Background: Plasma membrane potential (PMP) oscillations play a key role in glucose-stimulated insulin secretion.
Results: PMP oscillations and insulin secretion can be driven by pyruvate. Oscillations are not initiated by increases in mitochondrial membrane potential, NAD(P)H reduction, or ATP level.
Conclusion: PMP oscillations are not initiated by bioenergetic fluctuations.
Significance: The assumption that upstream glycolytic and bioenergetic oscillations are required for PMP oscillations may require re-examination.
Keywords: Calcium Imaging, Confocal Microscopy, Diabetes, Glucose, Mitochondria, Pyruvate, ATP, NAD(P)H, Clonal β Cells, Oscillations
Abstract
Oscillations in plasma membrane potential play a central role in glucose-induced insulin secretion from pancreatic β-cells and related insulinoma cell lines. We have employed a novel fluorescent plasma membrane potential (Δψp) indicator in combination with indicators of cytoplasmic free Ca2+ ([Ca2+]c), mitochondrial membrane potential (Δψm), matrix ATP concentration, and NAD(P)H fluorescence to investigate the role of mitochondria in the generation of plasma membrane potential oscillations in clonal INS-1 832/13 β-cells. Elevated glucose caused oscillations in plasma membrane potential and cytoplasmic free Ca2+ concentration over the same concentration range required for insulin release, although considerable cell-to-cell heterogeneity was observed. Exogenous pyruvate was as effective as glucose in inducing oscillations, both in the presence and absence of 2.8 mm glucose. Increased glucose and pyruvate each produced a concentration-dependent mitochondrial hyperpolarization. The causal relationships between pairs of parameters (Δψp and [Ca2+]c, Δψp and NAD(P)H, matrix ATP and [Ca2+]c, and Δψm and [Ca2+]c) were investigated at single cell level. It is concluded that, in these β-cells, depolarizing oscillations in Δψp are not initiated by mitochondrial bioenergetic changes. Instead, regardless of substrate, it appears that the mitochondria may simply be required to exceed a critical bioenergetic threshold to allow release of insulin. Once this threshold is exceeded, an autonomous Δψp oscillatory mechanism is initiated.
Introduction
The canonical pathway for glucose-stimulated insulin secretion from pancreatic β-cells links increased extracellular glucose availability to enhanced glycolysis and increased substrate availability for the mitochondria. Subsequently, mitochondria hyperpolarize, generating a raised cytoplasmic ATP/ADP ratio that inhibits plasma membrane ATP-sensitive K+ (KATP) channels. As a result, the plasma membrane is depolarized, Ca2+ enters via voltage-activated Ca2+ channels, and, finally, exocytosis of insulin-containing vesicles is triggered (1). Insulin secretion from isolated islets of Langerhans in response to elevated glucose is pulsatile, with a typical periodicity of 2–5 min (2). Pulsatile insulin secretion is also evident in the portal vein (3), with a periodicity similar to that exhibited by isolated islets. Importantly, loss of pulsatile insulin secretion is an early sign in the development of type 2 diabetes (4). This may reflect a dysfunction of the pancreatic β-cells or regulatory processes in islets.
At the single β-cell level, once the plasma membrane potential (Δψp) has depolarized in response to elevated glucose, complex fast and compound waves of further depolarization are initiated, lasting from seconds to a few minutes. They are associated with action potential firing and cytoplasmic Ca2+ ([Ca2+]c)3 elevation (5, 6). Electrophysiological capacitance studies have emphasized the importance of oscillatory plasma membrane depolarization both to reactivate desensitizing plasma membrane voltage-activated Ca2+ channels (7) and for granule recruitment (8).
In contrast to agreement on the mechanism of coupling of elevated glucose to the initial Δψp depolarization, there is currently no consensus as to the mechanisms controlling the subsequent fast and compound oscillations. In intact islets and dissociated β-cells, it has been suggested that glycolytic fluctuations, perhaps controlled by phosphofructokinase, may be responsible (9, 10). These are associated with oscillations of respiration (11), nicotinamide nucleotide reduction state (12), mitochondrial membrane potential (13), cytoplasmic ATP/ADP ratios (9, 14), and consequent activation and inactivation of the plasma membrane KATP channel.
Insulinoma-derived β-cell lines are amenable to metabolic manipulation, and have been extensively employed in investigations of the coupling of substrate metabolism to insulin secretion (15). The glucose-responsive INS-1 832/13 cell line is particularly robust and consistent (16). Unlike primary β-cells, most insulinoma-derived β-cell lines possess a plasma membrane monocarboxylate transporter and are thus able to metabolize exogenous pyruvate (17). Pyruvate is an effective secretagogue in these cells (18), and because it has direct access to the mitochondrion, bypassing glycolysis, its insulinotropic effect suggests that glycolysis-driven metabolic oscillations are not essential for insulin release from these clonal cells. Questions that remain include whether pyruvate induces Δψp oscillations similarly to glucose, whether these oscillations appear to be linked to insulin secretion, and whether they are initiated and driven by metabolic and/or bioenergetic oscillations.
In this work, we exploit a combination of fluorescent techniques to monitor changes in plasma membrane potential (Δψp), mitochondrial membrane potential (Δψm), cytoplasmic free Ca2+ ([Ca2+]c), matrix ATP, and NAD(P)H reduction in the presence of glucose or pyruvate to investigate these questions in INS-1 832/13 cells. The anionic plasma membrane potential indicator (PMPI) (19) readily detects and resolves oscillations in these cells separated by 20 s or more, and we have exploited PMPI together with fluorescent probes of Δψm, [Ca2+]c, NAD(P)H, and matrix ATP and image analysis to conclude that these clonal β-cells can maintain pyruvate-driven oscillations depolarizing Δψp and elevating [Ca2+]c that are not initiated by an increase in mitochondrial “function” (i.e. raised Δψm, NAD(P)H, or matrix ATP). Small downstream changes can be detected, but the implication is that regardless of substrate, the mitochondria may simply be required to exceed a critical bioenergetic threshold to allow these cells to release insulin and that once this threshold is surpassed, an autonomous oscillatory mechanism is initiated.
EXPERIMENTAL PROCEDURES
Materials
Tetramethylrhodamine methyl ester (TMRM), fluo-4 AM, fura-2 AM, Fura Red AM™, Hoechst 33342, Lipofectamine 2000, and Opti-MEM were from Invitrogen. PMPI is a component of a proprietary membrane potential assay kit (R-8042) from Molecular Devices Corp. (Sunnyvale, CA). AT1.03 constructs were a kind gift of Prof. Hiroyuki Noji (20) Unless otherwise indicated, all reagents were obtained from Sigma-Aldrich (St. Louis, MO).
Cell Culture
Clonal β-cells (INS-1 832/13) (16) were cultured in RPMI 1640 cell culture medium containing 11.1 mm glucose supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, 10 mm Hepes, 2 mm glutamine, 1 mm sodium pyruvate, and 50 μm β-mercaptoethanol at 37 °C in a humidified atmosphere containing 95% air and 5% CO2.
Insulin Secretion
Cells were seeded in 24-well plates in culture medium. At 24 h, medium was exchanged for culture medium containing 11 mm glucose. After a further 24 h, cells were transferred to secretion assay buffer containing 2.8 mm glucose, 114 mm NaCl, 4.7 mm KCl, 1.2 mm KH2PO4, 1.16 mm MgSO4, 25.5 mm NaHCO3, 20 mm Hepes, 2.5 mm CaCl2, and 0.2% BSA. After 2 h, cells were transferred to secretion assay buffer medium containing varying glucose concentrations or sodium pyruvate. Insulin released in the subsequent 60 min was determined with the Coat-a-Count radioimmunoassay kit (DPC, Los Angeles, CA).
Population Mitochondrial Membrane Potential Measurements
Cells were seeded in poly-D-lysine-coated 8-well chambered coverglasses (Lab-Tek, Naperville, IL) in culture medium and after 24 h were transferred for a further 24 h to culture medium containing 2.8 mm glucose. For Δψm measurements, the cells were loaded with 100 nm TMRM for 2 h in buffer A (2.8 mm glucose, 135 mm NaCl, 3.5 mm KCl, 0.5 mm MgSO4, 0.5 mm Na2HPO4, 5 mm NaHCO3, 10 mm Hepes, 1.5 mm CaCl2, 0.1 w/v% BSA (pH 7.4). Immediately prior to imaging the medium was changed again to buffer A without glucose. The chambered coverglass was inserted into a temperature-controlled (37 °C) and CO2-controlled (5%) incubation chamber on the stage of a Zeiss LSM510 inverted confocal fluorescence microscope with a ×40 air objective. The pinhole diameter was increased to give an optical slice of 10 μm to allow collection of the defocused signal from individual somata. TMRM was excited at 543 nm, and emission was detected with a 585-nm long-pass filter. All experiments were performed in quench mode (21) and confirmed by fluorescence dequenching upon a final addition of the protonophore FCCP (not shown in traces). Control experiments confirmed that changes in Δψp did not significantly affect short-term TMRM fluorescence. Further controls in the presence of 5 μm cyclosporine A confirmed that TMRM equilibration was not affected by multidrug transport activity.
