Abstract
Advances in the optimization of in vitro-transcribed mRNA are bringing mRNA-mediated therapy closer to reality. In cultured cells, we recently achieved high levels of translation with high-performance liquid chromatography (HPLC)-purified, in vitro-transcribed mRNAs containing the modified nucleoside pseudouridine. Importantly, pseudouridine rendered the mRNA non-immunogenic. Here, using erythropoietin (EPO)-encoding mRNA complexed with TransIT-mRNA, we evaluated this new generation of mRNA in vivo. A single injection of 100 ng (0.005 mg/kg) mRNA elevated serum EPO levels in mice significantly by 6 hours and levels were maintained for 4 days. In comparison, mRNA containing uridine produced 10–100-fold lower levels of EPO lasting only 1 day. EPO translated from pseudouridine-mRNA was functional and caused a significant increase of both reticulocyte counts and hematocrits. As little as 10 ng mRNA doubled reticulocyte numbers. Weekly injection of 100 ng of EPO mRNA was sufficient to increase the hematocrit from 43 to 57%, which was maintained with continued treatment. Even when a large amount of pseudouridine-mRNA was injected, no inflammatory cytokines were detectable in plasma. Using macaques, we could also detect significantly-increased serum EPO levels following intraperitoneal injection of rhesus EPO mRNA. These results demonstrate that HPLC-purified, pseudouridine-containing mRNAs encoding therapeutic proteins have great potential for clinical applications.
Introduction
Expressing a protein by delivering the encoding mRNA has many benefits over methods that use plasmid DNA or viral vectors. During mRNA transfection, the coding sequence of the desired protein is the only substance delivered to cells, thus avoiding all the side effects associated with plasmid backbones, viral genes, and viral proteins. Also, the cell-delivered mRNA can be instantly translated even in axon terminals located remotely from the nucleus, because mRNA only requires protein synthesis machinery, which is ubiquitous throughout the cell cytoplasm. More importantly, unlike DNA- and viral-based vectors, the mRNA does not carry the risk of being incorporated into the genome. Considering these benefits, it is surprising that mRNA-based transfection has not become a more common procedure to overexpress therapeutic proteins in vivo.
In 1992, Bloom and colleagues successfully rescued vasopressin-deficient rats by injecting in vitro-transcribed vasopressin mRNA into the hypothalamus.1 This represents the first and, until 2011, the only study where in vitro-transcribed mRNA is used for gene replacement in vivo. For many years, immunization with mRNA coding for cancer antigens has been the only field of application where direct injection of in vitro transcripts was successfully used, even reaching clinical trials.2,3,4,5 For this application, the low levels of translation and the immunogenicity of the mRNA were not limiting factors, but those two characteristics greatly restricted the exploitation of mRNA for many other in vivo and ex vivo applications.
As we and others have shown, in vitro transcribed RNA is immunogenic, activating cell surface, endosomal, and cytoplasmic RNA sensors, including Toll-like receptor (TLR) 3, TLR7, TLR8,6,7,8 retinoic acid-inducible gene I (RIG-I),9 RNA-dependent protein kinase (PKR),10,11 and the 2′-5′-oligoadenylate synthetase/RNase L axis.12 However, when modified nucleosides, such as pseudouridine, were incorporated into the RNA, it no longer activated TLR7, TLR8,8 RIG-I,9 PKR,11,13 and 2′-5′-oligoadenylate synthetase.12 More importantly, pseudouridine-containing mRNA (Ψ-mRNA) was not only translatable, but higher amounts of protein were produced compared to uridine-containing mRNA (U-mRNA).14 This was partly due to increased resistance of Ψ-mRNA to RNase L-mediated cleavage12 and a lack of PKR activation.11
Recently, Kormann et al. reported a successful in vivo use of modified nucleoside-containing mRNAs to express therapeutic proteins in mice.15 Incorporation of both 5-methylcytidine and 2-thiouridine into the mRNAs was critical for this accomplishment, since those reduced, although did not completely eliminate, immune activation. We achieved robust improvement in the translational capacity of Ψ-mRNA through high-performance liquid chromatography (HPLC) purification, which also completely eliminated immunogenicity when tested in vitro.16 Therefore, we were interested in testing the performance of these newly perfected mRNAs in vivo. We selected erythropoietin (EPO)-encoding mRNAs for our evaluation, since detecting the effect of EPO on red blood cell production, by measuring reticulocyte levels and the hematocrit from whole blood, is straightforward and well-established.
