Abstract
Embryonic stem cells (ESCs) are associated with a high degree of plasticity, which allows them to self-renew and differentiate into every somatic cell. During differentiation, ESCs follow a hierarchically organized pattern towards tissue specificity, which ultimately results in permanent cell cycle arrest and a loss of cellular plasticity. In contrast to their normal somatic counterparts, cancer cells retain elevated levels of plasticity that include switches between epithelial and mesenchymal phenotypes. Transitions between these cell stages have lately been linked to the reacquisition of stem cell features during cellular reprogramming and dedifferentiation in normal and neoplastic cells. In this review, we discuss the key factors and their interplay that is needed to regain a stem cell stage with a particular emphasis put on the impact of cell cycle regulation. Apart from mechanistic insights into the emerging fundamental processes of stem cell plasticity and capacity to transdifferentiate, we also highlight implications of these concepts for tissue biology, tumorigenesis, and cancer therapy.
Introduction
Embryonic stem cells (ESCs) derived from the inner cell mass of blastocyst stage embryos are able to self-renew and possess the potential to differentiate into any cell type of the three germ layers, namely neuroectoderm, endoderm, and mesoderm. During differentiation, ESCs follow a hierarchy of lineage-specific somatic stem cells and progenitors towards tissue-specific cells with specialized functions. The acquisition of a specific cellular shape and function is accompanied by limited lineage potential, ultimately results in terminal differentiation, and therefore a loss of cellular plasticity. The differentiation process is widely assumed to be unidirectional in normal mammalian cells, where natural dedifferentiation is limited and restricted to certain tissues like the liver. In somatic cells, terminal differentiation is largely controlled by a network of tumor suppressors, which guards the reentry into the cell cycle. In contrast to their normal somatic counterparts, cancer cells feature uncontrolled proliferation and display a high degree of plasticity that includes switching between epithelial and mesenchymal phenotypes. Such plasticity has recently been revealed to enable interconvertibility between stem-like and non-stem stages in cancer cells. Cancer stem-like cells (CSCs) are defined as a subpopulation of cancer cells that is able to self-renew, to initiate, and regenerate tumor growth, while possessing the differentiation potential for every cell present in the parental tumor. Recent reports also suggest that spontaneous dedifferentiation into stem-like stages can occur not only in cancer, but also in normal mammalian cells. While this is challenging the existing view on differentiation, the controlled reprogramming of somatic cells into induced pluripotent stem cells (iPSCs) using pluripotency transcription factors has already demonstrated that it is possible to reset the lineage potential of more differentiated cells. In the case of iPSCs, changing the lineage identity of a cell is generally seen as a two-step process that requires its initial dedifferentiation to an earlier progenitor stage, which is then followed by an activation of a developmental program that differentiates the cell into a new lineage. As outlined below, similar processes are involved in cancer development. The aim of this review is to assess the individual factors and their interplay regulating reprogramming processes that allow normal and malignant cells to regain a “stemness” stage, followed by a lineage conversion. Lineage conversion is also referred to as “transdifferentiation”. Notably, transdifferentiation is not necessarily accompanied by loss of developmental potential.
Epithelial Plasticity
Epithelial cell plasticity mainly involves two different stages: the epithelial and the mesenchymal phenotype. Transitions between these cell stages, namely epithelial-mesenchymal transition (EMT) and its reverse mesenchymal-epithelial transition (MET), have been accredited important roles in embryogenic development, tissue regeneration, cancer progression, and recently also the induction and maintenance of stem cell properties.1 Importantly, the phenotypic switches between epithelial and mesenchymal phenotypes are not irreversible, as they occur several times during formation of the complex three-dimensional structure of internal organs. Furthermore, EMT and MET are distinct from switches between lineages and rather define a change of a cell state within a lineage. The epithelial phenotype of cells is defined by an apicobasal polarization of cell membranes and the cytoskeleton, which leads to characteristic structures such as intercellular tight and adherens junctions that connect adjacent cells. Tight junctions seal the paracellular space close to the apical surface, resulting in a barrier function of epithelial cell layers.2 Cell adhesion between neighboring cells is initiated and maintained by components of adherens junctions, which are located just underneath the tight junctions.3 In addition to these extracellular adhesive features, tight and adherens junctions are closely linked to the intracellular cytoskeleton. Specialized components of these complexes (e.g., β-catenin and p120 catenin) also play important roles in cell signaling and the regulation of gene transcription.4 In contrast to epithelial cells, mesenchymal cells exhibit an irregular shape, which is based on unpolarized cytoskeletons and membranes. Further mesenchymal traits include the deposition of extracellular matrix components, increased motility, invasiveness, as well as elevated resistance to apoptosis and anoikis.1 EMT engages a series of events involving inter- and intracellular changes in affected cells. Importantly, not all of which have to occur during the transitional process. Often cells remain in stages referred to as an “incomplete” EMT, suggesting a spectrum of intermediate stages rather than a strict phenotypic conversion.5 Examples of such epithelial/mesenchymal (E/M) hybrid cells have been reported for multiple tissues including the ovarian surface epithelium,6 ovarian cancer,7 cells within the invasive front of breast cancer,8 as well as in normal epidermal tissue during wound healing.9
Repression of the Epithelial Phenotype
The EMT program is controlled on multiple levels including transcriptional repression, post-translational modifications, cell signaling, and epigenetic regulation. It can be initiated by a variety of extracellular signals and specifically, the crosstalk of several responding pathways initiates a complex network, which can force cells to acquire a mesenchymal phenotype. Moreover, sustained activation of EMT can lead to progressive epigenetic alterations, which induce inheritable effects that preserve the mesenchymal cell state even when EMT-inducing signals are vanished.10 However, one of the crucial steps in EMT is the transcriptional repression of the major epithelial adhesion molecule E-cadherin that is encoded by the CDH1 gene. E-cadherin repressors can be classified into two different groups based on their either indirect or direct repressive action. The group of direct repressors includes Snail1 and Snail2 (formerly known as Slug), Zeb1 and Zeb2, E47 (also known as TCF3), and Klf8. Examples for indirect E-cadherin repressors are transcription factors Twist, E2-2 (also known as TCF4), and FoxC2. Snail and ZEB factors as well as E47 directly bind to consensus E- or E2-boxes within the CDH1 promoter, while Klf8 targets GT boxes to repress E-cadherin expression (reviewed in ref. 1). In addition to the repression of CDH1, these factors also act as direct inducers of EMT by activation of mesenchymal genes such as N-cadherin, vimentin, fibronectin, and cyclin D1.11,12,13,14 Moreover, CDH1 repressors also affect the expression of other epithelial genes. Several tight junction components including occludin, multiple members of the claudin family, and zonula occludens-3 are actively repressed in a similar manner to E-cadherin.15,16 Thus, transcription factors like Snail, Zeb, and Twist can therefore be seen as repressors of the epithelial phenotype and potent inducers of EMT.
