Background: Cosmc regulates protein O-glycosylation by regulating biosynthesis of its client T-synthase.
Results: Cosmc forms a specific noncovalent complex with non-native T-synthase leading to enzyme activity and dissociation driven by additional free client.
Conclusion: Both non-native and native T-synthase regulate release of T-synthase from complexes with Cosmc.
Significance: This work gives insights into regulators of glycosyltransferases and endoplasmic reticulum/Golgi proteins.
Keywords: Chaperone Chaperonin, ER Quality Control, Glycoprotein Biosynthesis, Glycosyltransferases, Protein Folding, Cosmc, Specific Molecular Chaperone, T-synthase
Abstract
The interaction of the endoplasmic reticulum molecular chaperone Cosmc with its specific client T-synthase (Core 1 β1–3-galactosyltransferase) is required for folding of the enzyme and eventual movement of the T-synthase to the Golgi, but the mechanism of interaction is unclear. Here we show that the lumenal domain of recombinant Cosmc directly interacts specifically in either free form or covalently bound to solid supports with denatured T-synthase but not with the active dimeric form of the enzyme. This leads to formation of a relatively stable complex of Cosmc and denatured T-synthase accompanied by formation of reactivated enzyme in an ATP-independent fashion that is not regulated by redox, calcium, pH, or intermolecular disulfide bond formation. The partly refolded and active T-synthase remains tightly bound noncovalently to Cosmc. Dissociation of T-synthase from the complex is promoted by further interactions of the complex with free forms of either native or non-native T-synthase. Taken together, these results demonstrate a novel mechanism in which Cosmc cycles to bind non-native T-synthase, leading to enzyme activity and release in a client-driven process.
Introduction
Mucin-type O-glycosylation is one of the most common post-translational modifications to animal glycoproteins, and is important in many biological processes (1), but the overall regulation of this complex pathway is poorly understood. It is known that the first step in the pathway is catalyzed by a family of polypeptide-N-acetylgalactosaminyltransferases, which covalently modify Ser/Thr by the addition of N-acetylgalactosamine (GalNAc) (2). The resulting structure is known as the Tn antigen (GalNAcα1-Ser/Thr) and is the common precursor structure to all mucin-type O-glycans (3, 4). Although it is estimated that 20 different polypeptide-N-acetylgalactosaminyltransferases are involved in the first step (5), the common next step in biosynthesis in vertebrates is catalyzed by a single enzyme termed the T-synthase (core 1 β1–3-galactosyltransferase), which adds galactose onto the Tn antigen to form the core 1 disaccharide Galβ1–3GalNAcα1-Ser/Thr (T antigen) (3). The T antigen can be further modified by several downstream glycosyltransferases in the Golgi compartment to form complex O-glycans (3) that are critical in many cellular processes such as animal development, lymphocyte homing (6), and leukocyte trafficking (7–9).
The generation of functional T-synthase requires a specific molecular chaperone termed Cosmc, which promotes the folding and activity of T-synthase both in vivo and in vitro (10, 11). In the absence of functional Cosmc, enzymatically inactive T-synthase aggregates in the endoplasmic reticulum (ER)2 and retrotranslocates back to the cytosol where it is ubiquitinated and degraded by proteasomal machinery (12). Cells lacking Cosmc or T-synthase express the Tn and/or sialyl-Tn antigens, which are known as tumor-associated carbohydrate antigens found in many carcinomas (11, 12). Engineered mutations to delete Cosmc in cell lines uniformly causes formation of the Tn and/or sialyl-Tn antigen on multiple glycoprotein species (13). Acquired mutations in Cosmc give rise to Tn syndrome and Tn antigen in human cancers (12, 14, 15). Importantly, T-synthase activity is in most cell types and T-synthase is coordinately expressed with Cosmc (16). T-synthase is developmentally important and knock-out of this enzyme in mice is embryonic lethal (17), as seen also for Cosmc knock-out mice, which die at an embryonic stage (18).
In principle, chaperones typically interact specifically with unfolded but not with folded proteins, facilitating the formation of native structures (19) and are not part of the mature client protein (20). Several families of chaperones are responsible for protein folding in the ER; those most studied are the HSP70 family member BiP (Grp78), protein-disulfide isomerase, and calnexin/calreticulin families, all of which have different types of chaperone cycles for substrate binding and release (21, 22). Some ER molecular chaperones recognize many clients, but there are a large number of client-specific or client-restricted ER molecular chaperones (22).
Previous studies have shown that Cosmc is ER localized (16, 23, 24) and that Cosmc can promote the reactivation of denatured T-synthase; the accumulated information suggests that the T-synthase is a specific client for Cosmc (10). However, the mechanism of Cosmc function in the ER as a chaperone and its client-specific binding and release are not well understood. Here we present mechanistic insights on how Cosmc acts to promote formation of functional T-synthase and the conditions for binding and release of T-synthase after refolding. Our results support the hypothesis that Cosmc binds reversibly to non-native T-synthase and promotes its folding independent of other chaperones.
EXPERIMENTAL PROCEDURES
Preparation of Different Versions of Cosmc and T-synthase
Soluble N-terminal His6-tagged Cosmc (His-sCosmc) and soluble N-terminal HPC4-tagged T-synthase (HPC4-sT-syn) were prepared as described (10). His-sCosmc and HPC4-sT-syn were produced in pVL1393 vector as described (16). To obtain active T-synthase preparations, asialo-bovine submaxillary mucin (BSM) beads were prepared as described (25) and 500 ml of media containing secreted HPC4-sT-syn was produced as described (10) and mixed with 500 μl of asialo-BSM-conjugated beads in the presence of 20 mm MnCl2 and incubated for 3–4 h. Beads were washed with wash buffer containing 20 mm MnCl2 and protein was eluted by 1 m NaCl in buffer lacking Mn2+ and concentrated. The HPC4-sCosmc preparation was prepared as described (10, 16). His-sT-syn was prepared in pVL1393 using a similar strategy for making His-sCosmc as described in Ref. 10. For mutant T-synthase generation, see supplemental Experimental Procedures.
Preparation of Anti-HPC4 and Cosmc-conjugated Beads
Anti-HPC4 and His-sCosmc-conjugated UltraLink Biosupport beads were prepared following the manufacturer's protocol (Pierce) using ∼1.5 μg/μl of beads unless otherwise stated. In parallel, control beads without protein were prepared.
Characterization of Recombinant HPC4-sT-synthase Using Asialo-BSM Beads
HPC4-sT-syn (1.2 μg) was prepared in 300 μl of buffer (50 mm Tris-HCl, 20 mm MnCl2, 150 mm NaCl, 0.1% Triton X-100, pH 7.0). Two-thirds of the preparation was mixed with 10 μl of asialo-BSM beads and incubated for 20 min on ice. Beads were pelleted (200 × g) and the supernatant was mixed with 10 μl fresh asialo-BSM beads and the process was repeated four times. Supernatant (∼200 μl) was collected. All beads were pooled and washed. All washes were pooled and concentrated. A parallel experiment was conducted for beads alone. One-tenth of the preparation was assayed for T-synthase activity, which was carried out for 30 min, and one-fifth of the total reaction was assayed to determine the activity. One-tenth of the sample was directly boiled and used for SDS-PAGE and Western blotting.
In Vitro Reconstitution Experiments
Recombinant HPC4-sT-syn (0.25 μg) was denatured at ∼55 °C for 2 min in reconstitution buffer (10 mm HEPES, 12 mm MgCl2 at pH 7.8) and cooled to RT followed by addition of a 5 mm ATP final concentration. Renaturation was initiated by the addition of His-sCosmc to a final concentration of 2.27 μm unless otherwise stated and incubated for 45 min. T-synthase activity was measured as described (25, 26). Specific T-synthase activity of native T-synthase was considered 100% T-synthase activity, and all activities were calculated accordingly. For guanidinium hydrochloride refolding experiments of Cosmc, see supplemental Experimental Procedures.
In Vitro Reconstitution Experiments with Cosmc Beads and Pull-down Experiments
HPC4-sT-syn (0.45 μg) was denatured at ∼55 °C for 2 min in reconstitution buffer and cooled to RT. Cosmc beads (20 μl of 50% slurry) were diluted in 100 μl of buffer containing 0.1% Triton X-100 (pH 7.8) with 5 mm ATP or ATPγS final concentrations. In this preparation, renaturation was initiated by the addition of denatured T-synthase and incubated for 45 min at RT. Beads were pelleted (200 × g) and washed. Supernatant and all washes were concentrated. Input, bound, and unbound materials were analyzed by Western blotting against HPC4. Bound and unbound materials were assayed for activity. In this case total activity (bound, supernatant, and wash in relative fluorescence units (RFU) (counts/h)) of native T-synthase was considered 100% and the activity of all experiments was calculated accordingly. For in-solution refolding followed by pull-down experiments, see supplemental Experimental Procedures.