Using HPLC-purified EPO-encoding Ψ-mRNA complexed with TransIT-mRNA (TransIT), a nonliposomal cationic polymer/lipid formulation, we demonstrate that injection of submicrogram amounts into mice is sufficient to significantly increase the levels of reticulocytes and the hematocrit without an induction of circulating proinflammatory cytokines [interferon-α (IFN-α), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6)]. The presence of pseudouridine in the mRNA was critical, since HPLC-purified EPO U-mRNA induced IFN-α and had no significant effect on erythropoiesis. Extension of these preliminary studies into rhesus macaques similarly demonstrated the ability of systemically administered EPO-encoding Ψ-mRNA complexed with TransIT to produce significant amounts of circulating EPO without inducing proinflammatory cytokines. These results demonstrate that HPLC-purified, pseudouridine-containing mRNAs encoding therapeutic proteins have great potential for clinical applications.
Results
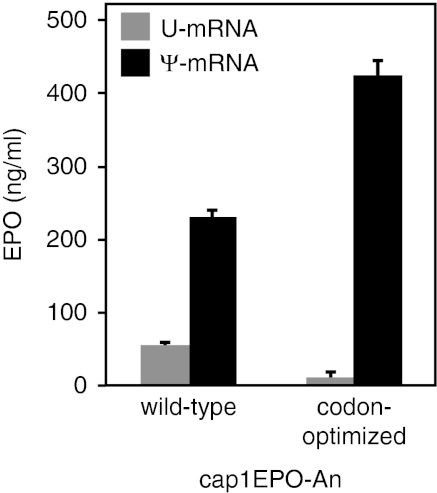
To identify the optimal murine EPO mRNA for use in vivo, we generated sets of mRNAs and tested their translational capacity in human dendritic cells (DCs). DCs efficiently translate exogenously delivered mRNA and express all known RNA sensors, such as TLR3, TLR7, TLR8, RIG-I, MDA-5, and PKR.17 All the features that are known to improve translation were incorporated into the transcripts. The mRNAs were HPLC purified and contained the 5′-UTR derived from the tobacco etch virus 5′ leader RNA, 3′-UTR of Xenopus β-globin mRNA, an enzymatically-generated cap1 structure, and a plasmid-encoded 51 nt-long poly(A) tail, which was extended to ~200 nt-long using poly(A) polymerase. Either pseudouridine or uridine was incorporated into the synthesized mRNAs. Codon optimization was used to increase translation further. The codon-optimized EPO mRNAs contained hybrids of AU- and GC-rich codons that have been reported to increase translation of human EPO mRNA.18 Indeed, the highest level of translation and a complete lack of proinflammatory cytokine secretion were achieved from pseudouridine-containing mRNA that was codon-optimized (Figure 1 and data not shown); therefore, we used this mRNA for subsequent in vivo analysis. For simplicity, we labeled this RNA as EPO Ψ-mRNA and the corresponding control mRNA that contained uridine as EPO U-mRNA.
Figure 1.
Codon-optimized, pseudouridine-containing murine erythropoietin (EPO) mRNA yields the highest level of translation. Human dendritic cells (DCs) were treated with TransIT-complexed in vitro-transcribed mRNA (0.1 µg/well) with the wild-type or codon-optimized sequence of murine EPO containing uridine (U-mRNA) or pseudouridine (Ψ-mRNA). The levels of EPO in the supernatants were measured by enzyme-linked immunosorbent assay (ELISA) 24 hours later. Error bars are standard errors of the mean (SEM). The data shown is one of three representative experiments.
