Abstract
Immune responses to transgene products may lead to rejection of transduced cells, limiting successful gene therapy for genetic diseases. While moderate dosages of chemotherapeutic agents such as busulfan may increase hematopoietic stem cells (HSC) engraftment, they are not immune suppressive and do not abrogate immune responses to transgene products. Studies focused on nonmyeloablative conditioning with busulfan ± fludarabine in a clinically relevant monkey model to induce immune suppression to allow cells expressing a foreign transgene product to persist. Bone marrow CD34+ HSC were transduced in two equal fractions using simian immunodeficiency virus (SIV)-based lentiviral vectors carrying a nonexpressed DNA sequence tag (NoN) and the green fluorescent protein (GFP) reporter gene. Post-transplant there was no evidence of elimination of cells containing the potentially immunogenic GFP gene; several recipients had stable persistence of cells, and no differences were detected with fludarabine, which was rapidly cleared. Antibodies and cellular immune responses to GFP developed in recipients with the highest levels of GFP-marked cells, although these cells were not eliminated. These studies establish a clinically relevant pediatric primate model to assess the effects of conditioning regimens on the engraftment of transduced HSC and the immune responses to cells expressing a foreign gene product.
Introduction
Genetic blood cell diseases, such as primary immune deficiencies, hemoglobinopathies, and lysosomal storage and metabolic diseases, may be treated by transplantation of hematopoietic stem cells (HSC) from a healthy allogeneic donor to the affected patient. Gene therapy using gene correction of autologous HSC is under development to treat these genetic blood cell diseases. Ideally, gene therapy will achieve equivalent clinical benefits for patients with these disorders, but with no risks for graft versus host disease, which can be a significant cause of morbidity and mortality with allogeneic HSC transplants. Initial gene therapy efforts using HSC did not administer cytoreductive conditioning to avoid the potential toxicities when benefits were unproven.1,2,3 However, in these early studies, essentially no clinical benefits were achieved and only extremely low levels of engrafted gene-corrected HSC were found. An important exception has been in trials for X-linked severe combined immune deficiency where the potent selective expansion of gene-corrected T lymphocytes allowed immune reconstitution to occur,4,5 although engraftment of gene-corrected HSC may not have occurred based on the absence of transduced myeloid cells beyond 1 year.6 Aiuti et al.7,8 advanced the field by using a nonmyeloablative regimen of busulfan as cytoreductive conditioning prior to gene therapy for patients with adenosine deaminase-deficient severe combined immune deficiency. By administering 4 mg/kg of busulfan, approximately one-fourth of the standard clinical dosage for full cytoablation of 16 mg/kg typically used, these authors achieved much higher levels of engraftment of gene-corrected HSC than previously observed, providing therapeutic benefits to the patients with essentially no clinical toxicity except transient myelosuppression.
Immune responses to transgene products may be a major factor limiting the successful replacement of missing gene products by gene therapy for genetic diseases. Immune responses to the transgene product, which is foreign in patients who congenitally lack expression of the protein, may lead to rejection of the transduced cells and loss of efficacy. The successful gene therapy in severe combined immune deficiency patients may, in part, reflect the inherent absence of immune-responsiveness in the recipients that could reject the gene-corrected cells. For most other genetic disorders considered for gene therapy, the recipients will have relatively normal immune function and may react immunologically against the transgene product, the vector, or excipients.9,10 While moderate dosages of chemotherapeutic agents such as busulfan or melphalan may increase the engraftment of gene-modified HSC, they are not significantly immune suppressive and thus do not abrogate immune responses to the transgene products. For these patients, it may be necessary to impose both myelosuppression as well as immune ablation to prevent rejection of the cells expressing the transferred gene product. Ideally, once the immune system reconstitutes from the gene-corrected HSC, a state of immunological tolerance will develop to the transgene product, as it is persistently expressed in the resultant cells and may include thymic dendritic cells and other cells that mediate tolerance. It may be expected that the majority of HSC and blood cells would not be from the transduced cells, but rather endogenous cells that were not ablated by the relatively low dose chemotherapy given. Nonetheless, persistent production of cells expressing the foreign transgene, even as a minority of the population, may provide sufficient antigen persistence to maintain a state of tolerance.
The studies described used an infant nonhuman primate model of gene transfer/bone marrow transplantation and reduced intensity conditioning11 to begin to address issues of transgene immunogenicity and induction of tolerance with a clinically acceptable conditioning regimen. The potential to induce tolerance to a foreign transgene product [green fluorescent protein (GFP)] was assessed by combining immunoablative dosages of fludarabine with nonmyeloablative marrow conditioning with busulfan before gene transfer/bone marrow transplantation. A competitive gene marking strategy with two simian immunodeficiency virus (SIV)-based lentiviral vectors was used to mark the CD34+ HSC in each recipient; one vector carried the nonexpressed neo (NoN) DNA sequence tag and the other included the GFP reporter gene. GFP has been reported to be immunogenic in mice and monkeys when introduced via transduced bone marrow cells without prior immune suppression,12,13,14,15,16,17 although full cytoablative conditioning may allow persistence of GFP-expressing cells.18 Thus, GFP was used as a test antigen to assess immune responses to the foreign transgene product and potential blunting by the preparative regimen. We also studied whether addition of a clinically acceptable immunosuppressive agent to conditioning with busulfan would induce sufficient immune suppression to allow cells expressing a foreign transgene product to engraft and persist. Fludarabine (9-β-D-arabinofuranosyl-2-fluoroadenine 5′-monophosphate) was developed as an antineoplastic reagent and is in widespread clinical use for the treatment of leukemia.19,20,21 Fludarabine has very potent anti-lymphocyte activity, providing efficacy in eradicating lymphocytic leukemia cells, but also results in significant lymphopenia and immune suppression. Fludarabine has been adopted for use in HSC transplant preconditioning regimens for its immune suppressive activity, and is often combined with busulfan, which is myeloablative, but not particularly immune suppressive, to allow engraftment of allogeneic HSC.22 Because of the absence of published data on the pharmacokinetics of fludarabine in infant rhesus monkeys, animals were treated in three successive cohorts where the fludarabine dosages were successively increased. The findings provide new information on the immunological responses to the GFP transgene product and may provide a platform for additional studies of immune responses and tolerance to novel transgene products in the context of gene therapy using HSC.
Results
Clinical observations
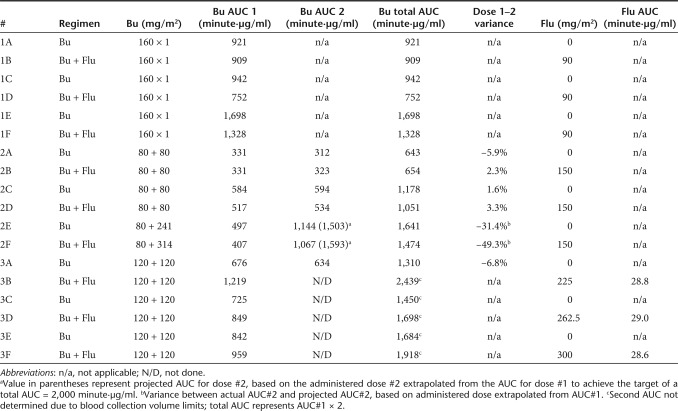
Infant rhesus monkeys (~3 months postnatal age; total N = 18) were transplanted in three series with escalating intensity of conditioning (Figure 1 and Table 1). Each received an infusion of autologous bone marrow CD34+ HSC, with one-half of the cells transduced with the potentially immunogenic GFP gene and the other half transduced with the NoN gene. The first group of six animals (Group 1, #1A–#1F) received busulfan as a single dose of 160 mg/m2; three of these animals also received fludarabine intravenously (i.v.) at 30 mg/m2/day × 3 days (total dose of 90 mg/m2). The second group of six animals (Group 2, #2A–#2F) received busulfan split into two doses, with 80 mg/m2 given on the first day and either 80 mg/m2 given on the third day (total of 160 mg/m2; #2A–#2D) or with a second tailored dose calculated based on the pharmacokinetics from the first dose to attempt to reach a net area under the curve (AUC) of 2,000 minute·µg/ml (for totals of 320 and 394 mg/m2 administered; #2E and #2F, respectively). Three of the animals from Group 2 were also administered fludarabine at 50 mg/m2/day × 3 days (150 mg/m2 total dose). The third group of six animals (Group 3, #3A–#3F) received busulfan split into two doses, each of 120 mg/m2 (total 240 mg/m2). Fludarabine was given to one of each of these monkeys at dosages of 75 mg/m2/day × 3 days (225 mg/m2 total dose, #3B), 87.5 mg/m2/day × 3 days (262.5 mg/m2 total dose, #3D), or 100 mg/m2/day × 3 days (300 mg/m2 total dose, #3F).
