Background: The vascular endothelial surface is highly sialylated.
Results: Vascular endothelia express catalytically active NEU1 and NEU3 sialidases, and NEU1 restrains the endothelial migratory response to wounding.
Conclusion: NEU1 regulates endothelial remodeling in response to injury.
Significance: Learning how NEU1 and NEU3 regulate sialylated molecules on the endothelial surface is key to understanding endothelial receptor-ligand, cell-cell, and host-pathogen interactions.
Keywords: Endothelial Dysfunction, Glycobiology, Lung, Signal Transduction, Vascular Biology, Migration, Neuraminidase, Sialic Acid, Sialidase, Wound Healing
Abstract
The microvascular endothelial surface expresses multiple molecules whose sialylation state regulates multiple aspects of endothelial function. To better regulate these sialoproteins, we asked whether endothelial cells (ECs) might express one or more catalytically active sialidases. Human lung microvascular EC lysates contained heat-labile sialidase activity for a fluorogenic substrate, 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid (4-MU-NANA), that was dose-dependently inhibited by the competitive sialidase inhibitor, 2,3-dehydro-2-deoxy-N-acetylneuraminic acid but not its negative control. The EC lysates also contained sialidase activity for a ganglioside mixture. Using real time RT-PCR to detect mRNAs for the four known mammalian sialidases, NEU1, -2, -3, and -4, NEU1 mRNA was expressed at levels 2700-fold higher that those found for NEU2, -3, or -4. Western analyses indicated NEU1 and -3 protein expression. Using confocal microscopy and flow cytometry, NEU1 was immunolocalized to both the plasma membrane and the perinuclear region. NEU3 was detected both in the cytosol and nucleus. Prior siRNA-mediated knockdown of NEU1 and NEU3 each decreased EC sialidase activity for 4-MU-NANA by >65 and >17%, respectively, and for the ganglioside mixture by 0 and 40%, respectively. NEU1 overexpression in ECs reduced their migration into a wound by >40%, whereas NEU3 overexpression did not. Immunohistochemical studies of normal human tissues immunolocalized NEU1 and NEU3 proteins to both pulmonary and extrapulmonary vascular endothelia. These combined data indicate that human lung microvascular ECs as well as other endothelia express catalytically active NEU1 and NEU3. NEU1 restrains EC migration, whereas NEU3 does not.
Introduction
The vascular endothelium lines the intravascular space and presents a selective barrier that actively regulates paracellular movement of circulating cells, macromolecules, and fluids into extravascular tissues and compartments (1, 2). In eukaryotic cells, glycoproteins and glycolipids expressed on the cell surface contain oligosaccharide chains whose outermost positions are terminated with sialic acids (3, 4). The sialic acid family of >50 closely related but structurally diverse sugars, with their terminal location and negative charge, are strategically positioned to influence intermolecular and cell-cell interactions through nonspecific steric hindrance and/or electrostatic repulsion. Vascular endothelia are highly sialylated (5). The sialic acid-bearing surface structures on endothelia together with other membrane-bound polyanionic macromolecules, constitute the endothelial cell (EC)2 glycocalyx (6, 7). This negatively charged zone can be extended through hydration and/or acquisition of plasma proteins. The glycocalyx might nonspecifically diminish cell-cell adhesion through either negative charge-mediated repulsion or the masking of specific adhesion molecules. However, sialyl residues also can serve as components of EC surface recognition motifs that participate in specific intermolecular interactions. For example, the selectins are C-type lectins that bind sialyl Lewis X-bearing molecules (1, 8). ECs can surface express or secrete selectins that recognize their sialylated ligands on circulating leukocytes (1, 8) and tumor cells (1, 8–10). Removal of sialic acid from the surface of HL60 myeloid cells diminishes their adhesion to EC-expressed E-selectin (9). Increased α2,6-sialylation of the EC surface increases endothelial adhesiveness for CD22-bearing B cells (11, 12) and lymphocyte function-associated antigen-1 expressing T cells (13). Vascular endothelial (VE)-cadherin is enriched to intercellular boundaries where it regulates EC-EC homophilic adhesion and paracellular pathway function (14). VE-cadherin is sialylated, and its desialylation results in its profound reorganization. Other sialylated molecules at the EC surface regulate components of both the clotting (15, 16) and complement (17) pathways. The sialylation state of these EC surface molecules influences their tertiary conformation, intermolecular interactions, resistance to proteolysis, and function (1, 9, 10, 12–14).
The state of sialylation of these EC- and leukocyte-associated glycoproteins are tightly and dynamically regulated through the net catalytic activities of both sialyltransferases (STs) (18, 19) and sialidases (20, 21). An ST family of at least 15 members, each containing the ∼50 amino acid sialyl motif thought to be involved in substrate binding, catalyzes the transfer of sialic acid molecules onto an acceptor substrate (18, 19). The endothelial surface reportedly expresses ST activity (11). Whether this same endothelial surface also expresses sialidase activity is unknown. At least four mammalian sialidases have been identified (20, 21). NEU1 is reportedly localized to lysosomes where it associates with cathepsin A- and β-galactosidase, which are required for correct sialidase conformation (22, 23). NEU2 is found in the cytosol (24), and NEU3 is associated with the plasma membrane (25, 26). The most recently described sialidase, NEU4, is associated with the mitochondria (27). As members of the neuraminidase/sialidase superfamily, each contains one or more of the conserved Asp box and an amino acid sequence, composed of SXDXGXTW, together with the (F/Y)RIP motif, composed of XPRP, where X represents variable residues (20, 21). Although the overall sequence identities between the four sialidases are low, their catalytic domains share a common “six-bladed β-propeller fold” architecture. Each of these enzymes selectively hydrolyzes specific glycosidic linkages between sialic acid molecules and the subterminal sugar of glycoconjugates.
We previously found that treatment of ECs with exogenous neuraminidase increased polymorphonuclear leukocyte (PMNL) adhesion to and migration across the endothelium and that preincubation of cells with the selective sialidase inhibitor, 2,3-dehydro-2-deoxy-N-acetyl-neuraminic acid, inhibited PMNL-mediated desialylation of the endothelial surface (28). In other studies we found that infusion of neutralizing anti-neuraminidase antibodies in mice blocked PMNL recruitment to the pulmonary microvasculature in response to either systemic complement activation or intratracheal interleukin-8 (IL-8) installation (29). These combined studies indicate that endogenous sialidase activity is operative during PMNL-EC interactions within the pulmonary microvasculature. However, the identity of the responsible sialidase molecule(s) is unknown. In this report we have asked whether human pulmonary microvascular endothelia and/or other vascular endothelia express one or more catalytically active sialidases and whether such expression regulates EC behavior.
EXPERIMENTAL PROCEDURES
Cell Culture
Human lung microvascular EC (HMVEC-Ls) and human pulmonary artery ECs (HPAECs) (Lonza, Rockland, ME) as well as HMEC-1 cells, a simian virus 40 T antigen-transformed human dermal microvascular EC line (Centers for Disease Control, Atlanta, GA) were cultured in EC growth medium (EBM-2, Lonza) containing 5% fetal bovine serum (Hyclone Laboratories, Logan, UT), human recombinant epidermal growth factor (EGF), human recombinant insulin-like growth factor-1, human basic fibroblast growth factor, vascular endothelial growth factor, hydrocortisone, ascorbic acid, gentamicin, and amphotericin B as described (30). HMVEC-Ls and HPAECs were studied in passages 2–7. Human bone marrow ECs (HBMEs) (31) and human umbilical vein retroviral telemorized ECs (Hrvts) were similarly cultured.
Fluorometric Assay for Sialidase Activity
Sialidase activity was measured in HMVEC-Ls, HPAECs, HBMEs, Hrvts, and HMEC-1s in the presence of 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid sodium salt hydrate (4-MU-NANA) (Sigma) as the substrate as described (32). ECs (∼106 cells/tube) were suspended in 200 μl of buffer containing 500 mm sodium acetate, 0.1% Triton X-100, supplemented with protease inhibitor mixture (Roche Applied Science) and then incubated for 1 h at 37 °C in the presence of 4-MU-NANA, mixing tubes every 15 min. The sialidase reaction was terminated by the addition of glycine-NaOH buffer, pH 10.3, containing 133 mm glycine, 60 mm NaCl, and 0.083 m Na2CO3, after which fluorescence intensity was measured with a Bio-Rad fluorometer (excitation at 355 nm; emission at 460 nm). In selected experiments EC lysates were boiled (100 °C, 10 min). In other experiments EC lysates were preincubated with the competitive neuraminidase inhibitor, 2-deoxy-NANA (5–500 μg/ml) (Calbiochem) (28, 29). A molecule with comparable molecular mass and charge to 2-deoxy-NANA, 2-keto-3-deoxyoctulosonic acid (KDO) (Sigma), was used as a negative control (28).
Sialidase Activity for Ganglioside Substrate
Because NEU3 preferentially hydrolyzes sialic acid linkages within gangliosides (25), sialidase activity for a ganglioside substrate was assayed as described (33). HMVEC-Ls were suspended in 200 μl of 500 mm sodium acetate, pH 4.4, containing 0.1% Triton X-100 and protease inhibitor mixture, and the total cell lysates were mixed with 25 μl of ganglioside mixture (2 mg/ml) (Calbiochem). The HMVEC-L ganglioside substrate mixtures were incubated for 1 h at 37 °C. The reaction was terminated by the addition of 25 μl glycine buffer, pH 10.3, containing 1.33 m glycine, 0.6 m NaCl, and 0.42 m Na2CO3. Released sialic acid in each reaction mixture was quantified by high pH anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) as described (29). For each assay, serial dilutions of known concentrations of pure sialic acid (Sigma) were measured, and a standard curve was generated. The sialic acid concentration in each sample was interpolated from the standard curve using GraphPad Prism 4 (GraphPad Software, Inc., La Jolla, CA). The concentration of sialic acid spontaneously released from the simultaneous cell-free ganglioside control, i.e. the background, was subtracted from each value.