Plasma Membrane Potential
An individual vial from a FLIPR® membrane potential assay kit, explorer format component A (Molecular Devices, catalog no. R-8042) containing a proprietary plasma membrane potential indicator that we have named “PMPI,” was reconstituted in 10 ml of distilled water, dispensed into 1-ml aliquots, and frozen (PMPI stock). Cells were cultured as above for 2–4 days in the presence of 11 mm glucose. Prior to imaging, cells were transferred for 2 h to 400 μl of buffer B containing 120 mm NaCl, 3.5 mm KCl, 1 mm MgCl2, 0.4 mm KH2PO4, 5 mm NaHCO3, 10 mm Na-Tes, 1.3 mm CaCl2, and either 0 or 2.8 mm glucose (pH 7.4). Immediately prior to imaging, 4 μl of PMPI stock was added. The chambered coverglass was transferred to the stage of an inverted Zeiss Pascal confocal microscope. Cells were excited at 514 nm, and emission was measured with a 530-nm long-pass filter. At the end of each experiment, the trace was calibrated by exchanging 74 μl of the incubation medium with 74 μl of high K+ buffer B (as low K+ buffer B but containing 123.5 mm KCl and omitting NaCl) to increase the K+ concentration to 25 mm (19).
Dual Plasma Membrane Potential and [Ca2+]c
Cells cultured in the presence of 11 mm glucose were transferred after 24 h to culture medium containing 2.8 mm glucose for a further 24 h before transferring to 400 μl of buffer A. After 1.5 h, 2 μm fluo-4 AM, 0.5 mm sulfinpyrazone (to inhibit multispecific organic anion transporters (22), and 16 μm bovine serum albumin were added, and the incubation was continued for a further 30 min. Prior to imaging, the incubation buffer was replaced by 400 μl of buffer A containing 0.5 mm sulfinpyrazone and 2 μl of PMPI stock, but without glucose or BSA, and the cells were loaded for 10 min prior to imaging. Fluo-4 was excited at 488 nm with emission at 505–530 nm, and PMPI was excited at 543 nm with emission at > 560 nm. Free cytoplasmic Ca2+ traces are displayed as arbitrary fluorescent units. PMPI fluorescence was normalized as described above.
Dual Mitochondrial Membrane Potential and [Ca2+]c
Cells were cultured for 2–4 days in the presence of 11.1 mm glucose in 8-well Lab-Tek chambers, seeding 4 × 105 cells/well. Prior to imaging, cells were transferred for 90 min to buffer B containing 2.8 mm glucose, 0.4 w/v % BSA, and 100 nm TMRM. At 90 min, 2 μm fura-2 AM and 0.5 mm sulfinpyrazone were added, and the incubation was continued for a further 30 min. For imaging, the incubation buffer was replaced by 500 μl of buffer B containing 0.5 mm sulfinpyrazone, 0.4 w/v % BSA, and 0 or 2.8 mm glucose. Epifluorescence microscopy was performed on a Nikon Eclipse Ti-PFS inverted microscope at 37 °C using a Cascade 512B camera (Photometrics, Tucson, AZ) with an S-Fluor 20×/0.75 air lens, a λ LB-LS17 Xe-arc light source (attenuated), 10-3 excitation and emission filter wheels (Sutter Instruments, Novato, CA), and an MS-2000 linear encoded motorized stage (ASI, Eugene, OR). The filter sets, given as excitation, dichroic mirror, emission in nm/bandwidth, were, for TMRM (at 75 ms exposure time), 543/22, 562, 617/73 and for fura-2, 340/26 (250 ms) and 387/11 (125 ms), 409, 510/84 (all from Semrock, Rochester, NY). Image acquisition was controlled by NIS Elements 3.22 (Nikon, Melville, NY). Oscillations were followed for 1 h starting 10 min after substrate addition. To reduce photodamage, 75 frames at 4-s intervals were acquired at one position and 12 distinct x,y positions were imaged successively using the “multipoint set acquisition” feature of the Elements. At the end of the Multipoint Set Acquisition, 10 μm FCCP was added to verify quench mode of TMRM, and fluorescence was followed by cyclic imaging of all 12 positions using “ND acquisition” in Elements. Finally, the deep red nuclear dye DRAQ5 (Biostatus, Shepshed, UK) was included, and all positions imaged previously were revisited and imaged at 628/40, 660, 692/40.
Image analysis was performed in Image Analyst MKII (Novato, CA). Briefly, mitochondrion-free areas of cells were determined on the basis of subtraction of TMRM from fura-2 images and image segmentation. For this, temporal maximum intensity projections rescaled between the 10th and 99th percentiles were used. These segments were assigned to individual cells on the basis of segmentation of fura-2 images using DRAQ5 staining as a seed of the watershed algorithm. TMRM and fura-2 fluorescence intensities corresponding to individual cells measured over the non-mitochondrial (typically nuclear) areas were analyzed further in Mathematica 8.0 (Wolfram Research, Champaign, IL). TMRM traces were corrected for any synchronous variations of intensities by normalizing with the mean whole cell intensity of TMRM fluorescence in the whole view field. Peaks of fura-2 ratio oscillations were determined on the basis of first and second temporal derivatives. To amplify the changes in TMRM fluorescence synchronous to [Ca2+]c oscillations, a stretch of the fura-2 ratio and normalized TMRM intensity time courses from 20 s before and 40 s after each peak were collected, their base lines were subtracted, and their means were calculated for each experimental run. Data are given as mean ± S.E. of these means, expressing variations between experiments. A range of different thresholds for fura-2 oscillation peak detection were examined, resulting in data consistent with the presented results.
Dual NAD(P)H Autofluorescence and Plasma Membrane Potential
Cells were incubated with PMPI as described above. Epifluorescence microscopy was performed on the Nikon Eclipse Ti-PFS microscope with the multipoint set acquisition/ND acquisition approach as described above but using an S-Fluor ×40/1.3 oil lens and the following filter sets for NAD(P)H autofluorescence (200 ms exposure time): 340/26, 409, 460/80 and for PMPI (150 ms) 500/24, 520, 542/27. Experiments were concluded by application of 2 μm FCCP followed by 2 μm rotenone, and images were cyclically captured in all positions. Using Image Analyst MKII, the PMPI projection images were segmented using DRAQ5 as seed. NAD(P)H autofluorescence and PMPI fluorescence were determined over each identified whole cell, and time courses were further analyzed in Mathematica. Autofluorescence traces were corrected for any synchronous variations of intensities, including photobleaching, by normalizing with the mean of all cells in each view field. Oscillations of NAD(P)H autofluorescence were expressed as percent change compared with the span of autofluorescence intensities measured between FCCP and rotenone additions.
Dual [ATP]m and [Ca2+]c
Cells were transfected with the mitochondrially targeted AT1.03 ATP sensor (ATeam) (20) using Lipofectamine 2000 in Opti-MEM medium at a 3:2 ratio of Lipofectamine (μl) to DNA (μg). 0.2 μg of DNA was transfected per well in 8-well Lab-Tek chambers. Then, cells were cultured for 3–4 days in the presence of 11.1 mm glucose. Prior to imaging, cells were transferred for 2 h to buffer B containing 2.8 mm glucose, 0.4 w/v % BSA. Then, 5 μm Fura Red AM, 0.5 mm sulfinpyrazone were added, and the incubation was continued for a further 30 min. Prior to imaging, the incubation buffer was replaced by buffer B containing 0.5 mm sulfinpyrazone, 0.4 w/v % BSA, and 2.8 mm glucose. Epifluorescence microscopy was performed on the Nikon Eclipse Ti-PFS microscope with the multipoint set acquisition/ND acquisition approach as described above but using the following filter sets for ratiometric ATeam, 438/24, 458, 483/32 (50-ms exposure time) or 542/27 (35 ms) and for ratiometric Fura Red, 438/24 (50 ms) or 500/24 (25 ms), 562, 641/75. Dynamic cross-bleed correction was performed to eliminate the effects of Fura Red on the 483-nm excitation of ATeam as described previously (23). At the end of the multipoint set acquisition, first glucose (16 mm total) and then 1 μg/ml oligomycin and 1 mm iodoacetate were added to maximize and then deplete ATP. These additions were followed by cyclic imaging of all 12 positions. Finally 10 μg/ml Hoechst 33342 nuclear dye was added and imaged at 340/26, 409, 460/80.
