Abstract
Melanomas contain distinct cell subpopulations. Several of these subpopulations, including one expressing CD20, may harbor stem cell-like or tumor-initiating characteristics. We hypothesized that patients at high risk of disease recurrence could benefit from an adjuvant anti-CD20 therapy. Therefore, we initiated a small pilot trial to study the effect of the anti-CD20 antibody rituximab in a group of melanoma patients with stage IV metastatic disease who had been rendered without evident disease by way of surgery, chemotherapy and/or radiation therapy. The major objective was safety, while secondary objectives were description of recurrence-free intervals (RFI) and overall survival (OS). Nine patients received rituximab at 375 mg/m2 qw for 4 weeks followed by a maintenance therapy every 8 weeks. Treatment was discontinued after 2 years or with disease recurrence. Treatment was well tolerated. After a median observation of 42 months, the median neither of RFI nor of OS has been reached. Despite therapy that ended after 2 years, six out of nine patients are still alive and five of them are recurrence-free. Though the patient number is too small for definitive conclusions, our data may represent a first example of the potential therapeutic value of targeting CD20+ cell populations—at least for a subset of patients.
Introduction
Once melanoma has spread to visceral sites, the usual outcome is bleak with a median survival of 7–10 months and a 10-year survival rate of around 10%.1 While significant therapeutic progress has been achieved with the development of targeted and novel immunomodulatory therapies,2,3 the majority of clinical responses are still incomplete and/or disappointingly short-lived. A subgroup of metastatic melanoma patients may benefit from complete metastasectomy. These patients have a reported 5–7 months median recurrence-free interval (RFI) and, at best, a 19–21 month median overall survival (OS) as observed in large prospective clinical trials and retrospective analyses from extensive patient databases.4,5,6,7,8 After complete metastasectomy, immunotherapies aimed at preventing disease recurrence have shown some promise in small, uncontrolled prospective clinical trials9,10 and are currently evaluated in larger prospective trials. So far, however, no adjuvant treatment of stage IV melanoma patients has proved preferable to close observation aimed at early detection and surgical management of disease recurrence.11
Melanomas, like other malignancies, contain distinct cell subpopulations.12,13 Work on these subpopulations originated with the description of so-called cancer stem cells, first in hematopoietic and brain tumors and more recently in many other tumors (reviewed in refs. 13,14). With their exceptional capacity to self-renew and differentiate into diverse cell populations, these cells may be key to the functional heterogeneity of cancer15 and may thus have major translational impact. In melanoma, several subpopulations with the capacity of self-renewal, differentiation, tumorigenicity and/or drug resistance have been described,14,16,17,18,19,20,21,22,23 including one expressing the B cell marker CD20.18 CD20 was initially identified on a small percentage of human melanoma cells when cultured in embryonic stem cell medium and found on nonadherent spheres. These CD20+ melanoma cells followed the definition of tumor stem cells,24 i.e., they self-renewed and differentiated into several cell lineages. CD20+ melanoma cells were highly tumorigenic in vivo after xenotransplantation, indicating that these cells exhibit tumor-initiating capacity.18 Consistently, Schmidt et al. observed in a preclinical cell-based xenograft model an inhibition of growth and recurrence of highly tumorigenic human melanoma cells by specific targeting of the CD20+ subpopulation with autologous T cells genetically engineered to express a chimeric CD3ζ/CD20 antigen receptor.25
We hypothesized that melanoma patients at high risk of disease recurrence could benefit from an adjuvant therapy specifically targeting this tumor-initiating subpopulation. We have therefore initiated a small pilot trial to study the effect of the anti-CD20 antibody rituximab on disease recurrence in a group of melanoma patients with stage IV metastatic disease1 who had been rendered disease-free by way of surgery, chemotherapy, and/or radiation therapy.