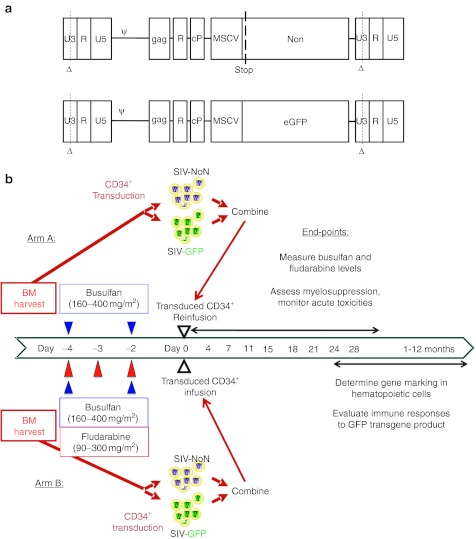
Figure 1.
Experimental approach. (a) Simian immunodeficiency virus (SIV)-based lentiviral vectors were used with either the nonexpressed neomycin transferase gene with a stop codon at the 5′ end (NoN) or the enhanced green fluorescent protein (GFP) gene. Vector backbones contain self-inactivating (SIN) deletions in the U3 regions of their long terminal repeats (Δ), as well as the SIV packaging sequence (ψ) which extends into the 5′ end of the gag gene, the rev-responsive element (R), and the central polypurine/central termination signal (cP), as well as the 5′ LTR from the murine stem cell virus (MSCV) as an internal promoter. (b) Overall experimental plan for nonmyeloablative conditioning, CD34+ cell transduction with lentiviral vectors, and autologous transplantation. Bone marrow was collected as described (see text), CD34+ cells were isolated, and for each recipient, half of the cells were transduced using the SIV-NoN lentiviral vector and half with the SIV-GFP lentiviral vector. Monkeys in both experimental arms (A and B) received nonmyeloablative conditioning with busulfan at the indicated dosages, and those in arm B also received fludarabine; blood levels of the drugs were measured. The CD34+ cells were combined and reinfused into their autologous donors for hematopoietic stem cells (HSC) transplants. Post-transplant follow-up included biweekly evaluations in the first month to detect acute toxicity then monthly evaluations to measure gene marking and immune responses for 6–12 months.
Table 1. Busulfan (Bu) and fludarabine (Flu) treatment regimen and measured area under the curve (AUC).
Serum chemistries were monitored twice weekly for the first month postconditioning (busulfan ± fludarabine) then monthly thereafter. There were no significant abnormalities of electrolytes, blood urea nitrogen, creatinine, bilirubin, or serum albumin. Serum levels of hepatic transaminases never exceeded >1.5-fold above the upper limit of the normative range for this age group (11–44 U/l) (historical controls N = 100). Similarly, there were no behavioral changes, anorexia, mucositis, hair loss, emesis, or other clinical manifestations from the chemotherapy dosages used.
Busulfan pharmacokinetics
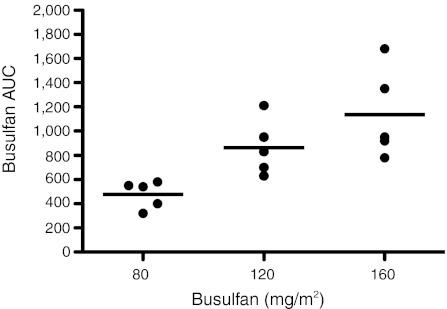
Busulfan levels in serum were measured following the first dose in all monkeys (Table 1). As expected, increasing administered dosages of busulfan led to increasing AUC for busulfan (Pearson correlative coefficient = 0.76, P = 0.0002) (Figure 2). However, there were significant interindividual variances in the AUC achieved per dosage, with greater than twofold differences in AUC measured in recipients of the same dosage. While busulfan dosages were determined based on body-surface area (e.g., 80 mg/m2), they were back-calculated to indicate the dosage based on recipient body mass (mg/kg). There was a similar correlation and degree of interindividual variation between the AUC of busulfan and the dosages administered based on body mass when compared to body surface area (data not shown).
Figure 2.
Busulfan pharmacokinetics after first dose. Busulfan was administered at dosages of either 80, 120, or 160 mg/m2 and serum levels of busulfan were measured at sequential time-points after the first dose. The area under the curve (AUC) of busulfan serum concentrations were calculated for each monkey. Horizontal bars indicate the mean value for each dosage group.
In seven of the transplanted infants, busulfan levels were measured after the first and second doses (Table 1). Five of these individuals received the same dosage of busulfan for the first and second infusions and two received a modified second infusion based on the level determined from the first dose as noted above in order to target a net AUC of 2,000 minute·µg/ml.11 For the five animals where the busulfan levels were measured after two identical dosages, there was high reproducibility of the levels achieved with the two doses in each individual (1.6–6.6% variation). For the two monkeys in which the second dosage was targeted based on the level measured from the initial dose, the second AUC levels were found to be lower than projected, leading to a total AUC of 18–26% below the target level of 2,000 minute·µg/ml (1,641 and 1,471 minute·µg/ml).
Fludarabine pharmacokinetics
One goal of these studies was to determine the potential effects from adding fludarabine to the busulfan treatment regimen, as a potential immuneablative component to potentially allow tolerance to a foreign protein expressed from the transplanted HSC. The sensitivity of young monkeys to fludarabine when coadministered with busulfan was not previously known, and there are no published measurements of fludarabine pharmacokinetics in rhesus monkeys, particularly in this age group. Therefore, to avoid possible adverse effects, the initial dosage was at the lower end of the clinical fludarabine dosing range (90 mg/m2). For the first series of animals, six monkeys were administered busulfan (160 mg/m2 total dose) and three of these monkeys were also administered fludarabine (30 mg/m2 i.v. × 3 days; 90 mg/m2 total). There was no discernible additive effect from fludarabine coadministration on the clinical outcomes. Most relevant, there was no induction of lymphopenia, which is the hallmark of fludarabine activity when used in humans at therapeutic dosages.23 Based on these findings, the next group of three monkeys was administered higher dosages of fludarabine at the upper end of the typical clinical range (50 mg/m2 × 3 days; 150 mg/m2 total). There was no additional clinical toxicity, lymphopenia, or other hematological toxicities, compared to the recipients receiving busulfan only. In the final group, fludarabine dosages were increased successively, such that one each received fludarabine at 75 mg/m2 × 3 days (225 mg/m2 total), 87.5 mg/m2 × 3 days (262.5 mg/m2 total), or 100 mg/m2 × 3 days (300 mg/m2 total). There were no clinical or hematological effects beyond those of the busulfan alone with this additional treatment regimen.
For the last three recipients, fludarabine plasma levels were serially measured following the first dose (1 minute, then 1, 3, and 12 hours postinfusion) and used to compute the peak concentrations (Cmax), the clearance (half-life, t1/2), and total exposure (AUC) (Supplementary Table S1). Despite dose escalation of fludarabine from 75 to 87.5 to 100 mg/m2, the fludarabine AUC were very similar. The fludarabine clearance in the monkey infants was markedly more rapid (t1/2 = 1.93 ± 0.17 hours) than that observed in human studies where the circulating half-life ranges from 8 to 20 hours.22,24 Although a drug–drug interaction between busulfan and fludarabine is possible, the concentrations of busulfan were not altered with the addition of fludarabine.
Hematological effects of the conditioning regimens
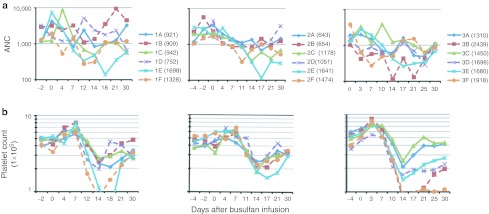
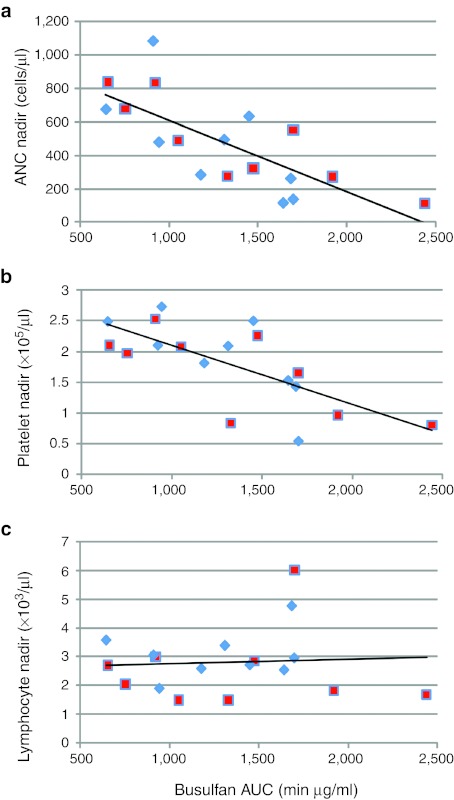
Transient neutropenia and thrombocytopenia occurred in all recipients (Figure 3). In the first two groups of animals (#1A–#1F and #2A–#2F), there were no discernible differences in the degree or time-course of myelosuppression that developed in recipients of busulfan alone compared to those receiving both busulfan and fludarabine. In the third group of animals (#3A–#3F), recipients of higher dosages of fludarabine with busulfan (dashed lines in Figure 3a, right panel) displayed earlier onsets of neutropenia (days 3–10) than did the recipients of busulfan only, suggesting that the fludarabine did have some suppressive effect on granulocytopoiesis in the time period immediately post-treatment. The nadir neutrophil and platelet counts were correlated inversely with the busulfan levels achieved (correlation coefficients = −0.74, P = 0.0004 and −0.71, P = 0.001, respectively) (Figure 4a,b). In contrast, there was no dosage-related effect of busulfan (without or with fludarabine) on total lymphocyte counts (correlation coefficient = 0.06, P = 0.81) (Figure 4c). Addition of fludarabine to the busulfan did not alter either the busulfan AUC achieved (P = 0.72 by two-sample t-test) or the nadir ANC levels reached (P = 0.56) compared to treatment with busulfan alone without fludarabine.
