Abstract
Interleukin-12 (IL-12) has potent antitumor activity, but its clinical application is limited by severe systemic toxicity, which might be alleviated by the use of membrane-anchored IL-12. In the present study, a new membrane-bound IL-12 containing murine single-chain IL-12 and B7-1 transmembrane and cytoplasmic domains (scIL-12-B7TM) was constructed and its efficacy in cancer treatment examined and its protective antitumor mechanism investigated. Surface expression of scIL-12-B7TM on colon adenocarcinoma cells significantly inhibited the growth of subcutaneous tumors, suppressed lung metastasis, and resulted in local and systemic suppression of unmodified tumors. Intratumoral injection of an adenoviral vector encoding scIL-12-B7TM not only resulted in complete regression of a majority of local tumors, but also significantly suppressed the growth of distant, untreated tumors. Moreover, mice that had been treated with scIL-12-B7TM developed memory responses against subsequent tumor challenge. Immunohistochemical staining and in vivo depletion of lymphocyte subpopulations demonstrated that both CD8+ T cells and CD4+ T cells contributed to the antitumor activity of scIL-12-B7TM. Importantly, the potent antitumor activities of scIL-12-B7TM were achieved with only negligible amounts of IL-12 in the circulation. Our data demonstrate that cancer immunotherapy using membrane-bound IL-12 has the advantage of minimizing systemic IL-12 levels without compromising its antitumor efficacy.
Introduction
Cytokine-based immunotherapy has been intensively investigated for the treatment of cancers. Among these cytokines, interleukin-12 (IL-12) is considered to be the most potent in triggering antitumor immune responses.1,2 IL-12 is a heterodimeric cytokine composed of a 35 kDa light chain and a 40 kDa heavy chain and is produced by activated phagocytic cells, B cells, and dendritic cells.3 IL-12 plays an important role in bridging innate and adaptive immune responses, is required for optimal differentiation of naive CD4 T cells into type 1 T helper cells, and promotes cell-mediated immunity.4
The potent antitumor activity of IL-12 has been demonstrated in many preclinical murine tumor models, including subcutaneous tumors, experimental metastasis, and spontaneous tumors.5,6,7 Mechanistic studies have shown that the generation of tumor-specific cytotoxic T lymphocytes and the enhancement of the cytotoxicity of natural killer (NK) cells and T cells play important roles in IL-12–mediated antitumor activity.8 IL-12 also stimulates T cells, NK cells, and natural killer T cells to produce interferon-γ (IFN-γ), which, through induction of IFN-γ inducible gene 10 and monokine-induced by IFN-γ, exert potent antiangiogenic activities.9,10,11 Clinical responses to IL-12 treatment have been reported in many types of tumors, such as cutaneous T cell lymphoma, non-Hodgkin's lymphoma, melanoma, renal cell carcinoma, and gastrointestinal carcinoma.7,12,13,14,15,16 However, systemic administration of IL-12 has been limited by its severe toxic effects.17,18 Alternative approaches, including local administration of recombinant IL-12,19 application of IL-12 gene-modified tumors, fibroblasts, or dendritic cells,20,21,22 or local injection of recombinant viruses expressing IL-12,23,24,25 have been explored to restrict IL-12 activity to the tumor site, with the goal of reducing systemic toxicity.
Secreted and intracellular proteins can be redirected to, and anchored on, the surface of mammalian cells. Local retention of bioactive immunomodulators has the potential to achieve superior immune responses without causing systemic toxicity.26 Several cytokines have been expressed on the surface of tumor cells as integral membrane proteins or as glycosylphosphatidylinositol (GPI)-anchored proteins by their conjugation, respectively, to a heterologous transmembrane domain sequence or GPI-anchored signal sequence.27 In general, these membrane-anchored cytokines, including IL-2, IL-4, IL-12, IL-21, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor-α, retain their biological activity and effectively stimulate immune cells and elicit protective antitumor immunity.28,29,30,31,32,33 As regards IL-12, the in vivo growth rate of tumors transduced with GPI-anchored IL-12 was significantly delayed and the selected tumor clones expressing GPI-IL-12 were completely eliminated.30 The antitumor effect of GPI-anchored IL-12 can be further enhanced by coexpression of GPI-anchored IL-2 on the same tumor cells.34 However, whether membrane-bound IL-12 can be used to treat more clinically relevant established tumors remains unexplored and the antitumor mechanisms of membrane-bound IL-12 have yet to be defined.
Previous studies have shown that the choice of a suitable transmembrane- or GPI-anchored sequence is extremely important in achieving high and stable expression of chimeric proteins on the plasma membrane, the highest level of surface expression being achieved when proteins were fused to the transmembrane domain and cytoplasmic tail of murine B7-1 (B7TM).35,36 The most commonly used GPI-anchored protein had been shown to be spontaneously released from the cell membrane due to shedding or proteolytic cleavage,37,38 raising concern about using a GPI anchor for applications that require stable surface expression. We therefore decided to use B7TM to modify mouse single-chain IL-12 (scIL-12-B7TM), and used two gene delivery systems, scIL-12-B7TM–transduced CT26 tumor cells and adenoviral vectors expressing scIL-12-B7TM, to explore the antitumor activity and protective antitumor mechanisms of this new membrane-bound form of IL-12.
Results
Construction and expression of scIL-12-B7TM
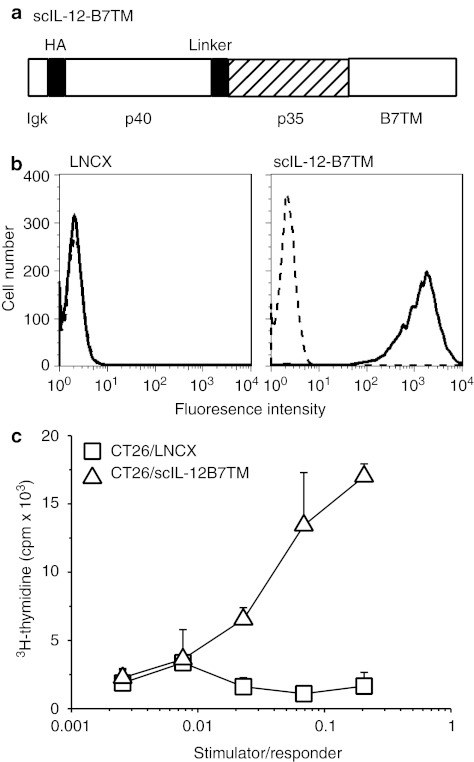
The complementary DNA coding for membrane-bound IL-12 was constructed by fusing the coding sequence of scIL-12 in the p40-35 orientation to murine B7TM,39 and subcloned into the retroviral vector pLNCX2. The resulting proviral construct pLNCX/scIL-12-B7TM encoded a protein containing the murine Igκ signal peptide, the influenza hemagglutinin (HA) epitope, murine scIL-12, and murine B7TM (Figure 1a). CT26 cells were infected with retroviral supernatant generated by transfecting pLNCX/scIL-12-B7TM into packaging cells and the selected G418-resistant cells were further enriched by fluorescence-activated cell sorting (FACS) using anti-HA monoclonal antibody (mAb) to select the 1% cells expressing the highest amounts of membrane IL-12, designated CT26/scIL-12-B7TM cells. IL-12 expression on the surface of CT26/scIL-12-B7TM cells was confirmed by binding of anti-mouse IL-12 p40/p70 mAb, using CT26/LNCX cells, which carry an empty LNCX vector,39 as the negative control (Figure 1b). To investigate the functional activity of scIL-12-B7TM, mitomycin C-treated CT26/scIL-12-B7TM or CT26/LNCX cells were cocultured with concanavalin A (Con A)-activated splenocytes in a proliferation assay. As shown in Figure 1c, the CT26/scIL-12-B7TM cells stimulated proliferation of Con A blasts in a dose-dependent manner, whereas the CT26/LNCX cells showed no activity.
Figure 1.