EMT and MET in Induction and Maintenance of Stem Cell Stages
The ultimate endpoint of a dedifferentiation process is the reacquisition of pluripotency, which resembles the differentiation potential of ESCs. Pluripotency in ESCs is maintained by a complex network of transcription factors and epigenetic regulators, which seems to be controlled by the core factors Oct4, Sox2, and Nanog. These three factors co-occupy promoters of numerous target genes,17 self-regulate their transcription levels, and build protein complexes with each other to activate and repress expression of target genes.18 Sox2 regulates Oct4 expression and is involved in self-renewal of stem cells, but depends on cofactors such as Oct4 in order to stably bind to DNA.19 Very recently, central roles for Oct4 and Sox2 as gatekeepers in early cell fate determination have also been revealed. Oct4 inhibits neural ectodermal differentiation and promotes differentiation into the mesendodermal germ layer, whereas Sox2 triggers the opposite effect. Importantly, together, Oct4 and Sox2 maintain an equilibrium in cell fate decision, which renders cells pluripotent.20 Furthermore, Oct4 and Sox2 drive the expression of Nanog,21 which has a critical role in the acquisition of pluripotency,22 whereas it seems to be dispensable for stem cell maintenance.23 Overall, a scenario has been proposed, where individual pluripotency factors function as classical lineage factors. This would lead to a constant competition among a multitude of factors attempting to direct differentiation towards their specific lineage, rather than cooperative inhibitory actions to block differentiation in general.24
How the lineage potential of a cell can be reset, once pluripotency is lost, can be studied by the controlled reprogramming of somatic cells into iPSCs. This approach, pioneered by Takahashi and Yamanaka using adult fibroblasts, utilizes certain transcription factors, such as Oct4, Klf4, Sox2, and c-Myc (OKSM), which drive dedifferentiation towards pluripotency.25 Notably, the generation of iPSCs from fibroblasts is a slow process (2–3 weeks) that requires major morphological changes.26 Fibroblasts are a product of EMT and successful reprogramming into iPSCs apparently demands their initial shift towards an epithelial phenotype.27,28 The epithelialization is promoted by Klf4, one of the OKSM factors, which directly induces expression of the major epithelial adhesion molecule E-cadherin.28,29 However, the role for Klf4 seems to be broader, as E-cadherin expression alone cannot replace Klf4 during the reprogramming process. Nevertheless, E-cadherin is essential for maintenance of pluripotency in ESCs and interestingly, can substitute for Oct4 during the generation of iPSCs.30 This implies that (cultured) epithelial cells might already harbor several factors that are critically needed for the induction of pluripotency. Supporting this notion, exogenous Klf4 is not needed for the generation of iPSCs from keratinocytes, as it is endogenously expressed at high levels. Furthermore, reprogramming of mammary epithelial cells or keratinocytes can be achieved faster, and with higher success rates when compared to nonepithelial cell types.28,31
A stem-like stage in immortalized human mammary epithelial cells can also be reached after induction of EMT due to ectopic expression of Snail or Twist, and culturing in the presence of transforming growth factor-β (TGF-β). In a similar manner, highly tumorigenic breast CSCs can be generated from more differentiated neoplastic cultures.32 This is interesting, as it seems to contradict the crucial function of epithelial properties that are needed to gain pluripotency in reprogramming processes. Importantly, shifts between epithelial and mesenchymal phenotypes occur gradually and the notion of complete transitions is likely to be simplistic in many cases.33 In this context, one has to keep in mind the epithelial phenotype as a starting point for the transitional process. A study from our laboratories recently identified that ovarian CSCs and progenitors simultaneously harbor features of epithelial and mesenchymal cells and remain in an E/M hybrid stage.34 Notably, the borders between these cell fractions were fluent and shifted to either the mesenchymal or epithelial side, for CSCs and progenitors, respectively. The gradual loss of epithelial features enabled CSCs to form tumors in immunocompromised mice with higher potency when compared to progenitors. In addition, CSCs gave rise to mesenchymal cells via passaging in vitro and to epithelial cells when injected into mice, indicating a high dependence on environmental cues for differentiation. Importantly, clonal cultures that were restricted to either epithelial or mesenchymal phenotypes, and therefore not able to switch cell states, were nontumorigenic. In an earlier study, genome-wide DNA expression profiling of these nontumorigenic clonal cultures identified EpCAM and CD44 as markers for epithelial and mesenchymal cells, respectively.7 Interestingly, the combination of these surface proteins is used to monitor CSCs in multiple tissues including prostate, pancreas, and colon, suggesting that the E/M hybrid stage might be a more general pattern for tumor-initiating cells.35,36 Moreover, a very recent study on trophoblast stem cells (TSCs) demonstrated, that these cells are locked in an E/M hybrid stage after the controlled induction of EMT.37 During EMT, TSCs lost several epithelial features and acquired an invasive character, while retaining pluripotency and the ability to self-renew. The metastable phenotype of TSCs was regulated on the epigenetic level via loss of histone H2A and H2B acetylations, which initially preserved the epithelial phenotype. Interestingly, the DNA expression profile of E/M hybrid TSCs was closely overlapping with that of breast CSCs of the claudin-low subtype. In summary, transitions between epithelial and mesenchymal cell stages play important roles in the acquisition of stem cell features. Strikingly, a shift towards the opposite phenotype is needed in order to induce dedifferentiation processes, while stem cells might remain in an E/M hybrid stage.
Cellular Dedifferentiation and Cell Cycle Control
Natural tissue regeneration can be achieved either by activation of somatic stem cells or by dedifferentiation.38 An example for activation of somatic cells is liver regeneration. Upon liver injuries due to drugs, toxins, resection, or acute viral diseases, mature, normally quiescent adult hepatocytes start to proliferate and regenerate the liver.39 G0–G1 transition in hepatocytes is triggered by cytokines while several secondary signals then stimulate progression through the cell cycle. Interestingly, the degree of hepatocytes proliferation is precisely controlled by the metabolic needs of the organism, such that the process stops once an appropriate liver to body weight ratio is achieved.
Dedifferentiation is a process that reverts terminally differentiated cells back to a less differentiated stage within its own lineage in order to let them proliferate. Hereafter, the resulting daughter cells redifferentiate to replace the lost tissue. An example for such dedifferentiation processes can be found in the limb regeneration of urodele amphibians, where a prominent role is accredited to the retinoblastoma protein (Rb). Rb is classically known as a tumor suppressor, largely due to its capacity to arrest cells in G1 by inhibiting E2F transcription factor activity and stabilization of the cell cycle inhibitor p27Kip1 (Figure 1). In addition, several reports suggest that Rb also functions as a transcriptional cofactor and as an adaptor protein for the recruitment of chromatin remodeling factors.40 During the limb regeneration process, Rb is inactivated due to hyperphosphorylation by cyclin-dependent kinase 4/6 (CDK4/6), which leads to dedifferentiation and subsequent cell cycle reentry in newt myotubes.41 Evolutionary differences in cellular dedifferentiation processes can be observed, when comparing the regenerative capacities between newt myotubes and their mammalian counterparts, where Rb inactivation alone does not translate to cell cycle reentry.42 Dedifferentiation in mammalian muscle cells is additionally blocked by ARF (alternate reading frame), one of three important tumor suppressors located in the CDKN2 locus on chromosome 9p21, which also includes the CDK4/6 inhibitors p16Ink4a, and p15Ink4b. ARF can mediate p53-dependent growth arrest43 and interestingly, is not present in regenerating vertebrates.44 Therefore, combined deactivation of Rb and ARF induces a serum-dependent loss of differentiation markers, as well as upregulation of cyclins D1 and E, which ultimately results in cell cycle reentry of initially postmitotic mouse muscle cells.45 The involvement of ARF in maintaining a more differentiated cell state has also been demonstrated for cells from the neural lineage. Inactivation of ARF reverts the properties of late-stage neural stem cells (NSCs) to those of early-stage cells, whereas ARF overexpression attenuates their ability to self-renew.46 The reversion to NSCs can be achieved in Ink4a/Arf-deficient primary murine astrocytes via enforced expression of Id4 (inhibitor of differentiation 4), which triggers a hyperproliferative phenotype due to nuclear cyclin E and inactivation of Rb.47 A positive regulator of an undifferentiated cell stage that targets the Ink4a/Arf locus is Polycomb group (PcG) member Bmi1. Overexpression of Bmi1 represses the transcription of p16Ink4a and ARF via direct interaction with the Ink4a/Arf locus.48 Notably, Bmi1 also regulates the cell cycle inhibitor p21Cip1 to mediate self-renewal of NSCs and proliferation throughout subsequent developmental cell stages.49 Furthermore, an intriguing report recently showed, that a knockout of p21Cip1 leads to enhanced tissue regeneration in mice, which gain the ability to close ear holes that are commonly used for life-long tagging.50
Figure 1.
Regulation of the cell cycle. Rb blocks cell cycle progression in G1 by binding E2F transcription factors. In order to enter S phase, cells must sequentially activate CDK4/6 and CDK2. These kinases are expressed throughout the cell cycle, but are only activated upon complex formation with their corresponding cyclins. During early G1 phase, mitogenic signals trigger activation of the CDK4/6-cyclin D complex, which partially deactivates Rb by phosphorylation. Subsequently released E2F factors mediate expression of pro-proliferative genes including cyclin E and Cdc25A. This transfers cells past the restriction (R) point, which commits for S-phase entry. Activation of the CDK2-cyclin E complex results in hyperphosphorylation of Rb and fully releases E2F. The expression of further E2F target genes, as cyclin A, facilitates progression through S phase. Upstream inhibitors, including members of the Ink4 and Cip/Kip families, inhibit the mitogenic action of CDKs. ARF and p53 can block cell cycle progression via p21Cip1. ARF, alternate reading frame; CDK, cyclin-dependent kinase; Rb, retinoblastoma.
