Abstract
Radiotherapy offers an effective treatment for advanced cancer but local and distant failures remain a significant challenge. Here, we treated melanoma and pancreatic carcinoma in syngeneic mice with ionizing radiation (IR) combined with the poly(ADP-ribose) polymerase inhibitor (PARPi) veliparib to inhibit DNA repair and promote accelerated senescence. Based on prior work implicating cytotoxic T lymphocytes (CTLs) as key mediators of radiation effects, we discovered that senescent tumor cells induced by radiation and veliparib express immunostimulatory cytokines to activate CTLs that mediate an effective antitumor response. When these senescent tumor cells were injected into tumor-bearing mice, an antitumor CTL response was induced which potentiated the effects of radiation, resulting in elimination of established tumors. Applied to human cancers, radiation-inducible immunotherapy may enhance radiotherapy responses to prevent local recurrence and distant metastasis.
Introduction
Patients with advanced cancer obtain significant benefit from radiotherapy but failure is frequent. Ongoing advances in radiation delivery and chemical radiosensitizers have improved local control in some cancers but approaches to prevent and treat metastasis remain elusive. Recent studies suggest an active host role in mediating the success of radiotherapy. Irradiated cells present danger signals,1,2 an altered antigenic peptide repertoire,3 enhanced major histocompatibility complex (MHC) class I expression,3 and actively secrete cytokines4 that attract dendritic antigen-presenting cells (DC) and stimulate CD8+ cytotoxic T cells (CTL) toward an antitumor immune response.1,5,6,7 Nonetheless, the challenge remains to maximize the potential for radiation to reliably induce a sustained antitumor immune response as a mechanism to prevent local relapse and/or reduce metastasis.
Poly(ADP-ribose) polymerase inhibitors (PARPi) are an emerging class of targeted agents that enhance radiation effects in vitro and in vivo by blocking DNA repair mechanisms.8,9 PARPi also have applications as immunomodulators in the treatment of inflammatory diseases. Recently, we linked the efficacy of PARP inhibition in combination with ionizing radiation (IR) to the persistence of DNA damage and the induction of accelerated senescence in tumor cells.10,11 Also called therapy induced senescence, accelerated senescence may be a critical determinant of success in cancer treatment.12,13,14 Recent attention has focused on links between the DNA damage response and the proinflammatory senescence-associated secretory phenotype (SASP) as a driver of tumor growth and metastasis.15,16,17 A requirement for PARP activity in the expression of the prometastatic SASP has been described.18
Here we report that the PARPi veliparib radiosensitizes tumor models through the induction of senescence characterized by a modified immunostimulatory SASP and activation of an antitumor adaptive immune response. Inoculation of senescent B16SIY melanoma tumor cells prevented formation of new tumors at distant sites and dramatically sensitized established tumors to IR. This work suggests a route to enhancing the benefits of radiotherapy, whereby driving cells toward senescence directs the immune response to target the tumor.
Results
Induction of senescence and inhibition of tumor growth by veliparib and radiation
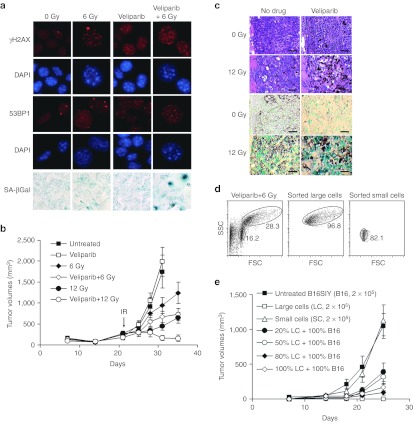
Our prior work combining PARPi with IR10,11 was limited to the analysis of human tumor cell lines in vitro and to xenograft tumors growing in immunodeficient athymic nude mice. To examine the influence of the adaptive immune system, we used the mouse melanoma tumor cell line B16SIY6,19,20 that grows rapidly after implantation into syngeneic C57BL/6 mice to form radiation-resistant tumors. As with human cell lines, treating B16SIY cells with IR and the PARPi veliparib induced persistence of γH2AX and 53BP1 foci at 24 hours and accelerated senescence at day 7, characterized by flattened cell morphology and enhanced senescence-associated β-galactosidase staining (SA-βGal, Figure 1a). While 6 or 12 Gy slowed tumor regrowth, combining IR with veliparib, 25 mg/kg twice daily for 2 days before IR and for 7 days thereafter (veliparib+IR), significantly delayed tumor regrowth (Figure 1b). Numerous enlarged senescent cells displaying intense SA-βGal staining were observed in veliparib+IR treated tumors compared to tumors treated with veliparib alone or IR alone (Figure 1c). These data suggested the hypothesis that senescent, growth-arrested B16SIY cells suppress tumor growth by affecting the proliferation or survival of non-senescent tumor cells. As a direct test, B16SIY cells were treated in vitro with veliparib+IR, incubated for 5 days, and surviving cells were sorted by size and granularity to obtain populations of large senescent cells and small non-senescent cells (Figure 1d). When injected into mice, small non-senescent cells formed tumors while large senescent cells did not (Figure 1e). Co-injection of untreated B16SIY cells with increasing numbers of large sorted senescent B16SIY cells progressively delayed tumor growth, suggesting direct suppression of tumor cell proliferation or survival.21
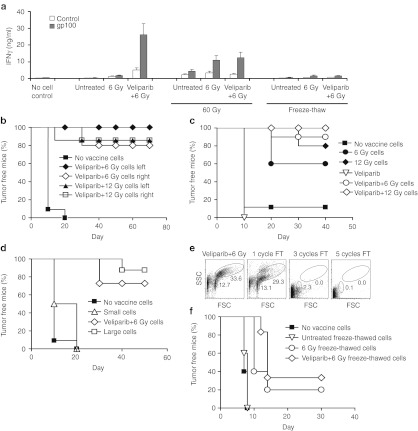
Figure 1.
