Abstract
Atypical ductal hyperplasia (ADH), flat epithelial atypia (FEA), and lobular neoplasia (LN) form a group of early precursor lesions that are part of the low-grade pathway in breast cancer development. This concept implies that the neoplastic disease process begins at a stage much earlier than in situ carcinoma. We have performed a review of the published literature for the upgrade risk to ductal carcinoma in situ or invasive carcinoma in open biopsy after a diagnosis of ADH, FEA, or LN in core needle biopsy. This has revealed the highest upgrade risk for ADH (28.2% after open biopsy), followed by LN (14.9%), and FEA (10.2%). With LN, the pleomorphic subtype is believed to confer a higher risk than classical LN. With all types of precursor lesions, careful attention must be paid to the clinicopathological correlation for the guidance of the clinical management. Follow-up biopsies are generally indicated in ADH, and if there is any radiological-pathological discrepancy, also in LN or FEA.
Key Words: Breast cancer, precursor; Atypical ductal hyperplasia (ADH); Flat epithelial atypia (FEA); Lobular neoplasia (LN)
Abstract
Die atypische duktale Hyperplasie (ADH), die flache epitheliale Atypie (FEA) und die lobuläre Neoplasie (LN) bilden eine Gruppe von frühen Vorläuferläsionen und gehören zum Low-Grade-Pathway des Mammakarzinoms. In diesem Modell beginnt der neoplastische Prozess bei diesen frühen Läsionen und nicht als In-Situ-Karzinom. Wir haben eine Literaturübersicht zum Risiko für ein Upgrade zum duktalen Carcinoma in situ oder invasiven Karzinom in der offenen Biopsie nach einer Diagnose des ADH, FEA oder LN in der Stanzbiopsie durchgeführt. Diese zeigt das höchste Risiko für ADH (28,2%), gefolgt von LN (14,9%) und FEA (10,2%). Der pleomorphen LN wird ein höheres Risiko zugeordnet als der klassischen LN. Bei allen Formen von Vorläuferläsionen ist der klinisch-pathologischen Korrelation große Aufmerksamkeit für das klinische Management zu widmen. Eine offene Biopsie ist bei Diagnose einer ADH in der Regel erforderlich, seltener auch bei LN oder FEA, insbesondere aber bei radiologisch-pathologischen Abweichungen.
Introduction
Improvements in clinical radiology, and the routine use of large core needle biopsies for the diagnosis of clinically occult breast lesions have led to an increased detection rate of early precursor lesions of the breast, such as atypical ductal hyperplasia (ADH) or lobular neoplasia (LN). This has provoked new questions regarding the role of these lesions in the evolution of breast cancer and their practical management [1]. The term ‘lesions of uncertain malignant potential’ that is used in screening mammography reflects the uncertainty regarding the behaviour of these lesions. Over the past years, our understanding on the pathology, classification, and molecular biology of early precursor lesions has substantially improved, however. This has led away from the risk analysis of proliferative breast disease in general [2] and permits a more detailed analysis of specific precursor lesions. The pathological assessment of precursor lesions relies on the use of a consistent nomenclature and reliable diagnostic criteria to allow for interinstitutional comparison. Unfortunately, the rate of inter-observer reproducibility of precursor lesions is relatively high [3], and this is related to controversial views about criteria and the biological behaviour and the lack of a unifying morphologic and molecular taxonomy of breast cancer in general [4]. With these caveats, we will try to summarise our current knowledge and understanding of low-grade precursor lesions and discuss the implications on their management.
Molecular Precursor Pathways
The currently favoured model of human breast cancer evolution includes a stepwise progression of very early, morphologically definable precursor lesions with cellular atypia to carcinoma in situ and invasive breast cancer [5]. The earliest lesions that are considered to carry a substantial risk for progression into carcinoma are classified into ADH, LN, and flat epithelial atypia (FEA) for columnar cell lesions with atypia. These early precursor lesions of the breast are characterised by relatively few somatic chromosomal alterations, with loss of 16q and gain of 1q being the most frequent findings [6]. Interestingly, these genetic changes are the same alterations that are also characteristic for low-grade ductal carcinoma in situ (DCIS) and for low-grade invasive carcinoma, such as tubular carcinoma [7, 8, 9]. Therefore, it is believed that a linear progression pathway exists from low-grade precursor lesions to low-grade invasive breast cancer [10].
The high expression of oestrogen receptors (ER) and progesterone receptors (PR) indicates a loss of regulative mechanisms in early precursor lesions and is a common denominator of these lesions. In contrast to this, much lower levels of ER and PR are seen in normal breast epithelia and typically vary from one cell to another [11]. This change in steroid receptor expression levels in the earliest precursor lesions points towards an important role of hormonal influences on the development of low-grade breast cancer. In addition to changes in steroid hormone receptor action, epigenetic changes have been implicated as an early carcinogenic event [12]. These features of early precursor lesions fit in the concept of a low-grade pathway in breast cancer development that was derived from genomic studies [6, 7]. In contrast to findings in the low-grade pathway, high-grade lesions such as high-grade DCIS show quite different molecular characteristics such as amplification of the HER2 gene [13] or (less frequently) p53 mutations [14].
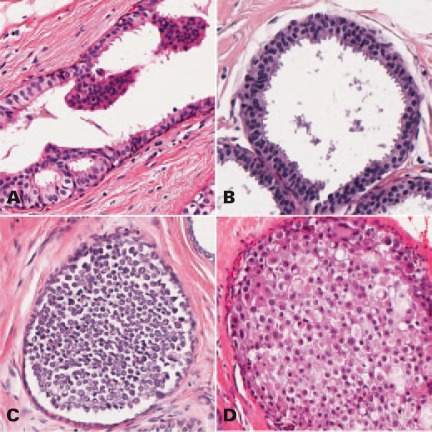
Morphologically, this concept of a low-grade pathway is supported by the fact that ADH, FEA, and LN share certain histological features (fig. 1), such as low-grade nuclear atypia, and often occur simultaneously in one biopsy specimen [15]. Also, lesions of the low-grade pathway can be observed in association with the spectrum of proliferative breast disease [16], and also with specific benign lesions such as radial scar [17] or intraductal papilloma [18]. Although proliferative breast disease carries a slightly elevated epidemiological risk on its own [19], these hyperplastic changes clearly are non-neoplastic and therefore are not considered precursor lesions. For all these reasons, low-grade precursor lesions (FEA, ADH, LN) are currently considered to be the earliest identifiable lesions of the low-grade pathway of breast cancer development. This, and the similarity of molecular findings in different histological types of breast cancer, has lead to a dualistic concept of breast cancer development, and has essentially replaced the historic concept of a ductal and lobular pathway in breast cancer development.