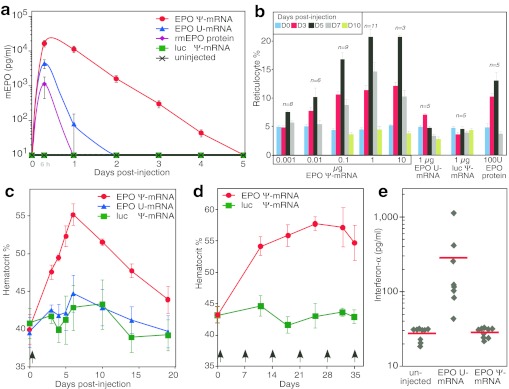
To determine if EPO mRNA administered in vivo can elevate blood EPO levels, adult mice were intraperitoneally (i.p.) injected with EPO mRNA (0.1 µg) complexed with TransIT. A sharp increase in serum EPO levels were measured, reaching a maximum at 6 hours postinjection (Figure 2a). Remarkably, EPO levels remained elevated up to 4 days after EPO Ψ-mRNA administration, while EPO U-mRNA resulted in much lower EPO levels that returned to pre-injection baseline levels by day 2. The injection of 3-µg recombinant murine EPO could not be detected beyond 6 hours postinjection, suggesting a rapid half-life, in agreement with studies that found a half-life for exogenously delivered EPO in mice of about 2 hours.19 Therefore, the high EPO levels we detected up to 4 days following a single injection are best explained by sustained translation of EPO Ψ-mRNA. When tenfold more (1.0 µg) EPO Ψ-mRNA complexed with TransIT was injected, a proportional tenfold higher EPO level was detected in the blood (data not shown). To determine if the injected EPO mRNA produces functional EPO protein, we counted reticulocytes in the treated animals. Reticulocytes are circulating immature red blood cells and their blood level is proportional to the rate of new red blood cell formation. In a dose escalation experiment, we gave EPO Ψ-mRNA (0.001–10 µg) complexed with TransIT to groups of mice and monitored blood reticulocyte levels. Significant increases in the levels of reticulocytes were measured as early as day 3 postinjection, reached a maximum at day 5, remained elevated at day 7, and declined to baseline (~5%) by day 10 (Figure 2b). Using human EPO protein, which is functional in mice, as a comparison, the reticulocyte level returned to baseline by day 7 (Figure 2b). There was a dose-dependent relationship between the amount of Ψ-mRNA injected, up to 1 µg, and the increase in circulating reticulocytes. It is remarkable that 0.001 µg EPO Ψ-mRNA was as potent as 1 µg of EPO U-mRNA in increasing reticulocyte counts (Figure 2b). It is worth mentioning that both subcutaneously and intramuscularly injected EPO Ψ-mRNA also raised reticulocyte counts significantly (data not shown), although not as potently as the i.p.-delivered mRNA. We found no elevation of reticulocyte counts at any time point when luciferase-encoding Ψ-mRNA complexed with TransIT was injected (Figure 2b), demonstrating that the effects were not due to Ψ-mRNA injection but to functional EPO translation from the exogenous EPO Ψ-mRNA (Figure 2b).
Figure 2.
Physiologic responses of mice injected with erythropoietin (EPO) mRNA. (a) Administration of EPO mRNA increases serum EPO levels. Adult mice were injected intraperitoneally (i.p.) with TransIT-complexed mRNA (0.1 µg) coding for murine EPO containing pseudouridine (EPO Ψ-mRNA) or containing uridine (EPO U-mRNA) or coding for firefly luciferase and containing pseudouridine (luc Ψ-mRNA) or with 3 µg of recombinant murine EPO (rmEPO) protein. Serum EPO levels were measured by enzyme-linked immunosorbent assay (ELISA) at the indicated time points. Five animals per condition were analyzed. (b) Increased reticulocyte count following a single injection of EPO mRNA. Mice received a single i.p. injection of the indicated amounts of EPO Ψ-mRNA, luc Ψ-mRNA, or EPO U-mRNA complexed with TransIT or human EPO protein (n = 3–11). Reticulocytes were counted at the indicated days using a 5 µl blood sample. Mice were injected with TransIT-complexed mRNA once with 1.0 µg RNA (c), or weekly (indicated by arrows) with 0.1 µg mRNA (d). Hematocrits were measured using 20 µl of blood. Five animals per group were analyzed. (e) Mice were injected with 1.0 µg of murine EPO mRNA that contained either pseudouridine or uridine, or were left uninjected. Murine IFN-α levels were measured in the plasma at 6 hours post-injection by ELISA. Individual data points and the group average (red line) are shown. Error bars are SEM.
Since ~1 µg EPO Ψ-mRNA produced a maximal effect, elevating the reticulocyte counts from a pre-injection level of 5% to 20% 5 days after injection, we used 1 µg mRNA to determine the physiological impact of EPO Ψ-mRNA on the hematocrit. As shown in Figure 2c, i.p. injection of TransIT-complexed EPO Ψ-mRNA led to a significant increase in the hematocrit as early as 3 days after injection and reached maximum at day 6. The hematocrit was still significantly elevated 2 weeks after injection. Injection of 1 µg of EPO U-mRNA or luc Ψ-mRNA did not result in a significant elevation of the hematocrit at any time point (Figure 2c), thus demonstrating pseudouridine incorporation is critical for efficient translation. We also found that an elevated hematocrit could be maintained with weekly injections of 0.1 µg EPO Ψ-mRNA complexed to TransIT. The preinjection hematocrit was raised, from 43.2 ± 1.5% up to 57.7 ± 1.1%, and maintained during the 5-week course of treatment (Figure 2d). Certain formulations of EPO protein given to humans20,21 and EPO gene therapy given to macaques22,23 have resulted in serious autoimmune reactions directed at EPO. Serum from mice that received 5 weekly injections of TransIT-complexed EPO Ψ-mRNA were analyzed for the presence of antibodies that bound to murine EPO protein in a sandwich enzyme-linked immunosorbent assay (ELISA) and none were detected. The assay could detect as little as 0.1 ng/ml of anti-EPO antibody in plasma.