N-Ethylmaleimide (NEM) Reaction
His-sCosmc (28 μm) and NEM (24 mm) were prepared in reaction buffer (100 mm phosphate, 150 mm NaCl, pH 7.2). After incubating overnight at 4 °C, the reaction was passed through a PD10 column equilibrated with 5 mm Tris-HCl (pH 7.8). The molecular mass of both NEM modified and unmodified Cosmc were determined using mass spectrometric analysis (MALDI-TOF).
Sodium Arsenite Reaction
His-sCosmc (15.5 μm) was incubated with DTT (290 μm) for 1 h. The reaction mixture was passed through a PD10 column equilibrated with 5 mm Tris-HCl (pH 7.8). This preparation of His-sCosmc (2.4 μg) was used to refold heat-denatured HPC4-sT-syn (0.25 μg) in the presence of 13 μm sodium arsenite.
Release Experiments
Reactivated HPC4-sT-syn associated with C-beads was prepared as described above using HPC4-sT-syn (2 μg) and 40 μl of His-sCosmc beads (50% slurry, 0.5 μg/μl of the beads). To this preparation, either 1.5 μg of native or heat-denatured His-sT-syn in 25 μl of reconstitution buffer was added. T-synthase was heated at 60 °C for ∼2 min. As a control, ∼3 μg of BSA, which was denatured at ∼65 °C for 15 min, was used. The reaction mixture was incubated at RT for 30 min, mixing every 4–5 min. Beads were centrifuged for 1 min at 1500 × g and allowed to settle on ice for 5 min. Four-fifths of the supernatant was allocated for SDS-PAGE and Western blotting for HPC4. Gels were silver stained to determine the amount of protein used for the elution. Beads were boiled and one-fifth of the mixture was used for SDS-PAGE and Western blot analysis. Dose studies were conducted using 0.2, 0.5, and 2.0 μg of heat-denatured His-sT-syn and ∼0.60 ng and ∼2.0 μg of native His-sT-syn. For preparation of native T-synthase passed through Cosmc beads and preparation of guanidinium hydrochloride treated T-synthase, see supplemental Experimental Procedures. For release experiments using these preparations, see supplemental Experimental Procedures.
Quantification of Western Blot Data
Western blot films were scanned using a Cannon scanner and bands were quantified using an Alphatech system.
Cross-linking
Proteins were prepared in 10 mm HEPES, 150 mm NaCl, 12 mm MgCl2 (pH 7.8) and cross-linking was carried out using bis(sulfosuccinimidyl)suberate (BS3) (Thermo Scientific) at 1 mm final concentration. The reaction was incubated for 30 min at RT and quenched using 1 m Tris-HCl for 15 min, which was followed by SDS-PAGE analysis and Western blot.
RESULTS
Cosmc Does Not Promote Activity of Native T-synthase but Promotes Refolding of Denatured T-synthase
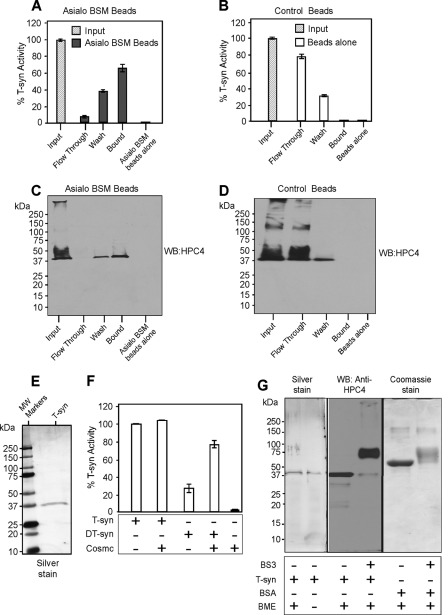
In our earlier studies on the interactions between Cosmc and T-synthase we found that His-tagged soluble Cosmc (His-sCosmc) containing only the lumenal domain of the protein directly promotes the activity of non-native soluble T-synthase (HPC4-sT-syn) (10). However, we and others noted that addition of recombinant Cosmc to a preparation of active T-synthase slightly increased its activity (10, 27), suggesting that either Cosmc may bind to native T-synthase and further activate it or T-synthase preparations may contain some non-native T-synthase. To address these possibilities, we purified enzymatically recombinant, active, HPC4-sT-syn by incubating it with immobilized asialo-BSM, an affinity support useful for purifying active T-synthase in the presence of Mn2+ (25). The majority of the HPC4-sT-syn bound to asialo-BSM beads (Fig. 1, A and C), whereas the HPC4-sT-syn preparation did not bind to control beads (Fig. 1, B and D). Analysis showed that the enzyme was purified to apparent homogeneity (Fig. 1E). As shown in Fig. 1F, His-sCosmc does not enhance the activity of this highly purified HPC4-sT-syn, whereas His-sCosmc significantly restored the activity of heat-denatured HPC4-sT-syn (DT-syn) (Fig. 1F). These results demonstrate that Cosmc does not affect purified native T-synthase and suggest that prior studies showing some enhancement of activity were due to the presence of non-native forms of T-synthase in such preparations.
FIGURE 1.
Characterization of recombinant HPC4-T-synthase and His-sCosmc. HPC4-sT-syn was co-expressed with wild type membrane-bound Cosmc in Hi-5 insect cells and His-sCosmc was also expressed in the same system. Both tagged proteins were purified from the media. A-D, HPC4-sT-syn preparation was characterized for its activity with asialo-BSM beads or beads alone. Pulldown experiments were carried out and HPC4-sT-syn bound to asialo-BSM beads as determined either by activity of T-synthase (A and B) or Western blotting (WB) against HPC4 (C and D). In A and B, two replicate experiments were performed, and the data represents the average of the two independent experiments. Error bars ± 1 S.D. from the average. C and D show a representative example of two independent experiments. E, recombinant HPC4-sT-syn (T-syn) purified with asialo-BSM beads was resolved by SDS-PAGE and visualized by silver stain. F, in vitro refolding shows that Cosmc restores the activity of asialo-BSM purified heat-denatured HPC4-sT-syn (DT-syn) but not the activity of the T-syn. Each experiment was performed in duplicate, two replicate experiments were performed, and the data represents the average of all experiments. Error bars ± 1 S.D. from the average. G, silver-stained gel of HPC4-T-synthase under reducing or non-reducing conditions. HPC4-T-synthase (0.25 μg) was cross-linked by using BS3 cross-linker followed by SDS-PAGE and Western blot for HPC4. In parallel, control BSA (6 μg) was treated with BS3 and analyzed by SDS-PAGE followed by Coomassie stain. BME, β-mercaptoethanol.
Our prior studies of T-synthase purified from rat liver showed that it occurred as both a disulfide-bonded dimer and as a monomer that was perhaps in equilibrium with a noncovalent dimeric species (25). To explore whether the soluble form of T-synthase exists as a dimer or monomer, we used a chemical cross-linking approach. In reducing or non-reducing SDS-PAGE the HPC4-sT-syn behaved as a monomeric species of ∼38 kDa, but addition of the cross-linker BS3 caused T-synthase to migrate as a species of ∼76 kDa, which is similar to the predicted size of the dimer (Fig. 1G). Control studies with BSA showed that BS3 caused a decrease in the electrophoretic mobility of BSA without generating dimeric species. A likely explanation for the reduction in mobility of cross-linked species of BSA could be the addition of several molecules of BS3 onto the BSA, which can alter its charge and mobility (28). These results suggest that the recombinant soluble form of T-synthase is primarily a noncovalent dimer in solution, and consistent with cross-linking and formation of dimeric species of native membrane-bound T-synthase in cells (16).