Fig. 1.
Examples of early precursor lesions. A typical ductal hyperplasia, B flat epithelial hyperplasia, C lobular neoplasia, classic type, D lobular neoplasia, pleomorphic-apocrina type.
Atypical Ductal Hyperplasia
Terminology
The term ADH has been defined to describe small atypical ductal lesions with insufficient criteria for a definite diagnosis of DCIS. There is no general agreement on diagnostic criteria to distinguish ADH from low-grade DCIS, and different definitions have been applied [20]. Commonly, ADH is either defined as partial involvement of the terminal ductal-lobular unit by monomorphic, low-grade atypical ductal epithelia with architectural disturbances such as rigid bridges or micropapillae but not completely filling the duct [21], or as an uni- or multifocal lesion that fulfils all criteria of low-grade DCIS except for a maximum size of 2–3 mm [16, 22]. Uncommon variants of ADH include atypical apocrine hyperplasia and atypical ductal proliferations developing within a pre-existing benign proliferative lesion such as sclerosing adenosis, usual-type ductal hyperplasia, or papilloma [20]. ADH may be distinguished pathologically from usual ductal hyperplasia by the use of basal cytokeratins, especially cytokeratin 5/6 [23]. There are two terminological problems with ADH: Firstly, ADH clearly is not a hyperplastic lesion but has all features of an early neoplastic process, and for this reason, ADH is also considered part of the classification system of ductal intraepithelial neoplasia [24]. Secondly, the term ‘atypical epithelial proliferation of ductal type’ is preferred over ADH in the European Screening Mammography guidelines [25], because it has been argued that a lesion with criteria of ADH on core biopsy may prove to be part of a larger low-grade DCIS on excision specimen, and a definitive diagnosis of ADH on core needle biopsies may not be possible. On the other hand the risk of DCIS after a diagnosis of ADH is well documented in the literature and the use of the term ‘atypical epithelial proliferation of ductal type’ may create the false impression of the presence of a lesion with lower risk. Therefore, we prefer to continue using the term ADH also in core biopsies, knowing that at the time of biopsy a substantial proportion will turn out to be low-grade DCIS.
Molecular Similarity of ADH and DCIS
The current concept of ADH being the immediate precursor of low-grade DCIS is based not only on clinical studies and morphologic similarities between both lesions, but in addition, on a high degree of genomic similarity with almost identical kinds of chromosomal imbalances [26, 27, 28]. A loss at chromosome 16q and 17p was concurrently observed when comparing ADH and DCIS lesions [26, 27]. Also in a study of 9 ADHs, a total of 18 copy number changes were identified with recurrent losses of 16q and 17p and frequent gains on chromosome 1q [26], similar to observations in low-grade DCIS. In view of the genomic similarity of low-grade DCIS and ADH one may question the validity of differentiating between both lesions. However, because of the prognostic differences of ADH and low-grade DCIS, it is fair to interpret the molecular data as supportive of the assumption that ADH is not just a small low-grade DCIS but a closely related precursor lesion [29].
Significance of ADH in Core Biopsy
Clinically, an excisional biopsy is recommended when ADH is identified in core needle biopsy or in a vacuum-assisted biopsy specimen [16, 25, 30]. This is because of the relatively high probability of underestimating a DCIS or invasive cancer on needle biopsy. The question about the risk of upgrade of an ADH lesion found in needle biopsy has been addressed in several studies (table 1) and has been reported to range between 22 and 56%. The high variability of these upgrade rates has been attributed to different biopsy techniques (i.e. core biopsy vs. vacuum-assisted biopsy) and to pathologic criteria used in these studies. Clearly, the diameter of the needle biopsy is one of the most important determinants of the probability of finding a higher-grade lesion but also the number of ADH foci in the needle biopsies. However, it has been concluded that neither by using 11- or 9-gauge needles nor by counting the ADH foci a group of patients that does not require excisional biopsy can be delineated with sufficient accuracy [31].
Table 1.
Upgrade rates after atypical ductal hyperplasia (ADH) diagnosis on vacuum-assisted or core needle biopsy and excision
| Author, year [ref.] | Cases, n | ADH lesions, n (%) | Excisional biopsy, n (%) | DCIS on excision, n (%) | Invasive carcinoma on excision, n (%) | Total upgrade rate, n (%) |
|---|---|---|---|---|---|---|
| Jackman et al. 1994 [57] | 450 | 19 (4.2) | 16 (84.2) | 6 (37.5) | 3 (18.8) | 9 (56.3) |
| Liberman et al. 1995 [58] | 264 | 25 (9.5) | 21 (84) | 8 (38.1) | 3 (14.3) | 11 (52.4) |
| Tocino et al. 1994 [59] | 358 | 18 (5) | 18 (100) | 5 (27.8) | 4 (22.2) | 9 (50) |
| Nguyen et al. 1996 [60] | 431 | 13 (3) | 13 (100) | ND | ND | 4 (30.8) |
| Burbank 1997 [61] | 868 | 26 (3) | 18 (69.2) | ND | ND | 8 (44.4) |
| Lee et al. 1997 [62] | 405 | 17 (4.2) | 15 (88.2) | 2 (13.3) | 3 (20) | 5 (33.3) |
| Liberman et al. 1997 [63] | 442 | 41 (9.3) | 37 (90.2) | 16 (43.2) | 4 (10.8) | 20 (54.1) |
| Moore et al. 1997 [64] | 510 | 23 (4.5) | 21 (91.3) | 7 (33.3) | ND | 7 (33.3) |
| Gadzala et al. 1997 [65] | 900 | 39 (4.3) | 36 (92.3) | 13 (36.1) | 4 (11.1) | 17 (47.2) |