During the reprogramming of somatic cells, the generation of iPSCs can critically be limited due to blockage of dedifferentiation by cell cycle repressors. Subsequently, several studies have shown that opposing cellular senescence by ablation of p53, ARF, p16Ink4a, or p21Cip1 greatly increases the reprogramming efficiency.51,52,53,54 Likewise, counteracting senescence by immortalization with SV40 large T antigen and/or telomerase reverse transcriptase (hTERT) dedifferentiates cells into a stage that dramatically increases their amenability to OKSM factors.55,56 Interestingly, loss of specific tumor suppressors alone can also lead to a stem cell stage in specific cell types. The transient inactivation of p53 in primary human keratinocytes has been shown to induce increased cell plasticity, which allowed for redifferentiation into neural lineages.57 Importantly, here the stem cell-like stage was achieved without forced expression of any of the transcription factors previously used in generation of iPSCs. Similar observations have been made for mouse embryonic fibroblasts that are devoid of all three Rb family members, Rb, p107, and p130. Triple-knockout of the Rb family genes results in failure of G1/S control that subsequently leads to loss of contact inhibition and immortalization.58 When triple-knockout cultures reach confluence, cells start to detach from the plastic dish to form floating spheres, which contain cell subfractions that mirror features of ESCs including the expression of OKSM factors and markers of the three germ layers.59 Analogous results were obtained with mouse embryonic fibroblasts only mutated in Rb. These cells remained contact inhibited in monolayers, but also formed spheres when cultured in suspension. Importantly, spheres derived from both triple-knockout and Rb-mutated cells, contained CD44high/CD24low CSCs that were able to form tumors in mice. Bonizzi et al. discussed recently that the increased frequency of mammary stem cells in p53-deficient breast tissues and mammospheres could be due to reprogramming of somatic cells as a consequence of p53 loss.60 Based on this, the authors speculate that p53 limits stem cell expansion, i.e., by imposing asymmetry of cell division. Notably, while it is more efficient to generate iPSCs from somatic cells with p53 mutations, these iPSCs are also characterized by increased genetic instability and malignant tumor formation potential.61
The ability to grow in suspension is a prerequisite of cells resistant to anoikis. This implies that loss of contact inhibition in combination with resistance to anoikis seems to have a positive impact on cellular dedifferentiation processes. Along this line is a report from Robert Weinberg's laboratory, suggesting, that human mammary epithelial cells can spontaneously dedifferentiate into CD44high/CD24low stem-like cells via detachment from the monolayer as floating cells.62 In contrast, cells in monolayers were mostly CD24high and rarely contained CD44high cells. Even though spontaneous dedifferentiation was also observed in untransformed primary cells, functional stem cell assays in this model were performed on cells immortalized with hTERT, which might have already primed them for dedifferentiation. However, floating cells injected into mice gave rise to milk duct-like structures that contained basal and luminal cells. The additional transformation with SV40 early region and H-Ras oncogenes gradually increased the frequencies of CD44high/CD24low cells within the floating cell fraction, resulting in the generation of tumorigenic CSCs. Furthermore, adherent cultures, mammospheres, and tumors derived from floating cells showed signs of EMT, demonstrated by morphological changes, markedly reduced levels of membrane-bound E-cadherin, and/or increased amounts of vimentin. EMT is a central process in the acquisition of anoikis resistance, and pathways targeting E-cadherin or ARF have recently been identified as mediators of action.63,64
Altogether, cellular dedifferentiation and reprogramming are linked to the deactivation of major cell cycle repressors. Whereas their loss or functional deregulation might be beneficial for the acquisition of a stem cell stage, such scenarios can also lead to genomic instability.65,66 Specifically during the generation of iPSCs, the enforced dedifferentiation can induce undesired genomic and chromosomal abnormalities, which preferentially target genes involved in cell cycle regulation and cancer.67,68 Consequently, reprogramming approaches that include the permanent ablation or sustained deactivation of cell cycle regulators should be handled with care. However, these studies also indicate that cancer might arise due to reprogramming-like processes. Understanding which mechanisms underlay events that render cells tumorigenic, could therefore eventually contribute to new cancer treatments.
Interconnection of “Stemness” Factors and Cell Cycle Regulators
Although a cellular “stemness” stage can be obtained by different means, the lineage background and differentiation status of the dedifferentiating cell play pivotal roles. Overall, pluripotency factors and EMT inducers can be seen as modulators of a “stemness” program, which is still subjected to regulation by the cell cycle machinery. It seems therefore critical to understand by which means individual “stemness” inducers are interconnected and also limited by guardians of the cell cycle. A central role in the regulation of EMT and EMT-related stem cell properties has p53, which drives the expression of micro RNA miR-200c. In human mammary epithelial cells, loss of p53 or miR-200c leads to elevated levels of EMT inducer Zeb1 as well as stem cell factors Bmi1 and Klf4, resulting in reduced E-cadherin expression and an increase in CD44high/CD24low stem-like cells.69 Interestingly, the cell surface marker CD44 also directly contributes to dedifferentiation processes via its cytoplasmic domain that acts as a transcription factor at several promoters including Nanog and c-Myc.70 Under stress conditions, CD44 can be repressed by p53, which enables p53-dependent cytostatic and apoptotic signals that would otherwise be blocked by the actions of CD44.71 Multiple reports also suggest that loss of p53 permits expansion of CSCs, whereas its presence represses more than 20 target genes involved in maintenance of tumor-initiating cells.72 The OKSM factor Klf4 can act as a transcriptional repressor of p53 and is indispensible for maintenance of breast CSCs and invasion.73,74 On the other hand, Klf4 is a strong inducer of the epithelial phenotype, which directly enforces E-cadherin expression.29 Moreover, Klf4 is required for terminal differentiation of epithelial cells and mediates suppression of EMT and metastasis in a breast cancer mouse model.75,76 This dual role for Klf4, to act either as an oncogene or tumor suppressor, seems to be context-dependent and critically linked to the activity of oncogenic Ras and p21Cip1.77 Interesting in this context is also the observation, that oncogenic Ras is a potent EMT inducer in epithelial cells, whereas in mesenchymal fibroblasts it enforces senescence.78,79 This again highlights a greater plasticity for cells harboring epithelial features, particularly when processes are induced that decide between dedifferentiation and senescence.
c-Myc is another OKSM factor and a known inducer of EMT in epithelial cells, whose transforming activity is dependent on the repression of E-cadherin.80 Potentially, c-Myc could therefore enforce a basic EMT signaling that would counteract Klf4-induced epithelialization during the generation of iPSCs. It was also recently demonstrated that c-Myc takes a central part in the induction of an ESC gene expression pattern and properties of CSCs in primary keratinocytes.81 Moreover, c-Myc is a classical target of Wnt, whose signaling contributes at least in part to EMT via activation of EMT transcription factors.82
A regulatory function in EMT has also been proposed for Rb. In mammary epithelial cells depletion of Rb induces rapid loss of epithelial markers and triggers expression of EMT transcription factors Snail2 and Zeb1.83 Furthermore, loss of Rb alone renders mouse mammary stem/progenitor cells tumorigenic and concomitant deletion of p53 specifically induces tumors that exhibit EMT.84 Similarly, the expression of SV40 large T, which is commonly used for immortalization purposes and simultaneously deregulates Rb and p53, leads to a Rb-dependent repression of E-cadherin and subsequent EMT in epithelial cells.85 The involvement of Rb in dedifferentiation processes is also observed during the generation of iPSCs, where Oct4 mediates its degradation via caspases 3 and 8 in order to induce pluripotency.86 Importantly, Rb has been accredited a unique role in controlling senescence, by repressing E2F target genes involved in DNA replication.87 In the absence of Rb, sustained loading of pre-replication complexes and aberrant DNA replication occurs in senescent cells, which can lead to proliferation and genomic instability upon loss of a second cell cycle regulator such as p53 or p21Cip1. Zeb transcription factors oppose p16Ink4a and p15Ink4b expression during TGF-β–mediated EMT to evade a senescence program induced by epidermal growth factor receptor overexpression in esophageal epithelial cells.88 Interestingly, the subsequent activation of p53 locks cells in a senescent state and abolishes the responsiveness to TGF-β, suggesting that senescent cells generally might be unable to undergo EMT.89 In addition to its EMT-inducing function, Zeb1 is also reported to repress “stemness”-inhibiting micro RNAs of the miR-200 family, which leads to upregulation of stem cell factors Klf4, Sox2, and Bmi1.90 Furthermore, this study showed that expression of Zeb1 is essential for the generation of pancreatic and colorectal CSCs, while repression of Zeb1-regulated micro RNAs contributes to maintenance of “stemness” in mouse ESCs. A regulatory role in the balance between pluripotency and differentiation in ESCs plays EMT inducer E47. In the absence of Wnt signaling, E47 acts as a repressor of “stemness” factors including Oct4 and Nanog, whereas sustained Wnt signaling or loss of E47 renders ESCs refractory to differentiation.91 Twist simultaneously deregulates the Rb and p53 pathways (by repression of p16Ink4a and p21Cip1) and thereby bypasses Ras-induced senescence in fibroblasts.92 During EMT and the generation of CSCs, Twist upregulates and functions together with Bmi1 to repress the expression of target genes such as E-cadherin and p16INK4a via chromatin remodeling.93 The connection of these two proteins is particularly interesting, because it also links EMT to Bmi1-mediated self-renewal in stem cells.94 In addition, the finding that Bmi1-modulating miR-200c is consistently downregulated in mammary stem/progenitor cells as well as in CSCs suggests, that the molecular regulation of “stemness” might be uniform to large extends in normal somatic stem cells and their tumorigenic counterparts.95
One major remaining question in the field of CSCs is, whether these cells are quiescent or proliferative. Their assumed resistance to therapy would imply a quiescent cell state, whereas most studies indicate that CSCs are actually highly proliferative.96 Interestingly, such discrepancy is mirrored, when one compares ESCs and somatic stem cells. ESCs possess an exceptional cell cycle pattern, in which G1/S control is largely absent. This enables cells to quickly progress through G1 in order to counteract differentiation processes and ensures a rapid growth of the early embryo. Repressors of the cell cycle are either transcriptionally downregulated or, as in the case of Rb, rendered inactive.97 Differences also exist among positive cell cycle regulators, as CDK2-cyclin E/A complexes are prominent throughout the cell cycle, while CDK4/6-cyclin D complexes are only expressed at basic levels. Upon differentiation, levels of cyclin E and D increase, whereas cyclin A expression is reduced. In order to arrest ESCs in G1, the absence of CDK2, accompanied by upregulation of p21Cip1 and p27Kip1, seems to be critical.98,99 Not surprisingly, the cell cycle in ESCs also is governed by core pluripotency factors including Oct4, Sox2, and Nanog, as well as by EMT inducer E47. These factors target major cell cycle regulators either by direct binding to their promoters or via micro RNAs.100,101,102 In contrast to ESCs, somatic stem cells are proposed to remain mostly in a quiescent state with the majority of cells arrested in G0/G1. Cell cycle entry in quiescent somatic (hematopoietic) stem cells is blocked by p53, p27Kip1, and p57Kip2, as extensive proliferation is thought to lead to stem cell exhaustion.103,104,105,106 However, it has recently been suggested, that separate pools of quiescent and proliferative somatic stem cells may coexist in adult tissues.107 Proliferation and maintenance of stem cell self-renewal is not contradictive. It is well described for stem cells in intestinal crypts, which are known to have high proliferation rates.108 Clearly, the proliferative stem cells could readily generate the appropriate tissue, whereas quiescent stem cells would be activated in response to loss of the proliferative pool. Importantly, this mechanism might also explain the earlier mentioned discrepancy in the phenotype of CSCs. A highly proliferative pool of CSCs would therefore support rapid tumor growth, while quiescent CSCs may be resistant to treatment. In this context, it should furthermore be considered, that most assays aimed to define CSCs preferentially select cells with a proliferative advantage. A quiescent pool of CSCs might consequently often remain hidden in non-stem cell fractions.
