Background: The A2BAR signals via cAMP. Cyclic AMP signaling has been shown to regulate MSC differentiation.
Results: A2BAR KO mice have reduced differentiation of osteoblasts, a mild osteopenic phenotype, and impaired fracture physiology. A2BAR activation increases the differentiation of osteoblasts.
Conclusion: The A2BAR regulates bone homeostasis.
Significance: A2BAR signaling is a component of bone homeostasis, particularly after injury.
Keywords: Adenosine Receptor, Bone, Cyclic AMP (cAMP), Mesenchymal Stem Cells, Osteoblasts
Abstract
The differentiation of osteoblasts from their precursors, mesenchymal stem cells, is an important component of bone homeostasis as well as fracture healing. The A2B adenosine receptor (A2BAR) is a Gαs/αq-protein-coupled receptor that signals via cAMP. cAMP-mediated signaling has been demonstrated to regulate the differentiation of mesenchymal stem cells (MSCs) into various skeletal tissue lineages. Here, we studied the role of this receptor in the differentiation of MSCs to osteoblasts. In vitro differentiation of bone marrow-derived MSCs from A2BAR KO mice resulted in lower expression of osteoblast differentiation transcription factors and the development of fewer mineralized nodules, as compared with WT mice. The mechanism of effect involves, at least partially, cAMP as indicated by experiments involving activation of the A2BAR or addition of a cAMP analog during differentiation. Intriguingly, in vivo, microcomputed tomography analysis of adult femurs showed lower bone density in A2BAR KO mice as compared with WT. Furthermore, A2BAR KO mice display a delay in normal fracture physiology with lower expression of osteoblast differentiation genes. Thus, our study identified the A2BAR as a new regulator of osteoblast differentiation, bone formation, and fracture repair.
Introduction
Bone health is a chief medical concern for the aging population, and the deterioration of bone is significantly correlated with fracture, disease, and a diminished quality of life (1). Existing osteoporosis therapies reduce the risk of fracture by only 30–40% (2), and there are currently no pharmacological treatments to promote fracture healing and accelerate recovery. However, it is estimated that 10% of all fractures in the United States show impaired healing (3), a figure that is likely higher in the aged population (4). A clinical study investigating the effect of the osteoporotic therapy teraparatide (recombinant parathyroid hormone (PTH))4 on fracture healing gave inconclusive results (5, 6).
The maintenance of bone is a complex balance of anabolism and catabolism, and the enhanced differentiation of new osteoblasts from their precursors, mesenchymal stem cells (MSCs), is one potential therapeutic target (7). PTH has been particularly well studied, because it is a major regulator of bone metabolism. PTH signals through a Gαs/αq-protein-coupled receptor and therefore signals, at least partially, through cAMP and PKA (8, 9). In studying the mechanism of PTH action, cAMP has come to the forefront as an important signaling modulator for bone homeostasis, in particular osteoblast differentiation. To study the role of cAMP in osteoblast differentiation, investigators have pharmacologically manipulated intracellular cAMP levels or PKA activity in a variety of cell lines and primary cells, showing somewhat contradicting results. A large number of these studies have shown cAMP to positively affect osteoblast differentiation (10–13), although several have shown cAMP to be inhibitory (14, 15). At the molecular level, these conflicting results are likely due to how cAMP is modulated, the concentration of intracellular cAMP achieved, and the duration of time that it remains elevated. For example, it was found that 10 μm forskolin, an adenylyl cyclase activator, inhibited the differentiation of rat calvarial cells, whereas 1 nm increased differentiation (16).
The A2BAR is also a Gαs/αq-protein-coupled receptor. This receptor is activated when adenosine concentrations are in the micromolar range. Injury to bone results in increased extracellular ATP (17) that is subsequently metabolized by cell surface ectonucleotidases to adenosine (18). In addition, the expression of the A2BAR is known to be up-regulated by stresses, such as inflammation (19) and oxidative stress (20). The A2BAR is expressed in MSCs (18, 21) and/or osteoblast progenitors (22).
Others have studied the role of adenosine receptors on MSC differentiation to osteoblasts. Cultured bone marrow cells derived from patients undergoing hip replacement showed the A2BAR to be expressed and that this increased during osteogenesis (18). Based on expression and measurements of cAMP levels after agonist treatments of rat MSCs, Gharibi et al. (21) concluded that the A2BAR is the dominant receptor, relative to other adenosine receptors, and that its expression increases during differentiation. In human bone marrow-derived MSCs, A2BAR activation increased osteoblast differentiation, as determined by an increase in alkaline phosphatase activity (18). Similarly, in rat bone marrow-derived MSCs, activation of the A2BAR increased Runx2 and alkaline phosphatase expression, as well as the staining of mineralized nodules with alizarin red (21). These studies, however, relied on pharmacological approaches with a focus on in vitro systems and no verification of bone homeostasis in vivo.
Here, we show that the differentiation of MSCs into osteoblasts is impaired in A2BAR KO mice and that pharmacological activation of the A2BAR in bone marrow-derived MSCs can promote osteoblast differentiation, at least partially via cAMP signaling. In accordance, base-line bone density and bone fracture physiology are compromised in A2BAR KO mice.
EXPERIMENTAL PROCEDURES
Animals
All of the procedures were performed according to the Guidelines for Care and Use of Laboratory Animals published by the National Institutes of Health. Throughout this study, all of the animals received humane care that was in agreement with the guidelines of and approved by the institutional animal care and use committee of the Boston University School of Medicine. A2BAR KO mice were originally generated in our laboratory, bred onto C57BL/6J, and confirmed, also in our laboratory, by Bax MaxPCR-based gene marker analysis using MAX-BAX (Charles River Laboratories) (19, 23). All in vivo studies were performed on adult males between the ages of 15 and 18 weeks old, unless otherwise indicated. Age-, strain-, and sex-matched wild type and A2BAR KO mice were used in all experiments. The time course of fracture healing had been established in this fracture model by both histological and selected candidate gene profiling (24, 25).
Cell Culture Conditions and Assessment of ex Vivo Mineralization
The mice were euthanized, and the marrow cavity contents were flushed with growth medium consisting of α-minimum essential medium supplemented with 10% FBS and 100 units/ml penicillin/streptomycin. The cells were plated at 6 × 106 cells/ml in growth medium. The cells were left undisturbed until day 4 of culture, when half of the medium was removed and replaced. On day 6 of culture, all of the medium was removed, and the cells were treated with osteoinductive reagents (10−8 m dexamethasone, 70 ng/ml l-ascorbic acid, and 8 mm β-glycerophosphate, disodium salt), as described in Ref. 26. The cells were grown at 37 °C with 5% CO2. The medium was changed every 2 days. To assess mineralized nodules, the culture plates were fixed with 10% formaldehyde. Following fixation, the cells were washed extensively with deionized water, and the cell layers were stained with alizarin red solution (Millipore) (26). Digital photographs of stained cell layers were taken from a fixed distance on a light box for illumination. Using Image-Pro Plus 5.1 software (Media-Cybernetics), an area of interest was created around the entire well. Using a histogram-based counting protocol at a fixed color setting, the stained area per well was quantified (26). The data are presented as the percentages of change in quantified staining area in A2BAR KO samples relative to WT or to the vehicle-treated wells.