| Meyer et al. 1998 [66] | 1,032 | 18 (1.7) | 18 (100) | 7 (38.9) | 3 (16.7) | 10 (55.6) |
| Lin et al. 1998 [67] | 539 | 21 (3.9) | 18 (85.7) | 2 (11.1) | ND | 2 (11.1) |
| Fuhrman et al. 1998 [68] | 1,440 | 67 (4.7) | 63 (94) | 24 (38.1) | 10 (15.9) | 34 (54) |
| Brem et al. 1999 [69] | 422 | 20 (4.7) | 16 (80) | 2 (12.5) | 2 (12.5) | 4 (25) |
| Burak et al. 2000 [70] | 851 | 43 (5.1) | 40 (93) | 1 (2.5) | 4 (10) | 5 (12.5) |
| Philpotts et al. 2000 [71] | 753 | 26 (3.5) | 26 (100) | 5 (19.2) | 1 (3.8) | 6 (23.1) |
| O'Hea and Tornos 2000 [72] | 590 | 27 (4.6) | 19 (70.4) | 4 (21.1) | 2 (10.5) | 6 (31.6) |
| Adrales et al. 2000 [73] | 1,081 | 90 (8.3) | 62 (68.9) | 7 (11.3) | 2 (3.2) | 9 (14.5) |
| Darling et al. 2000 [74] | 3,873 | 148 (3.8) | 139 (93.9) | 27 (19.4) | 11 (7.9) | 38 (27.3) |
| Cangierella et al. 2001 [75] | 160 | 10 (6.3) | 8 (80) | 2 (25) | ND | 2 (25) |
| Lai et al. 2001 [76] | 673 | 19 (2.8) | 12 (63.2) | ND | ND | 2 (16.7) |
| Jackman et al. 2002 [77] | 1,964 | 131 (6.7) | 104 (79.4) | 19 (18.3) | 3 (2.9) | 22 (21.2) |
| Liberman et al. 2002 [78] | 322 | 18 (5.6) | 16 (88.9) | 5 (31.3) | ND | 5 (31.3) |
| Zhao et al. 2003 [79] | 1,036 | 53 (5.1) | 39 (73.6) | 10 (25.6) | 1 (2.6) | 11 (28.2) |
| Lourenco et al. [80] | 1,223 | 95 (7.8) | 73 (76.8) | 18 (24.7) | 3 (4.1) | 21 (28.8) |
| Doren et al. 2008 [81] | ND | 51 | 51 (100) | 9 (17.6) | 8 (15.7) | 17 (33.3) |
| Wagoner et al. 2009 [82] | ND | 201 | 123 (61.2) | 22 (17.9) | ND | 22 (17.9) |
| Arora 2009 [83] | 1,072 | 35 (3.3) | 30 (85.7) | 3 (10) | 1 (3.3) | 4 (13.3) |
| Chae et al. 2009 [84] | 3,476 | 69 (2) | 45 (65.2) | 8 (17.8) | 2 (4.4) | 10 (22.2) |
| Eby et al. [85] | 991 | 141 (14.2) | 123 (87.2) | 21 (17.1) | 5 (4.1) | 26 (21.1) |
| Youk et al. [86] | 7,050 | 25 (0.4) | 21 (84) | 9 (42.9) | 4 (19) | 13 (61.9) |
| Kohr et al. [31] | 991 | 112 (11.3) | 101 (90.2) | 17 (16.8) | 3 (3) | 20 (19.8) |
| Total | 34,167 | 1,641 (4.8) | 1,342 (81.8) | 279 (20.8) | 86 (6.4) | 379 (28.2) |
ADH = Atypical ductal hyperplasia; DCIS = ductal carcinoma in situ; ND = not determined.
Flat Epithelial Atypia
Relationship to Other Columnar Cell Lesions
Although FEA has long been known to pathologists under varying names, we are currently just beginning to understand the role of these alterations in the development of breast carcinomas and their relationship with other hormone-dependent precursor lesions. Together with columnar cell metaplasia (CCM) and columnar cell hyperplasia (CCH), FEA are part of the spectrum of columnar cell changes (CCC) that at the benign end include alterations that have been called blunt duct adenosis. CCC without atypia are observed very frequently. Already in 1979, Azzopardi et al. [32] stressed the importance of distinguishing columnar cell lesions with nuclear atypia from benign changes and coined the term ‘clinging carcinoma, monomorphic type’ to describe a lesion known today as FEA.
Morphologic Criteria for the Diagnosis of FEA
Detailed morphologic criteria of CCM/CCH and FEA have been published by Schnitt et al. [33] and O'Malley et al. [34]. Briefly, CCC are comprised of columnar cells lining the ectatic acini of terminal ductal-lobular units. In CCM, only one or two layers of columnar cells are present while CCH shows several layers of stratified cells. Occasionally, small tufts but no well-formed micropapillae or rigid bridges may be formed. The nuclei typically are uniform, ovoid, or elongated and oriented along the basement membrane in a perpendicular fashion, but not atypical. FEA is defined as a lesion with architectural features of CCM/CCH showing low-grade nuclear atypia. In contrast to lesions without atypia, nuclei here typically are round, contain small nucleoli and display a loss of polarity. In a few cases, elongated, hyperchromatic nuclei with prominent stratification (similar to the pattern seen in colonic adenomas) may also be observed. Apical snouts and luminal secretions as well as microcalcifications are further characteristic features of both CCM/CCH and FEA. In fact, the vast majority of CCC is encountered in biopsy samples taken for microcalcifications [35, 36].
Relationship to the Low-Grade Pathway
Although the occasional coexistence of FEA with tubular carcinoma and foci of LN was already mentioned by Azzopardi et al. [32], the frequent association of these three alterations was reported in detail by Rosen [37] and has hence been entitled the ‘Rosen triad’ by some authors [38]. The neoplastic nature of FEA was first shown by Moinfar et al. [39]. Interestingly, the cytologic features of FEA with cuboid to columnar cells, low-grade nuclear atypia, and often prominent apical snouts closely resembles the cells of tubular carcinoma, and both alterations show a similar immunophenotype, leading pathologists to speculate on a possible precursor role [10, 40]. Using mitochondrial DNA sequencing and comptive assessment of allelic imbalances, we recently could confirm a direct clonal relationship between tubular carcinomas and topographically associated FEA in a high percentage of cases, indicating a precursor role for FEA in the development of this particular type of breast cancer [9]. Furthermore, in cases in which (low-grade) DCIS was present, FEA frequently was also clonally related to this while coexisting LN was clonally unrelated [9]. This observation is supported by the finding that LN (and invasive lobular carcinomas) frequently show genetic or epigenetic inactivation of the CDH1 gene, which is not observed in FEA, DCIS, or tubular carcinomas.