Real-time RT-PCR for NEU1, -2, -3, and -4
Total cellular RNA was extracted from HMVEC-Ls using the Qiagen RNA isolation kit according to the manufacturer's protocol (30). RNA purity was established with the 260/280-nm absorption ratio. For real time quantitative RT-PCR, total RNA was reverse-transcribed using avian myeloblastosis virus reverse transcriptase (Promega, Madison, WI) and poly-T primer, as recommended by the manufacturer. The resulting cDNA was quantified by using real-time PCR using SYBR Green PCR Master Mix (Applied Biosystems/Invitrogen) and ABI Prism 7900HT cycler. Primers for detection of NEU1, NEU2, NEU3, NEU4, and hypoxanthine phosphoribosyltransferase (HPRT) mRNAs were designed using the Primer Express 2.0 program (Applied Biosystems) and are indicated in Table 1. Relative gene expression was calculated using the ΔCt method, where Ct refers to the cycle number at which the PCR product for a particular gene is detected by the light cycler. The housekeeping gene, HPRT, was used as an internal control, and relative gene expression was normalized to the HPRT gene expression by the formula 1.8 exp[Cthousekeeping gene − Ctgene of interest]).
TABLE 1.
Oligonucleotide primers used for quantitative RT-PCR
F, forward primer; R, reverse prime.
| RNA target | Primer sequence | PCR product size | GenBankTM accession no. |
|---|---|---|---|
| NEU1 | F, 5′-TGTGACCTTCGACCCTGAGC-3′ | 124 | NM_000434 |
| R, 5′-TCGCAGGGTCAGGTTCACTC-3′ | |||
| NEU2 | F, 5′-AGTGGTCCACCTTTGCAGTG-3′ | 143 | NM_005383 |
| R, 5′-ATGGCTGAGGAAGCAGAAGG-3′ | |||
| NEU3 | F, 5′-AATGTGAAGTGGCAGAGGTGA-3′ | 148 | NM_006656 |
| R, 5′-TCACAGAGCTGTCGACTCAGG-3′ | |||
| NEU4 | F, 5′-TGCTGGTACCCGCCTACAC-3′ | 103 | NM_080741 |
| R, 5′-CCGTGGTCATCGCTGTAGAA-3′ | |||
| HPRT | F, 5′-ACCAGTCAACAGGGGACATAAAAG-3′ | 102 | NM_000194 |
| R, 5′-GTCTGCATTGTTTGCCAGTGTC-3′ |
Immunoblotting for NEU1 and -3
Cells were thoroughly rinsed with ice-cold HEPES buffer and solubilized with ice-cold lysis buffer containing 50 mm Tris-HC1, pH 8.0, 1% Nonidet P-40, 0.5% sodium dodecyl sulfate (SDS), 150 mm NaCl, 0.1 mm phenylmethylsulfonyl fluoride, 5 μg/ml leupeptin, 1 mg/ml pepstatin A, 1 mg/ml aprotinin, 1 mm vanadate, 1 mm sodium fluoride, 10 mm disodium pyrophosphate, 500 μm paranitrophenol, and 1 mm phenylarsine oxide (all purchased from Sigma) as described (29, 30). The cell lysates were assayed for protein concentration with a Bio-Rad Protein Assay Dye Reagent (Bio-Rad). Equal amounts of protein were resolved by electrophoresis on an 8–16% SDS-polyacrylamide gel (Novex, San Diego, CA) and transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA). The blots were blocked for 1 h using 5% nonfat milk in TBS-Tween buffer and probed with rabbit anti-human NEU1 antibody (Rockland Immunochemicals, Gilbertsville, PA) or anti-human NEU3 antibody (Strategic Diagnostics Inc., Newark, DE), each followed by horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody in 5% milk, TBS-T and developed with enhanced chemiluminescence (ECL) (Amersham Biosciences) (30). To confirm equivalent protein loading and transfer, blots were stripped with 100 mm 2-mercaptoethanol, 2% SDS, 62.5 mm Tris-HCI, pH 6.7, and reprobed with 0.5 ng/ml of murine anti-Physarum β-tubulin IgG2b (Roche Applied Science) followed by HRP-conjugated anti-mouse IgG (Transduction Laboratories) and again, developed with ECL. In selected experiments, NEU3 immunoblotting was performed with cytoplasmic and nuclear subcellular fractions isolated using the NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Fisher Scientific, Rockford, IL). To verify subcellular fractionation, the blot was stripped and reprobed for the cytoplasmic marker protein, IκBα, and the nuclear marker protein, lamin B1.
Flow Cytometry for NEU1 and -3 Expression in HMVEC-Ls
HMVEC-Ls were detached using 0.25% trypsin-EDTA, in some cases permeabilized with 0.1% Triton X-100, and incubated for 30 min at 4 °C with anti-human NEU1 or NEU3 antibodies or a species-matched control antibody (rabbit IgG, Invitrogen). The cells were washed and incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit antibody (BD Pharmingen). Antibody binding to the intact and permeabilized cells was evaluated using a flow cytometer (FACSCAN, BD Biosciences), and the data were analyzed with CELLQUEST Software (BD Biosciences) as described (29, 30).
Adenoviral Constructs Encoding for Epitope-tagged NEU1 and NEU3
To regulate NEU1 and NEU3 expression in HMVEC-Ls, recombinant adenovirus (Ad) encoding for human FLAG-tagged wild-type NEU1 (Ad-NEU1-FLAG) and human hemagglutinin (HA)-tagged wild-type NEU3 (Ad-NEU3-HA) were generated as described for another gene product (34). The human NEU1 (NM_000434.3) and NEU3 (NM_006656.5) sequences were cloned by RT-PCR using PCR primers synthesized by Primm Biotech (Cambridge, MA), after which the 3 × FLAG tag and HA tag sequences were inserted before the stop codon at the 3′ end of NEU1 and NEU3 sequences, respectively. The Ad-NEU1-FLAG and Ad-NEU3-HA were generated using the AdEasy Adenoviral Vector System (Stratagene, La Jolla, CA) according to the manufacturer's recommendation. Briefly, the NEU1-FLAG and NEU3-HA each were subcloned into a shuttle vector (pShuttle-IREs-hrGFP-1) using restriction enzyme digestion and ligation. Each resultant shuttle plasmid was linearized through Pmel digestion and, with the Ad backbone plasmid (pAdEasy-1, Qbiogene), was used to cotransform electrocompetent Escherichia coli BJ5183 cells to produce the recombinant plasmids, Ad-NEU1-FLAG and Ad-NEU3-HA. Recombinants were selected for kanamycin resistance and screened for recombination by Pac1 restriction enzyme analysis and agarose gel electrophoresis. The correct recombinant plasmids were used to transform XL10-Gold cells, and bacterial lysates were passed through Maxiprep columns (Qiagen) for purification. Ad-NEU1-FLAG and Ad-NEU3-HA each was linearized with Pac1 digestion and transfected, in the presence of Lipofectamine (Invitrogen), into AD-293 cells. After 7–10 days, cells were scraped off flasks with a rubber policeman and subjected to 3 freeze-thaw cycles, and virus was harvested in the supernatants for presentation to fresh AD-293 cells and titration in a plaque-forming assay. HMVEC-Ls were transiently infected with packaged Ad-NEU1-FLAG or Ad-NEU3-HA at increasing m.o.i. and after 48 h were lysed, and the lysates were processed for FLAG or HA immunoblotting. In selected experiments, Ad-GFP was used as a vector control as described (35).
Immunolocalization of NEU1 and -3 in HMVEC-Ls
HMVEC-Ls were cultured overnight in 8-well glass chamber slides (Nunc/Thermo Fisher, Waltham, MA), washed 3 times with PBS, fixed (4% paraformaldehyde in PBS, 10 min, room temperature), washed, permeabilized (0.5% Triton X-100 in HEPES buffer, 10 min, 4 °C), blocked (super-block buffer, 30 min), and incubated overnight at 4 °C separately with anti-human NEU1 or NEU3 antibodies (1:500 dilution, overnight, 4 °C) with 1% donkey serum. The chamber slides were then washed and incubated with Cy3-conjugated goat anti-rabbit antibody (Jackson ImmunoResearch). DAPI was used to counterstain nuclei. After immediate mounting with mounting media (Vectashield; Vector Laboratories), the immunostained HMVEC-Ls were analyzed and photographed using an Olympus FluoView 500 laser scanning confocal fluorescence microscope fitted with a 60×, N.A. 1.4 objective and standard excitation/emission filters for detection of DAPI and Cy3. Images were cropped and assembled into panels with Adobe Photoshop 4.0.
Knockdown of NEU1 and NEU3 through Small Interfering RNA (siRNA) Technology
First, HMVEC-Ls were transfected with siRNA duplex products designed to specifically target NEU1 or NEU3 or an irrelevant control siRNA duplex not corresponding to any known sequence in the human genome (Dharmacon, Lafayette, CO) as described (36). For transfection, 5 × 105 HMVEC-Ls were centrifuged (200 × g, 10 min), and the HMVEC-L pellet was resuspended in 100 μl of Nucleofactor solution (Amaxa Biosystems) with 2.7 μg of siRNA duplexes. The HMVEC-L-siRNA mixture was transferred to an Amaxa-certified cuvette and subjected to programmed electroporation (program S-005) (Amaxa Biosystems). The transfected cells were cultured for 24–72 h after which time they were lysed and the lysates processed for NEU1 and NEU3 immunoblotting. In other experiments, HMVEC-Ls were transiently infected with packaged Ad-NEU1-FLAG, Ad-NEU3-HA, or Ad-null vector control, each at an m.o.i. = 100. After 48 h, NEU1-targeting or control siRNAs were introduced into HMVEC-Ls overexpressing FLAG-tagged NEU1. Similarly, HMVEC-Ls overexpressing HA-tagged NEU3 were transfected with NEU3-targeting or control siRNAs. To exclude off-target effects, HMVEC-Ls overexpressing FLAG-tagged NEU1 were transfected with NEU3-targeting siRNAs, and HMVEC-Ls overexpressing HA-tagged NEU3 were transfected with NEU1-targeting siRNAs. After 24–72 h, the transfected cells were lysed and processed for immunoblotting with either murine monoclonal anti-FLAG antibody (Sigma) or rabbit anti-HA antibody (Cell Signaling, Danvers, MA). To confirm equivalent protein loading, blots were stripped and reprobed for β-tubulin. These siRNA-transfected HMVEC-Ls were studied for sialidase activity.