We recently demonstrated that mRNA complexed with TransIT does not induce IFN-α secretion from transfected human DCs, as long as the RNA contains pseudouridine and is purified by HPLC.16 It was therefore important for us to determine whether or not EPO Ψ-mRNA remains nonimmunogenic in vivo. We injected mice with TransIT-complexed EPO Ψ-mRNA or EPO U-mRNA (1 µg) i.p. and measured serum IFN-α, TNF-α and IL-6 levels at 6 hours postinjection. While the uridine-containing EPO mRNA was highly immunogenic, resulting in robust induction of IFN-α measured from plasma, IFN-α was not induced in mice injected with EPO Ψ-mRNA (Figure 2e). The levels of TNF-α and IL-6 in the plasma of EPO Ψ-mRNA and EPO U-mRNA injected mice were at background levels (data not shown).
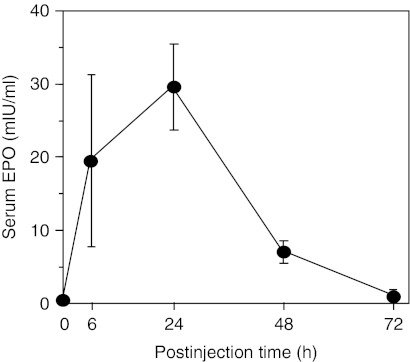
In a pilot study, we delivered a single injection of TransIT-complexed rhesus EPO-encoding Ψ-mRNA (rhEPO Ψ-mRNA) to macaques and measured circulating EPO levels. The i.p. injected rhEPO Ψ-mRNA elevated the levels of plasma EPO with kinetics similar to those observed in mice (Figure 3). No increase in the levels of serum type I IFNs, TNF-α, and IL-6 were detected after any dose of rhEPO Ψ-mRNA measured at 6–48 hours postinjection (data not shown).
Figure 3.
Pseudouridine-containing rhesus erythropoietin (EPO) mRNA increases circulating levels of EPO in macaques. Rhesus macaques (n = 4) were injected intraperitoneally (i.p.) with TransIT-complexed rhesus EPO Ψ-mRNA (0.012 mg/kg). Serum EPO levels were measured by enzyme-linked immunosorbent assay (ELISA). Error bars are SEM.
Discussion
Our study demonstrates the feasibility of using in vitro transcribed mRNA to express therapeutic proteins in vivo. As a model system, we used EPO-mediated induction of erythropoiesis to show the efficacy of mRNA-based therapy. We demonstrate in mice that a single dose of 0.1 µg EPO-encoding mRNA complexed with TransIT is sufficient to increase blood EPO levels as early as 6 hours postinjection and high levels are maintained for 4 days. As little as 0.01 µg EPO mRNA doubled the circulating reticulocyte count. We also demonstrate that the injected TransIT-complexed mRNA does not induce adverse side-effects or detectable increases in circulating proinflammatory cytokines. These remarkable features were achieved by a combination of incorporating pseudouridine into the mRNA and purifying by HPLC.
In our previous studies, we demonstrated that Ψ-mRNA possesses superior translational properties.14 The mechanisms underlying its enhanced translational quality include: (i) diminished PKR activation, thereby reducing translational inhibition,11 (ii) a stabilizing effect on the RNA by increasing resistance to RNase L-mediated cleavage,12 and (iii) a lack of 2′-5′-oligoadenylate synthetase activation and ribosomal RNA cleavage, thus avoiding a global reduction in translation.12 We also demonstrated that pseudouridine, similar to other modified nucleosides, reduced RNA-elicited innate immune responses.8 Recently, we further improved the quality of pseudouridine-containing RNA with HPLC purification.16 We found that HPLC removed double-stranded RNA and other contaminants from the in vitro-transcribed RNA, thereby maximizing its translational efficiency, as well as eliminating any residual immunogenicity. In this report, we demonstrate that HPLC-purified, pseudouridine-containing RNA complexed with TransIT, possessing very high translational capacity with no detectable immunogenicity, is not only effective in vivo but requires doses that will allow pharmacological development.