Agonist Treatment and cAMP Measurement
For cAMP analysis, the cells were treated with 1 unit/ml adenosine deaminase for 20 min prior to agonist treatment. The cells were treated with 200 μm papavarine (a phosphodiesterase inhibitor) for 10 min prior to agonist treatment. The cells were treated with 10 μm 5′-N-ethylcarboxamido adenosine (NECA) (27), 100 μm 8-bromoadenosine-3′,5′-cyclic monophosphate (8-br-cAMP) (28), or vehicle (Me2SO) for 10 min, collected, and assayed for cAMP as described by the manufacturer (Enzo Life Sciences). The protein was measured by Bradford assay. For monitoring the effect on gene expression, the agonists were added to the culture at the time of osteoinduction, and RNA was collected after 24 h. For monitoring the effect on nodule formation, agonists were added at the time of osteoinduction and at the second medium change. The nodules were counted on day 6 of osteoinduction.
Mouse Femoral Fracture Model
A closed mid-diaphyseal fracture of the left femur was generated by controlled blunt trauma using a scaled down modification of the apparatus described by Bonnarens and Einhorn (29). The mice were anesthetized, and a medial parapatellar skin incision was made, followed by a medial parapatellar incision into the quadriceps to the patellar ligament insertion, preserving the patellar ligament intact. An intramedullary pin entry point was created in the femur using a 25-gauge needle and then an intramedullary pin (the guide pin of a 25-gauge spinal needle) was inserted into the intramedullary space. The pin was cut, and the patellar ligament was returned to the original position. The skin was sutured with 3.0 suture. A subcutaneous dose of 0.5 mg/kg buprenorphine and 0.1 mg/kg penicillin was given to each mouse. For enrollment in our studies, the location and quality of fractures were assessed by x-ray analysis immediately after the fractures had been generated. A fracture whose configuration was inconsistent with our standardized placement criteria was not used in our studies. The mice were monitored and checked daily. The time course of fracture healing had been established in this fracture model by both histological and selected candidate gene profiling, as described in Refs. 24 and 25.
Specimen Harvesting
The procedures have been previously described (30). Briefly, the animals were euthanized by CO2 asphyxiation. The fractured femurs were collected, and those designated for microcomputed tomography (micro-CT; n = 5 WT and A2BAR KOs) were fixed in 4% formaldehyde for 7 days. Femurs designated for RNA extraction (n = 5 WT controls and A2BAR KOs) were cut at a distance of 5 mm on either side of the fracture. These fracture calluses were frozen in liquid nitrogen and then stored in −80 °C until they were used for RNA isolation. For base-line measurements, the femurs of WT and A2BAR KO mice were collected and fixed for micro-CT and histology, or the mid-diaphyseal region was dissected out and stored for RNA isolation. Histology was carried out on callus tissues obtained on days 14 and 21 postfracture. The femurs were decalcified in 14% (w/v) EDTA and embedded in paraffin, and 5-micron transverse sections were taken. The slides were stained with Fast Green and safranin O and counterstained with hematoxylin. The figures are composites of multiple images taken at 4× magnification. Immunohistochemistry for collagen type II, α1 (Col2a1) and aggrecan was performed after antigen retrieval with pepsin or chondroitinase, respectively. Primary antibodies were mouse anti-chicken Col2a1 and rabbit anti-mouse aggrecan (Millipore). Staining was detected using (3,3′-diaminobenzidine) peroxidase substrate kit for peroxidase (Vector Laboratories). For growth plate analysis, 4-week-old femurs were processed, sectioned, and stained as above. Histological measurements were made, as previously described (31), on the central three-fourths of the growth plate, and the lengths of the growth plate, proliferating zone, and hypertrophic zone were calculated as an average of 20 measurements per growth plate.
Quantitative Microcomputed Tomography
Micro-CT analysis of mouse femurs has been previously described (30). Fractured bones were prepared for scanning by removal of the intramedullary pins. The scans were performed using a Scanco μCT 40 system (Scanco Medical, Basserdorf, Switzerland) located in the Orthopaedic and Developmental Biomechanics Laboratory at Boston University. The scans were performed using a 12-micron voxel size resolution with 200 ms of integration time, under conditions of 55 E(KVp) and 145 I(μA). Reference lines were manually adjusted on each individual bone to include the entire callus area.
Transverse images scanned by the micro-CT were then traced manually with a computer program and stacked to render a three-dimensional image of the callus or midshaft in the case of the nonfractured bone. Subsequent analysis of the scans was carried out using the software program supplied by the manufacturer of the micro-CT instrument. Measurements of callus density (volume fraction and mineral density) were made directly from the micro-CT image data of each specimen. For these measurements, the callus (including the portion of the callus in the medullary canal) was first isolated using a semi-automated segmentation procedure that excludes the original cortex. A global threshold algorithm was then used that applies a fixed, constant threshold to all specimens. A constrained three-dimensional Gaussian filter (filter width = 0.8, filter support = 1 voxel) was used to partially suppress image noise. The total callus volume, mineralized volume fraction, and mineral density were then calculated using standard algorithms provided by the system manufacturer. The mineral density was calculated with the aid of a standard curve obtained from weekly scans of a set of hydroxyapatite phantoms of five different mineral densities.
To measure growth parameters, the right femurs of WT and A2BAR KO mice at 4, 8, and 15 weeks of age were fixed in 4% formaldehyde and then scanned and analyzed as above. For cortical bone analysis, 0.6 mm at the middle of the bone was analyzed, and for trabecular bone analysis, 1.0 mm at the distal metaphysis was analyzed.
Mineral Analysis
The mouse bone marrow cultures were grown in osteoinductive media for 6 days. The cultures were washed with PBS, lyophilized, and shipped to the Musculoskeletal Repair and Regeneration Core Center at the Hospital for Special Surgery in New York for analysis. Freshly dried (120 °C, 24 h) KBr (200 mg) was mixed with the samples, and pellets were made for spectroscopic analysis. For FTIR spectroscopy, samples from tissue culture wells were pooled. The specimens were scanned in transmittance on a Nicolet 4700 FTIR spectrometer. The spectra were collected from 400 to 2,000 cm−1. First, a base line for the spectra was determined, and the mineral to matrix ratio was determined as the area under the phosphate peak (900–1,200 cm−1) divided by the area under the amide I peak (1,585–1,720 cm−1) (32).
