Background: ApoE4 is a genetic risk factor for sporadic AD. PKC is involved in synaptogenesis and shows abnormalities in aging and AD.
Results: ApoE3 (not apoE4), acting through LRP1, protects synapses against ASPD by inducing PKCϵ.
Conclusion: ApoE3 stimulates synaptogenesis and protection against ASPD by increasing PKCϵ synthesis.
Significance: ApoE3 may reduce the risk for AD by stimulating PKCϵ synthesis.
Keywords: Alzheimer Disease, Amyloid, ApoE, Neuroprotection, Protein Kinase C (PKC), PKCϵ, Aβ
Abstract
Synaptic loss is the earliest pathological change in Alzheimer disease (AD) and is the pathological change most directly correlated with the degree of dementia. ApoE4 is the major genetic risk factor for the age-dependent form of AD, which accounts for 95% of cases. Here we show that in synaptic networks formed from primary hippocampal neurons in culture, apoE3, but not apoE4, prevents the loss of synaptic networks produced by amyloid β oligomers (amylospheroids). Specific activators of PKCϵ, such as 8-(2-(2-pentyl-cyclopropylmethyl)-cyclopropyl)-octanoic acid methyl ester and bryostatin 1, protected against synaptic loss by amylospheroids, whereas PKCϵ inhibitors blocked this synaptic protection and also blocked the protection by apoE3. Blocking LRP1, an apoE receptor on the neuronal membrane, also blocked the protection by apoE. ApoE3, but not apoE4, induced the synthesis of PKCϵ mRNA and expression of the PKCϵ protein. Amyloid β specifically blocked the expression of PKCϵ but had no effect on other isoforms. These results suggest that protection against synaptic loss by apoE is mediated by a novel intracellular PKCϵ pathway. This apoE pathway may account for much of the protective effect of apoE and reduced risk for the age-dependent form of AD. This finding supports the potential efficacy of newly developed therapeutics for AD.
Introduction
Alzheimer disease (AD)2 is a progressive age-related neurodegenerative disease accompanied by synaptic failure and neuronal loss in the brain. AD is characterized by accumulation of extracellular amyloid plaques and hyperphosphorylated Tau protein forming neurofibrillary tangles (1, 2). Several potential risk genes for AD have also been identified. The most consistent of these is apolipoprotein E (ApoE). ApoE exists in three major variant forms: apoE2, apoE3, and apoE4. Individuals with two apoE ϵ4 alleles are at 3–10 times greater risk in developing AD compared with individuals with two copies of the apoE ϵ3 allele (3). ApoE also co-localizes with extracellular amyloid deposits. Different variants of apoE interact differently with Aβ, with apoE4 reported to stabilize toxic Aβ oligomers (4), resulting in isoform-specific clearance (5–7). It has been also reported apoE4 modulates amyloid precursor protein recycling, resulting in increased Aβ production (8).
However, apoE3 in the presence of cholesterol is also a potent signal for synaptogenesis irrespective of any effect on Aβ. ApoE in the brain is responsible for cholesterol transport from astrocytes to neurons. It acts by binding to neuronal receptors, including VLDLR, apoER2, LDLR, and LRP-1 (low density lipoprotein receptor-related protein 1) (5–6). Transgenic mice expressing apoE4 in astrocytes exhibit impaired working memory (9) and impaired spatial memory (10) compared with mice expressing apoE3. Moreover, in the presence of cholesterol, apoE3 and apoE4 have different effects on neurite extension (11). Dendritic spine density is lower in apoE4 transgenic mice compared with apoE3 mice, indicating impaired synaptogenesis (12, 13). ApoE4 is less efficient than apoE3 in transporting brain cholesterol (14) and is also less effective than apoE3 in preventing apoptosis (15–16) and inducing synapse repair and neuritic growth (7).
Expression of some of the PKC isozymes decreases with aging (17). Neurotoxic Aβ also impairs PKC function (18, 19). These deficits in PKC may contribute to memory deficits in AD. PKCα and PKCϵ regulate amyloid precursor protein processing by the non-amyloidogenic pathway (20–24), and deficiency in these enzymes might lead to increased Aβ synthesis and accumulation. Moreover, PKC activation results in enhancement of synaptogenesis and may protect against neurodegeneration (25, 26). The PKC activator DCPLA methyl ester (DCPLA-ME) (Fig. 1E) is a derivative of DCPLA, which associates with the PKC phosphatidylserine-binding site and specifically activates PKCϵ (27, 28). Unlike diacylglycerol-binding PKC activators, phosphatidylserine-binding activators produce little or no down-regulation of PKC (29).
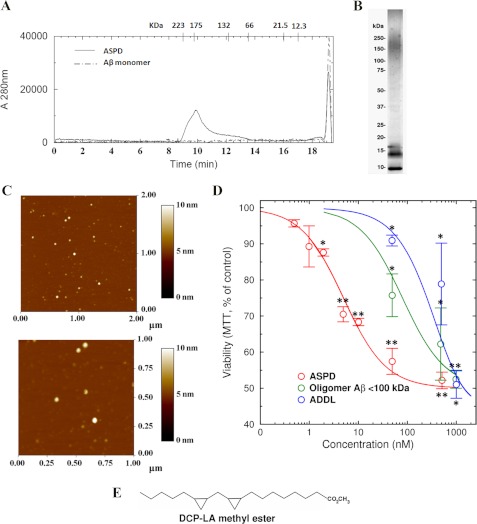
FIGURE 1.
Characterization and neurotoxic effect of different Aβ assemblies. ASPDs, ADDLs, and monomeric Aβ were prepared as described under “Experimental Procedures.” A, characterization of ASPD and Aβ monomers by size exclusion chromatography. ASPD showed a size in the range of 150–250 kDa. B, nondenaturing polyacrylamide electrophoresis of ASPDs. In denaturing gels containing SDS, the 150–200 kDa band is no longer visible (not shown). C, AFM examination showed that ASPDs are structures ∼10 nm in size. D, toxicity of different Aβ forms on cultured primary rat hippocampal neurons after 20 h estimated by the MTT assay. ASPDs represent the retentate from 100-kDa filtration, and oligomeric Aβ represents the filtrate. Cells treated with 1 μm Aβ monomer showed no change in viability compared with the vehicle-treated control cells. ASPDs were the most toxic. E, structure of DCPLA methyl ester. Values are mean ± S.E. (error bars) (Student's t test). *, p < 0.05; **, p < 0.005; ***, p < 0.0005. n = 6.
Previously, it was believed that Aβ fibrillar aggregates found in the plaques initiate neurodegeneration. However, recent findings point to prefibrillar soluble Aβ oligomers as responsible for synaptic dysfunction (30). Various Aβ assemblies ranging from 10 to >100 kDa have been isolated from AD brain (31). Different assemblies reported to be neurotoxic include protofibrils (32), Aβ-derived diffusible ligands (ADDLs) (33), nonamers and dodecamers (Aβ*56) (34), globulomers (35), 15–20-mer Aβ assemblies termed Aβ-oligomers (36), and amylospheroids (ASPDs) (37, 38). ASPDs were found to be unusually neurotoxic and were shown to activate GSK-3β, the enzyme responsible for hyperphosphorylation of Tau protein, thus making them potentially important in AD pathology. Therefore, we investigated the role of apoE3 in protecting neurons against Aβ. We found that ASPDs cause neuronal toxicity and synaptic loss at very low concentration at least in part by reducing the level of PKCϵ. The PKCϵ activator DCPLA-ME protected neurons from ASPD-induced damage and also restored PKCϵ levels. Similarly, apoE3 also prevented cell death caused by ASPD by an LRP-1-dependent mechanism, indicating a role for PKC in apoE signaling.
EXPERIMENTAL PROCEDURES
Materials
Cell culture media were obtained from Invitrogen (F12K, Neurobasal, and B27) and K.D. Medical (minimum Eagle's medium). Aβ(1–42) was purchased from Anaspec (San Jose, CA). Bryostatin 1 was purchased from Biomol International. DCPLA and DCPLA-ME were synthesized in our laboratory following the method described earlier (28). Primary antibodies (PKC-ϵ, β-actin, RACK1, synaptophysin, MAP-2, and PSD-95) were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-phospho-GSK-3β (Ser-9) and GSK-3β were from Cell Signaling Technology, and anti-β-tubulin was purchased from Millipore. All secondary antibodies were purchased from Jackson ImmunoResearch Laboratories. ApoE3, apoE4, PKCϵ translocation inhibitor (EAVSLKPT), and bisindolylmaleimide I (Go 6850) were procured from EMD Biosciences. All other reagents were purchased from Sigma-Aldrich.
Transgenic Mice
ApoE target replacement mice were purchased from Taconic Farms, Inc. In this strain (C57BL/6), the endogenous murine apoE gene has been replaced with human alleles of apoE3 (B6.129P2-Apoetm2(APOE*3)MaeN8) or apoE4 (B6.129P2-Apoetm3(APOE*4)MaeN8). All experiments were performed on age-matched male animals following an approved protocol.
