Background: Fibronectin harbors a cryptic antiadhesive site that is able to inactivate β-1 integrins.
Results: Spontaneous anoikis of nontransformed fibroblasts was caused by exposure of this antiadhesive site and its recognition by membrane-resident eEF1A.
Conclusion: eEF1A functions as a membrane receptor triggering cell detachment, resulting in anoikis.
Significance: The results demonstrate a new function of eEF1A that contributes to cell regulation, including anoikis.
Keywords: Apoptosis, Cell Adhesion, Fibronectin, Matrix Metalloproteinase (MMP), Translation Elongation Factors, Anoikis, Cell Detachment
Abstract
Anoikis, apoptosis because of loss of cell anchorage, is crucial for tissue homeostasis. Fibronectin not only provides a scaffold for cell anchorage but also harbors a cryptic antiadhesive site capable of inducing β1-integrin inactivation. In this study, this cryptic antiadhesive site is implicated in spontaneous induction of anoikis. Nontransformed fibroblasts (NIH3T3) adhering to a fibronectin substratum underwent anoikis during serum starvation culture. This anoikis was caused by proteolytic exposure of the cryptic antiadhesive site in fibronectin by matrix metalloproteinase. Eukaryotic elongation factor 1A (eEF1A) was identified as a membrane receptor for the exposed antiadhesive site. Serum starvation raised the membrane residence of eEF1A, and siRNA-based disruption of this increase rendered cells anoikis-resistant. By contrast, cells became more susceptible to anoikis in parallel with increased membrane residence of eEF1A by enforced expression. These results demonstrate that eEF1A acts as a membrane receptor for the cryptic antiadhesive site of fibronectin, which contributes to cell regulation, including anoikis, through negative regulation of cell anchorage.
Introduction
Cell adhesion to the extracellular matrix (ECM)2 is an essential biological event for cell survival and proliferation in multicellular organisms. Disruption of integrin-mediated cell adhesion leads to a specific type of apoptosis known as anoikis in most nontransformed cells (1, 2). Anoikis plays an important role in the physiological elimination of cells during development and maintenance of tissue homeostasis. It also functions in the pathogenesis of disease states, such as ischemia and tissue fibrosis (3, 4). The development of resistance to anoikis has been implicated in disordered tumor cell growth and metastasis (5, 6). In contrast to our understanding from numerous studies of the intracellular signaling for apoptosis that follows disruption of cell anchorage, the molecular basis underlying cell detachment is not fully understood. Moreover, little information is available regarding whether cell detachment is an active cellular process triggered by a distinct biochemical factor or a secondary process resulting from other biological events, such as degradation of the ECM.
The ECM provides not only a structural scaffold for cell anchorage but also a variety of biological signals for regulation of cellular processes such as survival, growth, differentiation, gene expression, and migration. It has become apparent that some of these signals are derived from cryptic functional sites within the ECM protein molecules that are exposed by proteolytic cleavage or conformational change in the ECM protein molecules (7, 8). Fibronectin, a key protein component of the ECM, plays a major role in survival and proliferation of many cell types through signal transduction via the fibronectin receptors (integrin α5β1 and α4β1) (9, 10). Several cryptic functional sites have also been found in fibronectin, particularly in the fibronectin type III repeat (FNIII) (11–14). We found previously that the amino acid sequence YTIYVIAL, buried within the structure of the 14th FNIII repeat, opposes cell adhesion to the extracellular matrix (15, 16). This antiadhesive site can be exposed by either proteolytic cleavage with matrix metalloproteinase (MMP)-2 or a conformational change in fibronectin (17). A 22-mer fibronectin peptide with the exposed antiadhesive site FNIII14 is capable of inhibiting cell adhesion to the ECM by inducing the conformational change in β1-integrins necessary for its functional inactivation (18, 19). On the basis of this antiadhesive effect, peptide FNIII14 influences a variety of anchorage-dependent cellular processes, such as cell survival (18, 20), differentiation (21, 22), and migration/invasion (19). The antiadhesive effect of the cryptic site appears to be expressed through an interaction with a membrane protein of 50 kDa (p50) (21, 23), although its identity has not been clarified.
In this study, we investigated the spontaneous induction of anoikis in nontransformed fibroblasts (NIH3T3) adhering to the fibronectin substratum, which occurs during serum starvation culture. Serum starvation culture has been used as an in vitro culture model to mimic the in vivo microenvironment of ischemic tissue and the tumor niche, which is characterized by nutritional starvation and reduced oxygen levels (24, 25). We first showed that apoptosis of NIH3T3 cells is due to spontaneous cell detachment from the fibronectin substratum. Anoikis during serum starvation culture was caused by proteolytic exposure of the cryptic antiadhesive site in fibronectin to which cells adhered. It is surprising that eukaryotic translation elongation factor 1A (eEF1A) was identified as a putative membrane receptor mediating the antiadhesive effect of this exposed site. eEF1A is a key player of the cytoplasm in polypeptide chain elongation during protein translation (26, 27). Although there have been no reports of the membrane localization of eEF1A, our results of immunofluorescence confocal microscopic and flow cytometric analyses and immunoprecipitation studies support the residence of eEF1A on the outer cell surfaces. Membrane residence of eEF1A was increased during serum starvation culture, and disruption of this increase by RNA interference rendered cells resistant to anoikis. Enforced expression also raised membrane residence of eEF1A, which rendered cells more susceptible to serum starvation-induced anoikis. Besides the canonical role as a cytosolic factor in polypeptide chain elongation during protein translation, our results provide evidence of a new function of eEF1A as a membrane receptor for triggering cell detachment from the ECM, which contributes to cell regulation, including anoikis.
EXPERIMENTAL PROCEDURES
Materials and Antibodies
BB-94 (Matimastat) was purchased from Tocris Bioscience and used at concentrations of 50–100 nm. The MMP-2/MMP-9 inhibitor II, (2R)-[(4-biphenylylsulfonyl)amino]-N-hydroxy-3-phenylpropionamide was obtained from Calbiochem. Peptide FNIII14 (TEATITGLEPGTEYTIYVIALC) and its inactive mutant peptide FNIII14mut (TEATITGLEPGTEYTAYVAALC) were purchased from Operon Biotechnology (Tokyo). Monoclonal antibodies recognizing the active conformation of the human (AG89) and mouse (9EG7) integrin β1 subunit were purchased from MBL and BD Biosciences, respectively. 9EG7 was used also for activation of mouse β1-integrins. Anti-human fibronectin monoclonal antibody (FNH3-8), recognizing the 12th type III repeat of the heparin-binding domain II, was obtained from TaKaRa Bio, Inc. Anti-EF1A monoclonal antibody (CBP-KK1) was from Upstate (Millipore). A function-blocking antibody against the antiadhesive site of fibronectin was prepared as follows. Rabbits were immunized with a synthetic peptide (CLEPGTEYTIYVIALK) containing the active sequence coupled with thyroglobulin. The IgG fraction of rabbit serum was applied to Sepharose beads coupled with the synthetic peptide immunogen. Eluted IgG was used as anti-FNIII14 antibody. Conjugation of peptide FNIII14 with ovalbumin (FNIII14-OVA) was prepared using maleimide-activated ovalbumin (Pierce) according to the manufacturer's protocol. Anti- GST monoclonal antibody was obtained from Abgent, Inc.