Using Image Analyst MKII, the ATeam images were segmented using Hoechst as seed. Fluorescence was determined over each identified whole cell. Using Mathematica, ATeam and Fura Red ratios were calculated after spectral unmixing. ATeam was calibrated by taking the maximal ratio at saturating (16 mm) glucose and minimal ratio at depleted ATP levels in the presence of oligomycin plus iodoacetate as above, and ratios were rescaled between 0 and 1. The concentration of ATP was calculated as ((1-R)Kd−n/R)−1/n, where R is the rescaled ATeam ratio, Kd = 3.3 mm, and n = 2.1 (20). Values above 10 mm ATP were considered as saturated. Peaks of Fura Red ratio oscillations were determined as above, and corresponding means of changes of Fura Red ratio and ATP concentration were calculated.
RESULTS
Calibration
The slow response of conventional fluorescent Δψp indicators such as bis-(1,3-dibutylbarbituric acid)-trimethine oxonol limits their utility to monitor changes in Δψp that occur with a subminute time course. The Δψp indicator we have termed PMPI for plasma membrane potential indicator (19) is a proprietary constituent of the Molecular Devices FLIPR® membrane potential assay kit and responds 14 times faster than bis-(1,3-dibutylbarbituric acid)-trimethine oxonol to changes in Δψp (24). The anionic indicator responds to a Δψp depolarization with an increased fluorescence as it enters the cell. External fluorescence is quenched by a proprietary agent. We have previously exploited PMPI to monitor changes in Δψp in cultured neurons in parallel with fluorescent monitoring of the mitochondrial membrane potential, Δψm (19). The resting Δψp of the related INS-1 cells under conditions of maximal KATP activation has been determined to be about −80 mV (25). Assuming a cytoplasmic K+ concentration of 120 mm, increasing the external [K+] to 25 mm would lower the K+ diffusion potential to −41 mV. This results in a mean enhancement of PMPI fluorescence of 4.43 ± 0.33-fold (S.E., n = 11). In subsequent figures, PMPI traces are normalized to the fluorescent enhancement span from fully polarized to that following oligomycin and 25 mm KCl.
Where indicated, approximate calibrations of the PMPI and TMRM traces in terms of Δψp and Δψm, respectively, were carried out using a previously published Excel program (19). The assumptions and calculations are detailed in the supplemental data.
Stochastic Δψp Depolarization and [Ca2+]c Transients in the Presence of Elevated Glucose
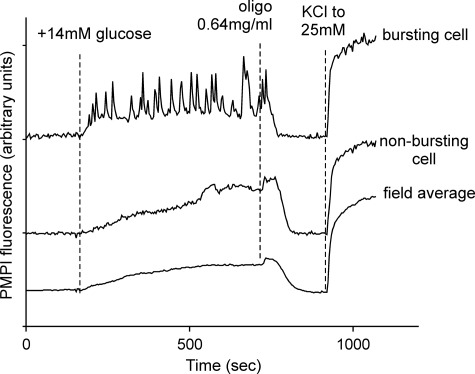
INS-1 832/13 cells kept in 2.8 mm glucose maintained a stable Δψp (Fig. 1). Increasing the glucose concentration to 16 mm initiated a Δψp depolarization. In contrast to intact islets, Δψp oscillations were not synchronized, and so the field average depolarization of ∼200 cells showed a slowly increasing average depolarization, reaching a plateau after about 7 min. Although every cell in the field responded to increased glucose by depolarizing, individual cells behaved heterogeneously, with some cells initiating Δψp oscillations with a periodicity of 20–120 s, whereas other cells progressively depolarized without showing oscillations (Fig. 1). It should be noted that a failure to oscillate did not reflect a failure to depolarize. Inhibition of the mitochondrial ATP synthase with oligomycin resulted in the expected uniform repolarization of all cells and a cessation of oscillations as oxidative phosphorylation was inhibited. This was consistently preceded by a brief period, typically 30–50 s, of enhanced depolarization.
FIGURE 1.
Δψp responses to 14 mm glucose. INS-1 832/13 cells were preincubated in buffer B in the presence of 2.8 mm glucose as described under “Experimental Procedures.” Where indicated, an additional 14 mm glucose was added, followed by oligomycin to inhibit the ATP synthase. Finally sufficient high K+ medium was substituted to increase the [K+] to 25 mm to calibrate the response. Changes in Δψp were monitored with PMPI, and the traces were normalized to equalize the change in fluorescence from the initial to final values in the experiment. The average response of the entire field is shown together with representative responses of bursting and non-bursting single cells.
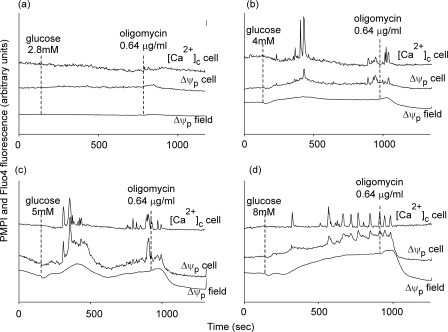
In Fig. 2, cells were preincubated in 2.8 mm glucose and deprived of glucose for 5 min following which the substrate was readded at increasing concentrations to estimate the threshold at which oscillations could be detected. The field average responses show depolarizations that increased with time and glucose concentration. Although the individual cell responses were heterogeneous, single cells were selected whose Δψp profile approximated the population average for each glucose concentration. At 2.8 mm glucose, cells did not oscillate, and no depolarization that could be reversed by ATP synthase inhibition by oligomycin was observed (Fig. 2a). Parallel monitoring of [Ca2+]c showed that the representative cell was quiescent. Increasing the glucose concentration to 4 mm produced a slight population depolarization and subsequent repolarization with oligomycin. A cell whose Δψp profile approximated the population average showed a brief Δψp burst accompanied by transient [Ca2+]c spiking (Fig. 2b). At 5 mm glucose, a pronounced biphasic depolarization was shown, with the selected cell showing clustered depolarization and [Ca2+]c spiking (Fig. 2c). Finally, exposure to 8 mm glucose resulted in a more sustained depolarization and prolonged Δψp bursting and [Ca2+]c spiking in the representative cell (Fig. 2d). It is notable that at each glucose concentration, the population and single cell Δψp depolarization persisted or was even enhanced for some 30 s after oligomycin addition.
FIGURE 2.
Glucose concentration-dependent responses. Cells were preincubated in buffer A in the presence of 2.8 mm glucose and transferred to glucose-free medium immediately before imaging. Plasma membrane potential (Δψp) and cytoplasmic free Ca2+ ([Ca2+]c) were monitored in parallel. Where indicated, varying concentrations of glucose were added, followed by oligomycin. The average Δψp response of the entire field (Δψp field) and of a selected cell (Δψp cell) is shown. In parallel, changes in [Ca2+]c in the same cell are reported. The Δψp traces are normalized to a KCl calibration (see “Experimental Procedures”).
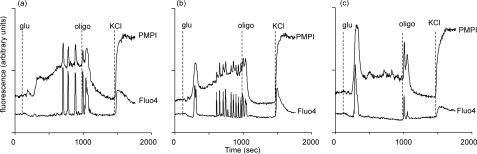
Although these experiments showed that there was a glucose threshold above which Δψp oscillations are initiated, it is important not to overinterpret these typical results because of the variable and stochastic nature of individual cell responses. In particular, the depolarization elicited by 5 mm glucose varied between days, suggesting that this concentration was close to a threshold for sustained and oscillatory depolarization. Even at 8 mm glucose, heterogeneous responses were seen. Fig. 3 shows examples of Δψp and [Ca2+]c response patterns in individual cells from the same field. Note the close parallel between individual Δψp and [Ca2+]c oscillations.
FIGURE 3.
Heterogeneous Δψp responses of individual cells to glucose. Cells were preincubated in buffer A in the presence of 2.8 mm glucose and transferred to glucose-free medium immediately before imaging. Where indicated, 8 mm glucose (glu), 640 ng/ml oligomycin (oligo) were added. KCl was added to 25 mm. Representative Δψp and [Ca2+]c responses of three individual cells are shown following addition of 8 mm glucose. Cell a had a long latent period before initiating Δψp and [Ca2+]c oscillations. Cell b showed an initial oscillation followed by a latent period. Cell c was quiescent after the initial oscillation until oligomycin was added.