Figure 3.
Absolute neutrophil and platelets counts after nonmyeloablative conditioning. Complete blood counts were assessed every 2–4 days for the month following conditioning and transplant. (a) Absolute neutrophil counts (ANC) and (b) platelet counts for each of the three groups of six recipients are shown. Monkeys from arm A (busulfan only) are plotted with solid lines and those from arm B (busulfan + fludarabine) are plotted with dashed lines.
Figure 4.
Nadir values of absolute neutrophils counts (ANC), platelet, and lymphocyte counts versus busulfan serum concentrations [area under the curve (AUC)]. The lowest value of (a) ANC (cells/µl) (R2 = 0.5502), (b) platelet counts (×105/µl) (R2 = 0.5005), and (c) lymphocyte counts (cells/µl) (R2 = 0.0036) recorded in the first month following conditioning and transplant are shown versus the busulfan AUC determined for each recipient. Recipients in arm A (busulfan only) are shown with diamond symbols and those in arm B (busulfan + fludarabine) are indicated with square symbols.
Lentiviral vector transduction of CD34+ cells
For all transplants, the autologous CD34+ cells isolated from bone marrow were divided into two equal portions, with one fraction transduced with the SIV-NoN marker vector and the other fraction transduced with the SIV-GFP vector. These cells were re-admixed and infused i.v. 48 hours after the last doses of chemotherapy had been given, to allow assessment of the relative survival of the GFP-transduced cells compared to the NoN-transduced cells. The data for the numbers of CD34+ cells that were transduced, the percentages of cells that expressed GFP by flow cytometry before transplantation, the vector copy numbers per cell [measured by real-time quantitative PCR (qPCR)], the numbers of colonies grown from each CD34+ cell fraction, and the percentage of colonies that expressed GFP for the third series of transplants are shown (Supplementary Table S2).
CD34+ cell dosages were between 3.7 and 6.8 × 106 cells (with average mass ~1 kg), which are within the range typically used in human transplants. GFP expression from cells transduced with the SIV-GFP vector ranged between a low of 6.2% to a high of 39.3%. The vector copies/cell measured from the CD34+ cells immediately after transduction ranged from 1.8 to 204 copies/cell. These high values are questionable and may in part represent nonintegrated reverse-transcription products and possibly residual vector plasmids from the packaging process present in or on the CD34+ cells shortly after transduction. Two of the marrow samples (#3A, #3C) grew very few colonies, whereas the other samples produced between 12 to 71 colony-forming unit-granulocyte macrophage from the plated sample. Between 47 and 57% of the colony-forming unit-granulocyte macrophage grown from the CD34+ cells transduced with the SIV-GFP vector expressed the GFP transgene.
To further characterize the relationship between vector copy numbers assessed immediately or after a 2-week short-term culture of the cells (to allow wash-out of nonintegrated vector DNA forms), CD34+ cells from a rhesus monkey donor of similar age were transduced with either the SIV-NoN and SIV-GFP vectors and analyzed for vector copy number 1–2 days after transduction and after a 2-week culture. We found that the vector copy number measured immediately after transduction grossly overestimated the gene transfer. (Supplemental Figure S1). We also plated colony forming units (CFUs) and determined the percentage that were vector provirus positive by PCR and the percentage of cells that expressed GFP by flow cytometry. 75% of the CFU grown from the CD34+ cells transduced with the GFP vector contained the vector as determined by PCR and 30% of the cells expressed GFP as determined by flow cytometry.
Gene marking of blood and bone marrow cells
Following autologous transplants, samples of peripheral blood and bone marrow were obtained monthly. Cell subpopulations were isolated by ficoll gradient separation and immunomagnetic methods [peripheral blood mononuclear cells (PBMCs); granulocytes from blood; CD34+ and CD34− cells from bone marrow]. DNA was extracted to measure the levels of cells containing each of the vectors, and assayed by qPCR using primers and probes to the NoN and GFP sequences. At the time of tissue harvest, large volumes of blood were obtained and additional leukocyte subsets were isolated and assayed by qPCR for the levels of gene marking.
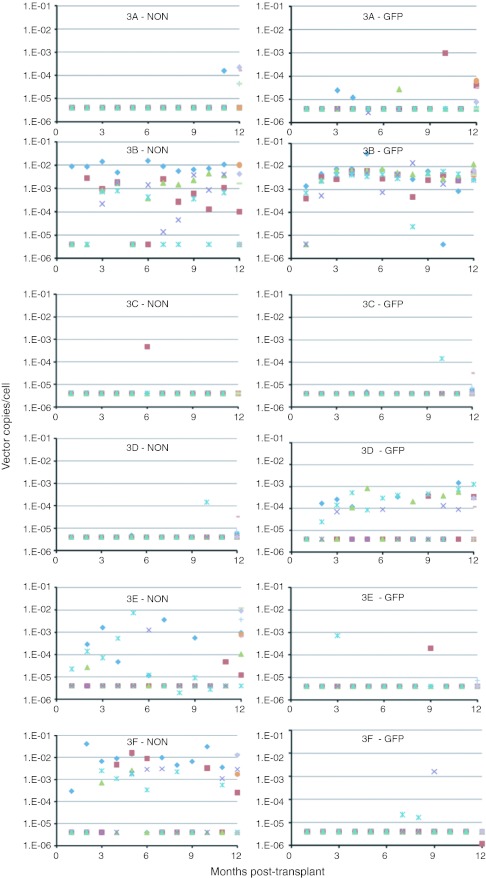
The gene marking data from the six recipients treated in Group 3 (#3A–#3F) are shown in Figure 5. Recipient #3B displayed the highest and most consistent gene marking in blood and bone marrow cells of any of the treated animals. In this recipient, the levels of GFP-marked cells consistently ranged between 0.001 and 0.01 vector copies/cell (equivalent to 0.1–1.0% of cells with a single copy of the vector). Recipient #3D had GFP marking at 0.0001–0.001 vector copies/cell (0.001–0.01%) but no NoN marking. Recipients #3E and #3F showed marking with the NoN vector at 0.001–0.01 vector copies/cell, but essentially no GFP-marked cells.
Figure 5.
Gene marking levels in blood and bone marrow cells. Peripheral blood and bone marrow samples were obtained from recipients #3A–#3F monthly post-transplant and cell subpopulations were isolated. Gene marking levels by the SIV-NoN and SIV-GFP vectors (vector copies/cell) were determined by quantitative PCR (qPCR). Mononuclear cell fractions were isolated from peripheral blood (Bld MNC; purple X) and bone marrow (BM MNC; green triangle), and the bone marrow mononuclear cells were further separated into CD34+ (BM CD34+; blue diamond) and CD34− (BM CD34−; brown square) cell fractions during each of the 12-month time points that were evaluated. At 1 year post-transplant, peripheral blood cells were fractionated further to produce populations of peripheral blood CD2+ T lymphocytes (Bld CD2+; blue cross), CD4+ T lymphocytes (Bld CD4+; red dash), CD8+ T lymphocytes (Bld CD8+; green dash), CD20+ B lymphocytes (Bld CD20+; purple diamond), and CD34+ progenitor cells (Bld CD34+; orange circle). Genomic DNA isolated from these samples was evaluated for vector copies per cell of both the NoN and GFP genes by qPCR. SIV, Simian immunodeficiency virus.