Despite much progress in the field, the immune activation caused by viral and plasmid vectors remains one of the primary reasons for failure in human gene therapy trials.24 Adverse immune reactions also emerge when administering therapeutic proteins.25 Examples of both include, respectively; induction of anti-EPO antibodies and severe autoimmune anemia when EPO-expressing viral vectors were used in monkeys,22,23 and patients treated with an altered formulation of recombinant EPO protein developed anti-EPO neutralizing antibodies and transfusion-dependent anemia.20,26 Thus, avoiding an immune reaction is essential for effective protein replacement, regardless if it is performed by directly supplying the protein or via an encoding vector. Using nucleoside-modified mRNA coding for the therapeutic protein seems the optimal choice. Kormann et al. recently used nucleoside-modified mRNA in vivo, and the innate immune reaction to the injected mRNA was limited but not completely abolished.15 Their mRNA construct still induced cytokine production, although at a lower level than unmodified mRNA. The residual immune activation was likely caused by transcription-related aberrant RNA contaminants; however, those contaminants, as we have found, are removable by HPLC-purification,16 thus making the nucleoside-modified mRNA nonimmunogenic (Figure 2e).
Generating constructs with codon-optimized sequences is another method to maximize protein expression.27 Mammalian codons usually have G or C in their third degenerative position, and such sequences are expressed more efficiently than those in which the codons end with A or T.28 High expression levels of human EPO were reported using a chimeric construct in which the N-terminal part of EPO was coded by sequences enriched in AT-ending codons, whereas the remaining part of EPO was coded by GC-ending codons.18 In the present study, we adopted this codon-optimization strategy for our murine EPO. Typically, codon optimizations are performed by comparing protein production from DNA plasmids carrying the wild-type sequence to the optimized sequence.28 In this setting, it is difficult to sort out if the increased protein expression from the codon-optimized construct is caused by enhanced transcription, increased RNA stability, or augmented translation. However, one study compared translation of cell-delivered wild-type and codon-optimized RNAs directly and, similar to our results with the Ψ-mRNAs (Figure 1), found an increase in protein production from the codon-optimized mRNA.29
As an extension to our analysis in mice, we were able to perform a short pilot study in rhesus macaques. HPLC-purified TransIT-complexed Ψ-mRNA encoding rhesus EPO was delivered i.p. at doses similar to those we used in mice, and we observed a substantial increase in circulating EPO. Due to limitations, including cost, time constraints, and the small cohort of macaques, we were unable to perform experiments where the hematological responses to EPO induction could have been measured. Regardless, and more importantly, no adverse effects were observed and no increase in levels of IFN-α, TNF-α, and IL-6 were found. Future studies are needed to optimize both the route of delivery and the formulation of complexing agent for primate studies. An initial evaluation in the macaques using subcutaneous injection, which is the preferred therapeutic route, found levels of circulating EPO at 48 hours that were similar to those measured at 24 hours, suggesting prolonged production and release.
mRNA therapy could be a promising approach for expressing proteins and the treatment of inherited genetic disorders. At present, extracellular proteins that are administered to patients, including type I IFNs, monoclonal antibodies, and adenosine deaminase, can successfully treat their conditions. However, delivering or replacing an intracellular protein requires alternative approaches, such as gene therapy. HPLC-purified, pseudouridine-modified mRNA offers a new approach to the therapeutic expression of both extra- and intracellular proteins. It avoids the two major adverse events found clinically with other gene therapy approaches, vector immunogenicity,30,31 and chromosomal integration.32 A limitation of mRNA delivery compared with integrative gene transfer is the transient duration of protein expression. However, there are many pathological disorders that can be treated with transient expression of therapeutic proteins. In addition, unless an integrative gene therapy approach targets the stem cells of interest, it will need to be repeated at a duration associated with the half-life of the cells being targeted. mRNA therapy has the added benefits that the dosing is scalable and the treatment stops when the RNA and the encoded protein degrade. Adapting mRNA delivery to therapeutics will require continued improvement in mRNA translational capacity, safe and efficient techniques to deliver mRNA to specific cells or tissues, a better understanding of the molecular mechanisms mediating mRNA uptake, and optimization of the large-scale production of mRNA for clinical use.
Materials and Methods
Cells. Human monocyte-derived DCs were produced as described previously.33
Animal experiments. All experiments were carried out with the approval of the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania. BALB/c female mice from Jackson Laboratory (Bar Harbor, ME), aged 6–12 weeks, were used. Macaques were housed and experiments were performed at Bioqual (Rockville, MD). They are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) and meet National Institutes of Health standards as described in the Guide for the Care and Use of Laboratory Animals. The University of Pennsylvania IACUC approved all experiments carried out by Bioqual.