Pyruvate Supports Oscillations in the Presence and Absence of Exogenous Glucose
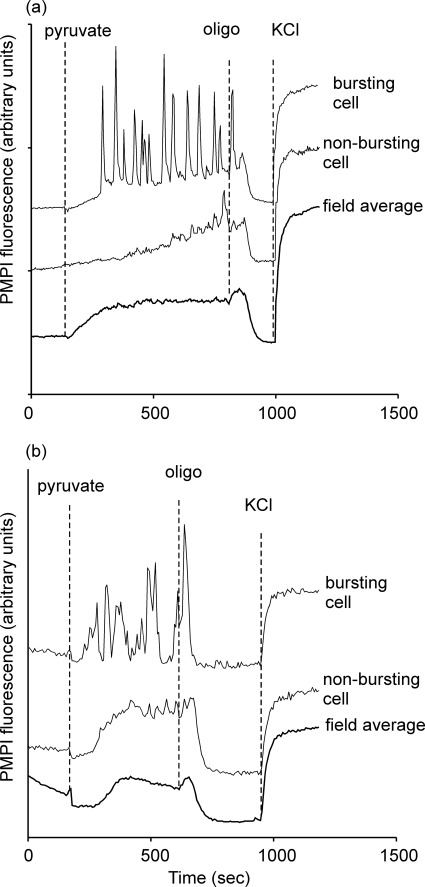
Exogenous pyruvate is established as an effective secretagogue for INS-1E and INS 832/13 cells at concentrations as low as 1 mm (17). Addition of 10 mm pyruvate to cells preincubated in 2.8 mm glucose (Fig. 4a) shows that the population depolarization developed more rapidly than with elevated glucose, consistent with the direct access of the substrate to the mitochondria. More than 80% of cells showed robust Δψp oscillations (see supplemental video), and the biphasic response to oligomycin seen with glucose was also present. Thus, pyruvate seems to be even more effective than glucose in inducing Δψp oscillations.
FIGURE 4.
Pyruvate-induced Δψp oscillations in the presence and absence of 2.8 mm glucose. a, INS-1 832/13 cells were preincubated in buffer A in the presence of 2.8 mm glucose. Where indicated, 10 mm Na-pyruvate was added, followed by 640 ng/ml oligomycin (oligo) and KCl to 25 mm. b, cells were preincubated in buffer B in the absence of exogenous substrate for 2 h prior to imaging.
In this experiment, it cannot be excluded that metabolic oscillations in glycolysis in cells kept at 2.8 mm exogenous glucose could control the plasma membrane potential oscillations even when the mitochondria are in the presence of excess pyruvate. To investigate this, cells were preincubated for 2 h in the absence of substrate (Fig. 4b). Addition of 1 mm pyruvate produced a biphasic Δψp response. An initial hyperpolarization was followed by a depolarization. A population of cells exhibited oscillations, and the addition of oligomycin caused a similar biphasic response as in the presence of glucose. Interestingly, the ability of the plasma membranes to repolarize and maintain this potential after ATP synthase inhibition suggests that the cells retained sufficient ATP to supply the plasma membrane Na+/K+-ATPase for this period.
Insulin Secretion Elicited by Glucose or Pyruvate
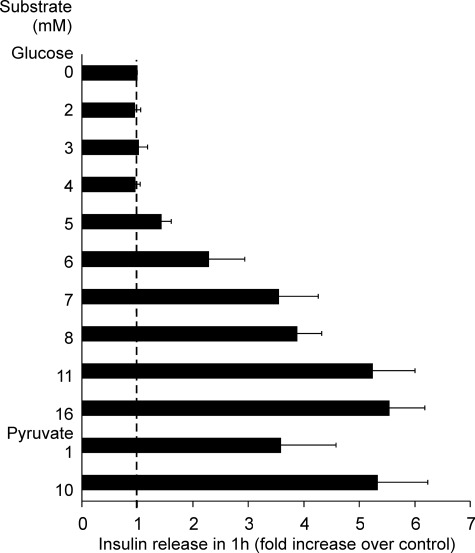
The threshold for insulin secretion by the 832/13 cells lay between 4 mm and 6 mm glucose (Fig. 5). In addition, as little as 1 mm pyruvate elicited a marked secretion of insulin.
FIGURE 5.
Insulin release in static 1-h incubations of INS-1 832/13 cells in the presence of the indicated concentrations of glucose or pyruvate relative to release in the absence of exogenous substrate. Data are mean ± S.E. (n = 3).
Mitochondrial Membrane Potential Change and Population Hyperpolarization
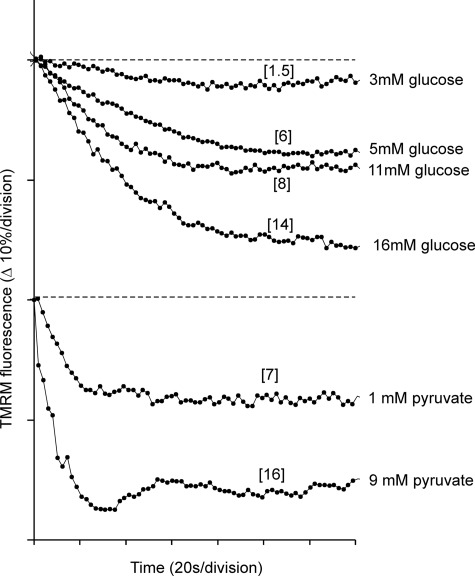
Equilibration of 832/13 cells with 100 nm TMRM allows changes in Δψm to be monitored in quench mode (21). Under these conditions, mitochondrial hyperpolarization produces a decrease in whole-cell fluorescence as the probe is accumulated into the mitochondrial matrix where its fluorescence is quenched (26). Fig. 6 shows the hyperpolarization as a function of glucose and pyruvate concentrations. The graded increase in Δψm as a function of glucose concentration was consistent with data from Heart et al. (27) for primary islet cells. It is notable that the onset of mitochondrial hyperpolarization with pyruvate was considerably more rapid than with glucose. This is consistent with the direct access of the substrate to the mitochondria. Using plausible initial conditions for Δψp and Δψm and values for the matrix volume as a fraction of total cell volume, it is possible to estimate the approximate extent of the Δψm hyperpolarization from the change in signal (supplemental Fig. S2). These values are shown in brackets in Fig. 6.
FIGURE 6.
Mitochondrial hyperpolarization induced by glucose or pyruvate. INS-1 832/13 cells were preincubated in buffer A in the presence of 2.8 mm glucose and 100 nm TMRM. Glucose-free medium with TMRM was substituted immediately before imaging. Field-average TMRM fluorescence is shown for 140 s following addition of the indicated concentration of glucose or pyruvate. Each division corresponds to a 10% decrease in signal. Because the experiment is performed in quench mode, a decrease in TMRM fluorescence corresponds to a mitochondrial hyperpolarization. The values in brackets are the estimated extents of hyperpolarization (in mV) calculated as described in the supplemental material. Note the rapid hyperpolarization induced by 9 mm pyruvate. Changes in Δψp do not affect the signal over this brief time period (21).
Two reports have shown that under certain conditions, Δψm oscillations can be detected in response to elevated glucose using rhodamine 123 (R123), either in individual (28) or clustered (29) β-cells. Although the results are difficult to compare with this study (primary cells versus insulinomas, glucose versus pyruvate), in one study the rising phase of an individual [Ca2+]c oscillation was associated with a slow Δψm hyperpolarization and the recovery with a more rapid depolarization (28), whereas in the second study the rising phase was accompanied by a Δψm depolarization and the recovery by repolarization. Neither study attempted to estimate the magnitude of the Δψm changes.
