Background: Exosome is a novel secretory pathway for HSPs, which induce antitumor responses.
Results: Anticancer drugs caused release of HSP-bearing exosomes by HepG2 cells and elicited efficient NK cell antitumor responses.
Conclusion: Exosomes derived from hepatocellular carcinoma cell-resistant anticancer drug-treated HepG2 cells conferred superior immunogenicity in inducing HSP-specific NK cell responses.
Significance: Exosomes provided a clue for finding an efficient vaccine for HCC immunotherapy.
Keywords: Anticancer Drug, Exosomes, Heat Shock Protein (HSP), Hepatocellular Carcinoma, Natural Killer (NK) Cell, HCC
Abstract
Failure of immune surveillance related to inadequate host antitumor immune responses has been suggested as a possible cause of the high incidence of recurrence and poor overall survival outcome of hepatocellular carcinoma. The stress-induced heat shock proteins (HSPs) are known to act as endogenous “danger signals” that can improve tumor immunogenicity and induce natural killer (NK) cell responses. Exosome is a novel secretory pathway for HSPs. In our experiments, the immune regulatory effect of the HSP-bearing exosomes secreted by human hepatocellular carcinoma cells under stress conditions on NK cells was studied. ELISA results showed that the production of HSP60, HSP70, and HSP90 was up-regulated in both cell lines in a stress-specific manner. After exposure to hepatocellular carcinoma cell-resistant or sensitive anticancer drugs (hereafter referred to as “resistant” or “sensitive” anticancer drug), the membrane microvesicles were actively released by hepatocellular carcinoma cells, differing in their ability to present HSPs on the cell surface, which were characterized as exosomes. Acting as a decoy, the HSP-bearing exosomes efficiently stimulated NK cell cytotoxicity and granzyme B production, up-regulated the expression of inhibitory receptor CD94, and down-regulated the expression of activating receptors CD69, NKG2D, and NKp44. Notably, resistant anticancer drugs enhanced exosome release and generated more exosome-carried HSPs, which augmented the activation of the cytotoxic response. In summary, our findings demonstrated that exosomes derived from resistant anticancer drug-treated HepG2 cells conferred superior immunogenicity in inducing HSP-specific NK cell responses, which provided a clue for finding an efficient vaccine for hepatocellular carcinoma immunotherapy.
Introduction
Worldwide, HCC4 is the sixth most common malignant tumor with increasing incidence. It is also the third leading cause of cancer-related deaths, with over 600,000 patients dying from this disease annually, especially in Southeast Asia and sub-Saharan Africa (1). At present, surgical treatment is regarded as the most effective standard therapy for HCC. Recent progresses in both diagnostic and surgical techniques have resulted in substantial improvement in the morbidity and mortality rates, but the overall outcome remains far from satisfactory. Traditional chemotherapy is widely performed and recognized as having a survival benefit, such as reducing HCC burden in patients with advanced disease or reducing the risk of recurrence after curative resection (2, 3). Unfortunately, its therapeutic efficacy appears disappointing in clinical trials, and a standard therapeutic method has not been established. Recently, attention has focused on a variety of vaccines investigated in experimental studies comprising patients with HCC, because they have been reported to greatly induce a tumor-specific immune response against tumor cells (4, 5). As a result, identifying and establishing a novel approach for the prevention of HCC development and recurrence (i.e. anticancer drug-based immunotherapy that targets antitumor immune response) has become the focus of researchers around the world.
HSPs were first discovered in 1962 (6) as a family of highly conserved proteins. HSPs play a crucial role as molecular chaperones by assisting the proper folding of newly synthesized and stress-denatured polypeptides, the assembly of multiprotein complexes, and the transport of proteins across cell membranes (7). The dual function of HSPs, depending on their intracellular and extracellular location, strongly increases the interest of these molecules in tumor therapy (8). Apart from their cytoprotective/antiapoptotic roles in the cytosol, HSPs have been found to provide danger signals for the host's cellular immune system when located in the extracellular space or on the plasma membrane (9, 10). These findings suggest that HSPs may be an ideal candidate for enhancing antitumor immunity. To develop a therapeutic vaccine, appropriate molecules for immune cells should be identified, and an adequate vehicle needs to be developed. One of the simplest vehicles for the therapeutic vaccine is tumor-derived exosome (Tex) that contains HSPs.
Exosomes are specialized 30–100-nm-sized lipid-rich membrane-bound microvesicles with a defined morphology and phenotype and are smaller and more homogeneous in size than membrane-shed vesicles (100–1000 nm). Exosomes are actively released into the extracellular environment from cells via the endosomal vesicle/multivesicular the body pathway by fusion with the plasma membrane under normal and pathological conditions (11–13). Many cells have the capacity to secrete exosomes, including epithelial cells (14), neurons (15), dendritic cells (16), T cells (17), and B cells (18). Depending on the cell types from which they are derived, exosomes play a role in diverse physiological and pathological processes, serving as a novel and more intricate form of cell-cell communication. Tumor cells also produce exosomes, evidently abundant in culture and malignant effusions (19, 20). Tex might represent ideal vehicles for immunomodulation with an impact on the immune system, and their influence should be taken into consideration when designing treatment for cancer patients (21).
In the present study, the identification of HSPs on the exosome surface and the known role of these molecules in the stimulation of resting NK cells prompted us to investigate whether anticancer drugs may efficiently up-regulate the expression of HSPs on the human hepatocellular carcinoma cell-derived exosomes and the ability of exosomal HSPs as a tumor vaccine to potentially induce NK cells responses that lead to eliciting an antitumor immune response in vitro. This was measured with the NK cell cytotoxic function, granzyme B secretion, and cell surface density of several NK cell receptors.
EXPERIMENTAL PROCEDURES
Cell Lines and Culture Conditions
The human hepatocellular carcinoma (HepG2 and PLC/PRF/5) and erythromyeloblastoid leukemia (K562) cell lines, purchased from the American Type Culture Collection (ATCC), were routinely cultured in complete DMEM culture medium (25 mm d-glucose, 4 mm l-glutamine, and 1 mm sodium pyruvate; Invitrogen). This medium was supplemented with 10% heat-inactivated exosome-depleted FBS (Biological Industries; previously accomplished by overnight ultracentrifugation at 100,000 × g at 4 °C) and penicillin (100 IU/ml) and streptomycin (100 μg/ml) (both from Sigma-Aldrich). The cells were kept at 37 °C in a humidified 95% air, 5% CO2 atmosphere incubator designated as culture at a steady-state condition. Cell viability was assessed using trypan blue exclusion test and routinely found to contain <5% dead cells.
Growth Inhibition
The in vitro growth-inhibitory effect of the anticancer drugs was measured by the MTT (Sigma-Aldrich) assay as described previously with slight modification (22). In brief, HepG2 and PLC/PRF/5 cells were seeded in 96-well flat bottom plates at a density of 4 × 103 cells/well (200 μl/well). After 24 h, cells were treated with different concentrations (6.25, 12.5, 25, 50, 100, and 200% test drug concentration (TDC)) of TAXOL (paclitaxel; Bristol-Myers Squibb Co.), Campto (irinotecan hydrochloride; Pfizer), Paraplatin (carboplatin; Bristol-Myers Squibb Co.), etoposide (Hengrui Medicine Co.), Militant (mitoxantrone hydrochloride; Sanjing Shenhe Pharmaceutical Co.), Pharmorubicin (epirubicin hydrochloride; Pfizer), cisplatin (Hansoh Pharmaceutical Co.), mitomycin (Hisun Pharmaceutical Co.), fluorouracil (Xudong Haipu Pharmaceutical Co.), Eloxatin (oxaliplatin; Sanofi-Aventis), or Gemzar (gemcitabine hydrochloride; Lilly) in 200 μl of fresh culture medium. After 72 h, medium was replaced with 200 μl of fresh culture medium containing MTT (0.5 mg/ml). After a 4-h incubation at 37 °C, MTT-containing medium was removed, and 150 μl of DMSO (Sigma-Aldrich) were added to each well. After gentle mixing for 15 min, the reduced purple formazan crystals were solubilized, and the absorbance was read at 490 nm using a spectrophotometric microplate reader (MK-3 microplate reader, Thermo Labsystems). The growth inhibition rate was calculated using the formula, growth inhibition rate (%) = (1 − ODdrug exposure/ODcontrol) × 100. For each single anticancer drug, the result of chemosensitivity was determined to be sensitive (100% TDC >90% and 50% TDC >70%) or resistant (100% TDC <70% and 50% TDC <50%) (Table 1).