PARP inhibition combined with irradiation delays tumor growth through accelerated tumor cell senescence. (a) Enhanced DNA-damaged foci persistence and senescence of B16SIY melanoma cells treated with veliparib+IR. Immunofluorescence reveals persistence of γH2AX and 53BP1 at IR-induced foci in cells 24 hours after treatment with veliparib ± 6 Gy. Nuclei indicated by blue DAPI staining. Cellular senescence was evaluated by SA-βGal staining at day 7. (b) Veliparib combined with IR delays B16SIY melanoma tumor growth compared to IR or veliparib alone (6 Gy, P = 0.033; 12 Gy, P = 0.004; n = 5–25 per group). (c) Veliparib combined with IR induces senescence in established B16SIY melanoma tumors. At day 7 after treatment, tumor tissue staining with H&E (upper panels) reveals numerous enlarged cells and intense SA-βGal staining (lower panels). Bar, 50 µm. (d) Enrichment of large senescent cells and small non-senescent cells from veliparib+IR treated B16SIY cells by flow sorting according to cell size and granularity. (e) Co-injection of untreated cells with increasing fractions of flow-sorted large senescent cells progressively inhibits the growth of B16SIY tumors (n = 5–10 per group). DAPI, 4′,6-diamidino-2-phenylindole; FSC, forward scatter; H&E, hematoxylin and eosin; IR, ionizing radiation; PARP, poly(ADP-ribose) polymerase; SA-βGal; senescence-associated β-galactosidase staining; SSC, side scatter.
An altered SASP after veliparib+IR
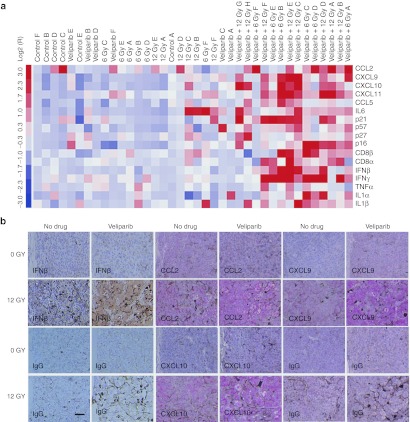
Senescent cells can affect non-senescent tumor cells and host cells via paracrine activity of the SASP.15,17 To examine the effect of veliparib+IR on the expression of cytokines and senescence markers, reverse transcription-quantitative PCR (RT-qPCR) was performed on tumor samples 7 days after treatment with veliparib and/or 0, 6 or 12 Gy (Figure 2a). Cluster analysis of gene expression confirmed that veliparib+IR-treated tumors displayed increased expression of senescence markers p21 and p16 compared to IR-treated tumors. Similarly, veliparib+IR treatment significantly increased the expression of SASP genes including interferon-β (IFN-β), CCL2, CCL5, CXCL9, CXCL10, and CXCL11, which correlated with elevation of CD8+ lymphocyte antigen and IFN-γ expression. Immunohistochemical staining for IFN-β, CCL2, CXCL9, and CXCL10 revealed that expression was localized to large senescent tumor cells (Figure 2b). Overall, these data suggested that veliparib+IR treatment shifts tumor cells toward senescence, although characterized by an altered, immunostimulatory SASP.
Figure 2.
PARP inhibition modifies immunoregulatory cytokine components in irradiated B16SIY tumors. (a) Cluster analysis of gene expression by qRT-PCR in tumor samples. Veliparib+IR treated tumors displayed increased expression of the senescence marker p21 (P = 0.024) and p16 (P = 0.021) and SASP components including IFN-β (P = 0.023), IFN-γ (P = 0.070), CCL2 (P = 0.029), CCL5, CXCL9, CXCL10, and CXCL11 (P = 0.004) compared to IR alone. (b) Immunohistochemistry reveals intense staining of IFN-β (brown), CXCL9 (red), CXCL10 (red), and CCL2 (red) in large senescent cells in tumors treated with veliparib+IR. The brown and black dots which appear in the control panels (IgG) are characteristic of melanin deposition. Representative images from tumors harvested from five mice. Bar, 50 µm. IFN, interferon; IR, ionizing radiation; PARP, poly(ADP-ribose) polymerase; qRT-PCR, quantitative reverse transcription-PCR; SASP, senescence-associated secretory phenotype.
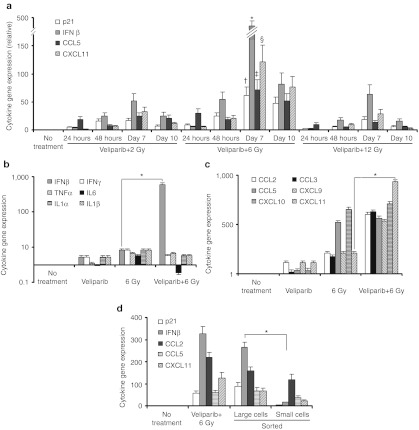
To distinguish the contribution of B16SIY cells to the elevated expression of immunoregulatory cytokines/chemokines and senescence markers in tumor samples, we cultured B16SIY cells in vitro, treated them with veliparib and/or IR and performed RT-qPCR analysis to examine expression of previously described components of the SASP.15,22,23 This analysis revealed time- and dose-dependent changes in the expression of multiple immunoregulatory cytokines in B16SIY cells treated with veliparib+IR compared to cells treated with IR or veliparib alone (Figure 3a–c). The most dramatic difference was observed 7 days after treatment with veliparib+6 Gy, when the cells also displayed the highest expression of the senescence marker p21 (Figure 3a), characteristic senescent morphology and positive SA-βGal staining (e.g., Figure 1a). The altered SASP exhibited increased transcription of IFN-β and decreased expression of interleukin-6 (IL-6) (Figure 3b). We detected upregulation of multiple chemokines that attract monocytes and DCs, natural killer cells (NKs) and CTLs, with CXCL11 being significantly upregulated (P = 0.006, Figure 3c). These data suggest a general effect of veliparib+IR on the SASP of senescent tumor cells. To confirm that the senescent cells contribute to antitumor immunity via the induction of immunoregulatory cytokine/chemokine expression, RT-qPCR analysis of large senescent cells versus small non-senescent cells was performed on size-sorted populations of veliparib+IR treated B16SIY cells. Sorted large senescent cells expressed higher levels of IFN-β, CCL5, CXCL11 and the senescence marker p21 compared to small non-senescent cells (Figure 3d).
