Abstract
Lens epithelium-derived growth factor (LEDGF/p75) is an essential cofactor of HIV integration. Both stable overexpression of the C-terminal part of LEDGF/p75 (LEDGF325–530) containing the integrase (IN)-binding domain (IBD) and stable knockdown (KD) of LEDGF/p75 are known to inhibit HIV infection in laboratory cell lines. Here, primary human CD4+ T-cells were transduced with lentiviral vectors encoding LEDGF325–530, the interaction-deficient mutant LEDGF325–530D366N, or a hairpin depleting LEDGF/p75 and challenged with HIV. Maximal protection of primary T-cells from HIV infection was obtained after LEDGF325–530 overexpression reducing HIV replication 40-fold without evidence of cellular toxicity. This strategy was subsequently evaluated in the NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mouse model. Threefold reduction in mean plasma viral load was obtained in mice engrafted with CD4+ T-cells expressing LEDGF325–530 in comparison with engraftment with LEDGF325–530D366N cells. Four weeks after transplantation with LEDGF325–530D366N cells, 70% of the CD4+ cells were lost due to ongoing HIV replication. However, in mice transplanted with LEDGF325–530 cells only a 20% decrease in CD4+ cells was measured. Liver and spleen sections of LEDGF325–530 mice contained less HIV than LEDGF325–530D366N mice as measured by p24 antigen detection. LEDGF325–530 overexpression potently inhibits HIV replication in vivo and protects against HIV mediated cell killing of primary CD4+ T-cells.
Introduction
During the last decade, the insight has grown that HIV engages several cellular proteins to serve as cofactors for its replication.1,2 Virus–host interactions are considered attractive targets for antiviral therapy since antiviral resistance development may be slower.
Lens epithelium-derived growth factor (LEDGF/p75) has been identified as a binding partner of HIV integrase (IN) in 2003.3 The interaction with IN is lentivirus specific and requires the IN-binding domain (IBD, amino acid 347–429) in the C-terminal part of LEDGF/p75.4 LEDGF/p75 orchestrates chromosomal tethering of HIV-1-IN.4 An ensemble of N-terminal motifs functions as the main chromatin tether (Figure 1). These motifs include the PWWP domain,4,5 AT-hook like motifs and three charged regions (CR1-3).6 No crystal structure of full-length HIV-IN or full-length LEDGF/p75 is available, but a crystal structure of the IN catalytic core domain in complex with the IBD revealed that two monomers of IBD interact with a dimer of the catalytic core domain of IN.7 Confirmation of the biological relevance of the co-crystal was obtained by subsequent mutagenesis studies.4
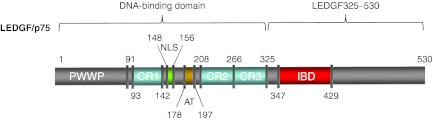
Figure 1.
Schematic representation of the LEDGF/p75 domain structure. LEDGF/p75 carries a conserved PWWP-domain and several charged regions (CR) at its N-terminal end. Together with the nuclear localization signal (NLS) and the AT-hook-like domains (AT), these elements form the DNA-binding domain of LEDGF/p75. The C-terminal IBD domain is responsible for the interaction with HIV-IN. D366 is an essential spot for the interaction with HIV-IN;17 mutation of this residue results in loss of interaction. AT, AT-hook; CR, charged region; IBD, integrase-binding domain; IN, integrase; LEDGF/p75, lens epithelium-derived growth factor; NLS, nuclear localization signal; PWWP, Pro-Trp-Trp-Pro domain.
The role of LEDGF/p75 in HIV replication was validated using RNA interference -mediated knockdown (KD), knockout and overexpression of truncation mutants. KD of LEDGF/p75 resulted in reduced viral replication and integration4 (Supplementary Figure S1, left panel). The central role of LEDGF/p75 in HIV replication was also demonstrated by transduction of LEDGF/p75 ablated mouse fibroblasts with HIV-derived vector.4 Overexpression of the LEDGF/p75 C-terminal end (amino acid 325-530; LEDGF325–530), which lacks the chromatin-binding domain, potently blocks HIV replication by competing with endogenous LEDGF/p75 for binding to HIV-IN (Supplementary Figure S1, right panel).4 Recently, IBD-mediated allosteric inhibition of integration has been proposed as an additional inhibitory mechanism.8,9 Moreover, depletion of LEDGF/p75 resulted in loss of preferential integration of HIV in the body of genes.4 Fusion proteins, in which the LEDGF/p75 chromatin interaction domain is replaced with alternative chromatin interaction domains, support viral replication and were shown to retarget integration towards regions bound by the specific chromatin-binding domain.10,11 Together, these results confirm that LEDGF/p75 tethers the lentiviral preintegration complex to cellular chromatin.4
To date highly active antiretroviral therapy (HAART) is the standard treatment for HIV-infected patients, combining three antiviral drugs blocking different steps in the replication cycle. HAART can efficiently suppress viral replication, but does not eradicate the virus and suffers from side effects. In addition, poor adherence often results in viral resistance development and treatment failure. As such, continuous development of new drugs, preferentially against new targets, is needed. Recently, we reported LEDGINs as first-in-class small molecule inhibitors targeting the LEDGF/p75–IN interaction and HIV-1 replication.12
Next to drug development, alternative strategies to treat and potentially cure HIV-infected people need to be explored. Gene therapy has the potential to protect natural target cells from HIV infection and could provide a lifelong treatment. Several gene therapeutic approaches have been developed for HIV/AIDS (for a review see refs. 13,14) that aim to create a reservoir of immune cells genetically modified to resist HIV infection in the patient through modification of CD4+ T-cells or hematopoietic stem cells. Different steps in the HIV replication cycle and both viral and cellular proteins can serve as targets for gene therapy and some approaches have been tested in a clinical setting,14 such as RNA decoys, transdominant proteins, ribozymes, and RNA interference targeting different viral proteins such as Tat, Rev, and gp 41.
Since LEDGF/p75 is an important cellular cofactor for HIV replication that acts before stable integration of the HIV-1 provirus in the host chromosomal DNA, we now have evaluated its potential as a target for HIV gene therapy. Here, we show that overexpression of LEDGF325–530 with or without additional LEDGF/p75 KD inhibits HIV replication in primary human CD4+ T-cells in vitro without cellular toxicity. In addition, LEDGF325–530 overexpression results in significant inhibition of HIV replication and protected primary T-cells from HIV-1 infection in a humanized mouse model.
Results
Construction of lentiviral vectors
To obtain maximal expression levels in primary CD4+ T-cells, we first compared transduction efficiency of human primary CD4+ T-cells by HIV-based lentiviral vectors driving the expression of enhanced green fluorescent protein (eGFP) from the cytomegalovirus immediate early promoter, the spleen focus-forming virus LTR promoter (SFFV)15 or the human cyclophilinA promoter15 (Supplementary Figure S2). Although cytomegalovirus immediate early promoter is generally described as a universal promoter, transduction efficiency in primary CD4+ T-cells was low compared to both other promoters. While the cyclophilinA promoter performed 15-fold better than cytomegalovirus immediate early promoter, the SFFV promoter outperformed both other promoters (200-fold and 13-fold, respectively). Comparable results were obtained in human T-cell lines (SupT1 and PM1; data not shown). Hence, all viral vector constructs in this study were designed to contain an internal SFFV LTR promoter.