TABLE 1.
100% TDC of anticancer drugs
| Anticancer drug | 100% TDC |
|---|---|
| μg/mol | |
| Paclitaxel | 13.6 |
| Etoposide | 48.0 |
| Carboplatin | 15.8 |
| Irinotecan hydrochloride | 14.0 |
| Mitoxantrone hydrochloride | 0.6 |
| Epirubicin hydrochloride | 0.5 |
| Cisplatin | 3.8 |
| Mitomycin | 0.23 |
| Fluorouracil | 22.5 |
| Oxaliplatin | 1.8 |
| Gemcitabine hydrochloride | 25.0 |
Drug Exposure
HepG2 and PLC/PRF/5 cells (3 × 105 cells/well) were plated in 6-well plates. After a 48-h incubation in complete DMEM culture medium with 10% heat-inactivated exosome-depleted FBS, cells reaching ∼80% confluence were exposed to 100% TDC of paclitaxel, carboplatin, etoposide, or irinotecan hydrochloride for different lengths of time. Untreated cells were used as controls. The culture media were harvested at 0, 2, 4, 8, 12, 18, 24, 36, 48, 72, and 96 h after treatment with anticancer drugs.
Heat Shock Experiment
HepG2 and PLC/PRF/5 cells were seeded in 6-well plates at a density of 3 × 105 cells/well in complete DMEM culture medium with 10% heat-inactivated exosome-depleted FBS. After a 24-h incubation period for attachment, cultured cells were heat-shocked by incubating them at 43 °C for 0.5, 1, 1.5, 2, or 3 h or kept at 37 °C as controls, followed by a recovery period of 24 h at 37 °C. The conditioned media were collected 24 h after the end of heat shock.
HSP ELISA
Cell culture supernatants were harvested at different time points from HepG2 and PLC/PRF/5 cells; exposed to heat shock, paclitaxel, carboplatin, etoposide, irinotecan hydrochloride, or control; and centrifuged at 900 × g for 15 min to remove cells. The concentrations of HSP60, HSP70, and HSP90 in each sample were measured using the human ultrasensitive heat shock protein 60/70/90 (HSP60/70/90) ELISA kit (CSB-E13498h/13463h/13497h, Cusabio Biotech), with subsequent assays done as recommended by the manufacturer's instructions. Absorbance at 450 nm was read by a spectrophotometer.
Exosome Isolation
Previously reported isolation protocols (23, 24) in other systems that used serial centrifugations were slightly modified to purify exosomes. Briefly, 12 ml of conditioned culture medium was collected from HepG2 cells at the indicated times; treated with heat shock, paclitaxel, carboplatin, etoposide, irinotecan hydrochloride, or control; and subjected to two consecutive centrifugations to remove residual cells and cellular debris: 800 × g for 10 min and 12,000 × g for 30 min at 4 °C. The pellet was discarded, and the supernatant was passed through a 0.22-μm pore diameter filter (Millipore), followed by ultracentrifugation at 110,000 × g for 3 h at 4 °C using SW41 Ti rotor (L-80XP, Beckman Coulter Instruments). The ultracentrifuged pellet was collected from the bottom of the tube, resuspended in sterile filtered PBS, and subsequently ultracentrifuged at 110,000 × g for 3 h again. Finally, the exosomal pellet was resuspended in 300 μl of PBS, and aliquots were stored at −80 °C for further use. Protein content of exosomes was measured using a BCA protein assay kit (Pierce).
Electron Microscopy
The morphology of exosome was examined by transmission electron microscopy using a method described previously (25). In brief, exosomes obtained after differential centrifugation were fixed in 1% glutaraldehyde. A 20-μl drop of the suspension was loaded onto Formvar-carbon-coated electron microscopy copper grids and allowed to stand for 10 min at room temperature. Excess fluid was drawn off with a piece of Whatman filter paper. The sample was then negative-stained on ice with a 20-μl drop of 1% uranyl acetate for 10 min and allowed to dry under an electric incandescent lamp for 10 min before viewing in a JEM-1400 transmission electron microscope (JEOL) operated at 80 kV.
Exosome Quantification
To quantify the amount of exosomes released, we assessed AChE activity, an enzyme that was specifically directed to these vesicles (26). AChE activity was assayed following a procedure described previously (27). Briefly, 40 μl of the exosome fraction was suspended in 110 μl of PBS, and a portion (37.5 μl) was then added to individual wells on a 96-well flat-bottomed microplate. Acetylthiocholine iodide (1.25 mm) and 5′,5′-dithio-bis(2-nitrobenzoic acid) (0.1 mm) (both from Sigma-Aldrich) were then added to exosome fractions in a final volume of 300 μl, and the absorbance change at 412 nm was monitored every 5 min.
Western Blotting
Western blot analysis of exosomal proteins was performed using methods described previously (28, 29). Exosomal pellets were solubilized in radioimmune precipitation assay buffer (Pierce) for 20 min on ice, and then equal volumes of reducing SDS loading buffer were added and boiled at 98 °C for 5 min. Total proteins (10 μg of protein/lane) were separated by SDS-PAGE on 12% polyacrylamide gels along with the PageRulerTM Plus prestained protein ladder (Fermentas) and electrophoretically transferred onto PVDF (Millipore) membranes. Following overnight blocking with 5% nonfat dry milk and 0.05% Tween 20 in PBS (PBST) containing 5% BSA at 4 °C, the blots were probed with the following primary mouse monoclonal antibodies (0.2 μg/ml) in PBST: anti-HSP60 (IgG1, clone LK1), anti-HSP70 (IgG1, clone C92F3A-5), anti-HSP90 (IgG2a, clone F-8) (all from Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or anti-CD63 (IgG1, clone MEM-259, Abcam). After a 1-h incubation at room temperature, the blots were then incubated with species-appropriate HRP-conjugated secondary antibody (1:10,000 dilution in PBST, Santa Cruz Biotechnology) for 1 h at room temperature. The corresponding bands were developed with SuperSignal West Pico Trial Kit (Pierce) and exposed to x-ray film. The Chemidoc EQ system with Quantity One software (Bio-Rad) was used for determining the density of protein bands.
Flow Cytometry
Flow cytometric analysis of HSP60, HSP70, and HSP90 surface expression on isolated exosomes was performed as described previously (24). Briefly, exosomes (30 μg) were incubated with 1.5 × 105 4-μm diameter aldehyde/sulfate latex microbeads (surfactant-free, ultraclean; Invitrogen) for 15 min at room temperature in a final volume of 100 μl of PBS. After overnight incubation at 4 °C under gentle agitation, the reaction was stopped by the addition of 100 mm glycine for 30 min to saturate any remaining free binding sites on the beads. Exosome-coated beads were stained with an appropriate concentration of primary mouse monoclonal antibodies directed against HSP60 (clone LK1), HSP70 (clone C92F3A-5), or HSP90 (clone F-8) for 1 h, washed twice, subjected to a 1-h incubation with Alexa Fluor 488-labeled (IgG (H+L), Molecular Probes) secondary antibody in darkness, and analyzed on a BD Biosciences FACScalibur flow cytometer using CELL-QuestTM data acquisition and analysis software.