Figure 3.
Veliparib modifies the SASP in irradiated B16SIY tumor cells. (a) Kinetics of IFN-β, CCL5, and CXCL11 expression in B16SIY tumor cells treated with veliparib+IR correlated with the expression of the senescence marker p21. At day 7, once cells had entered senescence, gene expression of p21 (†P = 0.016), IFN-β (*P = 0.011), CCL5 (‡P = 0.037), and CXCL11 (§P = 0.012) was significantly upregulated compared to 24 hours after IR. (b,c) Elevated expression of IFN-β (*P = 0.005) and chemokines such as CXCL11 (*P = 0.006) and decreased expression of IL-6 (P = 0.010) were observed in B16SIY tumor cells treated by veliparib+IR compared to IR alone. (d) Expression of cytokines in large senescent B16SIY cells versus small non-senescent cells sorted at day 7 following veliparib+IR treatment. A significant increase in IFN-β (*P = 0.015) was observed. IFN, interferon; IL, interleukin; IR, ionizing radiation; SASP, senescence-associated secretory phenotype.
Activated immune response to senescent tumor cells
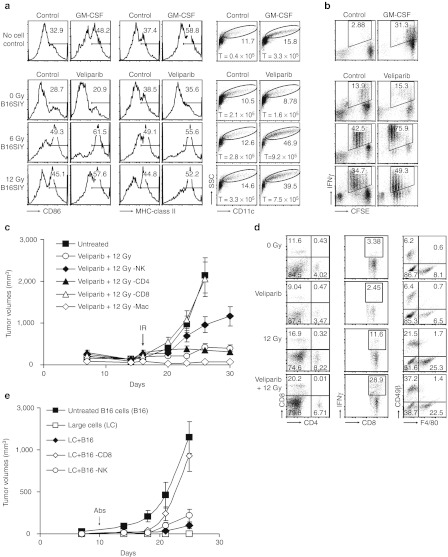
To detect if senescent cells activate antigen-presenting cells to stimulate CD8+ T cells, immature bone marrow dendritic cells (BMDCs) were cocultured with B16SIY cells after treatment with veliparib and/or 0, 6 or 12 Gy. Flow cytometry revealed an increase in CD11c+ cell proliferation, survival and maturation upon coculture with B16SIY cells treated with veliparib+6 Gy compared to coculture with B16SIY cells treated with 6 Gy alone or with granulocyte-macrophage colony-stimulating factor (control). BMDCs cocultured with B16SIY cells treated with veliparib+IR also yielded higher MHC-II+ and CD86+ fractions (Figure 4a) and enhanced CD8+ T cell proliferation, resulting in increased IFN-γ expression (Figure 4b).
Figure 4.
Veliparib+IR induces a CD8+ T cell-dependent antitumor response. (a) Coculture of BMDC with B16SIY cells pretreated with veliparib+IR (senescent cells) promoted DC proliferation, survival and maturation, demonstrated by an increase in large cells which express MHC-II and CD86 on CD11c+ cells; 1 × 105 immature BMDCs were placed in coculture. Five days later, the total (T) number of BMDC in the upper chamber was evaluated. (b) CD8+ T cell proliferation and IFN-γ production were stimulated by coculture with BMDCs previously cultured with senescent B16SIY cells induced by veliparib+IR treatment. (c) CD8+ T cells are required for tumor growth delay (P = 0.003), while NK cells may also contribute to antitumor effects (P = 0.009, n = 5–15 per group) of veliparib+IR. (d) Flow cytometry analysis of tumor-infiltrating cells in tumors treated with veliparib ± 12 Gy. Accumulation of CD4+ T cells, IFN-γ expressing CD8+ T cells, NK cells, and macrophages were analyzed. (e) CD8+ T cell-dependent inhibition of tumor growth by sorted large senescent cells. Veliparib+IR treated B16SIY cells were sorted, mixed with 2 × 105 untreated B16SIY cells at a ratio of 4:5 and injected subcutaneously into mice. CD8+ T cell or NK cell depletion was initiated at day 10. Tumor volume was determined. Abs, antibodies; BMDC, bone marrow dendritic cells; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IR, ionizing radiation; MHC, major histocompatibility complex; NK, natural killer cell; SSC, side scatter.
Antigen-specific CD8+ CTLs, which contribute to the antitumor effects of IR,6,20 might also be targets of the immunostimulatory SASP. Innate immune cells have been shown to participate in clearing senescent cells from tissue.24 Thus, we depleted CD4+ T cells, CD8+ T cells, NK cells or macrophages from tumor-bearing mice before treatment with veliparib+12 Gy. Strikingly, the benefits of veliparib+IR appeared to be CD8+ T cell dependent (P = 0.003, Figure 4c) and partly driven by NK cells (P = 0.009). CD4+ T cells had little effect (P = 0.257) while macrophages appeared to antagonize the effects of veliparib+IR, consistent with their effects on response to IR alone.19,24,25 Correspondingly, the proportion of IFN-γ–producing CD8+ T cells among tumor-infiltrating lymphocytes was increased by veliparib+IR (29%) compared to IR alone (12%). NK cells were also more abundant in veliparib+IR treated tumors compared to IR-treated tumors (37% versus 22%, Figure 4d).