Next, three different HIV-1-based lentiviral vectors were generated to interfere with LEDGF/p75 function during HIV infection (Supplementary Figure S3). All constructs expressed eGFP and truncated CD34 (tCD34)16 as reporter proteins. In order to obtain potent KD of the endogenous LEDGF/p75, we developed a miRNA-based KD vector (referred to as LV_KD) containing two miRNA-based shRNA sequences specifically recognizing LEDGF/p75 mRNA.10 We also generated a vector stably overexpressing the C-terminus of LEDGF/p75 fused to eGFP (LV_LEDGF325–530), for which we and others demonstrated its potential in cell culture.4 A third construct combined both strategies (LV_LEDGF325–530_KD). As controls we used LV_eGFP and LV_LEDGF325–530D366N (Supplementary Figure S3). Residue D366 in LEDGF/p75 is pivotal for the interaction with IN. Mutation of Asp into Asn at this position abolishes the interaction with IN.17
In a first step the potency of the respective constructs was evaluated in laboratory T-cell lines. We generated SupT1 cells stably transduced at high multiplicity of infection (MOI) (MOI >1) with the aforementioned lentiviral vectors. Transduction efficiency was measured at 72 hours by tCD34 flow cytometry, revealing >95% tCD34+ SupT1 cells for all vectors (data not shown). Protein expression of the respective constructs was evaluated by Western blot analysis (Supplementary Figure S4a): no LEDGF/p75 protein was detected in the KD cell line (KD), whereas expression of LEDGF325–530 or LEDGF325–530D366N resulted in protein bands migrating at the predicted MW (55 kDa). KD and overexpression levels were subsequently quantified by qPCR. In the KD cell line, LEDGF/p75 mRNA levels were suppressed by 87 ± 2% relative to wild-type (WT) cells (Supplementary Figure S4b), whereas LEDGF325–530 and LEDGF325–530D366N were overexpressed four- and sixfold, respectively, compared to endogenous LEDGF/p75 in WT cells (Supplementary Figure S4c). Growth curves of the different cell lines did not reveal differences in growth kinetics compared to control (data not shown).
Both LEDGF/p75 KD and LEDGF325–530 overexpression inhibit HIV replication in laboratory T-cell lines
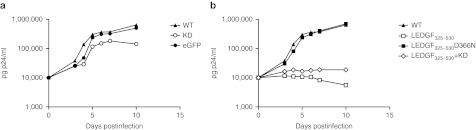
HIV-1NL4.3 replication in the SupT1-derived cell lines was monitored to evaluate the functionality of the constructs (Figure 2). Following challenge with HIV-1NL4.3 virus (500 pg p24) both WT SupT1 and SupT1 cells transduced with control LV_eGFP supported HIV-1NL4.3 replication (Figure 2a). In contrast, a 500-fold inhibition in p24 production was detected at day 11 in the LEDGF/p75 KD cells compared to WT cells, which is in accordance with previous data.18 Overexpression of LEDGF325–530 potently inhibited HIV replication (Figure 2b; 600-fold reduction compared to WT cells), whereas the interaction-deficient LEDGF325–530D366N control did not significantly affect HIV replication (Figure 2b). Combining LEDGF/p75 KD and LEDGF325–530 overexpression resulted in a twofold more potent inhibition of HIV replication compared to either strategy alone, suggesting a small additive effect. Similar results were obtained in other laboratory T-cell lines, such as PM1 cells19 (Supplementary Figure S5).
Figure 2.
Both LEDGF/p75 knockdown and LEDGF325–530 overexpression inhibit HIV-1NL4.3 infection in different T-cell lines. Transgenic SupT1 T-cell lines were infected with HIV-1NL4.3. HIV replication was monitored by p24 measurement using enzyme-linked immunosorbent assay (ELISA). (a) HIV breakthrough in SupT1 cells depleted for LEDGF/p75 (LEDGF/p75 KD) (open circle) and controls: untransduced WT cells (closed triangle) and cells transduced with an eGFP-encoding lentiviral vector (LV_eGFP) (closed circle). (b) Breakthrough in SupT1 cells expressing LEDGF325–530 (open square) and in cells expressing LEDGF325–530 in combination with LEDGF/p75 KD (open diamond), in WT (closed triangle) or in control SupT1 cells expressing LEDGF325–530D366N (closed square). All experiments were performed at least three times; a representative experiment is shown. eGFP, enhanced green fluorescent protein; LEDGF/p75, lens epithelium-derived growth factor; KD, knockdown; WT, wild-type.
Next to HIV-1NL4.3, we also evaluated replication of HIV-2 and HIV-1NDK, a highly cytopathic clade D strain, in the transgenic SupT1-derived cell lines. LEDGF/p75 KD potently inhibited HIV-2 replication, compared to control SupT1 cells transduced with LV_eGFP (Supplementary Figure S6a). Likewise HIV-2 replication was inhibited in SupT1 LEDGF325–530 cells (Supplementary Figure S6b), whereas the interaction-deficient control LEDGF325–530D366N cells supported WT levels of HIV-2 replication (Supplementary Figure S6b), in line with the results obtained for HIV-1NL4.3. The combination of LEDGF/p75 KD and LEDGF325–530 overexpression resulted in more potent inhibition of HIV-2 replication (Supplementary Figure S6b). Similar data were obtained with HIV-1NDK. Control SupT1 cells, transduced with LV_eGFP, supported HIV-1NDK breakthrough at day 7, whereas in LEDGF/p75 KD SupT1 cells a 500-fold inhibition was observed (Supplementary Figure S6c). A 20–150-fold inhibition could be detected in SupT1 cells overexpressing LEDGF325–530 alone or in combination with LEDGF/p75 KD (Supplementary Figure S6d), whereas overexpression of LEDGF325–530D366N in SupT1 cells supported HIV-1NDK replication (Supplementary Figure S6d). Together, these results show that both LEDGF/p75 KD and LEDGF325–530 overexpression inhibit virus replication of HIV-1NL4.3, HIV-2 and HIV-1NDK.
LEDGF325–530 overexpression potently inhibits HIV replication in primary CD4+ T-cells
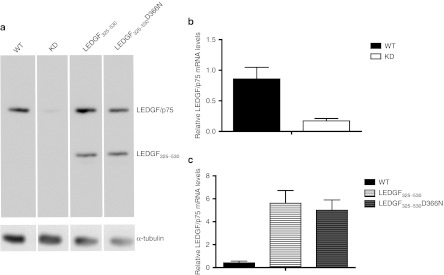
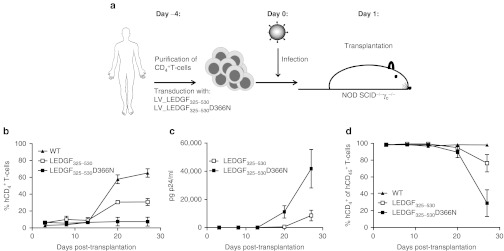
Next, we attempted to inhibit HIV replication in transgenic primary human CD4+ T-cells. Primary cells were purified and transduced with either LV_KD or LV_LEDGF325–530 or LV_LEDGF325–530_KD. Control primary CD4+ T-cells were transduced with LV_eGFP or LV_ LEDGF325–530D366N. LEDGF/p75 KD and overexpression of LEDGF325–530 or LEDGF325–530D366N was corroborated by western blot analysis (Figure 3a). Quantitative-PCR analysis for LEDGF/p75 mRNA levels demonstrated 80 ± 4% KD in KD cells (Figure 3b) and tenfold overexpression compared to endogenous LEDGF/p75 for LEDGF325–530 or LEDGF325–530D366N overexpression cells (Figure 3c).
Figure 3.