Results
Patient characteristics
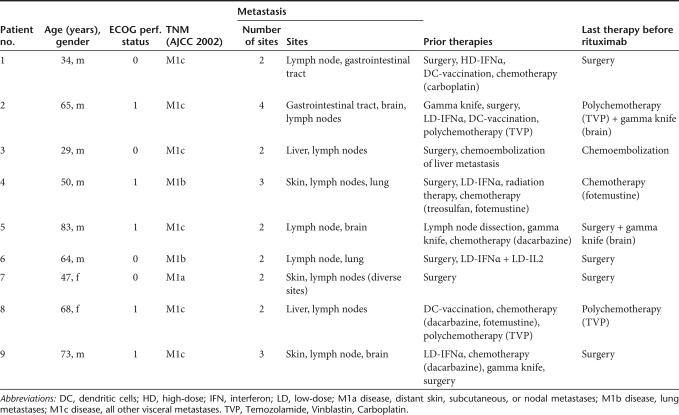
The study population consisted of nine patients (seven male, two female); baseline demographic and clinical characteristics are given in Table 1. All patients had clinical stage IV disease with metastatic lesions affecting at least two body sites. One patient presented with a history of M1a disease (distant skin, subcutaneous, or nodal metastases), two patients with a history of M1b disease (lung metastases) and six patients with a history of M1c disease (all other visceral metastases).1 Three patients had had brain metastases. Most patients had received multiple systemic and/or localized therapies including (multiagent) chemotherapy, various immunotherapies, radiation therapy and/or chemoembolization before inclusion into the trial. One patient had undergone only complete metastasectomy (Table 1). Of note, eight patients reported a disease history with at least one episode where all metastatic disease was initially fully responsive to conventional therapies and/or grossly resected, but disease recurred over time. The length of each of these RFIs is given in Figure 1. Some patients had experienced several of these episodes during stage IV disease (Figure 1, patients #1, #2, #6, #9).
Table 1. Baseline patients' demographic and clinical characteristics.
Figure 1.
Recurrence-free intervals (RFIs) in melanoma patents following conventional therapeutic strategies. Before inclusion into the trial, most patients had a stage IV metastatic disease history of at least one episode where all disease disappeared following standard therapies and/or was grossly resected, but recurred over time. The duration of each RFI is given in months. Consistent with published data, the median RFI was 6 months. Note, that patients #1, #2, #6, and #9 experienced several of these episodes, patient #7 experienced none.
Safety
Rituximab treatment was well tolerated, there were no doses-limiting toxicities. The majority of nonlaboratory adverse event were NCI-CTC (v. 3.0.) grade 1/2 (n = 58; mostly respiratory disorders such as pharyngitis, rhinitis), the one grade 3 event (thrombosis requiring anticoagulation therapy) was judged to be treatment-unrelated. Laboratory adverse events were exclusively CTC grade 1/2 (n = 125; mostly liver function and hematology), there were no CTC grade 3/4 events. Serious adverse events did not occur, virus serology remained unchanged.
Clinical results
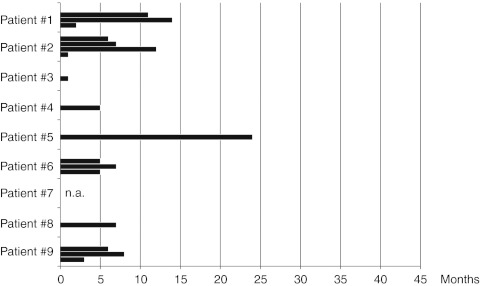
RFI and OS were defined as the time from initiation of therapy until documented recurrence of the disease and death, respectively. As of May 2011, the median follow up time for RFI and OS is 42 months. Despite therapy cessation after 2 years, six out of nine (66%) patients are still alive and five of them are recurrence-free. So far, the median neither of RFI nor of OS has been reached. The median of RFI is 42+ months (mean: 27.4+) as is the median of OS (42+ months, mean: 37.9+). One patient has been alive for 39+ months and showed only local recurrence of the disease. The other patients experienced a disease recurrence after 6–13 months of therapy and died from the disease later on (at 27–37 months, respectively). The duration of the RFIs following anti-CD20 therapy is given in Figure 2.
Figure 2.
Recurrence-free intervals (RFIs) in melanoma patents following anti-CD20 therapy. So far, the median of RFI has not been reached. Patients #1 to #5 are still tumor-free, although anti-CD20 therapy has been terminated after 2 years. The median RFI following anti-CD20 therapy is 42+ months (mean: 27.4+). Patients #6 to #9 experienced disease recurrence at the time points given in the graph, patients #7 to #9 died from the disease.