Cell Culture
Hippocampal neurons (NeuroPure, Genlantis) from 18-day-old embryonic Sprague-Dawley rat brains were plated on 24-well plates coated with poly-d-lysine (Sigma-Aldrich) in neurobasal medium supplemented with B-27 containing 0.5 μm glutamine and 25 μm glutamate (Invitrogen). The neuronal cells were grown under 5% CO2 for 14 days in an incubator maintained at 37 °C. All cell culture experiments included the B27 supplement, which is a standard component for neuronal cell culture, except for the experiment shown in Fig. 2B.
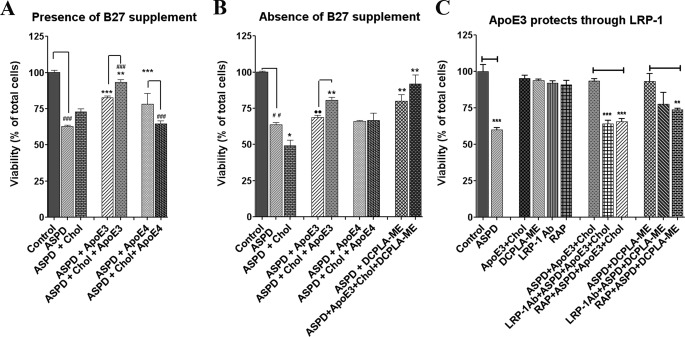
FIGURE 2.
Neuroprotective effect of apoE3. Rat hippocampal neurons were cultured in media (A) with B27 and (B) without B27 and treated with vehicle (Control), ASPD (50 nm), and apoE3 (10 nm), apoE4 (10 nm), or cholesterol alone (100 μm), apoE3 + cholesterol or apoE4 + cholesterol. C, apoE3 acts through LRP-1 receptor. Blocking LRP-1 receptor with LRP-1 antibody or RAP prevented apoE3-induced neuroprotection against ASPD (experiments were done in the presence of B27). Cell viability was measured using the MTT assay. Values are mean ± S.E. (error bars). Asterisks indicate significance with respect to ASPD-treated cells (Student's t test). *, p < 0.05; **, p < 0.005; ***, p < 0.0005. n = 6.
Cells were treated with ASPD, apoE3, apoE4, or PKC activators for 20 h. ApoE (10 nm) and cholesterol (100 μm) were added separately. For inhibition assays with PKC inhibitor or LRP-1 antibody, cells were pretreated with the inhibitor or antibody for 30 min.
Preparation of Different Aβ Oligomers
ASPDs and Aβ monomers were prepared following Noguchi et al. (37, 38). Briefly, Aβ(1–42) was dissolved in 1,1,1,3,3,3-hexafluoro-2-propanol and incubated overnight at 4 °C and then for 3 h at 37 °C. Finally, the dissolved Aβ(1–42) was lyophilized in 1.5-ml polypropylene centrifuge tubes at 40 nmol/tube concentration. For preparing the ASPDs, the lyophilized Aβ was dissolved in phosphate-buffered saline (PBS) without Ca2+ or Mg2+ at less than 50 μm concentration and rotated for 14 h at 4 °C. After incubation, the Aβ solution was purified using a 100-kDa molecular mass cut-off filter (Amicon Ultra, Millipore), and the high molecular weight fraction was saved to obtain the most toxic ASPDs. ADDLs were produced as described previously (33). Aβ(1–42) was solubilized at 5 mm in DMSO, diluted to 100 μm in F-12 medium, and incubated at 4 °C for 24 h. The solution was centrifuged at 14,000 × g for 10 min at 4 °C, and the supernatant was used as ADDL.
Size Exclusion Chromatography
Size exclusion chromatography was performed using an HPLC system (Shimadzu) connected with a TSKgel Super SW2000 column (Supelco). Molecular weight calibration was conducted using both high and low molecular weight proteins. Aβ assemblies were separated with buffer containing 0.1 m Na2PO4 and 0.1 m Na2SO4 adjusted to pH 6.65 with H3PO4, using a flow rate of 0.1 ml/min, with absorbance being monitored at 280 nm.
Atomic Force Microscopy (AFM)
AFM was performed by Polyinsight, LLC (Akron, OH). Samples were prepared by placing a small amount of the sample on freshly cleaved mica for a specific amount of time, spinning the sample to remove excess liquid, and then rinsing the sample with filtered, distilled water while the sample was spinning. The sample was then dried under a gentle stream of dry nitrogen before analysis with the AFM. The prepared samples were analyzed with a Veeco Instruments MultiMode AFM using an E scanner and a Nanoscope IV controller. The microscope was operated in tapping mode with height and phase images collected simultaneously. Platinum-coated silicon cantilevers with a nominal resonance frequency of 70 kHz (Olympus AC240TM ElectriLevers) were used, with medium light tapping forces as characterized by a 0.80 set point reduction ratio.
Native Gel Analysis of ASPDs
Native gel analysis was performed using 4–20% gradient Tris-glycine gel (Invitrogen) and Novex Tris-glycine native running buffer (Invitrogen) at 100 V in 4 °C. Gels were stained with Sypro Ruby Red stain (Invitrogen).
Viability Assay
Viability of cells was measured by an MTT assay (39). For the MTT assay, 3.2 × 104 primary hippocampal neurons from 18-day-old embryonic Sprague-Dawley rat brains were plated on each well of 24-well plates coated with poly-d-lysine. After treatment, the cells were washed with 1× PBS and were incubated with 200 μl of 1 mg/ml MTT solution (Sigma) at 37 °C for 2 h. Then the MTT solution was removed, and the cells were lysed with 200 μl of isopropyl alcohol containing 0.04 m HCl and 160 mm NaOH for 10 min. Finally, absorbance was measured at 570 and 630 nm. All of the samples were done in triplicate, and the data were represented as a percentage of control.
PKC Assay
For measurement of PKC activation by DCPLA-ME, activation of recombinant PKCα, PKCϵ, and PKCδ (Cell Signaling Technology) was used. DCPLA-ME-induced activation was measured in the absence of diacylglycerol and phosphatidylserine as described earlier (27, 28, 40). Individual enzymes were incubated for 15 min at 37 °C in the presence of 10 μm histones, 4.89 mm CaCl2, 10 mm MgCl2, 20 mm HEPES (pH 7.4), 0.8 mm EDTA, 4 mm EGTA, 4% glycerol, 8 μg/ml aprotinin, 8 μg/ml leupeptin, 2 mm benzamidine, and 0.5 μCi of [γ-32P]ATP. [32P]Phosphoprotein formation was measured by adsorption onto phosphocellulose.
Immunofluorescence and Confocal Microscopy
Cells were grown in two-chambered slides (Nunc) at low density. For immunofluorescence staining, the cells were washed with PBS (pH 7.4) and fixed with 4% paraformaldehyde for 4 min. Following fixation, cells were blocked and permeabilized with 5% serum and 0.3% Triton X-100 in 1× PBS for 30 min. Cells were washed three times with 1× PBS and incubated with primary antibodies for 1 h at 1:100 dilution. After the incubation, the slides were again washed three times in 1× PBS and were incubated with the FITC anti-mouse IgG and rhodamine anti-rabbit IgG for 1 h at 1:400 dilution. Cells were further washed and stained with DAPI (Thermo Scientific) to stain the nucleus. Finally, the slides were washed and mounted in Pro Long Gold antifade mounting solution (Invitrogen) and were viewed under an LSM 710 Meta confocal microscope (Zeiss) at 350-, 490-, and 540-nm excitation and 470-, 525-, and 625-nm emission for DAPI, FITC, and rhodamine, respectively. Six individual fields at 63× oil lens magnification were analyzed for the mean fluorescence intensity in each channel.
Cell Lysis and Western Blot Analysis
Cells were harvested in homogenizing buffer containing 10 mm Tris-HCl (pH 7.4), 1 mm phenylmethylsulfonyl fluoride (PMSF), 1 mm EGTA, 1 mm EDTA, 50 mm NaF, and 20 μm leupeptin and were lysed by sonication. The homogenate was centrifuged at 100,000 × g for 15 min at 4 °C to obtain the cytosolic fraction (supernatant) and membrane (pellet). The pellet was resuspended in the homogenizing buffer by sonication. For whole cell protein isolation from primary neurons, the homogenizing buffer contained 1% Triton X-100. Protein concentration was measured using the Coomassie Plus (Bradford) protein assay kit (Pierce). Following quantification, 20 μg of protein from each sample was subjected to SDS-PAGE analysis in 4–20% gradient Tris-glycine gels (Invitrogen). The separated protein was then transferred to nitrocellulose membrane. The membrane was blocked with 5% BSA at room temperature for 15 min and was incubated with primary antibody overnight at 4 °C. After the incubation, it was washed three times with TBS-T (Tris-buffered saline, Tween 20) and further incubated with alkaline phosphatase-conjugated secondary antibody (Jackson Immunoresearch Laboratories) at 1:10,000 dilution for 45 min. The membrane was finally washed 3 times with TBS-T and developed using the one-step nitro blue tetrazolium-5-bromo-4-chloro-3-indolyl phosphate substrate (Pierce). Blots were imaged in the ImageQuant RT-ECL (GE Healthcare), and densitometric quantification was performed using the IMAL software. For translocation assays, PKC activation was represented as the percentage of total protein in the membrane (membrane/(cytosol + membrane)).