Detection of knockdown and overexpression in primary CD4+ T-cells. (a) Analysis of protein expression in transgenic primary CD4+ T-cells and in wild-type (WT) cells. Equal loading was controlled by α-tubulin. (b) LEDGF/p75 KD (white bar) compared to WT (black bar) measured by quantitative reverse transcriptase (QRT)-PCR. (c) LEDGF325–530 (white bar, horizontal lines) and LEDGF325–530D366N (gray bar, horizontal lines) overexpression compared to WT (black bar) measured by QRT-PCR. mRNA levels were normalized for β-actin mRNA. The data are represented as mean + SD of at least three measurements. LEDGF/p75, lens epithelium-derived growth factor; KD, knockdown; WT, wild type.
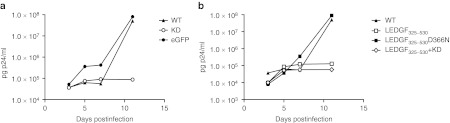
To evaluate the effect on HIV replication, transgenic cells were challenged with HIVNL4.3 virus (5,000 pg p24). Although LEDGF/p75 KD in T-cell lines resulted in potent inhibition of HIV replication, we only observed a moderate fivefold inhibition in the LEDGF/p75 KD cells compared to WT primary cells (Figure 4a), whereas LV_eGFP control cells supported HIV replication to the same extent as WT. Conversely, HIV replication was potently inhibited in the LEDGF325–530 expressing cells compared to WT cells (40-fold inhibition, Figure 4b), whereas no inhibition was observed in LEDGF325–530D366N cells. No additive effect of the KD to LEDGF325–530 overexpression could be detected when combining both strategies (Figure 4b). Altogether, these results demonstrate that in vitro HIV replication in primary human CD4+ T-cells is most potently inhibited by overexpression of LEDGF325–530.
Figure 4.
LEDGF/p75 KD and/or LEDGF325–530 overexpression inhibit HIV-1NL4.3 infection in primary CD4+ T-cells. Transgenic CD4+ T-cells were infected with HIV-1NL4.3. HIV replication was monitored over time by sampling the supernatant at indicated time-points postinfection, followed by p24 enzyme-linked immunosorbent assay (ELISA). (a) HIV breakthrough in LEDGF/p75 KD cells (open circle), WT CD4+ T-cells (closed triangle) or control cells expressing eGFP (closed circle). (b) HIV breakthrough in CD4+ T-cells expressing LEDGF325–530 (open square) and in cells expressing LEDGF325–530 in combination with LEDGF/p75 KD (open diamond), in WT (closed triangle) and in control LEDGF325–530D366N cells (closed square). Experiments were performed at least three times, a representative experiment is shown. eGFP, enhanced green fluorescent protein; KD, knockdown; LEDGF/p75, lens epithelium-derived growth factor; WT, wild-type.
Transgenic CD4+ T-cells display normal T-cell characteristics
In a step towards a gene therapeutic approach for HIV, we evaluated cell growth and T-cell characteristics for the transgenic CD4+ T-cells expressing LV_LEDGF325–530 and LV_LEDGF325–530_ KD with LV_LEDGF325–530D366N as control. No differences in cell growth between WT cells and the transgenic cells were detected (data not shown). The proliferative response to mitogenic stimulation by both phytohaemagglutinin and anti-CD28 antibody treatment was evaluated via monitoring of 3H thymidine incorporation (see Supplementary Materials and Methods and Supplementary Figure S7a; no difference compared to control cells, P > 0.05, two-tailed t-test). Additionally, the production of the cytokines interleukin-2, interleukin-5, and interferon-γ was evaluated in the cell culture supernatant (see Supplementary Materials and Methods and Supplementary Figure S7b–d). For none of the parameters checked, significant differences were detected between transgenic and WT cells.
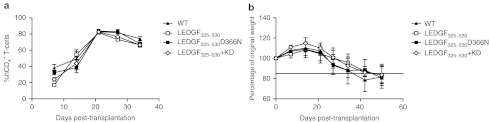
Furthermore, transgenic primary CD4+ T-cells were compared with WT CD4+ T-cells for their ability to engraft NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice. Therefore, primary human CD4+ T-cells were purified and transduced with the respective viral vectors, and after 5 days of culture, the cells were transplanted into NSG mice (n = 4 for each group). On a weekly basis, human CD4+ T-cell levels were monitored in the peripheral blood of the mice by flow cytometry. The percentage human CD4+ T-cells of total lymphocytes was analyzed as an estimate of human cell engraftment. Both WT and transgenic cells displayed similar engraftment kinetics, peaking at 3 weeks post-transplantation (80% human CD4+ T-cells/total lymphocytes) and leveling at ~65% human CD4+ T-cells at 5 weeks (Figure 5a).
Figure 5.
Transgenic primary CD4+ T-cells display a similar engraftment efficiency as WT CD4+ T-cells. WT (closed triangle) and transgenic primary CD4+ T-cells (transduced with LV_LEDGF325–530 (open square), LV_LEDGF325–530_KD (open diamond) or LV_LEDGF325–530 D366N (closed square) were transplanted into NSG mice (n = 4 for each group). (a) Human CD4+ T-cell levels were monitored in peripheral blood with flow cytometry and are depicted as percentage of human CD4+ cells of total lymphocytes. (b) Mice were weighed on a weekly basis. Average weight ± SD per treatment group is displayed. KD, knockdown; LEDGF/p75, lens epithelium-derived growth factor; NSG, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ; WT, wild-type.
Next to CD4+ T-cell levels, we also monitored the ability of WT and transgenic CD4+ T-cells to induce graft-versus-host disease in NSG mice. In general, mice are considered to suffer from graft-versus-host disease when their weight drops below 85% of the weight at the day of transplantation.20 The weight of the animals in the different groups decreased gradually until 80% after 42 days of transplantation, eventually resulting in death of the animals. This was comparable for the different groups (Figure 5b). Altogether, these results indicate that transduction with lentiviral vectors and permanent overexpression or KD of LEDGF/p75 in primary cells does not significantly influence T-cell characteristics.
Primary CD4+ T-cells expressing LEDGF325–530 are protected against HIV infection in a mouse model
We employed a human xenotransplant mouse model to evaluate whether transgenic primary cells are protected against HIV-1 infection. For our in vivo strategy the LEDGF325–530 approach was chosen because this construct demonstrated the strongest phenotype in primary T-cells in vitro. As displayed in Figure 6a, freshly prepared primary human CD4+ T-cells were transduced with LV_LEDGF325–530 or LV_LEDGF325–530D366N control vector at high MOI (MOI >1). After 4 days, transduction efficiency was measured by tCD34 flow cytometry. Transduction efficiency was comparable for all vectors used (70% tCD34+cells) (data not shown). Cells were infected with HIV-1NL4.3, and 1 day later transplanted into NSG mice (n = 4 per group). As an additional control, noninfected cells were also evaluated. On a weekly basis, peripheral blood was monitored for human CD4+ and human CD45+ cells and sampled for p24 as a measure for HIV replication. The experiment was halted when the mice showed signs of severe graft-versus-host disease.
Figure 6.
Primary CD4+ T-cells expressing LEDGF325–530 are protected against HIV infection in a mouse model. Five days after transduction with LV_LEDGF325–530 (open square) or LV_LEDGF325–530 D366N (closed square), primary CD4+ T-cells were debeaded and infected with HIV-1NL4.3 (100,000 pg p24). One day later cells were washed and transplanted into 6–10 weeks old NSG mice (n = 4 for each group). Non infected WT cells (closed triangle) were used as a control. Blood was sampled on a weekly basis. (a) Experimental set-up. (b) Percentage of human CD4+ T-cells of total lymphocytes over time. Means ± SEM for each group are shown. (c) HIV p24 levels over time in the plasma of the mouse blood [p24 enzyme-linked immunosorbent assay (ELISA)]. Means ± SEM for each group are shown. (d) Percentage human CD4+ T-cells of human CD45+ cells as a measurement for protection of transgenic cells. Means ± SEM for each group are shown. N = 4 for each group. LEDGF/p75, lens epithelium-derived growth factor; LV, lentivector; NSG, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ; WT, wild-type.