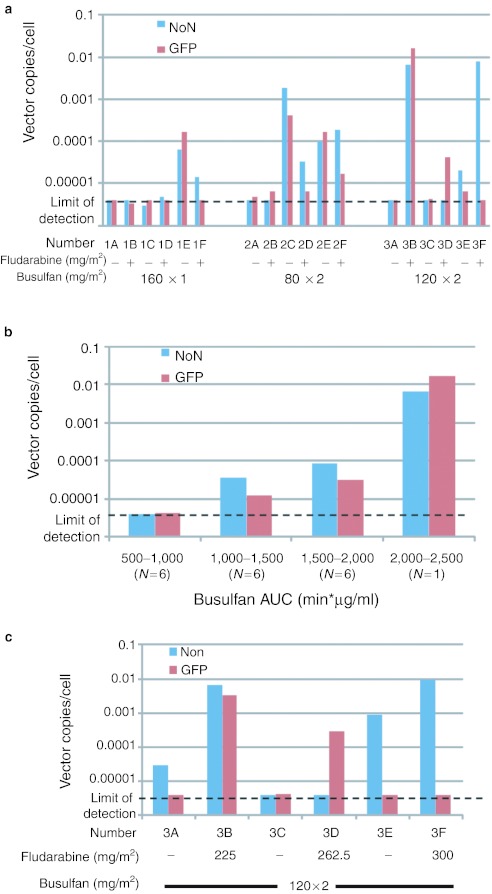
The average levels of gene marking in bone marrow CD34+ cells from months 4, 5, and 6 post-transplant for all 18 recipients are shown in Figure 6. While many of the monkeys had essentially no gene-marked CD34+ cells in their bone marrow during months 4–6 post-transplant, marking from 0.0001 to 0.01 vector copies/cell was achieved in several of the animals. In general, the levels of marking by both vectors were similar, independently of whether they had been treated with busulfan and fludarabine (#2F, #3B) or busulfan alone (#1E, #2C, #2E). There was no evidence of preferential loss of cells expressing GFP compared to those with the NoN gene suggesting the absence of a cytolytic immunologic response to cells expressing GFP. The levels of gene marking of bone marrow CD34+ cells in the recipients during months 4–6 were grouped, based on the busulfan levels that had been achieved during conditioning (Figure 6b). There were positive correlations between the busulfan AUC achieved and the levels of marking with both the NoN and GFP genes (Pearson correlation = 0.62, P = 0.006 and 0.57, P = 0.014, respectively).
Figure 6.
Gene marking levels in bone marrow CD34+ cells. (a) Average gene marking in bone marrow CD34+ cells during months 4–6. The levels (vector copy/cell) of NoN (blue bar) and GFP (red bar) genes measured in the bone marrow CD34+ cell samples from months 4–6 after transplant were averaged and are shown for all 18 recipients. The conditioning regimens each received prior to transplant are indicated on the x-axis (see Table 1). (b) Geometric mean gene marking in bone marrow CD34+ cells during months 4–6. The 18 recipients were clustered into four categories based upon the busulfan area under the curve (AUC) measured for each and the geometric means of the average levels of marking the NoN (blue bar) and GFP (red bar) vectors in bone marrow CD34+ cells were calculated. (c) Average gene marking in bone marrow CD34+ cells during months 7–12. The levels (vector copy/cell) of NoN (blue bar) and GFP (red bar) genes measured in the bone marrow CD34+ cell samples from months 7–12 after transplant were averaged and are shown for the six recipients in the Group 3 (#3A–#3F).
For the third series of transplants (#3A–#3F), observations were extended over 1 year from transplantation. The levels of marking in bone marrow CD34+ cells averaged for months 7–12 post-transplant were consistent with those measured over months 4–6 (Figure 6c), with #3B continuing to show relatively high levels of marking with both the GFP and the NoN genes, #3D with GFP, and #3E and #3F with the NoN genes.
Evaluation of immune responses to the GFP transgene product
In Group 3 recipients, humoral and cellular immune responses to the GFP transgene product were monitored monthly for 1 year post-transplant (Figure 7). Serum antibodies to recombinant GFP (rGFP) were quantified by enzyme-linked immunosorbent assay (ELISA) and the frequencies of PBMCs with antigen-specific responses to GFP by interferon γ (IFNγ) production were measured by enzyme-linked immunosorbent spot (ELISPOT). As a control to test the effects of the pretransplant conditioning regimen on immunity, all six monkeys were preimmunized with clinical grade tetanus toxoid at 1 and 2 months postnatal age (prior to gene transfer at 3 months) and titers of serum antibodies to tetanus were measured by ELISA and IFNγ responses were measured by ELISPOT. To test the ability of the monkeys to respond to a neo-antigen post-transplant, they were immunized with clinical grade hepatitis B surface antigen vaccine at 6 months after transplant and evaluated by ELISA and ELISPOT.
Figure 7.
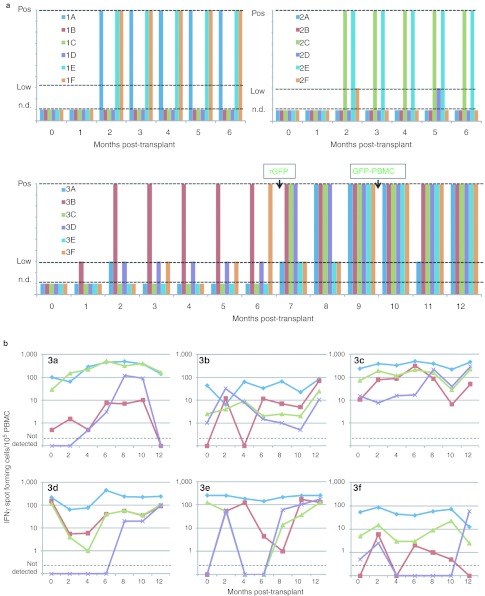
Evaluation of immune responses to the green fluorescent protein (GFP) transgene product. Plasma and peripheral blood mononuclear cells (PBMCs) were collected from subjects monthly after autologous transplant of CD34+ cells transduced with SIV-GFP and SIV-Non. Recipients in Group 3 were immunized with recombinant GFP (rGFP) at 6 months post-transplant and with autologous PBMCs transduced with the SIV-GFP lentiviral vector at 9 months post-transplant (GFP-PBMC). (a) Serum antibody levels were determined by ELISA to rGFP. n.d., not detected. (b) Cellular responses to GFP (red squares), tetanus toxoid (green triangles), hepatitis B surface antigen proteins (purple X), and to anti-CD3 monoclonal antibody (blue diamonds) were assessed by measuring the frequency of PBMCs producing interferon γ (IFNγ) by enzyme-linked immunosorbent spot (ELISPOT). SIV, Simian immunodeficiency virus.
The anti-tetanus antibody levels were stable in all six recipients over the entire year, with no decline seen immediately after conditioning (data not shown). All six recipients had a three-to tenfold increase in serum antibodies to hepatitis B vaccine after immunization at 6 months, indicating immune competence (data not shown). During the first six months after transplant, only recipient #3B with the highest level of GFP-marked blood cells developed a serological response to GFP (Figure 7a). There was no change in the levels of GFP-marked cells in this recipient following the increased levels of anti-GFP antibodies at 2 months post-transplant. Upon immunization with rGFP at 6 months post-transplant, the remaining five monkeys seroconverted with development of serum antibodies to GFP. ELISA for GFP antibodies were also performed for the first 12 recipients (Groups 1 and 2) (Figure 7a). In the first 6 months, positive ELISA measurements of antibodies to GFP were observed in each of the recipients that had GFP-marked cells detected consistently (#1E, #2C, #2E). Additionally, antibodies to GFP were present in #1A and #1F, neither of which had detectable GFP marking in bone marrow CD34+ cells.
Responses to antigens by IFNγ production were determined by ELISPOT using PBMCs that had been cryopreserved monthly and were all assayed simultaneously. The positive control for competence of the PBMCs to respond in the ELISPOT was a monoclonal antibody to CD3 (anti-CD3). All six recipients had stable frequencies of between 10 and 100 spot-forming cells/105 PBMCs in response to anti-CD3 (Figure 7b). Similarly, the responses to tetanus were relatively stable over time for each individual, ranging from 10 to 100% of the frequencies of spot-forming cells to anti-CD3. Tenfold increases in frequencies of IFNγ spot-forming cells to the hepatitis B surface antigen vaccine were seen in most of the recipients after 6 months. Tenfold or greater increases in the frequency of IFNγ spot-forming cells to GFP were also seen in most recipients after immunization at 6 months. In recipient #3B with the highest, persistent marking with GFP, relatively high levels of cells reactive to GFP developed after vaccination, and with no evidence of a decline in gene-marked cells.
Discussion
Large animal models have provided the essential preclinical platform for advancing approaches to improve the efficacy of gene transfer into HSC that have underpinned the recent successful clinical applications.25,26 Because of similarities of hematopoietic and immune systems of nonhuman primates when compared to humans, they also provide an important model in which to evaluate efforts to increase engraftment of gene-modified HSC and to modulate host immune responses against transgene products.
We have previously developed a model of gene therapy using HSC with nonmyeloablative conditioning in infant rhesus monkeys to examine the relationship between dosages and the engraftment of HSC after stable gene-modification by lentiviral vectors.11 In these studies, we determined that partial marrow cytoreduction with busulfan enhanced the engraftment of gene-modified HSC, and without significant toxicity. The present series of studies extends these prior findings. As shown previously, busulfan was safe and had no detectable acute toxicities at submyeloablative dosages, aside from the intended hematologic suppressive effects. These observations are consistent with the clinical experience using busulfan at similar nonmyeloablative dosages for gene therapy of adenosine deaminase-deficient severe combined immune deficiency.8,27 Consistent with prior findings, the monkey infants required dosages 3–4 times higher per kg than human patients to achieve AUC in the range targeted in clinical HSC transplantation (800–1,200 minute·µg/ml after a single ~1 mg/kg dose). Measurements of the pharmacokinetics of busulfan showed that there were dose-related increases of the AUC with increasing busulfan dosages. While there was at least 1.5-fold variability in the AUC among individual recipients at each dosage level, there were consistent intraindividual levels in subjects where multiple determinations were made, suggesting intrinsic variable clearance. Routinely splitting the busulfan dose, with the second dose based on first-dose pharmacokinetic measurements, would likely improve the consistency of the effects on engraftment of gene-marked HSC. Significant myelosuppressive effects (neutropenia and thrombocytopenia) were seen in recipients of the higher dosages of busulfan (120 and 160 mg/m2) with AUC >2,000 minute·µg/ml. In contrast, there was no significant lymphopenia associated with busulfan administration.