Significance of FEA in Core Biopsy
Although there is good evidence pointing towards a precursor role of FEA in the development of tubular (or other low-grade) carcinomas, management recommendations also have to consider the actual risk of progression. Looking at the follow-up data on patients with FEA, this risk appears to be rather low with only 4.7% of upgrade to DCIS in all published series of locally excised lesions (table 2). Following core needle biopsy alone, Martel et al. [41] observed 7 invasive recurrences in a series of 55 patients (12.7%) after an average follow-up time of 6.2 years. In 2 of these cases, additional biopsies were taken in the interval between FEA and invasive cancer, and both in retrospect contained foci of ADH. These findings would support a recommendation to perform a local excision when FEA is diagnosed on core needle biopsy. However, several authors have analysed concomitant alterations in excision biopsies performed in the setting of FEA on core needle biopsy (table 2) and reported frequencies of between 0 and 14% of in situ or invasive carcinomas. Taken together, there are data on excision biopsies for 337 systematically analysed patients with FEA (out of a total of 18,525 core needle biopsies, overall frequency 2.6%). The risk of concomitant DCIS in these studies was 4.7%, invasive carcinomas were observed in 5.5%. Considering the low risk and the long latency period between FEA and invasive or intraductal recurrences or secondary malignancies, experts consider FEA as a risk lesion in the great majority of cases [42]. Biopsy is always indicated, however, when suspicious microcalcifications or a mass lesion remain radiologically.
Table 2.
Upgrade rates after flat epithelial atypia (FEA) diagnosis on vacuum-assisted or core needle biopsy and excision
| Author, year [ref.] | Needle biopsies, n | FEA lesions, n (%) | Excisional biopsy, n (%) | DCIS on excision, n (%)a | Invasive carcinoma on excision, n (%) | Total upgrade rate, n (%) |
|---|---|---|---|---|---|---|
| Guerra-Wallace et al. 2004 [87] | 7,820 | 60 (0.8) | 60 (100) | 4 (6.7) | 3 (5) | 7 (11.7) |
| Kanji et al. 2007 [40] | 900 | 14 (1.6) | 12 (85.7) | 1 (8.3) | 2 (16.7) | 3 (25) |
| Martel et al. 2007 [41] | 1,751 | 63 (3.6) | 55 (87.3) | 0 (0) | 7 (12.7) | 7 (12.7) |
| Chivukula et al. 2009 [36] | 8,054 | 39 (0.5) | 35 (89.7) | 3 (8.6) | 2 (5.7) | 5 (14.3) |
| Piubello et al. 2009 [88] | 875 | 33 (3.8) | 20 (60.6) | 0 (0) | 0 (0) | 0 (0) |
| Hayes et al. 2009 [89] | 1,829 | 8 (0.4) | 8 (100) | 1 (12.5) | 0 (0) | 1 (12.5) |
| Noel et al. 2006 [90] | ND | 62 | 20 (32.3) | 0 (0) | 0 (0) | 0 (0) |
| Noske et al. 2010 [91] | 1,845 | 43 (2.3) | 30 (69.8) | 2 (6.7) | 0 (0) | 2 (6.7) |
| Ingegnoli et al. 2010 [92] | 476 | 15 (3.2) | 15 (100) | 1 (6.7) | 0 (0) | 1 (6.7) |
| Total | 18,525 | 337 (2.6) | 255 (75.7) | 12 (4.7) | 14 (5.5) | 26 (10.2) |
Lobular neoplasia and DCIS are not distinguished.
FEA = Flat epithelial atypia; DCIS = ductal carcinoma in situ; ND = not determined.
Lobular Neoplasia
Risk Factor or Precursor Lesion?
LN or lobular intraepithelial neoplasia (LIN) nowadays are the preferred terms for early neoplasia with lobular phenotype and include atypical lobular hyperplasia (ALH) and lobular carcinoma in situ (LCIS) [43]. For a long time, LN was considered to be just a risk indicator and not a precursor lesion for the subsequent development of carcinoma. This concept was derived from several observations. These are the relatively low frequency of subsequent invasive carcinoma together with a long interval to the manifest disease and the observation that carcinoma may develop ipsi- or contralaterally with relatively equal frequency and be of either tumour type, ductal, or lobular. If it was an immediate precursor, it would be expected that it leads to invasive lobular carcinoma developing in the same quadrant. More recently, these arguments have been put into question, and in view of recent molecular studies it is now believed that LN indeed is a non-obligatory precursor of invasive carcinoma, and at the same time a risk lesion for ipsi- and contralateral disease.
In a review of follow-up data from 252 women that had breast biopsies with LN between 1950 and 1985, Page et al. [44] showed that invasive carcinoma was three times more likely in the same breast that had been diagnosed with LN, than in the contralateral breast, and that the tumour type was much more likely to be lobular than ductal. Additionally, molecular studies have revealed a similar molecular profile of LN and synchronous invasive lobular carcinoma [45, 46], suggesting that in these cases LN indeed is the precursor lesion. Even more convincing is the fact that invasive lobular carcinoma indeed is clonally related to LN occurring years earlier [47]. Therefore, we now consider LN to be both a low-grade precursor lesion for carcinoma and an indicator lesion for ipsi-and contralateral disease. With extensive follow-up, an invasive carcinoma occurs in 35% of the patients 35 years after initial biopsy of the lesion [48].
Grading of Risk
Several different morphologic variants of LN have been described to more precisely evaluate the individual risk. Specifically, pleomorphic lobular carcinoma in situ (pLCIS) was shown to behave more aggressively compared to classical LN [49]. The distinction of pLCIS from classical LN relies on nuclear characteristics with pLCIS having larger, more pleomorphic nuclei with obvious nucleoli, and may show apocrine differentiation, necrosis, and microcalcifications. In this respect, pLCIS mimics DCIS, but characteristically it is associated with classical LN and not with DCIS. Also, molecular profiling studies have shown that pLCIS is similar to classical LN, supporting its role as a special form of LN. In another approach for risk assessment, a classification of LN into 3 different grades of severity (LIN 1–3) has been proposed, based on the extent of lobular cancerisation and cytologic features [50]; however, this has not been validated yet and therefore is not endorsed by the World Health Organisation (WHO) [22].
Significance of LN in Core Biopsy
The need for a follow-up surgical excision when LN is the most significant finding on core needle biopsy is a matter of debate, because LN has been regarded both a risk factor and a non-obligatory precursor for invasive carcinoma. Detection rates of more significant lesions in excision specimens vary greatly between 4 and 43% (table 3), and this is due to the fact that many of these studies are limited by size and may have a selection bias. On average, the upgrade rate is 14.9% for all studies listed in table 3. Interestingly, the average upgrade rate to invasive carcinoma is 7.1% and therefore similar to the rate of 6.4% for ADH (table 1). However, there is an obvious selection bias in the calculation of the upgrade rates for LN, because on average only 56% of all patients under went open biopsy, and it must be assumed that these patients had additional risk factors clinically or mammographically such as a mass lesion or microcalcifications. Therefore, the average upgrade rate of 14.6% as calculated in table 3 most likely is an overestimation with the true risk of a higher-grade lesion probably being less than 10%.
