Background: Cytotoxic agents are used to induce cell death to study apoptotic cell-induced immune regulation.
Results: Suppression of macrophage responses by actinomycin D-treated cells does not require phagocytosis but correlates with chemotherapeutic dose and phagocyte apoptosis.
Conclusion: Dead cells can deliver actinomycin D to interacting macrophages.
Significance: Actinomycin D tumor therapy may suppress secondary antitumor responses from immune cells.
Keywords: Anticancer Drug, Apoptosis, Cytokine Induction, Innate Immunity, Macrophages, Actinomycin D, Etoposide
Abstract
Immunosuppression via cell-cell contact with apoptotic cells is a well studied immunological phenomenon. Although the original studies of immune repression used primary cells, which undergo spontaneous cell death or apoptosis in response to irradiation, more recent studies have relied on chemotherapeutic agents to induce apoptosis in cell lines. In this work, we demonstrate that Jurkat cells induced to die with actinomycin D suppressed inflammatory cytokine production by macrophages, whereas cells treated with etoposide did not. This immune repression mediated by actinomycin D-treated cells did not require phagocytosis or cell-cell contact and thus occurs through a different mechanism from that seen with primary apoptotic neutrophils. Moreover, cells induced to die with etoposide and then treated for a short time with actinomycin D also suppressed macrophage responses, indicating that suppression was mediated by actinomycin D independent of the mechanism of cell death. Finally, phagocytosis of actinomycin D-treated cells caused apoptosis in macrophages, and suppression could be blocked by inhibition of caspase activity in the target macrophage. Together, these data indicate that apoptotic cells act as “Trojan horses,” delivering actinomycin D to engulfing macrophages. Suppression of cytokine production by macrophages is therefore due to exposure to actinomycin D from apoptotic cells and is not the result of cell-receptor interactions. These data suggest that drug-induced death may not be an appropriate surrogate for the immunosuppressive activity of apoptotic cells. Furthermore, these effects of cytotoxic drugs on infiltrating immune phagocytes may have clinical ramifications for their use as antitumor therapies.
Introduction
Billions of cells die in the human body each day as part of the normal mechanisms of turnover, repair, and replacement (1). Many of these constitutively dying cells undergo apoptosis, a programmed process characterized by chromatin condensation and fragmentation, loss of membrane symmetry, membrane blebbing, shrinkage of cellular volume, and exposure of “eat me” signals such as phosphatidylserine (2). Apoptotic cells are swiftly recognized and phagocytosed by neighboring cells or specialized phagocytes, including macrophages and dendritic cells. This timely removal of apoptotic cells is essential to maintaining organismal homeostasis. Efficient apoptotic cell clearance is also important in preserving immune tolerance, and defects in this process are associated with chronic inflammation and worsening of the pathology of many autoimmune diseases such as systemic lupus erythematosus, diabetes, and cystic fibrosis (3–6).
Apoptotic cell phagocytosis is complex, utilizing many different receptors, and the precise signaling pathways triggered by apoptotic cell recognition are not fully understood. However, through many studies, it is now well established that the engulfment of apoptotic cells can stimulate profound responses in immune phagocytes. Early studies reported that apoptotic cells suppressed production of inflammatory cytokines such as IL-1β, IL-8, TNF-α, and GM-CSF (7) by monocytes, macrophages, and dendritic cells. This was mediated in part by the production of anti-inflammatory agents, including TGF-β, IL-10, and other soluble mediators (7–10). Many of these initial studies used primary cells such as neutrophils, which die through constitutive apoptosis, or cells induced to die by irradiation. In contrast, cells that die through non-apoptotic mechanisms such as necrosis have been reported to promote inflammatory responses by phagocytes, suggesting that different forms of cell death might have different immunological outcomes.
Such differences in immune response to different forms of cell death have recently been highlighted in the context of cancer. The induction of widespread cell death in the tumor is an important end point of chemotherapeutic strategies. This not only serves to shrink the tumor mass but also provides abundant dead tumor cells, which can be captured and cross-presented by infiltrating antigen-presenting cells to prime antitumor immune responses (11). Recently, it has been reported that different chemotherapeutic drugs can induce either pro- or anti-inflammatory forms of apoptosis (12). Specifically, cells treated with actinomycin D, an antibiotic derived from Streptomyces that binds DNA and inhibits transcription, are anti-inflammatory, whereas cells that die after treatment with etoposide, a topoisomerase inhibitor derived from the herbaceous plant Podophyllum peltatum, are proinflammatory. The mechanisms by which these drugs elicit differential immune responses are not known.
Here, we took advantage of the observed differences in the immune response after exposure to actinomycin D- and etoposide-treated cells to explore what defines the suppressive versus inflammatory phenotype seen with these different types of dying cells. Our observations led us to conclude that apoptotic cells generated by treatment with different chemotherapeutics have the capacity to function as “Trojan horses,” delivering the drug to engulfing macrophages and thereby suppressing the inflammatory response.
EXPERIMENTAL PROCEDURES
Cell Cultures
J774A.1 murine primary macrophages and Jurkat T cells were obtained from American Type Culture Collection. J774 cells were grown in DMEM and GlutaMAXTM-I 10569 medium supplemented with 10% heat-inactivated FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. Jurkat T cells were grown in RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mm l-glutamine. All cell culture reagents were obtained from Invitrogen.