Quantitative RT-PCR
Total RNA isolation was prepared from (n = 5) fractured calluses harvested on days 0, 3, 7, 14, and 21 postinjury in the following manner. The specimens were powdered in liquid nitrogen using a mortar and pestle. Total RNA was extracted by TRIzol, as previously described (25). RNA was redissolved in TRIzol and extracted a second time. After isopropanol precipitation, RNA was dissolved in RNase-free water and further purified by using RNeasy mini columns (Qiagen). For cell culture, RNA was isolated with an RNeasy mini kit (Qiagen). RNA was reverse transcribed (Applied Biosystems), and gene expression was measured with Applied Biosystems reagents on an Applied Biosystems 7700 sequence detector. All of the gene expression assays used were TaqMan MGB expression assays with β-actin or 18 S rRNA used as endogenous controls. Gene expression relative to WT d0 or to vehicle treatment was calculated by the ΔΔCT method.
Statistical Analysis
The data are presented as the means ± standard deviation. n represents the number of individual experiments with mouse/group, and each experiment is typically done in duplicates. Statistical comparison was done using two-tail Student's t test assuming equal variance or, when appropriate, a two-way analysis of variance followed by the Bonferroni multiple comparison test was applied. The data are considered significant when p ≤ 0.05 (*). Analyses were performed with GraphPad Prism5 software.
RESULTS
MSC Differentiation to Osteoblasts Is Impaired in A2BAR KO Mice
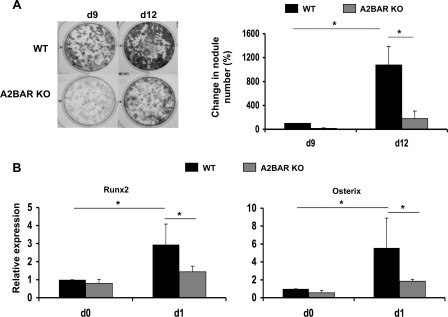
Because of the known effect that A2BAR activation has on cAMP levels (reviewed in Ref. 33) and the effect that cAMP levels have on osteoblast differentiation (10–15), we examined whether the loss of A2BAR would alter MSC differentiation to osteoblasts. MSCs were prepared from the bone marrow of A2BAR KO mice and age-, sex-, and strain-matched WT mice. They were then induced to undergo osteogenic differentiation by growing the cultures in standard osteoinductive media (see “Experimental Procedures”). Cultures from WT and A2BAR KO mice showed no differences in total protein at the time of osteoinduction or days later (supplemental Fig. S1). At 9 and 12 days after osteoinduction, bone marrow from A2BAR KO mice showed fewer mineralized nodules, demonstrating a reduction in osteoblast differentiation in the absence of the A2BAR (Fig. 1A). Using FTIR spectroscopic analysis (see “Experimental Procedures”), we validated that the accumulated mineral in both the WT and A2BAR KO cultures was apaptite. The data presented in supplemental Fig. S2 shows that the cultures accumulated mineral with typical characteristics of poorly crystalline hydroxyapatite.
FIGURE 1.
Genetic ablation of the A2BAR leads to impaired osteoblast differentiation. A, bone marrow cells were collected from WT and A2BAR KO mice and cultured in osteoinductive medium (see “Experimental Procedures”). The cultures were stained with alizarin red 9 and 12 days (d9 and d12, respectively) after osteoinduction to visualize mineralized nodules. The nodules were counted using ImagePro software. The data are presented relative to WT at day 0 and analyzed by analysis of variance (n = 3). *, p < 0.01. When day 9 WT versus A2BAR KO are analyzed by a paired t test, the difference between them is statistically significant: p < 0.01. B, measurement of osteoblast differentiation markers, Runx2 and Osterix, before adding the osteoinductive reagents (d0) or 24 h post-treatment (d1). Expression was measured by qRT-PCR and normalized to 18 S rRNA. The data are presented relative to WT at day 0 and analyzed by analysis of variance (n = 5). *, p < 0.05.
We next examined the expression of the two transcription factors that are central to osteoblast differentiation, Runx2 and Osterix, before and after osteoinduction. Prior to osteoinduction, the cells cultured from A2BAR KO mice have a trend toward reduced Runx2 and Osterix expression compared with control (a t test applied to this time point only shows significant difference in Osterix expression). With osteoinduction, the increase in the expression of both Runx2 and Osterix is significantly attenuated in the A2BAR KO cells (Fig. 1B).
A2BAR Activation Increases Osteoblast Differentiation
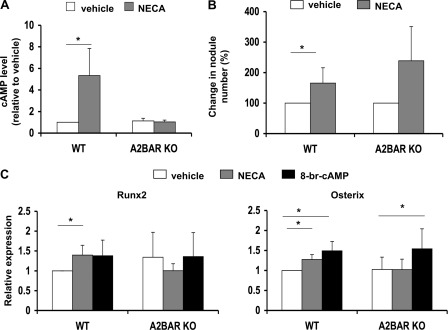
Because ablation of the A2BAR impairs osteoblast differentiation, we next tested whether activation of the A2BAR could increase differentiation. We treated bone marrow from WT and A2BAR KO mice with NECA, an A2-type receptor agonist, or vehicle, concurrently with osteoinduction. After 10 min of treatment, NECA significantly elevated cAMP levels in the WT cells, relative to treatment with vehicle (Fig. 2A). NECA had no significant effect on cAMP levels in the A2BAR KO cells, indicating that there is little contribution of the A2AAR, correlating with relatively low expression of the A2AAR in MSCs (18, 21). To test the effect of A2BAR activation on nodule formation, NECA or vehicle was added at the time of osteoinduction (day 0) and at the second medium change (day 2). Nodules were counted on day 6 of osteoinduction. We found that treatment with NECA increased cAMP levels and the number of mineralized nodules in the WT bone marrow. Although not statistically significant, treatment of A2BAR KO cells with NECA tended to increase the number of nodules (Fig. 2B). This is likely due to activation of other adenosine receptors after long term exposure to the ligand. NECA treatment of WT cells modestly increased the expression of Runx2 and Osterix as compared with treatment with vehicle (Fig. 2C). There was no effect of NECA on Runx2 or Osterix expression in the A2BAR KO cultures (Fig. 2C), indicating that the influence of NECA on gene transcription is due primarily to activation of the A2BAR receptor. Because the A2BAR can signal through activation of adenylyl cyclase and the up-regulation of cAMP, we predicted that cAMP is at least one of the signals responsible for up-regulation of Runx2 and Osterix. Treatment of WT cultures with the cAMP analog 8-br-cAMP highly increased intracellular cAMP levels (8-fold over vehicle-treated; data not shown), increased Osterix expression, and tended to augment Runx2 expression (Fig. 2C). Considering these latter data, we are not ruling out the possibility that signaling pathways other than cAMP are needed for full up-regulation of Runx2 and/or that finer tuning of cAMP levels is important for the control of Runx2 expression. Whereas treating A2BAR KO cultures with NECA did not up-regulate Osterix expression, treating these cultures with 8-br-cAMP, and therefore bypassing the receptor signaling, did significantly increase Osterix expression (Fig. 2C). This supports our hypothesis that cAMP signaling upon A2BAR activation is at least partially responsible for the augmented Osterix expression.