As shown in Figure 6b, transplantation of noninfected WT cells yielded ~60% human CD4+ cells of total lymphocytes at 3 weeks post-transplantation. In contrast, no human CD4+ cells were observed in mice transplanted with infected control LEDGF325–530D366N expressing cells. However, HIV-infected LEDGF325–530 expressing cells displayed an intermediate engraftment; 30% of total lymphocytes were human CD4+ cells at 3 weeks post-transplantation. In parallel, we monitored HIV replication by p24 antigen detection in the plasma of the respective animals (Figure 6c). Significant HIV-1 replication was demonstrated in animals carrying nonprotected, LEDGF325–530D366N cells, explaining the low level of human cells in the mouse blood. In contrast, a threefold reduction in mean plasma viral load was observed in mice engrafted with LEDGF325–530 expressing CD4+ T-cells compared to LEDGF325–530D366N control mice. When evaluating the percentage of human CD4+ T-cells in the total population of human cells (CD45+ cells), CD45+ cells remained 100% CD4 positive over the time course of the experiment in the mice transplanted with noninfected, control cells. In animals transplanted with LEDGF325–530 D366N expressing cells, ~70% of the CD4+ T-cells were lost by day 27, probably due to ongoing HIV replication (Figure 6d). In mice transplanted with LEDGF325–530 expressing cells, only a 20% decrease of CD4+ cells was observed (Figure 6d).
The experiment was repeated with blood of another donor. Transduction efficiency was comparable for all vectors used, resulting in a transduction efficiency of 30% tCD34+ cells for LV_LEDGF325–530 and LV_LEDGF325–530D366N (data not shown), about twofold lower than in the first experiment. Following infection with HIV-1NL4.3, cells were transplanted into NSG mice (n = 9 per group). Although engraftment was readily detectable for noninfected control cells (indicated by the percentage of human CD4+ cells in the peripheral blood), no significant increase of human CD4+ cells was detected at day 36 in animals transplanted with HIV-1-infected LEDGF325–530 or LEDGF325–530D366N-expressing cells (Supplementary Figure S8a). Nevertheless, a comparison of LEDGF325–530 and LEDGF325–530D366N-expressing CD4 positive T-cells evidenced a tenfold reduction in HIV-1 replication (Supplementary Figure S8b). Measuring the percentage of human CD4+ T-cells in the total human cell population (hCD45+ cells), hCD4+ T-cells remained at 100% of the hCD45+ cells in the mice transplanted with noninfected, control cells. In animals transplanted with LEDGF325–530D366N cells, 80% was lost by day 29 and 93% of the hCD4+ T-cells was lost by day 36 (Supplementary Figure S8c), in line with the previous experiment. However, in LEDGF325–530 mice 40% and 30% of the hCD45+ cells remained hCD4+ at day 29 or 36 post-transplantation (Supplementary Figure S8c). These results indicate that overexpression of LEDGF325–530 inhibits HIV-1 replication and protects primary cells against HIV-1 associated cytopathogenic effects.
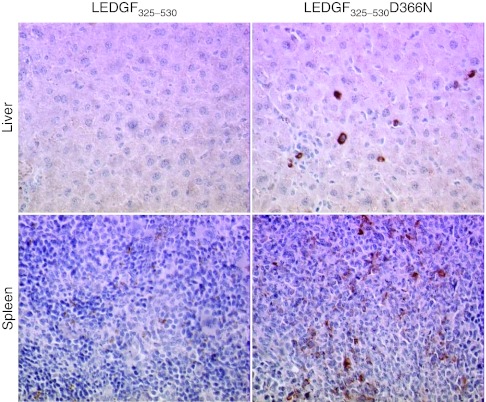
Further evidence for in vivo protection against HIV-1 infection by LEDGF325–530 overexpression was sought by examining the liver and the spleen of mice transplanted with HIV-1 infected LEDGF325–530 or LEDGF325–530D366N transgenic primary T-cells. Tissue sections of spleen and liver were examined for HIV-1 p24 antigen. p24 staining was observed in liver sections from animals engrafted with LV_LEDGF325–530D366N transduced CD4+ T-cells but not in liver from mice engrafted with CD4+ T-cells transduced with LV_LEDGF325–530 (Figure 7, upper panels). Similar results were obtained for tissue sections from spleen (Figure 7, lower panels). These experiments show that LEDGF325–530 overexpression prevents productive HIV-1 infection in vivo.
Figure 7.
p24 staining in liver and spleen from mice transplanted with CD4+ T-cells expressing LEDGF325–530. Paraffin-embedded sections of liver (upper panels) and spleen (lower panels) from mice transplanted with the LEDGF325–530-expressing human CD4+ T-cells (left panels) or with LEDGF325–530D366N cells (right panels) are shown. Sections were stained with anti-p24. All panels are at ×20 magnification. A representative section is shown. LEDGF/p75, lens epithelium-derived growth factor.
Discussion
Although HAART has decreased the mortality of HIV-infected patients, HIV infection is still not curable and lifelong pharmacotherapy remains necessary. In addition, toxic side effects and resistance development often urge changes in therapy regimens, which can finally result in multidrug resistance and therapy failure. The difficulties of controlling HIV-1 infection and the lack of an effective HIV vaccine fuel the thinking about alternatives, such as gene therapy. Ideally, gene therapy should potently suppress HIV-1 replication without eliciting viral resistance. While all steps of the viral life cycle are potential gene therapy targets, targeting the virus before integration into the host genomic DNA occurs, is essential to prevent the virus from becoming a heritable genetic element.
We report here for the first time on a gene therapy approach that utilizes LEDGF/p75, a cellular cofactor of HIV replication. LEDGF/p75, a cellular cofactor that is hijacked by the viral IN, orchestrates efficient chromosomal tethering and integration of the provirus into the host cell chromatin.3 Proviral integration is an attractive target because of its central role in the HIV replication cycle. The IN strand transfer inhibitor raltegravir was a recent successful addition to HAART. Although RNA interference and overexpression of truncation mutants in laboratory cell lines were employed to validate the pivotal role of LEDGF/p75 in HIV replication,4,21 the impact of LEDGF/p75 KD and/or LEDGF325–530 overexpression on HIV replication has not been studied in primary cells. In this study we examined the effect of LEDGF/p75 KD, LEDGF325–530 overexpression and the combination of both, on HIV replication in primary CD4+ T-cells. Viral vector constructs were first validated in laboratory T-cell lines. HIV replication was potently inhibited in LEDGF/p75 KD and in LEDGF325–530-expressing cells, as reported earlier.4 Combining both strategies even proved to be more potent (Figure 2 and Supplementary Figure S5), in line with results by Meehan and coworkers.21
In primary CD4+ T-cells, efficient inhibition of HIV-1 replication in vitro was achieved by overexpression of LEDGF325–530 (Figure 4), but not interaction-deficient control LEDGF325–530 D366N. The fact that KD in primary CD4+ T-cells fails to demonstrate a more pronounced effect on HIV replication is most probably due to the residual LEDGF/p75 protein, as observed in western analysis (lane 2, Figure 3a). Indeed, earlier studies revealed already that potent LEDGF/p75 KD is necessary to inhibit HIV replication, because residual protein levels are sufficient to support integration.18,22,23 In contrast with the T-cell line data, no additive effect was detected by combining the KD and overexpression strategies. This difference between laboratory T-cells and primary CD4+ T-cells can be due to the difference in KD levels, respectively 87% and 80% or possibly there is a difference in LEDGF/p75 dependency between laboratory and primary cells. Growth curves, T-cell characteristics, and engraftment of transgenic primary CD4+ T-cells expressing LEDGF325–530 or LEDGF325–530D366N were indistinguishable from nontreated primary cells ruling out that overexpression interferes with cell biology.