Construction, expression, and bioactivity of single-chain membrane-bound IL-12 (scIL-12-B7TM). (a) Schematic diagram of scIL- 12-B7TM, which is composed of the murine immunoglobulin κ-chain signal peptide, a 9 amino acid influenza virus HA epitope, the murine single-chain IL-12, and the murine B7-1 transmembrane domain with a cytosolic tail. The single-chain IL-12 was in the p40-p35 orientation, with the two subunits separated by a flexible 18 amino acid peptide linker. (b) CT26 cells were retrovirally transduced with LNCX (left panel) or scIL-12-B7TM (right panel). Stable CT26/scIL-12-B7TM cells were stained with anti-mouse IL-12 p40/p70 mAb (solid lines) or an isotype control mAb (dashed lines) and analyzed by flow cytometry. (c) Con A-activated lymphoblasts (responder), prepared as described in the Materials and Methods, were incubated for 60 hours with mitomycin-C treated CT26/scIL-12-B7TM or CT26/LNCX cells (stimulator) at the indicated stimulator to responder ratio and proliferation was assessed by [3H]-thymidine uptake during the last 18 hours of culture. The results are the mean ± SD for triplicate wells. Con A, concanavalin A; cpm, counts per minute; HA, hemagglutinin; IL, interleukin; mAb, monoclonal antibody.
Membrane-bound IL-12 reduces CT26 tumorigenicity
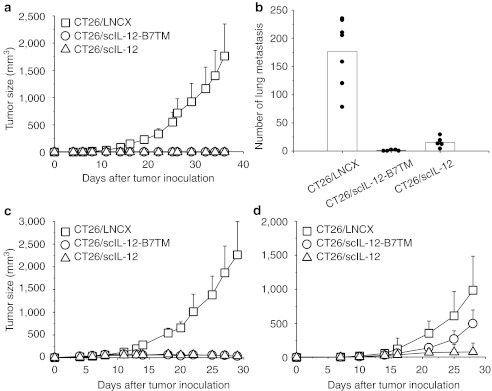
To investigate the antitumor effect of scIL-12-B7TM, groups of BALB/c mice (n = 10) were inoculated subcutaneously (s.c.) with 105 CT26/scIL-12-B7TM cells, while others received the same amount of CT26/LNCX cells (n = 10) as the negative control or CT26/scIL-12 cells (n = 10), which produce secreted scIL-12 and show potent antitumor activity as described in our previous study,39 as the positive control. Tumor growth in each group is shown in Figure 2a. CT26/scIL-12-B7TM and CT26/scIL-12 cells produced only palpable tumors at early time points after tumor implantation. All tumors were rejected by day 39 and all animals in these two groups remaining tumor-free for the entire 120-day observation period. By contrast, the tumors in the CT26/LNCX cell group grew progressively and all mice died or bore a large tumor and had to be euthanized by day 56.
Figure 2.
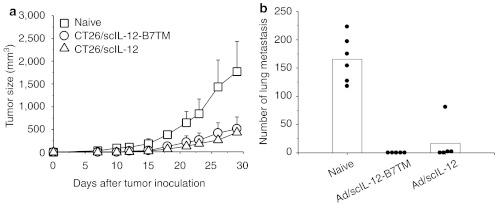
Antitumor activity of CT26/scIL-12-B7TM cells in BALB/c mice. (a) Groups of BALB/c mice (n = 10) were injected subcutaneously with 1 × 105 CT26/LNCX, CT26/scIL-12-B7TM, or CT26/scIL-12 cells and tumor growth monitored. (b) Groups of BALB/c mice (n = 5–7) were injected intravenously with 2 × 105 of the indicated tumors, then the animals were killed on day 16 and the number of lung metastases per mouse counted. (c) Groups of BALB/c mice (n = 5) were injected subcutaneously with 1 × 105 CT26 cells and 1 × 106 of the indicated tumor cells, and tumor growth monitored. (d) Groups of BALB/c mice (n = 10) were injected subcutaneously with 5 × 106 of the indicated tumor cells in the right flank and 1 × 105 CT26 cells in the left flank. Growth of CT26 in the left flank is shown. In a–c, the data are from one representative experiment of two performed. In a, c, and d, the results are the mean ± SD. In b, the metastatic foci of each mouse and the mean of each group are shown. IL, interleukin.
To evaluate the antimetastatic effect of membrane-bound IL-12, groups of BALB/c mice (n = 5–7) were injected intravenously (i.v.) with CT26/LNCX, CT26/scIL-12-B7TM, or CT26/scIL-12 cells and lung metastases were counted on day 16. As shown in Figure 2b, mice that received CT26/LNCX cells had a large number of metastatic nodules in their lungs (mean of 177, range 78–235). By contrast, the production of secreted IL-12 by CT26/scIL-12 cells greatly suppressed the number of metastatic nodules (mean of 15, range 4–29) and expression of membrane-bound IL-12 resulted in even fewer metastatic nodules (mean of 1, range 0–2, P = 0.004 versus the CT26/IL-12 group). More significantly, expression of membrane-bound IL-12 completely eliminated all injected tumor cells in 60% (3 of 5) of animals, while none of the animals in the CT26/scIL-12 cell group were free of lung metastasis.
To investigate whether expression of membrane-bound IL-12 could suppress unmodified neighboring tumor cells, CT26 cells were mixed with CT26/LNCX, CT26/scIL-12-B7TM, or CT26/scIL-12 cells and injected s.c into mice. As shown in Figure 2c, tumors in the group injected with CT26 cells plus CT26/LNCX cells grew progressively and all mice died or had to be euthanized by day 40. By contrast, tumor growth was completely suppressed in mice injected with CT26 cells mixed with CT26/scIL-12-B7TM or CT26/scIL-12 cells. We further examined whether membrane-bound IL-12 could elicit systemic antitumor responses against distant tumors. Mice were injected with CT26/LNCX, CT26/scIL-12-B7TM, or CT26/scIL-12 cells in the right flank and parental CT26 cells in the left flank. As shown in Figure 2d, both CT26/scIL-12-B7TM and CT26/scIL-12 group significantly suppressed the growth of distant CT26 tumors, the mean tumor volume on day 28 being 495 ± 203 mm3 in the CT26/scIL-12-B7TM group (P = 0.0005) and 81 ± 130 mm3 (P = 0.0000002) in the CT26/scIL-12 group, compared with 987 ± 345 mm3 in the CT26/LNCX group. Besides, 10% (1 of 10) of the mice in the CT26/scIL-12-B7TM group and 70% (7 of 10) of the mice in the CT26/scIL-12 group showed complete regression of the distant CT26 tumors. These results demonstrate that membrane-bound IL-12 is as effective as secreted IL-12 for bystander killing of local tumors but has less systemic effect against metastatic distant tumors.
Mechanisms of the antitumor effect of membrane-bound IL-12
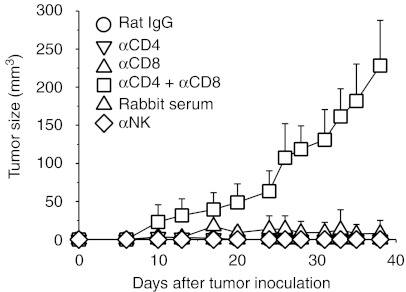
To dissect the roles of CD4+ T cells, CD8+ T cells, and NK cells in the membrane-bound IL-12–mediated antitumor effect, groups of BALB/c mice (n = 5) were depleted of these group of cells by treatment with anti-CD4, anti-CD8, anti-CD4 plus anti-CD8 mAbs, or anti-asialo GM1 antiserum before and after tumor challenge with animals treated with an irrelevant rat IgG or normal rabbit serum at the same dose and schedule being included as controls. As shown in Figure 3, depletion of CD4+ T cells, CD8+ T cells, or NK cells alone had little effect on the antitumor activity of membrane-bound IL-12; all mice had palpable tumors during the early stages, but these tumors were rejected by day 40. By contrast, depletion of both CD4+ and CD8+ T cells impaired the ability of scIL-12-B7TM to reject the tumors, resulting in tumor development in 100% of animals, although the tumor growth rate was much slower in these T cell-depleted mice than in wild-type mice similarly injected with CT26/LCNX cells (Figure 2a). These data suggest that CD4+ and CD8+ T cells were required for tumor rejection in the membrane-bound IL-12–mediated antitumor activity.
Figure 3.
Effect of selective depletion of different lymphocyte populations on subcutaneous CT26/scIL-12-B7TM tumor growth in BALB/c mice. Groups of BALB/c mice (n = 5) were injected subcutaneously with 1 × 105 CT26/scIL-12-B7TM cells on day 0 and anti-CD4 mAb and/or anti-CD8 mAb or anti-asialo GM1 antiserum was injected intraperitoneally on days −2, 0, 3, 5, 12, 19, and 26, then tumor size was monitored. Mice treated with the same dose and schedule of a monoclonal rat IgG or normal rabbit serum were included as controls. The results are the mean ± SD for the five mice. IL, interleukin; mAb, monoclonal antibody.