Quantitative RT-PCR
RNA was isolated from the cells using TRIzol reagent (Invitrogen) following the manufacturer's protocol. For quantitative RT-PCR, 500 ng of total RNA was reverse transcribed using oligo(dT) and Superscript III (Invitrogen) at 50 °C for 1 h. RT-PCR of the cDNA product was performed using a LightCycler 480 II (Roche Applied Science) machine and LightCycler 480 SYBR Green 1 master mix following the manufacturer's protocol. Primers were as follows: for PKCϵ, TGGCTGACCTTGGTGTTACTCC (forward) and GCTGACTTGGATCGGTCGTCTT (reverse); for PKCα, ACAACCTGGACAGAGTGAAACTC (forward) and CTTGATGGCGTACAGTTCCTCC (reverse); for PKCδ, ACATTCTGCGGCACTCCTGACT (forward) and CCGATGAGCATTTCGTACAGGAG (reverse) (Origene, Rockville, MD); and β-actin (Promega).
PKCϵ Knockdown Assay
PKCϵ knockdown was done using 29-mer shRNA constructs purchased from Origene. The shRNA constructs were transfected to the primary neurons using Lipofectamine 2000 (Invitrogen). Medium was changed after 4 h of Lipofectamine treatment. PKC expression was measured after 72 h of transfection.
Statistical Analysis
Each data point is the mean of 3–6 replications. Data are represented as mean ± S.E. Statistical analysis was performed by Student's t test using GraphPad Prism 5 software with p < 0.05 considered statistically significant.
RESULTS
Production and Size Determination of ASPDs and ADDLs
We prepared synthetic ASPDs from Aβ(1–42) monomers by slowly rotating a 50 μm solution of Aβ(1–42) for 14 h at 4 °C, following the method described by Noguchi et al. (38). ADDLs were generated by the procedure described by Lambert et al. (33). Before analyzing the toxicity of these ASPDs and ADDLs, we verified the size of 100-kDa retentates (ASPDs) and ADDLs by size exclusion chromatography. We found that the size of these ASPDs was ∼175 kDa (ranging from 150 to 220 kDa) (Fig. 1A) when compared with the size standards subjected to size exclusion chromatography, whereas ADDLs showed peaks at 18, 16, and 8 kDa. This was confirmed by static light scattering (488 nm), which showed an average molecular weight of 151,400 ± 4500. Native gel analysis also showed that the ASPDs were in the range of 150–220 kDa (Fig. 1B). The number of Aβ monomers in an ASPD particle was estimated by disassociating ASPD particles to monomers with 1,1,1,3,3,3-hexafluoro-2-propanol. The monomer concentration was estimated by densitometric analysis of Sypro Ruby-stained SDS-polyacrylamide gels. Analysis showed that the ASPD contained ∼23–32 Aβ monomers. AFM analysis showed that the predominant species were ∼8–10 nm in height (Fig. 1C), consistent with the previous findings (37, 38).
Neurotoxic Effect of Different Aβ(1–42) Oligomers (ASPDs; <100-kDa Filtrate of ASPD and ADDLs)
To assess the neurotoxic effect of different sized oligomers, we treated rat primary hippocampal neurons with different concentrations of these Aβ(1–42) species for 20 h. Viability of the treated cells was assessed using the MTT assay. We found that Aβ monomer at 1 μm concentration did not affect the viability of neurons (97.6 ± 1.3%), whereas 1 μm ADDL containing 12-mers to monomers significantly decreased the rate of MTT reduction (51.0 ± 3.8%, p = 0.0013), indicating a loss of viability. ASPDs caused a significant decrease in viability at 50 nm (57.1 ± 4.9%, p = 0.0028). The <100-kDa filtrate was significantly cytotoxic at 50 nm but less toxic than intact ASPDs (Fig. 1D). Further, we found that ASPD can cause significant loss of viability at concentrations as low as 5 nm. Thus, it can be concluded that the ASPDs are the most toxic oligomeric species, and 50 nm ASPDs causes damage equivalent to that from 1 μm ADDLs or 1 μm <100-kDa filtrate of ASPDs. We used a 50 nm concentration of ASPDs in all further experiments.
ApoE3 + Cholesterol Protects against ASPD Toxicity
ApoE3-containing lipoproteins are reported to protect neurons from apoptosis (16) and to act as a signal for synaptogenesis. Therefore, we studied the effect of apoE3 in the presence of cholesterol on ASPD-treated cells. ApoE3 + cholesterol protected the ASPD-treated neurons and provided 95.5 ± 6.0% viability compared with 60.5 ± 1.2% viability in ASPD-treated cells (p = 0.002). ApoE3 or apoE4 alone was less effective, increasing viability to 85.9 ± 3.0 and 82.9 ± 2.6%, respectively. ApoE4 + cholesterol did not protect against ASPD (Fig. 2A). To eliminate possible effects of the unsaturated fatty acids linoleic acid and linolenic acid, which are components of the B27-supplemented culture medium, we also measured the effects in the absence of B27 supplement. Under these conditions, apoE3 + cholesterol still protected against ASPDs (80.6 ± 2.0%, p = 0.005). The combination of apoE3-cholesterol + PKCϵ activator DCPLA-ME was the most effective (91.9 ± 3.5% viability), whereas apoE3 alone had only a small effect (68.8 ± 1.5% viability). ApoE4 or apoE4 + cholesterol was also not protective in the absence of B27 supplement. Only DCPLA-ME was found to be protective (79.9 ± 2.5%) (Fig. 2B). These results clearly show that apoE3 protects against Aβ toxicity and requires cholesterol to be maximally effective. Further, we found that if the apoE receptor LRP-1 is blocked by 30-min pretreatment with LRP-1 antibody or receptor-associated protein (RAP), DCPLA-ME and apoE3 + cholesterol did not protect against ASPDs. These results indicate that the protection involves LRP-1, suggesting an intracellular mechanism.
Blocking the LRP-1 receptor using RAP reduced the protective effect of DCPLA-ME (Fig. 2C). LRP-1 antibody showed a similar effect, but the effect was not statistically significant.
ASPD Causes Synaptic Damage
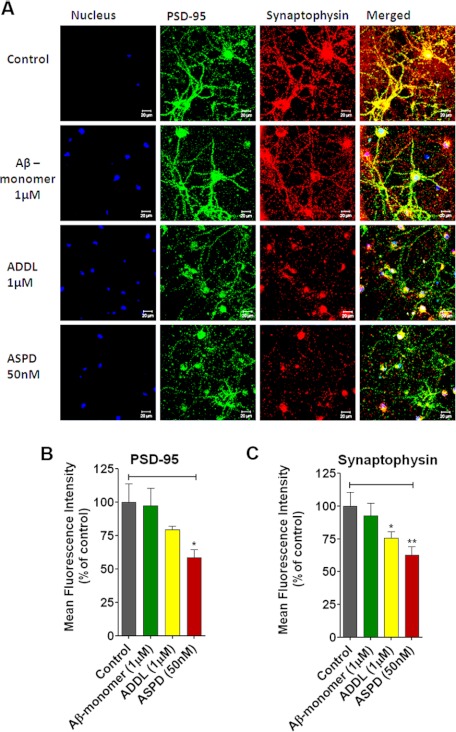
To estimate the synaptic damage caused by the ASPDs on primary hippocampal neurons, we measured the expression of the presynaptic marker synaptophysin and postsynaptic marker PSD-95 by immunofluorescence staining. Expression levels were calculated as change of percentage in mean fluorescence intensity compared with the untreated cells. It was found that, compared with the control, 50 nm ASPDs caused a ∼40% decrease in synaptophysin intensity (62.4 ± 6.7%, p = 0.007), and 1 μm ADDLs caused a 25% decrease (75.6 ± 4.8%, p = 0.033) (Fig. 3, A and C). PSD-95 expression also showed a 42% decrease in the ASPD-treated cells (Fig. 3B). Aβ(1–42) monomer at 1 μm concentration did not change the expression of synaptophysin or PSD-95. This indicates that ASPDs disrupt synaptic integrity even at nanomolar concentrations.
FIGURE 3.
ASPD-induced synaptic loss. A, confocal images of rat hippocampal primary neurons. Cells grown on chambered slides were treated with vehicle (Control), Aβ monomer (1 μm), ADDLs (1 μm), and 50 nm ASPD. Following a 20-h incubation, cells were stained for PSD-95 and synaptophysin. The first column represents the nucleus stained with DAPI (blue), the second column represents PSD-95 (green), the third column shows synaptophysin (red), and the fourth column is the merged image. Mean fluorescence intensity is expressed as a percentage of control (n = 6). Shown is a graphical representation of the expression level of PSD-95 (B) and synaptophysin (C). Values are mean ± S.E. (error bars) (Student's t test). *, p < 0.05; **, p < 0.005; ***, p < 0.0005.