Table 3.
Upgrade rates after lobular neoplasia (LN) diagnosis on vacuum-assisted or core needle biopsy and excision
| Author, year [ref.] | Needle biopsies, n | LN lesions, n (%) | Excisional biopsy, n (%) | DCIS on excision, n (%) | Invasive carcinoma on excision, n (%) | Total upgrade rate, n (%) |
|---|---|---|---|---|---|---|
| Libermann et al. 1999 [93] | 1,315 | 16 (1.2) | 13 (81.3) | 2 (15.4) | 1 (7.7) | 3 (23.1) |
| Burak 2000 [70] | 851 | 6 (0.7) | 6 (100) | 1 (16.7) | 0 (0) | 1 (16.7) |
| Philpotts et al. 2000 [71] | 158 | 6 (3.8) | 15 (250) | 5 (33.3) | 1 (6.7) | 6 (40) |
| Berg 2001 [94] | 1,400 | 25 (1.8) | 15 (60) | 1 (6.7) | 0 (0) | 1 (6.7) |
| O'Driscoll et al. 2001 [95] | 749 | 13 (1.7) | 7 (53.8) | 2 (28.6) | 1 (14.3) | 3 (42.9) |
| Renshaw 2002 [96] | 4,297 | 71 (1.7) | 15 (21.1) | 1 (6.7) | 0 (0) | 1 (6.7) |
| Irfan et al. 2002 [97] | 212 | 7 (3.3) | 7 (100) | 1 (14.3) | 0 (0) | 1 (14.3) |
| Shin and Rosen 2002 [54] | ND | 20 | 20 (100) | 1 (5) | 3 (15) | 0 (0) |
| Bauer et al. 2003 [98] | 1,460 | 13 (0.9) | 7 (53.8) | 0 (0) | 1 (14.3) | 1 (14.3) |
| Bonnett et al. 2003 [99] | ND | 24 | 24 (100) | 0 | 0 | 2 (8.3) |
| Crisi et al. 2003 [100] | ND | 31 | 20 (64.5) | 0 (0) | 2 (10) | 2 (10) |
| Dmyratz et al. 2003 [101] | 766 | 13 (1.7) | 7 (53.8) | 3 (42.9) | 0 (0) | 3 (42.9) |
| Middleton et al. 2003 [102] | 2,347 | 35 (1.5) | 17 (48.6) | 0 (0) | 6 (35.3) | 6 (35.3) |
| Yeh et al. 2003 [30] | 1,836 | 19 (1) | 12 (63.2) | 1 (8.3) | 0 (0) | 1 (8.3) |
| Arpino et al. 2004 [103] | 2,053 | 45 (2.2) | 21 (46.7) | 2 (9.5) | 1 (4.8) | 3 (14.3) |
| Elsheikh et al. 2005 [55] | ND | 39 | 33 (84.6) | 8 (24.2) | 1 (3) | 9 (27.3) |
| Karabakhtsian et al. 2007 [104] | ND | 92 | 92 (100) | 5 (5.4) | 5 (5.4) | 10 (10.9) |
| Hwang et al. 2008 [105] | ND | 277 | 87 (31.4) | 5 (5.7) | 4 (4.6) | 9 (10.3) |
| Menon et al. 2008 [106] | 14,597 | 49 (0.3) | 25 (51) | 1 (4) | 7 (28) | 8 (32) |
| Nagi et al. 2008 [56] | ND | 99 | 45 (45.5) | 1 (2.2) | 1 (2.2) | 2 (4.4) |
| Gao et al. 2010 [107] | 20,000 | 49 (0.2) | 49 (100) | 4 (8.2) | 4 (8.2) | 8 (16.3) |
| Total | 52,041 | 949 (0.7) | 537 (56.6) | 44 (8.2) | 38 (7.1) | 80 (14.9) |
LN = Lobular neoplasia; DCIS = ductal carcinoma in situ; ND = not determined.
Extensive Lobular Neoplasia
LN can be quite extensive filling almost all lobular units and extending into the ducts in vacuum biopsies or in an excisional specimen. The question how to handle this situation has not been addressed in clinical studies yet. In a retrospective study an increased risk for invasive carcinoma from 8 to 24% was reported when more than 10 lobular units are affected by LN [51]. Extensive LN has also been included in the definition of LIN 3 by Tavassoli [50], and there is a higher risk of microinvasion in these patients [52]. Clinically, extensive LN also may be associated with extensive microcalcifications or forming a tumour mass (tumour-like LN) [53].
Clinical Management of Lobular Neoplasia
In contrast to ADH, it is less clear if a follow-up excisional biopsy is beneficial to the outcome of a patient with the finding of LN in core biopsy, and therefore there is some disagreement if excision should be recommended as a rule or not. This is mainly due to the relative infrequency of LN as the most severe finding in core biopsies and the even lower number of excisional biopsies in this situation. Not surprisingly, these small studies have led to widely discrepant results and conflicting interpretations of published data. An excisional biopsy was recommended in fully developed LCIS because of an upgrade rate of greater than of 25% [54] or 16% [55], but results were inconclusive with lesions of lesser extent, namely ALH. The argument against a routine follow-up biopsy is that LN as the most significant pathology usually is an incidental finding in an otherwise benign core biopsy and if there is no other clinical or radiological detectable lesion, it is unlikely that an excisional biopsy could yield anything more significant [56]. This argument has to be taken seriously, but at least all cases with LCIS and a mass lesion should be followed up by a surgical biopsy. However, because of the reported upgrade rates in fully developed LCIS, the nature of these lesions as non-obligate precursors, and the risk of missing a radiologically occult invasive cancer, an open biopsy in classical LCIS should be considered as an option [55], especially if multiple lobules are involved. The management of LN in excisional biopsies by the pathologist requires attention to the following points: i) He should be aware of the risk of occult microinvasion and pay attention to the careful workup of the specimen; ii) In cases of pleomorphic LCIS, attention must be paid to the margin status like in low-grade DCIS to make sure that pleomorphic LN has been completely excised; iii) The metric extent of LN should be approximately determined by the pathologist since extensive LN may be associated with a higher risk and to help correlate the findings with the radiologic findings.
Conflict of Interest
None of these authors have personal financial interests or conflicts of interest to declare.