Cells and Transfection
Nontransformed mouse NIH3T3 fibroblast cells were cultured in DMEM containing 10% calf serum. Human fibrosarcoma-like cell line WI38VA and mouse colon cancer cell line Colon26M3.1 were grown in RPMI 1640 medium containing 10% fetal bovine serum. Human eEF1A cDNA was amplified by polymerase chain reaction using a cDNA library obtained from U937 cells and two primers (5′-CTGCGCGAATTCAAATGGGAAAGGAAAAGACT-3′ and 5′-ATTAGGGCGGCCGCTCATTTAGCCTTCTGAGCTTT-3′). The PCR product digested by EcoRI and NotI was subcloned into EcoRI-NotI-digested pCMV-Myc mammalian expression vector (Clontech). The identity of the inserted cDNA was confirmed by DNA sequencing with a 3730xl DNA analyzer (Applied Biosystems). Cells were washed and used at 48 h following transfection using LT-1 transfection reagent (TaKaRa) for NIH3T3 cells or Lipofectamine for WI38VA13 cells. siRNA of eEF1A1 (sense, GGAUGUCUACAAAAUUGGUtt and antisense, ACCAAUUUUGUAGACAUCCtg) (GenBankTM accession no. NM_001402) and the negative control siRNA were obtained from Ambion. The siRNAs were transfected into cells using NIH3T3 transfection reagent (Altogen Biosystems), and the cells were used at 48 h after transfection.
Antiadhesive Effect
The antiadhesive effect of peptide FNIII14 or FNIII14-OVA was evaluated by the inhibitory effects either on cell adhesion to fibronectin (19) or β1-integrin activation as follows. For cell adhesion, cells suspended with the serum-free medium with or without the sample to be tested were seeded onto a 96-well plate coated with fibronectin, incubated for 30–60 min, and then fixed with formalin. After washing off unadhered cells, cells adhered to the fibronectin substratum were counted as described previously (28). For β1-integrin activation, activation of β1 integrins on the cells was evaluated by flow cytometric analysis (FACS Aria, BD Biosciences) using 9EG7, a monoclonal antibody recognizing the active β1-integrin conformation-specific epitope (29), as described previously (18).
Cell Survival
Cell survival during serum starvation culture was determined as described previously (28). Briefly, cells suspended with serum-free medium were seeded onto a 96-well plate coated with fibronectin and cultured in the presence or absence of the sample to be tested for the indicated periods. The number of surviving cells was evaluated on the basis of the WST assay using the cell counting kit (Wako Pure Chemicals, Tokyo).
Affinity Labeling of p50 and Its Purification and Identification
A membrane protein, p50, that has specific binding affinity for peptide FNIII14 was affinity-labeled using biotinylated FNIII14 as described previously (23). Briefly, cells were incubated with biotinylated FNIII14 in the presence or absence of a 10-fold molar excess of unlabeled FNIII14 or an inactive control peptide, FNIII14mut. Biotinylated FNIII14 was prepared by labeling peptide FNIII14 with water-soluble, membrane-impermeable analog of NHS-LS-biotin, EZ-link sulfo-NHS-LC-biotin (Pierce) (30). Binding of biotinylated FNIII14 to p50 was covalently cross-linked with a water-soluble cross-linker, 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide (Pierce). Cell viability after affinity labeling was higher than 90%. p50 was detected by immunoblot analysis of cell lysates using peroxidase-labeled avidin. For the identification of p50 protein, 5 × 109 K562 cells was used for the affinity labeling, and the crude membrane-enriched fraction was prepared (31). The lysate of the membrane-enriched fraction was subjected to affinity chromatography using monomeric avidin-immobilized beads (Pierce). p50 bound to the beads was eluted with biotin and separated by SDS-PAGE. In-gel tryptic digestion, MALDI-TOF-MS, and protein identification by peptide mass fingerprinting were performed as described previously (32).
Immunofluorescence Confocal Microscopy
Cells were seeded on eight-well chamber slides coated with fibronectin and allowed to adhere for 3 h. Live cells were incubated without permeabilization with anti-eEF1A IgG (Upstate) (5 μg/ml) in PBS(-) containing 1% BSA at 4 °C for 2 h. After washing off the primary antibody, cells were fixed with 4% paraformaldehyde for 10 min, washed with PBS(-), and then blocked with 5% BSA-containing PBS(-). The primary antibody was labeled with FITC-conjugated goat anti-rabbit IgG at a dilution of 1:2000 with PBS(-) containing 1% BSA for 60 min. Alternatively, immunofluorescence staining was performed after fixation with paraformaldehyde and permeabilization with 0.1% Triton X-100. In either case, cells were observed by confocal laser scanning microscopy (Fluoview FV1000, Olympus). The images acquired were processed with FV10-ASW software (Olympus).
Flow Cytometry
Flow cytometry was performed on a FACScan cytometer (FACS Aria) according to the manufacturer's protocol. The cell suspension was incubated with anti-eEF1A IgG (2 μg/ml) (Upstate) or anti-β1 integrin 9EG7, followed by FITC-labeled anti-mouse IgG. In the analyses, propidium iodide-positive cells were excluded in our final flow cytometry analysis to avoid inaccuracies caused by dead or decaying cells.
Cell Surface Biotinylation and Immunoprecipitation Analysis
Subconfluent cells were incubated with cell membrane-impermeable EZ-Link sulfo-NHS-LC-biotin (Pierce) (30) or with PBS (control) for 60 min at 4 °C. Cell viability after biotinylation was higher than 95%. After washing, cell lysates were incubated with anti-biotin goat IgG, immunoprecipitated using protein G beads, and subjected to immunoblot analysis using anti-EF1A monoclonal antibody and peroxidase-labeled anti-mouse IgG.
Preparation of Recombinant eEF1A Protein
cDNAs encoding full-length human eEF1A was amplified by PCR with a specific primer set as follows. Sense, 5′-AAGGATCCATGGGAAAGGAAAAGACTCATATCAACAT-3′ and antisense, 5′-GAGAATTCTCATTTAGCCTTCTGAGCTTTCTGGG-3′. PCR products were sequenced and inserted into pGEX-2TK using the BamHI and EcoRI sites within the primer domain. Escherichia coli Rosetta-competent cells were transformed with pGEX-2TK containing human eEF1A1 DNA, and protein expression was induced with 0.1 mm isopropyl-β-d-thiogalactopyranoside. Cells were then collected and lysed by sonication in PBS containing 1% Triton X-100 and proteinase inhibitors. GST-tagged human eEF1A was trapped from the soluble fraction using glutathione-Sepharose 4B (GE Healthcare), and then trapped protein was eluted by 50 mm Tris HCl (pH 8.0) containing 5 mm glutathione (reduced form).