Generation of Apoptotic Cells
Apoptosis of Jurkat T cells was induced by culturing cells overnight at 1 × 106 cells/ml with either 100 μm etoposide or 0.078–5 μg/ml actinomycin D (both from Sigma). Where indicated, Jurkat T cells were also pretreated with 100 μm Caspase Inhibitor I (benzyloxycarbonyl-valyl-alanyl-aspartyl-(O-methyl)-fluoromethyl ketone (Z-VAD-fmk)5; EMD4Biosciences, San Diego, CA) for 3 h prior to induction of apoptosis overnight with actinomycin D at 2.5 μg/ml. Apoptosis of Jurkat T cells was confirmed via FACS analysis by staining cells with Alexa Fluor 488-labeled annexin V (AV; Invitrogen) and propidium iodide (PI; Sigma) according to the manufacturers' instructions. Live cells were defined as both AV- and PI-negative, apoptotic cells as AV-positive and PI-negative, and necrotic cells as both AV- and PI-positive. Where indicated, FACS analysis was gated on J774 cells only, stained for the macrophage marker F4/80 (eBioscience, San Diego, CA), therefore excluding all Jurkat cells. Apoptosis of this population was confirmed by staining with allophycocyanin-conjugated AV (BD Biosciences) and PI according to the manufacturers' instructions.
Macrophage and Apoptotic Jurkat T Cell Co-culture
J774 macrophages were plated at 1 × 105 cells/ml in a 48-well plate and left to adhere overnight. The following day, the medium was replaced with fresh DMEM, and J774 macrophages were co-cultured for 3 h with apoptotic Jurkat cells at a 5:1 ratio of Jurkat cells to macrophages. Cells were then stimulated with LPS (InvivoGen, San Diego, CA) at 20 ng/ml. Culture supernatants were collected after 18–20 h and stored at −80 °C until analysis of cytokine levels by ELISA (DuoSet ELISA system, R&D Systems). Experimental conditions were repeated in triplicate and run in duplicate on the ELISA plates. Where indicated, J774 macrophages were pretreated prior to co-culture with either the phagocytosis inhibitor cytochalasin D (Sigma) for 1 h at 10 μm or Caspase Inhibitor I for 3 h at 100 μm. For experiments in which cells were co-cultured in Transwell chambers, J774 macrophages were plated at 1.28 × 106 cells/ml in 6-well plates. The following day, apoptotic Jurkat T cells were added to the upper chamber of a 0.4-μm pore Transwell (Corning, Lowell, MA) in a total volume of 3 ml/well.
Statistical Analysis
The data were analyzed using one-way analysis of variance (ANOVA). The significance level was set as p < 0.05. All statistical analyses were performed using Microsoft Excel 2010. Data are presented as means ± S.E. from three independent experiments.
RESULTS
Actinomycin D-killed, but Not Etoposide-killed, Jurkat T Cells Suppress Cytokine Secretion from LPS-stimulated Macrophages
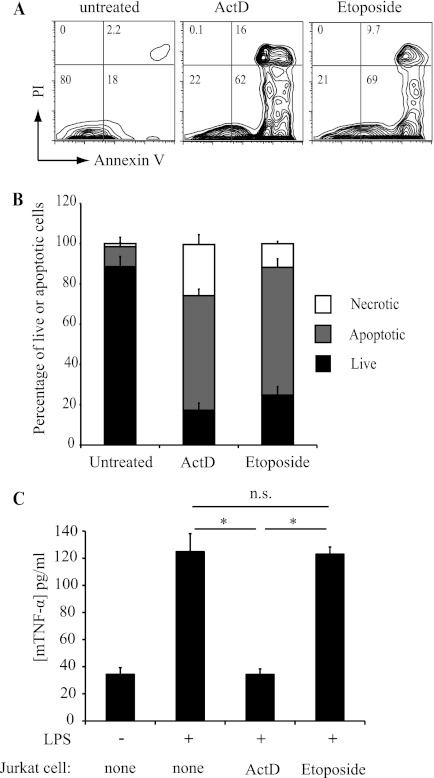
We first characterized apoptotic cell death in Jurkat T cells treated with actinomycin D and etoposide, which have been reported to induce anti-inflammatory and inflammatory cell death, respectively (12). After 16 h of treatment, we could define three populations based on FACS analysis with AV and PI staining: live cells that were AV- and PI-negative, apoptotic cells that were AV-positive and PI-negative, and necrotic cells that were AV- and PI-positive (Fig. 1, A and B). Both actinomycin D and etoposide induced similar levels of cell death (∼80%) in Jurkat T cells, although actinomycin D-treated cells included a slightly higher proportion of necrotic cells at 16 h (20% versus 10%) (Fig. 1B). We next tested the ability of apoptotic Jurkat cells to suppress inflammatory cytokine production by macrophages. Mouse J774 macrophages were cultured with actinomycin D- or etoposide-treated Jurkat T cells for 3 h and stimulated with LPS, and cytokine production was measured. Co-culture with actinomycin D-treated Jurkat cells completely suppressed LPS-induced TNF-α production by macrophages (Fig. 1C). This suppression is similar in magnitude to that seen with constitutively apoptotic neutrophils (7, 9). In contrast, etoposide-treated Jurkat cells had no effect on macrophage cytokine production (Fig. 1C). These results are consistent with published studies (12) and indicate that cells that die in response to different drugs can evoke distinct effects on inflammatory responses by engulfing macrophages.