References
- 1.Johnson NB, Collins LC. Update on percutaneous needle biopsy of nonmalignant breast lesions. Adv Anat Pathol. 2009;16:183–195. doi: 10.1097/PAP.0b013e3181a9d33e. [DOI] [PubMed] [Google Scholar]
- 2.Page DL, Jensen RA, Simpson JF, Dupont WD. Historical and epidemiologic background of human premalignant breast disease. J Mammary Gland Biol Neoplasia. 2000;5:341–349. doi: 10.1023/a:1009521726605. [DOI] [PubMed] [Google Scholar]
- 3.Schnitt SJ. Benign breast disease and breast cancer risk: morphology and beyond. Am J Surg Pathol. 2003;27:836–841. doi: 10.1097/00000478-200306000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Weigelt B, Reis-Filho JS. Histological and molecular types of breast cancer: is there a unifying taxonomy? Nat Rev Clin Oncol. 2009;6:718–730. doi: 10.1038/nrclinonc.2009.166. [DOI] [PubMed] [Google Scholar]
- 5.Allred DC, Mohsin SK, Fuqua SA. Histological and biological evolution of human premalignant breast disease. Endocr Relat Cancer. 2001;8:47–61. doi: 10.1677/erc.0.0080047. [DOI] [PubMed] [Google Scholar]
- 6.Reis-Filho JS, Simpson PT, Gale T, Lakhani SR. The molecular genetics of breast cancer: the contribution of comparative genomic hybridization. Pathol Res Pract. 2005;201:713–725. doi: 10.1016/j.prp.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Bürger H, Otterbach F, Simon R, Poremba C, Diallo R, Decker T, Riethdorf L, Brinkschmidt C, Dockhorn-Dworniczak B, Böcker W. Comparative genomic hybridization of ductal carcinoma in situ of the breast-evidence of multiple genetic pathways. J Pathol. 1999;187:396–402. doi: 10.1002/(SICI)1096-9896(199903)187:4<396::AID-PATH286>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Fatah T, Powe D, Hodi Z, Reis-Filho J, Lee A, Ellis I. Morphologic and molecular evolutionary pathways of low nuclear grade invasive breast cancers and their putative precursor lesions: further evidence to support the concept of low nuclear grade breast neoplasia family. Am J Surg Pathol. 2008;32:513–523. doi: 10.1097/PAS.0b013e318161d1a5. [DOI] [PubMed] [Google Scholar]
- 9.Aulmann S, Elsawaf Z, Penzel R, Schirmacher P, Sinn HP. Invasive tubular carcinoma of the breast frequently is clonally related to flat epithelial atypia and low-grade ductal carcinoma in situ. Am J Surg Pathol. 2009;33:1646–1653. doi: 10.1097/PAS.0b013e3181adfdcf. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Fatah TM, Powe DG, Hodi Z, Lee AH, Reis-Filho JS, Ellis IO. High frequency of coexistence of columnar cell lesions, lobular neoplasia, and low grade ductal carcinoma in situ with invasive tubular carcinoma and invasive lobular carcinoma. Am J Surg Pathol. 2007;31:417–426. doi: 10.1097/01.pas.0000213368.41251.b9. [DOI] [PubMed] [Google Scholar]
- 11.Allred DC, Brown P, Medina D. The origins of estrogen receptor alpha-positive and estrogen receptor alpha-negative human breast cancer. Breast Cancer Res. 2004;6:240–245. doi: 10.1186/bcr938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan L, Yang X, Davidson NE. Role of DNA meth-ylation and histone acetylation in steroid receptor expression in breast cancer. J Mammary Gland Biol Neoplasia. 2001;6:183–192. doi: 10.1023/a:1011308707512. [DOI] [PubMed] [Google Scholar]
- 13.Borg A, Linell F, Idvall I, Johansson S, Sigurdsson H, Ferno M, Killander D. Her2/neu amplification and comedo type breast carcinoma. Lancet. 1989;1:1268–1269. doi: 10.1016/s0140-6736(89)92365-9. [DOI] [PubMed] [Google Scholar]
- 14.Done S, Eskandarian S, Bull S, Redston M, Andrulis I. P53 missense mutations in micro-dissected high-grade ductal carcinoma in situ of the breast. J Nati Cancer Inst. 2001;93:700–704. doi: 10.1093/jnci/93.9.700. [DOI] [PubMed] [Google Scholar]
- 15.Leibl S, Regitnig P, Moinfar F. Rat epithelial atypia (din la, atypical columnar change): an un-derdiagnosed entity very frequently coexisting with lobular neoplasia. Histopathology. 2007;50:859–865. doi: 10.1111/j.1365-2559.2007.02700.x. [DOI] [PubMed] [Google Scholar]
- 16.Arpino G, Laucirica R, Elledge R. Premalignant and in situ breast disease: biology and clinical implications. Ann Intern Med. 2005;143:446–457. doi: 10.7326/0003-4819-143-6-200509200-00009. [DOI] [PubMed] [Google Scholar]
- 17.Sanders ME, Page DL, Simpson JF, Schuyler PA, Dale Plummer W, Dupont WD. Interdependence of radial scar and proliferative disease with respect to invasive breast carcinoma risk in patients with benign breast biopsies. Cancer. 2006;106:1453–1461. doi: 10.1002/cncr.21730. [DOI] [PubMed] [Google Scholar]
- 18.Page DL, Salhany KE, Jensen RA, Dupont WD. Subsequent breast carcinoma risk after biopsy with atypia in a breast papilloma. Cancer. 1996;78:258–266. doi: 10.1002/(SICI)1097-0142(19960715)78:2<258::AID-CNCR11>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs TW, Byrne C, Colditz G, Connolly JL, Schnitt SJ. Pathologic features of breast cancers in women with previous benign breast disease. Am J Clin Pathol. 2001;115:362–369. doi: 10.1309/UP07-K3KD-25NL-D3M8. [DOI] [PubMed] [Google Scholar]
- 20.Böcker W. A New Conceptual Approach to Proliferative Breast Disease. Munich: Saunders, Elsevier; 2006. Preneoplasia of the Breast. [Google Scholar]
- 21.Page D, Rogers L. Combined histologie and cytologie criteria for the diagnosis of mammary atypical ductal hyperplasia. Hum Pathol. 1992;23:1095–1097. doi: 10.1016/0046-8177(92)90026-y. [DOI] [PubMed] [Google Scholar]