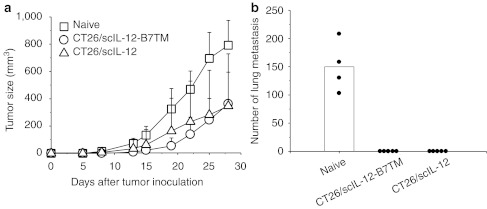
To investigate whether mice inoculated with CT26/scIL-12-B7TM cells developed memory immunity to wild-type tumors, we performed a series of tumor protection experiments. In the first experiment, mice that previously rejected a s.c. challenge of CT26/scIL-12-B7TM or CT26/scIL-12 cells were rechallenged with the parental CT26 tumor cells in the opposite flank, with age-matched naive mice inoculated with the same number of CT26 cells being included as controls. As shown in Figure 4a, all control animals developed rapidly growing s.c. tumors. By contrast, 63% (5 of 8) and 38% (3 of 8) of mice that had previously rejected CT26/scIL-12-B7TM and CT26/scIL-12 tumors, respectively, did not form tumors, and tumor growth suppression was also observed in the remaining tumor-bearing animals in each group. By day 28, the mean tumor volume in the tumor-bearing mice of the CT26/scIL-12-B7TM and CT26/scIL-12 groups was 363 ± 231 mm3 (P = 0.007) and 348 ± 382 mm3 (P = 0.01), respectively, compared with 793 ± 183 mm3 in the control group. In the second experiment, mice that rejected CT26/scIL-12-B7TM or CT26/scIL-12 tumors were i.v. rechallenged with CT26 tumor cells to evaluate the memory responses against lung metastasis. As shown in Figure 4b, all animals that had previously rejected CT26/scIL-12-B7TM or CT26/scIL-12 tumors showed a complete absence of metastatic lung foci, while the control mice presented a large number (mean of 150, range 103–208) of metastatic foci in their lungs. These results indicate that antitumor memory responses were induced by CT26/scIL-12-B7TM or CT26/scIL-12 cells. Indeed, we found that splenocytes from CT26/scIL-12-B7TM cell-injected mice showed dose-dependent cytotoxicity against the parental CT26 cells, but not the irrelevant P815 tumor cells (Supplementary Figure S1 and Supplementary Materials and Methods). Interestingly, splenocytes from CT26/scIL-12 cell-injected mice showed much less cytotoxicity against CT26 cells as compared with that induced by CT26/scIL-12-B7TM.
Figure 4.
Antitumor memory responses induced by CT26/scIL-12-B7TM. Groups of BALB/c mice (n = 4–8) that had previously rejected subcutaneous tumor cells as described in Figure 2a were rechallenged (a) subcutaneously with 1 × 105 CT26 tumors in the opposite flank or (b) intravenously with 2 × 105 CT26 cells. Age-matched naive BALB/c mice inoculated with the same number of CT26 tumors were included as controls. In a, the results are the mean ± SD for those mice with measurable tumors. In b, the animals were killed on day 21 after intravenous inoculation of CT26 cells and the metastatic foci of each mouse and the mean of each group are shown. IL, interleukin.
Treatment of established tumors using Ad/scIL-12-B7TM
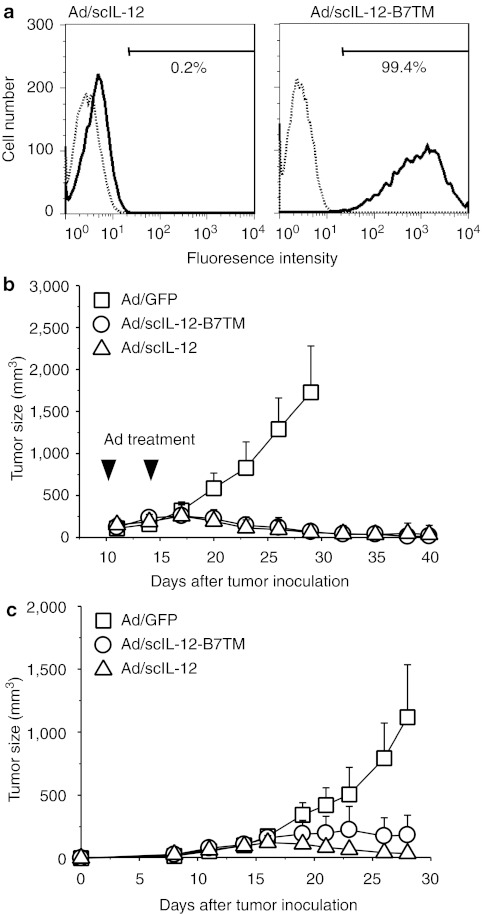
To investigate the therapeutic potential of membrane-bound IL-12 against pre-established tumors, we constructed an adenoviral vector encoding scIL-12-B7TM, designated Ad/scIL-12-B7TM. 3T3 cells were infected with Ad/scIL-12-B7TM or Ad/scIL-12, which produces secreted single-chain IL-12,40 and cells were harvested 48 hours postinfection. FACS analysis (Figure 5a) showed that Ad/scIL-12-B7TM, but not Ad/scIL-12, directed expression of IL-12 on the surface of the cells (99.4% positive using Ad/scIL-12-B7TM versus 0.2% using Ad/scIL-12).
Figure 5.
Treatment of established CT26 tumors with adenovirus expressing scIL-12-B7TM. (a) 3T3 cells were infected with Ad/scIL-12-B7TM or Ad/scIL-12 at 100 multiplicity of infection for 48 hours, then the cells were stained with anti-mouse IL-12 p40/p70 mAb (solid lines) or an isotype control mAb (dashed lines) and analyzed by flow cytometry. (b) Groups of BALB/c mice (n = 8–12) were injected subcutaneously with 5 × 105 CT26 cells, then treated with 1 × 109 pfu of Ad/scIL-12-B7TM, Ad/scIL-12, or Ad/GFP on days 10 and 14 by intratumoral injection, and tumor growth was measured. (c) Groups of BALB/c mice (n = 5) were injected subcutaneously with 5 × 105 CT26 cells in the right flank, and 1 × 105 CT26 cells in the left flank. The right flank tumor were treated with 1 × 109 pfu of Ad/scIL-12-B7TM, Ad/scIL-12, or Ad/GFP as described above, and the left flank tumor remained untreated. Tumor growth was monitored at the indicated times, and the results are presented as the mean ± SD. IL, interleukin; mAb, monoclonal antibody.
To determine the antitumor activity of Ad/scIL-12-B7TM, groups of BALB/c mice (n = 8–12) were injected s.c. with 5 × 105 CT26 cells, then, on days 10 and 14 after tumor implantation, 109 pfu of Ad/scIL-12-B7TM, Ad/scIL-12 (positive control), or Ad/GFP (negative control) was injected intratumorally. As shown in Figure 5b, both Ad/scIL-12-B7TM and Ad/scIL-12 showed potent therapeutic effects against established tumors, with tumor regression being observed within 1 week after treatment and the tumors being completely eradicated in 75% (6 of 8) of mice in the Ad/scIL-12-B7TM group and in 83% (10 of 12) in the Ad/scIL-12 group. By contrast, injection of Ad/GFP did not delay tumor growth as compared to phosphate-buffered saline (PBS)-treated controls (data not shown). We also performed a dose–response study to assess the efficacy of Ad/scIL-12-B7TM. Tumor-bearing BALB/c mice (n = 5) were injected intratumorally with different doses (109, 108, or 107 pfu per mouse) of Ad/scIL-12-B7TM or with the control Ad/GFP at the dose of 109 pfu per mouse. As shown in Supplementary Figure S2, Ad/scIL-12-B7TM clearly showed a dose-dependent antitumor effect, but, even at the low doses of 108 and 107 pfu, still significantly inhibited tumor growth compared to Ad/GFP.