Endothelial Cell Migration in Wounding Assay
HMVEC-Ls were transfected with NEU1-targeting, NEU3-targeting, or control siRNAs or infected with increasing m.o.i. of Ad-NEU1-FLAG, Ad-NEU3-HA, or Ad-GFP as the vector control. The transfected/infected cells were cultured to confluence in the wells of 24-well plates (Corning, Corning, NY). After 48 h, a sterile 200-μl pipette tip was used to place a single wound across the diameter of each monolayer, after which the medium was replaced to remove cell debris, as described (34, 36) with minor modifications. The cells were then incubated for 24 h with serum/growth factor-enriched medium. At 0, 2, 16, and 24 h, images of each monolayer were captured using a Nikon Eclipse TS100 microscope coupled to a Nikon Cool pix 4300 camera. Cell migration into the wound was calculated using ImageJ Software (Rasband, WS, ImageJ, National Institutes of Health, Bethesda, MD, rsb.info.nih.gov). Cell migration into each wound after 2, 16, and 24 h was compared with that observed in the same wounded monolayer at 0 h.
Immunostaining of NEU1 and NEU3 in Human Tissues
Human vascular tissues were harvested and processed through an Institutional Review Board-approved protocol at the University of Maryland, Baltimore. More specifically, vascular endothelia from lung, liver, and kidney as well as aorta and carotid and cerebral arteries were each obtained from normal tissues from two or more subjects. The sections were deparaffinized in xylene and rehydrated in graded series of ethanol. Sections were pretreated with heat-induced epitope retrieval using a pressure cooker and Target Retrieval Solution pH 6.1 (Dako TRS, S1699/1700) followed by endogenous peroxidase blocking for 5 min with 0.3% hydrogen peroxide. The sections were then incubated overnight with rabbit anti-NEU1 or anti-NEU3 antibodies at 1:250 dilution at 4 °C in a hydration chamber. Antibody detection was performed by incubation with biotinylated goat anti-rabbit secondary antibody (Dako, Carpinteria, CA) for 30 min at room temperature. Slides were developed for 5 min using diaminobenzidine as the chromagen (Dako) and were counterstained with hematoxylin. As a negative control, tissue sections were reacted with non-immune rabbit IgG plus the secondary antibody. For selected specimen, lung sections were probed with antibodies against CD31 (Ventana Med. Syst.; Tucson, AZ), a marker for endothelia (37). Staining was performed on a Dako automatic stainer using EnVision+ (Dako), a biotin-free detection system that consists of a secondary antibody covalently linked to peroxidase-coated dextrose polymers.
Statistical Methods
For those outcomes measured once per sample, Student's t test was used to compare experimental versus control groups. For those outcomes, measured more than once per sample, repeated measure analysis of variance was applied using SAS PROC MIXED with a random effects model (random intercept) and an unstructured covariance matrix to account for the serial autocorrelation of repeated measures from the same sample. A 2-tailed p < 0.05 was considered significant.
RESULTS
EC Sialidase Activity
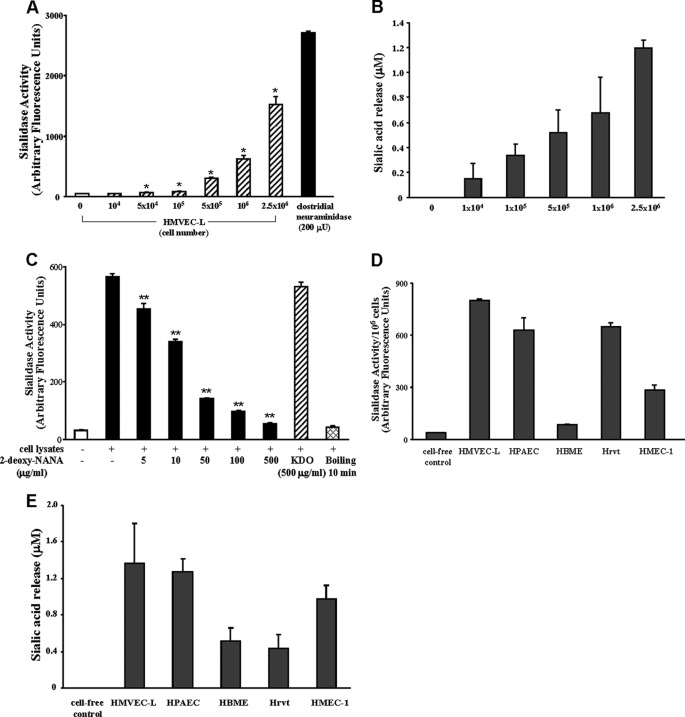
Dynamic and specific interactions between sialoproteins expressed on the endothelial surface and their counter ligands expressed on the PMNL surface regulate PMNL recruitment to inflamed extravascular tissues (1, 8). PMNLs express sialidase activity that influences their adhesion to and migration across the endothelial barrier (28, 29, 32). We asked whether the endothelium might also express such sialidase activity. Increasing HMVEC-L cell numbers were assayed for sialidase activity for the fluorogenic substrate, 4-MU-NANA (Fig. 1A) (n = 2), and for a ganglioside mixture (Fig. 1B) (n = 2). These ECs contained sialidase activity for 4-MU-NANA at ∼626 catalytic units/106 cells or ∼1.17 catalytic units/μg of cellular protein. For the ganglioside mixture, these same ECs expressed sialidase activity that released ∼0.677 μm sialic acid/106 cells or ∼0.001 μm sialic acid/μg of cellular protein. The lysates contained sialidase activity that was destroyed by boiling and was dose-dependently inhibited by the competitive sialidase inhibitor, 2-deoxy-NANA, but not the KDO negative control (n = 2) (Fig. 1C). To our knowledge this is the first report of sialidase activity in endothelia. We then asked whether sialidase activity was expressed in other endothelia. Equivalent numbers (106 cells) of HMVEC-Ls, HPAECs, HBMEs, Hrvts, and HMEC-1s were assayed for sialidase activity for the 4-MU-NANA substrate (n = 2) (Fig. 1D). Sialidase activity for the 4-MU-NANA substrate was detected in all endothelia studied, and their relative sialidase activities were HMVEC-L > Hrvt ≈ HPAEC > HMEC-1 > HBME. These same endothelia also contained sialidase activity for the ganglioside substrate (Fig. 1E) and here their relative sialidase activities were HMVEC-L ≈ HPAEC > HMEC-1 > HBME ≈ Hrvt. These findings indicate that sialidase activity could be detected in all endothelia tested and that HMVEC-Ls contain as much or more sialidase activity than did equivalent numbers of the other primary and immortalized endothelia studied. Furthermore, expression of sialidase activities for both 4-MU-NANA and ganglioside substrates were relatively abundant in pulmonary vascular endothelia.
FIGURE 1.
EC sialidase activity. Increasing HMVEC-L cell numbers were assayed for sialidase activity as liberation of sialic acid from the fluorogenic substrate, 4-MU-NANA (A) or from a ganglioside mixture (B). For comparison, sialidase activity for a known concentration of purified clostridial neuraminidase is indicated (A). In C, HMVEC-Ls were assayed for sialidase activity before and after boiling and in the presence of increasing concentrations (5–500 μg/ml) of the competitive sialidase inhibitor, 2-deoxy-NANA, or its negative control, KDO (500 μg/ml). Equivalent cell numbers of HMVEC-Ls, HPAECs, HBMEs, Hrvts, and HMEC-1s were assayed for sialidase activity for the 4-MU-NANA substrate (D) and the ganglioside mixture (E). In A–E, vertical bars represent the mean (±S.E.) sialidase activity, expressed as either arbitrary fluorescence units (A, C, and D) or sialic acid release in μm (B and E). The n for each experimental and control group is ≥2. *, significantly increased compared with background sialidase activity in the cell-free control at p < 0.05. **, significantly decreased compared with sialidase activity in total HMVEC-L lysates at p < 0.05.
EC Expression of NEU1, -2, -3, and -4
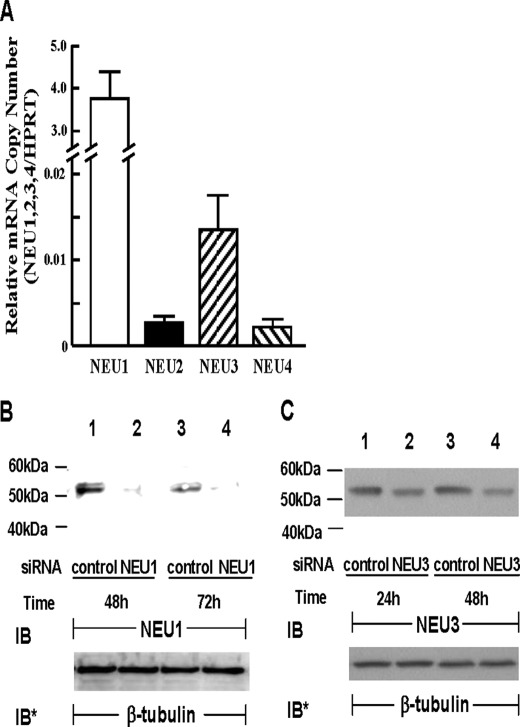
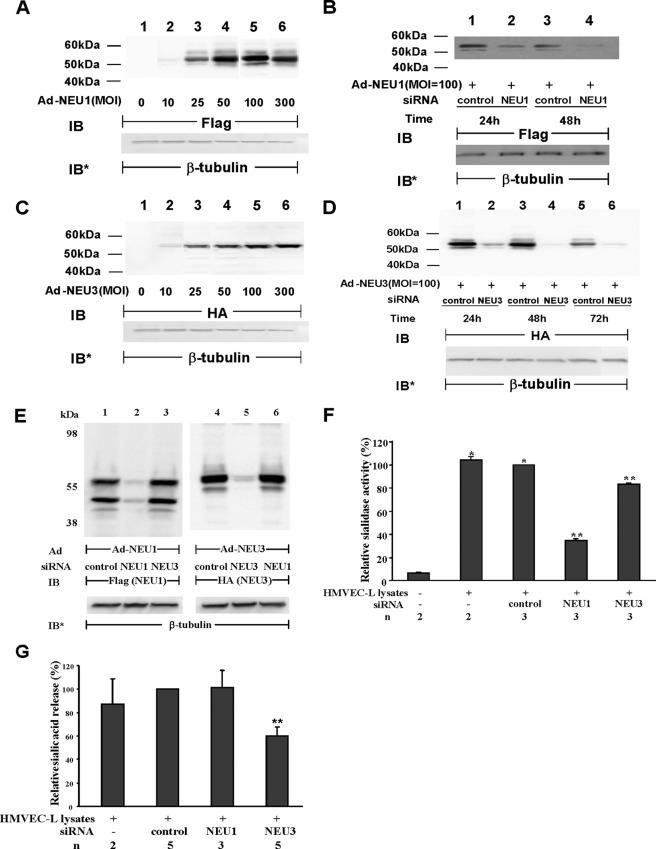
Because HMVEC-Ls contain sialidase activity (Fig. 1, A and B), we asked whether one or more of the already reported mammalian sialidases (20, 21) might be expressed in these same cells. In HMVEC-Ls, a real-time RT-PCR approach was adopted to detect mRNA for NEU1, -2, -3, and -4 (Fig. 2A). Each of the four sialidase mRNAs was normalized to HPRT mRNA in the same sample. NEU1 mRNA was expressed at the highest levels of 3.76 (±0.63) copies relative to HPRT expression. In contrast, the mRNAs for NEU2, -3, and -4 were each expressed at <0.002 copies relative to HPRT expression. Normalized NEU3 mRNA expression was >4-fold higher than was either NEU2 or NEU4 expression. Therefore, at the mRNA level, NEU1 was expressed at levels 2700-fold higher than were NEU2, -3, and -4, with NEU3 at a distant second. We then used antibodies specific for the NEU protein most abundantly expressed at the mRNA level, NEU1, to probe lysates of HMVEC-Ls transfected with NEU1-targeting or control siRNAs (Fig. 2B). NEU1 was detected at the protein level at its anticipated gel mobility (lanes 1 and 3) and prior siRNA-induced silencing reduced the intensity of this band (lanes 2 and 4). Although NEU3 mRNA was expressed at extremely low levels (Fig. 2A), HMVEC-Ls clearly contained sialidase activity for the ganglioside substrate (Fig. 1B). Accordingly, we processed lysates of HMVEC-Ls transfected with NEU3-targeting or control siRNAs for NEU3 immunoblotting (Fig. 2C). Like NEU1, NEU3 also could be detected at the protein level, again, at its anticipated gel mobility (lanes 1 and 3) and prior siRNA-induced silencing modestly but reproducibly reduced the intensity of this band (lanes 2 and 4). These findings clearly indicate that both NEU1 and NEU3 proteins are expressed in HMVEC-Ls.