- 22.Tavassoli FA, Devilee P. Tumours of the Breast and Female Genital Organs. Pathology and Genetics. Lyon: IARC Press; 2003. [Google Scholar]
- 23.Nofech-Mozes S, Holloway C, Hanna W. The role of cytokeratin 5/6 as an adjunct diagnostic tool in breast core needle biopsies. Int J Surg Pathol. 2008;16:399–406. doi: 10.1177/1066896908316901. [DOI] [PubMed] [Google Scholar]
- 24.Bratthauer GL, Tavassoli FA. Assessment of lesions coexisting with various grades of ductal intra-epithelial neoplasia of the breast. Virchows Arch. 2004;444:340–344. doi: 10.1007/s00428-004-0976-6. [DOI] [PubMed] [Google Scholar]
- 25.Perry N, Broeders M, Wolf CD. European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis. Luxembourg: European Commission; 2006. [DOI] [PubMed] [Google Scholar]
- 26.Gong G, DeVries S, Chew KL, Cha I, Ljung BM, Waldman FM. Genetic changes in paired atypical and usual ductal hyperplasia of the breast by comparative genomic hybridization. Clin Cancer Res. 2001;7:2410–2414. [PubMed] [Google Scholar]
- 27.Amari M, Suzuki A, Moriya T, Yoshinaga K, Amano G, Sasano H, Ohuchi N, Satomi S, Horii A. Loh analyses of premalignant and malignant lesions of human breast: frequent loh in 8p, 16q, and 17q in atypical ductal hyperplasia. Oncol Rep. 1999;6:1277–1280. doi: 10.3892/or.6.6.1277. [DOI] [PubMed] [Google Scholar]
- 28.Lakhani SR, Collins N, Stratton MR, Sloane JP. Atypical ductal hyperplasia of the breast: clonal proliferation with loss of heterozygosity on chromosomes 16q and 17p. J Clin Pathol. 1995;48:611–615. doi: 10.1136/jcp.48.7.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reis-Filho J, Lakhani S. The diagnosis and management of pre-invasive breast disease: genetic alterations in pre-invasive lesions. Breast Cancer Res. 2003;5:313–319. doi: 10.1186/bcr650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeh IT, Dimitrov D, Otto P, Miller AR, Kahlen-berg MS, Cruz A. Pathologic review of atypical hyperplasia identified by image-guided breast needle core biopsy. Correlation with excision specimen. Arch Pathol Lab Med. 2003;127:49–54. doi: 10.5858/2003-127-49-PROAHI. [DOI] [PubMed] [Google Scholar]
- 31.Kohr JR, Eby PR, Allison KH, Demartini WB, Gutierrez RL, Peacock S, Lehman CD. Risk of upgrade of atypical ductal hyperplasia after stere-otactic breast biopsy: effects of number of foci and complete removal of calcifications. Radiology. 2010;255:723–730. doi: 10.1148/radiol.09091406. [DOI] [PubMed] [Google Scholar]
- 32.Azzopardi JG, Ahmed A, Millis RR. Problems in Breast Pathology. Philadelphia, PA: Saunders; 1979. [PubMed] [Google Scholar]
- 33.Schnitt S, Vincent-Salomon A. Columnar cell lesions of the breast. Adv Anat Pathol. 2003;10:113–124. doi: 10.1097/00125480-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 34.O'Malley F, Mohsin S, Badve S, Bose S, Collins L, Ennis M, Kleer C, Pinder S, Schnitt S. Interobserver reproducibility in the diagnosis of flat epithelial atypia of the breast. Mod Pathol. 2006;19:172–179. doi: 10.1038/modpathol.3800514. [DOI] [PubMed] [Google Scholar]
- 35.Fraser J, Raza S, Chorny K, Connolly J, Schnitt S. Columnar alteration with prominent apical snouts and secretions: a spectrum of changes frequently present in breast biopsies performed for microcalcifications. Am J Surg Pathol. 1998;22:1521–1527. doi: 10.1097/00000478-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Chivukula M, Bhargava R, Tseng G, Dabbs DJ. Clinicopathologic implications of ‘Hat epithelial atypia’ in core needle biopsy specimens of the breast. Am J din Pathol. 2009;131:802–808. doi: 10.1309/AJCPLDG6TT7VAHPH. [DOI] [PubMed] [Google Scholar]
- 37.Rosen P. Columnar cell hyperplasia is associated with lobular carcinoma in situ and tubular carcinoma. Am J Surg Pathol. 1999;23:1561. doi: 10.1097/00000478-199912000-00017. [DOI] [PubMed] [Google Scholar]
- 38.Brandt SM, Young GQ, Hoda SA. The ‘Rosen triad’: tubular carcinoma, lobular carcinoma in situ, and columnar cell lesions. Adv Anat Pathol. 2008;15:140–146. doi: 10.1097/PAP.0b013e31816ff313. [DOI] [PubMed] [Google Scholar]
- 39.Moinfar F, Man Y, Bratthauer G, Ratschek M, Tavassoli F. Genetic abnormalities in mammary ductal intraepithelial neoplasia-flat type (‘clinging ductal carcinoma in situ’): a simulator of normal mammary epithelium. Cancer. 2000;88:2072–2081. [PubMed] [Google Scholar]
- 40.Kunju LP, Kleer CG. Significance of flat epithelial atypia on mammotome core needle biopsy: should it be excised? Hum Pathol. 2007;38:35–41. doi: 10.1016/j.humpath.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 41.Martel M, Barron-Rodriguez P, Tolgay Ocal I, Dotto J, Tavassoli FA. Rat din 1 (flat epithelial atypia) on core needle biopsy: 63 cases identified retrospectively among 1,751 core biopsies performed over an 8-year period (1992-1999) Virchows Arch. 2007;451:883–891. doi: 10.1007/s00428-007-0499-z. [DOI] [PubMed] [Google Scholar]
- 42.Pinder SE, Reis-Filho JS. Non-operative breast pathology: columnar cell lesions. J Clin Pathol. 2007;60:1307–1312. doi: 10.1136/jcp.2006.040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Contreras A, Sattar H. Lobular neoplasia of the breast: an update. Arch Pathol Lab Med. 2009;133:1116–1120. doi: 10.5858/133.7.1116. [DOI] [PubMed] [Google Scholar]