To examine whether local injection of Ad/scIL-12B7-TM to CT26 tumors was able to suppress CT26 tumors at distant locations, mice were inoculated with CT26 cells in both the right and left flanks, and as the tumors reached about 5 mm in diameter, the tumors on the right flank were treated with Ad/scIL-12-B7TM, Ad/scIL-12, or Ad/GFP as described above, and the tumors on the left flank remained untreated. Ad/scIL-12-B7TM and Ad/scIL-12 not only inhibited the growth of the treated tumors (data not shown), but also significantly suppressed the untreated distant tumors (Figure 5c). By day 28, the mean tumor volume of the distant tumors was 225 ± 158 mm3 (P = 0.001) and 37 ± 43 mm3 (P = 0.0002) in the Ad/scIL-12-B7TM and Ad/scIL-12 groups, respectively, compared to 1,118 ± 417 mm3 in the Ad/GFP group. Moreover, 20% (1 of 5) and 80% (4 of 5) of the mice in the Ad/scIL-12-B7TM and Ad/scIL-12 groups, respectively, showed complete regression of the distant tumors.
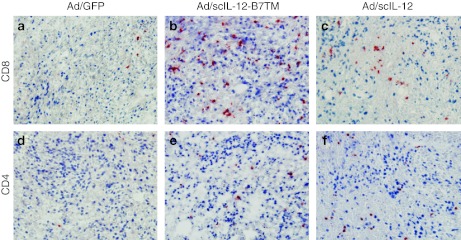
We then examined the phenotype of antitumor effector cells by immunohistochemical analysis of tumor tissues resected 10 days after adenoviral treatment. There was intense infiltration of CD8+ T cells in the tumor lesions treated with Ad/scIL-12-B7TM (Figure 6b) and to a lesser extent in those treated with Ad/scIL-12 (Figure 6c). Moderate levels of CD4+ T cells were also detected in tumors treated with Ad/scIL-12-B7TM (Figure 6e) or Ad/scIL-12 (Figure 6f). By comparison, CD8+ T cells (Figure 6a) and CD4+ T cells (Figure 6d) were only barely detectable in the tumors injected with the control Ad/GFP. These results indicate that T cells, in particular CD8+ T cells, might play an important role in IL-12–mediated tumor regression.
Figure 6.
Immunohistochemical evaluation of CT26 tumors treated with Ad/scIL-12-B7TM. BALB/c mice with established subcutaneous CT26 tumors were treated with Ad/scIL-12-B7TM, Ad/scIL-12, or Ad/GFP as described in Figure 5. Mice were killed on day 10 after adenovirus inoculation and the tumor tissues were removed for immunohistochemical analysis. Cryostat sections were stained with (a–c) anti-CD8 (d–f) or anti-CD4 mAb and then counterstained with hematoxylin. Original magnification, ×200. IL, interleukin; mAb, monoclonal antibody.
To investigate whether the tumor-infiltrating T cells can further develop into memory responses, mice that had been treated with Ad/scIL-12-B7TM or Ad/scIL-12 and eliminated the original tumors were subjected to s.c. or i.v. rechallenge of CT26 tumors, with age-matched naive mice inoculated with the same number of CT26 cells being included as controls. As shown in Figure 7a, mice that had been previously treated with Ad/scIL-12B7TM or Ad/scIL-12 significantly suppressed the growth of rechallenged CT26 tumors, with the mean tumor volume in the tumor-bearing mice being 514 ± 261 mm3 (2 of 5, P = 0.03) and 435 ± 37 mm3 (3 of 5, P = 0.007), respectively, compared to 1,770 ± 663 mm3 in the naive group by day 29. Similarly, in the i.v. challenge experiment, all mice (5 of 5) in the Ad/scIL-12-B7TM group (P = 0.000004 versus the naive group) and 40% (2 of 5) in the Ad/scIL-12 group (P = 0.00007 versus the naive group) were completely protected from tumor rechallenge, while all mice in the control group showed a large number (mean of 166, range 118–223) of metastatic foci in their lungs (Figure 7b).
Figure 7.
Antitumor memory responses induced by Ad/scIL-12-B7TM. Groups of BALB/c mice (n = 5–6) that had been previously treated with Ad/scIL-12-B7TM or Ad/scIL-12 and eliminated the original tumors were rechallenged (a) subcutaneously with 1 × 105 CT26 or (b) intravenously with 2 × 105 CT26 cells. Age-matched naive BALB/c mice were included as controls. In a, the results are the mean ± SD for those mice with measurable tumors. In b, the mean and individual metastatic foci of each group are presented. IL, interleukin.
Treatment of membrane-bound IL-12 results in lower systemic IL-12 levels
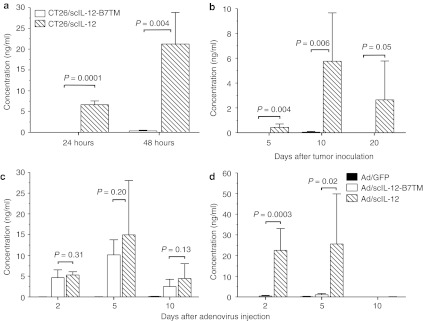
The main concern in the clinical application of IL-12 is its systemic side effects. To determine whether expression of scIL-12-B7TM on the cell surface was stable, IL-12 levels in the culture supernatants of CT26/scIL-12-B7TM and CT26/scIL-12 cells were measured by enzyme-linked immunosorbent assay. As shown in Figure 8a, significant amounts of IL-12 were released from CT26/scIL-12 cells (6.7 ± 0.9 ng/ml/5 × 104/24 hours; 21.2 ± 7.6 ng/ml/5 × 104/48 hours). By contrast, IL-12 levels in CT26/scIL-12-B7TM cell culture supernatants were undetectable at 24 hours and very low (0.4 ± 0.2 ng/ml) at 48 hours, 53-fold lower than in CT26/scIL-12 cell supernatants (P = 0.004). We also measured the in vivo release of IL-12 in mice inoculated with CT26/scIL-12-B7TM or CT26/scIL-12 cells. Because lower doses of both tumors were rapidly rejected after inoculation (Figure 2a), we increased the challenge dose to 107 cells per mouse in order to measure IL-12 release into the circulation. At this dose, CT26/scIL-12 tumors produced 0.4 ± 0.3 ng/ml of serum IL-12 on day 5 (mean tumor volume of 30 ± 13 mm3), 5.8 ± 3.9 ng/ml on day 10 (mean tumor volume of 109 ± 36 mm3), and 2.7 ± 3.0 ng/ml on day 20 (mean tumor volume of 92 ± 55 mm3) (Figure 8b). By contrast, CT26/scIL-12-B7TM tumors, even at this high challenge dose and with a mean tumor volume of 28 ± 6 mm3 on day 5, 88 ± 25 mm3 on day 10, and 3 ± 7 mm3 on day 20, did not produce detectable serum IL-12 during the 20-day observation period. These data demonstrate that expression of scIL-12-B7TM on the cell surface is stable both in vitro and in vivo.
Figure 8.
Systematic and local expression of IL-12. (a) 5 × 104 CT26/scIL-12-B7TM or CT26/scIL-12 cells were seeded on 6-well plate and the supernatants collected 24, or 48 hours later to measure the amount of IL-12 by ELISA. The values are presented as the mean ± SD of triplicate cultures. (b) Groups of BALB/c mice (n = 5) were injected subcutaneously with 1 × 107 CT26/scIL-12-B7TM, or CT26/scIL-12 cells, then serum samples were collected on the indicated days and assayed for the presence of IL-12. (c,d) Mice (n = 3–6) with established subcutaneous CT26 tumors as described in Figure 5 were injected intratumorally with 1 × 109 pfu of Ad/scIL-12-B7TM, Ad/scIL-12, or Ad/GFP on day 10. (c) Tumor and (d) serum samples were collected on the indicated days and assayed for the presence of IL-12 by ELISA. The results in b–d are the mean ± SD for the indicated number of mice. ELISA, enzyme-linked immunosorbent assay; IL, interleukin.