FIGURE 1.
Differential immune response of macrophages to cells killed by etoposide or actinomycin D. A, Jurkat T cells were treated overnight with etoposide (100 μm) or actinomycin D (ActD; 2.5 μg/ml), stained for AV and PI, and analyzed by FACS. Flow cytometry plots are of total cells, and numbers in quadrants indicate percentages of live (AV- and PI-negative), apoptotic (AV-positive and PI-negative), and necrotic (AV- and PI-positive) cells. B, bar graph representation of FACS data shown in A combined from three independent experiments (means ± S.E.). C, TNF-α production by J774 macrophages (mTNFα) co-incubated with either etoposide- or actinomycin D-treated Jurkat T cells for 3 h and then stimulated overnight with LPS (20 ng/ml). Data are presented as means ± S.E. from three independent experiments. *, statistically significant differences between the indicated values (p < 0.05; ANOVA). n.s., not statistically significantly different.
Apoptotic Cell-Macrophage Contact Is Not Required for Suppression of Cytokines by Actinomycin D-killed Jurkat T Cells
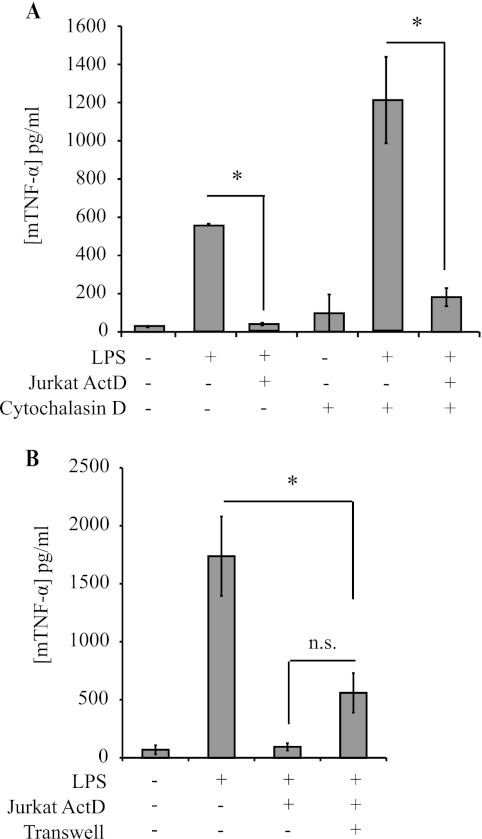
We, and others, have shown previously that the anti-inflammatory effects of neutrophils that undergo constitutive apoptosis require direct contact with macrophages and phagocytosis in the case of dendritic cells (7, 9, 10, 13). We therefore wished to determine whether the anti-inflammatory effect of actinomycin D-treated cells is similarly dependent on direct engagement by phagocytes. Phagocytosis of apoptotic Jurkat cells was blocked by pretreatment of macrophages with cytochalasin D, an inhibitor of actin polymerization (data not shown). However, even in the presence of cytochalasin D, actinomycin D-treated Jurkat cells suppressed TNF-α production by macrophages (Fig. 2A), indicating that phagocytosis was not necessary for the anti-inflammatory effects. To test whether cell-cell contact is required, macrophages and apoptotic Jurkat cells were co-cultured in Transwell chambers, separated by a membrane with 0.4-μm pores. Despite the lack of direct contact with actinomycin D-treated Jurkat cells, macrophage responses to LPS were significantly blunted, albeit less effectively than when cells were in contact (Fig. 2B). These data indicate that contact is not required and suggest that a soluble mediator is responsible for the suppressive phenotype. Moreover, these data suggest that suppression of the macrophage immune response mediated by actinomycin D-killed Jurkat T cells is distinct from the contact-dependent suppression mediated by apoptotic neutrophils that we have reported previously (9, 10, 13).
FIGURE 2.
Cell-to-cell contact is not necessary for inhibition of TNF-α production by macrophages. A, TNF-α production by J774 macrophages (mTNF-α) pretreated with the phagocytosis inhibitor cytochalasin D for 1 h, followed by co-culture with actinomycin D (ActD)-treated Jurkat T cells and LPS stimulation as described in the legend to Fig. 1. B, TNF-α production by J774 macrophages co-incubated directly with actinomycin D-treated Jurkat T cells (as in Fig. 1) or separated by filters with 0.4-μm pores (Transwell) and treated with LPS. Data are presented as means ± S.E. from three independent experiments. *, statistically significant differences between the indicated values (p < 0.05; ANOVA). n.s., not statistically significantly different.