FIGURE 2.
Activation of A2BAR increases osteoblast differentiation. A, bone marrow cells from WT or A2BAR KO mice were treated with vehicle (Me2SO) or 10 μm NECA for 10 min. cAMP was measured by ELISA and normalized to total protein (n = 4). *, p < 0.01. B, bone marrow cells from WT mice were treated with vehicle (Me2SO) or 10 μm NECA at the time of osteoinduction and at the next medium change. Six days after osteoinduction, the cultures were stained with alizarin red, and nodules were counted using ImagePro software (as in Fig. 1). The experiment was repeated with A2BAR KO mice. A t test was then applied to within each experimental group (WT or A2BAR KO; n = 4). *, p < 0.05. C, bone marrow cell cultures from WT mice were treated with vehicle (Me2SO), 10 μm NECA, or 100 μm 8-br-cAMP at the time of osteoinduction. Lower doses of 8-br-cAMP were also attempted, yielding variable results (data not shown). RNA was collected after 24 h. Runx2 and Osterix expression was measured by qRT-PCR and normalized to 18 S rRNA. The experiment was repeated with A2BAR KO mice. A t test was then applied to each experimental group (n = 4). *, p < 0.05.
Of note, despite an increase in Osterix expression after a 24-h exposure to 8-br-cAMP (Fig. 2C), longer treatment (at days 0 and 2 of osteoinduction) caused a significant decrease in nodule formation (supplemental Fig. S3A). However, we found that this long exposure led to a decrease in total cell number and in total protein within the culture (supplemental Fig. S3B), suggesting that the inhibition in nodule formation is due to a secondary inhibition of cell proliferation that masks the effect on Osterix up-regulation. These results are in agreement with previous studies showing high cAMP levels to inhibit proliferation (28). Taken together, these data show that A2BAR ablation has a profound effect on osteoblast differentiation (Fig. 1) and that A2BAR agonism can modulate osteoblast transcription factor expression and enhance the number of osteoblasts generated in vitro (Fig. 2).
A2BAR KO Mice Exhibit Impaired Bone Fracture Physiology
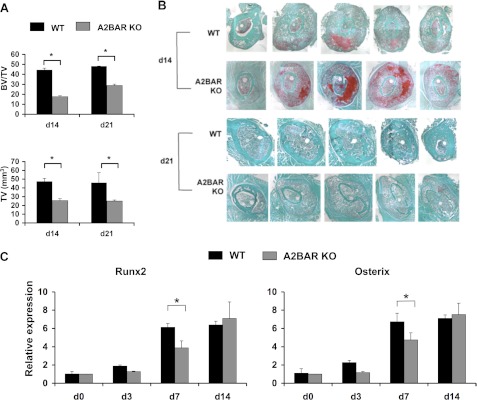
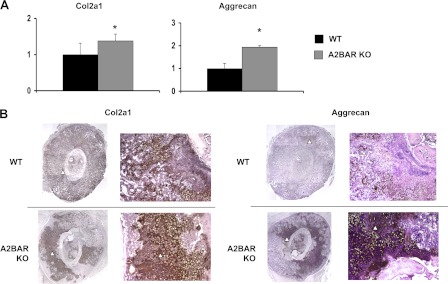
To assess the role of A2BAR in an injury response situation, we examined callus formation after fracture. Because fracture healing is dependent on the initial MSC differentiation into osteoblasts and chondrocytes (34), we hypothesized that the reduced potential of A2BAR KO MSCs to differentiate into osteoblasts would be manifested as reduced bone formation after fracture. The progression of tissue formation and mineralization within the callus was first quantified using micro-CT imaging. Micro-CT revealed that fracture calluses of A2BAR KO mice showed a smaller overall total volume, as well as a decrease in the ratio of bone volume to total callus volume at 14 and 21 days postfracture (Fig. 3A; differences at day 21 are significant with a t test applied to compare WT and KO at this time point only). Analysis of tissue sections supported these findings, because A2BAR KO sections had larger areas of cartilage staining that had not transitioned into mineralized bone tissue (Fig. 3B). In addition, tissue from the fracture callus was dissected and analyzed by qRT-PCR. As reported earlier for this injury model, a significant up-regulation of Osterix and Runx2 should be observed, both during early periods of MSC differentiation at day 3 postfracture and later starting on day 7 postfracture when mineralized cartilage resorption and primary bone formation are initiated (35). A2BAR KO mice tend to show decreased expression of Runx2 and Osterix at 3 days postfracture and show significantly decreased expression at 7 days postfracture, as compared with WT (Fig. 3C). Although the level of expression of these factors equalized in the WT and A2BAR KO samples by day 14 postfracture, there was still a difference in the ratio of mineralized tissue volume to total callus volume 14 and 21 days postfracture. Such findings suggest that the A2BAR is involved in aspects of the skeletal repair process, and its absence leads to delayed progression of bone development during fracture repair in the A2BAR KO mice. Interestingly, the day 14 postfracture sections from the A2BAR KO mice are dominated by cartilage, compared with WT (Fig. 3B), which is expected to delay normal fracture physiology (36). The increased abundance of cartilage seen in A2BAR KO sections was validated by assessing the expression levels of chondrocyte markers, Col2a1 and aggrecan, which are elevated in the A2BAR KO fractures (Fig. 4A). Further analysis with immunohistochemistry verified that the increased mRNA expression is translated into greater protein contents, based on the increased total amount of Col2a1 and aggrecan staining in the A2BAR KO as compared with WT (Fig. 4B and supplemental Fig. S4).
FIGURE 3.