We are aware that the trial patients represent a nonstandard population, reflecting the heterogeneity of the disease along with the low frequency of standard therapies to induce complete remissions of established metastatic stage IV disease. In our patients, NED was observed after (poly-) chemotherapy or obtained by complete metasasectomy, occasionally in combination with local radiation therapy of the brain (see also Table 1). The reported median of RFIs following these therapies varies from <4 months after chemotherapy26 to 5–7 months after complete metastasectomy.4,5,6,7,8 Consistently, the median of RFIs in our trial patients was 6 months (as reported before inclusion into the trial, see Figure 1). In contrast, the median of RFI in the very same patients following anti-CD20 therapy is 42+ (mean: 27.4+) months (Figure 2, P = 0.0008, logrank test).
Patients with or without disease recurrence were comparable for the time period from onset of stage IV disease until initiation of anti-CD20 therapy [patients with recurrence (median: 21, range 2–36 months) versus without recurrence (median: 24, range 3–48 months)] and established prognostic factors27 such as performance status [patients with recurrence (50% each ECOG grade 0 and 1) versus without recurrence (40% grade 0, 60% grade 1)], history of brain metastases [patients with recurrence (25%) versus without recurrence (40%)], previous disease progression according to M category1 [patients with recurrence (1 × M1a, 1 × M1b, 2 × M1c) versus patients without recurrence (2 × M1b, 4 × M1c)], and age at initiation of anti-CD20 therapy [patients with recurrence (mean: 63 years) versus without disease recurrence (mean: 52.2 years, Table 1)].
Biomarkers of therapy
In an attempt to identify biomarkers of anti-CD20 treatment in melanoma patients or correlation with the clinical course, we analyzed several phenotypic parameters. For immunophenotyping, paired peripheral blood mononuclear cells samples obtained before and during therapy were available from eight trial patients (one patient excluded due to a sampling failure).
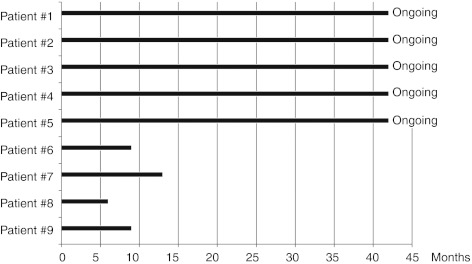
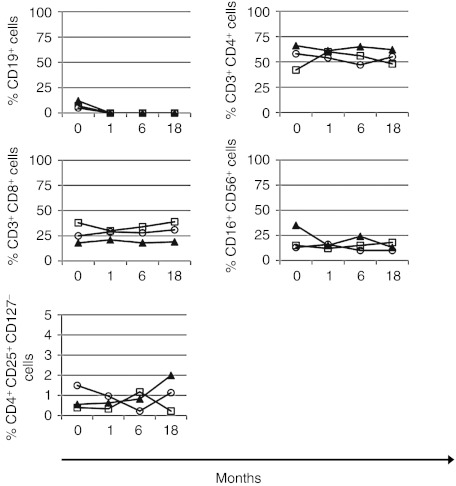
As expected, immunophenotyping of peripheral blood mononuclear cells showed a consistent loss of CD19+ B lymphocytes during anti-CD20 therapy, as demonstrated in samples collected at week 4 (Figure 3) and at 6 and 18 months (Figure 4). We did not observe any consistent changes in the absolute or relative numbers of CD3+CD4+, CD3+CD8+, CD16+CD56+, and CD4+CD25+CD127− lymphocytes during therapy, neither early at week 4 (Figure 3) nor later at 6 or 18 months (Figure 4).
Figure 3.
Short-term effects of anti-CD20 therapy-induced changes in peripheral blood mononuclear cells. Percentages of CD19+ B lymphocytes, CD3+CD4+, and CD3+CD8+ T cells, CD16+CD56+ NK cells and CD4+CD25+CD127− regulatory T cells. (a) Patients without disease recurrence (patients #1, open circle; #2, closed circle; #3, open square; #4, closed triangle; #5, closed square). (b) Patients with disease recurrence (patients #7, closed triangle; #8, open square; #9, open circle).
Figure 4.
Long-term effects of anti-CD20 therapy-induced changes in peripheral blood mononuclear cells. Long-term affects under therapy could be analyzed only in patients without disease recurrence. Percentages of CD19+ B lymphocytes, CD3+CD4+ and CD3+CD8+ T cells, CD16+CD56+ NK cells and CD4+CD25+CD127− regulatory T cells are shown for 3 patients (#1, open circle; #3: open square; #4: closed triangle) at 1, 6, and 18 months of therapy.