Inhibition of Cytokine Production in Response to Actinomycin D-killed Jurkat T Cells Is Proportional to Dosage of Actinomycin D
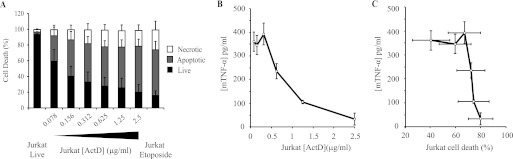
We then set out to understand better the relationship between actinomycin D-induced cell death and immunosuppression. Increasing doses of actinomycin D were used to produce apoptotic Jurkat T cells, which were then tested for their ability to suppress macrophage cytokine production. As expected, the proportion of dead cells (both apoptotic and necrotic) correlated closely with the concentration of actinomycin D used (Fig. 3A) and reached a plateau of 70–80% dead cells at 0.625 μg/ml actinomycin D. Above this concentration, the proportion of either apoptotic or necrotic cells did not increase significantly.
FIGURE 3.
Macrophage response is dependent on dose of actinomycin D used to induce Jurkat T cell death. A, induction of Jurkat cell death by increasing doses of actinomycin D. Jurkat T cells were treated overnight with increasing doses of actinomycin D (ActD; 0.078–2.5 μg/ml), and cell death was analyzed by FACS (as described in the legend to Fig. 1). B, TNF-α production by J774 macrophages (mTNFα) co-cultured with Jurkat T cells previously treated with the indicated amounts of actinomycin D, followed by LPS stimulation as in Fig. 1. C, combined data from A and B showing the relationship between the extent of Jurkat cell death and TNF-α production by co-cultured macrophages for increasing doses of actinomycin D. In all cases, data are presented as means ± S.E. from three independent experiments.
The degree of suppression of TNF-α production by macrophages incubated with dead Jurkat cells was also proportional to the dose of actinomycin D used to induce Jurkat cell death (Fig. 3B). Surprisingly, there appeared to be a closer correlation of macrophage response with the dose of actinomycin D used than with the degree of cell death itself (Fig. 3C). Hence, the biggest change in TNF-α production occurred between 0.312 and 2.5 μg/ml actinomycin D, whereas there was no statistically significant change in the number of apoptotic cells over this range (Fig. 3C). Therefore, it seemed likely that the suppression of macrophage responses by actinomycin D-treated Jurkat cells was due to an effect of actinomycin D, over and above the induction of cell death in the Jurkat cells.
Etoposide-killed Jurkat T Cells Suppress Cytokine Secretion When Transiently Loaded with Actinomycin D
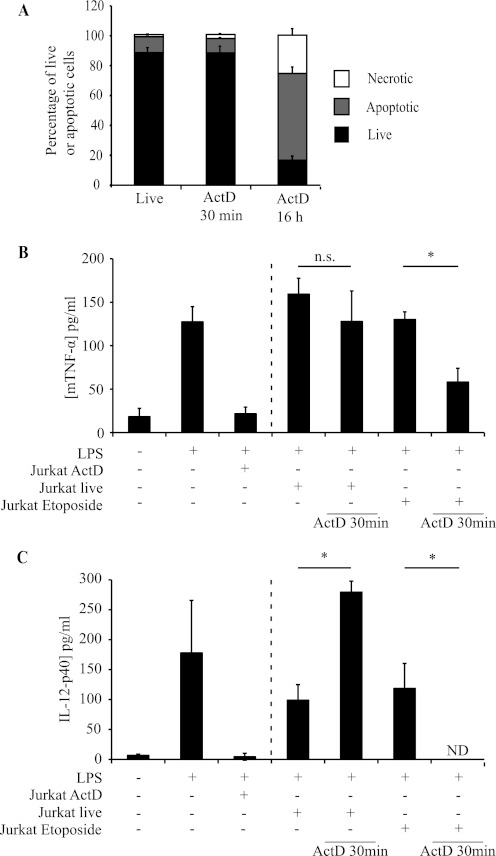
To test whether actinomycin D treatment of Jurkat cells suppresses macrophages through mechanisms independent of cell death, we performed two experiments. First, we treated Jurkat cells with actinomycin D for only 30 min before washing and co-culturing them with macrophages. This time period was not sufficient to induce death of the Jurkat cells (Fig. 4A), and these 30 min-treated Jurkat cells failed to inhibit TNF-α and IL-12p40 secretion by macrophages (Fig. 4B). We then treated Jurkat cells that had undergone apoptosis in response to etoposide with actinomycin D for 30 min and incubated them with macrophages. This short-term incubation with actinomycin D was sufficient to allow etoposide-treated Jurkat cells to suppress macrophage cytokine responses (Fig. 4, B and C).
FIGURE 4.
Etoposide-killed Jurkat T cells transiently exposed to actinomycin D inhibit TNF-α secretion. A, induction of Jurkat cell death by short (30 min) and long (16 h) exposures to actinomycin D (ActD; 2.5 μg/ml). Cell death was assessed by FACS as described in the legend to Fig. 1. B and C, TNF-α (B) and IL-12p40 (C) production by J774 macrophages co-incubated with Jurkat T cells treated as indicated, followed by LPS stimulation. Data are presented as means ± S.E. from three independent experiments. *, statistically significant differences between the indicated values (p < 0.05; ANOVA). n.s., not statistically significantly different; ND, none detectable.