NK Cell Isolation and Stimulation
Human peripheral blood mononuclear cells were isolated from peripheral venous blood drawn from healthy donors by density gradient centrifugation. Diluted blood was layered over Ficoll-Paque PREMIUM (1.077 g/ml; GE Healthcare) and centrifuged at 400 × g for 30 min. The interface layer was harvested and washed in PBS, followed by centrifugation at 800 × g for 10 min. NK cells were isolated from peripheral blood mononuclear cells, using an NK cell isolation kit (Miltenyi Biotec). Briefly, non-NK cells were magnetically labeled and depleted using a mixture of biotin-conjugated antibodies and the NK cell MicroBead mixture according to the manufacturer's protocol. The purity of isolated NK cells (CD3−CD56+) was analyzed with flow cytometric analysis by staining with anti-CD3-FITC/CD56-phycoerythrin (PE, IgG1, clone UCHT1/N901, Beckman Coulter) mouse monoclonal antibody and was ∼95%. Cell viability was determined by trypan blue exclusion test and always was found to be greater than 95%.
NK cells (1 × 106 cells/ml) were stimulated either with low dose IL-2 alone (100 IU/ml; PeproTech) or with IL-2 in combination with exosomal proteins (5, 10, or 20 μg/ml) at 37 °C in a humidified atmosphere containing 5% CO2 for 4 days. Cell surface density of different NK cell markers was determined on day 4 after stimulation using anti-NKG2D-PE (IgG1, clone 149810), anti-CD69-PE (IgG2a, clone 298614), anti-CD94-PE (IgG1, clone 131412), or anti-NKp44-PE (IgG2a, clone 253415) (all from R&D Systems) mouse monoclonal antibodies (10 μl/106 cells for 30 min at 4 °C) by flow cytometry using a standard protocol.
Cytotoxicity Assay
NK cell-mediated cytotoxic activity was determined in a colorimetric assay based on the measurement of lactate dehydrogenase activity released from the cytosol of lysed K562 or HepG2 target cells into the supernatant with the CytoTox 96® non-radioactive cytotoxicity assay (Promega) according to the manufacturer's instructions. K562 or HepG2 target cells were coincubated with NK cells, prestimulated for 4 days either with low dose IL-2 alone (100 IU/ml) or with IL-2 in combination with different amounts of exosomes (5, 10, or 20 μg/ml) at the indicated effector/target cell ratios of 5:1, 10:1, and 20:1. After a 4-h incubation period at 37 °C in 5% CO2, supernatants were harvested, and the percentage of specific lysis was calculated according to the equation, specific lysis (%) = (experimental − effector spontaneous − target spontaneous)/(target maximum − target spontaneous) × 100.
Granzyme B ELISA
Granzyme B released by NK cells during the stimulation period of 4 days, either with low dose IL-2 alone (100 IU/ml) or with IL-2 in combination with exosomal proteins (5, 10, or 20 μg/ml), was measured using a human granzyme B ELISA kit (CSB-E08718h, Cusabio Biotech) according to manufacturer's instructions. Plates were counted on an ELISA reader at 450 nm.
Statistical Analysis
Results were expressed as means ± S.D. Statistical significance of differences between the experimental and control groups was analyzed using the paired samples Student's t test or repeated measures analysis of variance where appropriate. Values of p < 0.05 were considered statistically significant.
RESULTS
Chemosensitivity of HepG2 and PLC/PRF/5 Cells to Anticancer Drug
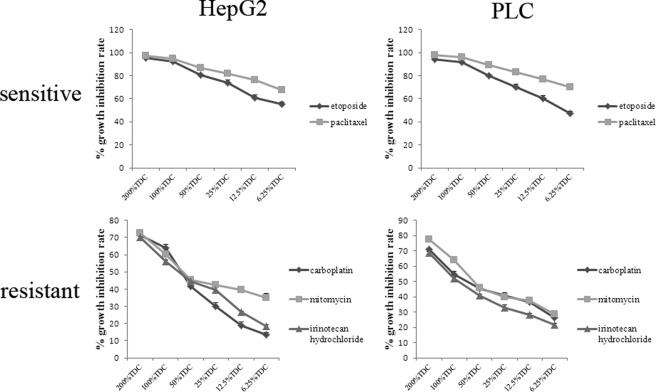
Chemosensitivity of HepG2 and PLC/PRF/5 cells was assessed by in vitro 72-h continuous exposure to each single agent (paclitaxel, irinotecan hydrochloride, carboplatin, etoposide, mitoxantrone hydrochloride, epirubicin hydrochloride, cisplatin, mitomycin, fluorouracil, oxaliplatin, or gemcitabine hydrochloride) at different concentrations (6.25, 12.5, 25, 50, 100, and 200% TDC). As can be seen in Fig. 1, HepG2 cells showed remarkably higher sensitivity to paclitaxel and etoposide (100% TDC >90% and 50% TDC >70%), with similar results obtained with both assays between two hepatocellular carcinoma cell lines. In contrast, HepG2 and PLC/PRF/5 cells exhibited resistance to irinotecan hydrochloride, carboplatin, and mitomycin (100% TDC <70 and 50% TDC <50%), and this was exemplified by both assays. Differences in growth inhibition rate under the same conditions among anticancer drugs reached the level of statistical significance (p < 0.05). For further investigation, paclitaxel, etoposide, irinotecan hydrochloride, and carboplatin were selected for treating HepG2 and PLC/PRF/5 cells as cellular stress.
FIGURE 1.
Chemosensitivity of HepG2 and PLC/PRF/5 cells to anticancer drugs. Dose-response curves for HepG2 and PLC/PRF/5 cells following continuous 72-h exposure to anticancer drugs at various concentrations using the MTT assay are depicted. For each single anticancer drug, chemosensitivity was determined to be sensitive (100% TDC >90% and 50% TDC >70%) or resistant (100% TDC <70% and 50% TDC <50%). HepG2 and PLC/PRF/5 cells showed significantly higher sensitivity to paclitaxel and etoposide compared with resistance to irinotecan hydrochloride, carboplatin, and mitomycin (p < 0.05). Data shown are representative of nine independent experiments for each drug and each cell line with similar results.
Secretion of HSP60, HSP70, and HSP90 by HepG2 and PLC/PRF/5 Cells Is Increased under Stress Conditions
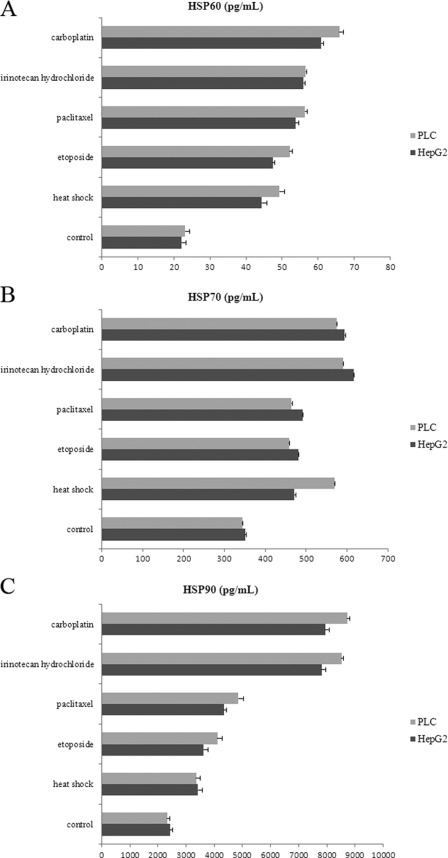
The production of HSP60, HSP70, and HSP90 by HepG2 and PLC/PRF/5 cells following heat shock or anticancer drugs was evaluated by ELISA. The results are summarized in Fig. 2. In agreement with previous studies, both HepG2 and PLC/PRF/5 cells constitutively released HSP60, HSP70, and HSP90; in addition, the levels were up-regulated after cellular stress (30, 31). The release of HSP60, HSP70, and HSP90 was approximately equally up-regulated by both types of stress in HepG2 and PLC/PRF/5 cells. No cell line-specific differences could be noted (p > 0.05). HSP60, HSP70, and HSP90 secretion were generally higher after heat shock or anticancer drug treatments, reaching statistical significance (p < 0.05). Moreover, our results demonstrated that HepG2 and PLC/PRF/5 cells secreted the highest levels of HSP60, HSP70, and HSP90 treated with carboplatin or irinotecan hydrochloride (resistant anticancer drugs) as opposed to those exposed to heat shock, paclitaxel, or etoposide (sensitive anticancer drugs). In HepG2 and PLC/PRF/5 cells, resistant anticancer drugs seemed to be more efficient in up-regulating HSP60, HSP70, and HSP90 production than sensitive anticancer drugs and heat shock.