mRNA synthesis. mRNAs were transcribed as previously described,14 using linearized plasmids encoding wild-type and codon-optimized murine EPO (pTEVwtEPO and pTEVoptEPO), firefly luciferase (pTEVLuc), and rhesus macaque EPO (pTEVrhEPO). The rhesus EPO and codon-optimized mouse EPO genes were synthesized by Entelechon (Bad Abbach, Germany). Codons for the mouse EPO gene were selected based on Entelechon's proprietary algorithm and codon-optimized human EPO.18 The Megascript T7 RNA polymerase kit (Ambion, Austin, TX) was used for transcription, and UTP was replaced with pseudouridine triphosphate (TriLink, San Diego, CA) to generate pseudouridine-containing mRNA. All mRNAs were transcribed to contain 51-nt long poly(A) tails. Additional poly(A) tail was added in a 3-hour incubation with yeast poly(A) polymerase (USB, Cleveland OH). To obtain cap1, RNA was processed using the m7G capping and 2′-O-methyltransferase kits (CellScript, Madison, WI). All mRNAs were HPLC-purified as described.16
Complexing of RNA. RNA was complexed to TransIT-mRNA (Mirus Bio, Madison, WI) according to the manufacturer. A ratio of mRNA (0.1 µg), TransIT-mRNA reagent (0.11 µl), and Boost reagent (0.07 µl) in a final volume of 10 µl Dulbecco's modified Eagle's medium was used. For complexing different amounts of mRNA the volumes of the reagents and the final volume were scaled proportionally.
i.p. injection of TransIT-complexed mRNA into mice. Blood, <25 µl, was drawn from the tail vein before treatments to establish baseline parameters. The mRNA and EPO proteins were injected into the peritoneal cavity with a 27-gauge needle using standard technique.
Measuring EPO levels and determining hematocrit. Translational levels of wild-type and codon-optimized EPO mRNAs were measured in human DCs. Cells were seeded into 96-well plates (1.5 × 105 cells/well) in 190 µl of complete medium and transfected by adding 0.1 µg mRNA complexed with TransIT in a 10-µl final volume. Mouse EPO levels were measured in the culture medium 24 hours post-transfection using a mouse EPO ELISA kit (R&D Systems, Minneapolis, MN). The same kit was used to measure EPO levels in the plasma of animals (5 mice/group) that were injected with either RNA complexed with TransIT or with 3 µg of recombinant mouse EPO protein (Sigma-Aldrich, St Louis, MO). Since blood was being drawn repeatedly, it was essential to minimize the volume so the sampling would not affect the hematological parameters of the animals. Eighteen microliter of blood was mixed with 2 µl EDTA (0.2 mol/l) and placed into a 20 µl Drummond microcaps glass microcapillary tube (Sigma-Aldrich). After sealing one end of the tubes with Cha-seal (Chase Scientific Glass, Rockwood, TN), the capillary tubes were centrifuged in IEC MB Microhematocrit Centrifuge (DAMON/IEC Division, Needham, MA) for 3 minutes at 14,000 rpm. Capillary tubes were scanned (ScanMaker; Microtek, Santa Fe, CA) and digital images of the tubes were imported into Canvas X (ADB System, Seattle, WA) and the packed cell volume ratio was determined. After determining the hematocrit, capillary tubes were snapped and the plasma was collected for the measurement of plasma EPO levels.
Reticulocyte counts. Five microliter of blood drawn from the tail vein of BALB/C mice (3–11 animals/group) injected with TransIT-complexed mRNA (0.001–10 µg) or with 100 U of recombinant human EPO (Epogen; Amgen, Thousands Oaks, CA) was mixed with 0.5 µl EDTA (0.2 mol/l) and analyzed using Retic-COUNT, a thiazole orange reagent (BD Biosciences, San Jose, CA), as recommended by the manufacturer. Stained cells were analyzed on a FACScalibur flow cytometer using CellQuest software (BD Biosciences). Values are expressed as the percentage of reticulocytes relative to total erythrocytes.
Measuring IFN-α, TNF-α, and IL-6. BALB/c mice injected with TransIT-complexed mRNA (1 µg) were processed 6 hours later by injecting 100 µl of heparin (Abraxis, Schaumburg, IL) i.p. and sacrificed. ELISA was used to measure the levels of IFN-α (R&D Systems), TNF-α and IL-6 (Cell Sciences, Sharon, MA) in undiluted plasma.
Analysis of EPO-specific antibody responses. A sandwich ELISA was used to measure the development of murine antibody responses to EPO protein. Plasma from mice injected weekly for 5 weeks with 0.1 µg of EPO or luc Ψ-mRNA was diluted 1:10 in phosphate-buffered saline and added to murine EPO protein coated plates. A positive control containing a rat anti-murine EPO mAb (R&D Systems) spiked into normal mouse plasma was used. Bound antibodies were detected with goat anti-mouse and rat Ig antisera labeled with peroxidase. A concentration of 0.1 ng/ml of anti-EPO antibody spiked into plasma-phosphate-buffered saline could be detected.