Next, transgenic primary CD4+ T-cells expressing LEDGF325–530 or LEDGF325–530D366N were infected with HIV-1NL4.3 and transplanted into NSG mice. Overexpression of LEDGF325–530 rendered primary T-cells more resistant to HIV infection compared to the D366N control, as illustrated by an engraftment up to 30% of total cells and a threefold reduction in the p24 antigen concentration in the circulating blood (Figure 6b,c respectively). In line with this result, p24 staining revealed less HIV in the liver and the spleen of mice transplanted with LEDGF325–530-expressing CD4+ T-cells compared to mice transplanted with LEDGF325–530D366N-expressing T-cells (Figure 7). Taken together, these results validate LEDGF/p75 as a novel antiviral target for HIV gene therapy.
The interest in gene therapeutic approaches to treat and potentially cure HIV infection has recently been fueled by the “Berlin case,” where an HIV-1 patient with acute myeloid leukemia received stem cells from a donor homozygous for a 32-base pair deletion in the CCR5 allele. The patient remained without viral rebound after transplantation and discontinuation of antiretroviral therapy24 and successful reconstitution of the systemic and gut-associated immune system was observed.25 Several gene therapeutic approaches have been developed for HIV/AIDS (for a review see refs. 13,14). Viral proteins (Rev, Tat, and Gag) as well as cellular proteins, such as the CCR5 coreceptor have been targeted using various strategies, including RNA decoys, ribozymes, siRNAs, and zinc-finger nucleases.26 Yet, each approach comes with specific concerns regarding toxicity,27 off-target effects,28 and viral resistance.27 Monotherapy readily gives rise to escape mutants, as demonstrated for RevM10 and several siRNA-based approaches, where a single nucleotide change is sufficient to overcome the RNA interference-mediated block of HIV replication.27 The surgical disruption of the CCR5 gene with zinc-finger nucleases is one of the most promising approaches to date (for a review see ref. 26). However, CCR5-independent viruses could be selected29 and the introduction of the CCR5Δ32 allele comes with a higher risk for West Nile virus infection.30 Additionally, the therapy only protects against HIV-R5 infection.31,32 Therefore, analogous efforts are underway to target CXCR4.33
We employ a fragment of a cellular cofactor to compete with the endogenous protein, LEDGF/p75, for the incoming viral IN. The first and most important advantage of targeting cellular proteins lies in the difficulty for resistance development. Theoretically, it is more challenging for the virus to develop resistance against an essential cofactor since mutations selected in the viral interacting protein will affect the interaction with the essential cellular cofactor. Although Hombrouck et al. selected viruses that were resistant to LEDGF325–530 overexpression, the two mutations selected in HIV-IN, A128T and E170G reduced the affinity of IN for LEDGF/p75 and replication kinetics were impaired in primary cells.34 Moreover, since the interaction between LEDGF/p75 and IN is important for replication of all lentiviruses, our strategy has as well the benefit to target all HIV-1 clades as well as HIV-235,36 as shown in Supplementary Figure S6 for HIV-1NDK and HIV-2.
The evaluation of new HIV therapies is limited by the lack of adequate animal models to evaluate HIV replication and pathogenesis. Here, we used NSG mice transplanted with acutely infected human CD4+ T-cells, which permitted high-level viremia and assessment of the effect of LEDGF325–530 expression on HIV-1 replication and CD4+ T-cell protection. Autotransfusion of ex vivo expanded CD4+ T-cells results in a clinical benefit for HIV-infected people.37 The main hurdle for HIV gene therapy lies in the large number of HIV-1 target cells (>1011). Since genetic modification of the entire target-cell population is impossible, only a limited number of the target cells can be genetically protected against de novo HIV infection. In a clinical setting, autologous CD4+ T-cells can be transduced at high efficiency and expanded ex vivo to >109 cells. Transfusion of transgenic cells will only result in a clinical benefit, when the genetically altered cells have a selective advantage (are protected) over the endogenous population and can accumulate over time. We evaluated the selective advantage of LEDGF325–530 transgenic PM1 cells, by mixing with WT PM1 cells (see Supplementary Materials and Methods and Supplementary Figure S9). A significant increase of the LEDGF325–530 expressing cells was observed over time, whereas no selection was observed in noninfected control cells or in interaction-deficient LEDGF325–530D366N cells. These results are comparable with the selective advantage reported for transgenic cells that are depleted for CCR5.38
A good gene therapy candidate combines low antigenicity with high efficacy. Since we use a fragment of a cellular cofactor, the protein fragment will not be recognized as foreign by the body. One disadvantage of cellular cofactors is the possible toxicity, since overexpression of an endogenous protein fragment might deregulate specific cellular interactions. The IBD of LEDGF/p75 does not only interact with HIV-IN, but is identified as a protein–protein interaction domain, ensuring interaction between LEDGF/p75 and several other cellular proteins, such as JPO2,39 pogZ,40 MLL/menin,41 and Cdc7-activator of S-phase kinase (Cdc7-ASK).42 Akin to its effect on HIV-1-IN, LEDGF/p75 orchestrates the chromatin-association of these proteins, with LEDGF/p75 acting as a multifunctional tether that can target a plethora of cellular machinery involved in expression and maintenance to specific loci in the chromatin. Overexpression of the IN-binding C-terminal end of the LEDGF/p75 protein, might affect these interactions and hence their downstream pathways. We performed several experiments to evaluate toxicity effects related to LEDGF325–530 overexpression in primary CD4+ T-cells (see Supplementary Materials and Methods). We compared transgenic cells and WT cells for growth, in vitro proliferative response (Supplementary Figure S7a) and production of IL-2, IL-5, and interferon-γ (Supplementary Figure S7b–d) following mitogenic stimulation. In addition, we evaluated engraftment capacity in NSG mice (Figure 5a) together with their ability to induce graft-versus-host disease (Figure 5b). No abnormalities were detected in these experiments.
In contrast to other cellular targets for gene therapy such as (co)receptors, inhibition of the LEDGF/p75-IN interaction tackles the last step before proviral integration preventing establishment of a latent reservoir. Ultimately, efficient HIV gene therapy would benefit by combining several potent approaches into one viral vector. As for HAART, combination of different strategies increases the potency and limits the likelihood for resistance development. In 2010 DiGiusto et al. reported on a phase I clinical trial using a triple punch gene therapeutic approach (Tat/Rev shRNA, TAR decoy and CCR5 ribozyme) to render HSC resistant to HIV infection. Low levels of genetically altered cells were detected up to 24 months after transplantation.43 Inclusion of potent fragments of cellular cofactors, such as LEDGF325–530, in a combinatorial gene therapeutic trial will prevent the HIV virus to become a stable, heritable element of the infected cell.
Materials and Methods
Ethics statement. Animal experiments were performed in compliance with the local animal experimentation guidelines and approved by the regional council (Regierungspräsidium, Darmstadt, Germany, protocol #F123/35). Human peripheral blood mononuclear cells were obtained from healthy anonymous donors, who provided written informed consent under protocols approved by the ethics committee of the Medical Faculty of the Johann-Wolfgang Goethe University Frankfurt, Protocol #81/10.