FIGURE 2.
Expression of NEU1–4 in endothelia. A, total RNA isolated from HMVEC-Ls was reverse-transcribed, and the resulting cDNA was used as a template for amplification with primers corresponding to NEU1, NEU2, NEU3, and NEU4 as well as HPRT as a housekeeping gene control. The mRNAs for each sialidase was normalized to HPRT (n = 2). B, HMVEC-Ls were transfected with NEU1-targeting or control siRNAs, cultured for 48 or 72 h, and lysed, and the lysates were processed for NEU1 immunoblotting. C, HMVEC-Ls were transfected with NEU3-targeting or control siRNAs, cultured for 24 or 48 h, and lysed, and the lysates were processed for NEU3 immunoblotting. B and C, to control for protein loading and transfer, blots were stripped and reprobed for β-tubulin. IB, immunoblot; IB*, immunoblot after stripping. Molecular mass in kDa is indicated on the left. Each blot is representative of three or more independent experiments.
Subcellular Localization of NEU1 and NEU3
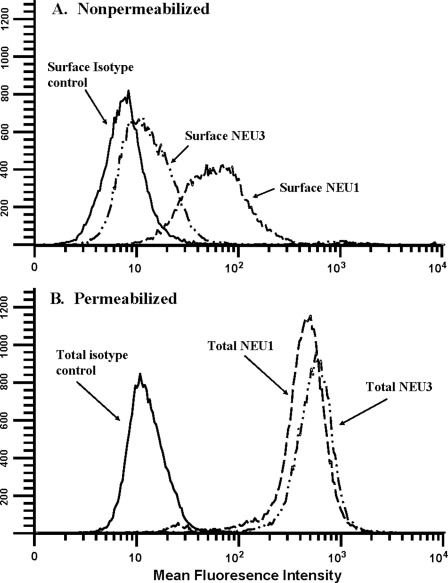
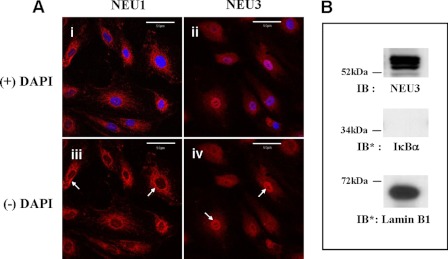
Because the mammalian sialidases, NEU1 and NEU3, have been previously immunolocalized in various host cells to specific subcellular compartments (23, 25, 26), we utilized flow cytometry of permeabilized and nonpermeabilized HMVEC-Ls (Fig. 3) together with confocal fluorescence microscopy (Fig. 4) to probe for NEU1 and -3 proteins. Flow cytometry of nonpermeabilized HMVEC-Ls detected greater surface expression of NEU1 with a mean fluorescence index (MFI) of 57.88 compared with NEU3 with an MFI of 13.97 (Fig. 3A). Simultaneous studies of these same nonpermeabilized cells with a species- and isotype-matched control antibody generated an MFI of 8.03. Flow cytometry of permeabilized HMVEC-Ls revealed large intracellular pools of both NEU1 with an MFI of 431.72 and NEU3 with an MFI of 550.86 (Fig. 3B). Here, incubation of these same permeabilized cells with the species- and isotype-matched antibody control generated an MFI of 12.1. These results indicate that HMVEC-Ls express both NEU1 and NEU3 proteins and that NEU1 is expressed on the EC surface at a higher level than is NEU3. Although it is NEU3 that is usually associated with the plasma membrane (25, 26), several reports have localized NEU1 to the cell surface (33, 38–40). More specifically, NEU1 has been detected on the surface of COS-7 cells (38), human aortic smooth muscle cells (39), human monocytes undergoing differentiation in response to a phorbol myristate acetate stimulus (40), and activated human T lymphocytes (33). Confocal fluorescence microscopy of immunostained HMVEC-Ls showed granular/punctuate staining for NEU1, with maximal signal in the perinuclear region (Fig. 4Ai). Punctate NEU3 immunostaining was present more uniformly throughout the cytosol (Fig. 4Aii). NEU1 was not detected within nuclei (Fig. 4Aiii, see arrows). In contrast, NEU3 signal was abundant within nuclei (Fig. 4Aiv, see arrows). To confirm nuclear expression of NEU3, immunoblots containing the nuclear fraction of HMVEC-Ls were probed with anti-NEU3 antibody, and NEU3 immunoreactivity was seen (Fig. 4B). These combined studies establish the presence of a relatively abundant intracellular pool of NEU3 in HMVEC-Ls together with localization to the nucleus. Relevant to this finding, Wang et al. (41) have demonstrated NEU3 within the inner nuclear membranes of both rodent and human neuroblastoma cell lines. These combined studies establish the presence of relatively abundant intracellular pools in HMVEC-Ls for both NEU1 and NEU3. NEU1 was more abundant on the HMVEC-L surface than was NEU3, whereas NEU3 could be immunolocalized to the nucleus.
FIGURE 3.
Surface and intracellular pools of NEU1 and NEU3. A, nonpermeabilized HMVEC-Ls were studied by flow cytometry for surface NEU1 and NEU3. B, permeabilized HMVEC-Ls were studied by flow cytometry for total NEU1 and NEU3. HMVEC-Ls, some of which were permeabilized with Triton X-100, were incubated with rabbit polyclonal antibodies raised against human NEU1 or NEU3 or a species-matched control antibody, washed, and incubated with FITC-conjugated goat anti-rabbit antibody. Each plot is representative of three or more independent experiments.
FIGURE 4.
Immunolocalization of NEU1 and -3 in HMVEC-Ls. Ai, subconfluent HMVEC-Ls were fixed, permeabilized, blocked, and incubated with rabbit polyclonal antibodies raised against NEU1 (i and iii) or NEU3 (ii and iv) and counterstained with DAPI (overlay in i and ii). Arrows indicate the perinuclear regions in NEU1-stained (iii) and nuclear localization in NEU3-stained (iv) cells. In each panel, the scale bar represents 50 μm. Each photomicrograph is representative of two or more independent experiments. B, the nuclear fraction of HMVEC-Ls was probed with anti-NEU3 antibody. To verify nuclear fractionation, the blot was stripped and reprobed for the cytoplasmic marker protein, IκBα, and the nuclear marker protein, lamin B1. IB, immunoblot; IB*, immunoblot after stripping. Molecular mass in kDa is indicated on the left. Each blot is representative of two independent experiments.
EC activation increases surface expression of the adhesion molecules, E-selectin and intercellular adhesion molecule-1 (ICAM-1) (1). We asked whether EC sialidase mRNA and/or activity might also be up-regulated upon activation. In HMVEC-Ls incubated for 1 h with TNFα (100 ng/ml), LPS (100 μg/ml), or medium alone, real time RT-PCR revealed no differences between these three conditions for either NEU1 mRNA or NEU3 mRNA (data not shown). Lysates of HMVEC-Ls preincubated for 0.5–2 h with TNFα (100 ng/ml), LPS (100 μg/ml), or media alone were assayed for sialidase activity for the 4-MU-NANA substrate. Total sialidase activity in activated ECs was not increased compared with the medium controls (data not shown). In another study PMNL sialidase activity could be mobilized to the plasma membrane upon activation (32). Accordingly, we asked whether EC sialidase activity might be translocated to the cell surface upon activation. Nonpermeabilized HMVEC-Ls were studied by flow cytometry for surface expression of NEU1 and NEU3 before and after exposure for increasing times to TNFα (100 ng/ml), LPS (100 μg/ml), or medium alone. No increases in surface expression of either NEU1 or NEU3 could be detected for up to 2 h after activation compared with the simultaneous medium controls (data not shown). Collectively, these combined data indicate that HMVEC-Ls express NEU1 and NEU3 both intracellularly and on the EC surface and that their total activity and surface expression was not altered in response to activation.