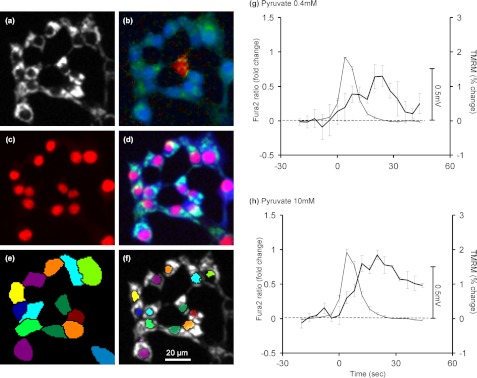
Preliminary experiments using TMRM in quench mode together with fluo-4 to monitor [Ca2+]c failed to detect any change in Δψm associated with the [Ca2+]c spikes (data not shown). A more sophisticated analysis was therefore devised to improve the sensitivity of the assay. In quench mode, the change in single cell TMRM fluorescence (Fig. 7a) in response to a small change in Δψm is restricted to the cytoplasm (21). Thus, removing the large invariant mitochondrial signal by restricting regions of interest to the mitochondria-poor (nuclear) regions of individual cells improves the signal-to-noise ratio. Secondly, a novel automated image analysis was devised, allowing this signal to be collected for each cell in the field (Fig. 7, e and f). Thirdly, the parallel fura-2 ratiometric signal (Fig. 7b) from each region was automatically processed to detect [Ca2+]c oscillations above a given threshold. Finally, the [Ca2+]c oscillations and the associated TMRM signals were collated and averaged from a large number of oscillations (Fig. 7, g and h). With both 0.4 mm and 10 mm pyruvate, the [Ca2+]c oscillations are followed by a small TMRM dequenching, indicative of a mitochondrial depolarization. By entering plausible starting values into the Excel computer simulation it is possible to predict the approximate relationship between cytoplasmic TMRM fluorescence and Δψm (supplemental Fig. S3). Using this to calibrate Fig. 7, we conclude that the mitochondria depolarize by less than 1 mV in response to a [Ca2+]c transient, and that, as in the case of the primary β-cell (28, 29), the depolarization follows, rather than precedes, the Ca2+ signal. We conclude that the magnitude of the change in Δψm is exceedingly small and trails behind the [Ca2+]c spike.
FIGURE 7.
Changes in Δψm associated with a [Ca2+]c spike in the presence of pyruvate. INS-1 832/13 cells were preincubated in buffer B in the presence of 2.8 mm glucose 100 nm TMRM (a) and 2 μm fura-2 (b, fura-2 340/380 fluorescence ratio is shown in pseudocolor). c, at the end of the recording, cells were stained with the nuclear marker DRAQ5. d, overlay image of TMRM (green), fura-2 (blue), and DRAQ5 (red) fluorescence micrographs. e, individual cells were identified by segmentation of fura-2 images using DRAQ5 as seed. f, mitochondrion-free (nuclear) areas were determined within each cell with further image segmentation. g and h, mean responses synchronized to [Ca2+]c spikes monitored with fura-2 (thin lines) and corresponding mean changes in single cell nuclear TMRM fluorescence (heavy lines) in the presence of 0.4 mm and 10 mm pyruvate in 2.8 and 0 mm glucose, respectively. Data points are mean ± S.E. of three experiments, compiled from total 516 and 564 oscillations for g and h, respectively. The mV scale bar represents the Δψm change approximated by modeling (supplemental Fig. S3).
NAD(P)H Autofluorescence Changes
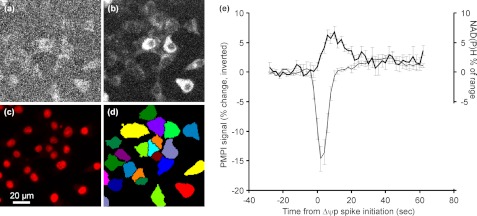
A limitation of the above analysis is that it takes no account of the additional contribution of the mitochondrial ΔpH to the total Δp. Because this parameter increases in response to elevated glucose (30), it is possible that Δp may change independently of Δψm. To investigate this, single cell NAD(P)H fluorescence (Fig. 8a) was monitored in parallel with PMPI (b). The redox span across complex I is in near equilibrium with Δp, so NADH autofluorescence may be used as a qualitative means to monitor changes in this parameter. The analysis is, however, complicated by any changes in NADPH. Emission originates from both NADH and NADPH, the former predominating in mitochondrial regions, whereas the NADPH signal is also cytoplasmic. This notwithstanding, after automated image processing of single cell autofluorescence (Fig. 8, c and d), a correlation was seen in the presence of 10 mm pyruvate between a Δψp depolarization and a delayed, small (∼7% of the mitochondrial NAD(H) pool determined by FCCP and rotenone) short-lived increase in NAD(P)H autofluorescence. If the following assumptions are made to maximize the bioenergetic interpretation of the NAD(P)H change, namely that the signal is due to mitochondrial NADH, that no compensatory change occurs in the ubiquinone redox state, and that the matrix NAD(H) pool is initially about 20% reduced, then a change to 25% reduction implies a 3-mV negative shift in the redox potential of the NAD+/NADH pool and, thus, an equivalent increase in the redox span across complex I. As four protons are extruded by complex I per two electrons, then this would correspond to an increase in Δp of less than 1 mV. In practice, the value would be even lower because changes in cytoplasmic NADP+ reduction may contribute.
FIGURE 8.
Temporal correlation between plasma membrane depolarization and NAD(P)H autofluorescence. INS-1 832/13 cells were preincubated in buffer B in the presence of 2.8 mm glucose and PMPI. NAD(P)H autofluorescence (a) and PMPI (b) intensities were detected on single cell basis by staining cultures by DRAQ5 (c) at the end of each recording and segmenting the PMPI images (d). e, mean responses synchronized to PMPI spikes in the presence of 10 mm pyruvate. PMPI signal (inverted, thin line) is reported as percentage change, and the NAD(P)H autofluorescence (heavy line) is reported as a percentage of the maximal span (FCCP to rotenone, see “Experimental Procedures”). Data points are mean ± S.E. of three experiments, compiled from a total of 800 oscillations.
Matrix ATP Changes
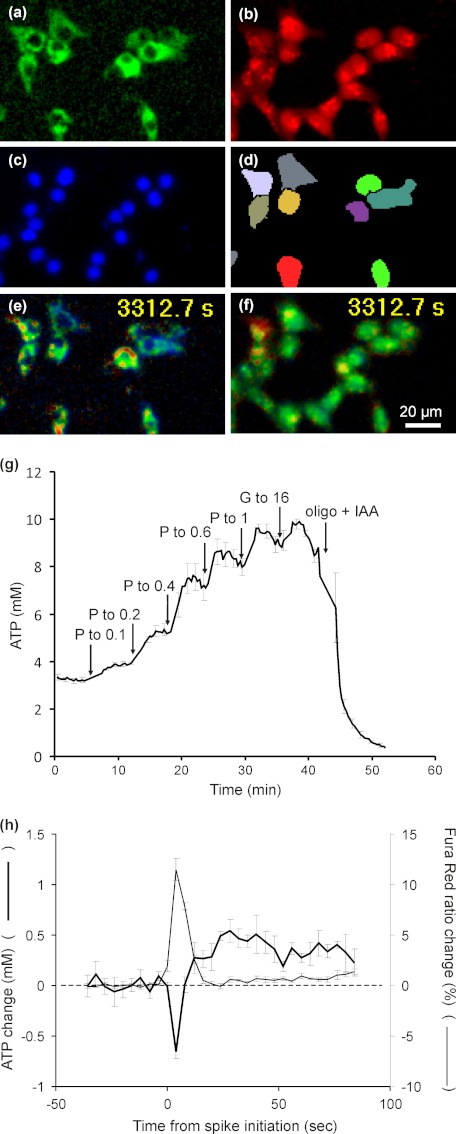
The targeted FRET-based ATeam probes for ATP, developed by Imamura et al. (20, 31), provide a means to monitor ATP concentrations in specific subcellular compartments. Although the affinity of AT 1.03, used in this study, is too high to reliably detect fluctuations in cytoplasmic ATP, the matrix-targeted probe (Fig. 9, a and e) is potentially capable of detecting any matrix ATP changes around the threshold at which Δψp oscillations are seen (g). Fig. 9h shows the correlation between matrix ATP concentration and [Ca2+]c oscillations detected with Fura Red (b and f), processed as described for Figs. 7 and 8 (Fig. 9, c and d). The initial response is a 0.6-mm drop in matrix ATP followed by a 0.5-mm rise. If these changes were entirely due to fluctuations in Δp and if thermodynamic equilibrium were maintained between Δp and the matrix ATP/ADP pool, the 12% drop and subsequent 10% rise in matrix ATP would correspond to a 1.2-mV drop and subsequent 1-mV increase in Δp. If, however, the changes in Δp were due to changes in extramitochondrial demand, the thermodynamic argument would not be valid.
FIGURE 9.