Binding Analysis of eEF1A Protein with Peptide FNIII14
The binding of peptide FNIII14 to GST-eEF1A fusion protein was confirmed by ELISA. In brief, anti-GST monoclonal antibody (0.5 μg/ml) was coated onto an ELISA plate, and the plate was blocked with Tris-buffered saline containing 0.1% Tween 20 and 2% BSA (blocking buffer). The wells were incubated with GST-eEF1A (500 nm) in blocking buffer at 37 °C for 2 h. After washing four times with blocking buffer, the wells were then incubated with the increasing concentrations of biotinylated FNIII14 or biotinylated FNIII14mut at 37 °C for 2 h in the presence or absence of unlabeled FNIII14 (10-fold molar excess of biotinylated FNIII14). After extensive washing, the amount of biotinylated FNIII14 bound to eEF1A was measured at 450 nm using streptavidin-peroxidase and o-phenylenediamine.
Statistics
Unpaired Student's t tests were used for statistical evaluation. Data are presented as mean ± S.D.
RESULTS
Proteolytic Exposure of the Cryptic Antiadhesive Site within the Fibronectin Molecule Causes Anoikis
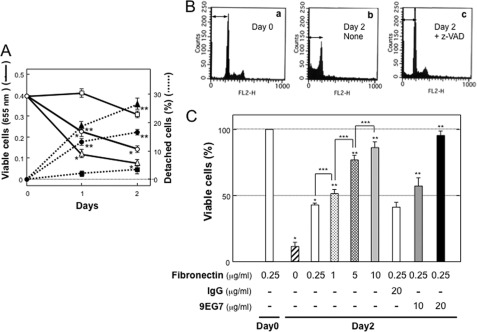
NIH3T3 cells adhere to the fibronectin substratum mainly via integrin α5β1 (28). When NIH3T3 cells adhering to the fibronectin were cultured under serum-starved conditions, the number of viable cells decreased in a time-dependent manner, which was in parallel with an increase in the detached cell number (Fig. 1A). This decrease in the viable cell number was accompanied by an increase in DNA fragmentation, which was blocked by addition of the general caspase inhibitor z-VAD fmk (Fig. 1, A and B). NIH3T3 cells became resistant to apoptosis depending on the coating concentration of fibronectin (Fig. 1C). Moreover, serum starvation-induced apoptosis was reduced by treatment with an anti-β1 integrin antibody, 9EG7, that is able to activate β1-integrins (29) (Fig. 1C). In contrast, the decrease in cell viability was accelerated by the RGD-containing peptide, an antagonist for integrin α5β1 (Fig. 1A). Thus, apoptosis of NIH3T3 cells adhering to the fibronectin substratum was shown to be anoikis.
FIGURE 1.
Serum starvation-induced anoikis of NIH3T3 cells adhering to the fibronectin substratum. NIH3T3 cells were seeded onto a 96-well plate coated with fibronectin (0.25 μg/ml or as indicated) and then cultured with serum-free medium. After culture for the indicated periods, the number of viable cells was evaluated by WST assay (see “Materials and Methods”). A, time-dependent decrease in cell survival (○, □, and △ with solid lines) and increase in cell detachment (●, ■, and ▴ with dashed lines) during serum starvation. ○ and ●, medium alone (control); △ and ▴, + peptide GRGDSP (200 μg/ml); □ and ■, + z-VAD fmk (100 μm). Each point represents the mean ± S.D. of triplicate determinations. One of three individual experiments is shown. *, p < 0.001 compared with viable cells for control on day 0; **, p < 0.001 compared with percentage of detached cells for control on day 0. B, DNA fragmentation in serum starvation culture. a, none (medium alone) on day 0 (sub-G1, 9%). b, none (medium alone) on day 2 (sub-G1, 63%). c, + z-VAD fmk on day 2 (sub-G1, 18%). DNA fragmentation was assayed using the cycle TEST PLUS DNA reagent kit. Data shown are representative of three individual experiments. C, effect of integrin activation on survival of NIH3T3 cells cultured with serum-free medium with or without the addition of IgG and/or 9EG7 on plates coated with the indicated concentration of fibronectin Each point represents the mean ± S.D. of triplicate determinations. One of three individual experiments is shown. *, p < 0.005 compared with the value on day 0; **, p < 0.01 compared with the value for fibronectin concentration of 0.25 μg/ml on Day 2; ***, p < 0.05 compared with the control.
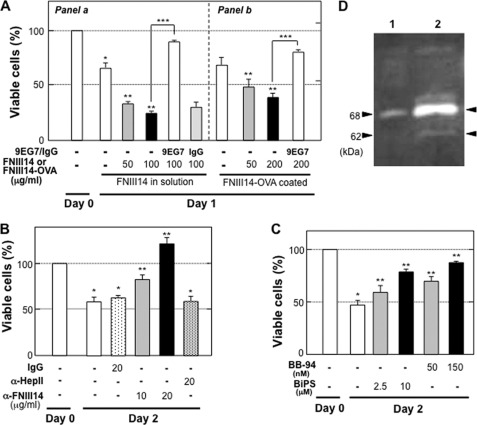
Peptide FNIII14 added in solution, which was capable of interfering with NIH3T3 cell adhesion to the fibronectin by inactivating β1-integrins (supplemental Fig. S1), accelerated anoikis in NIH3T3 cells adhering to the fibronectin substratum (Fig. 2A). Of note, peptide FNIII14 also accelerated anoikis when coated on the culture plate in combination with fibronectin (Fig. 2A). The peptide FNIII14 coated over the fibronectin substrate would mimic the exposed antiadhesive site in the fibronectin substratum to which cells adhere. Therefore, the results led to speculate that anoikis of NIH3T3 cells might be triggered by exposure of the cryptic antiadhesive site within the fibronectin molecule. To verify this, we prepared an antibody capable of blocking the antiadhesive effect of peptide FNIII14. A polyclonal antibody (α-FNIII14, see “Materials and Methods”) able to abrogate the antiadhesive effects of peptide FNIII14 was obtained (supplemental Fig. S1, A and B). Of importance, this function-blocking antibody was also capable of reducing anoikis of NIH3T3 cells (Fig. 2B). Preimmune control IgG and another antibody recognizing the heparin-binding domain II of fibronectin, which is located near the peptide FNIII14 region, had no effect (Fig. 2B). Furthermore, either a broad-spectrum MMP inhibitor (BB-94) or a specific inhibitor of MMP-2 and MMP-9 ((2R)-[(4-biphenylylsulfonyl)amino]-N-hydroxy-3-phenylpropionamide) also reduced anoikis of NIH3T3 cells (Fig. 2C). Supporting this result is the fact that gelatinolytic activity was detected in the culture medium at around 68 and 62 kDa (Fig. 2D), which corresponds to the molecular weight of the proform and active form of MMP-2, respectively. In fact, degradation of fibronectin molecules coated as the adhesion substratum was observed during the culture of NIH3T3 cells, in which the fibronectin molecule was degraded in a time-dependent manner, concomitant with increases in fibronectin fragments containing the epitope of the anti-FNIII14 antibody (supplemental Fig. S2). These results suggest that proteolytic exposure of the cryptic antiadhesive site in fibronectin was responsible for anoikis.
FIGURE 2.