Analysis of callus formation postfracture in A2BAR KO mice. A, micro-CT analysis of bone volume/total volume (BV/TV) and total volume (TV) of fracture calluses from WT and A2BAR KO mice 14 (d14) and 21 (d21) days postfracture. The data were analyzed by analysis of variance (n = 3). *, p < 0.01. B, representative sections of the fracture callus of WT and A2BAR KO mice at 14 and 21 days postfracture. The sections were stained with Fast Green (which depicts bone) and safranin O (which depicts cartilage) and counterstained with hematoxylin. The panel is a composite of 4× magnified images. C, the mid-diaphyseal region (d0) or the fracture callus (days 3 (d3), 7 (d7), and 14 (d14) postfracture) of WT and A2BAR KO mice were dissected, and qRT-PCR was performed for the osteoblast transcription factors Runx2 and Osterix and normalized to β-actin. The data are presented as fold changes relative to WT d0. Analysis of variance shows a clear difference between WT and A2BAR KO at day 7. *, p < 0.01, and a trend at day 3. In addition, the difference between days 0 or 3 versus days 7 or 14 was statistically significant for WT and A2BAR KO and days 7 versus 14 for A2BAR KO. p < 0.01 (n = 3).
FIGURE 4.
Analysis of chondrocyte markers in fracture calluses of WT and A2BAR KO mice. A, the fracture callus (14 days postfracture) of WT and A2BAR KO mice was dissected, and qRT-PCR was performed for the chondrocyte markers Col2a1 and aggrecan and normalized to β-actin (n = 5). *, p < 0.05. B, representative immunohistochemical staining of day 14 fracture calluses for Col2a1 and aggrecan. The arrowheads depict staining. Left panel, 4× magnified composite images; right panel, 10× magnified images. A larger image (for clarity) is also provided in supplemental Fig. S4.
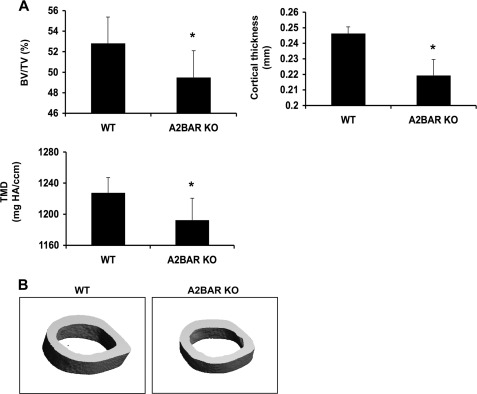
A2BAR KO Mice Exhibit an Osteopenic Phenotype
Because the differentiation of MSCs to osteoblasts and chondrocytes during the formation of the fracture callus recapitulates skeletogenesis (34), we explored the role of the A2BAR on bone development. There is no difference in body weight between 15-week-old WT and A2BAR KO mice (Table 1). Micro-CT analysis of the cortical bone of the mid-diaphyseal region of 15-week-old femurs shows a statistically significant reduction in cortical thickness (Fig. 5), which correlates with a lower bone volume relative to total volume, because total volume was not different (Table 1). Additionally, these mice display a reduction in tissue mineral density (Fig. 5A and Table 1). This shows that at 15 weeks of age, A2BAR KO mice display a mild osteopenic phenotype. Additionally, femurs of 15-week-old A2BAR KO mice were significantly shorter than WT, as measured with calipers (Table 1). Shorter femurs may be the result of impaired osteoblast differentiation during development; however, we cannot rule out other mechanisms, such as impairment in chondrocyte and/or growth plate development.
TABLE 1.
Analysis of WT and A2BAR KO bone growth and homeostasis
Bones were collected from male age-matched WT and A2BAR KO mice at 15 weeks of age. Micro-CT cortical analysis was done on the femur mid-diaphysis, and trabecular analysis was done on the distal metaphysis.
| 15 weeks |
|||
|---|---|---|---|
| WT | A2BAR KO | n/p value | |
| Body weight (g) | 30.92 ± 1.65 | 30.42 ± 1.03 | 5/0.58 |
| Femur length (mm) | 16.12 ± 0.14 | 15.50 ± 0.20 | 5/<0.001 |
| Cortical analysis | |||
| Total volume (mm3) | 1.32 ± 0.09 | 1.26 ± 0.02 | 3/0.30 |
| Bone volume (mm3) | 0.70 ± 0.06 | 0.64 ± 0.04 | 3/0.18 |
| Total volume/bone volume | 0.54 ± 0.03 | 0.51 ± 0.03 | 3/0.23 |
| Cortical thickness (mm) | 0.25 ± 0.01 | 0.23 ± 0.01 | 3/0.01 |
| Mean density (mg HA/ccm) | 1222.40 ± 23.92 | 1190.72 ± 25.78 | 3/0.19 |
| Minimum cross-sectional area (mm) | 1.17 ± 0.09 | 1.12 ± 0.09 | 3/0.53 |
| Maximum cross-sectional area (mm) | 1.52 ± 0.10 | 1.45 ± 0.12 | 3/0.49 |
| Trabecular analysis | |||
| Trabecular number | 4.73 ± 0.37 | 4.45 ± 0.65 | 3/0.50 |
| Trabecular thickness (mm) | 0.05 ± 0.01 | 0.05 ± 0.01 | 3/0.33 |
| Trabecular spacing (mm) | 0.21 ± 0.02 | 0.23 ± 0.03 | 3/0.40 |
FIGURE 5.
Effect of the A2BAR on cortical bone parameters. A, mid-diaphyseal cortical bones of 15-week-old WT and A2BAR KO mice were analyzed by micro-CT. A2BAR KO mice exhibit decreased cortical bone volume (BV) as a percentage of total volume (TV) of the mid-diaphyseal region analyzed, decreased cortical thickness, and decreased cortical tissue mineral density (TMD) (mg of hydroxyapatite (HA)/cm3) as compared with WT controls (n = 5). *, p < 0.05. B, representative micro-CT images of mid-diaphyseal cortical bone of WT and A2BAR KO mice, showing small differences in overall shape and size.
There are no significant differences in the cortical bone between WT and A2BAR KO mice at 4 or 8 weeks of age (supplemental Table S1). However, at 4 weeks of age, A2BAR KO mice have fewer trabeculae, a decrease in trabecular thickness, and an increase in trabecular spacing, as compared with WT mice (supplemental Table S1). Additionally, as in the 15-week-old mice, femurs of 4 and 8-week-old A2BAR KO mice are somewhat shorter, as compared with WT (supplemental Table S1).
Analysis of the growth plates of 4-week-old WT and A2BAR KO mice shows no statistically significant difference in the total growth plate thickness, proliferating zone thickness, or hypertrophic zone thickness, but A2BAR KO growth plates are slightly larger (supplemental Fig. S5 and Table S2). Together, our results demonstrate that the A2BAR has an important role in the promotion of osteoblast differentiation and bone formation with development and after fracture. This role is, at least partially, through the control of the expression of osteoblast differentiation transcription factors.