Cosmc Forms Stable Complex with Non-native but Not with Native T-Synthase
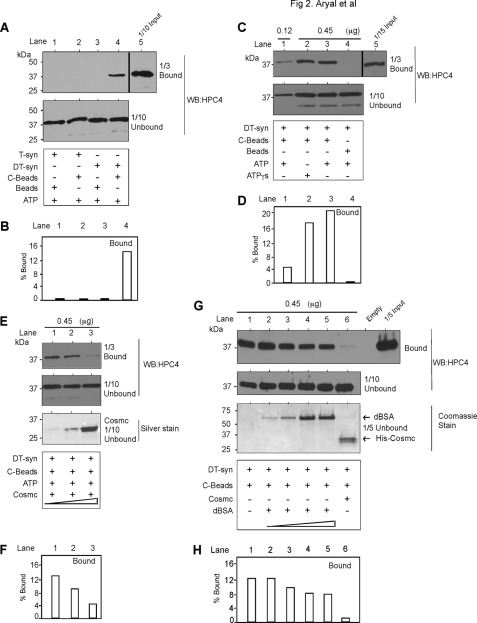
Molecular chaperones bind to non-native, but not native proteins, to form a stable complex leading to productive folding (20). Because Cosmc is a transmembrane protein in the ER, we immobilized Cosmc to approximate its interactions with T-synthase in the ER. For this we covalently coupled His-sCosmc to Ultralink beads (C-beads) and assayed for its direct interaction with DT-syn versus native HPC4-sT-syn (T-syn) in pulldown studies. We tested for association using the bead-associated T-syn by boiling the bead complex and performing Western blots on the released material using the anti-HPC4 monoclonal antibody. A portion of DT-syn was associated with C-beads (Fig. 2, A, lane 4, quantification in B, lane 4), whereas there was no association of native T-syn (Fig. 2A, lane 2). Neither DT-Syn (Fig. 2A, lane 3) nor T-syn (Fig. 2A, lane 1) bound nonspecifically to the control beads. Importantly, increasing the addition of DT-syn with C-beads showed that more was bound in a concentration-dependent manner (Fig. 2, C, lanes 1 and 3, quantification in D, lanes 1 and 3), and up to ∼20% of the input DT-syn was bound. There was no significant nonspecific binding of DT-syn to beads alone (Fig. 2C, lane 4). Furthermore, the binding of DT-syn with His-sCosmc-conjugated beads was competitive with free, His-sCosmc in a dose-dependent manner (Fig. 2, E, lanes 1–3, quantification in F, lanes 1–3). Importantly, as a control for specificity, the binding of DT-syn with His-sCosmc-conjugated beads was not competitive with increasing concentrations of free, denatured BSA (Fig. 2, G, lanes 2–5, quantification in H, lanes 2–5), as compared with free His-sCosmc (Fig. 2, G, lane 6, quantification in H, lane 6). These results show that denatured T-synthase is specifically bound by Cosmc and not perturbable by excesses of denatured BSA, and that immobilized Cosmc does not bind to native T-synthase.
FIGURE 2.
Cosmc directly interacts with denatured T-synthase but not with native T-synthase. Reconstitution was initiated by the addition of His-sCosmc-conjugated beads (C-beads) to the preparation of heat-denatured HPC4-sT-syn (DT-syn) and incubated for 45 min. After incubation, beads were pelleted, washed, and analyzed by Western blotting (WB) for HPC4. A parallel experiment was conducted with native HPC4-sT-syn (T-syn). A, DT-syn directly interacts with His-sCosmc but not with T-syn. Bound and unbound materials were analyzed to determine the amount of HPC4-sT-syn bound to C-beads. B, quantification of bound material from A. C, His-sCosmc directly interacts with DT-syn in a concentration-dependent manner. Two different concentrations of DT-syn were incubated with a constant amount of C-beads. After pulldown followed by washing, bound and unbound materials were analyzed by Western blotting (WB) for HPC4. D, quantification of bound material from C. E, His-sCosmc can compete with C-beads for binding to DT-syn. Reconstitution of DT-syn was initiated by the addition of C-beads in the presence of different concentrations of soluble His-sCosmc (4, 10, and 30 μg). F, quantification of bound material from E. G, denatured BSA (dBSA) cannot compete with C-beads for binding to DT-syn. Reconstitution of DT-syn was initiated as in E except for the double amount of C-beads in the presence of varied concentration of denatured BSA (buffer alone, 4, 10, 30, and 45 μg) and 30 μg of soluble Cosmc. H, quantification of bound material from G. Data are a representative example of two independent experiments. A vertical line separates the data from different parts of the same gel and of the same experiment. Input is considered 100% and accordingly % bound was calculated in all quantification data. C and E are from the same gel, therefore input for C and E are the same.
Cosmc Associates Tightly with Reconstituted Active T-synthase
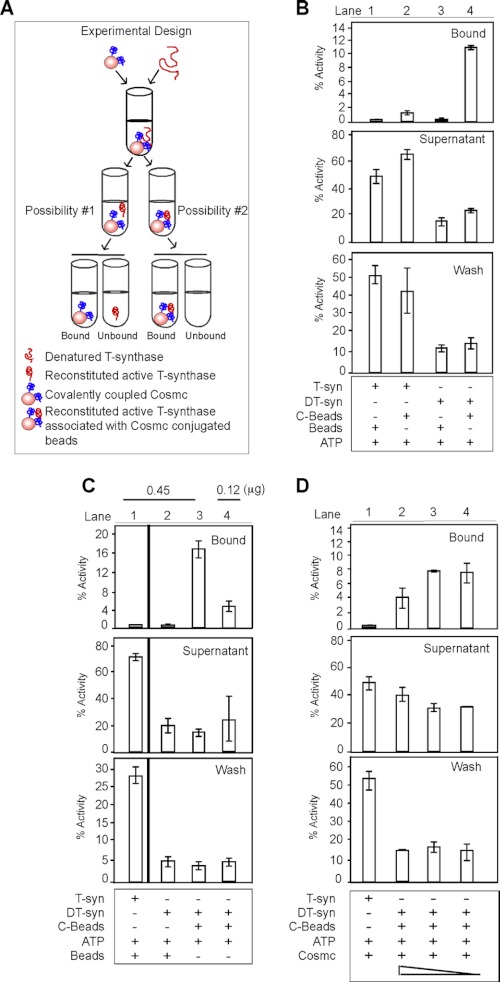
To explore the potential cycle of binding and release of T-synthase to Cosmc, we envisioned two possibilities. Cosmc may facilitate the refolding of non-native T-synthase to active enzyme that is subsequently released from Cosmc, or the refolded T-synthase may remain associated with Cosmc and require some other factors for its efficient release. To distinguish between these possibilities, we performed in vitro reconstitution of DT-syn with C-beads and analyzed both the bound and unbound fractions by assaying whether the C-beads functionally enhanced the activity and refolding of DT-syn and the effects on binding or release (Fig. 3A). The majority of the reconstituted active HPC4-sT-syn, but not native T-syn, was associated with C-beads (Fig. 3B, lanes 2 and 4). As controls, neither T-syn nor DT-syn bound nonspecifically to the beads as measured by T-synthase activity (Fig. 3B, lanes 1 and 3). These results demonstrate that immobilized Cosmc is a functional chaperone that can reactivate DT-syn, and that the reactivated DT-syn remains associated with Cosmc. Increasing the amount of DT-syn with a constant amount of C-beads resulted in increased activity associated with C-beads (Fig. 3C, lanes 3 and 4). Furthermore, increasing concentrations of His-sCosmc during the course of the reconstitution reaction decreased the activity of T-synthase associated with C-beads (Fig. 3D, lanes 2–4). These data demonstrate that immobilized Cosmc forms a relatively stable complex with catalytically active reactivated T-synthase and that dissociation of the enzyme from the complex may require some other step(s) or factor(s).
FIGURE 3.
Cosmc is associated with catalytically active T-synthase. A, schematic of experimental design depicting in vitro reconstitution followed by pulldown experiments. Renaturation of DT-syn followed by pulldown experiments was carried out as described in the legend to Fig. 2. Beads, supernatant, and wash were analyzed for T-synthase activity. B, His-sCosmc directly interacts with reactivated HPC4-sT-syn (lane 4) but not with T-syn (lane 2). A parallel experiment was carried out with native HPC4-sT-syn (T-syn). C, His-sCosmc reconstitutes DT-syn in a dose-dependent manner. A vertical line separates the activity of T-synthase determined separately. D, His-sCosmc competes with C-beads for reconstitution of DT-syn. Reconstitution of DT-syn was initiated by adding a constant amount of His-sCosmc beads containing different concentrations of His-sCosmc as described in the legend to Fig. 2E. A representative example of two independent experiments performed in duplicate is shown. Error bars ± 1 S.D. from the average of duplicate experiments.