Finding a clinically relevant agent that can reduce immune responses mounted against the novel transgene product expressed in gene-modified cells may be essential in most recipients of gene therapy, except those with severe immune deficiencies. The standard clinical pretransplant conditioning modalities that are potently immunesuppressive include total body irradiation, monoclonal antibodies, and other anti-sera such as anti-thymocyte globulin, and chemotherapeutic drugs such as cyclophosphamide and fludarabine. Total body irradiation is both myeloablative as well as immune suppressive, but may have significant short- and long-term toxicities. Clinically approved monoclonal antibodies to human immune cells may not crossreact with rhesus antigens or may have toxicities unique to nonhuman primates.28 Fludarabine is a nucleoside analogue initially developed as an anti-neoplastic agent that is now widely used in pretransplant conditioning regimens with busulfan. Fludarabine has been found to have highly active suppressive or ablative effects on recipient's immune systems to prevent rejection of the donor's cells. While the dosing regimens of fludarabine for pretransplant conditioning have been well defined,29 fludarabine dosages required to induce tolerance to an individual antigen rather than ablate the allo-responsivity of recipients to allogeneic HSC are currently unknown. We postulated that fludarabine could be added to the nonmyeloablative busulfan regimen with minimal toxicity, but with the potential to lymphoablate the recipient, leading to tolerance to a transgene product expressed in the HSC-derived leukocytes during post-transplant hematological and immunological reconstitution.
Our goals were to identify a fludarabine dosing regimen that is clinically tolerated, yet induces significant lymphopenia and immune ablation, and then determine whether this regimen could lead to immune tolerance to the foreign transgene product. The dosages of fludarabine used in this study were based on those used with human patients (based on body-surface area). Within this dose range, fludarabine showed minimal activity (no induced lymphopenia), even at the highest administered dosage (300 mg/m2). Pharmacokinetic analyses revealed clearance was very rapid in these young animals. The resulting subtherapeutic blood levels did not cause discernible clinical or immunological effects. Our finding that the AUC achieved in rhesus monkey infants was ~20-fold below that reported in humans suggests at least 20-fold higher dosages may be needed (e.g., 300–500 mg/m2 × 3–5 doses) to achieve net serum levels and lymphoablative effects achieved in standard clinical dosing. Additional studies with escalating dosages of fludarabine and pharmacokinetic assessments are needed to determine whether an effective dosing regimen can be developed that will reach the blood levels achieved in human patients, and to define the immunological effects.
Gene marking after transplant of lentiviral vector-transduced autologous CD34+ cells was highly variable among different subjects. Many of the recipients had only a few blood or marrow samples with any detectable gene-modified cells post-transplant during the entire observation period, whereas others showed stable persistence of cells marked with either the NoN gene or the GFP gene or both. Overall, the highest gene marking (0.1–1%) was achieved in those receiving the highest busulfan dosages with resulting high blood levels, and with the most severe neutropenia (ANC nadir <200), and after receiving the highest CD34+ cell doses (6 × 106/kg). Nevertheless, the overall levels of gene marking achieved were generally low, compared to those reported in several published studies on gene transfer to HSC in nonhuman primates using lentiviral vectors. It should be noted that we used minimally ablative conditioning with low dose busulfan, in contrast to essentially full cytoablation with total body irradiation as used in other published studies.23,30,31,32 Discordance in marking with the two different vectors in the same recipient (e.g., stable moderate levels of GFP-marked cells, but minimal NoN-marked cells) suggests that effective transduction or engraftment of gene-modified stem cells may be stochastic at limiting doses of gene-modified HSC. Increasing the CD34+ cell dose or using vectors of high titer at higher MOI may lead to more consistent engraftment of gene-modified HSC.
One of the primary goals of these studies was to determine whether humoral or cellular immune responses or tolerance were induced to GFP when it was produced from the transplanted cells. The dual marking strategy with the nonexpressed neomycin phosphotransferase gene and the GFP gene was designed to allow comparison of the relative expression of cells with these two markers. Overall, there were no differences in the levels of marking achieved. These findings suggest that persistence of gene marking was stochastic, rather than a function of the immunogenicity of the GFP transgene product. Immune responses to GFP were detected in some of the monkeys, mostly those with higher levels of GFP gene marking. Serum antibodies were detected in the first few months in those with long-term GFP gene marking, and in some with minimal gene marking detected. Seroconversion occurred in all animals that received booster immunization with rGFP and GFP-expressing autologous PBMCs (+6 and +9 months, respectively). This observation suggests that there was a lack of sufficient antigenic stimulation in some recipients by the GFP-transduced CD34+ cells, but they retained the potential to respond with antibody production once exposed to rGFP. Similarly, immunizations at six months led to increases in GFP-responsive T cells, as measured by IFNγ ELISPOT. Thus, there was no evidence that a state of immunological tolerance had been achieved, as both antibodies and T cells were induced with immunization. The control immunizations with tetanus toxoid prior to transplantation and the assessment of persistence of tetanus responsive cells and antibodies would be useful for monitoring immune ablation once effective dosages of fludarabine are achieved. In the experimental paradigm explored, it will also be important to document immune competence post-transplant because specific immune tolerance can only be deduced in the presence of good overall immune function.
Interestingly, the presence of the antibodies and presumed cytotoxic T lymphocytes to the GFP transgene was not associated with immunological elimination of GFP-marked cells. The antibodies may have been elicited by GFP released from transduced cells, but were unable to produce a cytolytic reaction against intracellular GFP within viable blood and marrow cells. It is also possible that there was insufficient expression of GFP in the cells that persisted due to transcriptional silencing, and to allow recognition by antibodies or T cells. We were unable to detect GFP expression in PBMCs from the recipient with the highest levels of GFP gene marking (~1% by qPCR) by either flow cytometry or reverse trancription-PCR (data not shown). Higher levels of GFP-expressing cells could lead to more vigorous immune responses that do lead to elimination of cells expressing the foreign transgene.
In conclusion, these studies begin to establish a novel clinically acceptable method to achieve engraftment of transduced HSC and persistence of cells expressing a new gene product. Further studies with higher dosages of fludarabine and, ideally, higher levels of transduced HSC, may allow exploration of the potential to achieve immune tolerance with a nonmyeloablative, but immune ablative conditioning regimen.
Materials and Methods
SIV-based lentiviral vectors. SIVmac1A11-based lentiviral vectors were produced using plasmids kindly provided by Arthur Nienhuis, St Jude Children's Research Hospital, Memphis, TN. The SIV transfer vector, pCL20c-SLFR-MSCV-eGFP (or SIV-GFP) expresses GFP from the murine stem cell virus (MSCV) LTR internal promoter and was constructed as previously described.33 The other vector (SIV-NoN) carries the neomycin-resistance gene from the Tn5 bacteriophage in which the translation start codon of neo was mutated into stop codon (CTG) to abolish neo gene expression (NoN)34 (Figure 1a). The NoN sequence was isolated from the Bgal-NeoB2-SK plasmid (generously provided by Cynthia Dunbar, National Institutes of Health, Bethesda, MD) and cloned into pCL20c-SLFR-MSCV-GFP in the place of the GFP sequences. The resulting plasmid was named pCL20c-SLFR-MSCV-NoN (or SIV-NoN).
Lentiviral vector packaging, concentration, and titer determination. The lentiviral vectors were produced by three-plasmid transient transfection of HEK293T cells (No. CRL-11268; American Type Culture Collection, Manassas, VA) as previously described.11,35 The SIV vector was packaged with 100 µg of transfer vector (SIV-GFP or SIV-NoN), 60 µg of SIV packaging plasmid (pCAG-SIVgprre), 20 µg of SIV packaging plasmid pCAG4-RTR-SIV, and 20 µg of envelope plasmid (pMD.G) expressing the vesicular stomatitis virus-glycoprotein (VSV-G) in 500 cm2 cell culture dishes coated with poly-L-lysine (Sigma Scientific, Brighton, MI). At 72 hours post-transfection, vector supernatants were harvested and clarified by passing through a 0.45-µm membrane, and concentrated approximately tenfold using a Centricon plus 70 ultrafiltration filter (Millipore, Bedford, MA). The ultrafiltrate concentrates were ultracentrifuged at 25,000g for 90 minutes and resuspended in ~0.1–1% of starting volume of phosphate-buffered saline (PBS), placed in aliquots, and stored at ≤−80 °C until use. To measure the vector titers, HT-29 cells (ATCC No. HTB-38) were transduced with serially diluted aliquots of concentrated vector supernatant using Polybrene at 8 µg/ml (Sigma). After 6 days of culture, the cells were harvested, genomic DNA was extracted, and the average vector copy per cell was determined by qPCR. Viral titers were quantified by comparing the copy number in transduced cells to a serially diluted standard as previously described.11 The primer and probe sequences for the SIV viral vectors were designed to amplify the psi region of the vector sequence. The forward primer had the sequence (5′-GCAGGGCAGAACCTCTGAAG-3′), the reverse primer was (5′- TGCCTAGCCCCAGCTTGA-3′), and the FAM-labeled probe was (6FAM-5′-CGCCACTCCTCCAAGCCTTCACC-3′-TAMRA).