To evaluate whether the use of membrane-bound IL-12 in adenovirus-mediated therapy could reduce systemic IL-12 levels, mice (n = 3–6) with established CT26 tumors were injected once intratumorally with 109 pfu of Ad/GFP, Ad/scIL-12-B7TM, or Ad/scIL-12 on day 10 after tumor inoculation and serum and tumor tissues were collected at different times to measure IL-12 levels. As shown in Figure 8c, intratumoral IL-12 levels in mice treated with Ad/scIL-12-B7TM or Ad/scIL-12 were comparable, being 4.3 ± 1.3 ng/mg for Ad/scIL-12-B7TM versus 5.3 ± 0.8 ng/mg for Ad/scIL-12 on day 2 (P = 0.17), 10.2 ± 3.6 ng/mg versus 15.0 ± 13.1 ng/mg on day 5 (P = 0.20), and 2.5 ± 1.7 ng/mg versus 4.4 ± 3.6 ng/mg on day 10 (P = 0.13). However, as shown in Figure 8d, Ad/scIL-12-B7TM injection resulted in only negligible IL-12 levels in the serum (0.4 ± 0.3 ng/ml on day 2 and 1.0 ± 0.6 ng/ml on day 5), significantly lower than those produced by Ad/scIL-12 (22.6 ± 10.5 ng/ml on day 2 (P = 0.0003) and 25.6 ± 24.2 ng/ml on day 5 (P = 0.02). No serum IL-12 was detectable in either group at day 10 after adenovirus therapy. In mice treated with Ad/GFP, IL-12 was not detectable in either the serum or tumor tissues. These results demonstrate that therapy with IL-12-B7TM can greatly reduce IL-12 release into the circulation without affecting the local cytokine concentration.
Discussion
IL-12 is a promising cytokine for cancer treatment but the clinical use of secreted or systemically applied IL-12 has been limited by its severe toxicity.17,18 Several reports have shown that modification of secreted cytokines to generate membrane-bound forms by adding transmembrane or GPI sequences can restrict cytokine activity to the site of injection and thus reduce systemic toxicity without compromising antitumor activity.26,27 The antitumor activity of a membrane-bound IL-12 as a GPI-anchored form had been demonstrated in previous studies.30,34 However, GPI-anchored protein had been shown to be spontaneously released from the cell membrane due to shedding or proteolytic cleavage.37,38 To reduce the potential leakage of IL-12 into the circulation, we constructed retroviral and adenoviral vectors that expressed a new membrane-bound IL-12 (scIL-12-B7TM, Figure 1a) by fusing mouse IL-12 to the transmembrane domain and cytoplasmic tail of murine B7-1, which had been shown to greatly increase the surface expression of chimeric proteins and reduce the spontaneous release of membrane proteins from the cell surface.35 Our data demonstrated that CT26 tumor cells transduced with the recombinant retroviral vector (Figure 1b) and 3T3 cells transduced with the recombinant adenoviral vector (Figure 5a) expressed high levels of IL-12 on the cell surface. The functional activity of IL-12 was maintained in the chimeric scIL-12-B7TM protein, as scIL-12-B7TM–transduced CT26 cells stimulated vigorous proliferation (Figure 1c) and IFN-γ production (data not shown) by Con A-activated lymphoblasts. More importantly, only very low levels of IL-12 were found in the culture supernatants of CT26/scIL-12-B7TM cells (Figure 8a) or in the serum of mice injected with high doses of CT26/scIL-12-B7TM cells (Figure 8b) or Ad/scIL-12-B7TM vector (Fig. 8d). By contrast, at the same treatment dose, a significant amount of unmodified scIL-12 was released into the circulation (Figure 8b,d). We are not able to directly compare the safety profile of the two forms of IL-12, because no apparent systemic toxicity was observed in mice treated with CT26 cells or adenoviral vectors expressing either scIL-12 or scIL-12-B7TM (data not shown). Nevertheless, our results demonstrate that modification of IL-12 with B7TM not only retains IL-12 bioactivity, but also greatly reduce its release into the circulation and thus is expected to produce much less systemic toxicity.
The antitumor activity of scIL-12-B7TM was first demonstrated using CT26 tumors transduced with the recombinant retroviral vector. Tumor growth of s.c. injected CT26/scIL-12-B7TM cells was greatly delayed, and all mice showed complete elimination of the tumors at later times and remained tumor-free for at least 120 days after tumor inoculation (Figure 2a). The suppression of tumor growth was mediated by the in vivo biological activity of scIL-12-B7TM, as the in vitro cell doubling time of the CT26/scIL-12-B7TM cells was similar to that of the control CT26/LNCX cells (data not shown), suggesting that membrane IL-12 expression did not affect the intrinsic tumor growth rate of CT26 cells. More importantly, we demonstrated that CT26/scIL-12-B7TM cells markedly inhibited the in vivo growth of unmodified parental CT26 tumors (Figure 2c), the activity being as good as that of CT26/scIL-12, suggesting that membrane-bound scIL-12-B7TM is as effective as secreted scIL-12 in causing bystander cell killing.41,42 Interestingly, we found that membrane-bound scIL-12-B7TM was significantly better than secreted scIL-12 in eliminating metastatic tumors in the lung, resulting in 60% of animals free of lung metastasis versus 0% in the secreted IL-12 group (Figure 2b). Surface expression of GPI-anchored IL-12 on tumor cells was also reported to significantly delay tumor growth, but most of the animals eventually developed tumors and all the escaped tumors lost expression of GPI-IL-12.30,34 In comparison, expression of scIL-12-B7TM on tumor cells resulted in complete rejection of all subcutaneous or lung metastatic tumors, highlighting the advantage of using B7TM over GPI-anchored sequence in making membrane-bound IL-12.
The therapeutic effect of scIL-12-B7TM was further demonstrated by treating pre-established tumors of about 5 mm in diameter with an adenoviral vector encoding scIL-12-B7TM. Intratumoral injection of 109 pfu of Ad/scIL-12-B7TM was as effective as Ad/scIL-12 in suppressing tumor growth (Figure 5b) and led to complete tumor eradication in most of the treated animals (75% in the scIL-12-B7TM group versus 83% in the scIL-12 group). The therapeutic effect of Ad/scIL-12-B7TM was dose-dependent, but, even at lower doses of 108 and 107 pfu, Ad/scIL-12-B7TM still significantly inhibited tumor growth (Supplementary Figure S2). Adenoviral vectors encoding secreted IL-12 have been used to treat patients with advanced digestive malignancies, but only achieved a mild therapeutic effect.16 Since the B7TM-modified IL-12 has the advantage of retaining its bioactivity locally (Figure 8c,d), we suggest that higher doses of Ad vectors producing membrane-bound IL-12 could be used to treat cancer patients, which may achieve a better therapeutic efficacy without systemic toxicity.
IL-12 exerts its antitumor activity either directly by activating NK or cytotoxic T lymphocytes, or indirectly through IFN-γ, which may inhibit angiogenesis or activate other cell types, such as macrophages.4,7 As regards the cellular antitumor mechanism of scIL-12-B7TM, our experiments in mice selectively depleted of various lymphocyte subpopulations suggested that both CD4+ T cells and CD8+ T cells were involved in the antitumor activity of scIL-12-B7TM, since the protective effect was lost in mice depleted of both of these cell types, but not in mice depleted of either cell population alone (Figure 3). This was further confirmed by immunohistochemical analysis which revealed massive infiltration of CD8+ T cells and moderate infiltration of CD4+ T cells in tumors treated with Ad/scIL-12B7TM (Figure 6). However, we noted that the growth of CT26/scIL-12-B7TM tumor in mice depleted of both CD4+ and CD8+ T cells was still significantly slower than that of control CT26/LNCX tumors, suggesting other IL-12–related antitumor mechanisms, such as antiangiogenesis,9,10,11 NK cells, or NKp46+ lymphoid tissue-inducer cells,43 might also be involved in the antitumor activity of scIL-12-B7TM. Our result showed that NK cell depletion did not affect the in vivo growth of CT26/scIL-12-B7TM cells, suggesting that NK cells are not required for or only play a minor role in the antitumor activity of scIL-12-B7TM in this CT26 tumor model.