To further confirm the observation that reactivated T-synthase associates with Cosmc, we initiated solution-based refolding of the DT-syn to address whether the conjugation of Cosmc to beads might affect T-syn binding and release from Cosmc, as well as whether the epitope tags themselves might alter the release function. Thus, we switched the epitope tags for each recombinant protein and generated His-sT-syn and HPC4-sCosmc; both were purified to apparent homogeneity (supplemental Fig. S1, B and C). The solution-based refolding experiments were conducted as shown schematically by first incubating HPC4-sCosmc with denatured His-sT-syn and allowing for refolding (supplemental Fig. S1A). This was followed by pulldown experiments using anti-HPC4 beads to define whether active HPC4-sT-syn remained associated with His-sCosmc. Consistent with our findings above, reconstituted active His-sT-syn remained associated with HPC4-sCosmc (supplemental Fig. S1D, lane 3). These data demonstrate that reconstitution of T-synthase activity is not affected by conjugation of Cosmc to beads versus in solution, and that the interaction is not affected by either type of epitope tag employed.
ATP Does Not Cause Release of Reconstituted Active T-synthase Associated with Cosmc
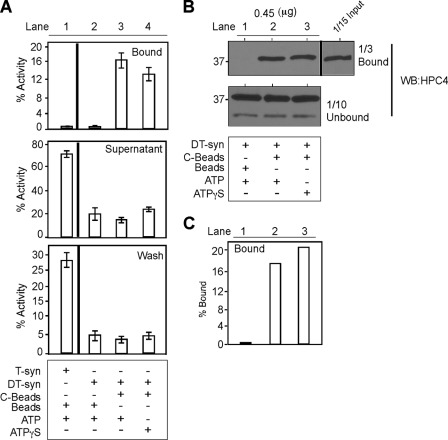
For many chaperones, ATP binding triggers a change in conformation of the chaperone leading to release of the substrate (19, 29). We reported previously that Cosmc binds to ATP, although neither ATP nor ATPγS had a significant difference in the in vitro refolding of heat-denatured HPC4-sT-syn by His-sCosmc (10), but we did not test whether the refolded T-synthase is released by ATP or ATPγS. Interestingly, we found that neither ATP nor ATPγS had a significant effect in either the activity or release of T-synthase (T-syn) (Fig. 4A, lanes 3 and 4). In a parallel experiment, we analyzed the amount of protein in the bound and unbound fractions by Western blotting for HPC4-sT-syn, however, we saw no significant differences (Fig. 4, B, lanes 2 and 3, quantification in C, lanes 2 and 3). We also considered the remote possibility that these results might be confounded by endogenous ATP bound to the His-sCosmc from the cells used for Cosmc expression. To remove potentially bound ATP, we denatured the purified His-sCosmc with 6 m guanidinium hydrochloride (GdmHCl) to cause unfolding. Interestingly, upon removal of GdmHCl, His-sCosmc refolded to an active form that was capable of promoting the reactivation of DT-syn (supplemental Fig. S2). These data show that in vitro Cosmc remains associated with reconstituted active T-synthase with or without ATP. Furthermore, the refolding of non-native His-sCosmc appears to be spontaneous and does not require other factors, in contrast to T-synthase, which shows no apparent spontaneous, independent folding and acquisition of enzyme activity after hours of incubation alone.
FIGURE 4.
ATP is not important in the functional cycle of Cosmc. Reconstitution of heat-denatured HPC4-sT-syn was carried out in the presence of ATP or ATPγS to investigate whether ATP regulates substrate binding and releasing. As described in the legend to Fig. 3A, in vitro reconstitution followed by pulldown experiments were carried out and analyzed by Western blot (WB) and activity. A, ATP does not cause the release of reactivated HPC4-sT-syn from C-beads. The graph shows a representative example of two independent experiments done in duplicate. Error bars ± 1 S.D. from the average of duplicate experiments. A vertical line separates the activity of T-synthase determined separately. B, ATP does not affect the binding of DT-syn to His-sCosmc, after reconstitution beads were pelleted, washed, and analyzed by Western blotting for HPC4. Representative example of two independent experiments. C, quantification of Western blot data in B.
Cosmc Folds T-synthase Independently of Intermolecular Disulfide Bond Formation
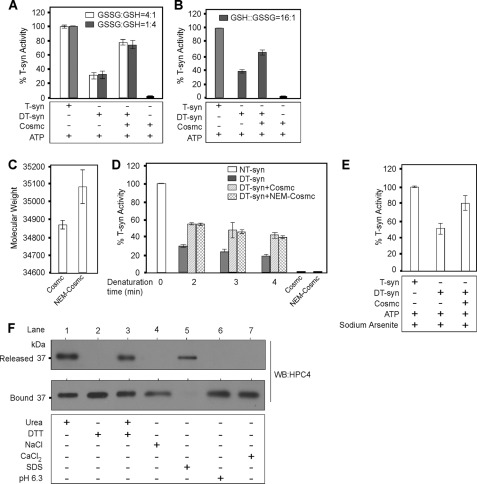
It has been speculated that Cosmc-dependent folding of the T-synthase might require disulfide bond formation (30). To test whether the reactivated T-synthase might covalently bind to Cosmc through disulfide bonds, thereby explaining its tight association after refolding, we performed refolding experiments in the millimolar range of reduced (GSH) and oxidized (GSSG) glutathione with a GSH:GSSG ratio ranging from reducing 4:1 to oxidizing 1:4 environment. Interestingly, we found that Cosmc functions in refolding DT-syn in either a reducing and oxidizing environment (Fig. 5A). Furthermore, even in an increased reducing environment (GSH:GSSG, 16:1), Cosmc restored the activity of DT-syn (Fig. 5B). We further tested the role of Cys residues by chemically alkylating all accessible Cys residues of His-sCosmc using NEM to prevent the potential reformation of disulfide bridges between Cosmc and T-synthase. Analysis by MALDI-TOF spectrometry showed that alkylation increased the size of Cosmc from 34,868 to 35,078 (a single NEM addition is predicted to add 125 mass), suggesting that Cosmc may have two free cysteines (Fig. 5C). Importantly, this NEM-derivatized form of His-sCosmc (NEM-Cosmc) was active in restoring the activity of DT-syn (Fig. 5D). Because Cosmc may have vicinal thiols, we used arsenite to test their potential role in the refolding of denatured T-synthase by Cosmc; arsenite reacts with dithiols and forms a relatively stable cyclic compound that blocks thiol availability. Treatment of Cosmc with arsenite did not affect its ability to cause reactivation of DT-syn (Fig. 5E). These results demonstrate that the refolding of heat-denatured T-synthase by His-sCosmc is not regulated by redox in the range tested and that Cosmc refolds denatured T-synthase without the formation of intermolecular disulfide bonds.
FIGURE 5.
Cosmc folds T-synthase independently of intermolecular disulfide bond formation. A–C, reconstitution of DT-syn was carried out with purified His-sCosmc in either an oxidizing environment or reducing environment for 45 min and directly assayed for T-synthase activity. A, reconstitution of DT-syn in reducing environment GSSG:GSH (1:4) or oxidizing environment GSSG:GSH (4:1). Data are the average of three different experiments each performed in duplicate. B, reconstitution was carried out in the reducing environment containing GSH:GSSG (16:1). Data are the average of duplicate experiments. C, mass spectrometry analysis of His-sCosmc with or without NEM modification. Data are the average of four independent mass spectrometry measurements. D, purified His-sCosmc or NEM modified His-sCosmc were incubated with DT-syn heated for different times at 55 °C and allowed to refold, and T-synthase activity was measured. Data are the average of two independent experiments performed in duplicate. E, refolding experiment carried out as in B, in the presence of 13 mm sodium arsenite. Data are the average of duplicate experiments. F, reactivated T-syn associated with C-beads was prepared as described in the legend to Fig. 3A except that reconstitution was carried out with 40 μl of His-sCosmc beads (50% slurry, ∼0.5 μg/μl of the beads). The bound materials were treated separately with 8 m urea, 10 mm DTT, 2 mm CaCl2, 1 m NaCl, 0.1% SDS (pH 6.3) reconstitution buffer for 45 min at RT. Unbound and bound fractions were analyzed by Western blotting (WB) for HPC4. The data are the representative examples of two independent experiments. Error bars ± 1 S.D. from the average.