FIGURE 2.
Secretion of HSP60, HSP70, and HSP90 by HepG2 and PLC/PRF/5 cells is increased under stress conditions. HSP60 (A), HSP70 (B), and HSP90 (C) secretion before and after heat shock and anticancer drugs was detected by ELISA. The findings showed no cell line-specific differences and enhancements of HSP60, HSP70, and HSP90 secretion into the extracellular medium under stress conditions. Resistant anticancer drugs (irinotecan hydrochloride and carboplatin) markedly increased HSP60, HSP70, and HSP90 production by HepG2 and PLC/PRF/5 cells compared with under basal (control) and other stress conditions (p < 0.05). The results are of one representative experiment of three. Mean values ± S.D. are calculated from triplicate experiments with similar results.
Identification and Characterization of Exosomes Secreted by HepG2 Cells under Basal Conditions
To characterize the features of the purified exosomal pellet, we performed transmission electron microscopy and Western blotting. As Fig. 3A shows, a pure exosomal population was present with typical cup-shaped morphology. It was surrounded by a two-layer lipid membrane and varied in size between 30 and 100 nm, with the majority around 70–90 nm. Besides morphology and size, we confirmed the presence of exosomes through its specific marker by Western blot with anti-CD63 antibody (Fig. 3B).
FIGURE 3.
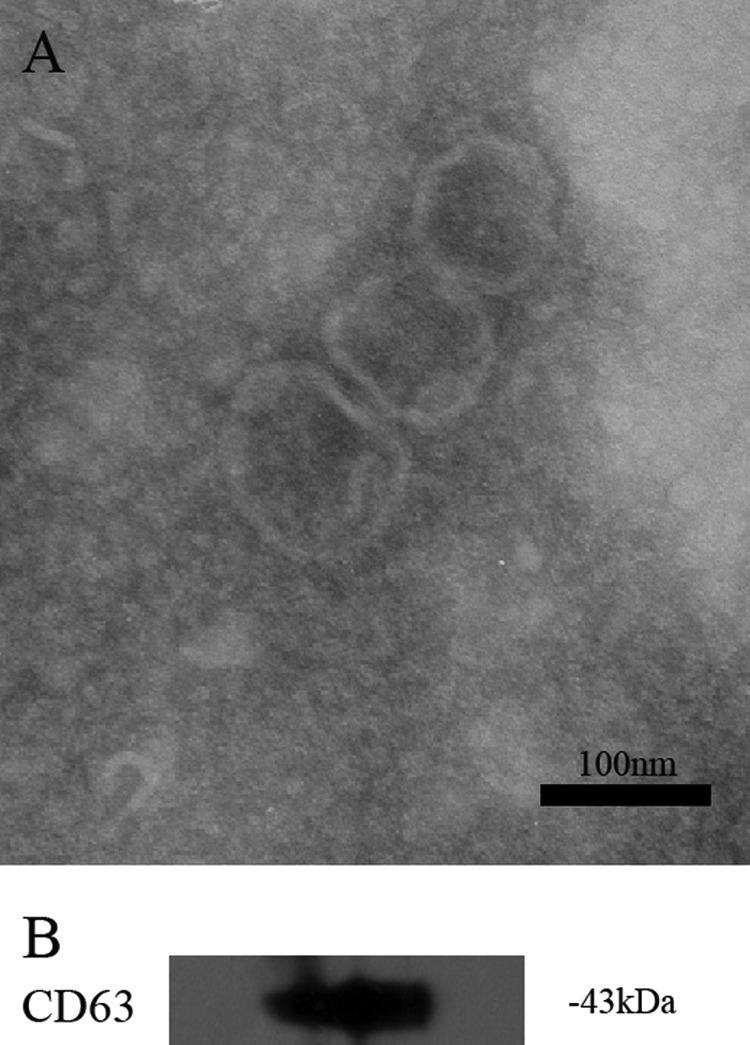
Identification and characterization of exosomes secreted by HepG2 cells under basal conditions. Exosomes were isolated by sequential centrifugations from supernatants of HepG2 cells under basal conditions. Shown is measurement of purified exosomal pellet by negative staining, showing a pure population with typical exosomal morphology (A), and Western blotting for the exosomal marker, tetraspan protein CD63 (B).
Heat Shock and Anticancer Drugs Significantly Increase Exosome Secretion by HepG2 Cells
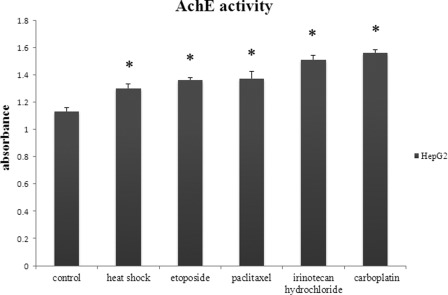
In the next step, we analyzed whether heat shock and anticancer drugs could also affect the quantity of exosomes secreted by HepG2 cells. We isolated exosomes from cell culture supernatants produced by HepG2 cells under both basal and stress-induced conditions. The amount of exosomes was quantified via the determination of AChE enzymatic activity, an enzyme specific to exosomes (26). As shown in Fig. 4, there was an increase in exosome release by HepG2 cells under stress-induced conditions compared with cells under basal conditions (control, p < 0.05). Importantly, the highest activity of AChE appeared in the culture medium of HepG2 cells that were exposed to irinotecan hydrochloride or carboplatin (resistant anticancer drugs) for 96 h. The greater the AChE enzymatic activity we observed, the greater the number of exosomes released. We concluded that resistant anticancer drugs could enhance exosome release to a higher degree than heat shock for 1.5 h and sensitive anticancer drugs for 36 h (paclitaxel and etoposide).
FIGURE 4.
Heat shock and anticancer drugs significantly increase exosome secretion by HepG2 cells. Exosomes were purified from untreated and stressed HepG2 cell culture medium and quantitated by measuring the AChE enzymatic activity as a marker for exosomes. Data are presented as the maximal activity measured at 30 min and are expressed as the mean ± S.D. (error bars) of nine independent experiments with similar results. *, p < 0.05 compared with control.
Effect of Anticancer Drugs on HSP60, HSP70, and HSP90 Expression in HepG2 Cell-derived Exosomes
The constitutive expression of HSPs in exosomes derived from reticulocytes (32), antigen-presenting cells (28, 33), and tumor cells (34, 35) has been reported previously. By ELISA, we showed that stress up-regulated the production of HSP60, HSP70, and HSP90 by HepG2 and PLC/PRF/5 cells. Through measurement of AChE enzymatic activity, we estimated that stress increased the amount of exosomes secreted by HepG2 cells. Summarizing the above experiments, it was logical to anticipate that the amount of exosomal HSP60, HSP70, and HSP90 under stress-induced conditions should also be enhanced. To prove this suggestion, HSP60, HSP70, and HSP90 expression in HepG2 cell-derived exosomes was assessed by Western blotting. The expressions of these molecules on the surface of exosomes were analyzed by flow cytometry based on total exosomal protein measurement by a BCA protein assay.