Another interesting observation of our study is that treatment of membrane-bound IL-12, either by CT26/scIL-12-B7TM or Ad/scIL-12-B7TM, can generate a protective effect against tumors at distant sites, although its effect is less effective than animals treated with CT26/scIL-12 or Ad/scIL-12 (Figures 2d and 5c), which secreted and maintained a significant amount of IL-12 in the circulation. By contrast, there were only negligible amounts of serum IL-12 in animals treated with membrane-bound IL-12 (Figure 8b,d), suggesting that its systemic antitumor activity is not likely due to the direct effect of IL-12. Instead, we believe that the antitumor immune responses induced by membrane-bound IL-12 treatment of the primary tumors could play a significant role in suppressing the development of disseminated tumors. Indeed, our data demonstrated that membrane-bound IL-12 had a potent adjuvant activity in eliciting tumor-specific T cell responses. Injection of BALB/c mice with CT26/scIL-12-B7TM cells induced strong cytotoxic T lymphocyte activity against the parental CT26 tumor cells but not the irrelevant P815 cells (Supplementary Figure S1). Moreover, mice previously treated with membrane-bound IL-12 developed memory immune responses and provided long-term protection against a subsequent challenge of patental CT26 cells (Figures 4 and 7). The potent adjuvant activity of membrane-bound IL-12 had also been demonstrated in a cancer DNA vaccine study using a plasmid encoding a similar B7TM-modified IL-12.44 Tumor cell vaccines expressing membrane-bound granulocyte-macrophage colony-stimulating factor were also found to induce strong antitumor immunity that protected against tumor challenge more effectively than analogous vaccines expressing secreted granulocyte-macrophage colony-stimulating factor.45 These results suggest that cancer immunotherapy using membrane-bound cytokines not only can reduce systemic toxicity but also has a further advantage of inducing strong antitumor immune responses, a fact that could be attributed to the colocalization on the same tumor cell of membrane cytokines and tumor antigens that are in close contact with, and thus effectively activate tumor-specific T cells.
Materials and Methods
Animals and cell lines. Female BALB/c mice (6- to 8-week-old) were purchased from the National Laboratory Animal Breeding and Research Center (Taipei, Taiwan). All animal studies were conducted in specific pathogen-free conditions and in accordance with guidelines approved by the Animal Care and Usage Committee of Academia Sinica (Taipei, Taiwan). The CT26 cell line is a N-nitroso-N-methylurethane–induced murine colon carcinoma in BALB/c mice.46 CT26 cells were maintained in Dulbecco's modified Eagle's medium (Sigma-Aldrich, St Louis, MO) supplemented with heat-inactivated 10% bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin at 37 °C in a 5% CO2 humidified incubator.
Construction of the membrane-bound 12 plasmid. To construct the plasmid encoding the membrane-bound IL-12, the forward primer 5′-CTGGGGCCCAGCCGGCCATGGCCATGTGGGAGCT-3′ and reverse primer 5′-GAGGTCGACGGCGGAGCTGACATAGCCCATCAC-3′ were used to amplify the murine single-chain IL-12 fragment from the plasmid pscIL-12.2,39 introducing a SfiI site at the 5′-end and a SalI site at the 3′-end. The PCR product was digested with SfiI and SalI restriction enzymes and cloned into the pHook-B7TM plasmid,35 which contains the murine B7-1 transmembrane and cytosolic domains. The scIL-12-B7TM fragment was then subcloned into the plasmids pLNCX2 (Clontech, Takara Bio, Mountain View, CA) and pAd-hPGK to generate pLNCX/scIL-12-B7TM and pAd-scIL-12-B7TM for production of retroviral and adenoviral vectors, respectively. The pAd-hPGK plasmid contains the human phosphoglycerate kinase promoter and the bovine growth hormone polyadenylation signal.47
Retroviral transduction. The production of retrovirally transduced tumor cells has been described previously.39 Briefly, retroviral supernatant was generated by transfecting the pLNCX/scIL-12-B7TM plasmid into the PT67 packaging cell line (Clontech) using lipofectamine 2000 (Invitrogen, Carlsbad, CA). CT26 cells were then infected with the retroviral supernatant and selected using G418 (0.5 mg/ml). The resistant clones were then screened for the expression of scIL-12-B7TM by FACS analysis. The transduced cells were enriched for high levels of scIL-12-B7TM expression by staining with rat anti-HA mAb (3F10; Boehringer, Mannheim, Germany) and fluorescein isothiocyanate-conjugated anti-rat IgG antibodies (Jackson ImmunoResearch, West Grove, PA) and sorting on a FACSVantage (BD Biosciences, San Jose, CA). The 1% of cells expressing the highest amount of scIL-12-B7TM, named CT26/scIL-12-B7TM cells, were selected for in vitro and in vivo studies. The generation of CT26/scIL-12 cells, which produce secreted scIL-12, and CT26/LNCX cells, infected with an empty pLNCX plasmid, has been described previously.39
Flow cytometry analysis of membrane bound IL-12. To screen for surface expression of scIL-12-B7TM, cells were incubated with biotin-conjugated anti-mouse IL-12 p40/p70 mAb (17.8; BD Biosciences Pharmingen, San Diego, CA), then with allophycocyanin-labeled streptavidin (BD Biosciences Pharmingen), and analyzed on a FACSCallibur (BD Biosciences). The data was processed using FlowJo software (Treestar, Ashland, OR).
Biofunctional assay of membrane-bound IL-12. An IL-12 proliferation assay was performed as described previously.48 Briefly, Con A-activated lymphoblasts were prepared by culturing mouse splenocytes in Roswell Park Memorial Institute (RPMI) medium containing 5% heat-inactivated fetal bovine serum, 0.5 mg/ml of human recombinant IL-2 and 2 µg/ml of Con A (Sigma-Aldrich) at a density of 1 × 106 cells/ml for 2 days. The lymphoblasts were then harvested and isolated using Lympholyte M (Cedarlane, Hornby, Ontario, Canada) centrifugation. The stimulator cells were prepared by treating CT26/scIL-12-B7TM and CT26/LNCX cells with 0.5 mg/ml of mitomycin C (Sigma-Aldrich) at 37 °C for 2 hours, then threefold serial dilutions of the inactivated stimulator cells were added in triplicate to individual wells containing 1 × 104 lymphoblasts and the cells cultured for 3 days, then pulsed for 18 hours with [3H]-thymidine (1 µCi per well) and the incorporated radioactivity measured on a TopCount (PerkinElmer,Wellesley, MA).
Tumor growth and metastasis in vivo. Exponentially growing tumor cells were harvested and used to induce s.c. tumors or metastases only if their viability exceeded 95%, as determined by Trypan blue staining. To measure the tumorigenicity of CT26/scIL-12-B7TM, CT26/scIL-12, and CT26/LNCX cells, groups of BALB/c mice were injected s.c. with 1 × 105 cells in 100 µl of Dulbecco's PBS (Sigma-Aldrich). To study the bystander antitumor effect, 1 × 105 wild-type CT26 tumors were mixed with 1 × 106 CT26/LNCX, CT26/scIL-12-B7TM, or CT26/scIL-12 cells and injected s.c. To study the systematic antitumor effect, mice were injected with 5 × 106 CT26/LNCX, CT26/scIL-12-B7TM, or CT26/scIL-12 cells in the right flank and 1 × 105 CT26 cells in the left flank. For in vivo antibody-depletion experiments, BALB/c mice were injected s.c. with 1 × 105 CT26/scIL-12-B7TM cells on day 0, and CD4+ T cells and/or CD8+ T cells or NK cells were depleted by intraperitoneal injection of anti-CD4 mAb (GK1.5, rat IgG2b) and/or anti-CD8 mAb (53-6.72, rat IgG2a) or rabbit anti-asialo-GM1 antiserum (Wako Pure Chemical Industries, Osaka, Japan), respectively, on day −2, 0, 3, 5, 12, 19, and 26.39 More than 95% of the respective lymphocyte populations was depleted using this dose and schedule (data not shown). Mice injected intraperitoneally with normal rat IgG or normal rabbit serum at the same dose and schedule were used as controls. Tumor growth was measured, and the mean volume in cubic millimeters and the SD for each group were calculated as described previously.39 The mice were euthanized when the tumor measured more than 3,000 mm3. To generate lung metastases, BALB/c mice were injected i.v. with 2 × 105 CT26/LNCX, CT26/scIL-12-B7TM, or CT26/scIL-12 cells and lung metastasis evaluated on day 16 later. The tumor nodules were contrasted using Black India ink (15% in PBS) before counting under a dissecting microscope. For some experiments, mice that had previously rejected the tumors were rechallenged s.c. with 1 × 105 CT26 cells in the other flank or i.v. with 2 × 105 CT26 cells to evaluate antitumor memory responses.