ApoE3 Protects Synapses from ASPD-induced Damage
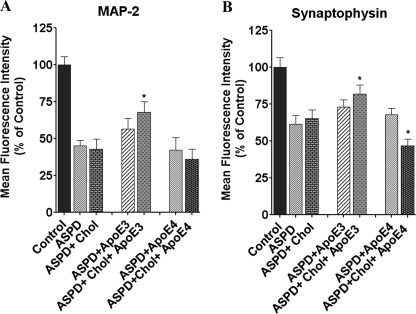
ApoE3 + cholesterol prevented the loss of MAP-2 and synaptophysin expression in ASPD-treated cells (Fig. 4). In ASPD + apoE3 + cholesterol-treated cells, MAP-2 expression was 67.7 ± 7.4% compared with 44.9 ± 3.6% in ASPD-treated cells (p < 0.0001, n = 6). ApoE3, apoE4 alone, and apoE4 + cholesterol did not show any significant change. Synaptophysin expression in ASPD + apoE3 + cholesterol-treated cells increased to 81.6 ± 6.3% compared with 61.3 ± 5.8% in cells treated with ASPD alone (p = 0.022, n = 6) (Fig. 4). ApoE3 and apoE4 alone showed no effect, whereas apoE4 + cholesterol significantly decreased synaptophysin staining in ASPD-treated cells, indicating that apoE3 + cholesterol prevents synaptic loss induced by ASPD.
FIGURE 4.
ApoE3 prevents synaptic damage. Cells grown on chambered slides were treated with vehicle (Control), 50 nm ASPD, 50 nm ASPD + apoE3 (10 nm), 50 nm ASPD + apoE4 (10 nm), 50 nm ASPD + cholesterol (100 μm), 50 nm ASPD + apoE3 (10 nm) + cholesterol (100 μm), or ASPD (50 nm) + apoE4 (10 nm) + cholesterol (100 μm). Following a 20-h incubation, the cells were stained for MAP-2 (A) and synaptophysin (B) as described under “Experimental Procedures.” Mean fluorescence intensity is expressed as a percentage of control (n = 6). ApoE3 + cholesterol prevented the synaptic loss caused by ASPD. *, significance with respect to ASPD-treated cells. Error bars, S.E. *, p < 0.05.
PKCϵ Activators Also Protect against ASPD-induced Neurotoxicity
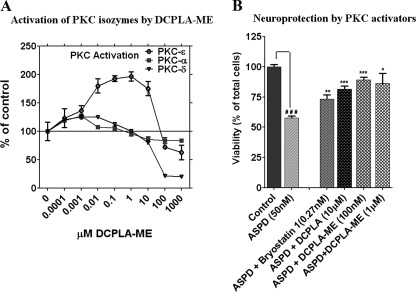
PKC activators are reported to provide neuroprotection against Aβ, possibly by activating TACE and Aβ-degrading enzymes, such as endothelin-converting enzyme, insulin-degrading enzyme, or neprilysin, or by stimulating synaptogenesis. We tested the neuroprotective efficacy of bryostatin 1 (25, 28), DCPLA (28), and the PKCϵ activator DCPLA-ME against ASPD-induced cytotoxicity. DCPLA-ME activated PKCϵ but not PKCα or PKCδ. DCPLA-ME activated PKCϵ by almost 100% in the 0.01–10 μm range, with maximum activation at 100 nm and 1 μm (Fig. 5A).
FIGURE 5.
Neuroprotection by PKC activators (bryostatin 1, DCPLA, and DCPLA-ME) against ASPD-induced toxicity. A, activation of PKCϵ by DCPLA-ME. Purified PKCα, PKCϵ, and PKCδ were preincubated with DCPLA-ME for 5 min at room temperature, and enzymatic activity was measured as described under “Experimental Procedures.” B, cell viability was measured using the MTT assay after PKC activator treatment in 50 nm ASPD-treated cultured primary rat hippocampal neurons. Among the PKC activators, DCPLA-ME (100 nm) was the most protective against ASPDs. Data represent mean ± S.E. (error bars). Asterisks indicate significance with respect to ASPD-treated cells (Student's t test). *, p < 0.05; **, p < 0.005; ***, p < 0.0005. n = 6.
PKCϵ activators were neuroprotective against 20-h treatment with ASPD. Primary neurons treated with 50 nm ASPD showed 57.6 ± 1.6% viability. Bryostatin 1(0.27 nm), DCPLA (10 μm), and DCPLA-ME (100 nm) treatment restored the viability to 73.2 ± 3.6% (p = 0.008, n = 6), 81.4 ± 2.8% (p = 0.0009, n = 6), and 89.2 ± 2.2% (p = 0.0002, n = 6), respectively (Fig. 5B), indicating that the neuroprotection against Aβ is mediated by PKCϵ activation. DCPLA-ME-treated cells were 8 and 16% more viable than DCPLA and bryostatin 1-treated cells. Thus, DCPLA-ME provides better neuroprotection than DCPLA and bryostatin 1.
DCPLA-ME Protects Neurons against ASPD-induced Synaptic Loss
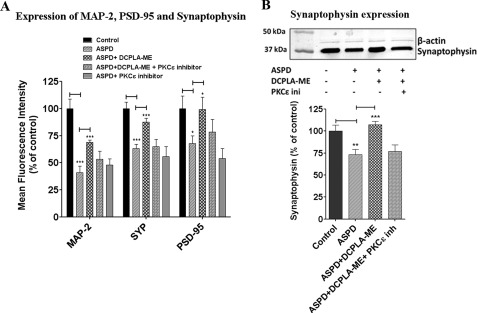
Our next aim was to find out if DCPLA-ME also protected the neurons from synaptic loss caused by the ASPDs. Primary neurons, treated and untreated, were immunostained for MAP-2, synaptophysin, and PSD-95 to determine the synaptic integrity. We found that the PSD-95 and synaptophysin staining of the neurites decreased on ASPD treatment, whereas DCPLA-ME treatment increased staining. DCPLA-ME treatment increased the expression (mean fluorescence intensity) of MAP-2 in ASPD-treated cells from 40.7 ± 6.2% to 68.9 ± 2.0% (p = 0.0007, n = 5), synaptophysin from 63.3 ± 3.8% to 87.5 ± 3.8% (p = 0.0005, n = 5), and PSD-95 from 67.6 ± 7.2% to 99.2 ± 11.3% (p = 0.02, n = 5) (Fig. 6A). These results suggest that DCPLA-ME not only protected the ASPD cells from cell death but also prevented the synaptic damage by increasing expression of synaptophysin, PSD-95, and MAP-2 in the synaptic networks. The expression of synaptophysin was confirmed by Western blot and showed that ASPD decreased the expression by ∼26% (73.3 ± 3.3%, p = 0.0035, n = 3), and DCPLA-ME treatment maintained the expression similar to control (Fig. 6B).
FIGURE 6.
DCPLA-ME protects against ASPD-induced synaptic loss. A, rat hippocampal primary neurons grown on chambered slides were treated with vehicle (Control), 50 nm ASPD, 50 nm ASPD + 100 nm DCPLA-ME, and 50 nm ASPD + 100 nm DCPLA-ME + 5 μm PKCϵ translocation inhibitor. PKCϵ inhibitor was added 30 min before adding ASPD and DCPLA-ME. Following a 20-h incubation, cells were stained for MAP-2, PSD-95, and synaptophysin as described under “Experimental Procedures.” Mean fluorescence intensity was calculated and was expressed as a percentage of control (n = 6). ASPD treatment produced a marked decrease in stained neurite processes, whereas DCPLA-ME protected against synaptic loss. B, Western blot analysis of synaptophysin expression in control and ASPD-, ASPD + DCPLA-ME-, and PKCϵ inhibitor + ASPD + DCPLA-ME-treated primary rat hippocampal neurons. Values are mean ± S.E. (error bars) (Student's t test). *, p < 0.05; **, p < 0.005; ***, p < 0.0005.
ApoE3 + Cholesterol and DCPLA-ME Activate PKCϵ, Leading to Neuroprotection
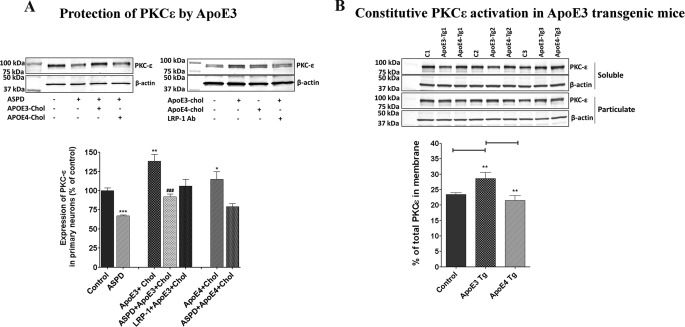
ApoE3 + cholesterol increased both PKCϵ protein level (Fig. 7A) and mRNA level (Fig. 8A) by ∼50% in untreated primary neurons and restored normal levels of PKCϵ in ASPD-treated neurons, whereas apoE4 had little or no effect (Figs. 7A and 8B). Moreover, blocking the LRP-1 receptor with LRP-1 antibody prevented the apoE3-induced PKCϵ expression (Figs. 7A and 8B).