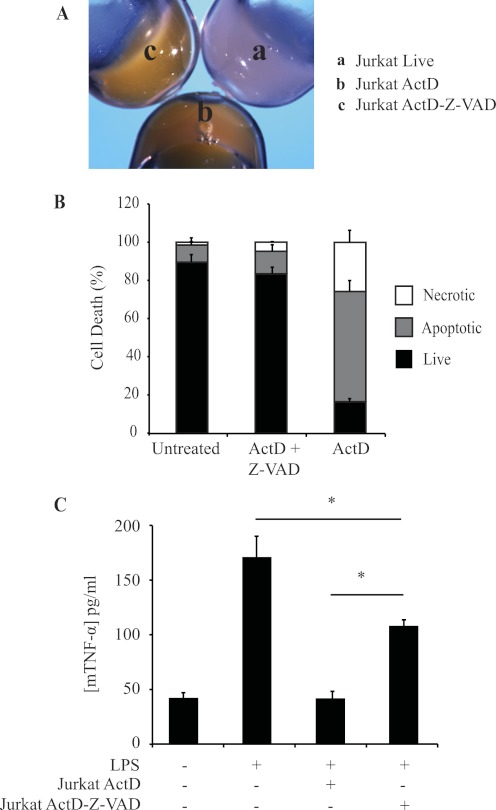
To study further the requirement for cell death in suppression of macrophage responses by actinomycin D-treated Jurkat cells, we blocked apoptosis using Z-VAD-fmk, a pan-caspase inhibitor. After a 16-h treatment with actinomycin D, both control and Z-VAD-fmk-treated Jurkat T cells were yellow (Fig. 5A), indicating that they were loaded with actinomycin D, which carries an orange/yellow coloring. However, in the presence of Z-VAD-fmk, the cells maintained their membrane integrity and did not become AV- or PI-positive (Fig. 5B); we termed these “undead” cells. Z-VAD-fmk-treated undead cells were much less efficient at suppressing macrophage cytokine production TNF-α than apoptotic Jurkat cells (Fig. 5C). Taken together, these data indicate that it is necessary for Jurkat cells to be both dead and actinomycin D-treated to suppress macrophage responses.
FIGURE 5.
Caspase Inhibitor I rescues inhibition of TNF-α secretion in pretreated actinomycin D-killed Jurkat T cells following incubation with macrophages and LPS. A, cell pellets containing 30 × 106 live (a), actinomycin D (ActD)-treated (b), and Caspase Inhibitor I (Z-VAD-fmk)/actinomycin D-treated (c) Jurkat cells. The yellow color of the cell pellets in b and c indicate that Z-VAD-fmk cells take up actinomycin D. B, induction of Jurkat cell death after a 3-h pretreatment with Z-VAD-fmk, followed by actinomycin D (2.5 μg/ml) overnight and analysis by FACS as described in the legend to Fig. 1. C, TNF-α production by J774 macrophages (mTNFα) co-cultured with Jurkat T cells previously treated with actinomycin D and Z-VAD-fmk as indicated, followed by LPS stimulation as in Fig. 1. Data are presented as means ± S.E. from three independent experiments. *, statistically significant differences between the indicated values (p < 0.05; ANOVA).
Actinomycin D Released from Membrane-permeable Apoptotic Jurkat T Cells Mediates Suppression of Cytokine Secretion
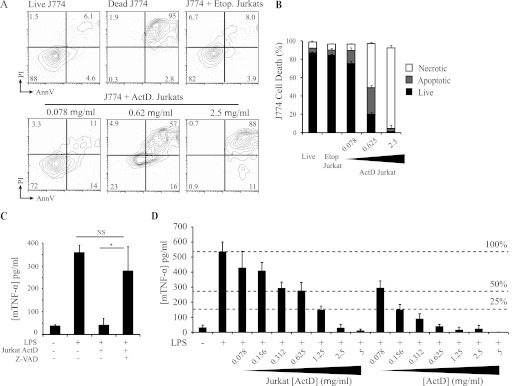
On the basis of these results and the close correlation between the dose of actinomycin D used to kill the Jurkat T cells and the level of suppression in macrophages, we hypothesized that the suppression of macrophage responses may be due to direct effects of actinomycin D delivered by Jurkat cells. To test this, we first assessed the extent of macrophage cell death following incubation with etoposide- or actinomycin D-treated Jurkat cells. Consistent with our hypothesis, actinomycin D-treated Jurkat cells triggered apoptosis of co-cultured macrophages, whereas etoposide-treated Jurkat cells did not (Fig. 6, A and B). To test whether macrophage death is responsible for the suppression of cytokine production, we blocked apoptosis in macrophages cultured with actinomycin D-treated Jurkat cells. Z-VAD-fmk treatment of macrophages both prevented cell death induced by actinomycin D-treated Jurkat cells and restored TNF-α production (Fig. 6C). These results strongly implicate apoptosis caused by delivery of actinomycin D from dead Jurkat cells to engulfing macrophages as the mechanism of inflammatory cytokine suppression.
FIGURE 6.