Rhesus monkeys. The overall experimental design is shown in Figure 1b. All animal procedures conformed to the requirements of the Animal Welfare Act, and protocols were approved prior to implementation by the Institutional Animal Care and Use Committee at the University of California, Davis, CA. Normally cycling, adult female rhesus monkeys (Macaca mulatta; N = 18) with a history of prior pregnancy were bred and identified as pregnant, using established methods.36 Activities related to animal care were performed as per California National Primate Research Center (CNPRC) standard operating procedures. Newborns were delivered by cesarean-section at term (160 ± 2 days gestation) using standardized protocols.37 Newborns were placed in incubators postdelivery and nursery-reared up through 3 months postnatal age. Animals were paired with another study animal for the duration of the study. Infant health, food intake, and body weights were recorded daily in the nursery and then on a routine basis (e.g., twice monthly) according to established protocols. Blood samples (~3–6 ml, dependent on age) were collected monthly from a peripheral vessel to monitor complete blood counts and serum chemistry panels.
Bone marrow aspiration and busulfan/fludarabine administration. At ~2 months postnatal age monkeys were sedated with telazol (5–8 mg/kg intramuscular, i.m.) for marrow collection. The iliac crest was shaved, lidocaine infused, and the site was aseptically prepared using standard protocols.38 Approximately 10 ml of bone marrow was collected into heparinized syringes using sterile technique. CD34+ cells were isolated and cryopreserved using a controlled rate cryopreservation protocol as described below. At ~3 months postnatal age, monkeys were sedated with telazol and supplemented with ketamine (10 mg/kg i.m.) for busulfan infusions. Busulfan (Busulfex; Otsuka America Pharmaceutical, Rockville, MD) was administered i.v. in a 20 ml volume over a 2-hour infusion period at each of the dosages studied (see Table 1). The dosages were calculated based on surface area (m2) by utilizing body weight and crown-rump length and a published surface area formula [weight (kg)0.5378 × height (cm)0.3964 × 0.024265].39 Preinfusion peripheral blood samples were collected, and an indwelling i.v. catheter was placed. After administering a prophylactic dose of phenytoin (3 mg/kg i.v.), the busulfan infusion was initiated using a Baxter FLO-GARD 6200 Volumetric Infusion Pump (Baxter Pharmaceuticals, Deerfield, IL). At the end of the 2-hour busulfan infusion period, the indwelling catheter was removed, a postinfusion dose of dilantin administered, and blood samples (~0.7 ml/time point) were collected from a peripheral vessel at 0.5, 1, 3, and 4 hours postinfusion to determine busulfan levels. Plasma was collected, frozen at ≤−80 °C, and then shipped frozen for analysis at the Clinical Chemistry Laboratory, Department of Pathology, Childrens Hospital Los Angeles. Plasma busulfan concentrations were determined as previously described11 and the AUC was calculated using trapezoidal estimation. Typically, the first busulfan infusion was performed on Monday and the second infusion performed on Wednesday.
Fludarabine (Bayer HealthCare Pharmaceutical, Wayne NJ) was reconstituted in 1 ml of sterile water and diluted in sterile saline to obtain 75, 87.5, and 100 mg/m2 doses. For animals that received fludarabine (5.5–8.4 ml) and busulfan, an i.v. injection of fludarabine was given prior to busulfan infusion on the first and third days (day −3 and −1), and fludarabine was administered alone on the second day under ketamine (day −2) (see Table 1, Figure 1b).
CD34+ cell isolation, transduction, and transplantation. CD34+ marrow cells were isolated using the mini-MACS immunomagnetic separation system (Miltenyi Biotec, Bergisch Gladbach, Germany) and cryopreserved as previously described.40 Following the initiation of conditioning as noted above, the CD34+ cells were thawed and transduced. Cells were plated at 105 cells/cm2 in nontissue culture-treated 25 cm2 flasks that had been coated with 4 µg/cm2 of the RetroNectin (Takara Bio, Otsu, Japan). Prestimulation was then performed overnight in X-Vivo 15 serum-free medium (Lonza Biologics, Hopkinton, MA) supplemented with 2 mmol/l L-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin, and containing 100 ng/ml each of the recombinant human cytokines: thrombopoietin, Flt3-ligand, and stem cell factor (R&D Systems, Minneapolis, MN). The next morning, the cells were resuspended in fresh cytokine-containing medium and transduced in separate portions with the SIV-NoN and SIV-GFP vector supernatants at final vector concentrations of 8 × 107 transducing unit /ml, with protamine sulfate at 4 µg/ml (American Pharmaceutical Partners, Schaumburg, IL). After 2 hours, the medium volume was doubled and cells were incubated at 37 °C with 5% CO2 for 6–8 hours. A second aliquot of each SIV vector supernatant was added to the cells and incubated overnight. The next morning, the cells were washed, then resuspended in PBS with 1% autologous serum (collected prior to marrow collection and busulfan infusion). Cell counts and viability were determined using trypan blue exclusion prior to each transplant. Small aliquots of cells were used for methylcellulose CFU assays, qPCR analysis, and flow cytometry for GFP expression. Autologous cells (~5 × 106 cells per animal) were injected i.v. into each animal in an ~1 ml volume, ~48 hours post-busulfan (±fludarabine) administration as noted above.
Post-transplant sample collection and analysis. Blood samples were collected for complete blood counts, serum chemistry panels, PBMCs, immunophenotyping (CD3, CD4, CD8, CD20, CD34), plasma, serum, and indirect ELISA as previously described.37 Blood (~6–10 ml depending on age) and bone marrow (~3 ml) were collected at monthly intervals beginning at 1 month post-transplantation. Mononuclear cells and granulocytes from peripheral blood samples were isolated by density gradient centrifugation over Histopaque (1.077 g/cm3; Sigma) at 400g for 30 minutes at 25 °C. Hematopoietic CFU assays were performed on mononuclear cells, as previously described.38,40 Briefly, mononuclear cells were resuspended in RPMI and washed. A total of 5 × 104 cells/plate (six plates, 3 × 105 mononuclear cells) from peripheral blood and 2 × 104 cells/plate (four plates, 8 × 104 mononuclear cells) from bone marrow were plated in 1 ml MethoCult GF+ H4435 containing human Epo, G-CSF, GM-CSF, stem cell factor, interleukin-3, and interleukin-6 (StemCell Technologies, Vancouver, British Columbia, Canada) in fibroblast-free 35-mm culture plates. After a standardized 10-day incubation period, erythroid (BFU-E) and myeloid progenitor colonies (colony-forming unit-granulocyte macrophage) were counted. Individual hematopoietic colonies were collected and then lysed and genomic DNA isolated using the QIAamp DNA blood kit (Qiagen, Valencia, CA), as recommended by the manufacturer for qPCR.
Animals were euthanized by an overdose of pentobarbital and tissue harvests were performed according to established protocols.37 Blood and marrow was collected, and all tissues were collected and fixed in 10% buffered formalin then embedded, sectioned (5–6 µm), and stained with hematoxylin and eosin for routine histopathology. Specimens were also placed in cryotubes and quick frozen over liquid nitrogen for qPCR.