Matrix ATP concentration in INS-1 832/13 cells as a function of substrate concentration and during [Ca2+]c spikes in the presence of 0.4 mm pyruvate. Cells transfected with matrix-targeted ATeam AT1.03 (a) were loaded with Fura Red (b) during preincubation in the presence of 2.8 mm glucose. Spectrally unmixed images are shown. At the end of each recording nuclei were stained by Hoechst 33342 (c), and the ATeam fluorescence images were segmented (d) from these seeds to identify individual cells. e and f, to evaluate [ATP] and [Ca2+]c, 542/483 emission and 438/500 excitation ratios were calculated, respectively, and are shown as pseudocolor scaled images. g, the response of matrix targeted AT1.03 to pyruvate was studied in cells not loaded with Fura Red. Where indicated pyruvate (P) was added to give final concentrations of 0.1, 0.2, 0.4, 0.6, and 1 mm, followed by glucose to 16 mm and 1 μm oligomycin (oligo) plus 1 mm iodoacate (IAA). ATP concentrations were calculated using the published data for the affinity for AT1.03 (20). Data points are mean ± S.E. of four experiments. h, mean responses synchronized to [Ca2+]c spikes monitored with Fura Red (thin line) and corresponding mean changes in [ATP] (heavy line) in cells exposed to 0.4 mm pyruvate. Data points are mean ± S.E. of four experiments, compiled from a total of 130 oscillations.
DISCUSSION
The complexity of the sequence of events associated with glucose-stimulated insulin secretion helps to explain why the process is still incompletely understood. If it is accepted that insulinoma cell lines are relevant models to investigate bioenergetic and metabolic aspects of insulin secretion, then a central core of events (the coupling of mitochondrial bioenergetics to ionic processes at the plasma membrane) can be investigated in isolation in these cells, bypassing glycolysis by the direct supply of exogenous pyruvate to the cell, monitoring multiple bioenergetic parameters in parallel with changes in Δψp and [Ca2+]c, and avoiding considerations of downstream coupling factors, insulin storage, and release.
We have attempted to separate two conditions. Firstly, addition of exogenous pyruvate to substrate-limited cells produces a robust tonic mitochondrial hyperpolarization (estimated to be up to 16 mV, Fig. 6) comparable with that induced by glucose (see also Ref. 17). This is associated with a maintained increase in matrix ATP concentration (Fig. 9) and a tonic plasma membrane depolarization (Figs. 1 and 3), whereas single cell [Ca2+]c remains at baseline values (or even slightly decreases, Fig. 3) until a plasma membrane Δψp oscillation occurs. Because insulin secretion is firmly linked to Ca2+ channel opening, this would suggest that secretion is dependent on these oscillations. Although we do not monitor insulin secretion at the single cell level in this study (but see Ref. 32), there is a reasonable association between the concentration thresholds for glucose or pyruvate for the initiation of oscillations (Fig. 2) and those for population insulin secretion (Fig. 5).
Nothing in this study argues against the extensive intact islet literature documenting slow oscillations in glucose utilization (33), respiration (11, 33), NAD(P)H reduction (12, 34), Δψm (13), ATP (9), and insulin release (35). However, in this simplified system, similar Δψp oscillations are seen in the presence of elevated glucose (Figs. 1–3), pyruvate in the presence of 2.8 mm glucose (Fig. 4a), or pyruvate alone (Fig. 4b). Indeed, pyruvate-induced Δψp oscillations (see supplemental video) appear to more robust than those induced by glucose.
Small oscillations in Δψm reported with rhodamine 123 have been observed in primary β-cells in the presence of elevated glucose (28, 29). Both studies hypothesized that elevated [Ca2+]c during a spike depressed Δψm and lowered the cytoplasmic ATP/ADP ratio sufficiently to open KATP channels and repolarize the plasma membrane. However, the highly sensitive bioenergetic assays of Δψm, NAD(P)H and matrix ATP in this study place a limit of about 1 mV on the change in Δp accompanying (or rather following) the Δψp oscillation. These observations contrast with the estimated 14- to 16-mV hyperpolarization of Δψm seen upon the initial addition of high glucose or pyruvate to the cells (Fig. 6) and argues against a direct mechanism in which the mitochondria in these cells act as synchronized bioenergetic oscillators, alternately increasing and decreasing cytoplasmic ATP/ADP ratios sufficiently to modulate KATP channel activity.
To arrive at this conclusion, we have developed or employed a range of techniques. Although metabolism-induced plasma membrane depolarization and oscillation are central to the canonical pathway of GSIS, fluorescent monitoring of Δψp oscillations have been hampered by the slow response of available oxonol probes. Although bis-(1,3-dibutylbarbituric acid)-trimethine oxonol (36), bis-(1,3-diethylthiobarbituric acid) trimethine oxonol (37), and bis-oxonol (15) have been employed previously in studies with insulin-secreting cells, their response times are slow. The proprietary Δψp probe that we have termed PMPI for plasma membrane potential indicator (19) responds 14-fold more rapidly than bis-(1,3-dibutylbarbituric acid)-trimethine oxonol (24) and was able to resolve the Δψp oscillations in the INS-1 832/13 cells. Although a redistributing indicator cannot compete with electrophysiological techniques in terms of time resolution, it does allow entire view fields (and hence cell-to-cell heterogeneity (Figs. 7–9) to be analyzed. This readily allows integration with parallel metabolic and bioenergetic investigations. Moreover, fluorescent techniques avoid the disturbance to cell metabolism inherent in cell-attached patch-clamp configurations. For these reasons, the approach described here may be considered a complement to the technically demanding perforated patch technique, which is required to maintain metabolism in electrophysiological examinations (15).
We and others have developed techniques for the fluorescent monitoring of changes in Δψm in intact cells (19, 21, 38). Single-cell TMRM fluorescence under quench conditions, produced by equilibrating the cells with TMRM concentrations in the range of 50–100 nm (21), is a relatively sensitive and fast-responding technique for monitoring significant changes in Δψm during the course of an experiment (supplemental data and Figs. S2 and S3). As long as quench conditions are maintained, the intramitochondrial fluorescence does not alter in response to a limited change in Δψm. Instead, the change in single-cell fluorescence is due to the increase (in response to a mitochondrial depolarization) or decrease (mitochondrial hyperpolarization) in the concentrations of TMRM in the cytoplasm. The initial substrate-limited loading conditions in this study, a combination of a high Δψp and low Δψm, maximizes the proportion of TMRM in the cytoplasm and, thus, improves sensitivity.
One criticism that can be leveled against techniques that monitor changes in Δψm is that they ignore the contribution of ΔpH to the full Δp. This is particularly important in the present context because changes in ΔpH have been reported in INS-1E cells in response to increased glucose (30). We have therefore supplemented the TMRM experiments with two independent techniques monitoring changes in parameters linked to the proton motive force, namely, NAD(P)H autofluorescence and matrix ATP concentration. A brief NAD(P)H fluorescence increase can be detected following the initiation of a Δψp depolarizing oscillation, amounting to 7% of the maximal range (defined as the span between maximal oxidation in the presence of FCCP and maximal reduction in the presence of rotenone). The increased NAD(P)H reduction lags behind the plasma membrane depolarization, suggesting that that might be a downstream consequence of the depolarization. The resolution of this study was insufficient to distinguish clearly between mitochondrial (mainly NADH) and cytoplasmic (mainly NADPH) signals, and so it is not possible to resolve whether the increased reduction was due to a bioenergetic response of the mitochondrion or a metabolically induced cytoplasmic NAD(P)H reduction, which has been proposed to play a key role in facilitating insulin secretion (39).
Ainscow and Rutter (14) were able to detect cytoplasmic ATP oscillations in a subset of single dissociated human and mouse primary β-cells in the presence of elevated glucose. However, the low sensitivity of the luciferase assay precluded parallel monitoring of [Ca2+]c at a single cell (rather than population) level. The ATP reporter AT1.03 used here proved to have too high an affinity to report cytoplasmic changes, but the lower matrix ATP/ADP ratio, a consequence of the electrogenic adenine nucleotide translocator (40), allowed the matrix-targeted probe to report pyruvate-dependent changes in combination with Fura Red as a [Ca2+]c indicator (Fig. 9g). The initial 12% drop in matrix ATP correlating with the [Ca2+]c spike (Fig. 9h) was larger and briefer than would be predicted from the minimal (∼0.5-mV) decrease in Δψm (Fig. 8a) and most likely reflected increased cytoplasmic ATP demand associated with the elevated [Ca2+]c. However, an alternative scenario for which there is precedent with skeletal muscle (41) is that accumulation of Ca2+ into the mitochondria produces a biphasic response: an immediate increased demand upon the proton circuit met largely by transient matrix ATP hydrolysis but partially by the Δψm depolarization followed immediately by Ca2+ activation of the tricarboxylic acid cycle, responsible for the increased NAD(P)H reduction (see also Ref. 42).
