S, Francis H, Gaudio E, Venter J, Franchitto A, Kopriva S, Onori P, Mancinelli R, Frampton G, Coufal M, Mitchell BM, Vaculin B, Alpini G. The endocannabinoid anandamide inhibits cholangiocarcinoma growth via activation of the non-canonical Wnt signaling pathway. Am J Physiol Gastrointest Liver Physiol 295: G1150–G1158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DeMorrow S, Glaser S, Francis H, Venter J, Vaculin B, Vaculin S, Alpini G. Opposing actions of endocannabinoids on cholangiocarcinoma growth: recruitment of fas and fas ligand to lipid rafts. J Biol Chem 282: 13098–13113, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Dickmeis T. Glucocorticoids and the circadian clock. J Endocrinol 200: 3–22, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Faropoulos K, Chroni E, Assimakopoulos SF, Mavrakis A, Stamatopoulou V, Toumpeki C, Drainas D, Grintzalis K, Papapostolou I, Georgiou CD, Konstantinou D. Altered occludin expression in brain capillaries induced by obstructive jaundice in rats. Brain Res 1325: 121–127, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Fernandez J, Escorsell A, Zabalza M, Felipe V, Navasa M, Mas A, Lacy AM, Gines P, Arroyo V. Adrenal insufficiency in patients with cirrhosis and septic shock: effect of treatment with hydrocortisone on survival. Hepatology 44: 1288–1295, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Francis H, Glaser S, Ueno Y, LeSage G, Marucci L, Benedetti A, Taffetani S, Marzioni M, Alvaro D, Venter J, Reichenbach R, Fava G, Phinizy JL, Alpini G. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol 41: 528–537, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Gaudio E, Barbaro B, Alvaro D, Glaser S, Francis H, Ueno Y, Meininger CJ, Franchitto A, Onori P, Marzioni M, Taffetani S, Fava G, Stoica G, Venter J, Reichenbach R, De Morrow S, Summers R, Alpini G. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology 130: 1270–1282, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Giljaca V, Poropat G, Stimac D, Gluud C. Glucocorticosteroids for primary sclerosing cholangitis. Cochrane Database Syst Rev CD004036, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Glaser S, Alvaro D, Francis H, Ueno Y, Marucci L, Benedetti A, De Morrow S, Marzioni M, Mancino MG, Phinizy JL, Reichenbach R, Fava G, Summers R, Venter J, Alpini G. Adrenergic receptor agonists prevent bile duct injury induced by adrenergic denervation by increased cAMP levels and activation of Akt. Am J Physiol Gastrointest Liver Physiol 290: G813–G826, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Glaser S, Benedetti A, Marucci L, Alvaro D, Baiocchi L, Kanno N, Caligiuri A, Phinizy JL, Chowdhury U, Papa E, LeSage G, Alpini G. Gastrin inhibits cholangiocyte growth in bile duct-ligated rats by interaction with cholecystokinin-B/gastrin receptors via d-myo-inositol 1,4,5-triphosphate-, Ca2+-, and protein kinase Cα-dependent mechanisms. Hepatology 32: 17–25, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Glaser SS, Rodgers RE, Phinizy JL, Robertson WE, Lasater J, Caligiuri A, Tretjak Z, LeSage GD, Alpini G. Gastrin inhibits secretin-induced ductal secretion by interaction with specific receptors on rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol 273: G1061–G1070, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Gonc EN, Kandemir N, Andiran N, Ozon A, Yordam N. Cholestatic hepatitis as a result of severe cortisol deficiency in early infancy: report of two cases and review of literature. Turk J Pediatr 48: 376–379, 2006 [PubMed] [Google Scholar]