Overall, cell cycle repressors, such as Rb, can restrict dedifferentiation processes towards the acquisition of a stem cell stage. These repressors actively counteract EMT inducers and pluripotency factors, which are aimed for their deactivation. Notably, the loss of individual cell cycle repressors due to mutations can severely limit a proper cell cycle control (Table 1). Theoretically, this enables an intrinsic tendency for dedifferentiation in cancer cells, which could be unleashed upon appropriate extrinsic signaling.
Table 1. Significant copy number alterations of “stemness” modulators in cancer.
Environmental Factors Regulating “Stemness” and Plasticity
Cancer cells and stem cells share many features, one of which is the ability for unlimited proliferation when cultured under specific conditions in vitro. Several signaling pathways have been implicated to play important roles in self-renewal of stem cells, as well as in cancer. These pathways include: the JAK/STAT pathway, Notch and Hedgehog signaling, the Map kinase/Erk and PI3K/AKT pathways, the NF-κB and Wnt pathways, as well as the TGF-β pathway.109,110 Because of their complexity, this review will only briefly discuss the Wnt and TGF-β pathways, which both have lately been identified to play critical roles in the induction and maintenance of mesenchymal and stem cell states in normal and neoplastic mammary epithelial cells.111 Scheel and colleagues showed, that “stemness”-granting EMT cannot be efficiently induced by a single signaling pathway and instead requires the interplay of several. According to their model, the TGF-β pathway must cooperate with canonical and noncanonical Wnt signaling in order to initiate EMT. It remains however elusive, whether the same EMT-inducing pathways function generally or rather tissue-specific. Nevertheless, after the transitional phase, the resulting mesenchymal or stem-like state can then be maintained in an autocrine fashion. Apparently, a number of EMT inducers are secreted by mammary epithelial cultures, but these fail in inducing the mesenchymal phenotype due to concomitantly released inhibitors. After an environment is created, which reduces such inhibitors, cells can undergo EMT and/or sustain the mesenchymal phenotype. Overall this study suggests, that interconversions between stem and non-stem cell stages can be entirely regulated by the microenvironment, to which normal and neoplastic cells respond in a similar manner.
In solid tumors, the microenvironment can reportedly direct cancer cell plasticity. Specifically, interactions between epithelial and stromal cells have been linked to the induction of EMT. In several human tumors and in animal xenograft models, EMT markers are predominantly expressed at the tumor-stroma interface.112,113,114,115 Similarly, CSCs of several lineages are reported to reside at the invasion front or close to the vasculature.112,116,117 However, contrasting reports also argue that CSCs can be spread more randomly throughout tumors.118,119 The effect of differentiated epithelial cells on cancer cells has also been studied. In co-culture experiments with hepatocytes, prostate cancer cells underwent an MET due to elevated E-cadherin expression.120 Apparently, the establishment of intercellular epithelial adherens junctions between cancer cells and hepatocytes triggered the differentiation towards the epithelial phenotype. E-cadherin takes a central role in the initiation of adherens junctions and its expression can lead to contact inhibition via activation of Rb, and concomitant cell cycle arrest in G1.121,122 Furthermore, E-cadherin forms complexes with growth factor receptors and inhibits their ligand-dependent signaling.123,124 Importantly, E-cadherin is a tumor suppressor that is often deleted in cancer (see Table 1). However, the E-cadherin impact on the cell cycle seems to be context-dependent, as highly proliferative ESCs also express E-cadherin, and its loss does not affect proliferation.125 A possible explanation for this discrepancy may lay in the specifics of the ESC cell cycle regulation. These include a low abundance of E-cadherin–induced growth suppressor p27Kip1, which is highly targeted for degradation by Skp2 in ESCs.126,127 Based on this, one could argue, that E-cadherin-mediated contact inhibition can confer stem cell quiescence, once cell cycle repression is no longer counteracted. It would also imply, that the activation of a proliferative stem cell program is linked to EMT in cells of epithelial origin.
Most somatic stem cells can only give rise to cell lineages present in the organ from which they are derived. In contrast, NSCs possess a broader capacity, allowing for differentiation into cell types of all three germ layers.128 The differentiation potentials of NSCs and ESCs seem therefore highly related, which is supported by the observation that ectopic expression of Oct4 alone can restore pluripotency in NSCs.129 Conversely, when cultured with E-cadherin–expressing feeder cells, ESCs lose Oct4 and differentiate into NSCs.130 Glioma CSCs were initially believed to derive directly from NSCs or astrocytes.131,132 However, it has also been suggested, that oligodendrocyte progenitors are the source for gliomas, as aberrant growth was not observed in NSCs or other NSC-derived lineages, even when initial mutations already occur in NSCs.133,134 NSCs reside next to endothelial cells in the subventricular zone, both during homeostasis and regeneration.135,136 Similarly, glioma CSCs preferentially locate to a “perivascular niche”, which allows them to self-renew and maintain stem cell properties.116,137 This scenario enables an angiogenic interplay between tumor and endothelial cells, in which tumor cell-derived vascular endothelial growth factor not only stimulates endothelial growth, but also contributes to cancer progression and survival.138,139 Interestingly, the high plasticity associated with NSCs, also allows for differentiation into endothelial cells.140 A similar degree of plasticity is now also accredited to glioma CSCs, as they are believed to induce their own niche in the absence of angiogenesis.141 Specifically, a number of recent studies show that p53-deficient glioma CSCs can generate endothelial cells and thereby contribute to tumor vasculature.142,143,144 Notably, transdifferentiation of CSCs into endothelial cells has been suggested before for a number of cancers including myeloma, neuroblastoma, and breast cancer.145,146,147 In glioma, the observed differentiation is a two-step process that involves the notch-1–mediated generation of endothelial progenitors, followed by maturation to endothelial cells via vascular endothelial growth factor signaling. Overall, these studies show, that glioma CSCs either maintain or reacquire a level of plasticity that is associated with their nontumorigenic counterparts. This plasticity might be facilitated by mutated cell cycle regulators, such as p53, which in turn trigger cellular reprogramming. Interestingly, the inhibition of either notch signaling or γ-secretase can abolish the transition to endothelial progenitors from glioma CSCs. Both of these actions are known to cause the loss of E-cadherin and a subsequent breakdown of adherens junctions,148,149 suggesting EMT-like processes as initial events for the transdifferentiation. Notably, while the abundance of E-cadherin in brain tissue is relatively low, it can be highly expressed in aggressive glioblastomas.150
Conclusions
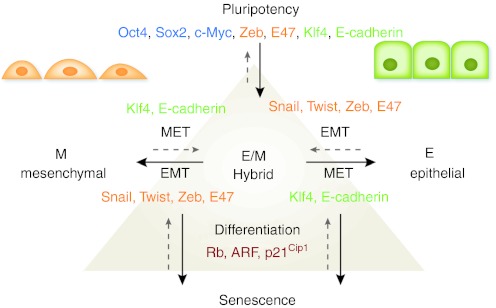
Transitions between epithelial and mesenchymal phenotypes are linked to the reacquisition of a stem cell stage. Whereas epithelial cells require EMT to gain features of stem cells, an MET is needed to successfully reprogram mesenchymal cells to a pluripotent stage. It seems therefore reasonable to assume, that stem cells possess an E/M hybrid phenotype that can be shifted to the epithelial or mesenchymal side to various degrees (Figure 2). The differentiation capacity of stem cells clearly depends on their lineage background. As in the case of NSCs and glioma CSCs, the differentiation potential might go beyond their own lineage, which enables transdifferentiation processes. In ESCs, pluripotency seems to be maintained by an equilibrium of individually signaling lineage factors. This allows for a high degree of cellular plasticity and results in gradual differentiation into a certain lineage upon specific extrinsic stimuli. The reacquisition of a less committed stem cell stage in more differentiated cells consequently requires their complementation with lacking lineage factors, concomitantly with the gain of epithelial or mesenchymal features. Simultaneously, the degree of differentiation and reentry into the cell cycle are modulated by major cell cycle repressors, such as Rb and p53. The expression and activity of these repressors is targeted by pluripotency factors and EMT inducers, which thereby act as highly interconnected “stemness” regulators.