Tumor therapy using adenoviral vectors. Replication-defective adenoviruses were produced by cotransfecting HEK-293 cells with pAd/scIL-12-TM and pJM17 as described previously.47 The recombinant virus, Ad/scIL-12-B7TM, was isolated from a single plaque, propagated in HEK-293 cells, and purified on a cesium chloride gradient. The construction and production of the recombinant Ad/scIL-12 and Ad/GFP vectors, encoding, respectively, secreted single-chain IL-12 and GFP have been reported previously.40 For tumor therapy, BALB/c mice were injected s.c. with 5 × 105 CT26 cells, then, 10 days later when the tumors were about 5 mm in diameter, Ad/scIL-12-B7TM, Ad/scIL-12, or Ad/GFP was directly injected into the established tumors at 1 × 109 pfu per mouse, in a volume of 50 µl diluted in PBS. The second injection of adenoviruses was given 4 days after the first. To study the systematic antitumor effect, mice were injected s.c. in the right flank with 5 × 105 CT26 cells and in the left flank with 1 × 105 CT26 cells. The right flank tumor received two injections of various adenoviral vectors as described above, and the left flank tumor remained untreated. Tumor growth was monitored as described above. For some experiments, mice that had been treated with Ad/scIL-12-B7TM or Ad/scIL-12 and eliminated the original tumors were rechallenged s.c. with 1 × 105 CT26 cells or i.v. with 2 × 105 CT26 cells to evaluate antitumor memory responses. Tumor growth and lung metastasis were monitored as described above.
Immunohistochemistry. Immunohistochemical analysis of tumor samples was performed as described previously.39 Briefly, cryostat sections (10 µm) were stained with rat mAb against CD8 (53-6.7; American Type Culture Collection, Manassas, VA) or CD4 (H129.19; BD Biosciences Pharmingen), followed by incubation with biotinylated rabbit anti-rat IgG (Dako, Carpinteria, CA), then the Vectastain ABC Elite Kit (Vector Laboratories, Burlingame, CA) and AEC+ substrate-chromogen (Dako) were added. The slides were counterstained with hematoxylin and analyzed by light microscopy at ×200 magnification.
IL-12 release assay. For the in vitro IL-12 release assay, 5 × 104 CT26/scIL-12-B7TM or CT26/scIL-12 cells were seeded per well on 6-well plates and cell-free supernatants collected at different time points. For the in vivo IL-12 release assay, BALB/c mice were injected s.c. with 1 × 107 CT26/scIL-12-B7TM or CT26/scIL-12 cells and serum samples collected at different time points. To evaluate local and secreted IL-12 during adenovirus therapy, BALB/c mice bearing established CT26 tumors were injected intratumorally with 1 × 109 pfu per mouse of Ad/scIL-12-B7TM, Ad/scIL-12, or Ad/GFP as described above and serum and tumor samples collected at different time points post-adenovirus treatment. Tumor lysates were prepared by sonication at 4 °C of tumor tissues in PBS supplemented with a complete protease inhibitor cocktail (Roche Applied Science, Mannheim, Germany) and the total protein concentration measured using a Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL) according to the manufacturer's instructions. IL-12 levels in serum and tumor tissue samples were measured with a sandwich enzyme-linked immunosorbent assay using mouse IL-12 p70 (R&D Systems, Minneapolis, MN).
Statistics. All data were analyzed for significance by Student's t-test. Findings were regarded as significant if the P value was ≤0.05.
SUPPLEMENTARY MATERIAL Figure S1. CTL activity induced by CT26/scIL-12-B7TM cells. Figure S2. Groups of BALB/c mice (n = 5) inoculated s.c. with 5 × 105 CT26 cells were treated with Ad/GFP or Ad/scIL-12-B7TM at the indicated dose on day 10. Materials and Methods.
Acknowledgments
We thank Daniel K Hsu and Dah-Jiun Fu (the Glycobiology Core, Institute of Biomedical Sciences, Academia Sinica) for technical help with immunohistochemistry. This work is supported by Academia Sinica and Grant NSC99-2320-B-001-015-MY3 from National Science Council (Taipei, Taiwan). The authors declared no conflict of interest.
Supplementary Material
CTL activity induced by CT26/scIL-12-B7TM cells.
Groups of BALB/c mice (n = 5) inoculated s.c. with 5 × 105 CT26 cells were treated with Ad/GFP or Ad/scIL-12-B7TM at the indicated dose on day 10.
REFERENCES
- Cavallo F, Signorelli P, Giovarelli M, Musiani P, Modesti A, Brunda MJ.et al. (1997Antitumor efficacy of adenocarcinoma cells engineered to produce interleukin 12 (IL-12) or other cytokines compared with exogenous IL-12 J Natl Cancer Inst 891049–1058. [DOI] [PubMed] [Google Scholar]
- Lo CH, Chang CM, Tang SW, Pan WY, Fang CC, Chen Y.et al. (2010Differential antitumor effect of interleukin-12 family cytokines on orthotopic hepatocellular carcinoma J Gene Med 12423–434. [DOI] [PubMed] [Google Scholar]
- Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U.et al. (1998The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses Annu Rev Immunol 16495–521. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR, Murphy M.et al. (1993Antitumor and antimetastatic activity of interleukin 12 against murine tumors J Exp Med 1781223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio K, Nicoletti G, Di Carlo E, Cavallo F, Landuzzi L, Melani C.et al. (1998Interleukin 12-mediated prevention of spontaneous mammary adenocarcinomas in two lines of Her-2/neu transgenic mice J Exp Med 188589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G.et al. (2007Interleukin-12: biological properties and clinical application Clin Cancer Res 134677–4685. [DOI] [PubMed] [Google Scholar]
- Colombo MP., and, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155–168. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- Voest EE, Kenyon BM, O'Reilly MS, Truitt G, D'Amato RJ., and, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst. 1995;87:581–586. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]
- Sgadari C, Angiolillo AL., and, Tosato G. Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood. 1996;87:3877–3882. [PubMed] [Google Scholar]
- Kanegane C, Sgadari C, Kanegane H, Teruya-Feldstein J, Yao L, Gupta G.et al. (1998Contribution of the CXC chemokines IP-10 and Mig to the antitumor effects of IL-12 J Leukoc Biol 64384–392. [DOI] [PubMed] [Google Scholar]
- Rook AH, Wood GS, Yoo EK, Elenitsas R, Kao DM, Sherman ML.et al. (1999Interleukin-12 therapy of cutaneous T-cell lymphoma induces lesion regression and cytotoxic T-cell responses Blood 94902–908. [PubMed] [Google Scholar]
- Ansell SM, Witzig TE, Kurtin PJ, Sloan JA, Jelinek DF, Howell KG.et al. (2002Phase 1 study of interleukin-12 in combination with rituximab in patients with B-cell non-Hodgkin lymphoma Blood 9967–74. [DOI] [PubMed] [Google Scholar]
- Bajetta E, Del Vecchio M, Mortarini R, Nadeau R, Rakhit A, Rimassa L.et al. (1998Pilot study of subcutaneous recombinant human interleukin 12 in metastatic melanoma Clin Cancer Res 475–85. [PubMed] [Google Scholar]
- Motzer RJ, Rakhit A, Schwartz LH, Olencki T, Malone TM, Sandstrom K.et al. (1998Phase I trial of subcutaneous recombinant human interleukin-12 in patients with advanced renal cell carcinoma Clin Cancer Res 41183–1191. [PubMed] [Google Scholar]
- Sangro B, Mazzolini G, Ruiz J, Herraiz M, Quiroga J, Herrero I.et al. (2004Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors J Clin Oncol 221389–1397. [DOI] [PubMed] [Google Scholar]
- Cohen J. IL-12 deaths: explanation and a puzzle. Science. 1995;270:908. doi: 10.1126/science.270.5238.908a. [DOI] [PubMed] [Google Scholar]
- Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB.et al. (1997Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production Blood 902541–2548. [PubMed] [Google Scholar]