- 21. Goodenough S, Davidson M, Kidd G, Matsumoto I, Wilce P. Cell death and immunohistochemistry of p53, c-Fos and c-Jun after spermine injection into the rat striatum. Exp Brain Res 131: 126–134, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Harry R, Auzinger G, Wendon J. The clinical importance of adrenal insufficiency in acute hepatic dysfunction. Hepatology 36: 395–402, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Heinrich S, Georgiev P, Weber A, Vergopoulos A, Graf R, Clavien PA. Partial bile duct ligation in mice: a novel model of acute cholestasis. Surgery 149: 445–451, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Hinz B, Hirschelmann R. Rapid non-genomic feedback effects of glucocorticoids on CRF-induced ACTH secretion in rats. Pharm Res 17: 1273–1277, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Jacobson L. Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinol Metab Clin North Am 34: 271–292, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev 12: 118–134, 1991 [DOI] [PubMed] [Google Scholar]
- 27. Jones MT, Hillhouse EW, Burden JL. Dynamics and mechanics of corticosteroid feedback at the hypothalamus and anterior pituitary gland. J Endocrinol 73: 405–417, 1977 [DOI] [PubMed] [Google Scholar]
- 28. Karnsakul W, Sawathiparnich P, Nimkarn S, Likitmaskul S, Santiprabhob J, Aanpreung P. Anterior pituitary hormone effects on hepatic functions in infants with congenital hypopituitarism. Ann Hepatol 6: 97–103, 2007 [PubMed] [Google Scholar]
- 29. Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr Rev 5: 1–24, 1984 [DOI] [PubMed] [Google Scholar]
- 30. Koslo RJ, Gmerek DE, Cowan A, Porreca F. Intrathecal bombesin-induced inhibition of gastrointestinal transit: requirement for an intact pituitary-adrenal axis. Regul Pept 14: 237–242, 1986 [DOI] [PubMed] [Google Scholar]
- 31. Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology 127: 1565–1577, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Leonard BE. HPA and immune axes in stress: involvement of the serotonergic system. Neuroimmunomodulation 13: 268–276, 2006 [DOI] [PubMed] [Google Scholar]
- 33. LeSage G, Glaser S, Alpini G. Regulation of cholangiocyte proliferation. Liver 21: 73–80, 2001 [DOI] [PubMed] [Google Scholar]
- 34. LeSage G, Glaser S, Ueno Y, Alvaro D, Baiocchi L, Kanno N, Phinizy JL, Francis H, Alpini G. Regression of cholangiocyte proliferation after cessation of ANIT feeding is coupled with increased apoptosis. Am J Physiol Gastrointest Liver Physiol 281: G182–G190, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Marzioni M, Glaser S, Francis H, Marucci L, Benedetti A, Alvaro D, Taffetani S, Ueno Y, Roskams T, Phinizy JL, Venter J, Fava G, LeSage GD, Alpini G. Autocrine/paracrine regulation of the growth of the biliary tree by the neuroendocrine hormone serotonin. Gastroenterology 128: 121–137, 2005 [DOI] [PubMed] [Google Scholar]
- 37. McNeilly AD, Macfarlane DP, O'Flaherty E, Livingstone DE, Mitic T, McConnell KM, McKenzie SM, Davies E, Reynolds RM, Thiesson HC, Skott O, Walker BR, Andrew R. Bile acids modulate glucocorticoid metabolism and the hypothalamic-pituitary-adrenal axis in obstructive jaundice. J Hepatol 52: 705–711, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miura T, Ouchida R, Yoshikawa N, Okamoto K, Makino Y, Nakamura T, Morimoto C, Makino I, Tanaka H. Functional modulation of the glucocorticoid receptor and suppression of NF-κB-dependent transcription by ursodeoxycholic acid. J Biol Chem 276: 47371–47378, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Morcos PA, Li Y, Jiang S. Vivo-morpholinos: a non-peptide transporter delivers morpholinos into a wide array of mouse tissues. Biotechniques 45: 613–618, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Muller J, Oertle M. Separate induction of the two isozymes of cytochrome P-450(11)-β in rat adrenal zona glomerulosa cells. J Steroid Biochem Mol Biol 47: 213–221, 1993 [DOI] [PubMed] [Google Scholar]