Exposure of the cryptic antiadhesive site in fibronectin is responsible for serum starvation-induced anoikis. NIH3T3 cells were cultured under various conditions in serum-free medium for 1 or 2 days, and cell viability was determined as described under “Materials and Methods.” A, NIH3T3 cell suspension with or without 9EG7 (20 μg/ml) were seeded onto a 96-well plate coated with either fibronectin alone (0.25 μg/ml) (a) or fibronectin (0.25 μg/ml) and FNIII14-OVA (0, 50, or 200 μg/ml) (b). Cells were cultured with serum-free medium in the presence (a) or absence (b) of peptide FNIII14 at the indicated concentrations (0–100 μg/ml). *, p < 0.01 compared with day 0. **, p < 0.05 compared with the value without FNIII14 on day 2; ***, p < 0.01 compared with control. B, cells were seeded onto a 96-well plate coated with fibronectin and then cultured with or without the following factors: IgG, preimmune normal IgG (20 μg/ml); α-HepII, anti-heparin-binding domain II of fibronectin (FNH3–8) (20 μg/ml); α-FNIII14, anti-FNIII14 function-blocking antibody (0, 10, or 20 μg/ml). *, p < 0.01 compared with day 0; **, p < 0.05 compared with the value without antibody on day 2. C, cells were seeded onto a 96-well plate coated with fibronectin and then cultured with or without the following factors: BB-94, a broad spectrum MMP inhibitor (50 or 150 nm); BiPS, (2R)-[(4-biphenylylsulfonyl)amino]-N-hydroxy-3-phenylpropionamide, a specific inhibitor of MMP-2 and MMP-9 (2.5 or 10 μm). *, p < 0.01 compared with day 0; **, p < 0.05 compared with the value without MMP inhibitors on day 2. Each point represents the mean ± S.D. of triplicate determinations. One of three or four individual experiments is shown. D, gelatin zymography of culture supernatant of NIH3T3 cells. NIH3T3 cells (1 × 104 cells in lane 1 and 5 × 104 cells in lane 2) were cultured on the fibronectin substrate with serum-free medium for 18 h. An aliquot of the supernatant was subjected to gelatin zymography. Data shown are representative of two individual experiments.
Identification of a Membrane Receptor for Triggering Cell Detachment
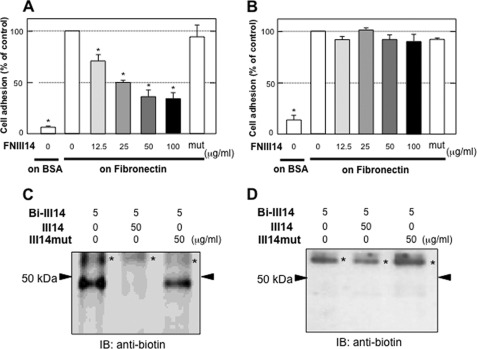
We previously detected a membrane protein of around 50 kDa (p50) that specifically binds to peptide FNIII14 with the exposed antiadhesive site (21, 23). p50 was detected on a number of cell lines, including NIH3T3 (Fig. 3, A and C) and human fibroblasts (WI38VA13) (supplemental Fig. S3), both of which were susceptible to the antiadhesive effect of peptide FNIII14, but not on other cell lines, including Colon26M3.1, which did not respond to peptide FNIII14 (Fig. 3, B and D). Taken together with the result that FNIII14 also exhibited an antiadhesive effect when coated on the culture plate, it appears that p50 might act as a membrane receptor associated with anoikis induction. To identify this putative membrane receptor, p50 affinity labeled with biotinylated FNIII14 was isolated (supplemental Fig. S4) and subjected to mass spectrometric analysis. Surprisingly, the sequences of four peptides derived from purified p50 were found to be identical to those of eEF1A (supplemental Fig. S4) (33).
FIGURE 3.
Correlation between cellular susceptibility to peptide FNIII14 and p50, a membrane protein with specific binding affinity to peptide FNIII14. A and B, effects of peptide FNIII14 (indicated concentrations) or inactive control peptide FNIII14mut (100 μg/ml) (mut) on adhesion of NIH3T3 (A) or Colon26M3.1 (B) cells were assayed. Each point represents the mean ± S.D. of triplicate determinations. One of three individual experiments is shown. *, p < 0.01 compared with the value without FNIII14 on fibronectin. C and D, p50 of NIH3T3 (C) and Colon26M3.1 (D) cells was detected by affinity labeling using biotinylated FNIII14 (Bi-III14) in the presence or absence of a 10-fold molar excess of unlabeled FNIII14 (III14) or inactive control peptide FNIII14mut (III14mut), as described under “Materials and Methods.” Bands with asterisks in C and D represent staining because of nonspecific binding of biotinylated FNIII14, which are detected in many other cell types (supplemental Fig. S3) (21, 23) but are not detected by immunoblot analysis (IB) using anti-EF1A antibody (supplemental Fig. S4). Data shown are representative of three individual experiments.
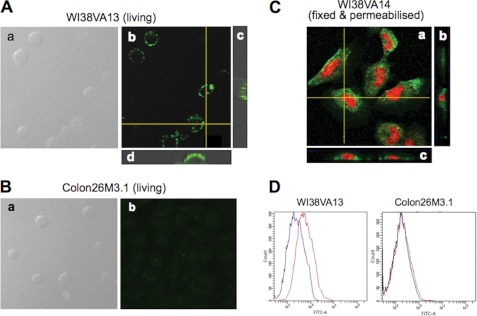
The canonical role of eEF1A in protein translation is associated with a cytoplasmic function (26, 27). To ascertain whether eEF1A is indeed resident on the outer surface of the plasma membrane, we sought to verify the location of eEF1A on the plasma membrane by immunofluorescence confocal microscopy and flow cytometry using a monoclonal antibody recognizing human eEF1A and WI38VA13 cells, which highly express eEF1A. In this series of analyses, we took particular care to avoid any inaccuracies that may be caused by dead or decaying cells. In confocal microscopic analysis, cells were immunostained alive without requiring fixation or permeabilization. With regard to WI38VA13 cells, immunoreactivity of anti-EF1A monoclonal antibody was detected along the cell periphery with no significant fluorescence inside the cell (Fig. 4A). When the images of longitudinal optical sections were reconstructed, the labeling along the cell periphery, but not inside, was evident (Fig. 4A). No specific fluorescence was detected in Colon26M3.1 cells (Fig. 4B), which did not respond to peptide FNIII14. Control cells incubated with preimmune IgG failed to exhibit specific staining. On the other hand, when cells were fixed and permeabilized, immunofluorescence was detected in the cytoplasm (Fig. 4C). In flow cytometric analysis, cells were also incubated with propidium iodide in parallel with the immunofluorescence staining to exclude dead and decaying cells from the final analysis. Flow cytometry patterns revealed cell surface residence of eEF1A on WI38VA13 cells but not on Colon26M3.1 cells (Fig. 4D).
FIGURE 4.