To further characterize the role of CD8+ T cells in senescent tumor cell-mediated antitumor immunity, we compared the effect of early and late depletion of CD8+ T cells on tumor growth and senescence induction in B16SIY tumor-bearing mice beginning 1 day before (early depletion) or 7 days after (late depletion) in mice treated with veliparib+12 Gy. As in early depletion that eliminated tumor regrowth delay induced by veliparib+IR treatment, late depletion attenuated the suppression of regrowth (Supplementary Figure S1a). We analyzed the effect of CD8+ T cell depletion on SA-βGal staining in tumor tissues. Tumors were excised at the indicated times and stained for SA-βGal. Histopathology revealed that early depletion of CD8+ T cells was associated with the loss of SA-βGal stained cells while late depletion partly reversed the senescent histology (Supplementary Figure S1b). Among the likely explanations for these observations, IFN-γ–producing CD8+ T cells may (i) enhance or reinforce senescence in veliparib+IR treated tumor cells, or (ii) inhibit tumor growth directly by targeting remaining, non-senescent tumor cells.
Thus, we sought to test whether the antitumor effects of large senescent cells are mediated by induction of innate and/or adaptive immunity to inhibit growth of non-senescent tumor cells. As before, we inoculated untreated control B16SIY cells with or without large senescent cells sorted from a veliparib+IR treated population. However, 10 days after inoculation, NK or CD8+ T cells were depleted from the mice. While NK depletion had minimal effects, loss of CD8+ T cells, even at this late time point, completely abrogated the tumor-suppressive effect of the large senescent cells (Figure 4e).
Senescent cell vaccination blocks tumor formation
Based on these results, we investigated the potential of senescent B16SIY cells to serve as a vaccine. Suggesting a strong immune response, T cells isolated from draining lymph nodes (DNLs) of mice inoculated with veliparib+IR treated B16SIY cells displayed markedly increased IFN-γ secretion upon stimulation with B16 tumor antigen gp100 (Figure 5a). To test if CTL response generated by veliparib+IR-induced senescent cells may be sufficient to prevent tumor formation, we treated B16SIY cells with veliparib+IR (6 or 12 Gy), incubated the cells for 7 days to induce senescence, and then implanted 5 × 105 cells on the right leg of C56BL/6 mice. Seven days later, untreated B16SIY cells were injected on both legs as a challenge. All control mice developed tumors on both legs within 2 weeks but more than 80% of mice inoculated with senescent cells failed to grow tumors on either leg (Figure 5b). By comparison, cells treated with 6 or 12 Gy alone displayed only partial inhibition, while veliparib treatment alone was ineffective (Figure 5c). To examine the population of cells in the inoculum, we flow-sorted veliparib+IR-treated B16SIY cells into large (senescent) and small (non-senescent) cells. Mice were injected with sorted, unsorted or untreated cells and 7 days later challenged with untreated cells (Figure 5d). An antitumor vaccine effect was observed only when senescent tumor cells were injected, with the greatest activity being observed with sorted large senescent cells. Cycles of freezing and thawing of veliparib+IR-treated B16SIY cells led to progressive depletion of the large senescent cell fraction as determined by flow cytometry (Figure 5e). Freeze-thaw treatment also lowered the ability of injected cells to stimulate gp100-inducible IFN-γ secretion in the DNL (Figure 5a) or prevent tumor formation (Figure 5f), suggesting intact cells are required for senescent cells to serve as an effective vaccine.
Figure 5.
PARP inhibition enhances the antitumor effects of irradiated tumor cells. (a) Inoculation of viable senescent B16SIY cells induced the greatest expression of IFN-γ in DNL when restimulated with tumor antigen gp100 in vitro as measured by ELISA (n = 6). (b) Injection of veliparib+IR treated senescent cells prevented the outgrowth of tumors after subsequent injection of untreated cells, suggesting systemic immune activation by senescent cells (n = 15–30). (c) Veliparib+IR treated cells prevented tumor formation more effectively than cells treated with 6 or 12 Gy alone. (d) Injection of flow-sorted cells revealed the antitumor effect was specific to large senescent cells (n = 10). (e) Repeated freeze-thawing reduced cell viability, with 3–5 cycles resulting in complete cellular destruction. (f) Freeze-thaw treatment markedly decreased the ability of irradiated or veliparib+IR treated cells to prevent tumor outgrowth, indicating a requirement for cell viability and/or integrity. ELISA, enzyme-linked immunosorbent assay; DNL, draining lymph node; FSC, forward scatter; IFN, interferon; IR, ionizing radiation; PARP, poly(ADP-ribose) polymerase; SSC, side scatter.
To determine if the vaccine effect of senescent cells might be specific to the B16SIY melanoma model, we examined two other murine tumors, P1048 pancreatic adenocarcinoma that over-expresses endogenous mouse Her2 and TUBO breast adenocarcinoma that over-expresses rat Her2/neu as a tumor antigen. Treating P1048 cells with veliparib+6 Gy induced senescence with an altered SASP (e.g., Supplementary Figure S2a,b), while TUBO cells displayed neither senescent morphology nor SA-βGal expression (data not shown). When injected into mice, veliparib+IR treated P1048 cells induced Her2-specific IFN-γ–producing T cells in the DNL (data not shown) and blocked tumor growth upon rechallenge with untreated P1048 cells (Supplementary Figure S3a). However, injection of veliparib+IR-treated TUBO cells failed to induce Her2-specific IFN-γ producing T cells (data not shown) and did not block tumor formation (Supplementary Figure S3b).