Cell culture. PM119 and SupT1 cells (both NIH AIDS reagents) were grown at 37°C in a humidified atmosphere containing 5% CO2 in RMPI (Gibco, Invitrogen, Merelbeke, Belgium) supplemented with 10% heat-inactivated fetal calf serum (Sigma-Aldrich, Bornem, Belgium) and 50 µg/ml gentamicin (Gibco). 293T cells (ATCC/LGC Standards, Molsheim Cedex France) were grown in Dulbecco's modified Eagle's medium with Glutamax (Gibco) supplemented with 8% fetal calf serum and 50 µg/ml gentamicin. Peripheral blood mononuclear cells were cultured in RPMI supplemented with 15% fetal calf serum, 1% nonessential amino acids (Gibco), 50 µg/ml gentamicin and recombinant human interleukin-2 (100 U/ml, Proleukin; Novartis, Vilvoorde, Belgium) at 37 °C with 5% CO2.
Plasmids and lentiviral vector production. All primers used are listed in Table 1. All enzymes used were obtained from Fermentas (St Leon-Rot, Germany).
Table 1. Primers used for plasmid cloning.
Transfer plasmid pSFFV_eGFP_Ires_tCD34 was used to produce LV_eGFP. First, pCMV_eGFP_Ires_tCD34 was cloned by replacing blasticidin with tCD34 in pCMV_eGFP_Ires_Bsd10 using primers tCD34s_BclI and tCD34as_SpeI to amplify tCD34. Next, CMV promoter of pCMV_eGFP_Ires_tCD34 was removed by XbaI and BamHI digestion and replaced by SFFV LTR promoter (PCR amplified using SFFVs_SpeI and SFFVas_XbaIBamHI primers and pFelix (kind gift of J. Luban Lab, Genève, Switzerland) as template).
Transfer plasmid SFFV_eGFP_LEDGF325–530_Ires_tCD34 was used to produce LV_LEDGF325–530. CMV promoter was replaced by SFFV LTR in CMV_eGFP_LEDGF325–530_Ires_Puro44 to create SFFV_eGFP_LEDGF325–530_Ires_Puro using the same protocol as described above. Ires_Puro was removed by BamHI and SpeI digestion and replaced by Ires_tCD34 (PCR amplified using primers: IRESs_BglII and tCD34as_SpeI and SFFV_eGFP_Ires_tCD34 as template). Same protocol was used to create SFFV_eGFP_LEDGF325–530_D366N_Ires_tCD34 which is the transfer plasmid for LV_LEDGF325–530D366N. Then CMV_eGFP_LEDGF325–530D366N_ Ires_Puro44 was used as starter plasmid.
SFFV_eGFP_LEDGF325–530_Ires_tCD34_mirLEDGF and SFFV_eGFP_Ires_tCD34_mirLEDGF were transfer plasmids for LV_LEDGF325–530_KD and LV_KD, respectively. SpeI, BclI, and NheI adaptor was cloned into NotI and XhoI digested pLNC_mirLEDGF_Zeo10 to create pLNC_MCS_mirLEDGF_Zeo. mirLEDGF was removed by digesting with NheI and XbaI and cloned into SpeI digested SFFV_eGFP_LEDGF325–530_Ires_tCD34 and SFFV_eGFP_Ires_tCD34 to create SFFV_eGFP_LEDGF325–530_Ires_tCD34_mirLEDGF and pSFFV_eGFP_Ires_tCD34_mirLEDGF.
Lentiviral vector production was performed as described earlier.45
Transduction. PM1 cells, SupT1 cells and primary CD4+ T-cells were transduced with lentiviral vectors at high MOI (>1) in RPMI medium with addition of protamine sulphate (Sigma-Aldrich) (8 µg/ml). After 72 hours of incubation medium was replaced.
Expression analysis. Quantitative-PCR and western blot was performed as previous described.10
Virus strains. The HIV-1 molecular clone, NL4.346 and NDK,47 and the HIV-2 molecular clone, ROD48 were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIH, contributed by Dr Malcolm Martin (Bethesda, MD). Virus stocks were produced on MT-4 cells following electroporation of viral plasmid at 250 V and 1500 mA. After 4 days the supernatants was harvested and aliquots were stored at −80 °C. Viral titers were determined by p24 enzyme-linked immunosorbent assay (Innotest HIV Antigen mAb; Innogenetics, Gent, Belgium).
HIV breakthrough assay. Primary CD4+ T-cells were seeded at 250 000 cells/well in a 48-well dish and infected 1 day later with 5,000 pg p24 of HIV (NL4.3). HIV replication was monitored by p24 enzyme-linked immunosorbent assay.
PM1 and SupT1 cells were seeded at 400 000 cells/well in a 12-well dish and infected the same day with 500 pg p24 of HIV (NL4.3), HIV-2 (ROD), and HIV Clade D (NDK).
T-cell purification. Peripheral blood mononuclear cells were purified from a buffy coat using density-gradient centrifugation (Lymphoprep; Axis-Shield PoC AS, Oslo, Norway). Primary CD4+ T-cells were isolated using negative selection (MACS; Miltenyi Biotec, Leiden, the Netherlands) and stimulated with CD2, CD3, CD28 beads (MACS).
FACS analysis. Transduced cells were fixed (2% PFA final) and analysed for eGFP expression by flow cytometry (FACSCalibur; BD Biosciences, Erembodegem, Belgium). Data were analyzed using CellQuest software. Likewise, tCD34 expression was analyzed. Cells were stained according to the manufacturer's protocol (Catnr 130-081-002, Miltenyi Biotec).
Evolution of human cell populations in the NSG mice were monitored by sampling blood (50 µl; retro-orbital bleeding) weekly. Blood was incubated with monoclonal antibody to mouse Fc-receptors (2.4G2; Bio Express, West Lebanon, NH) 15 minutes at room temperature. Cells were stained with PerCP-conjugated antihuman CD4 antibodies (clone SK3; BD-PharMingen, Heidelberg, Germany) and allophycocyanin-conjugated antihuman CD45 antibodies (clone HI30; BD-PharMingen) for 15 minutes at room temperature. Erythrocytes were lysed using BD PharmLyse (Heidelberg, Germany).
Virus replication assays in xenotransplanted NSG mice. NSG mice were obtained from Charles River Laboratories (Sulzfeld, Germany). Mice were bred and maintained under specific pathogen-free conditions in individually ventilated cages in the animal facilities of the Georg-Speyer-Haus. The experiments were performed in compliance with the local animal experimentation guidelines. On day 5 after transduction, human T lymphocytes were infected with HIVNL4.3. After 16 hours cells were harvested, washed with PBS and transplanted into adult NSG mice via intraperitoneal injection. Blood of the transplanted mice was regularly collected from the tail vein. Viral load in the serum of the infected mice was determined by p24 enzyme-linked immunosorbent assay. Mice were euthanized after anesthesia by cervical dislocation as soon as they showed signs of severe graft-versus-host disease.
Immunohistochemical analysis. Organs of the mice were assayed by immunohistochemistry as described in ref. 49 with minor modifications. Twenty-minute incubation at 98 °C in Tris-EDTA, pH 7.8 was used as heat-induced epitope retrieval. Sections were incubated overnight with the primary monoclonal anti-p24 antibody (Clone Kal-1, 1/5; Dako, Glostrup, Denmark) at 4 °C, followed by 30-minute incubation with secondary anti-mouse antibody (Envision Plus System Labeled Polymer HRP anti-mouse; Dako). Sections were finally treated with Liquid DAB Plus Substrate Chromogen System (Dako) for 30 minutes and counterstained with hematoxylin. Tissues were visualized with Leica DMR Biopoint 2 microscope (Leica Microsystems, Wetzlar, Germany). To assess nonspecific binding, tissue from uninfected transplanted mice was stained as a control.