Subcellular localization of eEF1A in WI38VA13 and Colon26M3.1 cells. Subcellular localization of eEF1A was investigated in living WI38VA13 (A) and Colon26M3.1 (B) cells or in WI38VA13 cells fixed with paraformaldehyde (C) as visualized by confocal microscopy. A and B, cells were incubated with anti-EF1A monoclonal antibody at 4 °C for 2 h (incubation at low temperature to avoid internalization of antigen-antibody complex) and then with FITC-labeled anti-mouse IgG antibody at room temperature for 1 h. a in A and B show differential interference contrast images. b, the images were constructed from a z-series of optical sections. c and d, the images were produced by vertically slicing the original z-series dataset; a consecutive series of longitudinal optical sections. C, WI38VA13 cells were fixed with 4% paraformaldehyde and then permeabilized with 0.03% Triton X-100. Cells were incubated with anti-eEF1A, FITC-labeled anti-mouse IgG antibody and then propidium iodide. a is the image of a cross-section. b and c show the images of longitudinal optical sections as above. D, flow cytometric analysis of membrane-resident eEF1A. WI38VA13 and Colon26M3.1 cells were incubated with anti-EF1A monoclonal antibody followed by FITC-labeled anti-mouse IgG. All data shown are representative of three individual experiments.
To define the molecular relationship between eEF1A and p50, a membrane protein with specific binding affinity for peptide FNIII14, p50, of WI38VA13 cells affinity-labeled with biotinylated FNIII14 was examined. p50 affinity labeled with biotinylated FNIII14 could be immunoprecipitated by anti-biotin antibody and detected by anti-EF1A antibody as a clear 50-kDa band (Fig. 5A, a). Similarly, p50 affinity-labeled with biotinylated FNIII14, which was immunoprecipitated by anti-EF1α antibody, could be detected by anti-biotin antibody (Fig. 5A, b). Furthermore, cell surface proteins were tagged with membrane-impermeable biotin (30) with no significant decrease in cell viability, and the cell lysate was then subjected to immunoprecipitation with anti-eEF1A antibody. As expected, a 50 kDa band was detected by anti-biotin antibody in the precipitate (Fig. 5B). The immunoprecipitation study also showed that p50 of NIH3T3 cells is immunochemically indistinguishable from eEF1A (supplemental Fig. S5).
FIGURE 5.
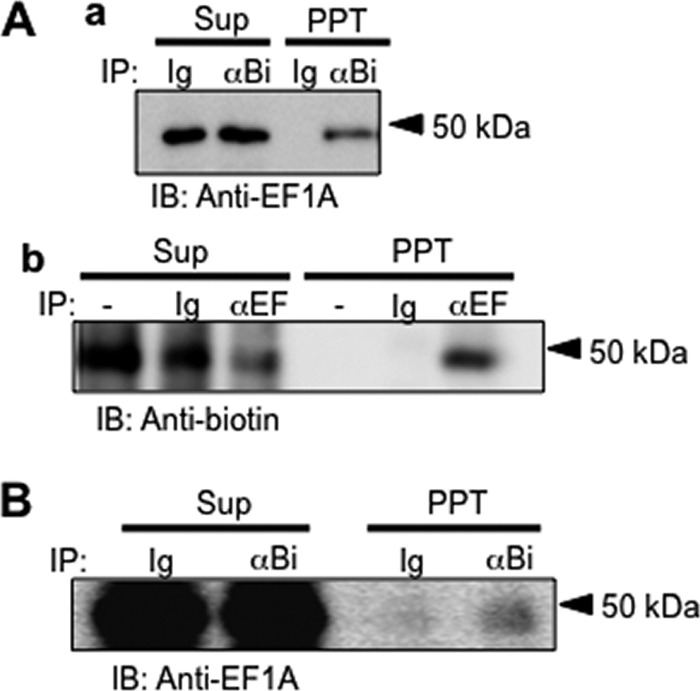
Molecular relationship between p50 and eEF1A. A, WI38VA13 cells were affinity-labeled with biotinylated FNIII14, and their lysates were immunoprecipitated (IP) using normal goat IgG (Ig) or anti-biotin goat IgG (αBi) and protein G beads. After centrifugation at 2,000 xg, Supernatants (Sup) and precipitates (PPT) were subjected to immunoblot analysis (IB) using anti-EF1A monoclonal antibody (a). b, immunoprecipitation was performed with anti-EF1A monoclonal antibody (αEF) and immunoblot analysis using peroxidase-labeled anti-biotin antibody. B, membrane proteins were labeled with a water-soluble, membrane-impermeable biotin without any remarkable decrease in cell viability. Cell lysates were immunoprecipitated with normal goat IgG or anti-biotin goat IgG and then subjected to immunoblot analysis using anti-EF1A monoclonal antibody as a primary antibody and peroxidase-labeled anti-mouse IgG as a secondary antibody. Data shown are representative of three to four individual experiments.
The binding between eEF1A and peptide FNIII14 was examined by ELISA. Purified GST-eEF1A fusion protein was immobilized onto an ELISA plate coated with anti-GST monoclonal antibody and then incubated with biotinylated FNIII14. As shown in supplemental Fig. S6, eEF1A had the ability to bind to biotinylated FNIII14, but not to biotinylated FNIII14mut, in a dose-dependent manner, and the binding of biotinylated FNIII14 to eEF1A was displaced competitively by unlabeled FNIII14. Thus, these results strongly suggest that eEF1A is resident on the outer surface of the plasma membrane and acts there as a specific binding protein for peptide FNIII14.
Functional Association between Membrane-resident eEF1A and Serum Starvation-induced Anoikis
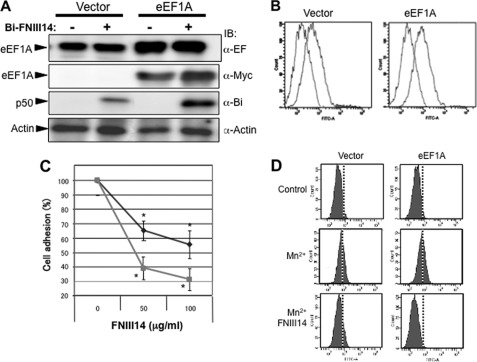
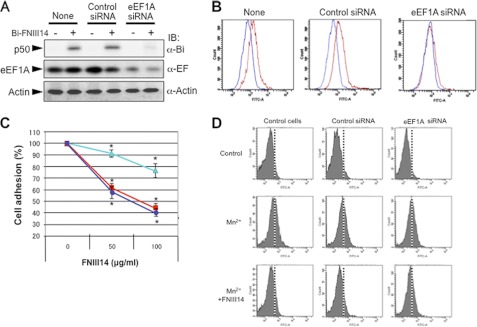
First, we examined the functional association between membrane-resident eEF1A and the antiadhesive effect of peptide FNIII14. Enforced overexpression of eEF1A caused an increase in the membrane residence of eEF1A, as detected by affinity labeling and flow cytometry (Fig. 6, A and B). This was accompanied by an increase in the membrane level of the FNIII14-binding protein p50 (Fig. 6A). Furthermore, inhibition of cell adhesion to the fibronectin (Fig. 6C) and β1-integrin activation (D) by FNIII14 became more remarkable in cells after overexpression of eEF1A. Conversely, siRNA-based down-regulation of eEF1A expression reduced the p50 band intensity and cell surface residence of eEF1A (Fig. 7, A and B). Down-regulation of eEF1A expression also made cells resistant to the antiadhesive effects of peptide FNIII14 (Fig. 7C). Thus, susceptibility of cells to the antiadhesive effect of FNIII14 was dependent on the level of membrane residence of its receptor, eEF1A.