NEU1 and NEU3 Sialidase Activity in Endothelia
On the basis of real-time RT-PCR (Fig. 2A) and immunoblotting experiments (Fig. 2, B and C), NEU1 and NEU3 are expressed in HMVEC-Ls. We asked whether HMVEC-L-associated sialidase activity might be, in part, explained through NEU1 and/or NEU3. Although transfection of the NEU1-targeting siRNA clearly knocked down NEU1 protein (Fig. 2B), NEU3 depletion was less convincing (Fig. 2C). To unambiguously establish siRNA-induced knockdown of NEU1 and NEU3, epitope-tagged proteins were ectopically expressed in HMVEC-Ls (Fig. 5, A and C). HMVEC-Ls overexpressing FLAG-tagged NEU1 were transfected with NEU1-targeting or control siRNAs, after which the cells were lysed, and the lysates were processed for FLAG tag immunoblotting (Fig. 5B). At 48 h, NEU1 was reduced >95% relative to control siRNA-transfected cells (lane 4 versus 3). When HMVEC-Ls overexpressing HA-tagged NEU3 were transfected with NEU3-targeting siRNA at 48 and 72 h, NEU3 was reduced >95% relative to control siRNA-transfected cells (Fig. 5D, lanes 4 versus 3 and 6 versus 5). Silencing of NEU1 did not knock down NEU3 (lane 6), and silencing of NEU3 did not influence NEU1 protein expression (lane 3) (Fig. 5E). These combined data clearly indicate successful and selective siRNA-induced depletion of either NEU1 or NEU3 in HMVEC-Ls. To establish whether the sialidase activity in HMVEC-Ls could be explained at least in part through either NEU1 or NEU3 expression, HMVEC-Ls transfected with NEU1-targeting, NEU3-targeting, or control siRNAs were lysed at 48 h, and the lysates were fluorometrically assayed for sialidase activity (Fig. 5F). Prior siRNA-mediated knockdown of NEU1 decreased sialidase activity for the 4-MU-NANA substrate by >65%. Prior siRNA-mediated NEU3 depletion diminished sialidase activity by ∼17%. In the ganglioside assay, prior siRNA-induced knockdown of NEU3 decreased sialidase activity for the ganglioside mixture by 40% (Fig. 5G). In contrast, prior knockdown of NEU1 did not alter sialidase activity for the ganglioside substrate. These findings indicate that much of the sialidase activity expressed in HMVEC-Ls can be ascribed to NEU1 and to a lesser degree, NEU3.
FIGURE 5.
NEU1 and NEU3 sialidase activity in HMVEC-Ls. A, HMVEC-Ls were transiently infected with increasing m.o.i. of Ad-NEU1-FLAG. At 48 h, cells were lysed, and the lysates were processed for immunoblotting with anti-FLAG antibodies. B, HMVEC-Ls were transiently infected with Ad-NEU1-FLAG at an m.o.i. of 100. After 48 h, the HMVEC-Ls overexpressing FLAG-tagged NEU1 were transfected with either NEU1-targeting or control siRNAs, cultured for 24 or 48 h, and lysed, and the lysates were processed for immunoblotting with anti-FLAG antibodies. C, HMVEC-Ls were transiently infected with increasing m.o.i. of Ad-NEU3-HA. At 48 h, cells were lysed, and the lysates were processed for immunoblotting with anti-HA antibodies. D, HMVEC-Ls were transiently infected with Ad-NEU3-HA at an m.o.i. = 100. After 48 h, the HMVEC-Ls overexpressing HA-tagged NEU3 were transfected with either NEU3-targeting or control siRNAs, cultured for 24, 48, or 72 h, and lysed, and the lysates were processed for immunoblotting with anti-HA antibodies. E, HMVEC-Ls were transiently infected with either Ad-NEU1-FLAG (lanes 1–3) or Ad-NEU3-HA (lanes 4–6), each at m.o.i. = 100. After 48 h, the cells were transfected with NEU1-targeting (lanes 2 and 6), NEU3-targeting (lanes 3 and 5), or control (lanes 1 and 4) siRNAs, cultured for 48 h, and lysed, and the lysates were processed for immunoblotting with either anti-FLAG (lanes 1–3) or anti-HA (lanes 4–6) antibodies. In A–E, to control for protein loading and transfer, blots were stripped and reprobed for β-tubulin. IB, immunoblot; IB* = immunoblot after stripping. Molecular mass in kDa is indicated on the left. Each blot is representative of three or more independent experiments. F and G, after transfection with NEU1-targeting, NEU3-targeting, and control siRNAs, HMVEC-Ls were fluorometrically assayed for sialidase activity for the 4-MU-NANA substrate (F) or the ganglioside mixture (G). In F and G, vertical bars represent mean (±S.E.) sialidase activity, expressed as either arbitrary fluorescence units (F) or sialic acid release in μm (G). The n for each experimental and control group is indicated below the bars. *, significantly increased compared with background sialidase activity in the cell-free control. **, significantly decreased compared with sialidase activity in lysates of control siRNA-transfected HMVEC-Ls at p < 0.05.
Effect of NEU1 and NEU3 Expression on HMVEC-L Migration in Wounding Assay
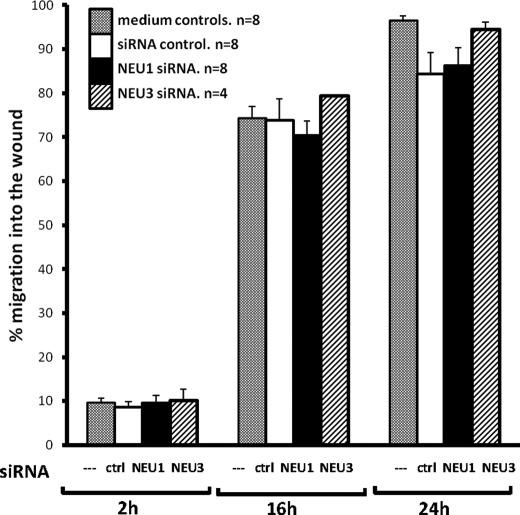
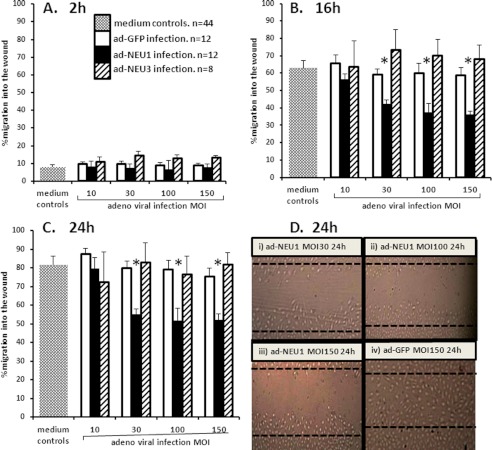
Because endothelia express multiple sialylated receptors that participate in cell migration (14), we asked whether changes in either NEU1 and/or NEU3 expression could be coupled to alterations in the injury-induced migratory response. HMVEC-Ls transfected with NEU1-targeting, NEU3-targeting, or control siRNAs (Fig. 6) or transiently infected with increasing m.o.i. of Ad-NEU1-FLAG, Ad-NEU3-HA, or Ad-GFP (Fig. 7), were studied for migration in a wounding assay. In cells transfected with either NEU1-targeting or NEU3-targeting siRNAs, migration was not different from control siRNA-transfected cells (Fig. 6). These results suggest that 1) neither NEU1 nor NEU3 is absolutely required for the EC migratory response to injury, 2) extremely low levels of either of these two molecules are sufficient, 3) the function of either sialidase overlaps with or is redundant with the function of the other, or 4) the resting EC surface is already maximally sialylated. In cells in which NEU1 was overexpressed, migration was dose-dependently inhibited by 29–44% compared with the simultaneous Ad-GFP controls (Fig. 7, A–C). At 2 h after wounding, no inhibition was seen (Fig. 7A). At both 16 h (Fig. 7B) and 24 h (Fig. 7C), transient infection with Ad-NEU1 at m.o.i. ≥ 30 inhibited EC migration compared with the Ad-GFP infected cells. In contrast, transient infection of cells with Ad-NEU3 at m.o.i. up to 150 did not diminish migration. Representative images of EC migration into the wound at 24 h using HMVEC-Ls infected with Ad-GFP at a m.o.i. of 150 (i) and HMVEC-Ls infected with Ad-NEU1-FLAG at m.o.i. of 30 (ii), 100 (iii), and 150 (iv) are presented (Fig. 7D). Again, of all the manipulations of NEU1 or NEU3 expression in HMVEC-Ls, only NEU1 overexpression altered cell migration into the wound. These combined data indicate that NEU1 restrains EC migration, whereas NEU3 does not.
FIGURE 6.
Effect of NEU1/NEU3 depletion on HMVEC-L migration in a wounding assay. HMVEC-Ls transfected with NEU1-targeting, NEU3-targeting, or control siRNAs were cultured to confluence, after which a single wound was made across each monolayer. At 0, 2, 16, and 24 h, images of each monolayer were captured, and cell migration into the wound after 2, 16, and 24 h was compared with that observed in the same wounded monolayer at 0 h. Vertical bars represent the mean (±S.E.) migration of cells into the wound at 2, 16, and 24 h. n, the number of independent experiments is indicated.
FIGURE 7.
Effect of NEU1/NEU3 overexpression on HMVEC-L migration in a wounding assay. HMVEC-Ls infected with increasing m.o.i. of Ad-NEU1-FLAG, Ad-NEU3-HA, or Ad-GFP were cultured to confluence, after which a single wound was made across each monolayer. At 0, 2, 16, and 24 h, images of each monolayer were captured, and cell migration into the wound after 2, 16, and 24 h was compared with that observed in the same wounded monolayer at 0h. In A, B, and C, vertical bars represent the mean (±S.E.) migration of cells into the wound at 2 h (A), 16 h (B), and 24 h (C). n, numbers of independent experiments are indicated. *, significantly decreased compared with the Ad-GFP infected cells at p < 0.05. D, at 24 h, representative images of wounded HMVEC-L monolayers are shown, including Ad-NEU1, m.o.i. = 30 (i), Ad-NEU1, m.o.i. = 100 (ii), Ad-NEU1, m.o.i. = 150 (iii), Ad-GFP, m.o.i. = 150 (iv). Dotted lines indicate wound boundaries at 0 h.