- 41. Papadimitriou A, Priftis KN. Regulation of the hypothalamic-pituitary-adrenal axis. Neuroimmunomodulation 16: 265–271, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Prince M, Christensen E, Gluud C. Glucocorticosteroids for primary biliary cirrhosis. Cochrane Database Syst Rev CD003778, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roberts SK, Ludwig J, LaRusso NF. The pathobiology of biliary epithelia. Gastroenterology 112: 269–279, 1997 [DOI] [PubMed] [Google Scholar]
- 44. Solomon S. POMC-derived peptides and their biological action. Ann NY Acad Sci 885: 22–40, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Stevens A, White A. ACTH: cellular peptide hormone synthesis and secretory pathways. Results Probl Cell Differ 50: 63–84, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Swain MG, Maric M. Defective corticotropin-releasing hormone mediated neuroendocrine and behavioral responses in cholestatic rats: implications for cholestatic liver disease-related sickness behaviors. Hepatology 22: 1560–1564, 1995 [PubMed] [Google Scholar]
- 47. Swain MG, Maric M, Carter L. Defective interleukin-1-induced ACTH release in cholestatic rats: impaired hypothalamic PGE2 release. Am J Physiol Gastrointest Liver Physiol 268: G404–G409, 1995 [DOI] [PubMed] [Google Scholar]
- 48. Swain MG, Patchev V, Vergalla J, Chrousos G, Jones EA. Suppression of hypothalamic-pituitary-adrenal axis responsiveness to stress in a rat model of acute cholestasis. J Clin Invest 91: 1903–1908, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tanaka H, Makino I. Ursodeoxycholic acid-dependent activation of the glucocorticoid receptor. Biochem Biophys Res Commun 188: 942–948, 1992 [DOI] [PubMed] [Google Scholar]
- 50. Tanaka H, Makino Y, Miura T, Hirano F, Okamoto K, Komura K, Sato Y, Makino I. Ligand-independent activation of the glucocorticoid receptor by ursodeoxycholic acid. Repression of IFN-γ-induced MHC class II gene expression via a glucocorticoid receptor-dependent pathway. J Immunol 156: 1601–1608, 1996 [PubMed] [Google Scholar]
- 51. Tsai MH, Peng YS, Chen YC, Liu NJ, Ho YP, Fang JT, Lien JM, Yang C, Chen PC, Wu CS. Adrenal insufficiency in patients with cirrhosis, severe sepsis and septic shock. Hepatology 43: 673–681, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Tsutsui K, Ukena K, Takase M, Kohchi C, Lea RW. Neurosteroid biosynthesis in vertebrate brains. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 124: 121–129, 1999 [DOI] [PubMed] [Google Scholar]
- 53. Westrin A. Stress system alterations and mood disorders in suicidal patients. Biomed Pharmacother 54: 142–145, 2000 [DOI] [PubMed] [Google Scholar]
- 54. White PC, Curnow KM, Pascoe L. Disorders of steroid 11β-hydroxylase isozymes. Endocr Rev 15: 421–438, 1994 [DOI] [PubMed] [Google Scholar]
- 55. Williams RD, Elliott DW, Zollinger RM. The effect of hypotension in obstructive jaundice. Arch Surg 81: 334–340, 1960 [DOI] [PubMed] [Google Scholar]
- 56. Wright G, Davies NA, Shawcross DL, Hodges SJ, Zwingmann C, Brooks HF, Mani AR, Harry D, Stadlbauer V, Zou Z, Williams R, Davies C, Moore KP, Jalan R. Endotoxemia produces coma and brain swelling in bile duct ligated rats. Hepatology 45: 1517–1526, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Zollinger RM, Williams RD. Surgical aspects of jaundice. Surgery 39: 1016–1030, 1956 [PubMed] [Google Scholar]