DISCUSSION
MSC differentiation to osteoblasts plays a necessary role in development and postnatal growth and homeostasis (reviewed in Ref. 37), as well as skeletal tissue repair after injuries such as fracture (38). Although the origin of the osteoblasts involved in fracture healing has not been demonstrated unequivocally, experimental evidence is accumulating to support the hypothesis that some of the MSCs that contribute to repair are harbored in the bone perivasculature (39) and upon receiving a signal (one of which is stromal derived factor-1 (38)) travel through the marrow capillaries (37) or neovasculature (40) to the site of injury or stress.
Here, we used genetic ablation to study the role of the A2BAR in bone homeostasis and repair in vivo and examined the potential mechanisms that were involved using in vitro studies of MSC differentiation. We present direct evidence that the A2BAR plays a role in osteoblast differentiation. We have also demonstrated that activation of this receptor with a pharmacological agonist can enhance osteoblast differentiation. This effect is likely due to the modulation of levels of the transcription factors essential for osteoblast differentiation, Runx2 and Osterix. This claim is supported by previous data demonstrating increased osteoblast differentiation with the overexpression of either Runx2 (41) or Osterix (42). Of note, however, the effect of A2BAR activation on osteoblast differentiation is much milder than the effect of this receptor ablation (compare Figs. 1 and 2). This raises the possibility that the receptor influences these processes independent of its activation, e.g. via its potential association with other regulatory pathways or proteins yet to be explored, which would be lost upon receptor deletion.
Genetic deletion of the receptor impacted bone fracture callus development and likely delayed normal fracture physiology. It had long been accepted that the low affinity A2BAR plays an important role in tissue injury, because extracellular adenosine levels increase after various types of stress/injury and in various organs (reviewed in Ref. 43). Many studies of bone have examined extracellular concentrations of ATP and ADP and the expression of their receptors, P2X and P2Y (17). Extracellular ATP concentrations increase after bone injury, and when exposed to hypoxic conditions, osteoblasts secrete ATP in the high nanomolar to micromolar range (44). This ATP is catabolized by ectonucleotidases that are also expressed on osteoblasts (18). Of note, it was recently demonstrated that genetic ablation of the ectonucleotidase CD73, an enzyme upstream of A2BAR signaling (by converting AMP to adenosine), results in osteopenia and decreased osteoblast differentiation (45). Although we too demonstrate diminished osteoblast differentiation ex vivo in A2BAR KO samples, it is likely that this explains only part of the reduced bone density in the fracture callus. Fracture healing is complex, and the A2BAR may have a role in other stages of the process, such as the inflammatory response. This possibility should be considered in the context of fracture healing because inflammatory cytokines, particularly TNF-α, have been shown to down-regulate the expression of the osteoblast differentiation genes Runx2 (46) and Osterix (47). Also, A2BAR KO mice tend to display a mild increase in the levels of inflammatory cytokines at base line, and more so postvascular injury, partially attributed to cAMP modulation of macrophage expression of cytokines (19, 23).
Our current study also points to a potential role for A2BAR signaling in the regulation of callus cartilage density postinjury. Others have documented A2BAR expression in chondrocytes (48), the source of cartilage, as well as the role of cAMP signaling in preventing (49) and promoting (50) their differentiation. It would be interesting in the future to examine the potential effect of A2BAR expression on the development of embryonic and adult chondrocytes.
Finally, our study is the first to describe a role for the A2BAR in bone development and homeostasis. Intriguingly, the bone phenotype of the A2BAR KO mouse is similar to observations made in the PTH receptor KO mouse (51), suggesting a possible synergy between the two receptors during bone development. We found that genetic ablation of the A2BAR causes a significant decrease in the early (4-week-old) development of trabecular bone; however, the differences normalize by 8 weeks of age. The genesis of trabecular bone depends on the differentiation of cells of multiple lineages, including osteoblasts, and the coordinated action of these cells (52). Impairment in osteoblast differentiation in the A2BAR KO mouse may impact the development of the perichondrium and at least partially explain the reduced trabecular bone formation in the A2BAR KO. The lack of difference in trabecular bone in older mice (8 and 15 weeks old) may be due to the diminished contribution of stem cell differentiation on trabecular bone homeostasis at these ages. In addition, our findings that the A2BAR KO femurs are shorter, with a trend toward increased height of the hypertrophic zone and entire growth plate, are consistent with the effects of removing the perichondrium during endochondral bone development (52). Therefore, A2BAR deletion may be delaying periosteal development with subsequent consequences on endochondral ossification and growth plate regulation. Future investigation of skeletal development in the A2BAR KO mouse could test these hypotheses. Taken together, our results identified the A2BAR as an important regulator of osteoblast transcription factor expression and of MSC differentiation to osteoblasts, as well as a regulator of bone homeostasis and fracture physiology.
Supplementary Material
Acknowledgments
We thank Dr. Adele Boskey (Musculoskeletal Integrity Core Center, New York Hospital for Special Surgery) for generous insight and for performing the FTIR spectroscopy. We thank Dr. Elise Morgan (Boston University Core) for help and guidance and for leading the analysis of the micro-CT analysis.
This work was supported, in whole or in part, by National Institutes of Health Grant HL93149 (to K. R.). This work was also supported in part by National Institutes of Health Grant AR046121.

This article contains supplemental Tables S1 and S2 and Figs. S1–S5.
- PTH
- parathyroid hormone
- MSC
- mesenchymal stem cell
- micro-CT
- microcomputed tomography
- NECA
- 5′-N-ethylcarboxamido adenosine
- 8-br-cAMP
- 8-bromoadenosine-3′,5′-cyclic monophosphate
- Col2a1
- collagen type II, α1
- qRT-PCR
- quantitative RT-PCR.