NEU1 and NEU3 Immunostaining in Vascular Endothelia in Human Tissues
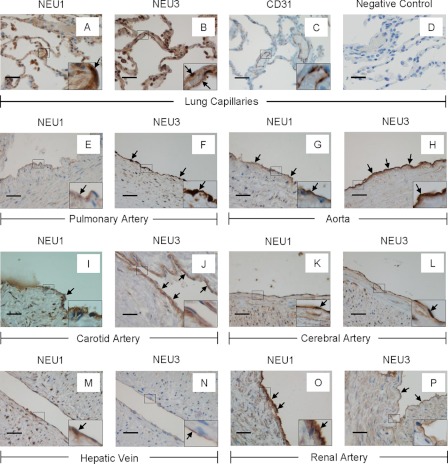
Sialidase activity can be detected in cultured ECs derived from various human endothelia (Fig. 1, D and E), and NEU1 and NEU3 are expressed in HMVEC-Ls (Figs. 2 and 5). We asked whether NEU1 and NEU3 proteins were also expressed in normal human tissues and other vascular endothelia using immunohistochemical techniques in human lung (A, B, E, and F), aorta (G and H), carotid (I and J), and cerebral (K and L) arteries, liver (M and N), and kidney (O and P) (Fig. 8). In the pulmonary microvasculature, NEU1 (A) and NEU3 (B) proteins and CD31 (C), a marker of endothelia (37), each was detected within alveolar septal wall capillaries, whereas sections probed with a species- and isotype-matched control antibody were completely nonreactive (D). Of note, NEU1 and NEU3 signals were also detected in the alveolar epithelium. We then asked whether NEU1 and/or NEU3 might be detected in larger caliber vessels within the same tissues. NEU1 (E) and NEU3 (F) each could be seen in the endothelium lining the pulmonary artery. Therefore, staining of neither NEU1 nor NEU3 was restricted to the endothelium of either microvascular or macrovascular endothelia. Immunostaining for NEU1 and NEU3 were both found in vascular endothelia within the aorta (G and H), carotid artery (I and J), cerebral artery (K and L), hepatic vein (E and F), and renal artery (O and P) (Fig. 8). In all the larger caliber vascular endothelia, neither NEU1 nor NEU3 signal could be detected in the subendothelial adventitia (E–P). Therefore, NEU1 and NEU3 proteins were selectively expressed in both pulmonary and extrapulmonary vascular endothelia, including vessels within the central nervous system (K and L).
FIGURE 8.
NEU1 and NEU3 immunostaining in vascular endothelia in human tissues. Sections of human tissues were probed with rabbit antibodies raised against either human NEU1 or NEU3 followed by biotinylated peroxidase-conjugated goat anti-rabbit IgG antibody and developed with diaminobenzidine and counterstained with hematoxylin. NEU1 and NEU3 immunostaining appears brown and is indicated by arrows. Shown are expanded pulmonary alveoli-NEU1 (A), expanded pulmonary alveoli-NEU3 (B), CD31 immunostaining of expanded pulmonary alveoli (C), a negative control in which sections of expanded lung were incubated with nonimmune rabbit IgG in lieu of the anti-NEU1/3 antibody followed by secondary goat anti-rabbit antibody (D), pulmonary artery-NEU1 (E), pulmonary artery-NEU3 (F), aorta-NEU1 (G), aorta-NEU3 (H), carotid artery-NEU1 (I), carotid artery-NEU3 (J), cerebral artery-NEU1 (K), cerebral artery-NEU3 (L), hepatic vein-NEU1 (M), hepatic vein-NEU3 (N), renal artery-NEU1 (O), and renal artery-NEU3 (P). Each tissue section was photographed at either 400× or 1000× (see the insets). In each panel the scale bar represents 50 μm. Arrows indicate immunostaining for either NEU1 or 3.
DISCUSSION
We now have demonstrated sialidase activity in endothelia (Fig. 1, A, B, D, and E). The EC-associated sialidase activity could be detected in the presence of either 4-MU-NANA or ganglioside substrates, was heat-labile, and was dose-dependently inhibited by the sialidase inhibitor, 2-deoxy-NANA, but not by its negative control, KDO (Fig. 1C). In HMVEC-Ls, NEU1 mRNA was expressed at far greater levels than were mRNAs for NEU2, NEU3, and NEU4 (Fig. 2A). At the protein level, NEU1 and NEU3 each was detected (Fig. 2, B and C). The application of flow cytometry to both permeabilized and nonpermeabilized cells localized more NEU1 than NEU3 signal to the cell surface (Fig. 3, A and B). Using confocal fluorescence microscopy, an intracellular pool of NEU1 could be immunolocalized to the perinuclear region (Fig. 4Ai), whereas NEU3 was detected in both the cytosol and nucleus (Fig. 4A, ii–iv). NEU3 protein was also detected in the nucleus through immunoblotting of nuclear fractions (Fig. 4B). Prior siRNA-induced knockdown of NEU1 and NEU3 diminished sialidase activity in the 4-MU-NANA assay by >65 and >17%, respectively (Fig. 5F) and in the ganglioside assay by 0 and 40%, respectively (Fig. 5G). Overexpression of NEU1 inhibited EC migration into a wound, whereas overexpression of NEU3 did not (Fig. 7). Finally, NEU1 and NEU3 proteins both were detected in the vascular endothelia of normal human tissues (Fig. 8). Our combined data indicate that HMVEC-Ls and likely all endothelia express sialidase activity that can be ascribed in part to NEU1, which restrains the EC migratory response to wounding, and to a lesser extent, to NEU3, which does not.
Although we are not aware of any previous studies of EC expression of one or more sialidase(s), there was a single report in 1988 of desialylation of transferrin by rat liver endothelium (42). However, sialidase activity in various organs, tissues, and cells has been described (20). It has been detected in brain, liver, intestines, skin, kidney, breast, and chorionic tissues (20). Sialidase activity has also been described in formed elements of the blood, including erythrocytes, neutrophils, monocytes, lymphocytes, and platelets (20, 32). In those studies where total tissue sialidase activity was determined, the relative activity of specific sialidases is unclear. Each sialidase has been localized to one or more discrete subcellular compartments where they display differential catalytic activity for various sialic acid linkages that can be further influenced by flanking residues. NEU1 or so-called lysosomal sialidase must operate within a multiprotein complex that includes cathepsin A, also known as protective protein, and β-galactosidase, for optimal catalytic activity (22, 23). The activity of membrane-bound NEU3 is enhanced by detergents (26). Divalent ions (20, 21), pH (20, 21, 26), and free sialic acid (29) each can influence sialidase activity. More recently, tissue surveys of mRNA expression of each of the four known mammalian sialidases have been reported (20, 21). NEU1 is usually expressed at much higher levels than either NEU3 or NEU4. NEU1 was expressed in pancreas > skeletal muscle > kidney > heart > placenta > liver > lung > brain (20, 21, 43). NEU3 and NEU4 expression each was ubiquitous (20, 21). NEU3 was expressed at higher levels in adrenal gland, skeletal muscle, testis, and thymus (26, 44). The highest expression of NEU4 was detected in liver (21). NEU2 was expressed at much lower levels, preferentially in muscle (45). The higher expression of NEU1 relative to the other three sialidases in endothelia (Figs. 2A and 5F) is compatible with these reported tissue surveys. We demonstrated both NEU1 and NEU3 staining in all endothelia examined (Fig. 8). Because vascular endothelia course through all organ systems, they surely contribute to the overall sialidase expression and activity detected in each of these various tissues.
The highly sialylated endothelial surface participates in a number of physiological processes and disease states. Multiple key ligand-counter ligand interactions that involve sialic acid binding lectins tightly regulate extravasation of circulating leukocytes into tissues (1). These EC-leukocyte interactions are central to the host response to septic and other inflammatory processes. Sialylated structures appear to also regulate endothelial adhesiveness for tumor cells (10). NEU1 and NEU3 sialidase-overexpressing tumor cells each displayed reduced experimental pulmonary metastasis (46–48). Metastatic potential did not correlate with overall cellular sialic acid content but in N17 mouse colon adenocarcinoma cells did so with sialyl Lewis X and ganglioside GM3 levels (48). Of interest, sialic acid-containing glycosphingolipids, ganglioside GD1a and GM3, each influence vascular endothelial growth factor (VEGF)-driven angiogenesis (49, 50). GD1a dramatically reduced the threshold for VEGF activation (49), whereas GM3 was anti-angiogenic (50). Components of the clotting (15, 16) and complement (17) pathways are sialylated and respond to changes in their sialylation states. Arterial sialic acid attenuates binding of low density lipoproteins to the vessel wall, and arterial desialylation is associated with increased smooth muscle cell proliferation and neointimal formation (51). It is conceivable that EC sialidases regulate one or more of these biological processes.
The mechanism(s) through which NEU1 overexpression might restrain HMVEC-L migration is unclear. EC migration requires responsiveness to multiple endogenous mediators, EC disengagement from adjacent ECs, and dynamic alternating adhesion/detachment from the underlying extracellular matrix (ECM) (52). Multiple sialoproteins known to participate in the EC migratory response are expressed both on the EC surface and within the underlying ECM (53–59). Potential NEU1 substrates might include the receptors for VEGFs, fibroblast growth factor (FGF), hepatocyte growth factor, insulin-like growth factor-1 (IGF-1), platelet-derived growth factor (PDGF), epidermal growth factor, angiopoietins, insulin growth factor, interleukins, tumor necrosis factor α, and platelet-activating factor (52). In fact, desialylation of the receptor for hepatocyte growth factor, c-Met, expressed on HCT116 colonic cancer cells reportedly abolishes their motility (58). Two other growth factor receptors, VEGF and FGF receptors, each is regulated by gangliosides (53, 60). However, in our wounding assays, overexpression of NEU3, which displays substrate preference for gangliosides (25), did not inhibit EC migration (Fig. 7, B and C). Other sialylated receptors extend across the paracellular space to homophilically interact with identical molecules on neighboring cells (11). The sialylation state of two such receptors, VE-cadherin and platelet endothelial cellular adhesion molecule-1 (PECAM-1), regulates their subcellular distribution (14, 59) and homophilic adhesion (59). ECM components such as collagens, laminins, fibronectin, and fibrin also regulate EC motility (52). During the early steps of angiogenesis, EC-expressed matrix metalloproteases proteolytically modify the ECM, initiating promigratory signals through either generation of proteolytic cleavage products and/or liberation of embedded stimulatory molecules (52). ECs also express multiple heterodimeric adhesion receptors or integrins that bind to and tether ECM proteins to the force-generating actin cytoskeleton (52). Actually, desialylation of β1 integrins has been shown to inhibit EC motility (54, 55). Endothelia express a migration-associated phenotype characterized by up-regulation of binding sites for the lectin, wheat germ agglutinin, and after neuraminidase treatment, peanut agglutinin, implicating sialic acid residues linked to galactose (61). Proinflammatory cytokines known to stimulate EC migration (52) also up-regulate α-2,6-sialyltransferase in ECs (11). Recently, NEU1 has been shown to desialylate the receptors for PDGF-BB and IGF-2 (56) and to physically associate with matrix metalloprotease-9 (62). NEU1 also desialylates integrin β4 in human colon cancer HT-29 cells and suppresses their metastasis (63). Finally, NEU1-mediated desialylation of insulin receptors and IGF-1R in arterial smooth muscle cells regulates their proliferative response to insulin (64). It is conceivable that NEU1-mediated inhibition of EC migration might be explained through such desialylation of one or more of these molecules.