Presence of CD20+ melanoma cells and lymphocytes in tumor samples
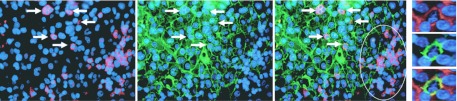
We had access to metastatic melanoma samples collected from seven patients before anti-CD20 treatment. These tissue samples were analyzed for the presence of the CD20+ melanoma cells and lymphocytes. CD20+ melanoma cells were identified by costaining for the β3 integrin subunit, because the expression of β3 integrin is restricted to tumor cells in human melanoma tissues and CD20+ melanoma spheres have been shown to coexpress the β3 integrin subunit.18 CD20+ β3 integrin+ cells were observed in five out of seven tumor lesions (from three patients with and two patients without disease recurrence), in two samples (from one patient with and one patient without disease recurrence) these cells could not be detected. CD20+ β3 integrin+ cells were distributed within the tumors either as single cells or as small clusters (Figure 5), in each sample the frequency was below 1% of tumor cells.
Figure 5.
Immunofluoresence imaging of a double immunostaining to detect CD20+ cells in human metastatic melanoma samples. Laser scan microscopy of immunostainings for CD20 (red, left panel), β3 integrin (green, middle left panel) and a merger of both (middle right panel). White arrows denote a melanoma subpopulation as identified by coexpression of CD20 and β3 integrin. In the right lower quadrant, red-stained collections of CD20+ β3 integrin− lymphocytes can be found. Right panel: close ups from another melanoma sample [from top to bottom: staining for CD20 (red), β3 integrin (green), merger]. Nuclear staining with DAPI (blue).
CD20+ lymphocytes were present in each tumor sample, preferentially grouped in small clusters at the rim of the tumor (Figure 5). Sometimes we observed single CD20+ lymphocytes between tumor cells. Consistent with previous reports,28,29 the frequency of CD20+ lymphocytes ranged between around 5 and 20% of tumor-infiltrating lymphocytes. We also had the chance to collect a post-treatment tumor sample from a patient with disease-recurrence. Here, neither CD20+ lymphocytes nor CD20+ melanoma cells could be detected.
Discussion
Whether heterogeneity of melanoma cells in phenotype and function is following a deterministic model driven by small subpopulations of cancer stem cells or a stochastic model resulting from the same probability of virtually all tumor cells to generate distinct subpopulations, or rather both models via bidirectional interconvertibility is still a matter of debate.12,13,14,30 All models, however, are consistent with a new understanding of the complex biology of the disease as a dynamic process mediated by generation of sporadically present subpopulations through epigenetic changes31 and/or microenvironmental factors that determine clonal dominance.32 Chemotherapeutic drugs, radiation treatment33,34 and presumably host immunity35 may impose a pressure to induce tumor-initiating subpopulations. Work on cancer stem cell niches further suggests that cytokines, soluble growth factors and extracellular matrix components such as osteopontin may provide a local microenvironment to sustain these subpopulations.32,36 As a result, patients who initially profited from conventional therapies will develop disease recurrence over time.
The recently identified CD20-expressing melanoma subpopulation is characterized by self-renewal, differentiation into several cell lineages and high tumorigenicity in cell-based in vitro and in vivo studies.18 Consistently, Schmidt et al. have described a highly tumorigenic human melanoma subpopulation that expressed high molecular weight melanoma-associated antigen/melanoma-associated chondroitin sulfate proteoglycan and contained a CD20+ population with a CD44+CD61+CD24−CD34− cancer stem cell phenotype.25 The possible physiological and therapeutic relevance of this subpopulation is underpinned by the recent observation that anti-CD20 immunotherapy can inhibit growth and recurrence25 of human melanoma cells in preclinical xenograft models and, perhaps more strikingly, by our own observations in a subset of melanoma patients subjected to CD20-immunotargeting. As we are about to learn more about the biological significance of tumor cell subpopulations, we expect that their targeting will become an integral part of future therapeutic strategies, either aimed at the prevention of recurrence or at the elimination of established disease.