FIGURE 7.
ApoE3 but not apoE4 induces PKCϵ and protects against neurotoxic ASPDs. A, left, immunoblot analysis of primary neurons treated with apoE3 (10 nm) + cholesterol (100 μm), apoE4 (10 nm) + cholesterol (100 μm), and LRP-1 antibody + apoE3-cholesterol. Right, 50 nm ASPD-treated primary neurons treated with apoE3 + cholesterol (10 nm apoE3 and 100 μm cholesterol) or apoE4 + cholesterol (10 nm apoE4 and 100 μm cholesterol). B, PKC activation in control, apoE3, and apoE4 transgenic mice. Data are mean ± S.E. (error bars) of three independent experiments. *, significance with respect to control; #, significance with respect to ASPD-treated cells (Student's t test). *, p < 0.05; **, p < 0.005; ***, p < 0.0005.
FIGURE 8.
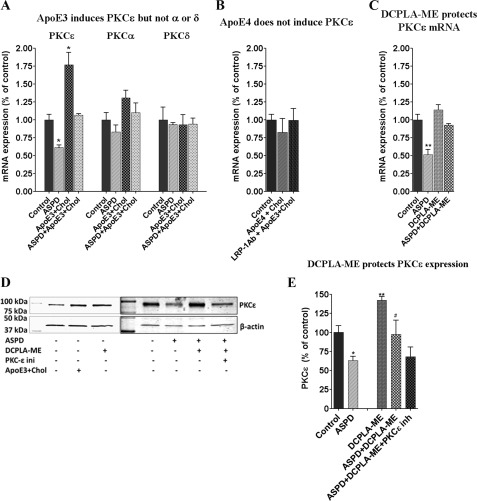
ASPD specifically down-regulates PKCϵ in primary rat hippocampal neurons. A, PKCϵ, PKCα, and PKCδ mRNA were quantified by quantitative RT-PCR in control and ASPD-, apoE3 + cholesterol-, and ASPD + apoE3 + cholesterol-treated cells. Individual cDNA was amplified for PKCϵ and β-actin, and the PKCϵ signal was normalized to β-actin. PKCα and PKCδ showed no significant change on ASPD or apoE3 + cholesterol treatment. ApoE3 blocked the down-regulation by ASPD. B, RT-PCR shows that apoE4 has no effect on PKCϵ mRNA levels. C, RT-PCR shows that DCPLA-ME protects PKCϵ mRNA levels. D and E, immunoblot analysis of primary neurons treated with apoE3 (10 nm) + cholesterol (100 μm), DCPLA-ME (100 nm), PKCϵ inhibitor (5 μm), or 50 nm ASPD. Data are mean ± S.E. (error bars) of three independent experiments. *, significance with respect to control; #, significance with respect to ASPD-treated cells (Student's t test). *, p < 0.05; **, p < 0.005; ***, p < 0.0005.
PKC Activation in ApoE Transgenic Mice
Mouse apoE behaves like human apoE3 (41). To compare the effects of apoE3 and apoE4 on PKCϵ expression, we obtained transgenic mice expressing human apoE3 or apoE4. In mice expressing human apoE3, PKC was constitutively more activated, as indicated by an increased percentage of total PKC in the particulate fraction (28.6 ± 1.1%, mean ± S.E.), compared with transgenic mice expressing human apoE4 (21.6 ± 1.0%) or wild-type mice (23.5 ± 0.5%) (Fig. 7B). These results are consistent with our finding that human apoE3 induces PKCϵ but apoE4 does not.
In cultured primary rat hippocampal neurons, ASPDs decreased PKCϵ mRNA by 40% as measured by RT-PCR (Fig. 8A). This effect was specific for PKCϵ, with only 20% inhibition observed for PKCα and 10% inhibition observed for PKCδ (Fig. 8A). RT-PCR of cells exposed to apoE4 showed that apoE4 had no effect (Fig. 8B). The inhibition of PKC mRNA synthesis by ASPDs was counteracted by the PKC activator DCPLA-ME (Fig. 8C). This protection was completely blocked by 5 μm PKCϵ translocation inhibitor EAVSLKPT (Fig. 8E; representative immunoblot shown in Fig. 8D).
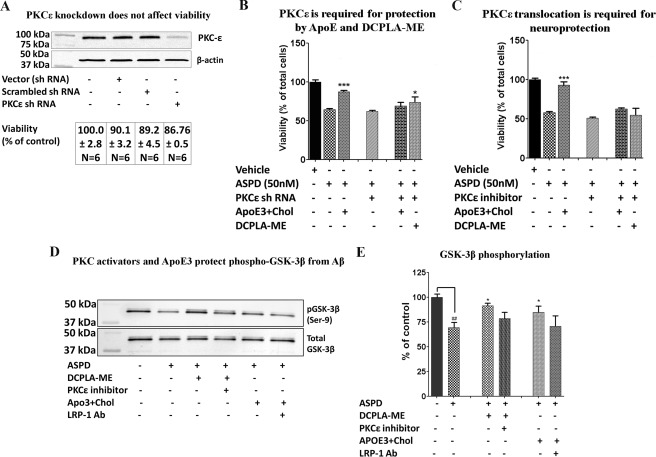
To confirm whether apoE3 + cholesterol acts through PKCϵ, we used RNA interference, a specific translocation inhibitor (EAVSLKPT), and the PKC inhibitor bisindolylmaleimide I. PKCϵ knockdown using PKCϵ shRNA did not impair the viability of the cells, as measured by the MTT assay (Fig. 9A). Protein and mRNA levels of PKCϵ were decreased by ∼60% in the PKCϵ shRNA-treated cells compared with untreated, vector only, or scrambled shRNA-treated cells (Fig. 9A). ApoE3 + cholesterol and DCPLA-ME were not protective against ASPDs in either the PKCϵ knockdown or inhibitor-pretreated cells (Fig. 9, B and C).
FIGURE 9.
ApoE3 acts through PKCϵ. A, immunoblot analysis and viability of primary neurons after shRNA transfection. PKCϵ shRNA reduced the expression of PKCϵ by 60% without significantly affecting the viability measured using the MTT assay. B, apoE3 and DCPLA-ME were not protective in neurons in which PKCϵ was knocked down. C, apoE3 and DCPLA-ME were not protective with PKCϵ inhibitor added. D, inhibition of GSK-3β phosphorylation by ASPDs. Shown is immunoblot analysis of phospho-GSK-3β (Ser-9) and total GSK-3β in total protein of rat hippocampal neurons treated with vehicle (Control), ASPD (50 nm), ASPD + DCPLA-ME (100 nm), ASPD + DCPLA-ME + PKCϵ inhibitor (5 μm), ASPD + apoE3 (10 nm) + cholesterol (100 μm), and LRP-1 antibody + ASPD + apoE3 (10 nm) + cholesterol (100 μm). E, phospho-GSK-3β expression was normalized against total GSK-3β expression. ASPD treatment decreased GSK-3β phosphorylation, whereas DCPLA-ME and apoE3 + cholesterol treatment increased it. Data are mean ± S.E. (error bars) (Student's t test). Asterisks indicate significance with respect to ASPD-treated cells. *, p < 0.05; **, p < 0.005; ***, p < 0.0005.
ApoE + Cholesterol and DCPLA-ME Inactivate GSK-3β in ASPD-treated Primary Neurons
DCPLA-ME treatment of ASPD-treated cells also restored the phosphorylation of the Ser-9 residue of GSK-3β to normal levels, as evidenced by an increased signal in anti-phospho-Ser-9 Western blots (Fig. 9, D and E). Phosphorylation of the Ser-9 residue by PKC is known to inhibit GSK-3β. Because GSK-3β is a key enzyme in the production of hyperphosphorylated Tau protein, increasing phosphorylation of GSK-3β at Ser-9 by PKC would also enhance the neuroprotective effect of DCPLA-ME. ApoE3 also increased phosphorylation of the Ser-9 residue, whereas blocking LRP-1 reversed the effect.
DISCUSSION
The primary role of apoE in brain is to transport cholesterol from astrocytes to neurons, where it is required for synaptic integrity and neuronal function (42). Cholesterol associated with apoE-lipoprotein particles is secreted by astrocytes and is essential for formation of mature synapses through functional apoE receptors (43–44). It acts by enhancing the synapse's structural stability and controlling synaptic vesicle formation and transport (42, 43) and axon growth (44).