EC-derived sialidase(s) may not only desialylate carbohydrate structures on the endothelium but may also modify such structures on neighboring cells in a paracrine manner. In fact, plasma membrane-associated NEU3 has been shown to modify gangliosides on adjacent cells (65). Furthermore, we have found that activated PMNLs can directly desialylate the endothelial surface (28). Each of the two sialidases expressed in human lung microvascular endothelia, NEU1 and NEU3, either singularly or in concert may provide an additional level of control over sialic acid-based intermolecular and intercellular interactions that occur at the endothelial surface.
Acknowledgments
We thank Shirley Taylor for excellent secretarial support and Kimberly Tuttle for immunohistochemistry expertise.
This work was supported, in whole or in part, by National Institutes of Health Grants HL086933 (to A. S. C.) and HL084223 (to S. E. G.).
- EC
- endothelial cell
- 4-MU-NANA
- 2′-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid
- Ad
- adenovirus
- HBME
- human bone marrow endothelial
- HMVEC-L
- human lung microvascular endothelial cell
- HPAEC
- human pulmonary artery endothelial cell
- HPRT
- hypoxanthine phosphoribosyltransferase
- Hrvt
- human umbilical vein retroviral telemorized endothelial cell
- KDO
- 2-keto-3-deoxyoctulosonic acid
- MFI
- mean fluorescence index
- m.o.i.
- multiplicity of infection
- PMNL
- polymorphonuclear leukocyte
- ST
- sialyltransferase
- VE-cadherin
- vascular endothelial-cadherin
- 2-deoxy-NANA
- 2,3-dehydro-2-deoxy-N-acetyl-neuraminic acid
- ECM
- extracellular matrix
- GM3
- NeuAcα 2,3Galβ1,4Glc-ceramide or N-acetylneuraminylgalactosylceramide
- GD1a
- NeuAcα2-3Galβ1-3GalNAcβ1-4(NeuAcα2-3)Galβ1-4Glcβ1-1Cer.
REFERENCES
- 1. Smith C. W. (2008) Adhesion molecules and receptors. J. Allergy Clin. Immunol. 121, S375–S379 [DOI] [PubMed] [Google Scholar]
- 2. Stevens T., Garcia J. G., Shasby D. M., Bhattacharya J., Malik A. B. (2000) Mechanisms regulating endothelial cell barrier function. Am. J. Physiol. Lung Cell. Mol. Physiol. 279, L419–L422 [DOI] [PubMed] [Google Scholar]
- 3. Varki A. (2008) Sialic acids in human health and disease. Trends Mol. Med. 14, 351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schauer R. (2009) Sialic acids as regulators of molecular and cellular interactions. Curr. Opin. Struct. Biol. 19, 507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Born G. V., Palinski W. (1985) Unusually high concentrations of sialic acids on the surface of vascular endothelia. Br. J. Exp. Pathol. 66, 543–549 [PMC free article] [PubMed] [Google Scholar]
- 6. Constantinescu A. A., Vink H., Spaan J. A. (2003) Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler. Thromb. Vasc. Biol. 23, 1541–1547 [DOI] [PubMed] [Google Scholar]
- 7. Pahakis M. Y., Kosky J. R., Dull R. O., Tarbell J. M. (2007) The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem. Biophys. Res. Commun. 355, 228–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Varki A. (2007) Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature 446, 1023–1029 [DOI] [PubMed] [Google Scholar]
- 9. Phillips M. L., Nudelman E., Gaeta F. C., Perez M., Singhal A. K., Hakomori S., Paulson J. C. (1990) ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science 250, 1130–1132 [DOI] [PubMed] [Google Scholar]
- 10. Walz G., Aruffo A., Kolanus W., Bevilacqua M., Seed B. (1990) Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science 250, 1132–1135 [DOI] [PubMed] [Google Scholar]
- 11. Hanasaki K., Varki A., Stamenkovic I., Bevilacqua M. P. (1994) Cytokine-induced β-galactoside α-2,6-sialyltransferase in human endothelial cells mediates α-2,6-sialylation of adhesion molecules and CD22 ligands. J. Biol. Chem. 269, 10637–10643 [PubMed] [Google Scholar]
- 12. Hanasaki K., Varki A., Powell L. D. (1995) CD22-mediated cell adhesion to cytokine-activated human endothelial cells. Positive and negative regulation by α 2–6-sialylation of cellular glycoproteins. J. Biol. Chem. 270, 7533–7542 [DOI] [PubMed] [Google Scholar]
- 13. Weber K. S., Alon R., Klickstein L. B. (2004) Sialylation of ICAM-2 on platelets impairs adhesion of leukocytes via LFA-1 and DC-SIGN. Inflammation 28, 177–188 [DOI] [PubMed] [Google Scholar]
- 14. Geyer H., Geyer R., Odenthal-Schnittler M., Schnittler H. J. (1999) Characterization of human vascular endothelial cadherin glycans. Glycobiology 9, 915–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Conway E. M., Van de Wouwer M., Pollefeyt S., Jurk K., Van Aken H., De Vriese A., Weitz J. I., Weiler H., Hellings P. W., Schaeffer P., Herbert J. M., Collen D., Theilmeier G. (2002) The lectin-like domain of thrombomodulin confers protection from neutrophil-mediated tissue damage by suppressing adhesion molecule expression via nuclear factor κB and mitogen-activated protein kinase pathways. J. Exp. Med. 196, 565–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ellies L. G., Ditto D., Levy G. G., Wahrenbrock M., Ginsburg D., Varki A., Le D. T., Marth J. D. (2002) Sialyltransferase ST3Gal-IV operates as a dominant modifier of hemostasis by concealing asialoglycoprotein receptor ligands. Proc. Natl. Acad. Sci. U.S.A. 99, 10042–10047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ram S., Sharma A. K., Simpson S. D., Gulati S., McQuillen D. P., Pangburn M. K., Rice P. A. (1998) A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J. Exp. Med. 187, 743–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Broquet P., Baubichon-Cortay H., George P., Louisot P. (1991) Glycoprotein sialyltransferases in eucaryotic cells. Int. J. Biochem. 23, 385–389 [DOI] [PubMed] [Google Scholar]
- 19. Rifat S., Kang T. J., Mann D., Zhang L., Puche A. C., Stamatos N. M., Goldblum S. E., Brossmer R., Cross A. S. (2008) Expression of sialyltransferase activity on intact human neutrophils. J. Leukoc. Biol. 84, 1075–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Achyuthan K. E., Achyuthan A. M. (2001) Comparative enzymology, biochemistry, and pathophysiology of human exo-α-sialidases (neuraminidases). Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 129, 29–64 [DOI] [PubMed] [Google Scholar]
- 21. Monti E., Preti A., Venerando B., Borsani G. (2002) Recent development in mammalian sialidase molecular biology. Neurochem. Res. 27, 649–663 [DOI] [PubMed] [Google Scholar]
- 22. Pshezhetsky A. V., Richard C., Michaud L., Igdoura S., Wang S., Elsliger M. A., Qu J., Leclerc D., Gravel R., Dallaire L., Potier M. (1997) Cloning, expression, and chromosomal mapping of human lysosomal sialidase and characterization of mutations in sialidosis. Nat. Genet. 15, 316–320 [DOI] [PubMed] [Google Scholar]
- 23. Bonten E. J., d'Azzo A. (2000) Lysosomal neuraminidase. Catalytic activation in insect cells is controlled by the protective protein/cathepsin A. J. Biol. Chem. 275, 37657–37663 [DOI] [PubMed] [Google Scholar]
- 24. Monti E., Preti A., Nesti C., Ballabio A., Borsani G. (1999) Expression of a novel human sialidase encoded by the NEU2 gene. Glycobiology 9, 1313–1321 [DOI] [PubMed] [Google Scholar]
- 25. Miyagi T., Wada T., Iwamatsu A., Hata K., Yoshikawa Y., Tokuyama S., Sawada M. (1999) Molecular cloning and characterization of a plasma membrane-associated sialidase specific for gangliosides. J. Biol. Chem. 274, 5004–5011 [DOI] [PubMed] [Google Scholar]
- 26. Monti E., Bassi M. T., Papini N., Riboni M., Manzoni M., Venerando B., Croci G., Preti A., Ballabio A., Tettamanti G., Borsani G. (2000) Identification and expression of NEU3, a novel human sialidase associated to the plasma membrane. Biochem. J. 349, 343–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Comelli E. M., Amado M., Lustig S. R., Paulson J. C. (2003) Identification and expression of Neu4, a novel murine sialidase. Gene 321, 155–161 [DOI] [PubMed] [Google Scholar]
- 28. Sakarya S., Rifat S., Zhou J., Bannerman D. D., Stamatos N. M., Cross A. S., Goldblum S. E. (2004) Mobilization of neutrophil sialidase activity desialylates the pulmonary vascular endothelial surface and increases resting neutrophil adhesion to and migration across the endothelium. Glycobiology 14, 481–494 [DOI] [PubMed] [Google Scholar]
- 29. Cross A. S., Sakarya S., Rifat S., Held T. K., Drysdale B. E., Grange P. A., Cassels F. J., Wang L. X., Stamatos N., Farese A., Casey D., Powell J., Bhattacharjee A. K., Kleinberg M., Goldblum S. E. (2003) Recruitment of murine neutrophils in vivo through endogenous sialidase activity. J. Biol. Chem. 278, 4112–4120 [DOI] [PubMed] [Google Scholar]
- 30. Gong P., Angelini D. J., Yang S., Xia G., Cross A. S., Mann D., Bannerman D. D., Vogel S. N., Goldblum S. E. (2008) TLR4 signaling is coupled to SRC family kinase activation, tyrosine phosphorylation of zonula adherens proteins, and opening of the paracellular pathway in human lung microvascular endothelia. J. Biol. Chem. 283, 13437–13449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. D'Souza D. R., Salib M. M., Bennett J., Mochin-Peters M., Asrani K., Goldblum S. E., Renoud K. J., Shapiro P., Passaniti A. (2009) Hyperglycemia regulates RUNX2 activation and cellular wound healing through the aldose reductase polyol pathway. J. Biol. Chem. 284, 17947–17955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cross A. S., Wright D. G. (1991) Mobilization of sialidase from intracellular stores to the surface of human neutrophils and its role in stimulated adhesion responses of these cells. J. Clin. Invest. 88, 2067–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nan X., Carubelli I., Stamatos N. M. (2007) Sialidase expression in activated human T lymphocytes influences production of IFN-γ. J. Leukoc. Biol. 81, 284–296 [DOI] [PubMed] [Google Scholar]