Our study represents a first example of the potential value of this strategy in the clinics, but also of its current limitations. Targeting a single subpopulation may not be sufficient to completely inhibit human melanoma growth in xenotransplantation models25 or to prevent recurrence in more than a subset of patients. In our trial, we could not differentiate between patients with disease recurrence from those without it by use of known prognostic factors. It can thus be concluded that the development not only of more effective (combination) therapies, but also of biomarkers for identification of patients who may potentially benefit from this kind of therapy is essential. Data from Schmidt et al.25 have given a first clue about the nature of a potential biomarker, i.e., the frequency of CD20+ melanoma cells in pretreatment tumor specimens. Consistently, we detected CD20+ cells in pretreatment melanoma lesions. Unfortunately, the low frequency of CD20+ melanoma cells, the small number of patients and the heterogeneity of the patient cohort did not allow any definitive statements beyond the purely descriptive observations provided here. Further biomarker studies accompanying future clinical trials in patients with established disease may help to evaluate the value of this particular biomarker and/or to develop alternative ones. Immunotherapy may represent one promising approach to target subpopulations,22,25 however, as with cancer stem cells, some of the melanoma-initiating subpopulations share remarkable phenotypic and functional similarities with normal stem cells.15 Therefore, it may be difficult to develop systemic immunotherapeutic approaches to specifically kill melanoma subpopulations. For the time being, CD20 seems to be an especially attractive target as its expression is highly restricted. Additionally, well-characterized antibodies (Abs) against CD20 are available, allowing even prolonged therapies with good tolerability and feasibility.
As expected, we also observed depletion of B lymphocytes in the peripheral blood. B lymphocytes are the central component of the humoral immune system and play important roles in immunity via Ab-dependent and Ab-independent mechanisms. B lymphocytes may also contribute to tumor initiation as initially hypothesized by comparison of wild-type with B cell-deficient mice.37 Most recently, animal models of de novo carcinogenesis identified two underlying mechanisms, i.e., promotion of chronic inflammation through circulating B cells via IgG-mediated stimulation of activating FcγR on tumor-resident and recruited myeloid cells38 and secretion of proinflammatory cytokines by tumor-infiltrating B cells.39 Consistently, most melanoma patients mount tumor-specific autoantibody responses as identified by serological cloning methods40 and melanoma tumors frequently contain CD20+ lymphocytes.28 While resting B cells do not activate resting T cells,41 tumor-infiltrating B cells have documented antitumorigenic effects through mechanisms such as direct or indirect antigen-presentation, enhancement of T cell responses by secretion of stimulatory cytokines, direct Ab-independent cytotoxicity, or secretion of chemokines promoting the formation of tertiary lymphoid structures (reviewed in ref. 42). Given this functional heterogeneity, the role of B cells in the cancer context is still to be defined. Accordingly, CD20 depletion in preclinical in vivo models has resulted in enhancement of43 as well as protection against44 the growth of transplanted tumors. While we did not find evidence for melanoma-promoting effects of anti-CD20 therapy in our patient cohort, we also did not for the induction of antitumor immunity, neither in phenotypic analyses nor by occurrence of clinical autoimmune phenomena such as induction of vitiligo.
We conclude that adjuvant immunotargeting of CD20 with mAbs offers an attractive and immediately available therapeutic option with an excellent safety profile, even in heavily pretreated melanoma patients. However, the patient cohort enrolled in this study is highly heterogeneous and this may have affected the observed clinical results. Thus, application of these initial and preliminary clinical observations through carefully designed trials is highly warranted and may open up a new perspective for a more effective and better tolerated treatment option for at least a subset of patients suffering from high-risk or metastatic melanoma.
Materials and Methods
Patient eligibility criteria. Eligibility criteria were: age ≥18 years; biopsy-confirmed nonocular metastatic melanoma, clinical stage IV according to AJCC 2002,45 no detectable disease after therapeutic intervention. Exclusion criteria were: prior treatment with an anti-CD20 antibody, ECOG performance status ≤2, radiation or chemo-/immunotherapy < 4 weeks prior to study entry; LDH- and S100- or MIA-serology >upper limit of normal; active infection incl. HIV, hepatitis B and C infection; pregnant and lactating females; history of other invasive cancers within the past 5 years.
Study design. This study (EudraCT number: 2007-005125-30) was an open label, single-arm, investigator-initiated pilot phase I trial. All patients were enrolled at the Medical University of Vienna under a protocol approved by the institutional review board (457/2007) and the Austrian health authority. The study was conducted according to the principles embodied in the Declaration of Helsinki Principles and supervised by a Data and Safety Monitoring Board of the Medical University of Vienna. All patients provided written informed consent. All patients had had documented biopsy-proven clinical stage IV melanoma and all disease had to be either responsive to systemic or localized therapeutic interventions or grossly resected within 8 weeks before enrollment into the trial. At the start of rituximab treatment, all patients had no evidence of disease (NED) as documented by tumor imaging, physical examination and LDH- and S100- or MIA-serology. Patients with a history of successfully treated brain metastases could be included.