FIGURE 6.
Effect of enforced expression of eEF1A on cellular susceptibility to peptide FNIII14. WI38VA13 cells were transfected with an empty vector (Vector) or eEF1A and then examined as follows. A, cells were affinity labeled with (+) or without (-) biotinylated FNIII14 and then subjected to immunoblot (IB) analysis using anti-EF1A (α-EF), anti-myc (α-Myc), anti-biotin (α-Bi), and anti-actin (α-Actin) antibodies. B, cells treated as indicated were incubated with preimmune normal IgG (blue lines) or anti-EF1A monoclonal antibody (red lines) as primary antibody and with FITC-labeled anti-mouse IgG and then subjected to flow cytometry. C, inhibition of cell adhesion to the fibronectin substratum by peptide FNIII14 was assayed using cells transfected with an empty vector (blue line with ♦) or with eEF1A (red line with ■). Each point represents the mean ± S.D. of triplicate determinations. One of three or four individual experiments is shown. *, p < 0.01 compared with control (without FNIII14). D, inhibition of Mn2+-dependent β1-integrin activation by peptide FNIII14 was assayed by flow cytometry using AG89, a monoclonal antibody against an active conformation of β1-integrins. Data shown in A, B, and D are representative of three individual experiments.
FIGURE 7.
Effect of down-regulation of eEF1A expression on cellular susceptibility to peptide FNIII14. NIH3T3 cells were transfected with control siRNA or eEF1A siRNA and then examined as follows. A, cells were affinity-labeled with (+) or without (-) biotinylated FNIII14 and then subjected to immunoblot analysis (IB) using anti-biotin (α-Bi), anti-EF1A (α-EF), and anti-actin (α-Actin) antibodies. B, cells treated as indicated were incubated with preimmune normal IgG (blue lines) or anti-EF1A monoclonal antibody (red lines) as primary antibody and then subjected to flow cytometric analysis. C, inhibition of cell adhesion to the fibronectin substratum by peptide FNIII14 was assayed using untreated cells (blue line with ●) or cells transfected with control siRNA (red line with ■) or with eEF1A siRNA (pale blue line with ▴). Each point represents the mean ± S.D. of triplicate determinations. One of three or four individual experiments is shown. *, p < 0.05 compared with control (without FNIII14). D, inhibition of Mn2+-dependent β1-integrin activation by peptide FNIII14 was assayed by flow cytometry as in Fig. 6D. Data shown in A, B, and D, are representative of three individual experiments.
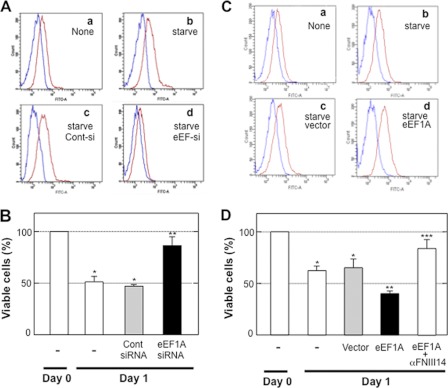
Finally, the functional association between membrane-resident eEF1A and anoikis was examined. As shown in Fig. 8A, the membrane-resident level of eEF1A was increased prior to anoikis. When this increase was disrupted by RNA interference (Fig. 8A), cells became more resistant to anoikis (B). Conversely, overexpression of eEF1A, which caused an increase in the level of membrane residence of eEF1A (Fig. 8C), rendered cells more susceptible to anoikis (D). This increased susceptibility to anoikis was reversed by the α-FNIII14 function-blocking antibody (Fig. 8D). These results suggest that an increase in the membrane residence of eEF1A, as well as proteolytic exposure of the cryptic antiadhesive site, was responsible for cell detachment from the fibronectin substratum, resulting in anoikis.
FIGURE 8.
Functional association between membrane-resident eEF1A and serum starvation-induced anoikis. In down-regulation of eEF1A expression, NIH3T3 cells were transfected with control siRNA (Cont-si or cont siRNA) or eEF1A siRNA (eEF-siR or eEF1A siRNA) (a–d in A). In up-regulation of eEF1A, cells were transfected with empty vector (vector) or eEF1A (eEF1A) (a–d in C). After culture for 48 h, parts of cells were subjected to evaluation of membrane-resident eEF1A by flow cytometry as in Fig. 6B, and other parts were subjected to cell survival assay (B and D). Data shown in A and C are representative of four individual experiments. Each point in B and D represents the mean ± S.D. of triplicate determinations. One of four or five individual experiments is shown. *, p < 0.01 compared with control; **, p < 0.01 compared with cells transfected with control siRNA or vector; ***, p < 0.01 compared with lack of antibody.
DISCUSSION
Exposure of the Antiadhesive Site and Increased Membrane Residence of eEF1A Cause Induction of Anoikis
Cell detachment from the ECM is a trigger for the induction of anoikis. It has been presumed that two biochemical processes, pericellular proteolysis and retraction of ECM proteins, are mainly responsible for cell detachment in vivo (34). However, considering the central role of integrins in cell adhesion, we propose that a change in the ligand-binding activity of integrins is also involved. Integrins switch between an active, high ligand-binding affinity conformation and an inactive, low ligand-binding affinity conformation (35, 36). Integrin-mediated cell regulation is characterized by the presence of this inactive state. It is therefore likely that inactivation of β1-integrins causes anoikis by triggering cell detachment. However, little is known about endogenous factors capable of inducing integrin inactivation. We have previously demonstrated the presence of the cryptic antiadhesive site within the fibronectin molecule. In this study, we have shown that this cryptic antiadhesive site, once exposed, can play a decisive role in anoikis induction. Serum starvation appeared to stimulate MMP-2 excretion by NIH3T3 cells, which contributed to exposure of the cryptic antiadhesive site within the fibronectin molecule. Surprisingly, eEF1A was identified as a membrane receptor that mediates the anti-adhesive effect of this exposed site. An increase in the membrane resident level of eEF1A also caused anoikis, although its mechanism remains unclear. Thus, serum starvation served as an inducer for the emergence of key determinants (an extracellular stimulus and its receptor) necessary for cell detachment. Serum starvation culture mimics the in vivo microenvironment of ischemic tissue and tumors (37, 38). Besides nutritional starvation, reduced oxygen is characteristic of ischemia and the tumor niche (38). It would be interesting to investigate the effect of hypoxia on membrane residence of eEF1A and exposure of the cryptic antiadhesive site within the fibronectin molecule.
Membrane Localization of the Translation Elongation Factor
This is not the first demonstration that the translation elongation factor is expressed on the cell surface. The membrane location of EF-Tu, the prokaryotic homolog of eEF1A, has been demonstrated in several prokaryotic cells (39–43). Interestingly, it was reported that EF-Tu expressed on the outer membrane surface has binding affinity toward ECM components, including fibronectin. Several bacterial pathogens, including Mycoplasma pneumoniae and Pseudomonas aeruginosa, utilize the ECM-binding affinity of membrane-resident EF-Tu to infect mammals and to spread to other organ sites. The amino acid sequences of EF-Tu and eEF1A from various species are highly similar. Probably because of this structural similarity, they share many biochemical functions (26–27). Binding to ECM components, including fibronectin, may be a common characteristic for membrane-resident EF-Tu and eEF1A, although their biological significances are entirely different.