Enhancement of radiation-mediated antitumor effects by senescent cells
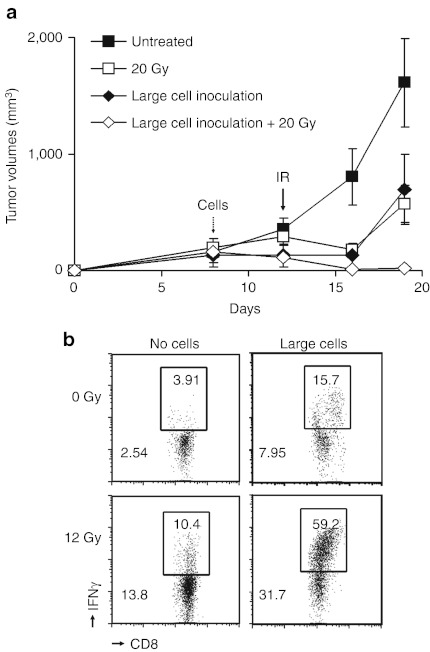
Taken together, our results raised the hypothesis that senescent cells induced by veliparib+IR might be able to stimulate CD8+ T cells to target established tumors. Thus, to establish tumors, untreated B16SIY cells were injected into the right leg. On day 7, sorted large senescent cells or buffer was injected in the left leg. On day 12, tumors were treated with 0 or 20 Gy. Treatment with IR alone or senescent cells alone significantly delayed tumor outgrowth (P = 0.016, P = 0.038), while their combination almost entirely eradicated the primary tumors (P = 0.003, Figure 6a). Importantly, analysis of tumor-infiltrating lymphocytes revealed a marked increase in the fraction of IFN-γ–producing CD8+ T cells when tumors were treated by injection of senescent cells followed by 20 Gy versus treatment with senescent cells alone or IR alone (Figure 6b). Considered together, these results demonstrate proof-of-principle for radiation-inducible immunotherapy, wherein senescent cells induce IFN-γ–producing CD8+ T cells that can be targeted to the tumor by irradiation.
Figure 6.
Senescent cells block tumor formation and sensitize tumors to irradiation. (a) Mice bearing B16SIY tumors on the right leg were inoculated with flow-sorted large senescent cells on the left leg. Significant tumor growth delay was observed in mice injected with senescent cells compared to untreated mice (P = 0.038), similar to treatment with 20 Gy alone (P = 0.016). However, inoculation with senescent cells followed by 20 Gy almost completely eliminated tumor growth (P = 0.003, n = 5 per group). (b) Combining senescent cell inoculation with irradiation markedly enhanced IFN-γ–expressing tumor-infiltrating CD8+ T cells compared to treatment with senescent cells alone or IR alone, consistent with an antitumor adaptive immune response mediated by radiation-inducible immunotherapy. IFN, interferon; IR, ionizing radiation.
Discussion
In prior work,10,11 we reported that the PARPi veliparib prolongs DNA damage persistence and promotes onset of senescence after irradiation. Here, we observed that senescent cells induced by irradiation in the presence of the PARPi veliparib display an altered SASP with significantly increased levels of IFN-β. Senescent cells express a characteristic secretome that is considered to be a major determinant of their role within normal and tumor tissue.15,17,22,26 The SASP has been considered alternatively beneficial, through a role in reinforcing senescence and blocking emergence of malignant clones, or deleterious, by promoting chronic inflammation, neoplastic progression, or both.15,17,18,26,27 PARP serves critical roles both in the DNA damage response and in inflammatory signaling.18,28 Veliparib may exert dual effects by promoting cellular senescence and altering the SASP following irradiation. Indeed, secretion of a prometastatic SASP by senescent melanoma cells was shown to depend on PARP activity.18 In summary veliparib is not necessary to form senescent cells or to create the vaccine effect. However, veliparib appears to markedly enhance the formation of senescent cells and may enhance the efficacy of irradiated cells as a vaccine, potentially via an effect on secreted factors.
A role for the innate immune system in the elimination of senescent cells from tumors upon re-expression of p53 has been reported.24,29 In the context of the adaptive immune system, CD4+ T cells mediate antitumor effects by inducing senescence in MYC-activated tumor cells.25 Here, using B16SIY murine melanoma tumors in syngeneic mice, we found that senescent cells formed by irradiation in the presence of veliparib stimulate a robust antitumor response mediated by the activation and infiltration of a CD8+ T cells and NK cells. By inducing B16SIY cell senescence and an altered SASP, veliparib+IR promoted DC proliferation, maturation, and function, which led to the activation of tumor-specific IFN-γ–expressing CD8+ T cells. Validating their critical role, both DCs and CD8+ T cells have been implicated as key mediators of the radiation response and determinants of immunogenic tumor regression.1,5,6,20,30 We observed that the block to tumor regrowth after irradiation of B16SIY tumors and the ability of flow-sorted senescent B16SIY cells to prevent tumor formation each require the presence of CD8+ T cells. These results suggest that senescent cells can efficiently stimulate an antitumor cytotoxic T-cell response. Previously, cell-based cancer vaccines have been prepared by lethal irradiation, freeze-thawing, and other approaches.31,32,33 Irradiated tumor cells effectively prime DC-mediated immunity, while freeze-thaw–disrupted tumor cells appear to inhibit CTL activity in vitro31,32. These reports are consistent with our model that viable senescent cells represent the critical component responsible for antitumor effects.