The major objective of this pilot trial was to determine safety, because rituximab—an immunosuppressive agent—was given to a vulnerable patient collective, namely (in most cases heavily) pretreated patients suffering from a highly immunogenic tumor. Secondary objectives were description of RFI and OS. Rituximab was administered at a dose and schedule established in follicular lymphoma patients,46 i.e., induction treatment with 375 mg/m2 qw for 4 weeks followed by maintenance therapy with 375 mg/m2 every 8 weeks. Treatment was stopped after 2 years or with recurrence of disease (as to tumor imaging, physical examination, or LDH- and S100- or MIA-serology >upper limit of normal performed every 8 weeks). After 2 years, recurrence-free patients were followed only by physical examination and LDH-, S100-, MIA-serology every month as well as tumor imaging every 3 months. Patients with progressive disease received salvage therapies (including chemotherapy, radiation therapy, experimental vaccination) and were followed for survival.
Study assessments. Safety evaluations were conducted at baseline and at each visit thereafter and consisted of history taking and physical examination. CBC, serum biochemistry, baseline coagulation, coombs testing, quantitative immunoglobulins, complement C3/C4/CH50 levels and virus serology were evaluated at baseline and every 8 weeks in the first 2 years and graded according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 3.0. Radiologic assessments included complete tumor imaging (computed tomography scan or magnetic resonance imaging of chest, abdomen/pelvis, and brain or whole body positron emission tomography/computed tomography) and were performed every 8 weeks in the first 2 years, thereafter every 3 months (follow-up). Scans were read by the study-radiologist/nuclear medicine physician, who decided on continuation of the therapy.
Immunological analyses. Peripheral blood mononuclear cells were analyzed by four-color flow cytometry at indicated time points. B cells, CD4+ T cells, CD8+ T cells, NK cells and regulatory T cells were gated on a two-laser flow cytometer (FACSCalibur; BD Biosciences, San Jose, CA) as CD19+, CD3+CD4+, CD3+CD8+, CD16+CD56+, and CD4+CD25+CD127− lymphocytes, respectively. Fluorochrome-labeled monoclonal antibodies directed against CD3, CD4, CD8, CD16, CD25, CD56, and CD127 were obtained from BD Biosciences. Percentages of positive cells were calculated.
Immunostainings of pretreatment tumor tissues. Immunostainings of human tumor tissues were performed essentially as described.47 Briefly, formalin-fixed paraffin-embedded tissue sections were subjected to epitope-retrieval by target retrieval solution (Dako, Glostrup, Denmark) and CD20 and β3 integrin expression visualized with fluorochromes Alexa633 and Alexa488, respectively. Antibodies were mouse monoclonal anti-CD20 (clone L26, Dako) and mouse monoclonal anti-β3 integrin (clone 23C6, BD Biosciences). Secondary antibodies were Alexa633-conjugated goat anti-mouse IgG2A and Alexa488-conjugated goat anti-mouse IgG1 (both Life Technologies, Grand Island, NY). Normal mouse serum was substituted for the primary antibody in each case as a negative control. Nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole). The stained sections were read by confocal scanning microscopy (LSM510; Zeiss, Jena, Germany).
Acknowledgments
We thank the patients and their families who participated in this trial; the clinical staff members of the Division of Immunology, Allergy and Infectious Diseases, Department of Dermatology, Medical University of Vienna; Drs. Ulrich Jäger, Winfried Pickl, and Franz Trautinger for serving in the Data and Safety Monitoring Board, Drs Peter Bauer, Claus Garbe, and Axel Hauschild for conceptual contributions and Dr. Wolfgang Schreiner for statistical advice. This work has been funded by grants of the Vienna Hans Mayr-Fund and the Vienna Science and Technology Fund (WWTF) through project LS11-045 to S.N.W. We thank Susanna Stopka for editorial, language, and writing assistance. This work was done in Vienna, Austria.