- 34. Hyun S. W., Anglin I. E., Liu A., Yang S., Sorkin J. D., Lillehoj E., Tonks N. K., Passaniti A., Goldblum S. E. (2011) Diverse injurious stimuli reduce protein-tyrosine phosphatase-μ expression and enhance epidermal growth factor receptor signaling in human airway epithelia. Exp. Lung Res. 37, 327–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang C., Cirielli C., Capogrossi M. C., Passaniti A. (1995) Adenovirus-mediated wild-type p53 expression induces apoptosis and suppresses tumorigenesis of prostatic tumor cells. Cancer Res. 55, 4210–4213 [PubMed] [Google Scholar]
- 36. Liu A., Garg P., Yang S., Gong P., Pallero M. A., Annis D. S., Liu Y., Passaniti A., Mann D., Mosher D. F., Murphy-Ullrich J. E., Goldblum S. E. (2009) Epidermal growth factor-like repeats of thrombospondins activate phospholipase Cγ and increase epithelial cell migration through indirect epidermal growth factor receptor activation. J. Biol. Chem. 284, 6389–6402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Muller W. A., Ratti C. M., McDonnell S. L., Cohn Z. A. (1989) A human endothelial cell-restricted, externally disposed plasmalemmal protein enriched in intercellular junctions. J. Exp. Med. 170, 399–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lukong K. E., Seyrantepe V., Landry K., Trudel S., Ahmad A., Gahl W. A., Lefrancois S., Morales C. R., Pshezhetsky A. V. (2001) Intracellular distribution of lysosomal sialidase is controlled by the internalization signal in its cytoplasmic tail. J. Biol. Chem. 276, 46172–46181 [DOI] [PubMed] [Google Scholar]
- 39. Hinek A., Pshezhetsky A. V., von Itzstein M., Starcher B. (2006) Lysosomal sialidase (neuraminidase-1) is targeted to the cell surface in a multiprotein complex that facilitates elastic fiber assembly. J. Biol. Chem. 281, 3698–3710 [DOI] [PubMed] [Google Scholar]
- 40. Liang F., Seyrantepe V., Landry K., Ahmad R., Ahmad A., Stamatos N. M., Pshezhetsky A. V. (2006) Monocyte differentiation up-regulates the expression of the lysosomal sialidase, Neu1, and triggers its targeting to the plasma membrane via major histocompatibility complex class II-positive compartments. J. Biol. Chem. 281, 27526–27538 [DOI] [PubMed] [Google Scholar]
- 41. Wang J., Wu G., Miyagi T., Lu Z. H., Ledeen R. W. (2009) Sialidase occurs in both membranes of the nuclear envelope and hydrolyzes endogenous GD1a. J. Neurochem. 111, 547–554 [DOI] [PubMed] [Google Scholar]
- 42. Irie S., Kishimoto T., Tavassoli M. (1988) Desialation of transferrin by rat liver endothelium. J. Clin. Invest. 82, 508–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bonten E., van der Spoel A., Fornerod M., Grosveld G., d'Azzo A. (1996) Characterization of human lysosomal neuraminidase defines the molecular basis of the metabolic storage disorder sialidosis. Genes Dev. 10, 3156–3169 [DOI] [PubMed] [Google Scholar]
- 44. Wada T., Yoshikawa Y., Tokuyama S., Kuwabara M., Akita H., Miyagi T. (1999) Cloning, expression, and chromosomal mapping of a human ganglioside sialidase. Biochem. Biophys. Res. Commun. 261, 21–27 [DOI] [PubMed] [Google Scholar]
- 45. Monti E., Preti A., Rossi E., Ballabio A., Borsani G. (1999) Cloning and characterization of NEU2, a human gene homologous to rodent soluble sialidases. Genomics 57, 137–143 [DOI] [PubMed] [Google Scholar]
- 46. Tokuyama S., Moriya S., Taniguchi S., Yasui A., Miyazaki J., Orikasa S., Miyagi T. (1997) Suppression of pulmonary metastasis in murine B16 melanoma cells by transfection of a sialidase cDNA. Int. J. Cancer 73, 410–415 [DOI] [PubMed] [Google Scholar]
- 47. Kato T., Wang Y., Yamaguchi K., Milner C. M., Shineha R., Satomi S., Miyagi T. (2001) Overexpression of lysosomal-type sialidase leads to suppression of metastasis associated with reversion of malignant phenotype in murine B16 melanoma cells. Int. J. Cancer 92, 797–804 [DOI] [PubMed] [Google Scholar]
- 48. Sawada M., Moriya S., Saito S., Shineha R., Satomi S., Yamori T., Tsuruo T., Kannagi R, Miyagi T. (2002) Reduced sialidase expression in highly metastatic variants of mouse colon adenocarcinoma 26 and retardation of their metastatic ability by sialidase overexpression. Int. J. Cancer 97, 180–185 [DOI] [PubMed] [Google Scholar]
- 49. Liu Y., McCarthy J., Ladisch S. (2006) Membrane ganglioside enrichment lowers the threshold for vascular endothelial cell angiogenic signaling. Cancer Res. 66, 10408–10414 [DOI] [PubMed] [Google Scholar]
- 50. Mukherjee P., Faber A. C., Shelton L. M., Baek R. C., Chiles T. C., Seyfried T. N. (2008) Thematic review series. Sphingolipids. Ganglioside GM3 suppresses the proangiogenic effects of vascular endothelial growth factor and ganglioside GD1a. J. Lipid Res. 49, 929–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cuniberti L. A., Martinez V., Schachter J., Magariños G., Meckert P. C., Laguens R. P., Levenson J., Werba J. P. (2005) Sialic acid as a protective barrier against neointima development. Atherosclerosis 181, 225–231 [DOI] [PubMed] [Google Scholar]
- 52. Lamalice L., Le Boeuf F., Huot J. (2007) Endothelial cell migration during angiogenesis. Circ. Res. 100, 782–794 [DOI] [PubMed] [Google Scholar]
- 53. Lin H. Y., Moustakas A., Knaus P., Wells R. G., Henis Y. I., Lodish H. F. (1995) The soluble exoplasmic domain of the type II transforming growth factor (TGF)-β receptor. A heterogeneously glycosylated protein with high affinity and selectivity for TGF-β ligands. J. Biol. Chem. 270, 2747–2754 [DOI] [PubMed] [Google Scholar]
- 54. Semel A. C., Seales E. C., Singhal A., Eklund E. A., Colley K. J., Bellis S. L. (2002) Hyposialylation of integrins stimulates the activity of myeloid fibronectin receptors. J. Biol. Chem. 277, 32830–32836 [DOI] [PubMed] [Google Scholar]
- 55. Seales E. C., Jurado G. A., Brunson B. A., Wakefield J. K., Frost A. R., Bellis S. L. (2005) Hypersialylation of β1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Res. 65, 4645–4652 [DOI] [PubMed] [Google Scholar]
- 56. Hinek A., Bodnaruk T. D., Bunda S., Wang Y., Liu K. (2008) Neuraminidase-1, a subunit of the cell surface elastin receptor, desialylates and functionally inactivates adjacent receptors interacting with the mitogenic growth factors PDGF-BB and IGF-2. Am. J. Pathol. 173, 1042–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nightingale T. D., Frayne M. E., Clasper S., Banerji S., Jackson D. G. (2009) A mechanism of sialylation functionally silences the hyaluronan receptor LYVE-1 in lymphatic endothelium. J. Biol. Chem. 284, 3935–3945 [DOI] [PubMed] [Google Scholar]
- 58. Qian J., Zhu C. H., Tang S., Shen A. J., Ai J., Li J., Geng M. Y., Ding J. (2009) α2,6-Hyposialylation of c-Met abolishes cell motility of ST6Gal-I-knockdown HCT116 cells. Acta Pharmacol. Sin. 30, 1039–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kitazume S., Imamaki R., Ogawa K., Komi Y., Futakawa S., Kojima S., Hashimoto Y., Marth J. D., Paulson J. C., Taniguchi N. (2010) α2,6-Sialic acid on platelet endothelial cell adhesion molecule (PECAM) regulates its homophilic interactions and downstream antiapoptotic signaling. J. Biol. Chem. 285, 6515–6521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rusnati M., Tanghetti E., Urbinati C., Tulipano G., Marchesini S., Ziche M., Presta M. (1999) Interaction of fibroblast growth factor-2 (FGF-2) with free gangliosides. Biochemical characterization and biological consequences in endothelial cell cultures. Mol. Biol. Cell 10, 313–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Augustin-Voss H. G., Pauli B. U. (1992) Migrating endothelial cells are distinctly hyperglycosylated and express specific migration-associated cell surface glycoproteins. J. Cell Biol. 119, 483–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jayanth P., Amith S. R., Gee K., Szewczuk M. R. (2010) Neu1 sialidase and matrix metalloproteinase-9 cross-talk is essential for neurotrophin activation of Trk receptors and cellular signaling. Cell. Signal. 22, 1193–1205 [DOI] [PubMed] [Google Scholar]
- 63. Uemura T., Shiozaki K., Yamaguchi K., Miyazaki S., Satomi S., Kato K., Sakuraba H., Miyagi T. (2009) Contribution of sialidase NEU1 to suppression of metastasis of human colon cancer cells through desialylation of integrin β4. Oncogene 28, 1218–1229 [DOI] [PubMed] [Google Scholar]
- 64. Arabkhari M., Bunda S., Wang Y., Wang A., Pshezhetsky A. V., Hinek A. (2010) Desialylation of insulin receptors and IGF-1 receptors by neuraminidase-1 controls the net proliferative response of L6 myoblasts to insulin. Glycobiology 20, 603–616 [DOI] [PubMed] [Google Scholar]
- 65. Papini N., Anastasia L., Tringali C., Croci G., Bresciani R., Yamaguchi K., Miyagi T., Preti A., Prinetti A., Prioni S., Sonnino S., Tettamanti G., Venerando B., Monti E. (2004) The plasma membrane-associated sialidase MmNEU3 modifies the ganglioside pattern of adjacent cells supporting its involvement in cell-to-cell interactions. J. Biol. Chem. 279, 16989–16995 [DOI] [PubMed] [Google Scholar]