Mitochondrial ATP generation (estimated by the decrease in respiration upon addition of oligomycin) and, hence, cellular ATP utilization, is accelerated when INS-1 832/13 cells are exposed to 16.7 mm glucose (43). Little is known about the ATP-consuming processes in these cells, although these will be as important as ATP-generating pathways for regulating cytoplasmic ATP/ADP ratios. Local subplasmalemmal ATP in MIN-6 cells, detected with targeted luciferase (44), shows kinetics distinct from the bulk cytoplasm in response to elevated glucose, suggesting that the sites of ATP generation and utilization may be important for regulating plasma membrane KATP channels.
Pyruvate has long been recognized to be an effective secretagogue in insulinoma cells possessing increased monocarboxylate transporter levels (17, 45, 46). As well as pyruvate, the cell-permeant methyl succinate (37, 45), dihydroxy acetone (17), β-hydroxybutyrate (47), acetoacetate (47), α-ketoisocaproate (46), and glycerol (17), separately or in combination, are effective secretagogues. This study supports the conclusion of Antinozzi et al. (17) that the potency of individual secretagogues correlates with their ability to hyperpolarize the mitochondria in intact cells independent of the levels of proposed signaling glycolytic intermediates (17). Whether Ca2+ (48) and possible metabolic coupling factors such as glutamate (49) or cytoplasmic NADPH (50) function independently of mitochondrial bioenergetics remains to be investigated. Such an approach will help to establish whether the putative coupling factors act upstream or downstream of the plasma membrane potential response and, accordingly, may be categorized as triggering or amplifying signals.
The scope of this study does not extend to an investigation into the mechanisms generating the Δψp oscillations. However, their relatively simple waveform (e.g. Figs. 4a and 8e) contrasting with the complex compound patterns seen in the intact islet (51) may provide some insight into the control of insulin secretion in this model.
Supplementary Material
Acknowledgments
We thank Dr. H. E. Hohmeier for the gift of INS-1 832/13 cells and Drs. H. Imamura and H. Noji for the gift of the ATeam mitochondrial and non-targeted AT 1.03 constructs.
This work was supported, in whole or in part, by National Institutes of Health Grants P01 AG025901 and P30 AG025708. This work was also supported by the Swedish Research Council and the Knut and Alice Wallenberg Foundation (to I. G., S. S., and H. M.).

This article contains supplemental Figs. S1–S4 and a movie.
- [Ca2+]c
- cytoplasmic Ca2+
- Δψp
- plasma membrane potential
- Δψm
- mitochondrial membrane potential
- AM
- acetoxymethyl ester
- FCCP
- carbonyl cyanide-p-trifluoromethoxy-phenylhyadrazone
- PMPI
- plasma membrane potential indicator
- TMRM
- tetramethylrhodamine methyl ester.
REFERENCES
- 1. Ashcroft F. M., Proks P., Smith P. A., Ammälä C., Bokvist K., Rorsman P. (1994) Stimulus-secretion coupling in pancreatic β cells. J. Cell Biochem. 55, 54–65 [DOI] [PubMed] [Google Scholar]
- 2. Dahlgren G. M., Kauri L. M., Kennedy R. T. (2005) Substrate effects on oscillations in metabolism, calcium and secretion in single mouse islets of Langerhans. Biochim. Biophys. Acta 1724, 23–36 [DOI] [PubMed] [Google Scholar]
- 3. Song S. H., McIntyre S. S., Shah H., Veldhuis J. D., Hayes P. C., Butler P. C. (2000) Direct measurement of pulsatile insulin secretion from the portal vein in human subjects. J. Clin. Endocrinol. Metab. 85, 4491–4499 [DOI] [PubMed] [Google Scholar]
- 4. Pørksen N., Hollingdal M., Juhl C., Butler P., Veldhuis J. D., Schmitz O. (2002) Pulsatile insulin secretion. Detection, regulation, and role in diabetes. Diabetes 51, S245–S254 [DOI] [PubMed] [Google Scholar]
- 5. Gilon P., Henquin J. C. (1992) Influence of membrane potential changes on cytoplasmic Ca2+ concentration in an electrically excitable cell, the insulin-secreting pancreatic B-cell. J. Biol. Chem. 267, 20713–20720 [PubMed] [Google Scholar]
- 6. Beauvois M. C., Merezak C., Jonas J. C., Ravier M. A., Henquin J. C., Gilon P. (2006) Glucose-induced mixed ]Ca2+[c oscillations in mouse β-cells are controlled by the membrane potential and the SERCA3 Ca2+-ATPase of the endoplasmic reticulum. Am. J. Physiol. Cell Physiol. 290, C1503–C1511 [DOI] [PubMed] [Google Scholar]
- 7. Jing X., Li D. Q., Olofsson C. S., Salehi A., Surve V. V., Caballero J., Ivarsson R., Lundquist I., Pereverzev A., Schneider T., Rorsman P., Renström E. (2005) CaV2.3 calcium channels control second-phase insulin release. J. Clin. Invest. 115, 146–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kanno T., Ma X., Barg S., Eliasson L., Galvanovskis J., Göpel S., Larsson M., Renström E., Rorsman P. (2004) Large dense-core vesicle exocytosis in pancreatic β-cells monitored by capacitance measurements. Methods 33, 302–311 [DOI] [PubMed] [Google Scholar]
- 9. Nilsson T., Schultz V., Berggren P. O., Corkey B. E., Tornheim K. (1996) Temporal patterns of changes in ATP/ADP ratio, glucose 6-phosphate and cytoplasmic free Ca2+ in glucose-stimulated pancreatic β-cells. Biochem. J. 314, 91–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Civelek V. N., Deeney J. T., Fusonie G. E., Corkey B. E., Tornheim K. (1997) Oscillations in oxygen consumption by permeabilized clonal pancreatic β-cells (HIT) incubated in an oscillatory glycolyzing muscle extract. Roles of free Ca2+, substrates, and the ATP/ADP ratio. Diabetes 46, 51–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bergsten P. (2002) Role of oscillations in membrane potential, cytoplasmic Ca2+, and metabolism for plasma insulin oscillations. Diabetes 51, S171-S176 [DOI] [PubMed] [Google Scholar]
- 12. Luciani D. S., Misler S., Polonsky K. S. (2006) Ca2+ controls slow NAD(P)H oscillations in glucose-stimulated mouse pancreatic islets. J. Physiol. 572, 379–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nunemaker C. S., Satin L. S. (2004) Comparison of metabolic oscillations from mouse pancreatic β cells and islets. Endocrine 25, 61–67 [DOI] [PubMed] [Google Scholar]
- 14. Ainscow E. K., Rutter G. A. (2002) Glucose-stimulated oscillations in free cytosolic ATP concentration imaged in single islet β-cells. Evidence for a Ca2+-dependent mechanism. Diabetes 51, S162–S170 [DOI] [PubMed] [Google Scholar]
- 15. Merglen A., Theander S., Rubi B., Chaffard G., Wollheim C. B., Maechler P. (2004) Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology 145, 667–678 [DOI] [PubMed] [Google Scholar]
- 16. Hohmeier H. E., Mulder H., Chen G., Henkel-Rieger R., Prentki M., Newgard C. B. (2000) Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49, 424–430 [DOI] [PubMed] [Google Scholar]
- 17. Antinozzi P. A., Ishihara H., Newgard C. B., Wollheim C. B. (2002) Mitochondrial metabolism sets the maximal limit of fuel-stimulated insulin secretion in a model pancreatic β cell. A survey of four fuel secretagogues. J. Biol. Chem. 277, 11746–11755 [DOI] [PubMed] [Google Scholar]
- 18. Ishihara H., Wang H., Drewes L. R., Wollheim C. B. (1999) Overexpression of monocarboxylate transporter and lactate dehydrogenase alters insulin secretory responses to pyruvate and lactate in β cells. J. Clin. Invest. 104, 1621–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nicholls D. G. (2006) Simultaneous monitoring of ionophore- and inhibitor-mediated plasma and mitochondrial membrane potential changes in cultured neurons. J. Biol. Chem. 281, 14864–14874 [DOI] [PubMed] [Google Scholar]