Figure 2.
Regulatory network of cellular plasticity and differentiation. Pluripotency in ESCs is maintained by a variety of factors including Oct4 and Sox2. Several regulators that are critically required for maintenance of pluripotency in ESCs are also accredited to epithelial (Klf4, E-cadherin) and mesenchymal cells (Zeb, E47). Epithelial cells (E) are defined by an apicobasal polarization of their cytoskeleton and membranes, whereas mesenchymal cells (M) possess an unpolarized, irregular shape. Reversible transitions between these phenotypes are regulated by MET (Klf4, E-cadherin) or EMT inducers (Snail, Twist, Zeb, E47). In order to switch from one phenotype to the other, cells progress through an intermediate E/M hybrid stage that is associated with the ability to regain stem cell features. Cellular differentiation and senescence are regulated by the activity of major cell cycle repressors, such as Rb, ARF, and p21Cip1. The absence of these repressors enables dedifferentiation processes towards pluripotency. ARF, alternate reading frame; EMT, epithelial-mesenchymal transition; ESCs, embryonic stem cells; MET, mesenchymal-epithelial transition; Rb, retinoblastoma.
Whereas the cell cycle control in normal cells and somatic stem cells is intact, in cancer cells it appears to be deregulated at various levels. Depending on the acquired mutations, which preferentially occur in cell cycle repressors, cancer cells therefore harbor the potential to dedifferentiate towards a stem cell stage with different potencies. Based on this, one also could estimate, that CSCs most likely are not able to differentiate properly. Instead, CSCs might be forced into differentiation processes and cell cycle arrest due to a lack of growth factors and by signaling from adjacent cells via adhesion molecules as E-cadherin. This would furthermore imply, that cancer cells could readily dedifferentiate upon reignited signaling that might arise after changes in their microenvironment. In addition, sustained signaling in CSCs also can redirect differentiation into other lineages, such as endothelial cells. It remains however elusive, to which degree such differentiation processes can be completed in cells with mutated cell cycle repressors. The high plasticity and heterogeneity that is consequently associated with CSCs also dramatically complicates treatment strategies, as certain signaling pathways are only active in specific cellular phenotypes. Furthermore, the proliferative nature of CSCs in combination with the absence of cell cycle repressors that help maintaining genome integrity, can give rise to mutants that escape targeted therapies. Despite the great challenge to treat cancer in the face of such enormous “adaptability”, we believe that the emerging deeper understanding of “stemness” can inspire innovative and potent therapeutic strategies. One could for example selectively try to prevent EMT in epithelial tumors and MET in mesenchymal neoplasms, and thereby focus on blocking the route to the powerful E/M hybrid stage of cancer cells, possibly combined with standard-of-care genotoxic treatment modalities. This combination might prevent the otherwise life-threatening renewal of, and dedifferentiation into CSCs, and at the same time minimize the pool of cancer cells from which treatment resistant variants could otherwise be selected that might lead to recurrent tumor growth and eventual lethal spread.
Acknowledgments
We are thankful to Christoffel Dinant, Matthias Altmeyer, and Monika Mortensen for useful discussions. Furthermore, we apologize to all researchers whose work could not be cited due to space limitations. Research on mechanisms regulating cellular “stemness” in our laboratories is funded by the Danish Cancer Society, the Danish National Research Foundation, the Novo Nordisk Foundation, the Danish Medical Research Council, the Czech Ministry of Health (grants NS10282-3/2009 and NT/11065-5/2010), the Lundbeck Foundation, and the European Commission 7th FP (projects Infla-Care, Biomedreg, and DDResponse). The authors declared no conflict of interest.
REFERENCES
- Thiery JP, Acloque H, Huang RY., and, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Turksen K., and, Troy TC. Barriers built on claudins. J Cell Sci. 2004;117 Pt 12:2435–2447. doi: 10.1242/jcs.01235. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Stepniak E, Radice GL., and, Vasioukhin V. Adhesive and signaling functions of cadherins and catenins in vertebrate development. Cold Spring Harb Perspect Biol. 2009;1:a002949. doi: 10.1101/cshperspect.a002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen JJ., and, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- Wong AS., and, Auersperg N. Normal ovarian surface epithelium. Cancer Treat Res. 2002;107:161–183. doi: 10.1007/978-1-4757-3587-1_7. [DOI] [PubMed] [Google Scholar]
- Strauss R, Sova P, Liu Y, Li ZY, Tuve S, Pritchard D.et al. (2009Epithelial phenotype confers resistance of ovarian cancer cells to oncolytic adenoviruses Cancer Res 695115–5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côme C, Magnino F, Bibeau F, De Santa Barbara P, Becker KF, Theillet C.et al. (2006Snail and slug play distinct roles during breast carcinoma progression Clin Cancer Res 125395–5402. [DOI] [PubMed] [Google Scholar]
- Arnoux V, Come C, Kusewitt D, Hudson L., and, Savagner P.2005Cutaneous wound reepithelization: a partial and reversible EMTIn: Savagner, P (ed.). Rise and Fall of Epithelial Phenotype: Concepts of Epithelial-Mesenchymal Transition Springer: Berlin; pp. 111–134. [Google Scholar]
- Dumont N, Wilson MB, Crawford YG, Reynolds PA, Sigaroudinia M., and, Tlsty TD. Sustained induction of epithelial to mesenchymal transition activates DNA methylation of genes silenced in basal-like breast cancers. Proc Natl Acad Sci USA. 2008;105:14867–14872. doi: 10.1073/pnas.0807146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C.et al. (2004Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis Cell 117927–939. [DOI] [PubMed] [Google Scholar]
- Perez-Moreno MA, Locascio A, Rodrigo I, Dhondt G, Portillo F, Nieto MA.et al. (2001A new role for E12/E47 in the repression of E-cadherin expression and epithelial-mesenchymal transitions J Biol Chem 27627424–27431. [DOI] [PubMed] [Google Scholar]
- Zhao J, Bian ZC, Yee K, Chen BP, Chien S., and, Guan JL. Identification of transcription factor KLF8 as a downstream target of focal adhesion kinase in its regulation of cyclin D1 and cell cycle progression. Mol Cell. 2003;11:1503–1515. doi: 10.1016/s1097-2765(03)00179-5. [DOI] [PubMed] [Google Scholar]
- Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG.et al. (2000The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression Nat Cell Biol 276–83. [DOI] [PubMed] [Google Scholar]
- Ikenouchi J, Matsuda M, Furuse M., and, Tsukita S. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci. 2003;116 Pt 10:1959–1967. doi: 10.1242/jcs.00389. [DOI] [PubMed] [Google Scholar]
- Vandewalle C, Comijn J, De Craene B, Vermassen P, Bruyneel E, Andersen H.et al. (2005SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell-cell junctions Nucleic Acids Res 336566–6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP.et al. (2005Core transcriptional regulatory circuitry in human embryonic stem cells Cell 122947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X.et al. (2006The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells Nat Genet 38431–440. [DOI] [PubMed] [Google Scholar]
- Kondoh H., and, Kamachi Y. SOX-partner code for cell specification: Regulatory target selection and underlying molecular mechanisms. Int J Biochem Cell Biol. 2010;42:391–399. doi: 10.1016/j.biocel.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Thomson M, Liu SJ, Zou LN, Smith Z, Meissner A., and, Ramanathan S. Pluripotency factors in embryonic stem cells regulate differentiation into germ layers. Cell. 2011;145:875–889. doi: 10.1016/j.cell.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B, Ng HH.et al. (2005Transcriptional regulation of nanog by OCT4 and SOX2 J Biol Chem 28024731–24737. [DOI] [PubMed] [Google Scholar]
- Silva J, Nichols J, Theunissen TW, Guo G, van Oosten AL, Barrandon O.et al. (2009Nanog is the gateway to the pluripotent ground state Cell 138722–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers I, Silva J, Colby D, Nichols J, Nijmeijer B, Robertson M.et al. (2007Nanog safeguards pluripotency and mediates germline development Nature 4501230–1234. [DOI] [PubMed] [Google Scholar]