We infer that our observations with murine tumors may have direct relevance to the treatment of human cancers. Of immediate significance, we propose that some of the beneficial effects of the experimental PARPi such as olaparib, veliparib, and iniparib observed in ongoing clinical trials34 and in combination with chemotherapy or radiation may depend, in part, on the accumulation of senescent tumor cells to activate host antitumor responses. More broadly, this work may predict success for a new approach to tumor vaccines, wherein patients might be inoculated with senescent cells derived from their tumors and then treated with radiotherapy to target an antitumor immune response to the primary tumor and/or to gross metastases.35 By enhancing the potential of radiotherapy to activate antitumor CTLs, this strategy may not only improve local control but also suppress the emergence of metastases. Successful translation of this radiation-inducible senescence-mediated immunotherapy to the clinic would signal a paradigm shift, extending the value of radiotherapy beyond local tumor control and toward a role in the elimination of systemic disease.
Materials and Methods
Syngeneic tumors and experimental therapy. The care and treatment of experimental animals was conducted in accordance with institutional guidelines. Tumor cell inoculation, various treatments, and analysis of T-cell recruitment were conducted as previously described.19,20 Mouse melanoma cell line B16SIY,36 a generous gift of Thomas F Gajewski, expressing the SIY epitope SIYRYYGL as a fusion to enhanced green fluorescent protein (EGFP)37 was maintained in complete medium supplemented with 10% fetal bovine serum. To induce tumors, 5 × 105 cells were injected subcutaneously on the leg of 6- to 8-week-old C57BL/6 mice (Harlan, Madison, WI). Tumors were allowed to grow to a volume of 100–150 mm3 (∼2 weeks) before treatment with X-ray irradiation using a Phillips orthovoltage X-ray generator operating at 250 kV/15 mA and/or with 25 mg/kg veliparib ((R)-2-(2-methylpyrrolidin-2-yl)-1H-benzo[d]imidazole-4-carboxamide, ABT-888; ChemieTek, Indianapolis, IN) dissolved in water and administered twice daily by oral gavage.10 CD8+ T cells, CD4+ T cells, or NK cells were depleted by injection of 100 µg/kg of anti-CD8 (clone 2.43.1), anti-CD4 (clone GK1.5) or anti-NK1.1 (clone PK136, Fitch Monoclonal Antibody Facility, University of Chicago), respectively, 1 day before IR. Depletion was confirmed by flow cytometry of peripheral blood. Mice were treated with liposomal clodronate beginning 1 day before irradiation as described.19 Depletion of macrophages was confirmed by flow cytometry of splenocytes and tumor samples collected 2–7 days after IR. Flow cytometry was performed on a BD LSRII, gating forward and side scatter to examine CD4+ and CD8+ T cells versus NK cells or macrophages. Percentage of each cell type was determined using fluorescence-labeled antibodies purchased from BD Pharmingen (San Diego, CA) for CD4+, CD8+, and NK cell markers respectively.
Immunofluorescence. Immunofluorescence was performed as previously described.11,12 Antibodies used were rabbit anti-phospho-H2AX Ser139 diluted 1:500 (γH2AX; Cell Signaling Technology, Beverly, MA) and rabbit anti-53BP1 diluted 1:500 (Novus Biologicals, Littleton, CO), detected by Texas Red anti-rabbit IgG diluted 1:1,000 (Vector Laboratories, Burlingame, CA). Images were captured on a Zeiss Axiovert 200M controlled by OpenLab software.
Analysis of cells and tissues. Histopathology, immunohistochemistry, and real-time RT-qPCR were performed as previously described.19,20 Tumors were harvested 7 days post-treatment and formalin-fixed or snap-frozen for analysis. SA-βGal assays were performed using the Senescence β-galactosidase Staining Kit (Cell Signaling Technology) as described.10 Formalin-fixed paraffin-embedded tissues were sectioned, deparaffinized in xylene, rehydrated with ethanol, treated with 0.3% H2O2/methanol to quench endogenous peroxidase activity and then treated in ethylenediaminetetraacetic acid (EDTA) buffer to unmask antigens. Slides were incubated with biotinylated polyclonal goat anti-mouse CCL2, CCL5, CXCL9, CXCL10 or IFN-β antibodies (R&D Systems, Minneapolis, MN) at a 1:5 dilution. Immunoreactivity was detected with avidin conjugates to polymerized horseradish peroxidase or alkaline phosphatase (Vector Laboratories) and 3,3′-diaminobenzidine or Vulcan Red as chromogenic substrates, respectively. Slides were counterstained with hematoxylin.19 Negative controls used isotype-matched primary antibodies. Micrographs were captured on an Axiovert 200M microscope (Zeiss, Thornwood, NY) using an Axiocam color digital camera using OpenLab software. RT-qPCR was performed with primers shown in Supplementary Table S1 as previously described and analyzed by unsupervised clustering using dChip.20
Senescent cell preparation and assay of tumor resistance and sensitization. To evaluate tumor formation by senescent cells, 5 × 105 B16SIY cells treated with veliparib±IR and incubated for 5 days were injected subcutaneously on the right leg. Alternatively, B16SIY cells were treated with veliparib+6 Gy in vitro and incubated for 5 days. Cells were flow sorted into populations of large (enriched for senescent) or small (enriched for non-senescent) cells according to size and granularity.38 Flow-sorted cells were injected directly into mice or mixed with untreated B16SIY cells before injection. Tumor incidence and growth were evaluated and measured. In the tumor prevention vaccine model, 5 days after injection of 5 × 105 treated, flow sorted or control cells, the mice were challenged by injection of 5 × 105 untreated B16SIY cells and tumor growth was measured.35 In the therapeutic vaccine setting, 7 days after injection of 5 × 105 untreated B16SIY cells on the right leg, 5 × 105 treated, flow sorted or untreated B16SIY cells were injected on the left leg. Five days later, mice were treated with 0 or 20 Gy to the right leg only and tumor growth was measured. To evaluate immunogenicity, B16SIY cells treated with veliparib+6 Gy, 6 Gy alone or untreated were inoculated in the foot pad of C57BL/6 mice, DNL were collected, digested into single cell suspension and cultured in the presence of the tumor specific antigen gp100 to analyze antigen-specific immune response. Three to five days later, supernatants were analyzed for IFN-γ secretion by ELISA (R&D Systems).20 Then, the same cells, treated with veliparib+6 Gy, 6 Gy alone or untreated, were exposed to 60 Gy or subjected to 5 cycles of freeze-thaw before injection into the foot pad.