- 20. Imamura H., Nhat K. P., Togawa H., Saito K., Iino R., Kato-Yamada Y., Nagai T., Noji H. (2009) Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc. Natl. Acad. Sci. U.S.A. 106, 15651–15656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ward M. W., Rego A. C., Frenguelli B. G., Nicholls D. G. (2000) Mitochondrial membrane potential and glutamate excitotoxicity in cultured cerebellar granule cells. J. Neurosci. 20, 7208–7219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cunningham M. D., Cunningham S. M. (1999) High-throughput functional detection of NMDA receptor activity. Methods Mol. Biol. 128, 179–188 [DOI] [PubMed] [Google Scholar]
- 23. Gerencser A. A., Mark K. A., Hubbard A. E., Divakaruni A. S., Mehrabian Z., Nicholls D. G., Polster B. M. (2009) Real-time visualization of cytoplasmic calpain activation and calcium deregulation in acute glutamate excitotoxicity. J. Neurochem. 110, 990–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baxter D. F., Kirk M., Garcia A. F., Raimondi A., Holmqvist M. H., Flint K. K., Bojanic D., Distefano P. S., Curtis R., Xie Y. (2002) A novel membrane potential-sensitive fluorescent dye improves cell-based assays for ion channels. J. Biomol. Screen. 7, 79–85 [DOI] [PubMed] [Google Scholar]
- 25. Ullrich S., Abel K. B., Lehr S., Greger R. (1996) Effects of glucose, forskolin and tolbutamide on membrane potential and insulin secretion in the insulin-secreting cell line INS-1. Pfluegers Arch. 432, 630–636 [DOI] [PubMed] [Google Scholar]
- 26. Duchen M. R. (1992) Ca(2+)-dependent changes in the mitochondrial energetics in single dissociated mouse sensory neurons. Biochem. J. 283, 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heart E., Corkey R. F., Wikstrom J. D., Shirihai O. S., Corkey B. E. (2005) Glucose-dependent increase in mitochondrial membrane potential, but not cytoplasmic calcium, correlates with insulin secretion in single islet cells. Am. J. Physiol. Endocrinol. Metab. 290, 143–148 [DOI] [PubMed] [Google Scholar]
- 28. Kindmark H., Köhler M., Brown G., Bränström R., Larsson O., Berggren P. O. (2001) Glucose-induced oscillations in cytoplasmic free Ca2+ concentration precede oscillations in mitochondrial membrane potential in the pancreatic β-cell. J. Biol. Chem. 276, 34530–34536 [DOI] [PubMed] [Google Scholar]
- 29. Krippeit-Drews P., Düfer M., Drews G. (2000) Parallel oscillations of intracellular calcium activity and mitochondrial membrane potential in mouse pancreatic β-cells. Biochem. Biophys. Res. Commun. 267, 179–183 [DOI] [PubMed] [Google Scholar]
- 30. Wiederkehr A., Park K. S., Dupont O., Demaurex N., Pozzan T., Cline G. W., Wollheim C. B. (2009) Matrix alkalinization. A novel mitochondrial signal for sustained pancreatic β-cell activation. EMBO J. 28, 417–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakano M., Imamura H., Nagai T., Noji H. (2011) Ca2+ regulation of mitochondrial ATP synthesis visualized at the single cell level. ACS Chem. Biol. 6, 709–715 [DOI] [PubMed] [Google Scholar]
- 32. Rutter G. A., Leclerc I., Tsuboi T., Xavier Gda S., Diraison F., Qian Q. (2004) Imaging glucose-regulated insulin secretion and gene expression in single islet β-cells. Control by AMP-activated protein kinase. Cell Biochem. Biophys. 40, 179–190 [DOI] [PubMed] [Google Scholar]
- 33. Jung S. K., Kauri L. M., Qian W. J., Kennedy R. T. (2000) Correlated oscillations in glucose consumption, oxygen consumption, and intracellular free Ca(2+) in single islets of Langerhans. J. Biol. Chem. 275, 6642–6650 [DOI] [PubMed] [Google Scholar]
- 34. Pralong W. F., Spät A., Wollheim C. B. (1994) Dynamic pacing of cell metabolism by intracellular Ca2+ transients. J. Biol. Chem. 269, 27310–27314 [PubMed] [Google Scholar]
- 35. Gilon P., Shepherd R. M., Henquin J. C. (1993) Oscillations of secretion driven by oscillations of cytoplasmic Ca2+ as evidences in single pancreatic islets. J. Biol. Chem. 268, 22265–22268 [PubMed] [Google Scholar]
- 36. Hjortoe G. M., Hagel G. M., Terry B. R., Thastrup O., Arkhammar P. O. (2004) Functional identification and monitoring of individual α and β cells in cultured mouse islets of Langerhans. Acta Diabetol. 41, 185–193 [DOI] [PubMed] [Google Scholar]
- 37. Heart E., Yaney G. C., Corkey R. F., Schultz V., Luc E., Liu L., Deeney J. T., Shirihai O., Tornheim K., Smith P. J., Corkey B. E. (2007) Ca2+, NAD(P)H and membrane potential changes in pancreatic β-cells by methyl succinate. Comparison with glucose. Biochem. J. 403, 197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Duchen M. R., Biscoe T. J. (1992) Relative mitochondrial membrane potential and [Ca2+]i in type I cells isolated from the rabbit carotid body. J. Physiol. 450, 33–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ivarsson R., Quintens R., Dejonghe S., Tsukamoto K., in 't Veld P., Renström E., Schuit F. C. (2005) Redox control of exocytosis. Regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes 54, 2132–2142 [DOI] [PubMed] [Google Scholar]
- 40. Klingenberg M. (2008) The ADP and ATP transport in mitochondria and its carrier. Biochim. Biophys. Acta 1778, 1978–2021 [DOI] [PubMed] [Google Scholar]
- 41. Liu T., O'Rourke B. (2009) Regulation of mitochondrial Ca2+ and its effects on energetics and redox balance in normal and failing heart. J. Bioenerg. Biomembr. 41, 127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Magnus G., Keizer J. (1997) Minimal model of β-cell mitochondrial Ca2+ handling. Am. J. Physiol. 273, C717–C733 [DOI] [PubMed] [Google Scholar]
- 43. Malmgren S., Nicholls D. G., Taneera J., Bacos K., Koeck T., Tamaddon A., Wibom R., Groop L., Ling C., Mulder H., Sharoyko V. V. (2009) Tight coupling between glucose and mitochondrial metabolism in clonal β-cells is required for robust insulin secretion. J. Biol. Chem. 284, 32395–32404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kennedy H. J., Pouli A. E., Ainscow E. K., Jouaville L. S., Rizzuto R., Rutter G. A. (1999) Glucose generates sub-plasma membrane ATP microdomains in single islet β-cells. Potential role for strategically located mitochondria. J. Biol. Chem. 274, 13281–13291 [DOI] [PubMed] [Google Scholar]
- 45. MacDonald M. J. (2007) Synergistic potent insulin release by combinations of weak secretagogues in pancreatic islets and INS-1 cells. J. Biol. Chem. 282, 6043–6052 [DOI] [PubMed] [Google Scholar]
- 46. MacDonald M. J., Longacre M. J., Stoker S. W., Brown L. J., Hasan N. M., Kendrick M. A. (2008) Acetoacetate and β-hydroxybutyrate in combination with other metabolites release insulin from INS-1 cells and provide clues about pathways in insulin secretion. Am. J. Physiol. Cell Physiol. 294, C442–C450 [DOI] [PubMed] [Google Scholar]
- 47. Hasan N. M., Longacre M. J., Stoker S. W., Boonsaen T., Jitrapakdee S., Kendrick M. A., Wallace J. C., MacDonald M. J. (2008) Impaired anaplerosis and insulin secretion in insulinoma cells caused by small interfering RNA-mediated suppression of pyruvate carboxylase. J. Biol. Chem. 283, 28048–28059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wiederkehr A., Szanda G., Akhmedov D., Mataki C., Heizmann C. W., Schoonjans K., Pozzan T., Spät A., Wollheim C. B. (2011) Mitochondrial matrix calcium is an activating signal for hormone secretion. Cell Metab. 13, 601–611 [DOI] [PubMed] [Google Scholar]
- 49. Maechler P., Wollheim C. B. (1999) Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature 402, 685–689 [DOI] [PubMed] [Google Scholar]
- 50. Brown L. J., Longacre M. J., Hasan N. M., Kendrick M. A., Stoker S. W., Macdonald M. J. (2009) Chronic reduction of the cytosolic or mitochondrial NAD(P)-malic enzyme does not affect insulin secretion in a rat insulinoma cell line. J. Biol. Chem. 284, 35359–35367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bertram R., Sherman A., Satin L. S. (2007) Metabolic and electrical oscillations: partners in controlling pulsatile insulin. Am. J. Physiol. Endocrinol. Metab. 56, E890–E900 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.