Interestingly, T-synthase, but not Cosmc, contains a thioredoxin (CXXS)-like motif (31). We asked whether this motif might have a role in the refolding process. For this purpose, we expressed and purified a mutant version of T-synthase (HPC4-mT-syn) (CXXS to SXXS) (mT-syn) (supplemental Fig. S3A). The in vitro refolding studies show that His-sCosmc can restore the activity of the denatured mT-syn (dmT-syn) (supplemental Fig. S3B). Taken together, these results demonstrate that the CXXS motif of T-synthase is not involved in the refolding process in vitro and further confirm that disulfide bonds do not form between Cosmc and T-synthase during the refolding process.
To further define whether there is some other potential covalent interaction between Cosmc and reactivated T-syn, we treated the complex of the proteins with either 8 m urea, 10 mm DTT, 1 m NaCl, or 0.1% SDS. We found that treatment by either 8 m urea or 0.1% SDS caused the release of T-synthase protein from C-beads (Fig. 5F, lanes 1 and 5), whereas neither 10 mm DTT nor 1 m NaCl had an effect (Fig. 5F, lanes 2 and 4). Because some ER chaperones, such as Hsp47 and RAP, are regulated by the physiological pH of Golgi and ER (32, 33), and other chaperones such as Hsp90, protein-disulfide isomerase, ERp72, Calreticulin, and p50 are regulated by calcium (34), we tested the potential role of physiological pH and calcium to release reactivated T-synthase from His-sCosmc. We found that Cosmc and renatured T-synthase remained associated upon treatment at pH 6.3 or with excess calcium (Fig. 5F, lanes 6 and 7). These results demonstrate that the interaction between reactivated T-synthase and Cosmc is noncovalent and not regulated by either pH or calcium in the physiologic range.
Both Native and Denatured T-synthase Can Cause Release of Reconstituted Active T-synthase Bound to Cosmc
The interaction of Cosmc with T-synthase is highly specific, and Cosmc does not bind to native T-synthase. Thus, we considered two major possibilities. One is that release of reactivated T-synthase from its complex with Cosmc might be induced by an additional client protein in a client-dependent manner (possibility 1). Such a process has been observed for other chaperones, such as AHSP (α-hemoglobin stabilizing protein), which acts in a reversible fashion to bind and release α-Hb to β-Hb to form αβ-Hb and recycles to rebind more α-Hb (35, 36). The alternative possibility is that more T-synthase binds to Cosmc, and no release of bound T-synthase is observed (possibility 2).
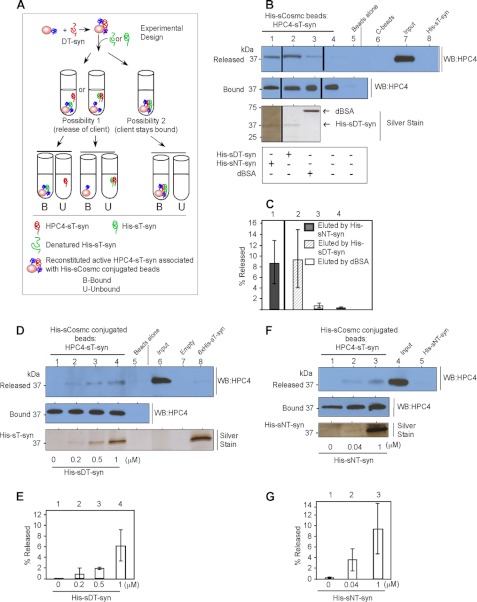
The experimental design to test these two possibilities is shown in Fig. 6A. First, we asked whether the addition of denatured T-synthase to C-beads preloaded with reactivated T-synthase promoted dissociation of the bound client using two different versions of T-synthase: HPC4-sTsyn and His-sT-syn. First, we prepared reactivated HPC4-sT-syn associated with C-beads using the in vitro reconstitution experiments. We then incubated this preparation of reactivated HPC4-sT-syn associated with C-beads with either native or denatured His-sT-syn and determined whether any HPC4-sT-syn was released. We found that both native and denatured His-sT-syn promoted the release of HPC4-sT-syn from C-beads, as determined by Western blot against HPC4 (Fig. 6, B, lanes 1 and 2 released, quantification in C, lanes 1 and 2). As controls, denatured BSA and buffer alone were ineffective in causing release of HPC4-sT-syn from C-beads (Fig. 6, B, lanes 3 and 4 released, quantification in C, lanes 3 and 4). The release was dose dependent with increasing amounts of either DT-syn (Fig. 6, D, lanes 2–4, quantification in E, lanes 2–4) or NT-syn (Fig. 6, F, lanes 2 and 3, quantification in G, lanes 2 and 3). The release of HPC4-sT-syn from Cosmc beads was not enhanced by incubating denatured His-sT-syn for a longer time, up to 4 hours (supplemental Fig. S4). These data demonstrate that reactivated T-synthase associated with Cosmc is dissociated from Cosmc upon interaction with additional T-synthase in a client-dependent and -specific manner.
FIGURE 6.
Both native and denatured T-synthase can cause release of the reconstituted active T-synthase from Cosmc. A, schematic diagram of in vitro release experiment. B, both native and denatured T-synthase can alter the equilibrium between Cosmc and reconstituted active T-synthase but not of denatured BSA or buffer alone. Reactivated T-syn associated with Cosmc beads were incubated with native, heat-denatured His-sT-syn, heat-denatured BSA, or buffer alone. Supernatant and bound fractions were analyzed by Western blotting (WB) against HPC4. The silver-stained gel shows the amount of T-synthase and BSA used in the experiment. A vertical line separates the data taken from different parts of the same gel and the same experiment except the vertical line between 1 and 2, which separates the experiment done at different times. C, quantification of eluted fraction (Released) of Western blot data as compared with Input from B. Data are an average of three independent experiments. Error bars ± 1 S.D. from the average of three independent experiments. D, denatured His-sT-syn releases reactivated HPC4-sT-syn from Cosmc in a concentration-dependent manner. Reactivated T-syn associated with Cosmc beads was incubated with different concentrations of DT-syn. Eluted fractions (Released) and the amount of protein remained on the beads (Bound) were analyzed by Western blotting for HPC4. The silver-stained gel shows the amount of heat-denatured His-sT-syn used. F, native His-sT-syn releases reactivated HPC4-sT-syn from Cosmc in a concentration-dependent manner. As described in D, in vitro reconstitution followed by pulldown experiments were carried out and analyzed by Western blot to determine the amount released and the silver-stained gel to show the amount of His-sT-syn used for elution. E and G, quantification of eluted fraction (Released) as compared with Input. Data are an average of two independent experiments. Error bars ± 1 S.D. from the average of two independent experiments.
Because these results show that both native and non-native T-synthase can cause release of Cosmc-associated reactivated T-synthase, we considered the possibility that the native T-synthase preparation might have some denatured T-synthase within it, and conversely, that the heat-denatured T-synthase preparation might have some portion of native T-synthase. To address this, we prepared an inactive form of T-synthase using GdmHCl to denature the enzyme (10). This preparation, which had <5% initial enzyme activity, was effective in causing release of the reactivated T-synthase associated with Cosmc beads (supplemental Fig. S5, lane 4). Moreover, we incubated the native T-synthase with Cosmc beads to remove any forms of T-synthase that might be non-native and capable of binding Cosmc. This native T-synthase, which was not bound by preincubation with Cosmc beads, was equivalently potent to the GdmHCl-denatured T-synthase in causing release of the bound reactivated T-synthase (supplemental Fig. S5, lane 3). These results show that both native and denatured T-synthase client molecules can cause release of reactivated T-synthase from its complex with Cosmc and demonstrate that the interaction between Cosmc and reactivated T-synthase is reversible during the chaperone cycle and client-driven.
DISCUSSION
Our results show that soluble Cosmc directly interacts in a specific manner with denatured, but not native, T-synthase to form a noncovalent and reversible complex that results in the acquisition of T-synthase catalytic activity. In addition, the release of the T-synthase from the Cosmc-T-synthase complex in vitro is promoted by both non-native and native T-synthase, whereas ATP, calcium, and pH, which are known regulators of several ER chaperones, do not promote release. These results provide new information about the specific mechanisms of Cosmc function, and also provide insight into the quality control systems for generation of functional T-synthase.