There are several theories about the role of apoE in AD. One theory is that a deficiency of apoE3 causes dysfunctional Aβ clearance. ApoE3 also has a signaling role through LRP-1 and other receptors and indirectly inactivates GSK-3β by activating PKC and PKB, which phosphorylate the Ser-9 residue. Differences have been reported in the biphasic activation-deactivation kinetics of GSK-3β by apoE3 and -4 (45). ApoE3-containing lipoproteins protect against apoptosis, whereas apoE4 is less protective (15, 16). ApoE3 has also been reported to activate neprilysin, an important Aβ-degrading protease (46).
We found that apoE3 in the presence of cholesterol protected primary neurons against ASPD-induced cell death, whereas apoE4 + cholesterol did not. ApoE3 or apoE4 without cholesterol was protective in the presence of B27 supplement, but in absence of B27 supplement, neither apoE3 nor apoE4 protected against Aβ. This is consistent with previous reports that the effects of apoE isoforms are determined by their lipidation state. For example, there is no difference in Aβ binding between the nonphysiological delipidated forms of apoE3 and apoE4, but in the presence of cholesterol, apoE3 binds Aβ with 2–3-fold higher affinity than apoE4 (47–50). The effects of B27 supplement could be due to the presence of linoleic acid, linolenic acid, or other factors in the B27 supplement, which may facilitate the formation of intact lipoprotein particles. In the presence of cholesterol, apoE3 forms a stable structure that efficiently transports cholesterol to the cell. ApoE3 may also facilitate Aβ degradation by extracellular proteases, such as insulin-degrading enzyme, or facilitate export of Aβ from the brain and its transport to the liver. Although apoE4 in the presence of cholesterol is a less efficient transporter, it also helps in stabilizing Aβ oligomeric structures (4), thus potentiating its toxicity. We found that apoE3 also prevents the loss of synaptic proteins, confirming that apoE3 helps maintain synaptic integrity. ApoE3 acts through LRP-1, indicating that it acts intracellularly. Therefore, apoE must be acting by some other mechanism instead of or in addition to enhancing the clearance of Aβ.
In apoE3 transgenic mice, PKCϵ was constitutively activated compared with apoE4 or control mice, consistent with the finding that human apoE3 induces PKCϵ but human apoE4 does not. We also found that apoE3, but not apoE4, induces PKCϵ transcription and increases PKCϵ levels in both control and ASPD-treated cells. Blocking LRP-1 prevented apoE3 from increasing PKCϵ expression. When PKCϵ was knocked down or inhibited, apoE3 failed to protect the neurons against Aβ. Thus, apoE3 acts through PKCϵ and LRP-1 to induce neuroprotection and synaptogenesis (Fig. 10). This is consistent with the finding that apoE binding to LRP-1 can prevent apoptosis by inducing PKC-δ, which inactivates GSK-3β (15, 16). Mehta et al. (51) also showed that PKCϵ induces LDL receptor transcription. This suggests that apoE may act through PKCϵ to induce its own receptors.
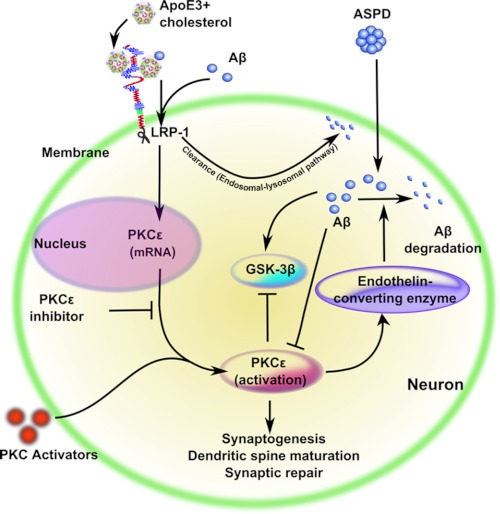
FIGURE 10.
PKCϵ regulation in neuroprotection against ASPD. ASPD reduces PKCϵ expression in rat hippocampal neurons and causes synaptic loss. ASPD also dephosphorylates and activates GSK-3β, which is responsible for hyperphosphorylation of Tau protein, forming neurofibrillary tangles. PKCϵ activators, such as DCPLA-ME, restore PKCϵ expression and prevent Aβ-induced synaptic loss. Endogenous PKC activators, such as arachidonic acid or Ca2+ from neuronal signaling, would have a similar effect. ApoE3 + cholesterol also protects against Aβ induced neurotoxicity. LRP-1 antibody and PKCϵ-specific inhibitors block the apoE3-mediated protection against ASPD. ApoE3 bound to cholesterol acts through the PKCϵ signaling pathway via LRP-1 to induce neuroprotection and synaptogenesis.
It has been reported that soluble Aβ reduces the levels of PKC isozymes (19) and down-regulates PKC by direct binding (18). Pharmacological restoration of the impaired PKC function results in an enhanced memory capacity and synaptic remodeling/repair and synaptogenesis and therefore represents a potentially important strategy for the treatment of memory disorders, such as Alzheimer disease (25, 28, 52). Here we showed that PKC activators protect against Aβ toxicity. DCPLA-ME, a new PKC activator that is specific for PKCϵ, provides greater protection than DCPLA or bryostatin 1. Therefore, PKCϵ is involved directly or indirectly in protection of neuronal survival against Aβ. We also showed that PKCϵ was reduced by 40% in the ASPD-treated cells, indicating that Aβ not only inactivates PKC enzymatically, as observed previously (23), but also blocks the synthesis of new PKC. Thus, our data suggest that ASPDs could be more pathologically relevant than other forms of Aβ because ASPDs can inhibit PKC activation and affect the cellular viability at nanomolar concentrations compared with the micromolar concentration needed by ADDLs.
PKCϵ is relatively brain-specific and is known to induce neuritic outgrowth (53), maintain the synaptic structure (25–26), and lower Aβ levels by activating α-secretase-mediated amyloid precursor protein cleavage (24, 54) and activating Aβ degradation by endothelin-converting enzyme (28). Therefore, PKCϵ activators such as DCPLA-ME exhibit at least two mechanisms that are potentially useful in treating AD: inducing synaptogenesis/repair and reducing Aβ levels. However, other mechanisms cannot be ruled out. DCPLA has also been reported to stimulate AMPA receptor exocytosis by inhibiting protein phosphatase-1 (40) and to enhance synaptic vesicle stability and stimulate neurotransmitter release (55).
DCPLA-ME also prevented the loss of synaptophysin and PSD-95 in ASPD-treated cells. Loss of synaptophysin by Aβ indicates a loss of synaptic vesicles, which would impair synaptic plasticity and reduce long-term potentiation (56–57). Aβ oligomers may act presynaptically, suppressing spontaneous synaptic activity by inhibition of P/Q-type calcium current (58) or by disrupting synaptic vesicle endocytosis (59). It is also reported that inhibition of PKC signaling impairs synaptic plasticity by disrupting synaptic vesicle recycling (60). These effects are all consistent with a principal target of Aβ being the disruption of synaptic integrity. Our results also show that ASPDs activate GSK-3β and that a PKCϵ activator prevents the effect. Conversely, apoE3 inhibited GSK-3β in a manner dependent on the LRP-1 receptor. Interestingly, we found that blocking the LRP-1 receptor using RAP also reduced the protective effect of DCPLA-ME. This could be explained by facilitation of DCPLA-ME uptake by LRP-1 or by low levels of endogenous apoE3 + cholesterol or other inducers of PKC synthesis in the medium. Another possibility is that Aβ may be imported through LRP-1 as described by Fuentealba et al. (61), leading to degradation of Aβ by PKC-activated proteases, such as endothelin-converting enzyme (28). Our findings demonstrate the importance of intracellular signaling in mediating the effects of apoE and represent a novel mechanism by which apoE3 can prevent synaptic damage independent of its effects on Aβ clearance. Activation of this pathway using PKCϵ activators or LRP-1 agonists may have therapeutic value in protecting against Aβ-induced synaptic loss.
Footnotes
- AD
- Alzheimer disease
- DCPLA
- 8-(2-(2-pentyl-cyclopropylmethyl)-cyclopropyl)-octanoic acid
- DCPLA-ME
- DCPLA methyl ester
- Aβ
- amyloid β
- ADDL
- Aβ-derived diffusible ligand
- ASPD
- amylospheroid
- AFM
- atomic force microscopy
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- RAP
- receptor-associated protein.