Caspase Inhibitor I rescues inhibition of TNF-α secretion in pretreated macrophages following incubation with actinomycin D-killed Jurkat T cells and LPS. A and B, induction of cell death in J774 macrophages co-cultured with Jurkat cells treated with etoposide (Etop.) or the indicated doses of actinomycin D (ActD). A, FACS analysis was gated on J774 cells only (by staining for the macrophage marker F4/80) and excluded all Jurkat cells. Representative untreated J774 cells (Live J774) and J774 cells induced to die by direct treatment with actinomycin D (Dead J774) are shown for comparison. AnnV, annexin V. B, combined data from three independent co-cultures of J774 and Jurkat cells. C, TNF-α production by J774 macrophages (mTNF-α) pretreated with Z-VAD-fmk or untreated, followed by co-culture with Jurkat T cells and LPS stimulation as described in the legend to Fig. 1. Data are presented as means ± S.E. from three independent experiments. *, statistically significant differences between the indicated values (p < 0.05; ANOVA). NS, not statistically significantly different. D, TNF-α production by J774 macrophages treated directly with actinomycin D ([ActD]) or co-cultured with Jurkat T cells previously treated with actinomycin D (Jurkat [ActD]), followed by overnight stimulation with LPS. Dashed lines represent response of untreated macrophages (100%) and suppression of macrophage response to 50 and 25% of untreated levels.
Our results were consistent with suppression of macrophage responses being mediated by actinomycin D that leaked from permeable apoptotic Jurkat T cells and that compromised the viability of the engulfing macrophages. To test this, we treated macrophages with actinomycin D directly and compared their response to macrophages co-cultured with actinomycin D-treated Jurkat cells. Actinomycin D-inhibited macrophage TNF-α production was similar to that seen with actinomycin D-treated Jurkat cells (Fig. 6D). However, 10-fold less actinomycin D was required to achieve equivalent suppression of cytokine production in direct treatment of macrophages compared with actinomycin D delivery through Jurkat cells (Fig. 6D). Taken together, these data indicate that actinomycin D not only induces cell death in Jurkat T cells treated directly with the chemical but also compromises the viability of phagocytes that encounter or engulf the dying cells.
DISCUSSION
Here, we investigated the mechanisms by which cells induced to die with different chemotherapeutic drugs elicit differential immunological responses. Recent work has demonstrated that although phagocytosed equally well by macrophages, Jurkat T cells killed by etoposide are proinflammatory, whereas those killed by actinomycin D are immunosuppressive (12). However, the mechanisms by which actinomycin D-killed cells suppressed the immune response of the engulfing cells could not be determined.
In an attempt to understand the mechanisms of immunosuppression seen with apoptotic cells, we have focused on the response to apoptotic cell death induced using these two drugs, etoposide and actinomycin D. Consistent with observations made by Kozmar et al. (12), our results indicate that although these drugs induce comparable levels of cell death, the immunological outcomes are strikingly different: etoposide-killed cells do not suppress the secretion of the proinflammatory cytokines TNF-α and IL-12p40, whereas actinomycin D-killed cells actively suppress TNF-α and IL-12p40 secretion in a dose-dependent manner. We have also observed similar suppression of primary mouse bone marrow macrophages. Furthermore, this phenomenon is not limited to mammalian immune cells, as Drosophila S2 cells treated with actinomycin D suppressed both S2 cell and human 293T cell inflammatory responses, whereas no suppression was seen with S2 cells killed using etoposide or other drugs.6 Hence, the ability of actinomycin D-killed cells to inhibit the immune response is conserved across species.
In an effort to establish the mechanism by which actinomycin D-killed cells modify macrophages, we first addressed whether direct contact or phagocytosis is required. Our results show that neither phagocytosis nor direct cell-cell contact is required for the suppression of LPS-mediated TNF-α secretion by actinomycin D-killed cells. These data led us to hypothesize that a soluble mediator released from the dying cells was responsible for the suppression. Further experiments aimed at characterizing this factor showed that actinomycin D itself is the likely mediator of the suppressive phenotype. Confirming this, etoposide-killed cells loaded with actinomycin D became suppressive. However, the cells did need to lose membrane integrity to deliver the toxic agent. Furthermore, by using Z-VAD-fmk to block the effects of actinomycin D on the macrophages, we confirmed that actinomycin D is the soluble factor responsible for the suppressive effects rather than the apoptotic cell per se. Although we propose that actinomycin D leaks directly into the culture medium, given our transmembrane co-culture system, we cannot exclude the possibility that some actinomycin D is transferred via microvesicles smaller than 400 nm.
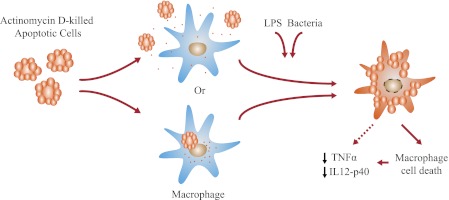
Taken together, our data identify one mechanism by which apoptotic cells can interact with macrophages and modulate their immune response. In this model, apoptotic Jurkat T cells killed by actinomycin D not only die but also retain significant quantities of the drug despite extensive washing. Once the dead cells come into proximity with macrophages, we propose that actinomycin D leaks through the compromised plasma membrane into the culture supernatant or directly into the engulfing cells. This in turn compromises the viability of the surrounding macrophages (Fig. 7). It is notable that actinomycin D exerts its effects by binding to double- and single-stranded DNAs (14, 15). The cell nucleus therefore provides a large potential “sink” for DNA-binding compounds. Furthermore, as nuclear material is preferentially exposed on the surface of dying cells, cytotoxic drugs that directly target DNA may be more efficiently presented to interacting phagocytes. These two reasons may explain the differences we report between actinomycin D and etoposide, which causes DNA damage indirectly by inhibiting topoisomerase function (16, 17).