The T-synthase is a typical type II transmembrane protein with a short N-terminal cytoplasmic domain, a 26-amino acid transmembrane domain, and a 331-amino acid functional lumenal domain (37). Type II membrane proteins fold in the ER during co-translational insertion where they encounter many co-translational controls, which include simultaneous tethering of their N- and C-terminal sequences during biosynthesis, translation rate, interactions with chaperones, and post-translational modifications, including N-glycosylation and formation of disulfide bonds (22). In all of these processes there are multiple quality control checkpoints to limit accumulation of misfolded or misassembled proteins, but the overall nature of the quality control system in the ER is poorly understood. Intermolecular interactions between proteins of identical sequence, so-called domain or strand swapping, can lead to protein aggregation and misfolding (38, 39). Misfolding or misassembly of proteins in the ER can result in their retrotranslocation back into the cytoplasm, where they become ubiquitinated and degraded by the 26 S proteasome, as we showed previously for the T-synthase in the absence of Cosmc (16).
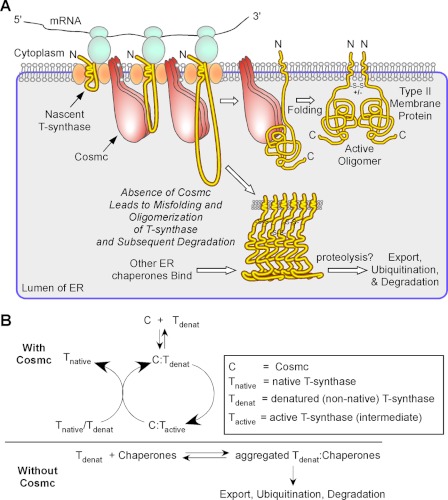
The physiological importance of these processes, and in particular the role of Cosmc in T-synthase folding, was initially revealed by studies in patients with Tn syndrome, who exhibit acquired loss-of-function mutations in the X-linked Cosmc gene, resulting in loss of functional T-synthase (14, 40). Studies in cell lines showed that loss of Cosmc results in efficient ubiquitin/proteasomal degradation of T-synthase, and that drug blockade of degradation results in the accumulation of inactive aggregates of T-synthase. The in vitro studies reported here and elsewhere (16, 18, 23, 24), together with recent evidence that Cosmc is required in cells for co-translational biosynthesis of T-synthase (30), suggests a model for T-synthase biosynthesis shown in Fig. 7. We propose that the interaction between Cosmc and unfolded T-synthase in the ER results in the formation of a stable noncovalent complex, and that within this complex the T-synthase is prevented from forming oligomeric complexes where folding and maturation to active dimeric enzyme are hindered. Thus, in the absence of Cosmc the T-synthase will accumulate and eventually be exported from the ER and degraded in a ubiquitin/proteasome-dependent manner. We also showed previously that the accumulated and misfolded T-synthase oligomers are co-precipitable with ER chaperones (16), such as BiP, which are probably involved in the unfolded protein response to the accumulated T-synthase and may be important in its eventual exit from the ER. By contrast, in the presence of Cosmc, the stable complex between T-synthase and Cosmc results in folding of T-synthase to an active enzyme, perhaps a monomer, which is released from the complex through competition with additional unfolded or newly synthesized T-synthase and forms active dimers themselves or by the assistance from Cosmc. Additionally, released monomers could form dimers with reactivated T-synthase associated with Cosmc, thereby promoting the release of reactivated T-synthase.
FIGURE 7.
A model of Cosmc function in the biosynthesis of T-synthase. A, a working model is presented for Cosmc function in T-synthase biosynthesis. It is proposed that the ER-localized Cosmc directly interacts with co-translationally translocated non-native T-synthase and promotes its refolding independently of other factors. Cosmc forms a transient complex with active T-synthase and active T-synthase is released from Cosmc complex when Cosmc interacts with other co-translationally translocated non-native T-synthase or native T-synthase for another cycle of refolding. Active dimeric T-synthase exits to the Golgi apparatus. In the absence of functional Cosmc, the T-synthase aggregates and is degraded by proteasomal machinery. B, simplified equilibria and folding kinetics of T-synthase in the presence and absence of Cosmc relative to the fate of correctly or incorrectly folded T-synthase.
This dimerization process for the T-synthase could happen spontaneously or with the assistance from Cosmc. In cells lacking Cosmc no dimeric forms of T-synthase accumulate, and only high molecular aggregates, prior to their proteasomal degradation, accumulate (16). Interestingly, Cosmc and T-synthase share a potential common ancestor, and show 26% overall sequence identity. Whether this similarity in sequence is related in some way to their potential interactions is unknown, because Cosmc does not bind to native T-synthase. Such information about the potential dimeric interfaces of active T-synthase will hopefully be forthcoming upon crystallization of the protein in the future. It is also possible that T-synthase might have two independent binding domains, one for Cosmc and the other for its dimerization. In such a case, as soon as dimeric T-synthase forms, stable dimeric T-synthase dissociates from Cosmc. Furthermore, the current data suggest that at least in vitro, Cosmc alone is sufficient to bind to and facilitate folding of T-synthase independent of other factors. Thus, in the absence of Cosmc in patients with Tn syndrome, other ER chaperones are not able to rescue T-synthase, but are more likely involved in its degradation and removal. Such a specific requirement of Cosmc to make functional T-synthase both in vitro and in vivo might be partly explained by their nature of similarity.
The ability of Cosmc to discriminate between native T-synthase, to which it does not bind, and non-native T-synthase, to which it binds tightly, suggest that there may be unique structural elements exposed in the non-native structure. The dissociation of the Cosmc complex with denatured T-synthase by urea, but not by DTT and other dissociative reagents, show that the interactions are noncovalent. The exact nature of these structural elements within T-synthase remain to be identified, but they are likely to be inherent in vertebrate T-synthase, which requires Cosmc for function, but absent in invertebrate T-synthases, as for Caenorhabditis elegans (41) or Drosophila (42, 43), which do not require Cosmc for function. In addition, unlike the invertebrate T-synthases (41), the vertebrate T-synthase is not N-glycosylated, so ER folding pathways involving N-glycan recognition are not involved in vertebrate T-synthase folding (44). The ability of Cosmc to associate with non-native T-synthase appears to be similar in general to that of other molecular chaperones including Hsp70 (45), Cpn60 (GroEL) (46), and DnaK (47) with their substrates, although these chaperones are not client-specific. Interestingly, studies by others (27), as well as our own (10), had shown that recombinant Cosmc slightly enhances the activity of T-synthase preparations, which suggested the remote possibility that Cosmc and T-synthase may form a weak heterodimer or hetero-oligomer for maximal activity. However, our results definitively show that highly purified T-synthase, obtained by affinity chromatography on asialo-BSM, does not interact with Cosmc and addition of Cosmc does not affect the activity of the highly purified T-synthase. Thus, no such heterodimer form of mature T-synthase and Cosmc occurs.
Unexpectedly, we found that Cosmc and heat or chemically denatured T-synthase form a relatively stable complex, consistent with a role for Cosmc in preventing irreversible aggregation of non-native T-synthase. Similar stable complexes between chaperones and their substrates have been reported, such as α-crystalline and carbonic anhydrase (48), and GroEL and ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) (46). We also showed that Cosmc remains associated with the reconstituted catalytically active form of T-synthase. Such a stable complex might appear to be inconsistent with one of the characteristics of molecular chaperones, which is that they do not form part of the final functional structure (20). However, our finding that further addition of denatured or native T-synthase to the complex of Cosmc with reconstituted T-synthase causes dissociation of the bound T-synthase, strongly suggests that the Cosmc-associated T-synthase represents a transitional stage in the folding process, because the native form of T-synthase does not bind to Cosmc. Such transitional intermediates in protein folding in association with chaperones are difficult to define and further work in this direction is needed.
We demonstrated that Cosmc facilitates reacquisition of activity in denatured T-synthase even in reducing environments, suggesting that Cosmc is not a redox-regulated chaperone. Although ATP regulates many chaperone cycles and Cosmc binds to ATP (16, 49), we saw no effect of either ATP or ATPγS in our in vitro experiments. Furthermore, refolded Cosmc following GdmHCl denaturation could refold independently and could reactivate heat-denatured T-synthase, further suggesting that Cosmc action in vitro is not dependent on ATP. Interestingly, some ER chaperones, such as Hps47, are regulated by pH; the chaperone binds and releases the substrate by exploiting the change in pH from the ER to the Golgi environment (33). Additionally, chaperones such as Hsp90, protein-disulfide isomerase, ERp72, Calreticulin, and p50 are regulated by calcium (34). However, our results show that within the physiological range of pH and calcium, the complex of Cosmc and reconstituted T-synthase do not easily dissociate, which demonstrates that Cosmc is not regulated by either pH or calcium in its chaperone functions.