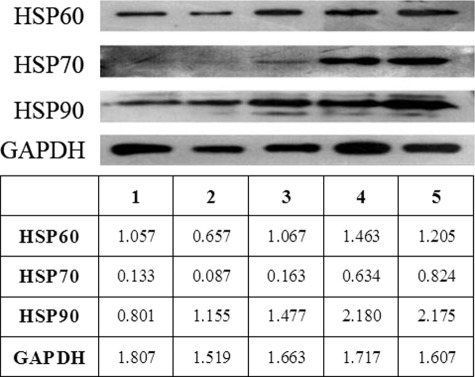
The exosomes were isolated from cell culture supernatants produced by HepG2 cells under both basal and stress-induced conditions. The total amounts of exosomal HSP60, HSP70, and HSP90 were measured by densitometric analysis of Western blot bands. Fig. 5 shows the expression levels of these HSPs in HepG2 cell-derived exosomes under basal conditions, with high constitutive levels of HSP60 and HSP90 and markedly less (∼6–8-fold) expression of HSP70. Following treatment with anticancer drugs, an increased band density of HSP90 occurred, reaching a 2.7-fold increase above its constitutive level after 96 h by resistant anticancer drugs (irinotecan hydrochloride and carboplatin) and a 1.6-fold increase after 36 h by sensitive anticancer drugs (paclitaxel and etoposide). Meanwhile, resistant anticancer drugs induced a 5.5-fold increase in HSP70 and a 1.3-fold increment in HSP60 versus basal conditions, respectively. In contrast to resistant anticancer drugs, sensitive anticancer drugs did not alter HSP60 and HSP70 expression. Therefore, it seems that resistant anticancer drugs can markedly increase the total amount of exosomal HSPs, especially HSP70.
FIGURE 5.
The effect of anticancer drugs on HSP60, HSP70, and HSP90 expression in HepG2 cell-derived exosomes. Exosomes, collected from HepG2 cell culture medium under basal conditions (1) or in the presence of paclitaxel (2), etoposide (3), irinotecan hydrochloride (4), or carboplatin (5), were separated on 12% SDS gel and examined by Western blotting using antibodies specific for HSP60, HSP70, and HSP90. Exposure of HepG2 cells to resistant anticancer drugs (irinotecan hydrochloride and carboplatin) considerably increased the content of HSP60, HSP70, and HSP90 compared with cells exposed to sensitive anticancer drugs (paclitaxel and etoposide) or under basal conditions (p < 0.05). Control loading is shown by GADPH. Densities of the bands are indicated at the bottom. One representative experiment of three is shown.
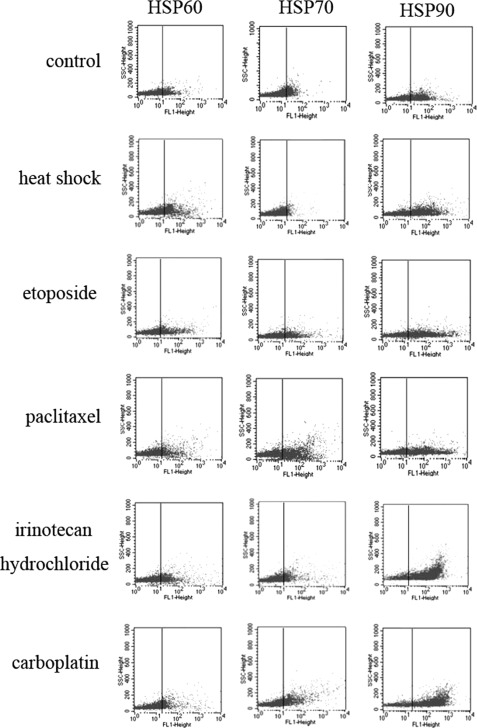
Assessment of HSP60, HSP70, and HSP90 Expression on Surface of Exosomes Secreted by HepG2 Cells under both Basal and Stress-induced Conditions
We next examined whether stress-induced conditions also elevated HSP60, HSP70, and HSP90 expression on the surface of exosomes produced by HepG2 cells. Cell culture supernatants were collected from HepG2 cells; exposed to resistant anticancer drugs (irinotecan hydrochloride and carboplatin) for 96 h, sensitive anticancer drugs (paclitaxel and etoposide) for 36 h, heat shock for 1.5 h, or basal conditions; and ultracentrifuged to isolate exosomes. The expression of exosome surface molecules was assessed by mean fluorescence intensity measurements of the exosome-bead complexes. The obtained results were depicted in Fig. 6. Staining with anti-HSP antibodies revealed that exosomes produced by HepG2 cells expressed HSP60, HSP70, and HSP90 on their surface under basal conditions (control) and exhibited a higher intensity under stress-induced conditions (p < 0.05). On the surface of HepG2 cell-derived exosomes, the highest up-regulation was observed with resistant anticancer drugs for HSP70 (5.6-fold), followed by HSP90 (4.4-fold) and HSP60 (3.8-fold). At the same time, incubation with sensitive anticancer drugs modestly caused a 4-fold increase in HSP70 exosome surface expression, a 2.7-fold increase in HSP60 expression, and a 3.6-fold increase in HSP90 expression. Conversely, heat shock only slightly affected HSP60, HSP70, and HSP90 expression on the surface of exosomes secreted by HepG2 cells. These results were consistent with the Western blot data reported previously and suggested that resistant anticancer drugs seemed to enhance HSP60, HSP70, and HSP90 exosome surface expression to a higher degree than sensitive anticancer drugs and heat shock, especially HSP70. No significant difference in the effect of two types of resistant anticancer drugs or sensitive anticancer drugs was observed.
FIGURE 6.
Assessment of HSP60, HSP70, and HSP90 expression on the surface of exosomes secreted by HepG2 cells under both basal and stress-induced conditions. Exosomes, derived from HepG2 cells subjected to heat shock, anticancer drugs, or under basal conditions, were subsequently immobilized onto latex beads, and surface HSP expression was analyzed by flow cytometry. Elevated levels of HSP60, HSP70, and HSP90 were observed under stress-induced conditions as compared with basal conditions (p < 0.05). The values for the mean fluorescence intensity (MFI) are presented as the mean ± S.D. of three independent experiments with similar results.
Anticancer Drugs Enhance Positive Effect of HSP-bearing Exosomes on NK Cell-mediated Cytotoxic Response through Granzyme B Release Concomitant with Altered Cell Surface Density of Several NK Cell Receptors
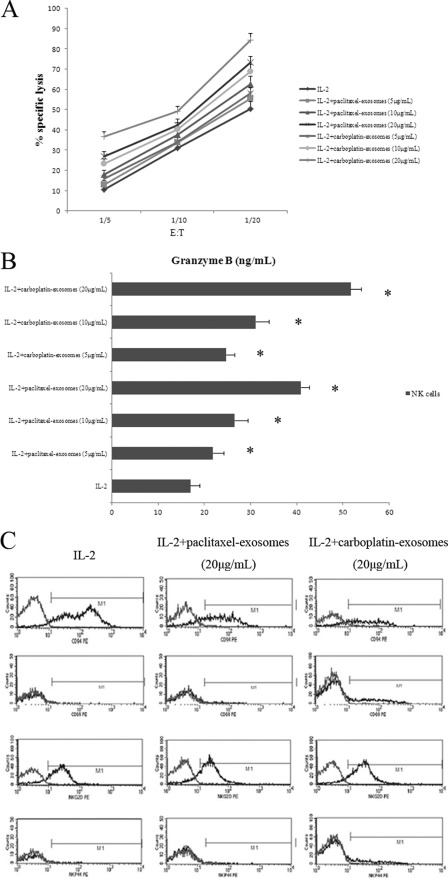
Finally, we sought to determine whether the secreted form of HSPs was biologically active. NK cells were incubated either with low dose IL-2 alone or with IL-2 in combination with exosomal proteins for 4 days. These exosomal proteins were isolated from HepG2 cells under paclitaxel or carboplatin treatment. The NK cell-mediated cytotoxic function, granzyme B production, and cell surface density of several NK cell receptors were investigated.