- van Herpen CM, van der Laak JA, de Vries IJ, van Krieken JH, de Wilde PC, Balvers MG.et al. (2005Intratumoral recombinant human interleukin-12 administration in head and neck squamous cell carcinoma patients modifies locoregional lymph node architecture and induces natural killer cell infiltration in the primary tumor Clin Cancer Res 111899–1909. [DOI] [PubMed] [Google Scholar]
- Tahara H, Zeh HJ., 3rd, , Storkus WJ, Pappo I, Watkins SC, Gubler U.et al. (1994Fibroblasts genetically engineered to secrete interleukin 12 can suppress tumor growth and induce antitumor immunity to a murine melanoma in vivo Cancer Res 54182–189. [PubMed] [Google Scholar]
- Tahara H, Zitvogel L, Storkus WJ, Zeh HJ., 3rd, , McKinney TG, Schreiber RD.et al. (1995Effective eradication of established murine tumors with IL-12 gene therapy using a polycistronic retroviral vector J Immunol 1546466–6474. [PubMed] [Google Scholar]
- Nishioka Y, Hirao M, Robbins PD, Lotze MT., and, Tahara H. Induction of systemic and therapeutic antitumor immunity using intratumoral injection of dendritic cells genetically modified to express interleukin 12. Cancer Res. 1999;59:4035–4041. [PubMed] [Google Scholar]
- Meko JB, Yim JH, Tsung K., and, Norton JA. High cytokine production and effective antitumor activity of a recombinant vaccinia virus encoding murine interleukin 12. Cancer Res. 1995;55:4765–4770. [PubMed] [Google Scholar]
- Bramson JL, Hitt M, Addison CL, Muller WJ, Gauldie J., and, Graham FL. Direct intratumoral injection of an adenovirus expressing interleukin-12 induces regression and long-lasting immunity that is associated with highly localized expression of interleukin-12. Hum Gene Ther. 1996;7:1995–2002. doi: 10.1089/hum.1996.7.16-1995. [DOI] [PubMed] [Google Scholar]
- Toda M, Martuza RL, Kojima H., and, Rabkin SD. In situ cancer vaccination: an IL-12 defective vector/replication-competent herpes simplex virus combination induces local and systemic antitumor activity. J Immunol. 1998;160:4457–4464. [PubMed] [Google Scholar]
- Cheng TL., and, Roffler S. Membrane-tethered proteins for basic research, imaging, and therapy. Med Res Rev. 2008;28:885–928. doi: 10.1002/med.20127. [DOI] [PubMed] [Google Scholar]
- Kim YS. Tumor Therapy Applying Membrane-bound Form of Cytokines. Immune Netw. 2009;9:158–168. doi: 10.4110/in.2009.9.5.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Li J, Holmes LM, Burgin KE, Yu X, Wagner TE.et al. (2002Glycoinositol phospholipid-anchored interleukin 2 but not secreted interleukin 2 inhibits melanoma tumor growth in mice Mol Cancer Ther 11019–1024. [PubMed] [Google Scholar]
- Kim YS, Sonn CH, Paik SG., and, Bothwell AL. Tumor cells expressing membrane-bound form of IL-4 induce antitumor immunity. Gene Ther. 2000;7:837–843. doi: 10.1038/sj.gt.3301175. [DOI] [PubMed] [Google Scholar]
- Nagarajan S., and, Selvaraj P. Glycolipid-anchored IL-12 expressed on tumor cell surface induces antitumor immune response. Cancer Res. 2002;62:2869–2874. [PubMed] [Google Scholar]
- Zhao F, Dou J, Wang J, Chu L, Tang Q, Wang Y.et al. (2010Investigation on the anti-tumor efficacy by expression of GPI-anchored mIL-21 on the surface of B16F10 cells in C57BL/6 mice Immunobiology 21589–100. [DOI] [PubMed] [Google Scholar]
- Soo Hoo W, Lundeen KA, Kohrumel JR, Pham NL, Brostoff SW, Bartholomew RM.et al. (1999Tumor cell surface expression of granulocyte-macrophage colony-stimulating factor elicits antitumor immunity and protects from tumor challenge in the P815 mouse mastocytoma tumor model J Immunol 1627343–7349. [PubMed] [Google Scholar]
- Marr RA, Addison CL, Snider D, Muller WJ, Gauldie J., and, Graham FL. Tumour immunotherapy using an adenoviral vector expressing a membrane-bound mutant of murine TNF alpha. Gene Ther. 1997;4:1181–1188. doi: 10.1038/sj.gt.3300528. [DOI] [PubMed] [Google Scholar]
- Ji J, Li J, Holmes LM, Burgin KE, Yu X, Wagner TE.et al. (2004Synergistic anti-tumor effect of glycosylphosphatidylinositol-anchored IL-2 and IL-12 J Gene Med 6777–785. [DOI] [PubMed] [Google Scholar]
- Chou WC, Liao KW, Lo YC, Jiang SY, Yeh MY., and, Roffler SR. Expression of chimeric monomer and dimer proteins on the plasma membrane of mammalian cells. Biotechnol Bioeng. 1999;65:160–169. doi: 10.1002/(sici)1097-0290(19991020)65:2<160::aid-bit5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Liao KW, Chou WC, Lo YC., and, Roffler SR. Design of transgenes for efficient expression of active chimeric proteins on mammalian cells. Biotechnol Bioeng. 2001;73:313–323. doi: 10.1002/bit.1064. [DOI] [PubMed] [Google Scholar]
- Metz CN, Brunner G, Choi-Muira NH, Nguyen H, Gabrilove J, Caras IW.et al. (1994Release of GPI-anchored membrane proteins by a cell-associated GPI-specific phospholipase D EMBO J 131741–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poloso NJ, Nagarajan S, Mejia-Oneta JM., and, Selvaraj P. GPI-anchoring of GM-CSF results in active membrane-bound and partially shed cytokine. Mol Immunol. 2002;38:803–816. doi: 10.1016/s0161-5890(02)00005-6. [DOI] [PubMed] [Google Scholar]
- Lo CH, Lee SC, Wu PY, Pan WY, Su J, Cheng CW.et al. (2003Antitumor and antimetastatic activity of IL-23 J Immunol 171600–607. [DOI] [PubMed] [Google Scholar]
- Ye YL, Lee YL, Chuang ZJ, Lai HJ, Chen CC, Tao MH.et al. (2004Dendritic cells modulated by cytokine-expressing adenoviruses alleviate eosinophilia and airway hyperresponsiveness in an animal model of asthma J Allergy Clin Immunol 11488–96. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Tahara H, Robbins PD, Storkus WJ, Clarke MR, Nalesnik MA.et al. (1995Cancer immunotherapy of established tumors with IL-12. Effective delivery by genetically engineered fibroblasts J Immunol 1551393–1403. [PubMed] [Google Scholar]
- Curti A, Parenza M., and, Colombo MP. Autologous and MHC class I-negative allogeneic tumor cells secreting IL-12 together cure disseminated A20 lymphoma. Blood. 2003;101:568–575. doi: 10.1182/blood-2002-03-0991. [DOI] [PubMed] [Google Scholar]
- Eisenring M, vom Berg J, Kristiansen G, Saller E., and, Becher B. IL-12 initiates tumor rejection via lymphoid tissue-inducer cells bearing the natural cytotoxicity receptor NKp46. Nat Immunol. 2010;11:1030–1038. doi: 10.1038/ni.1947. [DOI] [PubMed] [Google Scholar]
- Chakrabarti R, Chang Y, Song K., and, Prud'homme GJ. Plasmids encoding membrane-bound IL-4 or IL-12 strongly costimulate DNA vaccination against carcinoembryonic antigen (CEA) Vaccine. 2004;22:1199–1205. doi: 10.1016/j.vaccine.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Yei S, Bartholomew RM, Pezzoli P, Gutierrez A, Gouveia E, Bassett D.et al. (2002Novel membrane-bound GM-CSF vaccines for the treatment of cancer: generation and evaluation of mbGM-CSF mouse B16F10 melanoma cell vaccine Gene Ther 91302–1311. [DOI] [PubMed] [Google Scholar]
- Belnap LP, Cleveland PH, Colmerauer ME, Barone RM., and, Pilch YH. Immunogenicity of chemically induced murine colon cancers. Cancer Res. 1979;39:1174–1179. [PubMed] [Google Scholar]
- Chang TC, Huang CJ, Tam K, Chen SF, Tan KT, Tsai MS.et al. (2005Stabilization of hypoxia-inducible factor-1{alpha} by prostacyclin under prolonged hypoxia via reducing reactive oxygen species level in endothelial cells J Biol Chem 28036567–36574. [DOI] [PubMed] [Google Scholar]
- Schoenhaut DS, Chua AO, Wolitzky AG, Quinn PM, Dwyer CM, McComas W.et al. (1992Cloning and expression of murine IL-12 J Immunol 1483433–3440. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CTL activity induced by CT26/scIL-12-B7TM cells.
Groups of BALB/c mice (n = 5) inoculated s.c. with 5 × 105 CT26 cells were treated with Ad/GFP or Ad/scIL-12-B7TM at the indicated dose on day 10.