Immunizations. Group 3 animals (#3A–#3F, N = 6) (see Table 1) were immunized with clinical-grade tetanus toxoid vaccine (Sanofi Pasteur, Swiftwater, PA; ~0.5 ml i.m.) before transplantation and at 1 and 2 months postnatal age (prior to transplantation). Animals were reimmunized with tetanus at 6 months post-transplant, as well as with a single dose of recombinant hepatitis B vaccine as a neo-antigen (Recombivax HB; Merck & Co., Whitehouse Station, NJ) and with the test antigen (25 µg of rGFP, 0.5 ml i.m.; BioVision, Mountain View, CA). At nine months post-transplant, autologous PBMCs (1 × 106) were obtained from collected blood samples and activated using the T Cell Activation Kit (Miltenyi Biotec) containing 300 IU/ml recombinant human interleukin-2 for 2 days. Cells were transduced with the SIV-GFP lentiviral vector overnight. Transduced PBMCs (2 × 106) were washed three times in sterile PBS and injected i.m. (~0.3–0.5 ml) at 9 months post-transplant under ketamine or telazol.
qPCR. To detect proviral sequences, genomic DNA from mononuclear cells and negative cell fractions were isolated using the QIAamp DNA Blood Mini kit (Qiagen), as recommended by the manufacturer. The positive cell fractions were lysed in 50 mmol/l Tris–HCl, pH = 7.4 (Sigma, St Louis, MO), containing 0.25 mg/ml of Proteinase K (Invitrogen, Carlsbad, CA) solution by incubating at 65 °C for 1 hour and then for 15 minutes at 95 °C. For qPCR analysis, primers and probes were designed using Primer Express software (Perkin-Elmer, Foster City, CA). The primers for GFP were as follows: forward 5′- GCAGTGCTTCAGCCGCTAC-3′, reverse 5′-AAGAAGATGGTGCGCTCCTG-3′, and probe 5′-FAM-CCGACCACA TGAAGCAGCACGACTT-TAMSp-3′. The primers for the neomycin (neo) gene sequences were as follows: forward 5′-TGCGGCGGCTGCAT-3′, reverse 5′- TTCGCTTGGTGGTCGAATG-3′, and probe 5′-FAM-CGCT TGATCCGGCTACCTGCCC-TAMSp-3′. The ɛ-globin system was utilized as an internal control for DNA isolation and PCRs. The primer sequences for ɛ-globin were as follows: forward 5′- TGGCAAGGAGTTCACCCCT-3′, reverse 5′-AATGGCGACAGCAGACACC-3′, and probe 5′-FAM-TGCA GGCTGCCTGGCAGAAGC-TAMSp-3′. Real-time qPCR analysis was carried out in 96-well optical plates using the 7900 ABI Sequence Detection System and the TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA) according to the manufacturer's protocols. PCRs were run in duplicate in separate wells and contained 1× TaqMan universal master mix with 400 nmol/l of forward and reverse primers and 100 nmol/l probe in a 25-µl reaction volume. The PCR protocol consisted of one cycle of 2 minutes at 50 °C, 15 minutes at 95 °C, followed by 40 cycles at 15 seconds at 95 °C, and 60 seconds at 60 °C.
ELISPOT and ELISA. Immune responses of bulk PBMCs to recall antigens were assessed by measuring IFNγ production by ELISPOT. Recombinant proteins used for ex vivo restimulation were the same as those used for in vivo immunizations: recombinant hepatitis B antigen (Recombivax HB), tetanus toxoid, and rGFP. The Monkey IFN-γ ELISpot PLUS kit (HRP) (Mabtech, Mariemont, OH) was used for coating and developing the plates. A multiscreen PDVF plate (Millipore) was pre-wetted with 70% ethanol, washed five times with water, then coated overnight at 4 °C with anti-rhesus IFNγ capture antibody (GZ-4 Mabtech kit) diluted in PBS at 15 µg/ml final concentration. After overnight incubation, the coated plate was washed and then blocked for 2 hours with 0.5% fetal bovine serum in PBS. All antigens were diluted in RPMI 10% fetal bovine serum (R10) to reach a final working concentration of 1 µg/ml; 200 µl aliquots of each antigen dilution were placed in duplicate on the coated and blocked ELISPOT plate.
Cryopreserved PBMCs collected from experimental animals (and cryopreserved colony control PBMCs) were counted, resuspended in R10, and added in duplicate (1–2 × 105 cells/well) to individual wells containing antigen. The plates were incubated for 72 hours at 37 °C, then cell suspensions were discarded and the plate washed in PBS. Biotinylated anti-rhesus IFN-γ detecting antibody (7-B6-1 Mabtech kit) was diluted to 1 µg/ml with 0.5% fetal bovine serum in PBS and added to each well of the plate. After incubating for 2 hours at room temperature in the dark and then washing as stated above, streptavidine-HRP (diluted 1:1,000 in 0.5% fetal bovine serum in PBS) was added to the plate. After incubating for 1 hour at room temperature and washing, 100 µl of tetramethylbenzidine undiluted substrate was added. The plates were incubated 15–30 minutes in the dark, then washed in running water and allowed to dry overnight. Spots were analyzed and counted by an Immunospot Analyzer (CTL, Shaker Heights, OH) in the UCLA Immuno/BioSpot Core.
ELISAs were used to measure serum antibodies to rGFP, tetanus toxoid, and hepatitis B antigen. Polystyrene flat-bottom ELISA plates (96-wells, Nunc) were coated with 100 µl of 0.125 µg/ml of rGFP, tetanus toxoid, or hepatitis B antigen diluted in carbonate buffer (pH = 9.6) and incubated overnight at 4 °C. The plates were washed three times with PBS and 0.05% Tween-20 (PBST). Blocking of nonspecific binding was achieved by addition of 300 µl/well of 1% wt/vol BSA/PBS to the plate followed by a 2-hour incubation at room temperature. The plates were then washed three times with PBST as described above, and monkey serum diluted 1:100 in 1% BSA/PBST was added at 100 µl/well to the plates followed by 1-hour incubation at room temperature. The plates were washed again 3 times with PBST and 100 µl/well of HRP-coupled goat anti-monkey IgG antibody (KPL, Gaithersburg, MD) diluted 1:100,000 in 1% BSA/PBST which was added to the plate and allowed to incubate for 1 hour at room temperature. The plates were then washed for a final time as before and the color reaction was started by addition of 100 µl/well tetramethylbenzidine substrate (Sigma) and allowed to incubate at room temperature for 30 minutes. The reaction was stopped by addition of 50 µl/well 0.5 mol/l H2S04. Five minutes after stopping the reaction, absorbance at A450 was read using a microplate reader (BioRad, Hercules, CA).
Statistical analysis. The correlations between busulfan dosages and busulfan AUC, between busulfan dosages and nadir values of neutrophils, platelets, and lymphocytes, and between busulfan AUC and levels of gene marking in bone marrow cells were determined by the Pearson correlation coefficient. The log transformation was used for the levels of gene marking in blood to account for skewing to normalize their values prior to computing the correlation coefficients. Effects of the addition of fludarabine to busulfan on the busulfan AUC and the nadir ANC values were evaluated with the two-sample t-test, with P values <0.05 considered statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. Bone marrow CD34+ cells were thawed and plated at 105 cells/cm2 in nontissue culture-treated 25 cm2 flasks that had been coated with 4 µg/cm2 of the RetroNectin (Takara Bio). Table S1. Fludarabine (Flu) pharmacokinetic data. Table S2. CD34+ cell dosages, gene transduction, and colony-forming unit (CFU) assay.
Acknowledgments
The authors thank the animal care staff at the CNPRC for expert technical assistance. These studies were performed through the support of the National Heart, Lung, and Blood Institute (NHLBI) Center for Fetal Monkey Gene Transfer for Heart, Lung, and Blood Diseases (grant #HL85794 to A.F.T), grant #2P01 HL73104 (to D.B.K.), and the CNPRC base operating grant (#RR00169). The authors declared no conflict of interest.
Supplementary Material
Bone marrow CD34+ cells were thawed and plated at 105 cells/cm2 in nontissue culture-treated 25 cm2 flasks that had been coated with 4 µg/cm2 of the RetroNectin (Takara Bio).
Fludarabine (Flu) pharmacokinetic data.
CD34+ cell dosages, gene transduction, and colony-forming unit (CFU) assay.