REFERENCES
- 1. Ray N. F., Chan J. K., Thamer M., Melton L. J., 3rd (1997) Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995. Report from the National Osteoporosis Foundation. J. Bone Miner. Res. 12, 24–35 [DOI] [PubMed] [Google Scholar]
- 2. Kawai M., Mödder U. I., Khosla S., Rosen C. J. (2011) Emerging therapeutic opportunities for skeletal restoration. Nat. Rev. Drug Discov. 10, 141–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jørgensen N. R., Schwarz P. (2011) Effects of anti-osteoporosis medications on fracture healing. Curr. Osteoporos. Rep. 9, 149–155 [DOI] [PubMed] [Google Scholar]
- 4. Gruber R., Koch H., Doll B. A., Tegtmeier F., Einhorn T. A., Hollinger J. O. (2006) Fracture healing in the elderly patient. Exp. Gerontol. 41, 1080–1093 [DOI] [PubMed] [Google Scholar]
- 5. Aspenberg P., Johansson T. (2010) Teriparatide improves early callus formation in distal radial fractures. Acta Orthop. 81, 234–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aspenberg P., Genant H. K., Johansson T., Nino A. J., See K., Krohn K., García-Hernández P. A., Recknor C. P., Einhorn T. A., Dalsky G. P., Mitlak B. H., Fierlinger A., Lakshmanan M. C. (2010) Teriparatide for acceleration of fracture repair in humans. A prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J. Bone Miner. Res. 25, 404–414 [DOI] [PubMed] [Google Scholar]
- 7. Mauney J. R., Volloch V., Kaplan D. L. (2005) Role of adult mesenchymal stem cells in bone tissue engineering applications. Current status and future prospects. Tissue Eng. 11, 787–802 [DOI] [PubMed] [Google Scholar]
- 8. Naot D., Cornish J. (2008) The role of peptides and receptors of the calcitonin family in the regulation of bone metabolism. Bone 43, 813–818 [DOI] [PubMed] [Google Scholar]
- 9. Datta N. S., Abou-Samra A. B. (2009) PTH and PTHrP signaling in osteoblasts. Cell Signal. 21, 1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghayor C., Ehrbar M., San Miguel B., Grätz K. W., Weber F. E. (2009) cAMP enhances BMP2-signaling through PKA and MKP1-dependent mechanisms. Biochem. Biophys. Res. Commun. 381, 247–252 [DOI] [PubMed] [Google Scholar]
- 11. Lo K. W., Kan H. M., Ashe K. M., Laurencin C. T. (2012) The small molecule PKA-specific cyclic AMP analogue as an inducer of osteoblast-like cells differentiation and mineralization. J. Tissue Eng. Regen. Med. 6, 40–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siddappa R., Martens A., Doorn J., Leusink A., Olivo C., Licht R., van Rijn L., Gaspar C., Fodde R., Janssen F., van Blitterswijk C., de Boer J. (2008) cAMP/PKA pathway activation in human mesenchymal stem cells in vitro results in robust bone formation in vivo. Proc. Natl. Acad. Sci. U.S.A. 105, 7281–7286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sugama R., Koike T., Imai Y., Nomura-Furuwatari C., Takaoka K. (2006) Bone morphogenetic protein activities are enhanced by 3′,5′-cyclic adenosine monophosphate through suppression of Smad6 expression in osteoprogenitor cells. Bone 38, 206–214 [DOI] [PubMed] [Google Scholar]
- 14. Siddappa R., Mulder W., Steeghs I., van de Klundert C., Fernandes H., Liu J., Arends R., van Blitterswijk C., de Boer J. (2009) cAMP/PKA signaling inhibits osteogenic differentiation and bone formation in rodent models. Tissue Eng. Part A 15, 2135–2143 [DOI] [PubMed] [Google Scholar]
- 15. Nagata A., Tanaka T., Minezawa A., Poyurovsky M., Mayama T., Suzuki S., Hashimoto N., Yoshida T., Suyama K., Miyata A., Hosokawa H., Nakayama T., Tatsuno I. (2009) cAMP activation by PACAP/VIP stimulates IL-6 release and inhibits osteoblastic differentiation through VPAC2 receptor in osteoblastic MC3T3 cells. J. Cell. Physiol. 221, 75–83 [DOI] [PubMed] [Google Scholar]
- 16. Turksen K., Grigoriadis A. E., Heersche J. N., Aubin J. E. (1990) Forskolin has biphasic effects on osteoprogenitor cell differentiation in vitro. J. Cell. Physiol. 142, 61–69 [DOI] [PubMed] [Google Scholar]
- 17. Hoebertz A., Arnett T. R., Burnstock G. (2003) Regulation of bone resorption and formation by purines and pyrimidines. Trends Pharmacol. Sci. 24, 290–297 [DOI] [PubMed] [Google Scholar]
- 18. Costa M. A., Barbosa A., Neto E., Sá-e-Sousa A., Freitas R., Neves J. M., Magalhães-Cardoso T., Ferreirinha F., Correia-de-Sá P. (2011) On the role of subtype selective adenosine receptor agonists during proliferation and osteogenic differentiation of human primary bone marrow stromal cells. J. Cell. Physiol. 226, 1353–1366 [DOI] [PubMed] [Google Scholar]
- 19. Yang D., Zhang Y., Nguyen H. G., Koupenova M., Chauhan A. K., Makitalo M., Jones M. R., St Hilaire C., Seldin D. C., Toselli P., Lamperti E., Schreiber B. M., Gavras H., Wagner D. D., Ravid K. (2006) The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J. Clin. Invest. 116, 1913–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. St Hilaire C., Koupenova M., Carroll S. H., Smith B. D., Ravid K. (2008) TNF-α upregulates the A2B adenosine receptor gene. The role of NAD(P)H oxidase 4. Biochem. Biophys. Res. Commun. 375, 292–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gharibi B., Abraham A. A., Ham J., Evans B. A. (2011) Adenosine receptor subtype expression and activation influence the differentiation of mesenchymal stem cells to osteoblasts and adipocytes. J. Bone Miner. Res. 26, 2112–2124 [DOI] [PubMed] [Google Scholar]
- 22. Evans B. A., Elford C., Pexa A., Francis K., Hughes A. C., Deussen A., Ham J. (2006) Human osteoblast precursors produce extracellular adenosine, which modulates their secretion of IL-6 and osteoprotegerin. J. Bone Miner. Res. 21, 228–236 [DOI] [PubMed] [Google Scholar]
- 23. Yang D., Koupenova M., McCrann D. J., Kopeikina K. J., Kagan H. M., Schreiber B. M., Ravid K. (2008) The A2b adenosine receptor protects against vascular injury. Proc. Natl. Acad. Sci. U.S.A. 105, 792–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gerstenfeld L. C., Cullinane D. M., Barnes G. L., Graves D. T., Einhorn T. A. (2003) Fracture healing as a post-natal developmental process. Molecular, spatial, and temporal aspects of its regulation. J. Cell. Biochem. 88, 873–884 [DOI] [PubMed] [Google Scholar]
- 25. Kon T., Cho T. J., Aizawa T., Yamazaki M., Nooh N., Graves D., Gerstenfeld L. C., Einhorn T. A. (2001) Expression of osteoprotegerin, receptor activator of NF-κB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J. Bone Miner. Res. 16, 1004–1014 [DOI] [PubMed] [Google Scholar]