SUPPLEMENTARY MATERIAL Figure S1. Gene therapeutic approaches using LEDGF/p75. Figure S2. Promoter study. Figure S3. Lentiviral vector constructs. Figure S4. Detection of knockdown and overexpression in SupT1 cells. Figure S5. LEDGF/p75 KD and/or LEDGF325–530 overexpression inhibits HIV-1NL4.3 infection in PM1 cells. Figure S6. LEDGF/p75 KD and/or LEDGF325–530 overexpression inhibits lentivirus infection. Figure S7. Transgenic CD4+ T-cells display normal T-cell characteristics in vitro. Figure S8. Primary CD4+ T-cells expressing LEDGF325–530 inhibit HIV infection in a mouse model. Figure S9. Selective advantage of LEDGF325–530-expressing PM1 cells. Materials and Methods.
Acknowledgments
We thank Barbara Van Remoortel and Paulien Van de Velde for excellent technical assistance and Anne-Sophie Van Rompuy, MD for excellent assistance with immunohistochemical stainings. S.V. is funded by the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen). Work of A.V. was funded by the Ernst-Schering-Foundation. J.D.R. had a Mathilde-Krim postdoctoral fellowship from amfAR. R.S. is a doctoral fellow of the Flemish Fund for Scientific Research (FWO Vlaanderen). This work was supported by KU Leuven Research Council (grant OT/09/047); the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen) CellCoVir SBO grant (60813); the Flanders Research Foundation (FWO) grant (G.0530.08); European Commission THINC grant [HEALTH-F3-2008-201032] to Z.D.
Supplementary Material
Gene therapeutic approaches using LEDGF/p75.
Promoter study.
Lentiviral vector constructs.
Detection of knockdown and overexpression in SupT1 cells.
LEDGF/p75 KD and/or LEDGF325–530 overexpression inhibits HIV-1NL4.3 infection in PM1 cells.
LEDGF/p75 KD and/or LEDGF325–530 overexpression inhibits lentivirus infection.
Transgenic CD4+ T-cells display normal T-cell characteristics in vitro.
Primary CD4+ T-cells expressing LEDGF325–530 inhibit HIV infection in a mouse model.
Selective advantage of LEDGF325–530-expressing PM1 cells.
REFERENCES
- Van Maele B, Busschots K, Vandekerckhove L, Christ F., and, Debyser Z. Cellular co-factors of HIV-1 integration. Trends Biochem Sci. 2006;31:98–105. doi: 10.1016/j.tibs.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Spearman P. Cellular cofactors involved in HIV assembly and budding. Curr Opin HIV AIDS. 2006;1:200–207. doi: 10.1097/01.COH.0000221592.49412.1f. [DOI] [PubMed] [Google Scholar]
- Cherepanov P, Maertens G, Proost P, Devreese B, Van Beeumen J, Engelborghs Y.et al. (2003HIV-1 integrase forms stable tetramers and associates with LEDGF/p75 protein in human cells J Biol Chem 278372–381. [DOI] [PubMed] [Google Scholar]
- Llano M, Morrison J., and, Poeschla EM. Virological and cellular roles of the transcriptional coactivator LEDGF/p75. Curr Top Microbiol Immunol. 2009;339:125–146. doi: 10.1007/978-3-642-02175-6_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix J, Gijsbers R, De Rijck J, Voet A, Hotta J, McNeely M.et al. (2011The transcriptional co-activator LEDGF/p75 displays a dynamic scan-and-lock mechanism for chromatin tethering Nucleic Acids Res 391310–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlure F, Maertens G, Rahman S, Cherepanov P., and, Engelman A. A tripartite DNA-binding element, comprised of the nuclear localization signal and two AT-hook motifs, mediates the association of LEDGF/p75 with chromatin in vivo. Nucleic Acids Res. 2006;34:1653–1665. doi: 10.1093/nar/gkl052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherepanov P, Ambrosio AL, Rahman S, Ellenberger T., and, Engelman A. Structural basis for the recognition between HIV-1 integrase and transcriptional coactivator p75. Proc Natl Acad Sci USA. 2005;102:17308–17313. doi: 10.1073/pnas.0506924102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee CJ, Kessl JJ, Shkriabai N, Dar MJ, Engelman A., and, Kvaratskhelia M. Dynamic modulation of HIV-1 integrase structure and function by cellular lens epithelium-derived growth factor (LEDGF) protein. J Biol Chem. 2008;283:31802–31812. doi: 10.1074/jbc.M805843200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeely M, Hendrix J, Busschots K, Boons E, Deleersnijder A, Gerard M.et al. (2011In vitro DNA tethering of HIV-1 integrase by the transcriptional coactivator LEDGF/p75 J Mol Biol 410811–830. [DOI] [PubMed] [Google Scholar]
- Gijsbers R, Ronen K, Vets S, Malani N, De Rijck J, McNeely M.et al. (2010LEDGF hybrids efficiently retarget lentiviral integration into heterochromatin Mol Ther 18552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris AL, Wu X, Hughes CM, Stewart C, Smith SJ, Milne TA.et al. (2010Lens epithelium-derived growth factor fusion proteins redirect HIV-1 DNA integration Proc Natl Acad Sci USA 1073135–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ F, Voet A, Marchand A, Nicolet S, Desimmie BA, Marchand D.et al. (2010Rational design of small-molecule inhibitors of the LEDGF/p75-integrase interaction and HIV replication Nat Chem Biol 6442–448. [DOI] [PubMed] [Google Scholar]
- Kitchen SG, Shimizu S., and, An DS. Stem cell-based anti-HIV gene therapy. Virology. 2011;411:260–272. doi: 10.1016/j.virol.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Laer D, Baum C., and, Protzer U. Antiviral gene therapy. Handb Exp Pharmacol. 2009. pp. 265–297. [DOI] [PubMed]
- Neagu MR, Ziegler P, Pertel T, Strambio-De-Castillia C, Grütter C, Martinetti G.et al. (2009Potent inhibition of HIV-1 by TRIM5-cyclophilin fusion proteins engineered from human components J Clin Invest 1193035–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehse B, Richters A, Putimtseva-Scharf K, Klump H, Li Z, Ostertag W.et al. (2000CD34 splice variant: an attractive marker for selection of gene-modified cells Mol Ther 15 Pt 1448–456. [DOI] [PubMed] [Google Scholar]
- Cherepanov P, Sun ZY, Rahman S, Maertens G, Wagner G., and, Engelman A. Solution structure of the HIV-1 integrase-binding domain in LEDGF/p75. Nat Struct Mol Biol. 2005;12:526–532. doi: 10.1038/nsmb937. [DOI] [PubMed] [Google Scholar]
- Llano M, Saenz DT, Meehan A, Wongthida P, Peretz M, Walker WH.et al. (2006An essential role for LEDGF/p75 in HIV integration Science 314461–464. [DOI] [PubMed] [Google Scholar]
- Lusso P, Cocchi F, Balotta C, Markham PD, Louie A, Farci P.et al. (1995Growth of macrophage-tropic and primary human immunodeficiency virus type 1 (HIV-1) isolates in a unique CD4+ T-cell clone (PM1): failure to downregulate CD4 and to interfere with cell-line-tropic HIV-1 J Virol 693712–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffee BD., and, Claman HN. Chronic graft-versus-host disease (GVHD) as a model for scleroderma. I. Description of model systems. Cell Immunol. 1983;77:1–12. doi: 10.1016/0008-8749(83)90001-1. [DOI] [PubMed] [Google Scholar]