FIGURE 7.
Actinomycin D mode of action. Shown is the proposed model of cytokine inhibition by macrophages incubated with actinomycin D-killed Jurkat T cells as presented under “Discussion.”
Importantly, although actinomycin D-treated cells superficially mimic the immunosuppressive effect seen with constitutively apoptotic neutrophils, they are distinct. Our previous work demonstrated that cell contact is both necessary and sufficient for the immunosuppressive effect of apoptotic neutrophils on macrophages (13). We therefore proposed a “kiss and tell” model in which apoptotic neutrophils first engage with molecular receptors present on the surface of macrophages to establish direct cell-cell contact and stimulate phagocytosis and also activate the production of anti-inflammatory cytokines, including TGF-β (13). Thus, for neutrophils, suppression occurs without exposure to a drug and involves both cell-cell contact and TGF-β secretion. In contrast, in this system, apoptotic cells serve as a mechanism to concentrate and deliver cytotoxic drugs to macrophages. Therefore, although we have made an important discovery as to how some apoptotic cells might modulate immune responses, this is not the mechanism by which neutrophils and other constitutively apoptotic cells exert their suppressive effects. Importantly, this study highlights a potential pitfall in considering drug-induced apoptotic cells as surrogates for cells that have undergone constitutive apoptosis, particularly when considering their effects on phagocytes.
Both actinomycin D and etoposide are used in chemotherapy to treat a variety of cancers. In this context, the ideal anticancer therapy should not only eradicate tumor cells but also have the potential to harness the power of the adaptive immune system to eliminate micrometastases and tumor stem cells. Recent work by Obeid et al. (11, 18) has shown that most cell death inducers that cause damage to DNA, mitochondria, lysosomes, or the endoplasmic reticulum (E9R) do not elicit an immunogenic response, whereas other chemicals belonging to the family of anthracyclines, as well as γ-irradiation and UVC light, do. The mechanisms for these responses are unknown. Immunogenic cell death is characterized by the translocation of calreticulin to the cell surface of apoptotic cells, where it behaves as an “eat me” signal to dendritic cells (11, 19). This exposure leads to apoptotic cell recognition via LRP1 (lipoprotein receptor-related protein-1), tumor antigen presentation, and ultimately tumor-specific T cell-dependent responses (19). As such, immunogenic cell death would be more favorable when eradicating tumors and eliciting antitumor immune responses. We suggest that although actinomycin D does kill tumor cells, it might negatively impact the surrounding environment by infiltrating immune cells. Specifically, by functioning as Trojan horses, actinomycin D-loaded cells may disrupt the establishment of antitumor immunity.
In summary, the details of many aspects of the interaction between apoptotic cells and professional phagocytes remain elusive. Various reports now argue that the immunological responses to cell death vary depending on the type of cell death, the death stimulus used, the type of cell undergoing death, the phagocytic cell that comes in contact with it, and the surrounding environment in which the interaction occurs. Understanding how different ways of dying affect immunological outcomes is key to many aspects of health and disease, including cancer management, differential responses to viral and bacterial infections, inflammation, and ultimately the development of autoimmune diseases. Our studies have emphasized the importance of understanding the mechanisms that underlie how different modes of cell death impact the immunological outcome. Moreover, how this might impact choices for chemotherapy is clinically relevant. As an example, future studies will be required to test whether actinomycin D-treated cells might contribute to tolerance in vivo by acting as Trojan horses that impair the function of infiltrating immune cells and to establish how important this balance may be for efficient cancer immunization therapies.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1AI79198 and U24A1082660 (to L. M. S.), NIH R25GM076321 (to R. S.), and 5RO1 GM069541 (to K. W.) and a National Science Foundation Grant EHR0412390 (to R. S.). Additionally, N. P. was supported by NIH/NERCE Grant U54AI057159, and H. P. was supported by UK Medical Research Council Grant G0802069.
B. Zhai, K. White, N. Paquette, and L. M. Stuart, unpublished data.
- Z-VAD-fmk
- benzyloxycarbonyl-valyl-alanyl-aspartyl-(O-methyl)-fluoromethyl ketone
- AV
- annexin V
- PI
- propidium iodide
- ANOVA
- analysis of variance.