- 26. Edgar C. M., Chakravarthy V., Barnes G., Kakar S., Gerstenfeld L. C., Einhorn T. A. (2007) Autogenous regulation of a network of bone morphogenetic proteins (BMPs) mediates the osteogenic differentiation in murine marrow stromal cells. Bone 40, 1389–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ravid K., Smith-Mungo L. I., Zhao Z., Thomas K. M., Kagan H. M. (1999) Upregulation of lysyl oxidase in vascular smooth muscle cells by cAMP. Role for adenosine receptor activation. J. Cell. Biochem. 75, 177–185 [DOI] [PubMed] [Google Scholar]
- 28. Xu Y., Ravid K., Smith B. D. (2008) Major histocompatibility class II transactivator expression in smooth muscle cells from A2b adenosine receptor knock-out mice. Cross-talk between the adenosine and interferon-gamma signaling. J. Biol. Chem. 283, 14213–14220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bonnarens F., Einhorn T. A. (1984) Production of a standard closed fracture in laboratory animal bone. J. Orthop. Res. 2, 97–101 [DOI] [PubMed] [Google Scholar]
- 30. Wigner N. A., Luderer H. F., Cox M. K., Sooy K., Gerstenfeld L. C., Demay M. B. (2010) Acute phosphate restriction leads to impaired fracture healing and resistance to BMP-2. J. Bone Miner. Res. 25, 724–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Börjesson A. E., Lagerquist M. K., Liu C., Shao R., Windahl S. H., Karlsson C., Sjögren K., Movérare-Skrtic S., Antal M. C., Krust A., Mohan S., Chambon P., Sävendahl L., Ohlsson C. (2010) The role of estrogen receptor α in growth plate cartilage for longitudinal bone growth. J. Bone Miner. Res. 25, 2690–2700 [DOI] [PubMed] [Google Scholar]
- 32. Pleshko N., Boskey A., Mendelsohn R. (1991) Novel infrared spectroscopic method for the determination of crystallinity of hydroxyapatite minerals. Biophys. J. 60, 786–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aherne C. M., Kewley E. M., Eltzschig H. K. (2011) The resurgence of A2B adenosine receptor signaling. Biochim. Biophys. Acta 1808, 1329–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shapiro F. (2008) Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur. Cell Mater. 15, 53–76 [DOI] [PubMed] [Google Scholar]
- 35. Jepsen K. J., Price C., Silkman L. J., Nicholls F. H., Nasser P., Hu B., Hadi N., Alapatt M., Stapleton S. N., Kakar S., Einhorn T. A., Gerstenfeld L. C. (2008) Genetic variation in the patterns of skeletal progenitor cell differentiation and progression during endochondral bone formation affects the rate of fracture healing. J. Bone Miner. Res. 23, 1204–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Evans K., Refshauge K. M., Adams R. (2006) Measurement of active rotation in standing. Reliability of a simple test protocol. Percept Mot Skills 103, 619–628 [DOI] [PubMed] [Google Scholar]
- 37. Khosla S., Westendorf J. J., Mödder U. I. (2010) Concise review. Insights from normal bone remodeling and stem cell-based therapies for bone repair. Stem Cells 28, 2124–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Granero-Moltó F., Weis J. A., Miga M. I., Landis B., Myers T. J., O'Rear L., Longobardi L., Jansen E. D., Mortlock D. P., Spagnoli A. (2009) Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells 27, 1887–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shi S., Gronthos S. (2003) Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J. Bone Miner. Res. 18, 696–704 [DOI] [PubMed] [Google Scholar]
- 40. Maes C., Kobayashi T., Selig M. K., Torrekens S., Roth S. I., Mackem S., Carmeliet G., Kronenberg H. M. (2010) Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 19, 329–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhao Z., Zhao M., Xiao G., Franceschi R. T. (2005) Gene transfer of the Runx2 transcription factor enhances osteogenic activity of bone marrow stromal cells in vitro and in vivo. Mol. Ther. 12, 247–253 [DOI] [PubMed] [Google Scholar]
- 42. Tai G., Polak J. M., Bishop A. E., Christodoulou I., Buttery L. D. (2004) Differentiation of osteoblasts from murine embryonic stem cells by overexpression of the transcriptional factor osterix. Tissue Eng. 10, 1456–1466 [DOI] [PubMed] [Google Scholar]
- 43. Fredholm B. B. (2007) Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 14, 1315–1323 [DOI] [PubMed] [Google Scholar]
- 44. Orriss I. R., Knight G. E., Utting J. C., Taylor S. E., Burnstock G., Arnett T. R. (2009) Hypoxia stimulates vesicular ATP release from rat osteoblasts. J. Cell. Physiol. 220, 155–162 [DOI] [PubMed] [Google Scholar]
- 45. Takedachi M., Oohara H., Smith B. J., Iyama M., Kobashi M., Maeda K., Long C. L., Humphrey M. B., Stoecker B. J., Toyosawa S., Thompson L. F., Murakami S. (2012) CD73-generated adenosine promotes osteoblast differentiation. J. Cell. Physiol. 227, 2622–2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gilbert L., He X., Farmer P., Rubin J., Drissi H., van Wijnen A. J., Lian J. B., Stein G. S., Nanes M. S. (2002) Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2α A) is inhibited by tumor necrosis factor-α. J. Biol. Chem. 277, 2695–2701 [DOI] [PubMed] [Google Scholar]
- 47. Lu X., Gilbert L., He X., Rubin J., Nanes M. S. (2006) Transcriptional regulation of the osterix (Osx, Sp7) promoter by tumor necrosis factor identifies disparate effects of mitogen-activated protein kinase and NF-κB pathways. J. Biol. Chem. 281, 6297–6306 [DOI] [PubMed] [Google Scholar]
- 48. Koolpe M., Pearson D., Benton H. P. (1999) Expression of both P1 and P2 purine receptor genes by human articular chondrocytes and profile of ligand-mediated prostaglandin E2 release. Arthritis Rheum. 42, 258–267 [DOI] [PubMed] [Google Scholar]
- 49. Iwamoto T., Nakamura T., Doyle A., Ishikawa M., de Vega S., Fukumoto S., Yamada Y. (2010) Pannexin 3 regulates intracellular ATP/cAMP levels and promotes chondrocyte differentiation. J. Biol. Chem. 285, 18948–18958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tsuda M., Takahashi S., Takahashi Y., Asahara H. (2003) Transcriptional co-activators CREB-binding protein and p300 regulate chondrocyte-specific gene expression via association with Sox9. J. Biol. Chem. 278, 27224–27229 [DOI] [PubMed] [Google Scholar]
- 51. Lanske B., Amling M., Neff L., Guiducci J., Baron R., Kronenberg H. M. (1999) Ablation of the PTHrP gene or the PTH/PTHrP receptor gene leads to distinct abnormalities in bone development. J. Clin. Invest. 104, 399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Colnot C., Lu C., Hu D., Helms J. A. (2004) Distinguishing the contributions of the perichondrium, cartilage, and vascular endothelium to skeletal development. Dev. Biol. 269, 55–69 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.