REFERENCES
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR.et al. (2009Final version of 2009 AJCC melanoma staging and classification J Clin Oncol 276199–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA.et al. (2010Inhibition of mutated, activated BRAF in metastatic melanoma N Engl J Med 363809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB.et al. (2010Improved survival with ipilimumab in patients with metastatic melanoma N Engl J Med 363711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essner R, Lee JH, Wanek LA, Itakura H., and, Morton DL. Contemporary surgical treatment of advanced-stage melanoma. Arch Surg. 2004;139:961–6; discussion 966. doi: 10.1001/archsurg.139.9.961. [DOI] [PubMed] [Google Scholar]
- Hsueh EC, Essner R, Foshag LJ, Ollila DW, Gammon G, O'Day SJ.et al. (2002Prolonged survival after complete resection of disseminated melanoma and active immunotherapy with a therapeutic cancer vaccine J Clin Oncol 204549–4554. [DOI] [PubMed] [Google Scholar]
- Leo F, Cagini L, Rocmans P, Cappello M, Geel AN, Maggi G.et al. (2000Lung metastases from melanoma: when is surgical treatment warranted Br J Cancer 83569–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RP, Hanish SI, Haney JC, Miller CC 3rd, Burfeind WR, Jr, Tyler DS.et al. (2007Improved survival with pulmonary metastasectomy: an analysis of 1720 patients with pulmonary metastatic melanoma J Thorac Cardiovasc Surg 133104–110. [DOI] [PubMed] [Google Scholar]
- Othus M, Moon J., and, Margolin K. Melanoma's deadly march to the brain: by what route, and can it be stopped. Cancer. 2011;117:1560–1563. doi: 10.1002/cncr.25716. [DOI] [PubMed] [Google Scholar]
- Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK.et al. (2008Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma J Clin Oncol 265896–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitler LE, Grossbard ML, Ernstoff MS, Silver G, Jacobs M, Hayes FA.et al. (2000Adjuvant therapy of stage III and IV malignant melanoma using granulocyte-macrophage colony-stimulating factor J Clin Oncol 181614–1621. [DOI] [PubMed] [Google Scholar]
- Fecher LA., and, Flaherty KT. Where are we with adjuvant therapy of stage III and IV melanoma in 2009. J Natl Compr Canc Netw. 2009;7:295–304. doi: 10.6004/jnccn.2009.0022. [DOI] [PubMed] [Google Scholar]
- Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM., and, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton M, Quintana E, Fearon ER., and, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822–829. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Frank NY, Schatton T., and, Frank MH. The therapeutic promise of the cancer stem cell concept. J Clin Invest. 2010;120:41–50. doi: 10.1172/JCI41004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MF., and, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124:1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP.et al. (2010Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271 Nature 466133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyaratanakornkit JB, Yue L, Strachan LR, Scalapino KJ, LeBoit PE, Lu Y.et al. (2010Selection of tumorigenic melanoma cells using ALDH J Invest Dermatol 1302799–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S.et al. (2005A tumorigenic subpopulation with stem cell properties in melanomas Cancer Res 659328–9337. [DOI] [PubMed] [Google Scholar]
- Held MA, Curley DP, Dankort D, McMahon M, Muthusamy V., and, Bosenberg MW. Characterization of melanoma cells capable of propagating tumors from a single cell. Cancer Res. 2010;70:388–397. doi: 10.1158/0008-5472.CAN-09-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupas V, Weishaupt C, Siepmann D, Kaserer ML, Eickelmann M, Metze D.et al. (2011RANK is expressed in metastatic melanoma and highly upregulated on melanoma-initiating cells J Invest Dermatol 131944–955. [DOI] [PubMed] [Google Scholar]
- Monzani E, Facchetti F, Galmozzi E, Corsini E, Benetti A, Cavazzin C.et al. (2007Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential Eur J Cancer 43935–946. [DOI] [PubMed] [Google Scholar]
- Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M.et al. (2008Identification of cells initiating human melanomas Nature 451345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CE, Paratore C, Dours-Zimmermann MT, Rochat A, Pietri T, Suter U.et al. (2006Neural crest-derived cells with stem cell features can be traced back to multiple lineages in the adult skin J Cell Biol 1751005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF., and, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Schmidt P, Kopecky C, Hombach A, Zigrino P, Mauch C., and, Abken H. Eradication of melanomas by targeted elimination of a minor subset of tumor cells. Proc Natl Acad Sci USA. 2011;108:2474–2479. doi: 10.1073/pnas.1009069108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajetta E, Del Vecchio M, Bernard-Marty C, Vitali M, Buzzoni R, Rixe O.et al. (2002Metastatic melanoma: chemotherapy Semin Oncol 29427–445. [DOI] [PubMed] [Google Scholar]