REFERENCES
- 1. Selkoe D. J. (2001) Alzheimer's disease results from the cerebral accumulation and cytotoxicity of amyloid β-protein. J. Alzheimers Dis. 3, 75–80 [DOI] [PubMed] [Google Scholar]
- 2. Selkoe D. J. (2001) Alzheimer's disease. Genes, proteins, and therapy. Physiol. Rev. 81, 741–766 [DOI] [PubMed] [Google Scholar]
- 3. Ballard C., Gauthier S., Corbett A., Brayne C., Aarsland D., Jones E. (2011) Alzheimer's disease. Lancet 377, 1019–1031 [DOI] [PubMed] [Google Scholar]
- 4. Cerf E., Gustot A., Goormaghtigh E., Ruysschaert J. M., Raussens V. (2011) High ability of apolipoprotein E4 to stabilize amyloid-β peptide oligomers, the pathological entities responsible for Alzheimer's disease. FASEB J. 25, 1585–1595 [DOI] [PubMed] [Google Scholar]
- 5. Kim J., Castellano J. M., Jiang H., Basak J. M., Parsadanian M., Pham V., Mason S. M., Paul S. M., Holtzman D. M. (2009) Overexpression of low density lipoprotein receptor in the brain markedly inhibits amyloid deposition and increases extracellular Aβ clearance. Neuron 64, 632–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim J., Basak J. M., Holtzman D. M. (2009) The role of apolipoprotein E in Alzheimer's disease. Neuron 63, 287–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fagan A. M., Bu G., Sun Y., Daugherty A., Holtzman D. M. (1996) Apolipoprotein E-containing high density lipoprotein promotes neurite outgrowth and is a ligand for the low density lipoprotein receptor-related protein. J. Biol. Chem. 271, 30121–30125 [DOI] [PubMed] [Google Scholar]
- 8. Ye S., Huang Y., Müllendorff K., Dong L., Giedt G., Meng E. C., Cohen F. E., Kuntz I. D., Weisgraber K. H., Mahley R. W. (2005) Apolipoprotein (apo) E4 enhances amyloid β peptide production in cultured neuronal cells. ApoE structure as a potential therapeutic target. Proc. Natl. Acad. Sci. U.S.A. 102, 18700–18705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hartman R. E., Wozniak D. F., Nardi A., Olney J. W., Sartorius L., Holtzman D. M. (2001) Behavioral phenotyping of GFAP-apoE3 and -apoE4 transgenic mice. ApoE4 mice show profound working memory impairments in the absence of Alzheimer's-like neuropathology. Exp. Neurol. 170, 326–344 [DOI] [PubMed] [Google Scholar]
- 10. Bour A., Grootendorst J., Vogel E., Kelche C., Dodart J. C., Bales K., Moreau P. H., Sullivan P. M., Mathis C. (2008) Middle-aged human apoE4-targeted-replacement mice show retention deficits on a wide range of spatial memory tasks. Behav. Brain Res. 193, 174–182 [DOI] [PubMed] [Google Scholar]
- 11. Nathan B. P., Bellosta S., Sanan D. A., Weisgraber K. H., Mahley R. W., Pitas R. E. (1994) Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science 264, 850–852 [DOI] [PubMed] [Google Scholar]
- 12. Ji Y., Gong Y., Gan W., Beach T., Holtzman D. M., Wisniewski T. (2003) Apolipoprotein E isoform-specific regulation of dendritic spine morphology in apolipoprotein E transgenic mice and Alzheimer's disease patients. Neuroscience 122, 305–315 [DOI] [PubMed] [Google Scholar]
- 13. Dumanis S. B., Tesoriero J. A., Babus L. W., Nguyen M. T., Trotter J. H., Ladu M. J., Weeber E. J., Turner R. S., Xu B., Rebeck G. W., Hoe H. S. (2009) ApoE4 decreases spine density and dendritic complexity in cortical neurons in vivo. J. Neurosci. 29, 15317–15322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rapp A., Gmeiner B., Hüttinger M. (2006) Implication of apoE isoforms in cholesterol metabolism by primary rat hippocampal neurons and astrocytes. Biochimie 88, 473–483 [DOI] [PubMed] [Google Scholar]
- 15. Hayashi H., Campenot R. B., Vance D. E., Vance J. E. (2009) Protection of neurons from apoptosis by apolipoprotein E-containing lipoproteins does not require lipoprotein uptake and involves activation of phospholipase Cγ1 and inhibition of calcineurin. J. Biol. Chem. 284, 29605–29613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hayashi H., Campenot R. B., Vance D. E., Vance J. E. (2007) Apolipoprotein E-containing lipoproteins protect neurons from apoptosis via a signaling pathway involving low density lipoprotein receptor-related protein-1. J. Neurosci. 27, 1933–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cole G., Dobkins K. R., Hansen L. A., Terry R. D., Saitoh T. (1988) Decreased levels of protein kinase C in Alzheimer brain. Brain Res. 452, 165–174 [DOI] [PubMed] [Google Scholar]
- 18. Lee W., Boo J. H., Jung M. W., Park S. D., Kim Y. H., Kim S. U., Mook-Jung I. (2004) Amyloid β peptide directly inhibits PKC activation. Mol. Cell Neurosci. 26, 222–231 [DOI] [PubMed] [Google Scholar]
- 19. Favit A., Grimaldi M., Nelson T. J., Alkon D. L. (1998) Alzheimer's-specific effects of soluble β-amyloid on protein kinase C-α and -γ degradation in human fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 95, 5562–5567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kinouchi T., Sorimachi H., Maruyama K., Mizuno K., Ohno S., Ishiura S., Suzuki K. (1995) Conventional protein kinase C (PKC)-α and novel PKC epsilon, but not -δ, increase the secretion of an N-terminal fragment of Alzheimer's disease amyloid precursor protein from PKC cDNA transfected 3Y1 fibroblasts. FEBS Lett. 364, 203–206 [DOI] [PubMed] [Google Scholar]
- 21. Slack B. E., Nitsch R. M., Livneh E., Kunz G. M., Jr., Eldar H., Wurtman R. J. (1993) Regulation of amyloid precursor protein release by protein kinase C in Swiss 3T3 fibroblasts. Ann. N.Y. Acad. Sci. 695, 128–131 [DOI] [PubMed] [Google Scholar]
- 22. Jolly-Tornetta C., Wolf B. A. (2000) Regulation of amyloid precursor protein (APP) secretion by protein kinase Cα in human ntera 2 neurons (NT2N). Biochemistry 39, 7428–7435 [DOI] [PubMed] [Google Scholar]
- 23. Lanni C., Mazzucchelli M., Porrello E., Govoni S., Racchi M. (2004) Differential involvement of protein kinase C α and ϵ in the regulated secretion of soluble amyloid precursor protein. Eur. J. Biochem. 271, 3068–3075 [DOI] [PubMed] [Google Scholar]
- 24. Yeon S. W., Jung M. W., Ha M. J., Kim S. U., Huh K., Savage M. J., Masliah E., Mook-Jung I. (2001) Blockade of PKC ϵ activation attenuates phorbol ester-induced increase of α-secretase-derived secreted form of amyloid precursor protein. Biochem. Biophys. Res. Commun. 280, 782–787 [DOI] [PubMed] [Google Scholar]
- 25. Hongpaisan J., Sun M. K., Alkon D. L. (2011) PKC ϵ activation prevents synaptic loss, Aβ elevation, and cognitive deficits in Alzheimer's disease transgenic mice. J. Neurosci. 31, 630–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hongpaisan J., Alkon D. L. (2007) A structural basis for enhancement of long-term associative memory in single dendritic spines regulated by PKC. Proc. Natl. Acad. Sci. U.S.A. 104, 19571–19576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kanno T., Yamamoto H., Yaguchi T., Hi R., Mukasa T., Fujikawa H., Nagata T., Yamamoto S., Tanaka A., Nishizaki T. (2006) The linoleic acid derivative DCP-LA selectively activates PKC-ϵ, possibly binding to the phosphatidylserine binding site. J. Lipid Res. 47, 1146–1156 [DOI] [PubMed] [Google Scholar]
- 28. Nelson T. J., Cui C., Luo Y., Alkon D. L. (2009) Reduction of β-amyloid levels by novel protein kinase Cϵ activators. J. Biol. Chem. 284, 34514–34521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nelson T. J., Alkon D. L. (2009) Neuroprotective versus tumorigenic protein kinase C activators. Trends Biochem. Sci. 34, 136–145 [DOI] [PubMed] [Google Scholar]
- 30. Sakono M., Zako T. (2010) Amyloid oligomers. Formation and toxicity of Aβ oligomers. FEBS J 277, 1348–1358 [DOI] [PubMed] [Google Scholar]
- 31. Kuo Y. M., Emmerling M. R., Vigo-Pelfrey C., Kasunic T. C., Kirkpatrick J. B., Murdoch G. H., Ball M. J., Roher A. E. (1996) Water-soluble Aβ (N-40, N-42) oligomers in normal and Alzheimer's disease brains. J. Biol. Chem. 271, 4077–4081 [DOI] [PubMed] [Google Scholar]
- 32. Walsh D. M., Hartley D. M., Kusumoto Y., Fezoui Y., Condron M. M., Lomakin A., Benedek G. B., Selkoe D. J., Teplow D. B. (1999) Amyloid β-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J. Biol. Chem. 274, 25945–25952 [DOI] [PubMed] [Google Scholar]