The identified role of Cosmc as a specific regulator of T-synthase formation and acquisition of activity reflects the growing understanding of molecular regulators in forming glycosyltransferases, which are mostly type II transmembrane proteins. The formation of this class of enzymes in the ER and their movements to other organelles in the secretory pathway may require a suite of chaperones and additional proteins for correct folding/localization/activity, as shown recently for chitin synthase III, which requires Chs7p to prevent oligomerization and allow COPII vesicle export from the ER (50), the Drosophila Golgi β4GalNAcTB, which requires GABPI (51), the ER proteins POMT1/POMT2, which are both required for functional O-mannosyltransferase (52), Golgi EXT1/EXT2, which are both required for functional heparin sulfate biosynthesis (53), Golgi β4GalT-V and -VI, which require membrane factors to function in transferring Gal from UDP-Gal to Glc (54), and the Golgi GnT1IP, which is an inhibitory protein of GnT1 and is required for expression of high mannose-type N-glycans in testis (55). However, mechanistically little is known about such systems, and it is possible that other glycosyltransferases may need chaperones or co-factors to regulate their activities, but clearly more research is needed. Our studies on Cosmc and its specific interactions with T-synthase provide detailed insights into the specific mechanisms of how such a specific chaperone functions to generate its active client enzyme and may be relevant to studies of biosynthesis of other glycosyltransferases and ER/Golgi resident proteins.
Supplementary Material
Acknowledgment
We thank Dr. Jamie Heimburg-Molinaro for manuscript editing.
This work was supported, in whole or in part, by National Institutes of Health Grant RO1 GM068559 (to R. D. C.).

This article contains supplemental “Experimental Procedures” and Figs. S1–S5.
- ER
- endoplasmic reticulum
- NEM
- N-ethylmaleimide
- HPC
- human protein C
- Cosmc
- core 1 β3-galactosyltransferase specific molecular chaperone
- T-synthase
- core 1 β1–3-galactosyltransferase
- GdmHCl
- guanidinium hydrochloride
- BiP
- binding protein
- His-sCosmc
- soluble N-terminal His6-tagged Cosmc
- HPC4-sT-syn
- soluble N-terminal HPC4-tagged T-synthase
- BSM
- bovine submaxillary mucin
- DT-syn
- heat-denatured HPC4-sT-syn
- BS3
- bis(sulfosuccinimidyl)suberate
- C-beads
- His-sCosmc covalently coupled to ultralink beads.
REFERENCES
- 1. Hang H. C., Bertozzi C. R. (2005) The chemistry and biology of mucin-type O-linked glycosylation. Bioorg. Med. Chem. 13, 5021–5034 [DOI] [PubMed] [Google Scholar]
- 2. Brockhausen I., Schachter H., Stanley P. (2009) in Essentials of Glycobiology (Varki A., Cummings R. D., Esko J. D., Freeze H. H., Stanley P., Certozzi C. R., Hart G. W., Etzler M. E., eds) pp. 115–128, Cold Spring Harbor Laboratory, Cold Spring Harbor, New York: [PubMed] [Google Scholar]
- 3. Ju T., Otto V. I., Cummings R. D. (2011) The Tn antigen-structural simplicity and biological complexity. Angew. Chem. Int. Ed. Engl. 50, 1770–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brockhausen I., Schutzbach J., Kuhns W. (1998) Glycoproteins and their relationship to human disease. Acta Anat. 161, 36–78 [DOI] [PubMed] [Google Scholar]
- 5. Gill D. J., Clausen H., Bard F. (2011) Location, location, location. New insights into O-GalNAc protein glycosylation. Trends Cell Biol. 21, 149–158 [DOI] [PubMed] [Google Scholar]
- 6. Rosen S. D. (2004) Ligands for L-selectin. Homing, inflammation, and beyond. Annu. Rev. Immunol. 22, 129–156 [DOI] [PubMed] [Google Scholar]
- 7. McEver R. P., Cummings R. D. (1997) Role of PSGL-1 binding to selectins in leukocyte recruitment. J. Clin. Invest. 100, S97–103 [PubMed] [Google Scholar]
- 8. Ley K., Laudanna C., Cybulsky M. I., Nourshargh S. (2007) Getting to the site of inflammation. The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 [DOI] [PubMed] [Google Scholar]
- 9. Zarbock A., Ley K., McEver R. P., Hidalgo A. (2011) Leukocyte ligands for endothelial selectins. Specialized glycoconjugates that mediate rolling and signaling under flow. Blood 118, 6743–6751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aryal R. P., Ju T., Cummings R. D. (2010) The endoplasmic reticulum chaperone Cosmc directly promotes in vitro folding of T-synthase. J. Biol. Chem. 285, 2456–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ju T., Cummings R. D. (2002) A unique molecular chaperone Cosmc required for activity of the mammalian core 1 β3-galactosyltransferase. Proc. Natl. Acad. Sci. U.S.A. 99, 16613–16618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ju T., Lanneau G. S., Gautam T., Wang Y., Xia B., Stowell S. R., Willard M. T., Wang W., Xia J. Y., Zuna R. E., Laszik Z., Benbrook D. M., Hanigan M. H., Cummings R. D. (2008) Human tumor antigens Tn and sialyl Tn arise from mutations in Cosmc. Cancer Res. 68, 1636–1646 [DOI] [PubMed] [Google Scholar]
- 13. Steentoft C., Vakhrushev S. Y., Vester-Christensen M. B., Schjoldager K. T., Kong Y., Bennett E. P., Mandel U., Wandall H., Levery S. B., Clausen H. (2011) Mining the O-glycoproteome using zinc finger nuclease-glycoengineered SimpleCell lines. Nat. Methods 8, 977–982 [DOI] [PubMed] [Google Scholar]
- 14. Ju T., Cummings R. D. (2005) Protein glycosylation. Chaperone mutation in Tn syndrome. Nature 437, 1252. [DOI] [PubMed] [Google Scholar]
- 15. Schietinger A., Philip M., Yoshida B. A., Azadi P., Liu H., Meredith S. C., Schreiber H. (2006) A mutant chaperone converts a wild-type protein into a tumor-specific antigen. Science 314, 304–308 [DOI] [PubMed] [Google Scholar]
- 16. Ju T., Aryal R. P., Stowell C. J., Cummings R. D. (2008) Regulation of protein O-glycosylation by the endoplasmic reticulum-localized molecular chaperone Cosmc. J. Cell Biol. 182, 531–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xia L., Ju T., Westmuckett A., An G., Ivanciu L., McDaniel J. M., Lupu F., Cummings R. D., McEver R. P. (2004) Defective angiogenesis and fatal embryonic hemorrhage in mice lacking core 1-derived O-glycans. J. Cell Biol. 164, 451–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Y., Ju T., Ding X., Xia B., Wang W., Xia L., He M., Cummings R. D. (2010) Cosmc is an essential chaperone for correct protein O-glycosylation. Proc. Natl. Acad. Sci. U.S.A. 107, 9228–9233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fink A. L. (1999) Chaperone-mediated protein folding. Physiol. Rev. 79, 425–449 [DOI] [PubMed] [Google Scholar]
- 20. Ellis R. J. (1990) The molecular chaperone concept. Semin. Cell Biol. 1, 1–9 [PubMed] [Google Scholar]
- 21. Kriegenburg F., Ellgaard L., Hartmann-Petersen R. (2012) Molecular chaperones in targeting misfolded proteins for ubiquitin-dependent degradation. FEBS J. 279, 532–542 [DOI] [PubMed] [Google Scholar]
- 22. Braakman I., Bulleid N. J. (2011) Protein folding and modification in the mammalian endoplasmic reticulum. Annu. Rev. Biochem. 80, 71–99 [DOI] [PubMed] [Google Scholar]
- 23. Narimatsu Y., Ikehara Y., Iwasaki H., Nonomura C., Sato T., Nakanishi H., Narimatsu H. (2008) Immunocytochemical analysis for intracellular dynamics of C1GalT associated with molecular chaperone, Cosmc. Biochem. Biophys. Res. Commun. 366, 199–205 [DOI] [PubMed] [Google Scholar]