- Korn EL, Liu PY, Lee SJ, Chapman JA, Niedzwiecki D, Suman VJ.et al. (2008Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials J Clin Oncol 26527–534. [DOI] [PubMed] [Google Scholar]
- Hillen F, Baeten CI, van de Winkel A, Creytens D, van der Schaft DW, Winnepenninckx V.et al. (2008Leukocyte infiltration and tumor cell plasticity are parameters of aggressiveness in primary cutaneous melanoma Cancer Immunol Immunother 5797–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein MR, Elsers DA, Fadel SA., and, Omar AE. Immunohistological characterisation of tumour infiltrating lymphocytes in melanocytic skin lesions. J Clin Pathol. 2006;59:316–324. doi: 10.1136/jcp.2005.028860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga-Kalabis M, Roesch A., and, Herlyn M. From cancer stem cells to tumor maintenance in melanoma. J Invest Dermatol. 2011;131:1600–1604. doi: 10.1038/jid.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A.et al. (2010A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth Cell 141583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PB, Chaffer CL., and, Weinberg RA. Cancer stem cells: mirage or reality. Nat Med. 2009;15:1010–1012. doi: 10.1038/nm0909-1010. [DOI] [PubMed] [Google Scholar]
- Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA.et al. (2009Identification of selective inhibitors of cancer stem cells by high-throughput screening Cell 138645–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF.et al. (2008Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy J Natl Cancer Inst 100672–679. [DOI] [PubMed] [Google Scholar]
- Schatton T., and, Frank MH. Antitumor immunity and cancer stem cells. Ann N Y Acad Sci. 2009;1176:154–169. doi: 10.1111/j.1749-6632.2009.04568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voog J., and, Jones DL. Stem cells and the niche: a dynamic duo. Cell Stem Cell. 2010;6:103–115. doi: 10.1016/j.stem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Divekar AA, Hilchey SP, Cho HM, Newman CL, Shin SU.et al. (2005Increased rejection of primary tumors in mice lacking B cells: inhibition of anti-tumor CTL and TH1 cytokine responses by B cells Int J Cancer 117574–586. [DOI] [PubMed] [Google Scholar]
- Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S.et al. (2010FcRgamma activation regulates inflammation-associated squamous carcinogenesis Cancer Cell 17121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammirante M, Luo JL, Grivennikov S, Nedospasov S., and, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–305. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuschenbach M, von Knebel Doeberitz M., and, Wentzensen N. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother. 2009;58:1535–1544. doi: 10.1007/s00262-009-0733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassila O, Vainio O., and, Matzinger P. Can B cells turn on virgin T cells. Nature. 1988;334:253–255. doi: 10.1038/334253a0. [DOI] [PubMed] [Google Scholar]
- Nelson BH. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol. 2010;185:4977–4982. doi: 10.4049/jimmunol.1001323. [DOI] [PubMed] [Google Scholar]
- DiLillo DJ, Yanaba K., and, Tedder TF. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. J Immunol. 2010;184:4006–4016. doi: 10.4049/jimmunol.0903009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abès R, Gélizé E, Fridman WH., and, Teillaud JL. Long-lasting antitumor protection by anti-CD20 antibody through cellular immune response. Blood. 2010;116:926–934. doi: 10.1182/blood-2009-10-248609. [DOI] [PubMed] [Google Scholar]
- Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG.et al. (2001Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma J Clin Oncol 193635–3648. [DOI] [PubMed] [Google Scholar]
- Vidal L, Gafter-Gvili A, Salles G, Dreyling MH, Ghielmini M, Hsu Schmitz SF.et al. (2011Rituximab maintenance for the treatment of patients with follicular lymphoma: an updated systematic review and meta-analysis of randomized trials J Natl Cancer Inst 1031799–1806. [DOI] [PubMed] [Google Scholar]
- Robertson D, Savage K, Reis-Filho JS., and, Isacke CM. Multiple immunofluorescence labelling of formalin-fixed paraffin-embedded (FFPE) tissue. BMC Cell Biol. 2008;9:13. doi: 10.1186/1471-2121-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]