As reported in Fig. 7A, stimulation with exosomes brought about a considerable rise in cytotoxic activity of NK cells against K562 target cells compared with IL-2 alone (p < 0.05). A similar trend was observed in the studies of the effects of exosomes on cytotoxicity of NK cells against HepG2 target cells (data not shown). In addition, this effect was concentration-dependent. There was a significant up-regulation of the cytotoxic response in the presence of exosomal proteins (20 μg/ml) derived from HepG2 cells pretreated with paclitaxel for 36 h. Moreover, NK cells exhibited a stronger cytolytic capacity when stimulated with the same amounts of exosomes isolated from HepG2 cells preactivated with carboplatin for 96 h. Altogether, our cytotoxicity experiments indicated that exosomes derived from resistant anticancer drug-treated HepG2 cells were highly efficient in stimulating HSP reactivity in NK cells. Accordingly, an interesting observation made was that resistant anticancer drugs caused the highest increase of exosome secretion, as illustrated in Fig. 4. The highest HSP levels in and on the surface of exosomes are shown in Figs. 5 and 6.
FIGURE 7.
Anticancer drugs enhance the positive effect of HSP-bearing exosomes on NK cell-mediated cytotoxic response through granzyme B release concomitant with an altered cell surface density of several NK cell receptors. NK cells were incubated either with low dose IL-2 alone or with IL-2 plus exosomes derived from HepG2 cells, treated with paclitaxel or carboplatin, for 4 days. A, NK cell-mediated cytotoxic activity was measured in a standard lactate dehydrogenase release assay. A comparable, strong lysis was detected against K562 target cells if NK cells were stimulated with IL-2 plus exosomes. After stimulation with IL-2 alone, cytotoxic activity was weaker (p < 0.05). Data are from one experiment representative of seven separate experiments with similar results (means ± S.D.). B, after 4 days, culture supernatants were harvested, and the amount of released granzyme B was estimated by ELISA. Columns, mean values of three independent experiments; error bars, S.D. *, statistical significance (p < 0.05). C, after 4 days, NK cells were stained with anti-NKG2D, anti-CD69, anti-CD94, or anti-NKp44 antibodies and analyzed by flow cytometry. The geometric MFI of NK cells stimulated with IL-2 plus exosomes was significantly different from IL-2-stimulated controls (p < 0.05). Data represent mean values ± S.D. (error bars) from three independent experiments with similar results.
Measurement of granzyme B release in response to the appropriate target is useful for evaluating NK cell-mediated cytotoxicity. The result shown in Fig. 7B suggested that substantial release of granzyme B was induced when NK cells were stimulated with 20 μg/ml exosomes (51.7 ng/ml) derived from carboplatin-treated HepG2 cells. Identical amounts of exosomes derived from paclitaxel-treated HepG2 cells resulted in a markedly weaker release of granzyme B (40.95 ng/ml). Only 16.96 ng/ml granzyme B was secreted if NK cells were stimulated with IL-2 alone. This effect occurred in a dose-dependent manner.
The antitumor activity of NK cells is regulated by two main receptor systems involving inhibitory and activating receptors (36, 37). Concomitantly, the cell surface densities of activating receptors CD69, NKG2D, and NKp44 were intensely enhanced after incubation of NK cells with exosomes; CD94, one of the NK cell-inhibitory receptors, was strikingly down-regulated in a concentration-dependent manner (p < 0.05; Fig. 7C). In addition, following stimulation with 20 μg/ml exosomes derived from carboplatin-treated HepG2 cells, the geometric mean fluorescence intensity of CD69 (68.23%), NKG2D (32.18%), and NKp44 (44.26%) was sharply higher than that of CD69 (59.01%), NKG2D (28.27%), and NKp44 (36.56%) incubated with exosomal proteins (20 μg/ml) derived from HepG2 cells exposed to paclitaxel. Conversely, stimulation with exosomal proteins (20 μg/ml) derived from HepG2 cells exposed to carboplatin, the geometric mean fluorescence intensity of CD94 (53.53%) was significantly lower than that of CD94 (65.73%) incubated with 20 μg/ml paclitaxel-treated HepG2 cell-derived exosomes.
DISCUSSION
In this report, we have used HepG2 and PLC/PRF/5 cell lines as models for study of HSP-bearing exosome secretion by hepatocellular carcinoma cells under stress conditions for the following reasons: (a) several studies have shown that NK cells from HCC patients are defective in their cytotoxic function (38, 39), and failure of immunological surveillance caused by inadequate NK cell function may be correlated with rapid HCC progression and poor prognosis; (b) conventional cytotoxic or cytostatic chemotherapy of HCC, which exposes the body to massive cellular stress, is toxic and relatively ineffective; (c) secretion of exosomes is a constitutive feature of many human tumors; (d) Tex are known to express HSPs and enhance the cytolytic activity of NK cells.
HSPs are highly evolutionarily conserved proteins that inhabit nearly all subcellular compartments. According to their molecular weights, mammalian HSPs have been classified into five families: HSP100, HSP90, HSP70, HSP60, and the small HSPs. They are ubiquitously expressed at a basal level but are specifically induced in response to various stress stimuli. Anticancer drugs, collectively known as stress stimuli, considerably enhanced the production of HSP60, HSP70, and HSP90 by hepatocellular carcinoma cells, especially resistant anticancer drugs (Fig. 2). Extracellularly located or plasma membrane-bound HSPs elicit potent antitumor immune responses mediated either by innate or adaptive immunity. Apart from chaperoning tumor-specific antigens (7), HSPs per se provide activation signals for the innate immune system.
Exosomes have been described as potent export vehicles for HSPs from the early endosomal compartment into the extracellular environment (40). Morphological and biochemical properties identified pellet secreted by HepG2 cells as exosomes (Fig. 3). Notably, we found that hepatocellular carcinoma cells constitutively secreted exosomes, and the exosome secretion was also substantially increased by anticancer drugs, in particular resistant anti-cancer drugs (Fig. 4). Based on assessment of the results described above, it is conceivable that the exosome-mediated secretion of HSPs under stress-induced conditions should also be enhanced. Indeed, hepatocellular carcinoma cell-derived exosomes carried more HSP60, HSP70, and HSP90 under treatment with anticancer drugs. Resistant anticancer drugs caused induction of exosome-carried HSPs release at a level remarkably higher than sensitive anticancer drugs, especially HSP70 (Figs. 5 and 6). Considering this differential reaction pattern, it is tempting to speculate that treatment with resistant anticancer drugs for a relatively long drug exposure time might be suitable for further usage.
The immune impact of Tex has been a controversial issue. Much of the previous work has been interested in exosomes as a novel cell-free source to exert a broad array of detrimental effects on the immune responses, including inducing apoptosis of T lymphocytes, suppressing lymphocyte proliferation, and impairing NK cell cytotoxicity by down-regulating NKG2D receptor expression (25, 29, 41, 42). On the other hand, mounting clinical and experimental evidence is available to suggest that Tex can also be involved in immunity activation (34, 43). Importantly, although the functional relevance of many Tex-associated proteins is not entirely understood at present, it has been repeatedly shown that HSPs on Tex enable these vesicles to augment NK cell cytotoxic responses. For example, Gastpar et al. (35) demonstrated that Hsp70 present at the exosome surface, originating from Hsp70/Bag-4 membrane-positive tumor cells, could directly trigger NK cell activation, supporting migration and cytotoxicity in vitro. Similarly, a study by other researchers found that human melanoma cell-released bioactive HSP70-positive exosomes could promote the activation of mouse NK cells, resulting in a diminished tumor growth and suppression of metastatic disease (44).