- Loh KM., and, Lim B. A precarious balance: pluripotency factors as lineage specifiers. Cell Stem Cell. 2011;8:363–369. doi: 10.1016/j.stem.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Takahashi K., and, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Hochedlinger K., and, Plath K. Epigenetic reprogramming and induced pluripotency. Development. 2009;136:509–523. doi: 10.1242/dev.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samavarchi-Tehrani P, Golipour A, David L, Sung HK, Beyer TA, Datti A.et al. (2010Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming Cell Stem Cell 764–77. [DOI] [PubMed] [Google Scholar]
- Li R, Liang J, Ni S, Zhou T, Qing X, Li H.et al. (2010A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts Cell Stem Cell 751–63. [DOI] [PubMed] [Google Scholar]
- Yori JL, Johnson E, Zhou G, Jain MK., and, Keri RA. Kruppel-like factor 4 inhibits epithelial-to-mesenchymal transition through regulation of E-cadherin gene expression. J Biol Chem. 2010;285:16854–16863. doi: 10.1074/jbc.M110.114546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmer T, Diecke S, Grigoryan T, Quiroga-Negreira A, Birchmeier W., and, Besser D. E-cadherin is crucial for embryonic stem cell pluripotency and can replace OCT4 during somatic cell reprogramming. EMBO Rep. 2011;12:720–726. doi: 10.1038/embor.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F.et al. (2008Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes Nat Biotechnol 261276–1284. [DOI] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY.et al. (2008The epithelial-mesenchymal transition generates cells with properties of stem cells Cell 133704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., and, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Strauss R, Li ZY, Liu Y, Beyer I, Persson J, Sova P.et al. (2011Analysis of epithelial and mesenchymal markers in ovarian cancer reveals phenotypic heterogeneity and plasticity PLoS ONE 6e16186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader JE., and, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- Marhaba R, Klingbeil P, Nuebel T, Nazarenko I, Buechler MW., and, Zoeller M. CD44 and EpCAM: cancer-initiating cell markers. Curr Mol Med. 2008;8:784–804. doi: 10.2174/156652408786733667. [DOI] [PubMed] [Google Scholar]
- Abell AN, Jordan NV, Huang W, Prat A, Midland AA, Johnson NL.et al. (2011MAP3K4/CBP-regulated H2B acetylation controls epithelial-mesenchymal transition in trophoblast stem cells Cell Stem Cell 8525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jopling C, Boue S., and, Izpisua Belmonte JC. Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat Rev Mol Cell Biol. 2011;12:79–89. doi: 10.1038/nrm3043. [DOI] [PubMed] [Google Scholar]
- Riehle KJ, Dan YY, Campbell JS., and, Fausto N. New concepts in liver regeneration. J Gastroenterol Hepatol. 2011;26 ( suppl. 1:203–212. doi: 10.1111/j.1440-1746.2010.06539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhart DL., and, Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat Rev Cancer. 2008;8:671–682. doi: 10.1038/nrc2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka EM, Gann AA, Gates PB., and, Brockes JP. Newt myotubes reenter the cell cycle by phosphorylation of the retinoblastoma protein. J Cell Biol. 1997;136:155–165. doi: 10.1083/jcb.136.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarda G, Siepi F, Pajalunga D, Bernardini C, Rossi R, Montecucco A.et al. (2004A pRb-independent mechanism preserves the postmitotic state in terminally differentiated skeletal muscle cells J Cell Biol 167417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ, Bertwistle D, DEN Besten W, Kuo ML, Sugimoto M, Tago K.et al. (2005p53-Dependent and -independent functions of the Arf tumor suppressor Cold Spring Harb Symp Quant Biol 70129–137. [DOI] [PubMed] [Google Scholar]
- Brockes JP., and, Kumar A. Comparative aspects of animal regeneration. Annu Rev Cell Dev Biol. 2008;24:525–549. doi: 10.1146/annurev.cellbio.24.110707.175336. [DOI] [PubMed] [Google Scholar]
- Pajcini KV, Corbel SY, Sage J, Pomerantz JH., and, Blau HM. Transient inactivation of Rb and ARF yields regenerative cells from postmitotic mammalian muscle. Cell Stem Cell. 2010;7:198–213. doi: 10.1016/j.stem.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao M, Campbell K, Burns K, Kuan CY, Trumpp A., and, Nakafuku M. Coordinated control of self-renewal and differentiation of neural stem cells by Myc and the p19ARF-p53 pathway. J Cell Biol. 2008;183:1243–1257. doi: 10.1083/jcb.200807130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon HM, Jin X, Lee JS, Oh SY, Sohn YW, Park HJ.et al. (2008Inhibitor of differentiation 4 drives brain tumor-initiating cell genesis through cyclin E and notch signaling Genes Dev 222028–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken AP, Kleine-Kohlbrecher D, Dietrich N, Pasini D, Gargiulo G, Beekman C.et al. (2007The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells Genes Dev 21525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano CA, Dimos JT, Ivanova NB, Lowry N, Lemischka IR., and, Temple S. shRNA knockdown of Bmi-1 reveals a critical role for p21-Rb pathway in NSC self-renewal during development. Cell Stem Cell. 2007;1:87–99. doi: 10.1016/j.stem.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Bedelbaeva K, Snyder A, Gourevitch D, Clark L, Zhang XM, Leferovich J.et al. (2010Lack of p21 expression links cell cycle control and appendage regeneration in mice Proc Natl Acad Sci USA 1075845–5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T, Suzuki J, Wang YV, Menendez S, Morera LB, Raya A.et al. (2009Linking the p53 tumour suppressor pathway to somatic cell reprogramming Nature 4601140–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Collado M, Villasante A, Strati K, Ortega S, Cañamero M.et al. (2009The Ink4/Arf locus is a barrier for iPS cell reprogramming Nature 4601136–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M.et al. (2009Suppression of induced pluripotent stem cell generation by the p53-p21 pathway Nature 4601132–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marión RM, Strati K, Li H, Murga M, Blanco R, Ortega S.et al. (2009A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity Nature 4601149–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utikal J, Polo JM, Stadtfeld M, Maherali N, Kulalert W, Walsh RM.et al. (2009Immortalization eliminates a roadblock during cellular reprogramming into iPS cells Nature 4601145–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA.et al. (2008Reprogramming of human somatic cells to pluripotency with defined factors Nature 451141–146. [DOI] [PubMed] [Google Scholar]
- Li SC, Jin Y, Loudon WG, Song Y, Ma Z, Weiner LP.et al. (2011Increase developmental plasticity of human keratinocytes with gene suppression Proc Natl Acad Sci USA 10812793–12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage J, Mulligan GJ, Attardi LD, Miller A, Chen S, Williams B.et al. (2000Targeted disruption of the three Rb-related genes leads to loss of G(1) control and immortalization Genes Dev 143037–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Clem B, Zuba-Surma EK, El-Naggar S, Telang S, Jenson AB.et al. (2009Mouse fibroblasts lacking RB1 function form spheres and undergo reprogramming to a cancer stem cell phenotype Cell Stem Cell 4336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzi G, Cicalese A, Insinga A., and, Pelicci PG. The emerging role of p53 in stem cells. Trends Mol Med. 2012;18:6–12. doi: 10.1016/j.molmed.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Sarig R, Rivlin N, Brosh R, Bornstein C, Kamer I, Ezra O.et al. (2010Mutant p53 facilitates somatic cell reprogramming and augments the malignant potential of reprogrammed cells J Exp Med 2072127–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO.et al. (2011Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state Proc Natl Acad Sci USA 1087950–7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit MA., and, Peeper DS. Zeb1 is required for TrkB-induced epithelial-mesenchymal transition, anoikis resistance and metastasis. Oncogene. 2011;30:3735–3744. doi: 10.1038/onc.2011.96. [DOI] [PubMed] [Google Scholar]
- Kumar S, Park SH, Cieply B, Schupp J, Killiam E, Zhang F.et al. (2011A pathway for the control of anoikis-sensitivity by E-cadherin and epithelial-to-mesenchymal transition Mol Cell Biol 314036–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harn T, Foijer F, van Vugt M, Banerjee R, Yang F, Oostra A.et al. (2010Loss of Rb proteins causes genomic instability in the absence of mitogenic signaling Genes Dev 241377–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Hollstein M., and, Xu Y. p53 gain-of-function cancer mutants induce genetic instability by inactivating ATM. Nat Cell Biol. 2007;9:573–580. doi: 10.1038/ncb1571. [DOI] [PubMed] [Google Scholar]
- Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J.et al. (2011Somatic coding mutations in human induced pluripotent stem cells Nature 47163–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayshar Y, Ben-David U, Lavon N, Biancotti JC, Yakir B, Clark AT.et al. (2010Identification and classification of chromosomal aberrations in human induced pluripotent stem cells Cell Stem Cell 7521–531. [DOI] [PubMed] [Google Scholar]
- Chang CJ, Chao CH, Xia W, Yang JY, Xiong Y, Li CW.et al. (2011p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs Nat Cell Biol 13317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YJ, Lai HM, Chang YW, Chen GY., and, Lee JL. Direct reprogramming of stem cell properties in colon cancer cells by CD44. EMBO J. 2011;30:3186–3199. doi: 10.1038/emboj.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godar S, Ince TA, Bell GW, Feldser D, Donaher JL, Bergh J.et al. (2008Growth-inhibitory and tumor- suppressive functions of p53 depend on its repression of CD44 expression Cell 13462–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerry DJ, Tao L., and, Yan H. Regulation of cancer stem cells by p53. Breast Cancer Res. 2008;10:304. doi: 10.1186/bcr2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland BD, Bernards R., and, Peeper DS. The KLF4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nat Cell Biol. 2005;7:1074–1082. doi: 10.1038/ncb1314. [DOI] [PubMed] [Google Scholar]
- Yu F, Li J, Chen H, Fu J, Ray S, Huang S.et al. (2011Kruppel-like factor 4 (KLF4) is required for maintenance of breast cancer stem cells and for cell migration and invasion Oncogene 302161–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yori JL, Seachrist DD, Johnson E, Lozada KL, Abdul-Karim FW, Chodosh LA.et al. (2011Krüppel-like factor 4 inhibits tumorigenic progression and metastasis in a mouse model of breast cancer Neoplasia 13601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW.et al. (2002The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon Development 1292619–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland BD., and, Peeper DS. KLF4, p21 and context-dependent opposing forces in cancer. Nat Rev Cancer. 2006;6:11–23. doi: 10.1038/nrc1780. [DOI] [PubMed] [Google Scholar]
- Janda E, Lehmann K, Killisch I, Jechlinger M, Herzig M, Downward J.et al. (2002Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways J Cell Biol 156299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D., and, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Cowling VH., and, Cole MD. E-cadherin repression contributes to c-Myc-induced epithelial cell transformation. Oncogene. 2007;26:3582–3586. doi: 10.1038/sj.onc.1210132. [DOI] [PubMed] [Google Scholar]
- Wong DJ, Liu H, Ridky TW, Cassarino D, Segal E., and, Chang HY. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell. 2008;2:333–344. doi: 10.1016/j.stem.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neth P, Ries C, Karow M, Egea V, Ilmer M., and, Jochum M. The Wnt signal transduction pathway in stem cells and cancer cells: influence on cellular invasion. Stem Cell Rev. 2007;3:18–29. doi: 10.1007/s12015-007-0001-y. [DOI] [PubMed] [Google Scholar]
- Arima Y, Inoue Y, Shibata T, Hayashi H, Nagano O, Saya H.et al. (2008Rb depletion results in deregulation of E-cadherin and induction of cellular phenotypic changes that are characteristic of the epithelial-to-mesenchymal transition Cancer Res 685104–5112. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Deng T, Jones R, Li H, Herschkowitz JI, Liu JC.et al. (2010Rb deletion in mouse mammary progenitors induces luminal-B or basal-like/EMT tumor subtypes depending on p53 status J Clin Invest 1203296–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel C, Harper F, Cereghini S, Noë V, Mareel M., and, Crémisi C. Inactivation of retinoblastoma family proteins by SV40 T antigen results in creation of a hepatocyte growth factor/scatter factor autocrine loop associated with an epithelial-fibroblastoid conversion and invasiveness. Cell Growth Differ. 1997;8:165–178. [PubMed] [Google Scholar]
- Li F, He Z, Shen J, Huang Q, Li W, Liu X.et al. (2010Apoptotic caspases regulate induction of iPSCs from human fibroblasts Cell Stem Cell 7508–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicas A, Wang X, Zhang C, McCurrach M, Zhao Z, Mert O.et al. (2010Dissecting the unique role of the retinoblastoma tumor suppressor during cellular senescence Cancer Cell 17376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi S, Natsuizaka M, Wong GS, Michaylira CZ, Grugan KD, Stairs DB.et al. (2010Epidermal growth factor receptor and mutant p53 expand an esophageal cellular subpopulation capable of epithelial-to-mesenchymal transition through ZEB transcription factors Cancer Res 704174–4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit MA., and, Peeper DS. Epithelial-mesenchymal transition and senescence: two cancer-related processes are crossing paths. Aging (Albany NY) 2010;2:735–741. doi: 10.18632/aging.100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A.et al. (2009The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs Nat Cell Biol 111487–1495. [DOI] [PubMed] [Google Scholar]
- Cole MF, Johnstone SE, Newman JJ, Kagey MH., and, Young RA. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev. 2008;22:746–755. doi: 10.1101/gad.1642408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C.et al. (2008Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence Cancer Cell 1479–89. [DOI] [PubMed] [Google Scholar]
- Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY, Yang WH.et al. (2010Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition Nat Cell Biol 12982–992. [DOI] [PubMed] [Google Scholar]
- Martin A., and, Cano A. Tumorigenesis: Twist1 links EMT to self-renewal. Nat Cell Biol. 2010;12:924–925. doi: 10.1038/ncb1010-924. [DOI] [PubMed] [Google Scholar]
- Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D.et al. (2009Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells Cell 138592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floor S, van Staveren WC, Larsimont D, Dumont JE., and, Maenhaut C. Cancer cells in epithelial-to-mesenchymal transition and tumor-propagating-cancer stem cells: distinct, overlapping or same populations. Oncogene. 2011;30:4609–4621. doi: 10.1038/onc.2011.184. [DOI] [PubMed] [Google Scholar]
- Neganova I., and, Lako M. G1 to S phase cell cycle transition in somatic and embryonic stem cells. J Anat. 2008;213:30–44. doi: 10.1111/j.1469-7580.2008.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárta T, Vinarský V, Holubcová Z, Dolezalová D, Verner J, Pospísilová S.et al. (2010Human embryonic stem cells are capable of executing G1/S checkpoint activation Stem Cells 281143–1152. [DOI] [PubMed] [Google Scholar]
- Neganova I, Vilella F, Atkinson SP, Lloret M, Passos JF, von Zglinicki T.et al. (2011An important role for CDK2 in G1 to S checkpoint activation and DNA damage response in human embryonic stem cells Stem Cells 29651–659. [DOI] [PubMed] [Google Scholar]
- Marson A, Levine SS, Cole MF, Frampton GM, Brambrink T, Johnstone S.et al. (2008Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells Cell 134521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card DA, Hebbar PB, Li L, Trotter KW, Komatsu Y, Mishina Y.et al. (2008Oct4/Sox2-regulated miR-302 targets cyclin D1 in human embryonic stem cells Mol Cell Biol 286426–6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Neganova I, Przyborski S, Yang C, Cooke M, Atkinson SP.et al. (2009A role for NANOG in G1 to S transition in human embryonic stem cells through direct binding of CDK6 and CDC25A J Cell Biol 18467–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Takeishi S, Kanie T, Susaki E, Onoyama I, Tateishi Y.et al. (2011p57 is required for quiescence and maintenance of adult hematopoietic stem cells Cell Stem Cell 9262–271. [DOI] [PubMed] [Google Scholar]
- Zou P, Yoshihara H, Hosokawa K, Tai I, Shinmyozu K, Tsukahara F.et al. (2011p57(Kip2) and p27(Kip1) cooperate to maintain hematopoietic stem cell quiescence through interactions with Hsc70 Cell Stem Cell 9247–261. [DOI] [PubMed] [Google Scholar]
- Orford KW., and, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9:115–128. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- Liu Y, Elf SE, Miyata Y, Sashida G, Liu Y, Huang G.et al. (2009p53 regulates hematopoietic stem cell quiescence Cell Stem Cell 437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., and, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons BD., and, Clevers H. Stem cell self-renewal in intestinal crypt. Exp Cell Res. 2011;317:2719–2724. doi: 10.1016/j.yexcr.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Dreesen O., and, Brivanlou AH. Signaling pathways in cancer and embryonic stem cells. Stem Cell Rev. 2007;3:7–17. doi: 10.1007/s12015-007-0004-8. [DOI] [PubMed] [Google Scholar]