Macaque experiments. Two- to three-year-old Rhesus macaques (n = 4) were bled and injected with TransIT-complexed mRNA i.p. using standard procedures. Plasma for rhesus EPO was analyzed undiluted in a human EPO-specific ELISA kit that crossreacts with rhesus EPO (R&D Systems). The ELISA underestimates rhesus EPO by approximately fourfold.34 The uncorrected ELISA readings are given. Rhesus-specific ELISAs were used to measure the levels of IFN-α (PBL Interferon Source, Piscataway, NJ), TNF-α, and IL-6 (Cell Sciences) in undiluted plasma.
Acknowledgments
We thank Houping Ni for technical assistance, Magdolna Sebestyén (Roche Madison, Madison, WI) for advice on the macaque study, and Mark Lewis and Bioqual, Inc, Rockville, MD for performing the macaque studies. This work was supported by National Institutes of Health (grant number R01NS029331 and R42HL87688 to K.K.; R01AI50484 and R21DE019059 to D.W.). K.K. and D.W. have formed a small biotech company RNARx that receives funding from the National Institutes of Health (R42HL87688) to explore the use of nucleoside-modified mRNA for gene therapy.
REFERENCES
- Jirikowski GF, Sanna PP, Maciejewski-Lenoir D., and, Bloom FE. Reversal of diabetes insipidus in Brattleboro rats: intrahypothalamic injection of vasopressin mRNA. Science. 1992;255:996–998. doi: 10.1126/science.1546298. [DOI] [PubMed] [Google Scholar]
- Weide B, Carralot JP, Reese A, Scheel B, Eigentler TK, Hoerr I.et al. (2008Results of the first phase I/II clinical vaccination trial with direct injection of mRNA J Immunother 31180–188. [DOI] [PubMed] [Google Scholar]
- Weide B, Pascolo S, Scheel B, Derhovanessian E, Pflugfelder A, Eigentler TK.et al. (2009Direct injection of protamine-protected mRNA: results of a phase ½ vaccination trial in metastatic melanoma patients J Immunother 32498–507. [DOI] [PubMed] [Google Scholar]
- Rittig SM, Haentschel M, Weimer KJ, Heine A, Muller MR, Brugger W.et al. (2011Intradermal vaccinations with RNA coding for TAA generate CD8+ and CD4+ immune responses and induce clinical benefit in vaccinated patients Mol Ther 19990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiter S, Diken M, Selmi A, Türeci Ö., and, Sahin U. Tumor vaccination using messenger RNA: prospects of a future therapy. Curr Opin Immunol. 2011;23:399–406. doi: 10.1016/j.coi.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Karikó K, Ni H, Capodici J, Lamphier M., and, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279:12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- Diebold SS, Kaisho T, Hemmi H, Akira S., and, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- Karikó K, Buckstein M, Ni H., and, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Hornung V, Ellegast J, Kim S, Brzózka K, Jung A, Kato H.et al. (20065′-Triphosphate RNA is the ligand for RIG-I Science 314994–997. [DOI] [PubMed] [Google Scholar]
- Nallagatla SR, Hwang J, Toroney R, Zheng X, Cameron CE., and, Bevilacqua PC. 5′-triphosphate-dependent activation of PKR by RNAs with short stem-loops. Science. 2007;318:1455–1458. doi: 10.1126/science.1147347. [DOI] [PubMed] [Google Scholar]
- Anderson BR, Muramatsu H, Nallagatla SR, Bevilacqua PC, Sansing LH, Weissman D.et al. (2010Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation Nucleic Acids Res 385884–5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BR, Muramatsu H, Jha BK, Silverman RH, Weissman D., and, Karikó K. Nucleoside modifications in RNA limit activation of 2′-5′-oligoadenylate synthetase and increase resistance to cleavage by RNase L. Nucleic Acids Res. 2011;39:9329–9338. doi: 10.1093/nar/gkr586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallagatla SR., and, Bevilacqua PC. Nucleoside modifications modulate activation of the protein kinase PKR in an RNA structure-specific manner. RNA. 2008;14:1201–1213. doi: 10.1261/rna.1007408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karikó K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S.et al. (2008Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability Mol Ther 161833–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kormann MS, Hasenpusch G, Aneja MK, Nica G, Flemmer AW, Herber-Jonat S.et al. (2011Expression of therapeutic proteins after delivery of chemically modified mRNA in mice Nat Biotechnol 29154–157. [DOI] [PubMed] [Google Scholar]