- 24. Sun Q., Ju T., Cummings R. D. (2011) The transmembrane domain of the molecular chaperone Cosmc directs its localization to the endoplasmic reticulum. J. Biol. Chem. 286, 11529–11542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ju T., Cummings R. D., Canfield W. M. (2002) Purification, characterization, and subunit structure of rat core 1 β1,3-galactosyltransferase. J. Biol. Chem. 277, 169–177 [DOI] [PubMed] [Google Scholar]
- 26. Ju T., Xia B., Aryal R. P., Wang W., Wang Y., Ding X., Mi R., He M., Cummings R. D. (2011) A novel fluorescent assay for T-synthase activity. Glycobiology 21, 352–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alexander W. S., Viney E. M., Zhang J. G., Metcalf D., Kauppi M., Hyland C. D., Carpinelli M. R., Stevenson W., Croker B. A., Hilton A. A., Ellis S., Selan C., Nandurkar H. H., Goodnow C. C., Kile B. T., Nicola N. A., Roberts A. W., Hilton D. J. (2006) Thrombocytopenia and kidney disease in mice with a mutation in the C1galt1 gene. Proc. Natl. Acad. Sci. U.S.A. 103, 16442–16447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang B. X., Kim H. Y., Dass C. (2004) Probing three-dimensional structure of bovine serum albumin by chemical cross-linking and mass spectrometry. J. Am. Soc. Mass. Spectrom. 15, 1237–1247 [DOI] [PubMed] [Google Scholar]
- 29. Wegele H., Müller L., Buchner J. (2004) Hsp70 and Hsp90, A relay team for protein folding. Rev. Physiol. Biochem. Pharmacol. 151, 1–44 [DOI] [PubMed] [Google Scholar]
- 30. Narimatsu Y., Kubota T., Furukawa S., Shimojima M., Iwasaki H., Tozawa Y., Tachibana K., Narimatsu H. (2011) Co-translational function of Cosmc, core 1 synthase-specific molecular chaperone, revealed by a cell-free translation system. FEBS Lett. 585, 1276–1280 [DOI] [PubMed] [Google Scholar]
- 31. Fomenko D. E., Gladyshev V. N. (2002) CxxS, fold-independent redox motif revealed by genome-wide searches for thiol/disulfide oxidoreductase function. Protein Sci. 11, 2285–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li Y., Lu W., Schwartz A. L., Bu G. (2002) Receptor-associated protein facilitates proper folding and maturation of the low-density lipoprotein receptor and its class 2 mutants. Biochemistry 41, 4921–4928 [DOI] [PubMed] [Google Scholar]
- 33. Saga S., Nagata K., Chen W. T., Yamada K. M. (1987) pH-dependent function, purification, and intracellular location of a major collagen-binding glycoprotein. J. Cell Biol. 105, 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nigam S. K., Goldberg A. L., Ho S., Rohde M. F., Bush K. T., Sherman M. (1994) A set of endoplasmic reticulum proteins possessing properties of molecular chaperones includes Ca2+-binding proteins and members of the thioredoxin superfamily. J. Biol. Chem. 269, 1744–1749 [PubMed] [Google Scholar]
- 35. Szolajska E., Chroboczek J. (2011) Faithful chaperones. Cell Mol. Life Sci. 68, 3307–3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brillet T., Baudin-Creuza V., Vasseur C., Domingues-Hamdi E., Kiger L., Wajcman H., Pissard S., Marden M. C. (2010) α-Hemoglobin stabilizing protein (AHSP), a kinetic scheme of the action of a human mutant, AHSPV56G. J. Biol. Chem. 285, 17986–17992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ju T., Brewer K., D'Souza A., Cummings R. D., Canfield W. M. (2002) Cloning and expression of human core 1 β1,3-galactosyltransferase. J. Biol. Chem. 277, 178–186 [DOI] [PubMed] [Google Scholar]
- 38. Betts S., King J. (1999) There's a right way and a wrong way. In vivo and in vitro folding, misfolding, and subunit assembly of the P22 tailspike. Structure 7, R131–139 [DOI] [PubMed] [Google Scholar]
- 39. Liu Y., Gotte G., Libonati M., Eisenberg D. (2001) A domain-swapped RNase A dimer with implications for amyloid formation. Nat. Struct. Biol. 8, 211–214 [DOI] [PubMed] [Google Scholar]
- 40. Crew V. K., Singleton B. K., Green C., Parsons S. F., Daniels G., Anstee D. J. (2008) New mutations in C1GALT1C1 in individuals with Tn positive phenotype. Br J. Haematol. 142, 657–667 [DOI] [PubMed] [Google Scholar]
- 41. Ju T., Zheng Q., Cummings R. D. (2006) Identification of core 1 O-glycan T-synthase from Caenorhabditis elegans. Glycobiology 16, 947–958 [DOI] [PubMed] [Google Scholar]
- 42. Müller R., Hülsmeier A. J., Altmann F., Ten Hagen K., Tiemeyer M., Hennet T. (2005) Characterization of mucin-type core 1 β1,3-galactosyltransferase homologous enzymes in Drosophila melanogaster. FEBS J. 272, 4295–4305 [DOI] [PubMed] [Google Scholar]
- 43. Yoshida H., Fuwa T. J., Arima M., Hamamoto H., Sasaki N., Ichimiya T., Osawa K., Ueda R., Nishihara S. (2008) Identification of the Drosophila core 1 β1,3-galactosyltransferase gene that synthesizes T antigen in the embryonic central nervous system and hemocytes. Glycobiology 18, 1094–1104 [DOI] [PubMed] [Google Scholar]
- 44. Ellgaard L., Helenius A. (2003) Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 4, 181–191 [DOI] [PubMed] [Google Scholar]
- 45. Palleros D. R., Shi L., Reid K. L., Fink A. L. (1994) Hsp70-protein complexes. Complex stability and conformation of bound substrate protein. J. Biol. Chem. 269, 13107–13114 [PubMed] [Google Scholar]
- 46. Goloubinoff P., Christeller J. T., Gatenby A. A., Lorimer G. H. (1989) Reconstitution of active dimeric ribulose-bisphosphate carboxylase from an unfoleded state depends on two chaperonin proteins and Mg-ATP. Nature 342, 884–889 [DOI] [PubMed] [Google Scholar]
- 47. Schmid D., Baici A., Gehring H., Christen P. (1994) Kinetics of molecular chaperone action. Science 263, 971–973 [DOI] [PubMed] [Google Scholar]
- 48. Rao P. V., Horwitz J., Zigler J. S., Jr. (1993) α-Crystallin, a molecular chaperone, forms a stable complex with carbonic anhydrase upon heat denaturation. Biochem. Biophys. Res. Commun. 190, 786–793 [DOI] [PubMed] [Google Scholar]
- 49. Inoue S., Sano H., Ohta M. (2000) Growth suppression of Escherichia coli by induction of expression of mammalian genes with transmembrane or ATPase domains. Biochem. Biophys. Res. Commun. 268, 553–561 [DOI] [PubMed] [Google Scholar]
- 50. Trilla J. A., Durán A., Roncero C. (1999) Chs7p, a new protein involved in the control of protein export from the endoplasmic reticulum that is specifically engaged in the regulation of chitin synthesis in Saccharomyces cerevisiae. J. Cell Biol. 145, 1153–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Johswich A., Kraft B., Wuhrer M., Berger M., Deelder A. M., Hokke C. H., Gerardy-Schahn R., Bakker H. (2009) Golgi targeting of Drosophila melanogaster β4GalNAcTB requires a DHHC protein family-related protein as a pilot. J. Cell Biol. 184, 173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Manya H., Chiba A., Yoshida A., Wang X., Chiba Y., Jigami Y., Margolis R. U., Endo T. (2004) Demonstration of mammalian protein O-mannosyltransferase activity. Coexpression of POMT1 and POMT2 required for enzymatic activity. Proc. Natl. Acad. Sci. U.S.A. 101, 500–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McCormick C. D., Bearden W. H., Hunts J. H., Anderson R. L. (2004) Cerebral vasospasm and ischemia after orbital decompression for graves ophthalmopathy. Ophthal. Plast. Reconstr. Surg. 20, 347–351 [DOI] [PubMed] [Google Scholar]
- 54. Kumagai T., Sato T., Natsuka S., Kobayashi Y., Zhou D., Shinkai T., Hayakawa S., Furukawa K. (2010) Involvement of murine β1,4-galactosyltransferase V in lactosylceramide biosynthesis. Glycoconj. J. 27, 685–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huang H. H., Stanley P. (2010) A testis-specific regulator of complex and hybrid N-glycan synthesis. J. Cell Biol. 190, 893–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.