Generation of BMDC, selection of CD8+ T cells and coculture with veliparib+IR, IR or untreated tumors cells in a transwell system. BMDC isolation and coculture with tumor cells were performed as described previously.19,20 Briefly, B16SIY cells maintained in complete RPMI medium supplemented with 10% fetal calf serum were treated with 10 µm veliparib for 1 hour before irradiation with 6 or 12 Gy (Gammacell 1000; MDS Nordion, Ottawa, Ontario, Canada), incubated for 4 days, and then cocultured in Transwell plates (Corning Life Science, Lowell, MA) with immature BMDCs. BMDCs were isolated from the bone marrow of C57BL/6 mice and incubated for 5 days with granulocyte-macrophage colony-stimulating factor. Three days later, BMDCs were analyzed by flow cytometry using fluorescently labeled antibodies for maturation markers and intracellular cytokines (BD Pharmingen). Splenocytes were harvested from C57BL/6 mice 7–10 days after inoculation with B16SIY cells, and CD8+ T cells were positively selected by magnetic beads using CD8 MicroBeads (Miltenyi Biotec, Boston, MA). CD8+ T cell purity was routinely >95% as analyzed by flow cytometry. Selected CD8+ T cells were labeled with CFSE and cocultured with BMDC generated/matured after coculture with veliparib+IR treated B16SIY cells, IR-treated cells or untreated cells. After 4–5 days of coculture, floating CD8+ T cells were collected. Following fixation and permeabilization, anti-IFNγ (BD Pharmingen) was added to the cells. With gating on CD8+ T cells and using CFSE dilution, cell proliferation and intracellular IFNγ expression were analyzed.
Statistical analysis. One-way analysis of variance with Dunnett's post-test was performed using GraphPad InStat, version 3.05 (GraphPad Software, La Jolla, CA) to compare differences between mean tumor volumes of different experimental groups. Two-tailed Student's t-test were performed and significance determined. P < 0.05 was accepted for significance. Error bars represent ±SD.
SUPPLEMENTARY MATERIAL Figure S1. Effect of CD8+ T cells on tumor regrowth and induction of tumor cellular senescence. Figure S2. Induction of senescence and cytokines in pancreatic cancer p1048 cell line. Figure S3. Vaccine effect of veliparib+IR treated cells in pancreatic cancer and breast cancer models. Table S1. Primer sequences used for quantitative RT-PCR.
Acknowledgments
The authors acknowledge Michael T Spiotto and Nikolai N Khodarev for helpful scientific discussion and thank Juan C Barreto-Andrade, Hua Liang and Michael A Beckett for generous help and support. This research was funded by the Ludwig Center for Metastasis Research (Y-X.F., S.J.K., and R.R.W.), NIH NCI grants CA138365 and CA164492 (S.J.K. and R.R.W.) and NIGMS grant GM60443 (S.J.K.), The Chicago Center for Radiation Therapy (R.R.W.), Grant Achatz and the Alinea Team (S.J.K. and R.R.W.) and a generous gift from The Foglia Foundation and Mr and Mrs Vincent Foglia (Y-X.F., S.J.K., and R.R.W.). The University of Chicago has sought protection as intellectual property for use of senescent cells as cancer vaccines, with inventors Y.M., E.V.E., S.J.K., and R.R.W.
Supplementary Material
Effect of CD8+ T cells on tumor regrowth and induction of tumor cellular senescence.
Induction of senescence and cytokines in pancreatic cancer p1048 cell line.
Vaccine effect of veliparib+IR treated cells in pancreatic cancer and breast cancer models.
Primer sequences used for quantitative RT-PCR.