REFERENCES
- Bordignon C, Notarangelo LD, Nobili N, Ferrari G, Casorati G, Panina P.et al. (1995Gene therapy in peripheral blood lymphocytes and bone marrow for ADA-immunodeficient patients Science 270470–475. [DOI] [PubMed] [Google Scholar]
- Kohn DB, Weinberg KI, Nolta JA, Heiss LN, Lenarsky C, Crooks GM.et al. (1995Engraftment of gene-modified umbilical cord blood cells in neonates with adenosine deaminase deficiency Nat Med 11017–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malech HL, Maples PB, Whiting-Theobald N, Linton GF, Sekhsaria S, Vowells SJ.et al. (1997Prolonged production of NADPH oxidase-corrected granulocytes after gene therapy of chronic granulomatous disease Proc Natl Acad Sci USA 9412133–12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay JP.et al. (2002Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy N Engl J Med 3461185–1193. [DOI] [PubMed] [Google Scholar]
- Gaspar HB, Parsley KL, Howe S, King D, Gilmour KC, Sinclair J.et al. (2004Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector Lancet 3642181–2187. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Hauer J, Lim A, Picard C, Wang GP, Berry CC.et al. (2010Efficacy of gene therapy for X-linked severe combined immunodeficiency N Engl J Med 363355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiuti A, Slavin S, Aker M, Ficara F, Deola S, Mortellaro A.et al. (2002Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning Science 2962410–2413. [DOI] [PubMed] [Google Scholar]
- Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L.et al. (2009Gene therapy for immunodeficiency due to adenosine deaminase deficiency N Engl J Med 360447–458. [DOI] [PubMed] [Google Scholar]
- Riddell SR, Elliott M, Lewinsohn DA, Gilbert MJ, Wilson L, Manley SA.et al. (1996T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients Nat Med 2216–223. [DOI] [PubMed] [Google Scholar]
- Tuschong L, Soenen SL, Blaese RM, Candotti F., and, Muul LM. Immune response to fetal calf serum by two adenosine deaminase-deficient patients after T cell gene therapy. Hum Gene Ther. 2002;13:1605–1610. doi: 10.1089/10430340260201699. [DOI] [PubMed] [Google Scholar]
- Kahl CA, Tarantal AF, Lee CI, Jimenez DF, Choi C, Pepper K.et al. (2006Effects of busulfan dose escalation on engraftment of infant rhesus monkey hematopoietic stem cells after gene marking by a lentiviral vector Exp Hematol 34369–381. [DOI] [PubMed] [Google Scholar]
- Stripecke R, Carmen Villacres M, Skelton D, Satake N, Halene S., and, Kohn D. Immune response to green fluorescent protein: implications for gene therapy. Gene Ther. 1999;6:1305–1312. doi: 10.1038/sj.gt.3300951. [DOI] [PubMed] [Google Scholar]
- Skelton D, Satake N., and, Kohn DB. The enhanced green fluorescent protein (eGFP) is minimally immunogenic in C57BL/6 mice. Gene Ther. 2001;8:1813–1814. doi: 10.1038/sj.gt.3301586. [DOI] [PubMed] [Google Scholar]
- Andersson G, Illigens BM, Johnson KW, Calderhead D, LeGuern C, Benichou G.et al. (2003Nonmyeloablative conditioning is sufficient to allow engraftment of EGFP-expressing bone marrow and subsequent acceptance of EGFP-transgenic skin grafts in mice Blood 1014305–4312. [DOI] [PubMed] [Google Scholar]
- Rosenzweig M, Connole M, Glickman R, Yue SP, Noren B, DeMaria M.et al. (2001Induction of cytotoxic T lymphocyte and antibody responses to enhanced green fluorescent protein following transplantation of transduced CD34(+) hematopoietic cells Blood 971951–1959. [DOI] [PubMed] [Google Scholar]
- Berger C, Huang ML, Gough M, Greenberg PD, Riddell SR., and, Kiem HP. Nonmyeloablative immunosuppressive regimen prolongs in vivo persistence of gene-modified autologous T cells in a nonhuman primate model. J Virol. 2001;75:799–808. doi: 10.1128/JVI.75.2.799-808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Conerly M, Thomasson B, Storek J, Riddell SR., and, Kiem HP. Induction of cytotoxic T-lymphocyte responses to enhanced green and yellow fluorescent proteins after myeloablative conditioning. Blood. 2004;103:492–499. doi: 10.1182/blood-2003-07-2324. [DOI] [PubMed] [Google Scholar]
- Donahue RE, Wersto RP, Allay JA, Agricola BA, Metzger ME, Nienhuis AW.et al. (2000High levels of lymphoid expression of enhanced green fluorescent protein in nonhuman primates transplanted with cytokine-mobilized peripheral blood CD34(+) cells Blood 95445–452. [PubMed] [Google Scholar]
- Hutton JJ, Von Hoff DD, Kuhn J, Phillips J, Hersh M., and, Clark G. Phase I clinical investigation of 9-beta-D-arabinofuranosyl-2-fluoroadenine 5'-monophosphate (NSC 312887), a new purine antimetabolite. Cancer Res. 1984;44:4183–4186. [PubMed] [Google Scholar]
- Von Hoff DD. Phase I clinical trials with fludarabine phosphate. Semin Oncol. 1990;17 5 Suppl 8:33–38. [PubMed] [Google Scholar]
- Goodman ER, Fiedor PS, Fein S, Athan E., and, Hardy MA. Fludarabine phosphate: A DNA synthesis inhibitor with potent immunosuppressive activity and minimal clinical toxicity. Am Surg. 1996;62:435–442. [PubMed] [Google Scholar]
- Bonin M, Pursche S, Bergeman T, Leopold T, Illmer T, Ehninger G.et al. (2007F-ara-A pharmacokinetics during reduced-intensity conditioning therapy with fludarabine and busulfan Bone Marrow Transplant 39201–206. [DOI] [PubMed] [Google Scholar]
- Von Hoff DD. Phase I clinical trials with fludarabine phosphate. Semin Oncol. 1990;17:33–38. [PubMed] [Google Scholar]
- Avramis VI, Champagne J, Sato J, Krailo M, Ettinger LJ, Poplack DG.et al. (1990Pharmacology of fludarabine phosphate after a phase I/II trial by a loading bolus and continuous infusion in pediatric patients Cancer Res 507226–7231. [PubMed] [Google Scholar]
- Wu T, Kim HJ, Sellers SE, Meade KE, Agricola BA, Metzger ME.et al. (2000Prolonged high-level detection of retrovirally marked hematopoietic cells in nonhuman primates after transduction of CD34+ progenitors using clinically feasible methods Mol Ther 1285–293. [DOI] [PubMed] [Google Scholar]
- Kiem HP, Andrews RG, Morris J, Peterson L, Heyward S, Allen JM.et al. (1998Improved gene transfer into baboon marrow repopulating cells using recombinant human fibronectin fragment CH-296 in combination with interleukin-6, stem cell factor, FLT-3 ligand, and megakaryocyte growth and development factor Blood 921878–1886. [PubMed] [Google Scholar]
- Shaw, KL, Sokolic R., and, Choi, C. Immune reconstitution after gene therapy for adenosine deaminase deficient severe combined immune deficiency (ADA-SCID) Mol Ther. 2009;17:S138. [Google Scholar]
- Hale G, Hoang T, Prospero T, Watt SM., and, Waldmann H. Removal of T cells from bone marrow for transplantation. Comparison of rat monoclonal anti-lymphocyte antibodies of different isotypes. Mol Biol Med. 1983;1:305–319. [PubMed] [Google Scholar]
- Long-Boyle JR, Green KG, Brunstein CG, Cao Q, Rogosheske J, Weisdorf DJ.et al. (2011High fludarabine exposure and relationship with treatment-related mortality after nonmyeloablative hematopoietic cell transplantation Bone Marrow Transplant 4620–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Kim YS, Larochelle A, Renaud G, Wolfsberg TG, Adler R.et al. (2009Sustained high-level polyclonal hematopoietic marking and transgene expression 4 years after autologous transplantation of rhesus macaques with SIV lentiviral vector-transduced CD34+ cells Blood 1135434–5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An DS, Kung SK, Bonifacino A, Wersto RP, Metzger ME, Agricola BA.et al. (2001Lentivirus vector-mediated hematopoietic stem cell gene transfer of common gamma-chain cytokine receptor in rhesus macaques J Virol 753547–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trobridge GD, Beard BC, Gooch C, Wohlfahrt M, Olsen P, Fletcher J.et al. (2008Efficient transduction of pigtailed macaque hematopoietic repopulating cells with HIV-based lentiviral vectors Blood 1115537–5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawa H, Hematti P, Keyvanfar K, Metzger ME, Krouse A, Donahue RE.et al. (2004Efficient gene transfer into rhesus repopulating hematopoietic stem cells using a simian immunodeficiency virus-based lentiviral vector system Blood 1034062–4069. [DOI] [PubMed] [Google Scholar]
- Hanazono Y, Brown KE, Handa A, Metzger ME, Heim D, Kurtzman GJ.et al. (1999In vivo marking of rhesus monkey lymphocytes by adeno-associated viral vectors: direct comparison with retroviral vectors Blood 942263–2270. [PubMed] [Google Scholar]
- Shaw KL, Pais E, Ge S, Hardee C, Skelton D, Hollis RP.et al. (2009Lentiviral vectors with amplified beta cell-specific gene expression Gene Ther 16998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantal, AF.2005Ultrasound Imaging in Rhesus and Long-tailed Macaques: Reproductive and Research Applications Woolf-Coote, S.ed). The Laboratory Primate Elsevier Academic Press; 317–351. [Google Scholar]
- Tarantal AF, McDonald RJ, Jimenez DF, Lee CC, O'Shea CE, Leapley AC.et al. (2005Intrapulmonary and intramyocardial gene transfer in rhesus monkeys (Macaca mulatta): safety and efficiency of HIV-1-derived lentiviral vectors for fetal gene delivery Mol Ther 1287–98. [DOI] [PubMed] [Google Scholar]
- Tarantal AF, Goldstein O, Barley F., and, Cowan MJ. Transplantation of human peripheral blood stem cells into fetal rhesus monkeys (Macaca mulatta) Transplantation. 2000;69:1818–1823. doi: 10.1097/00007890-200005150-00015. [DOI] [PubMed] [Google Scholar]
- DuBois, D., and, DuBois E. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–871. [Google Scholar]
- Lee CC, Fletcher MD., and, Tarantal AF. Effect of age on the frequency, cell cycle, and lineage maturation of rhesus monkey (Macaca mulatta) CD34+ and hematopoietic progenitor cells. Pediatr Res. 2005;58:315–322. doi: 10.1203/01.PDR.0000169975.30339.32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bone marrow CD34+ cells were thawed and plated at 105 cells/cm2 in nontissue culture-treated 25 cm2 flasks that had been coated with 4 µg/cm2 of the RetroNectin (Takara Bio).
Fludarabine (Flu) pharmacokinetic data.
CD34+ cell dosages, gene transduction, and colony-forming unit (CFU) assay.