NK cells as an important component of the cytotoxic lymphocyte compartment substantially contribute to antitumor immune responses (37, 45). NK cells provide the body's first line of defense against transformed cells by releasing cytotoxic granules, producing cytokines and causing cytotoxicity, and this capacity is dependent on a dynamic balance between the inhibitory and activating receptors (46). Granzyme B is a serine protease stored in the cytoplasmic granules of NK cells. When granzyme B is actively secreted into the interspace between the cytotoxic cell and the target cell, granzyme B works along with perforin to induce apoptosis in target cells by forming transmembrane pores and through cleavage of effector caspases (47, 48). Here, our study revealed that contact of NK cells with the increased amount of HSP-bearing exosomes augmented cytolytic activity against K562 or HepG2 target cells through granzyme B release; down-regulation of activating receptors CD69, NKG2D, and NKp44; and up-regulation of inhibitory receptor CD94 (Fig. 7). Furthermore, the extent of cytotoxic effect of the HSP-expressing exosomes was correlated with their concentration, reaching a maximum effect at 20 μg/ml. More importantly, the effects of paclitaxel- and carboplatin-treated HepG2 cell-derived exosomes on NK cell activation appeared to be different. The studies described above indicated that this anticancer drug-inducible, increased exosome-carried HSP surface density was associated with an enhanced sensitivity toward NK cell-mediated cytotoxicity.
In conclusion, the results presented in this report confirm and reinforce the importance of the HSP-expressing Tex as stimulatory vehicles mediating NK cell bioactivity. Furthermore, we demonstrated that resistant anticancer drugs caused the highest level of Tex secretion in general. They also caused the greatest increase of exosome-carried HSPs in particular, resulting in the most potent antitumor response of NK cells. In addition, Tex is very stable and can be cryopreserved for more than 6 months at −80 °C with a preserved phenotype and function. Perhaps most importantly, HSP-enriched exosomes are easy to obtain from hepatocellular carcinoma cell lines and do not have the limitations of requiring surgical tissues. Anticancer drugs, especially resistant anticancer drug-based immunotherapy, which specifically stimulates immune response using HSP-expressing Tex, have emerged as a promising alternative approach for the innovative and effective treatment of HCC. However, HSP-bearing Tex function in vivo remains unclear. More studies are needed to identify whether the HSP-bearing Tex used to vaccinate animals could protect against established tumors and to test its safety, feasibility, and efficacy.
Acknowledgment
We thank Elizabeth Barrington of the University of Pittsburgh for language assistance.
This work was supported by National High Technology Research and Development Program of China (863 Program) Grant 2007AA02Z117 and National Natural Science Foundation of China Grants 30571805, 30672036, 30671987, and 81000065.
- HCC
- hepatocellular carcinoma
- HSP
- heat shock protein
- NK
- natural killer
- NKG2D
- natural killer group 2 member D
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- AChE
- acetylcholinesterase
- Tex
- tumor-derived exosome(s)
- TDC
- test drug concentration
- PE
- phycoerythrin.
REFERENCES
- 1. Jemal A., Siegel R., Ward E., Hao Y., Xu J., Murray T., Thun M. J. (2008) Cancer statistics, 2008. CA Cancer J. Clin. 58, 71–96 [DOI] [PubMed] [Google Scholar]
- 2. Yeo W., Mok T. S., Zee B., Leung T. W., Lai P. B., Lau W. Y., Koh J., Mo F. K., Yu S. C., Chan A. T., Hui P., Ma B., Lam K. C., Ho W. M., Wong H. T., Tang A., Johnson P. J. (2005) A randomized phase III study of doxorubicin versus cisplatin/interferon α-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J. Natl. Cancer Inst. 97, 1532–1538 [DOI] [PubMed] [Google Scholar]
- 3. Boige V., Taïeb J., Hebbar M., Malka D., Debaere T., Hannoun L., Magherini E., Mignard D., Poynard T., Ducreux M. (2006) Irinotecan as first-line chemotherapy in patients with advanced hepatocellular carcinoma. A multicenter phase II study with dose adjustment according to baseline serum bilirubin level. Eur. J. Cancer 42, 456–459 [DOI] [PubMed] [Google Scholar]
- 4. Kuang M., Peng B. G., Lu M. D., Liang L. J., Huang J. F., He Q., Hua Y. P., Totsuka S., Liu S. Q., Leong K. W., Ohno T. (2004) Phase II randomized trial of autologous formalin-fixed tumor vaccine for postsurgical recurrence of hepatocellular carcinoma. Clin. Cancer Res. 10, 1574–1579 [DOI] [PubMed] [Google Scholar]
- 5. Boozari B., Mundt B., Woller N., Strüver N., Gürlevik E., Schache P., Kloos A., Knocke S., Manns M. P., Wirth T. C., Kubicka S., Kühnel F. (2010) Antitumoral immunity by virus-mediated immunogenic apoptosis inhibits metastatic growth of hepatocellular carcinoma. Gut 59, 1416–1426 [DOI] [PubMed] [Google Scholar]
- 6. Ritossa P. (1962) [Problems of prophylactic vaccinations of infants]. Riv. Ist. Sieroter. Ital. 37, 79–108 [PubMed] [Google Scholar]
- 7. Hartl F. U., Hayer-Hartl M. (2002) Molecular chaperones in the cytosol. From nascent chain to folded protein. Science 295, 1852–1858 [DOI] [PubMed] [Google Scholar]
- 8. Schmitt E., Gehrmann M., Brunet M., Multhoff G., Garrido C. (2007) Intracellular and extracellular functions of heat shock proteins. Repercussions in cancer therapy. J. Leukoc. Biol. 81, 15–27 [DOI] [PubMed] [Google Scholar]
- 9. Pockley A. G. (2003) Heat shock proteins as regulators of the immune response. Lancet 362, 469–476 [DOI] [PubMed] [Google Scholar]
- 10. Hickman-Miller H. D., Hildebrand W. H. (2004) The immune response under stress. The role of HSP-derived peptides. Trends Immunol. 25, 427–433 [DOI] [PubMed] [Google Scholar]
- 11. Théry C., Zitvogel L., Amigorena S. (2002) Exosomes. Composition, biogenesis, and function. Nat. Rev. Immunol. 2, 569–579 [DOI] [PubMed] [Google Scholar]
- 12. Février B., Raposo G. (2004) Exosomes. Endosomal-derived vesicles shipping extracellular messages. Curr. Opin. Cell Biol. 16, 415–421 [DOI] [PubMed] [Google Scholar]
- 13. Schorey J. S., Bhatnagar S. (2008) Exosome function. From tumor immunology to pathogen biology. Traffic 9, 871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kapsogeorgou E. K., Abu-Helu R. F., Moutsopoulos H. M., Manoussakis M. N. (2005) Salivary gland epithelial cell exosomes. A source of autoantigenic ribonucleoproteins. Arthritis Rheum. 52, 1517–1521 [DOI] [PubMed] [Google Scholar]
- 15. Fauré J., Lachenal G., Court M., Hirrlinger J., Chatellard-Causse C., Blot B., Grange J., Schoehn G., Goldberg Y., Boyer V., Kirchhoff F., Raposo G., Garin J., Sadoul R. (2006) Exosomes are released by cultured cortical neurons. Mol. Cell Neurosci. 31, 642–648 [DOI] [PubMed] [Google Scholar]