On the other hand, colonization of mucosal surfaces is the key initial step in most bacterial infections (44). The accelerated turnover and shedding of mucosal epithelial cells, so-called exfoliation, is implicated in the innate defense mechanism against bacterial infection (45, 46). Attachment of bacteria seems to causally induce exfoliation of the infected epithelial cells. It was demonstrated recently that some bacterial pathogens use special tactics to keep their infectious foothold. They prevent detachment of infected epithelial cells through stabilization of cell adhesion either by raising cell surface levels of β1-integrins or by stimulating β1-integrin activation (46, 47). Thus, the exfoliation of infected epithelial cells seems to be controlled through an active cellular process stimulated by a distinct biochemical factor(s). It would be interesting to determine whether membrane-resident eEF1A of host cells or EF-Tu on pathogens is involved in the detachment of mucosal epithelial cells. In any case, future studies should focus on the molecular mechanisms underlying membrane transport of eEF1A and inactivation of β1-integrins by the membrane-resident eEF1A.
This work was supported in part by the Vehicle Racing Commemorative Foundation.

This article contains supplemental Figs. S1–S6.
- ECM
- extracellular matrix
- FN
- fibronectin
- FNIII
- fibronectin type III
- MMP
- matrix metalloproteinase
- eEF
- eukaryotic elongation factor.
REFERENCES
- 1. Meredith J. E., Jr., Fazeli B., Schwartz M. A. (1993) The extracellular matrix as a cell survival factor. Mol. Biol. Cell 4, 953–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frisch S. M., Francis H. (1994) Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 124, 619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gavrieli Y., Sherman Y., Ben-Sasson S. A. (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 119, 493–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gabbiani G. (2003) The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 200, 500–503 [DOI] [PubMed] [Google Scholar]
- 5. Stupack D. G., Cheresh D. A. (2002) Get a ligand, get a life. Integrins, signaling and cell survival. J. Cell Sci. 115, 3729–3738 [DOI] [PubMed] [Google Scholar]
- 6. Ruoslahti E. (1999) Fibronectin and its integrin receptors in cancer. Adv. Cancer Res. 76, 1–20 [DOI] [PubMed] [Google Scholar]
- 7. Davis G. E., Bayless K. J., Davis M. J., Meininger G. A. (2000) Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am. J. Pathol. 156, 1489–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schenk S., Quaranta V. (2003) Tales from the crypt[ic] sites of the extracellular matrix. Trends Cell Biol. 13, 366–375 [DOI] [PubMed] [Google Scholar]
- 9. Zhang Z., Vuori K., Reed J. C., Ruoslahti E. (1995) The α 5 β 1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc. Natl. Acad. Sci. U.S.A. 92, 6161–6165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee J. W., Juliano R. L. (2000) α5β1 integrin protects intestinal epithelial cells from apoptosis through a phosphatidylinositol 3-kinase and protein kinase B-dependent pathway. Mol. Biol. Cell 11, 1973–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morla A., Zhang Z., Ruoslahti E. (1994) Superfibronectin is a functionally distinct form of fibronectin. Nature 367, 193–196 [DOI] [PubMed] [Google Scholar]
- 12. Zhong C., Chrzanowska-Wodnicka M., Brown J., Shaub A., Belkin A. M., Burridge K. (1998) Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J. Cell Biol. 141, 539–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Erickson H. P. (2002) Stretching fibronectin. J. Muscle. Res. Cell Motil. 23, 575–580 [DOI] [PubMed] [Google Scholar]
- 14. Shinde A. V., Bystroff C., Wang C., Vincent P. A., Vogelezang M. G., Hynes R. O., Van De Water L. (2008) Identification of the peptide sequences within the EIIIA (EDA) segment of fibronectin that mediate integrin α9β1-dependent cellular activities. J. Biol. Chem. 283, 2858–2870 [DOI] [PubMed] [Google Scholar]
- 15. Fukai F., Takahashi H., Habu Y., Kubushiro N., Katayama T. (1996) Fibronectin harbors anticell adhesive activity. Biochem. Biophys. Res. Commun. 220, 394–398 [DOI] [PubMed] [Google Scholar]
- 16. Fukai F., Hasebe S., Ueki M., Mutoh M., Ohgi C., Takahashi H., Takeda K., Katayama T. (1997) Identification of the anti-adhesive site buried within the heparin-binding domain of fibronectin. J. Biochem. 121, 189–192 [PubMed] [Google Scholar]
- 17. Watanabe K., Takahashi H., Habu Y., Kamiya-Kubushiro N., Kamiya S., Nakamura H., Yajima H., Ishii T., Katayama T., Miyazaki K., Fukai F. (2000) Interaction with heparin and matrix metalloproteinase 2 cleavage exposes a cryptic anti-adhesive site of fibronectin. Biochemistry 39, 7138–7144 [DOI] [PubMed] [Google Scholar]
- 18. Matsunaga T, Fukai F., Miura S., Nakane Y., Owaki T., Kodama H., Tanaka M., Nagaya T., Takimoto R., Takayama T., Niitsu Y. (2008) Combination therapy of an anticancer drug with the FNIII14 peptide of fibronectin effectively overcomes cell adhesion-mediated drug resistance of acute myelogenous leukemia. Leukemia 22, 353–360 [DOI] [PubMed] [Google Scholar]
- 19. Kato R., Ishikawa T., Kamiya S., Oguma F., Ueki M., Goto S., Nakamura H., Katayama T., Fukai F. (2002) A new type of antimetastatic peptide derived from fibronectin. Clin. Cancer Res. 8, 2455–2462 [PubMed] [Google Scholar]
- 20. Fukai F., Mashimo M., Akiyama K., Goto T., Tanuma S., Katayama T. (1998) Modulation of apoptotic cell death by extracellular matrix proteins and a fibronectin-derived antiadhesive peptide. Exp. Cell Res. 242, 92–99 [DOI] [PubMed] [Google Scholar]
- 21. Kamiya S., Kato R., Wakabayashi M., Tohyama T., Enami I., Ueki M., Yajima H., Ishii T., Nakamura H., Katayama T., Takagi J., Fukai F. (2002) Fibronectin peptides derived from two distinct regions stimulate adipocyte differentiation by preventing fibronectin matrix assembly. Biochemistry 41, 3270–3277 [DOI] [PubMed] [Google Scholar]
- 22. Kato R., Kamiya S., Ueki M., Yajima H., Ishii T., Nakamura H., Katayama T., Fukai F. (2001) The fibronectin-derived antiadhesive peptides suppress the myofibroblastic conversion of rat hepatic stellate cells. Exp. Cell Res. 265, 54–63 [DOI] [PubMed] [Google Scholar]