- Karikó K, Muramatsu H, Ludwig J., and, Weissman D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011;39:e142. doi: 10.1093/nar/gkr695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M, Schulte BM, Eleveld-Trancikova D, van Hout-Kuijer M, Toonen LW, Tel J.et al. (2010Cross-talk between human dendritic cell subsets influences expression of RNA sensors and inhibits picornavirus infection J Innate Immun 2360–370. [DOI] [PubMed] [Google Scholar]
- Kim CH, Oh Y., and, Lee TH. Codon optimization for high-level expression of human erythropoietin (EPO) in mammalian cells. Gene. 1997;199:293–301. doi: 10.1016/s0378-1119(97)00384-3. [DOI] [PubMed] [Google Scholar]
- Lee DE, Son W, Ha BJ, Oh MS., and, Yoo OJ. The prolonged half-lives of new erythropoietin derivatives via peptide addition. Biochem Biophys Res Commun. 2006;339:380–385. doi: 10.1016/j.bbrc.2005.11.034. [DOI] [PubMed] [Google Scholar]
- Casadevall N, Nataf J, Viron B, Kolta A, Kiladjian JJ, Martin-Dupont P.et al. (2002Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin N Engl J Med 346469–475. [DOI] [PubMed] [Google Scholar]
- Bennett CL, Cournoyer D, Carson KR, Rossert J, Luminari S, Evens AM.et al. (2005Long-term outcome of individuals with pure red cell aplasia and antierythropoietin antibodies in patients treated with recombinant epoetin: a follow-up report from the Research on Adverse Drug Events and Reports (RADAR) Project Blood 1063343–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Lebherz C, Weiner DJ, Grant R, Calcedo R, McCullough B.et al. (2004Erythropoietin gene therapy leads to autoimmune anemia in macaques Blood 1033300–3302. [DOI] [PubMed] [Google Scholar]
- Chenuaud P, Larcher T, Rabinowitz JE, Provost N, Cherel Y, Casadevall N.et al. (2004Autoimmune anemia in macaques following erythropoietin gene therapy Blood 1033303–3304. [DOI] [PubMed] [Google Scholar]
- Hedman M, Hartikainen J., and, Ylä-Herttuala S. Progress and prospects: hurdles to cardiovascular gene therapy clinical trials. Gene Ther. 2011;18:743–749. doi: 10.1038/gt.2011.43. [DOI] [PubMed] [Google Scholar]
- Kromminga A., and, Schellekens H. Antibodies against erythropoietin and other protein-based therapeutics: an overview. Ann N Y Acad Sci. 2005;1050:257–265. doi: 10.1196/annals.1313.027. [DOI] [PubMed] [Google Scholar]
- Kharagjitsingh AV, Korevaar JC, Vandenbroucke JP, Boeschoten EW, Krediet RT, Daha MR, NECOSAD Study Group et al. Incidence of recombinant erythropoietin (EPO) hyporesponse, EPO-associated antibodies, and pure red cell aplasia in dialysis patients. Kidney Int. 2005;68:1215–1222. doi: 10.1111/j.1523-1755.2005.00514.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson C, Govindarajan S., and, Minshull J. Codon bias and heterologous protein expression. Trends Biotechnol. 2004;22:346–353. doi: 10.1016/j.tibtech.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Zhong F, Cao W, Chan E, Tay PN, Cahya FF, Zhang H.et al. (2005Deviation from major codons in the Toll-like receptor genes is associated with low Toll-like receptor expression Immunology 11483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngumbela KC, Ryan KP, Sivamurthy R, Brockman MA, Gandhi RT, Bhardwaj N.et al. (2008Quantitative effect of suboptimal codon usage on translational efficiency of mRNA encoding HIV-1 gag in intact T cells PLoS ONE 3e2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP.et al. (2003Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer Mol Genet Metab 80148–158. [DOI] [PubMed] [Google Scholar]
- Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM., and, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P.et al. (2003LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1 Science 302415–419. [DOI] [PubMed] [Google Scholar]
- Weissman D, Ni H, Scales D, Dude A, Capodici J, McGibney K.et al. (2000HIV gag mRNA transfection of dendritic cells (DC) delivers encoded antigen to MHC class I and II molecules, causes DC maturation, and induces a potent human in vitro primary immune response J Immunol 1654710–4717. [DOI] [PubMed] [Google Scholar]
- Rivera VM, Gao GP, Grant RL, Schnell MA, Zoltick PW, Rozamus LW.et al. (2005Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer Blood 1051424–1430. [DOI] [PubMed] [Google Scholar]