- Polyak K., and, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- Scheel C, Eaton EN, Li SH, Chaffer CL, Reinhardt F, Kah KJ.et al. (2011Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast Cell 145926–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson V, Hilmarsdottir B, Sigmundsdottir H, Fridriksdottir AJ, Ringnér M, Villadsen R.et al. (2011Endothelial induced EMT in breast epithelial cells with stem cell properties PLoS ONE 6e23833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., and, Zhou BP. Inflammation: a driving force speeds cancer metastasis. Cell Cycle. 2009;8:3267–3273. doi: 10.4161/cc.8.20.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan KM, Gulmann C, Eichler GS, Weinstein JN, Barrett HL, Kay EW.et al. (2008Signal pathway profiling of epithelial and stromal compartments of colonic carcinoma reveals epithelial-mesenchymal transition Oncogene 27323–331. [DOI] [PubMed] [Google Scholar]
- Francí C, Takkunen M, Dave N, Alameda F, Gómez S, Rodríguez R.et al. (2006Expression of Snail protein in tumor-stroma interface Oncogene 255134–5144. [DOI] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B.et al. (2007A perivascular niche for brain tumor stem cells Cancer Cell 1169–82. [DOI] [PubMed] [Google Scholar]
- He X, Marchionni L, Hansel DE, Yu W, Sood A, Yang J.et al. (2009Differentiation of a highly tumorigenic basal cell compartment in urothelial carcinoma Stem Cells 271487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulmann S, Waldburger N, Penzel R, Andrulis M, Schirmacher P., and, Sinn HP. Reduction of CD44(+)/CD24(-) breast cancer cells by conventional cytotoxic chemotherapy. Hum Pathol. 2010;41:574–581. doi: 10.1016/j.humpath.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Todaro M, Iovino F, Eterno V, Cammareri P, Gambara G, Espina V.et al. (2010Tumorigenic and metastatic activity of human thyroid cancer stem cells Cancer Res 708874–8885. [DOI] [PubMed] [Google Scholar]
- Yates CC, Shepard CR, Stolz DB., and, Wells A. Co-culturing human prostate carcinoma cells with hepatocytes leads to increased expression of E-cadherin. Br J Cancer. 2007;96:1246–1252. doi: 10.1038/sj.bjc.6603700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrais M, Chen X, Perez-Moreno M., and, Gumbiner BM. E-cadherin homophilic ligation inhibits cell growth and epidermal growth factor receptor signaling independently of other cell interactions. Mol Biol Cell. 2007;18:2013–2025. doi: 10.1091/mbc.E06-04-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day ML, Zhao X, Vallorosi CJ, Putzi M, Powell CT, Lin C.et al. (1999E-cadherin mediates aggregation-dependent survival of prostate and mammary epithelial cells through the retinoblastoma cell cycle control pathway J Biol Chem 2749656–9664. [DOI] [PubMed] [Google Scholar]
- Bryant DM, Wylie FG., and, Stow JL. Regulation of endocytosis, nuclear translocation, and signaling of fibroblast growth factor receptor 1 by E-cadherin. Mol Biol Cell. 2005;16:14–23. doi: 10.1091/mbc.E04-09-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Karpova T, Sheppard AM, McNally J., and, Lowy DR. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J. 2004;23:1739–1748. doi: 10.1038/sj.emboj.7600136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soncin F, Mohamet L, Eckardt D, Ritson S, Eastham AM, Bobola N.et al. (2009Abrogation of E-cadherin-mediated cell-cell contact in mouse embryonic stem cells results in reversible LIF-independent self-renewal Stem Cells 272069–2080. [DOI] [PubMed] [Google Scholar]
- Egozi D, Shapira M, Paor G, Ben-Izhak O, Skorecki K., and, Hershko DD. Regulation of the cell cycle inhibitor p27 and its ubiquitin ligase Skp2 in differentiation of human embryonic stem cells. FASEB J. 2007;21:2807–2817. doi: 10.1096/fj.06-7758com. [DOI] [PubMed] [Google Scholar]
- St Croix B, Sheehan C, Rak JW, Flørenes VA, Slingerland JM., and, Kerbel RS. E-Cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27(KIP1) J Cell Biol. 1998;142:557–571. doi: 10.1083/jcb.142.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Karlström H.et al. (2000Generalized potential of adult neural stem cells Science 2881660–1663. [DOI] [PubMed] [Google Scholar]
- Kim JB, Sebastiano V, Wu G, Araúzo-Bravo MJ, Sasse P, Gentile L.et al. (2009Oct4-induced pluripotency in adult neural stem cells Cell 136411–419. [DOI] [PubMed] [Google Scholar]
- Moore RN, Cherry JF, Mathur V, Cohen R, Grumet M., and, Moghe PV. E-cadherin-expressing feeder cells promote neural lineage restriction of human embryonic stem cells. Stem Cells Dev. 2012;21:30–41. doi: 10.1089/scd.2010.0434. [DOI] [PubMed] [Google Scholar]
- Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE., and, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000;25:55–57. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- Bruggeman SW, Hulsman D, Tanger E, Buckle T, Blom M, Zevenhoven J.et al. (2007Bmi1 controls tumor development in an Ink4a/Arf-independent manner in a mouse model for glioma Cancer Cell 12328–341. [DOI] [PubMed] [Google Scholar]
- Liu C, Sage JC, Miller MR, Verhaak RG, Hippenmeyer S, Vogel H.et al. (2011Mosaic analysis with double markers reveals tumor cell of origin in glioma Cell 146209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg N, Kastemar M, Olofsson T, Smits A., and, Uhrbom L. Oligodendrocyte progenitor cells can act as cell of origin for experimental glioma. Oncogene. 2009;28:2266–2275. doi: 10.1038/onc.2009.76. [DOI] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B.et al. (2008A specialized vascular niche for adult neural stem cells Cell Stem Cell 3279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK.et al. (2008Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions Cell Stem Cell 3289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S.et al. (2009Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells Cancer Cell 15501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knizetova P, Ehrmann J, Hlobilkova A, Vancova I, Kalita O, Kolar Z.et al. (2008Autocrine regulation of glioblastoma cell cycle progression, viability and radioresistance through the VEGF-VEGFR2 (KDR) interplay Cell Cycle 72553–2561. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB.et al. (2006Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor Cancer Res 667843–7848. [DOI] [PubMed] [Google Scholar]
- Wurmser AE, Nakashima K, Summers RG, Toni N, D'Amour KA, Lie DC.et al. (2004Cell fusion-independent differentiation of neural stem cells to the endothelial lineage Nature 430350–356. [DOI] [PubMed] [Google Scholar]
- Sakariassen PO, Prestegarden L, Wang J, Skaftnesmo K-O, Mahesparan R, Molthoff C.et al. (2006Angiogenesis-independent tumor growth mediated by stem-like cancer cells Proc Natl Acad Sci USA 10316466–16471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A.et al. (2010Glioblastoma stem-like cells give rise to tumour endothelium Nature 468829–833. [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T.et al. (2010Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells Nature 468824–828. [DOI] [PubMed] [Google Scholar]
- Soda Y, Marumoto T, Friedmann-Morvinski D, Soda M, Liu F, Michiue H.et al. (2011Transdifferentiation of glioblastoma cells into vascular endothelial cells Proc Natl Acad Sci USA 1084274–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigolin GM, Fraulini C, Ciccone M, Mauro E, Bugli AM, De Angeli C.et al. (2006Neoplastic circulating endothelial cells in multiple myeloma with 13q14 deletion Blood 1072531–2535. [DOI] [PubMed] [Google Scholar]
- Pezzolo A, Parodi F, Corrias MV, Cinti R, Gambini C., and, Pistoia V. Tumor origin of endothelial cells in human neuroblastoma. J Clin Oncol. 2007;25:376–383. doi: 10.1200/JCO.2006.09.0696. [DOI] [PubMed] [Google Scholar]
- Bussolati B, Grange C, Sapino A., and, Camussi G. Endothelial cell differentiation of human breast tumour stem/progenitor cells. J Cell Mol Med. 2009;13:309–319. doi: 10.1111/j.1582-4934.2008.00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlgren C, Gustafsson MV, Jin S, Poellinger L., and, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci USA. 2008;105:6392–6397. doi: 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V.et al. (2002A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions EMBO J 211948–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis-Tuffin LJ, Rodriguez F, Giannini C, Scheithauer B, Necela BM, Sarkaria JN.et al. (2010Misregulated E-cadherin expression associated with an aggressive brain tumor phenotype PLoS ONE 5e13665. [DOI] [PMC free article] [PubMed] [Google Scholar]