REFERENCES
- 1. Stark M. A., Huo Y., Burcin T. L., Morris M. A., Olson T. S., Ley K. (2005) Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 22, 285–294 [DOI] [PubMed] [Google Scholar]
- 2. Kroemer G., Galluzzi L., Vandenabeele P., Abrams J., Alnemri E. S., Baehrecke E. H., Blagosklonny M. V., El-Deiry W. S., Golstein P., Green D. R., Hengartner M., Knight R. A., Kumar S., Lipton S. A., Malorni W., Nuñez G., Peter M. E., Tschopp J., Yuan J., Piacentini M., Zhivotovsky B., Melino G. (2009) Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 16, 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herrmann M., Voll R. E., Zoller O. M., Hagenhofer M., Ponner B. B., Kalden J. R. (1998) Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 41, 1241–1250 [DOI] [PubMed] [Google Scholar]
- 4. Ren Y., Tang J., Mok M. Y., Chan A. W., Wu A., Lau C. S. (2003) Increased apoptotic neutrophils and macrophages and impaired macrophage phagocytic clearance of apoptotic neutrophils in systemic lupus erythematosus. Arthritis Rheum. 48, 2888–2897 [DOI] [PubMed] [Google Scholar]
- 5. Vandivier R. W., Fadok V. A., Ogden C. A., Hoffmann P. R., Brain J. D., Accurso F. J., Fisher J. H., Greene K. E., Henson P. M. (2002) Impaired clearance of apoptotic cells from cystic fibrosis airways. Chest 121, suppl., 89S [DOI] [PubMed] [Google Scholar]
- 6. O'Brien B. A., Fieldus W. E., Field C. J., Finegood D. T. (2002) Clearance of apoptotic β-cells is reduced in neonatal autoimmune diabetes-prone rats. Cell Death Differ. 9, 457–464 [DOI] [PubMed] [Google Scholar]
- 7. Fadok V. A., Bratton D. L., Konowal A., Freed P. W., Westcott J. Y., Henson P. M. (1998) Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Invest. 101, 890–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Voll R. E., Herrmann M., Roth E. A., Stach C., Kalden J. R., Girkontaite I. (1997) Immunosuppressive effects of apoptotic cells. Nature 390, 350–351 [DOI] [PubMed] [Google Scholar]
- 9. Lucas M., Stuart L. M., Savill J., Lacy-Hulbert A. (2003) Apoptotic cells and innate immune stimuli combine to regulate macrophage cytokine secretion. J. Immunol. 171, 2610–2615 [DOI] [PubMed] [Google Scholar]
- 10. Stuart L. M., Lucas M., Simpson C., Lamb J., Savill J., Lacy-Hulbert A. (2002) Inhibitory effects of apoptotic cell ingestion upon endotoxin-driven myeloid dendritic cell maturation. J. Immunol. 168, 1627–1635 [DOI] [PubMed] [Google Scholar]
- 11. Obeid M., Tesniere A., Ghiringhelli F., Fimia G. M., Apetoh L., Perfettini J. L., Castedo M., Mignot G., Panaretakis T., Casares N., Métivier D., Larochette N., van Endert P., Ciccosanti F., Piacentini M., Zitvogel L., Kroemer G. (2007) Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 13, 54–61 [DOI] [PubMed] [Google Scholar]
- 12. Kozmar A., Greenlee-Wacker M. C., Bohlson S. S. (2010) Macrophage response to apoptotic cells varies with the apoptotic trigger and is not altered by a deficiency in LRP expression. J. Innate Immun. 2, 248–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lucas M., Stuart L. M., Zhang A., Hodivala-Dilke K., Febbraio M., Silverstein R., Savill J., Lacy-Hulbert A. (2006) Requirements for apoptotic cell contact in regulation of macrophage responses. J. Immunol. 177, 4047–4054 [DOI] [PubMed] [Google Scholar]
- 14. Waring M. J. (1981) DNA modification and cancer. Annu. Rev. Biochem. 50, 159–192 [DOI] [PubMed] [Google Scholar]
- 15. Pan J. X., Liu Y., Zhang S. P., Tu T. C., Yao S. D., Lin N. Y. (2001) Photodynamic action of actinomycin D: an EPR spin trapping study. Biochim. Biophys. Acta 1527, 1–3 [DOI] [PubMed] [Google Scholar]
- 16. Burden D. A., Osheroff N. (1998) Mechanism of action of eukaryotic topoisomerase II and drugs targeted to the enzyme. Biochim. Biophys. Acta 1400, 139–154 [DOI] [PubMed] [Google Scholar]
- 17. Imbert T. F. (1998) Discovery of podophyllotoxins. Biochimie 80, 207–222 [DOI] [PubMed] [Google Scholar]
- 18. Obeid M., Panaretakis T., Joza N., Tufi R., Tesniere A., van Endert P., Zitvogel L., Kroemer G. (2007) Calreticulin exposure is required for the immunogenicity of γ-irradiation and UVC light-induced apoptosis. Cell Death Differ. 14, 1848–1850 [DOI] [PubMed] [Google Scholar]
- 19. Gardai S. J., McPhillips K. A., Frasch S. C., Janssen W. J., Starefeldt A., Murphy-Ullrich J. E., Bratton D. L., Oldenborg P. A., Michalak M., Henson P. M. (2005) Cell surface calreticulin initiates clearance of viable or apoptotic cells through transactivation of LRP on the phagocyte. Cell 123, 321–334 [DOI] [PubMed] [Google Scholar]