REFERENCES
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A.et al. (2007Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy Nat Med 131050–1059. [DOI] [PubMed] [Google Scholar]
- Ma Y, Kepp O, Ghiringhelli F, Apetoh L, Aymeric L, Locher C.et al. (2010Chemotherapy and radiotherapy: cryptic anticancer vaccines Semin Immunol 22113–124. [DOI] [PubMed] [Google Scholar]
- Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK.et al. (2006Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy J Exp Med 2031259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiao SL., and, Coussens LM. The tumor-immune microenvironment and response to radiation therapy. J Mammary Gland Biol Neoplasia. 2010;15:411–421. doi: 10.1007/s10911-010-9194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG., and, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol. 2008;180:3132–3139. doi: 10.4049/jimmunol.180.5.3132. [DOI] [PubMed] [Google Scholar]
- Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y.et al. (2009Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment Blood 114589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L.et al. (2004Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated Int J Radiat Oncol Biol Phys 58862–870. [DOI] [PubMed] [Google Scholar]
- Schreiber V, Dantzer F, Ame JC., and, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7:517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- Ashworth A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol. 2008;26:3785–3790. doi: 10.1200/JCO.2008.16.0812. [DOI] [PubMed] [Google Scholar]
- Efimova EV, Mauceri HJ, Golden DW, Labay E, Bindokas VP, Darga TE.et al. (2010Poly(ADP-ribose) polymerase inhibitor induces accelerated senescence in irradiated breast cancer cells and tumors Cancer Res 706277–6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto-Andrade JC, Efimova EV, Mauceri HJ, Beckett MA, Sutton HG, Darga TE.et al. (2011Response of human prostate cancer cells and tumors to combining PARP inhibition with ionizing radiation Mol Cancer Ther 101185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardella C, Clohessy JG, Alimonti A., and, Pandolfi PP. Pro-senescence therapy for cancer treatment. Nat Rev Cancer. 2011;11:503–511. doi: 10.1038/nrc3057. [DOI] [PubMed] [Google Scholar]
- Kahlem P, Dörken B., and, Schmitt CA. Cellular senescence in cancer treatment: friend or foe. J Clin Invest. 2004;113:169–174. doi: 10.1172/JCI20784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roninson IB. Tumor cell senescence in cancer treatment. Cancer Res. 2003;63:2705–2715. [PubMed] [Google Scholar]
- Rodier F, Coppé JP, Patil CK, Hoeijmakers WA, Muñoz DP, Raza SR.et al. (2009Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion Nat Cell Biol 11973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppé JP, Desprez PY, Krtolica A., and, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F., and, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohanna M, Giuliano S, Bonet C, Imbert V, Hofman V, Zangari J.et al. (2011Senescent cells develop a PARP-1 and nuclear factor-{kappa}B-associated secretome (PNAS) Genes Dev 251245–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Beckett MA, Liang H, Mauceri HJ, van Rooijen N, Cohen KS.et al. (2010Blockade of tumor necrosis factor alpha signaling in tumor-associated macrophages as a radiosensitizing strategy Cancer Res 701534–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Mauceri HJ, Khodarev NN, Darga TE, Pitroda SP, Beckett MA.et al. (2010Ad.Egr-TNF and local ionizing radiation suppress metastases by interferon-beta-dependent activation of antigen-specific CD8+ T cells Mol Ther 18912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue LY, Butler NJ, Makrigiorgos GM, Adelstein SJ., and, Kassis AI. Bystander effect produced by radiolabeled tumor cells in vivo. Proc Natl Acad Sci USA. 2002;99:13765–13770. doi: 10.1073/pnas.182209699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orjalo AV, Bhaumik D, Gengler BK, Scott GK., and, Campisi J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci USA. 2009;106:17031–17036. doi: 10.1073/pnas.0905299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakova Z, Hubackova S, Kosar M, Janderova-Rossmeislova L, Dobrovolna J, Vasicova P.et al. (2010Cytokine expression and signaling in drug-induced cellular senescence Oncogene 29273–284. [DOI] [PubMed] [Google Scholar]
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V.et al. (2007Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas Nature 445656–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhra K, Bachireddy P, Zabuawala T, Zeiser R, Xu L, Kopelman A.et al. (2010CD4(+) T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivation Cancer Cell 18485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J., and, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Evan GI., and, d'Adda di Fagagna F. Cellular senescence: hot or what. Curr Opin Genet Dev. 2009;19:25–31. doi: 10.1016/j.gde.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Aguilar-Quesada R, Muñoz-Gámez JA, Martín-Oliva D, Peralta-Leal A, Quiles-Pérez R, Rodríguez-Vargas JM.et al. (2007Modulation of transcription by PARP-1: consequences in carcinogenesis and inflammation Curr Med Chem 141179–1187. [DOI] [PubMed] [Google Scholar]
- Krizhanovsky V, Xue W, Zender L, Yon M, Hernando E., and, Lowe SW. Implications of cellular senescence in tissue damage response, tumor suppression, and stem cell biology. Cold Spring Harb Symp Quant Biol. 2008;73:513–522. doi: 10.1101/sqb.2008.73.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C.et al. (2009Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors Nat Med 151170–1178. [DOI] [PubMed] [Google Scholar]
- Strome SE, Voss S, Wilcox R, Wakefield TL, Tamada K, Flies D.et al. (2002Strategies for antigen loading of dendritic cells to enhance the antitumor immune response Cancer Res 621884–1889. [PubMed] [Google Scholar]
- Tirapu I, Lewis A, Kreutz M, McLinden H., and, Diebold SS. Freeze-and-thaw-disrupted tumour cells impair the responsiveness of DC to TLR stimulation. Eur J Immunol. 2008;38:2740–2750. doi: 10.1002/eji.200838284. [DOI] [PubMed] [Google Scholar]
- Schnurr M, Scholz C, Rothenfusser S, Galambos P, Dauer M, Röbe J.et al. (2002Apoptotic pancreatic tumor cells are superior to cell lysates in promoting cross-priming of cytotoxic T cells and activate NK and gammadelta T cells Cancer Res 622347–2352. [PubMed] [Google Scholar]
- Powell C, Mikropoulos C, Kaye SB, Nutting CM, Bhide SA, Newbold K.et al. (2010Pre-clinical and clinical evaluation of PARP inhibitors as tumour-specific radiosensitisers Cancer Treat Rev 36566–575. [DOI] [PubMed] [Google Scholar]
- Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K.et al. (1993Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity Proc Natl Acad Sci USA 903539–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T.et al. (2004PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells Cancer Res 641140–1145. [DOI] [PubMed] [Google Scholar]
- Spiotto MT, Yu P, Rowley DA, Nishimura MI, Meredith SC, Gajewski TF.et al. (2002Increasing tumor antigen expression overcomes “ignorance” to solid tumors via crosspresentation by bone marrow-derived stromal cells Immunity 17737–747. [DOI] [PubMed] [Google Scholar]
- Passos JF., and, von Zglinicki T. Methods for cell sorting of young and senescent cells. Methods Mol Biol. 2007;371:33–44. doi: 10.1007/978-1-59745-361-5_4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of CD8+ T cells on tumor regrowth and induction of tumor cellular senescence.
Induction of senescence and cytokines in pancreatic cancer p1048 cell line.
Vaccine effect of veliparib+IR treated cells in pancreatic cancer and breast cancer models.
Primer sequences used for quantitative RT-PCR.