- Meehan AM, Saenz DT, Morrison J, Hu C, Peretz M., and, Poeschla EM. LEDGF dominant interference proteins demonstrate prenuclear exposure of HIV-1 integrase and synergize with LEDGF depletion to destroy viral infectivity. J Virol. 2011;85:3570–3583. doi: 10.1128/JVI.01295-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove L, Christ F, Van Maele B, De Rijck J, Gijsbers R, Van den Haute C.et al. (2006Transient and stable knockdown of the integrase cofactor LEDGF/p75 reveals its role in the replication cycle of human immunodeficiency virus J Virol 801886–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielske SP., and, Stevenson M. Modest but reproducible inhibition of human immunodeficiency virus type 1 infection in macrophages following LEDGFp75 silencing. J Virol. 2006;80:7275–7280. doi: 10.1128/JVI.02470-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hütter G, Nowak D, Mossner M, Ganepola S, Müssig A, Allers K.et al. (2009Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation N Engl J Med 360692–698. [DOI] [PubMed] [Google Scholar]
- Allers K, Hütter G, Hofmann J, Loddenkemper C, Rieger K, Thiel E.et al. (2011Evidence for the cure of HIV infection by CCR5?32/?32 stem cell transplantation Blood 1172791–2799. [DOI] [PubMed] [Google Scholar]
- Cannon P., and, June C. Chemokine receptor 5 knockout strategies. Curr Opin HIV AIDS. 2011;6:74–79. doi: 10.1097/COH.0b013e32834122d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden D, Pusch O., and, Ramratnam B. Overcoming HIV-1 resistance to RNA interference. Front Biosci. 2007;12:3104–3116. doi: 10.2741/2298. [DOI] [PubMed] [Google Scholar]
- Pattanayak V, Ramirez CL, Joung JK., and, Liu DR. Revealing off-target cleavage specificities of zinc-finger nucleases by in vitro selection. Nat Methods. 2011;8:765–770. doi: 10.1038/nmeth.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BM, Foulke JS, Flinko R, Heredia A, DeVico A., and, Reitz M. An alteration of human immunodeficiency virus gp41 leads to reduced CCR5 dependence and CD4 independence. J Virol. 2008;82:5460–5471. doi: 10.1128/JVI.01049-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass WG, McDermott DH, Lim JK, Lekhong S, Yu SF, Frank WA.et al. (2006CCR5 deficiency increases risk of symptomatic West Nile virus infection J Exp Med 20335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R.et al. (1996Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection Cell 86367–377. [DOI] [PubMed] [Google Scholar]
- Mummidi S, Ahuja SS, Gonzalez E, Anderson SA, Santiago EN, Stephan KT.et al. (1998Genealogy of the CCR5 locus and chemokine system gene variants associated with altered rates of HIV-1 disease progression Nat Med 4786–793. [DOI] [PubMed] [Google Scholar]
- Wilen CB, Wang J, Tilton JC, Miller JC, Kim KA, Rebar EJ.et al. (2011Engineering HIV-resistant human CD4+ T cells with CXCR4-specific zinc-finger nucleases PLoS Pathog 7e1002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombrouck A, De Rijck J, Hendrix J, Vandekerckhove L, Voet A, De Maeyer M.et al. (2007Virus evolution reveals an exclusive role for LEDGF/p75 in chromosomal tethering of HIV PLoS Pathog 3e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare S, Shun MC, Gupta SS, Valkov E, Engelman A., and, Cherepanov P. A novel co-crystal structure affords the design of gain-of-function lentiviral integrase mutants in the presence of modified PSIP1/LEDGF/p75. PLoS Pathog. 2009;5:e1000259. doi: 10.1371/journal.ppat.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busschots K, Vercammen J, Emiliani S, Benarous R, Engelborghs Y, Christ F.et al. (2005The interaction of LEDGF/p75 with integrase is lentivirus-specific and promotes DNA binding J Biol Chem 28017841–17847. [DOI] [PubMed] [Google Scholar]
- Levine BL, Bernstein WB, Aronson NE, Schlienger K, Cotte J, Perfetto S.et al. (2002Adoptive transfer of costimulated CD4+ T cells induces expansion of peripheral T cells and decreased CCR5 expression in HIV infection Nat Med 847–53. [DOI] [PubMed] [Google Scholar]
- Perez EE, Wang J, Miller JC, Jouvenot Y, Kim KA, Liu O.et al. (2008Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases Nat Biotechnol 26808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomeeusen K, De Rijck J, Busschots K, Desender L, Gijsbers R, Emiliani S.et al. (2007Differential interaction of HIV-1 integrase and JPO2 with the C terminus of LEDGF/p75 J Mol Biol 372407–421. [DOI] [PubMed] [Google Scholar]
- Bartholomeeusen K, Christ F, Hendrix J, Rain JC, Emiliani S, Benarous R.et al. (2009Lens epithelium-derived growth factor/p75 interacts with the transposase-derived DDE domain of PogZ J Biol Chem 28411467–11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama A., and, Cleary ML. Menin critically links MLL proteins with LEDGF on cancer-associated target genes. Cancer Cell. 2008;14:36–46. doi: 10.1016/j.ccr.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S, Jenkins V, Dar MJ, Engelman A., and, Cherepanov P. Transcriptional co-activator LEDGF interacts with Cdc7-activator of S-phase kinase (ASK) and stimulates its enzymatic activity. J Biol Chem. 2010;285:541–554. doi: 10.1074/jbc.M109.036491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiusto DL, Krishnan A, Li L, Li H, Li S, Rao A.et al. (2010RNA-based gene therapy for HIV with lentiviral vector-modified CD34(+) cells in patients undergoing transplantation for AIDS-related lymphoma Sci Transl Med 236ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rijck J, Vandekerckhove L, Gijsbers R, Hombrouck A, Hendrix J, Vercammen J.et al. (2006Overexpression of the lens epithelium-derived growth factor/p75 integrase binding domain inhibits human immunodeficiency virus replication J Virol 8011498–11509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraerts M, Michiels M, Baekelandt V, Debyser Z., and, Gijsbers R. Upscaling of lentiviral vector production by tangential flow filtration. J Gene Med. 2005;7:1299–1310. doi: 10.1002/jgm.778. [DOI] [PubMed] [Google Scholar]
- Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A.et al. (1986Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone J Virol 59284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spire B, Sire J, Zachar V, Rey F, Barré-Sinoussi F, Galibert F.et al. (1989Nucleotide sequence of HIV1-NDK: a highly cytopathic strain of the human immunodeficiency virus Gene 81275–284. [DOI] [PubMed] [Google Scholar]
- Clavel F, Guyader M, Guétard D, Sallé M, Montagnier L., and, Alizon M. Molecular cloning and polymorphism of the human immune deficiency virus type 2. Nature. 1986;324:691–695. doi: 10.1038/324691a0. [DOI] [PubMed] [Google Scholar]
- Klaritsch P, Mayer S, Sbragia L, Toelen J, Roubliova X, Lewi P.et al. (2010Albumin as an adjunct to tracheal occlusion in fetal rats with congenital diaphragmatic hernia: a placebo-controlled study Am J Obstet Gynecol 202198.e1–198.e9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gene therapeutic approaches using LEDGF/p75.
Promoter study.
Lentiviral vector constructs.
Detection of knockdown and overexpression in SupT1 cells.
LEDGF/p75 KD and/or LEDGF325–530 overexpression inhibits HIV-1NL4.3 infection in PM1 cells.
LEDGF/p75 KD and/or LEDGF325–530 overexpression inhibits lentivirus infection.
Transgenic CD4+ T-cells display normal T-cell characteristics in vitro.
Primary CD4+ T-cells expressing LEDGF325–530 inhibit HIV infection in a mouse model.
Selective advantage of LEDGF325–530-expressing PM1 cells.