- 16. Segura E., Nicco C., Lombard B., Véron P., Raposo G., Batteux F., Amigorena S., Théry C. (2005) ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood 106, 216–223 [DOI] [PubMed] [Google Scholar]
- 17. Blanchard N., Lankar D., Faure F., Regnault A., Dumont C., Raposo G., Hivroz C. (2002) TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/ζ complex. J. Immunol. 168, 3235–3241 [DOI] [PubMed] [Google Scholar]
- 18. Knight A. M. (2008) Regulated release of B cell-derived exosomes. Do differences in exosome release provide insight into different APC function for B cells and DC? Eur. J. Immunol. 38, 1186–1189 [DOI] [PubMed] [Google Scholar]
- 19. Andre F., Schartz N. E., Movassagh M., Flament C., Pautier P., Morice P., Pomel C., Lhomme C., Escudier B., Le Chevalier T., Tursz T., Amigorena S., Raposo G., Angevin E., Zitvogel L. (2002) Malignant effusions and immunogenic tumor-derived exosomes. Lancet 360, 295–305 [DOI] [PubMed] [Google Scholar]
- 20. Koga K., Matsumoto K., Akiyoshi T., Kubo M., Yamanaka N., Tasaki A., Nakashima H., Nakamura M., Kuroki S., Tanaka M., Katano M. (2005) Purification, characterization, and biological significance of tumor-derived exosomes. Anticancer Res. 25, 3703–3707 [PubMed] [Google Scholar]
- 21. Clayton A., Mason M. D. (2009) Exosomes in tumor immunity. Curr. Oncol. 16, 46–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B. (1987) Evaluation of a tetrazolium-based semiautomated colorimetric assay. Assessment of chemosensitivity testing. Cancer Res. 47, 936–942 [PubMed] [Google Scholar]
- 23. Pisitkun T., Shen R. F., Knepper M. A. (2004) Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. U.S.A. 101, 13368–13373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J. J., Lötvall J. O. (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 [DOI] [PubMed] [Google Scholar]
- 25. Liu C., Yu S., Zinn K., Wang J., Zhang L., Jia Y., Kappes J. C., Barnes S., Kimberly R. P., Grizzle W. E., Zhang H. G. (2006) Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J. Immunol. 176, 1375–1385 [DOI] [PubMed] [Google Scholar]
- 26. Savina A., Vidal M., Colombo M. I. (2002) The exosome pathway in K562 cells is regulated by Rab11. J. Cell Sci. 115, 2505–2515 [DOI] [PubMed] [Google Scholar]
- 27. Savina A., Furlán M., Vidal M., Colombo M. I. (2003) Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J. Biol. Chem. 278, 20083–20090 [DOI] [PubMed] [Google Scholar]
- 28. Clayton A., Turkes A., Navabi H., Mason M. D., Tabi Z. (2005) Induction of heat shock proteins in B-cell exosomes. J. Cell Sci. 118, 3631–3638 [DOI] [PubMed] [Google Scholar]
- 29. Clayton A., Mitchell J. P., Court J., Mason M. D., Tabi Z. (2007) Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 67, 7458–7466 [DOI] [PubMed] [Google Scholar]
- 30. Lindquist S. (1986) The heat-shock response. Annu. Rev. Biochem. 55, 1151–1191 [DOI] [PubMed] [Google Scholar]
- 31. Parsell D. A., Lindquist S. (1993) The function of heat-shock proteins in stress tolerance. Degradation and reactivation of damaged proteins. Annu. Rev. Genet. 27, 437–496 [DOI] [PubMed] [Google Scholar]
- 32. Géminard C., Nault F., Johnstone R. M., Vidal M. (2001) Characteristics of the interaction between Hsc70 and the transferrin receptor in exosomes released during reticulocyte maturation. J. Biol. Chem. 276, 9910–9916 [DOI] [PubMed] [Google Scholar]
- 33. Wubbolts R., Leckie R. S., Veenhuizen P. T., Schwarzmann G., Möbius W., Hoernschemeyer J., Slot J. W., Geuze H. J., Stoorvogel W. (2003) Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J. Biol. Chem. 278, 10963–10972 [DOI] [PubMed] [Google Scholar]
- 34. Dai S., Wan T., Wang B., Zhou X., Xiu F., Chen T., Wu Y., Cao X. (2005) More efficient induction of HLA-A*0201-restricted and carcinoembryonic antigen (CEA)-specific CTL response by immunization with exosomes prepared from heat-stressed CEA-positive tumor cells. Clin. Cancer Res. 11, 7554–7563 [DOI] [PubMed] [Google Scholar]
- 35. Gastpar R., Gehrmann M., Bausero M. A., Asea A., Gross C., Schroeder J. A., Multhoff G. (2005) Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 65, 5238–5247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lanier L. L. (1998) NK cell receptors. Annu. Rev. Immunol. 16, 359–393 [DOI] [PubMed] [Google Scholar]
- 37. Moretta L., Moretta A. (2004) Unraveling natural killer cell function. Triggering and inhibitory human NK receptors. EMBO J. 23, 255–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jinushi M., Takehara T., Tatsumi T., Hiramatsu N., Sakamori R., Yamaguchi S., Hayashi N. (2005) Impairment of natural killer cell and dendritic cell functions by the soluble form of MHC class I-related chain A in advanced human hepatocellular carcinomas. J. Hepatol. 43, 1013–1020 [DOI] [PubMed] [Google Scholar]
- 39. Cai L., Zhang Z., Zhou L., Wang H., Fu J., Zhang S., Shi M., Zhang H., Yang Y., Wu H., Tien P., Wang F. S. (2008) Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin. Immunol. 129, 428–437 [DOI] [PubMed] [Google Scholar]
- 40. Lancaster G. I., Febbraio M. A. (2005) Exosome-dependent trafficking of HSP70. A novel secretory pathway for cellular stress proteins. J. Biol. Chem. 280, 23349–23355 [DOI] [PubMed] [Google Scholar]
- 41. Abusamra A. J., Zhong Z., Zheng X., Li M., Ichim T. E., Chin J. L., Min W. P. (2005) Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol. Dis. 35, 169–173 [DOI] [PubMed] [Google Scholar]
- 42. Clayton A., Mitchell J. P., Court J., Linnane S., Mason M. D., Tabi Z. (2008) Human tumor-derived exosomes down-modulate NKG2D expression. J. Immunol. 180, 7249–7258 [DOI] [PubMed] [Google Scholar]
- 43. Wolfers J., Lozier A., Raposo G., Regnault A., Théry C., Masurier C., Flament C., Pouzieux S., Faure F., Tursz T., Angevin E., Amigorena S., Zitvogel L. (2001) Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 7, 297–303 [DOI] [PubMed] [Google Scholar]
- 44. Elsner L., Muppala V., Gehrmann M., Lozano J., Malzahn D., Bickeböller H., Brunner E., Zientkowska M., Herrmann T., Walter L., Alves F., Multhoff G., Dressel R. (2007) The heat shock protein HSP70 promotes mouse NK cell activity against tumors that express inducible NKG2D ligands. J. Immunol. 179, 5523–5533 [DOI] [PubMed] [Google Scholar]
- 45. Lanier L. L. (2005) NK cell recognition. Annu. Rev. Immunol. 23, 225–274 [DOI] [PubMed] [Google Scholar]
- 46. Taylor D. D., Gerçel-Taylor C. (2005) Tumor-derived exosomes and their role in cancer-associated T-cell signaling defects. Br. J. Cancer 92, 305–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lord S. J., Rajotte R. V., Korbutt G. S., Bleackley R. C. (2003) Granzyme B. A natural born killer. Immunol. Rev. 193, 31–38 [DOI] [PubMed] [Google Scholar]
- 48. Trapani J. A., Sutton V. R. (2003) Granzyme B. Pro-apoptotic, antiviral, and antitumor functions. Curr. Opin. Immunol. 15, 533–543 [DOI] [PubMed] [Google Scholar]