- 23. Miura S., Kamiya S., Saito Y., Wada S., Hayashi R., Taira J., Kodama H., Yajima H., Ueki M., Fukai F. (2007) Antiadhesive sites present in the fibronectin type III-like repeats of human plasma fibronectin. Biol. Pharm. Bull. 30, 891–897 [DOI] [PubMed] [Google Scholar]
- 24. Bialik S., Cryns V. L., Drincic A., Miyata S., Wollowick A. L., Srinivasan A., Kitsis R. N. (1999) The mitochondrial apoptotic pathway is activated by serum and glucose deprivation in cardiac myocytes. Circ. Res. 85, 403–414 [DOI] [PubMed] [Google Scholar]
- 25. Carmeliet P., Jain R. K. (2000) Angiogenesis in cancer and other diseases. Nature 407, 249–257 [DOI] [PubMed] [Google Scholar]
- 26. Kinzy T. G., Goldman E. (2000) in Translational Control of Gene Expression (Hersy J. W. B., Mathews M. B., Sonenberg N., eds) pp. 973–997, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 27. Ejiri S. (2002) Moonlighting functions of polypeptide elongation factor 1. From actin bundling to zinc finger protein R1-associated nuclear localization. Biosci. Biotechnol. Biochem. 66, 1–21 [DOI] [PubMed] [Google Scholar]
- 28. Saito Y., Imazeki H., Miura S., Yoshimura T., Okutsu H., Harada Y., Ohwaki T., Nagao O., Kamiya S., Hayashi R., Kodama H., Handa H., Yoshida T., Fukai F. (2007) A peptide derived from tenascin-C induces β1 integrin activation through syndecan-4. J. Biol. Chem. 282, 34929–34937 [DOI] [PubMed] [Google Scholar]
- 29. Bazzoni G., Shih D. T., Buck C. A., Hemler M. E. (1995) Monoclonal antibody 9EG7 defines a novel β 1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J. Biol. Chem. 270, 25570–25577 [DOI] [PubMed] [Google Scholar]
- 30. Baqui M., Botero D., Gereben B., Curcio C., Harney J. W., Salvatore D., Sorimachi K., Larsen P. R., Bianco A. C. (2003) Human type 3 iodothyronine selenodeiodinase is located in the plasma membrane and undergoes rapid internalization to endosomes. J. Biol. Chem. 278, 1206–1211 [DOI] [PubMed] [Google Scholar]
- 31. Nishiumi S., Ashida H. (2007) Rapid preparation of a plasma membrane fraction from adipocytes and muscle cells. Application to detection of translocated glucose transporter 4 on the plasma membrane. Biosci. Biotechnol. Biochem. 71, 2343–2346 [DOI] [PubMed] [Google Scholar]
- 32. Sabarth N., Lamer S., Zimny-Arndt U., Jungblut P. R., Meyer T. F., Bumann D. (2002) Identification of surface proteins of Helicobacter pylori by selective biotinylation, affinity purification, and two-dimensional gel electrophoresis. J. Biol. Chem. 277, 27896–27902 [DOI] [PubMed] [Google Scholar]
- 33. Brands J. H., Maassen J. A., van Hemert F. J., Amons R., Möller W. (1986) The primary structure of the α subunit of human elongation factor 1. Structural aspects of guanine-nucleotide-binding sites. Eur. J. Biochem. 155, 167–171 [DOI] [PubMed] [Google Scholar]
- 34. Michel J. B. (2003) Anoikis in the cardiovascular system. Known and unknown extracellular mediators. Arterioscler. Thromb. Vasc. Biol. 23, 2146–2154 [DOI] [PubMed] [Google Scholar]
- 35. Chan J. R., Hyduk S. J., Cybulsky M. I. (2001) Chemoattractants induce a rapid and transient up-regulation of monocyte α4 integrin affinity for vascular cell adhesion molecule 1 which mediates arrest. An early step in the process of emigration. J. Exp. Med. 193, 1149–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shimaoka M., Takagi J., Springer T. A. (2002) Conformational regulation of integrin structure and function. Annu. Rev. Biophys. Biomol. Struct. 31, 485–516 [DOI] [PubMed] [Google Scholar]
- 37. Bonavita F., Stefanelli C., Giordano E., Columbaro M., Facchini A., Bonafè F., Caldarera C. M., Guarnieri C. (2003) H9c2 cardiac myoblasts undergo apoptosis in a model of ischemia consisting of serum deprivation and hypoxia. Inhibition by PMA. FEBS Lett. 536, 85–91 [DOI] [PubMed] [Google Scholar]
- 38. Rennebeck G., Martelli M., Kyprianou N. (2005) Anoikis and survival connections in the tumor microenvironment. Is there a role in prostate cancer metastasis? Cancer Res. 65, 11230–11235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jacobson G. R., Rosenbusch J. P. (1976) Abundance and membrane association of elongation factor Tu in E. coli. Nature 261, 23–26 [DOI] [PubMed] [Google Scholar]
- 40. Dallo S. F., Kannan T. R., Blaylock M. W., Baseman J. B. (2002) Elongation factor Tu and E1 β subunit of pyruvate dehydrogenase complex act as fibronectin binding proteins in Mycoplasma pneumoniae. Mol. Microbiol. 46, 1041–1051 [DOI] [PubMed] [Google Scholar]
- 41. Granato D., Bergonzelli G. E., Pridmore R. D., Marvin L., Rouvet M., Corthésy-Theulaz I. E. (2004) Cell surface-associated elongation factor Tu mediates the attachment of Lactobacillus johnsonii NCC533 (La1) to human intestinal cells and mucins. Infect. Immun. 72, 2160–2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kunert A., Losse J., Gruszin C., Hühn M., Kaendler K., Mikkat S., Volke D., Hoffmann R., Jokiranta T. S., Seeberger H., Moellmann U., Hellwage J., Zipfel P. F. (2007) Immune evasion of the human pathogen Pseudomonas aeruginosa. Elongation factor Tuf is a factor H and plasminogen binding protein. J. Immunol. 179, 2979–2988 [DOI] [PubMed] [Google Scholar]
- 43. Balasubramanian S., Kannan T. R., Baseman J. B. (2008) The surface-exposed carboxyl region of Mycoplasma pneumoniae elongation factor Tu interacts with fibronectin. Infect. Immun. 76, 3116–3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peason J. P., Brownlee I. A. (2005) in Colonization of mucosal surfaces (Nataro J. P., Cohen P. S., Mobeley H. L. T., Weiser J. N., eds) pp. 3–16, ASM Press, Washington, DC [Google Scholar]
- 45. Mulvey M. A., Schilling J. D., Martinez J. J., Hultgren S. J. (2000) Bad bugs and beleaguered bladders. Interplay between uropathogenic Escherichia coli and innate host defenses. Proc. Natl. Acad. Sci. U.S.A. 97, 8829–8835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cliffe L. J., Humphreys N. E., Lane T. E., Potten C. S., Booth C., Grencis R. K. (2005) Accelerated intestinal epithelial cell turnover. A new mechanism of parasite expulsion. Science 308, 1463–1465 [DOI] [PubMed] [Google Scholar]
- 47. Kim M., Ogawa M., Fujita Y., Yoshikawa Y., Nagai T., Koyama T., Nagai S., Lange A., Fässler R., Sasakawa C. (2009) Bacteria hijack integrin-linked kinase to stabilize focal adhesions and block cell detachment. Nature 459, 578–582 [DOI] [PubMed] [Google Scholar]