- 33. Lambert M. P., Barlow A. K., Chromy B. A., Edwards C., Freed R., Liosatos M., Morgan T. E., Rozovsky I., Trommer B., Viola K. L., Wals P., Zhang C., Finch C. E., Krafft G. A., Klein W. L. (1998) Diffusible, nonfibrillar ligands derived from Aβ 1–42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. U.S.A. 95, 6448–6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lesné S., Koh M. T., Kotilinek L., Kayed R., Glabe C. G., Yang A., Gallagher M., Ashe K. H. (2006) A specific amyloid-β protein assembly in the brain impairs memory. Nature 440, 352–357 [DOI] [PubMed] [Google Scholar]
- 35. Barghorn S., Nimmrich V., Striebinger A., Krantz C., Keller P., Janson B., Bahr M., Schmidt M., Bitner R. S., Harlan J., Barlow E., Ebert U., Hillen H. (2005) Globular amyloid β-peptide oligomer. A homogenous and stable neuropathological protein in Alzheimer's disease. J. Neurochem. 95, 834–847 [DOI] [PubMed] [Google Scholar]
- 36. Deshpande A., Mina E., Glabe C., Busciglio J. (2006) Different conformations of amyloid β induce neurotoxicity by distinct mechanisms in human cortical neurons. J. Neurosci. 26, 6011–6018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hoshi M., Sato M., Matsumoto S., Noguchi A., Yasutake K., Yoshida N., Sato K. (2003) Spherical aggregates of β-amyloid (amylospheroid) show high neurotoxicity and activate Tau protein kinase I/glycogen synthase kinase-3β. Proc. Natl. Acad. Sci. U.S.A. 100, 6370–6375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Noguchi A., Matsumura S., Dezawa M., Tada M., Yanazawa M., Ito A., Akioka M., Kikuchi S., Sato M., Ideno S., Noda M., Fukunari A., Muramatsu S., Itokazu Y., Sato K., Takahashi H., Teplow D. B., Nabeshima Y., Kakita A., Imahori K., Hoshi M. (2009) Isolation and characterization of patient-derived, toxic, high mass amyloid β-protein (Aβ) assembly from Alzheimer disease brains. J. Biol. Chem. 284, 32895–32905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dinamarca M. C., Cerpa W., Garrido J., Hancke J. L., Inestrosa N. C. (2006) Hyperforin prevents β-amyloid neurotoxicity and spatial memory impairments by disaggregation of Alzheimer's amyloid-β deposits. Mol. Psychiatry 11, 1032–1048 [DOI] [PubMed] [Google Scholar]
- 40. Kanno T., Yaguchi T., Nagata T., Tanaka A., Nishizaki T. (2009) DCP-LA stimulates AMPA receptor exocytosis through CaMKII activation due to PP-1 inhibition. J. Cell. Physiol. 221, 183–188 [DOI] [PubMed] [Google Scholar]
- 41. Raffai R. L., Dong L. M., Farese R. V., Jr., Weisgraber K. H. (2001) Introduction of human apolipoprotein E4 “domain interaction” into mouse apolipoprotein E. Proc. Natl. Acad. Sci. U.S.A. 98, 11587–11591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pfrieger F. W. (2003) Role of cholesterol in synapse formation and function. Biochim. Biophys. Acta 1610, 271–280 [DOI] [PubMed] [Google Scholar]
- 43. Mauch D. H., Nägler K., Schumacher S., Göritz C., Müller E. C., Otto A., Pfrieger F. W. (2001) CNS synaptogenesis promoted by glia-derived cholesterol. Science 294, 1354–1357 [DOI] [PubMed] [Google Scholar]
- 44. Bu G. (2009) Apolipoprotein E and its receptors in Alzheimer's disease. Pathways, pathogenesis, and therapy. Nat. Rev. Neurosci. 10, 333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cedazo-Mínguez A., Popescu B. O., Blanco-Millán J. M., Akterin S., Pei J. J., Winblad B., Cowburn R. F. (2003) Apolipoprotein E and β-amyloid (1–42) regulation of glycogen synthase kinase-3β. J. Neurochem. 87, 1152–1164 [DOI] [PubMed] [Google Scholar]
- 46. Jiang Q., Lee C. Y., Mandrekar S., Wilkinson B., Cramer P., Zelcer N., Mann K., Lamb B., Willson T. M., Collins J. L., Richardson J. C., Smith J. D., Comery T. A., Riddell D., Holtzman D. M., Tontonoz P., Landreth G. E. (2008) ApoE promotes the proteolytic degradation of Aβ. Neuron 58, 681–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tokuda T., Calero M., Matsubara E., Vidal R., Kumar A., Permanne B., Zlokovic B., Smith J. D., Ladu M. J., Rostagno A., Frangione B., Ghiso J. (2000) Lipidation of apolipoprotein E influences its isoform-specific interaction with Alzheimer's amyloid β peptides. Biochem. J. 348, 359–365 [PMC free article] [PubMed] [Google Scholar]
- 48. Strittmatter W. J., Weisgraber K. H., Huang D. Y., Dong L. M., Salvesen G. S., Pericak-Vance M., Schmechel D., Saunders A. M., Goldgaber D., Roses A. D. (1993) Binding of human apolipoprotein E to synthetic amyloid β peptide. Isoform-specific effects and implications for late-onset Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 90, 8098–8102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Strittmatter W. J., Saunders A. M., Schmechel D., Pericak-Vance M., Enghild J., Salvesen G. S., Roses A. D. (1993) Apolipoprotein E. High-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 90, 1977–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. LaDu M. J., Falduto M. T., Manelli A. M., Reardon C. A., Getz G. S., Frail D. E. (1994) Isoform-specific binding of apolipoprotein E to β-amyloid. J. Biol. Chem. 269, 23403–23406 [PubMed] [Google Scholar]
- 51. Mehta K. D., Radominska-Pandya A., Kapoor G. S., Dave B., Atkins B. A. (2002) Critical role of diacylglycerol- and phospholipid-regulated protein kinase C epsilon in induction of low-density lipoprotein receptor transcription in response to depletion of cholesterol. Mol. Cell. Biol. 22, 3783–3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sun M. K., Alkon D. L. (2009) Protein kinase C activators as synaptogenic and memory therapeutics. Arch. Pharm. (Weinheim) 342, 689–698 [DOI] [PubMed] [Google Scholar]
- 53. Ling M., Trollér U., Zeidman R., Stensman H., Schultz A., Larsson C. (2005) Identification of conserved amino acids N-terminal of the PKC ϵ C1b domain crucial for protein kinase C ϵ-mediated induction of neurite outgrowth. J. Biol. Chem. 280, 17910–17919 [DOI] [PubMed] [Google Scholar]
- 54. Zhu G., Wang D., Lin Y. H., McMahon T., Koo E. H., Messing R. O. (2001) Protein kinase C ϵ suppresses Aβ production and promotes activation of α-secretase. Biochem. Biophys. Res. Commun. 285, 997–1006 [DOI] [PubMed] [Google Scholar]
- 55. Shimizu T., Kanno T., Tanaka A., Nishizaki T. (2011) α,β-DCP-LA selectively activates PKC-ϵ and stimulates neurotransmitter release with the highest potency among 4 diastereomers. Cell Physiol. Biochem. 27, 149–158 [DOI] [PubMed] [Google Scholar]
- 56. Fernández-Chacón R., Südhof T. C. (1999) Genetics of synaptic vesicle function. Toward the complete functional anatomy of an organelle. Annu. Rev. Physiol. 61, 753–776 [DOI] [PubMed] [Google Scholar]
- 57. Janz R., Südhof T. C., Hammer R. E., Unni V., Siegelbaum S. A., Bolshakov V. Y. (1999) Essential roles in synaptic plasticity for synaptogyrin I and synaptophysin I. Neuron 24, 687–700 [DOI] [PubMed] [Google Scholar]
- 58. Nimmrich V., Grimm C., Draguhn A., Barghorn S., Lehmann A., Schoemaker H., Hillen H., Gross G., Ebert U., Bruehl C. (2008) Amyloid β oligomers (Aβ(1–42) globulomer) suppress spontaneous synaptic activity by inhibition of P/Q-type calcium currents. J. Neurosci. 28, 788–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kelly B. L., Ferreira A. (2007) β-Amyloid disrupted synaptic vesicle endocytosis in cultured hippocampal neurons. Neuroscience 147, 60–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Volmer R., Monnet C., Gonzalez-Dunia D. (2006) Borna disease virus blocks potentiation of presynaptic activity through inhibition of protein kinase C signaling. PLoS Pathog. 2, e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fuentealba R. A., Liu Q., Zhang J., Kanekiyo T., Hu X., Lee J. M., LaDu M. J., Bu G. (2010) Low-density lipoprotein receptor-related protein 1 (LRP1) mediates neuronal Aβ42 uptake and lysosomal trafficking. PLoS One 5, e11884. [DOI] [PMC free article] [PubMed] [Google Scholar]