- 44.Page DL, Schuyler PA, Dupont WD, Jensen RA, Plummer WD, Jr, Simpson JF. Atypical lobular hyperplasia as a unilateral predictor of breast cancer risk: a retrospective cohort study. Lancet. 2003;361:125–129. doi: 10.1016/S0140-6736(03)12230-1. [DOI] [PubMed] [Google Scholar]
- 45.Hwang E, Nyante S, Yi Chen Y, Moore D, DeVries S, Korkola J, Esserman L, Waldman F. Qonality of lobular carcinoma in situ and synchronous invasive lobular carcinoma. Cancer. 2004;100:2562–2572. doi: 10.1002/cncr.20273. [DOI] [PubMed] [Google Scholar]
- 46.Vos CB, Cleton-Jansen AM, Berx G, de Leeuw WJ, ter Haar NT, van Roy F, Cornelisse CJ, Peterse JL, van de Vijver MJ. E-cadherin inacti-vation in lobular carcinoma in situ of the breast: an early event in tumorigenesis. Br J Cancer. 1997;76:1131–1133. doi: 10.1038/bjc.1997.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aulmann S, Penzel R, Longerich T, Funke B, Schirmacher P, Sinn HP. Clonality of lobular carcinoma in situ (LCIS) and metachronous invasive breast cancer. Breast Cancer Res Treat. 2008;107:331–335. doi: 10.1007/s10549-007-9557-0. [DOI] [PubMed] [Google Scholar]
- 48.Bodian CA, Perzin KH, Lattes R. Lobular neoplasia. Long term risk of breast cancer and relation to other factors. Cancer. 1996;78:1024–1034. doi: 10.1002/(SICI)1097-0142(19960901)78:5<1024::AID-CNCR12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 49.Chivukula M, Haynik DM, Brufsky A, Carter G, Dabbs DJ. Pleomorphic lobular carcinoma in situ (PLCIS) on breast core needle biopsies: clinical significance and immunoprofile. Am J Surg Pathol. 2008;32:1721–1726. doi: 10.1097/PAS.0b013e31817dc3a6. [DOI] [PubMed] [Google Scholar]
- 50.Bratthauer GL, Tavassoli FA. Lobular intraepi-thelial neoplasia: previously unexplored aspects assessed in 775 cases and their clinical implications. Virchows Arch. 2002;440:134–138. doi: 10.1007/s00428-001-0541-5. [DOI] [PubMed] [Google Scholar]
- 51.Ottesen GL, Graversen HP, Blichert-Toft M, Zedeler K, Andersen JA. Lobular carcinoma in situ of the female breast. Short-term results of a prospective nationwide study. The Danish breast cancer cooperative group. Am J Surg Pathol. 1993;17:14–21. doi: 10.1097/00000478-199301000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Rosen PP. Rosen's Breast Pathology. 2nd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2001. [Google Scholar]
- 53.Stein LF, Zisman G, Rapelyea JA, Schwartz AM, Abell B, Brem RF. Lobular carcinoma in situ of the breast presenting as a mass. AJR Am J Roentgenol. 2005;184:1799–1801. doi: 10.2214/ajr.184.6.01841799. [DOI] [PubMed] [Google Scholar]
- 54.Shin SJ, Rosen PP. Excisional biopsy should be performed if lobular carcinoma in situ is seen on needle core biopsy. Arch Pathol Lab Med. 2002;126:697–701. doi: 10.5858/2002-126-0697-EBSBPI. [DOI] [PubMed] [Google Scholar]
- 55.Elsheikh TM, Silverman JF. Follow-up surgical excision is indicated when breast core needle biopsies show atypical lobular hyperplasia or lobular carcinoma in situ: a correlative study of 33 patients with review of the literature. Am J Surg Pathol. 2005;29:534–543. doi: 10.1097/01.pas.0000152566.78066.d1. [DOI] [PubMed] [Google Scholar]
- 56.Nagi CS, O'Donnell JE, Tismenetsky M, Bleiweiss IJ, Jaffer SM. Lobular neoplasia on core needle biopsy does not require excision. Cancer. 2008;112:2152–2158. doi: 10.1002/cncr.23415. [DOI] [PubMed] [Google Scholar]
- 57.Jackman RJ, Nowels KW, Shepard MJ, Finkelstein SI, Marzoni FA., Jr Stereotaxic large-core needle biopsy of 450 nonpalpable breast lesions with surgical correlation in lesions with cancer or atypical hyperplasia. Radiology. 1994;193:91–95. doi: 10.1148/radiology.193.1.8090927. [DOI] [PubMed] [Google Scholar]
- 58.Liberman L, Cohen MA, Dershaw DD, Abramson AF, Hann LE, Rosen PP. Atypical ductal hyperplasia diagnosed at stereotaxic core biopsy of breast lesions: an indication for surgical biopsy. AJR Am J Roentgenol. 1995;164:1111–1113. doi: 10.2214/ajr.164.5.7717215. [DOI] [PubMed] [Google Scholar]
- 59.Tocino I, Garcia BM, Carter D. Surgical biopsy findings in patients with atypical hyperplasia diagnosed by stereotaxic core needle biopsy. Ann Surg Oncol. 1996;3:483–488. doi: 10.1007/BF02305767. [DOI] [PubMed] [Google Scholar]
- 60.Nguyen M, McCombs MM, Ghandehari S, Kim A, Wang H, Barsky SH, Love S, Bassett LW. An update on core needle biopsy for radiologically detected breast lesions. Cancer. 1996;78:2340–2345. [PubMed] [Google Scholar]
- 61.Burbank F. Stereotactic breast biopsy: comparison of 14- and 11-gauge mammotome probe performance and complication rates. Am Surg. 1997;63:988–995. [PubMed] [Google Scholar]
- 62.Lee CH, Egglin TK, Philpotts L, Mainiero MB, Tocino I. Cost-effectiveness of stereotactic core needle biopsy: analysis by means of mammographie findings. Radiology. 1997;202:849–854. doi: 10.1148/radiology.202.3.9051045. [DOI] [PubMed] [Google Scholar]
- 63.Liberman L, Dershaw DD, Glassman JR, Abramson AF, Morris EA, LaTrenta LR, Rosen PP. Analysis of cancers not diagnosed at stereotactic core breast biopsy. Radiology. 1997;203:151–157. doi: 10.1148/radiology.203.1.9122384. [DOI] [PubMed] [Google Scholar]
- 64.Moore MM, Hargett CW, 3rd, Hanks JB, Fajardo LL, Harvey JA, Frierson HF, Jr, Slingluff CL., Jr Association of breast cancer with the finding of atypical ductal hyperplasia at core breast biopsy. Ann Surg. 1997;225:726–731. doi: 10.1097/00000658-199706000-00010. discussion 731-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gadzala DE, Cederbom GJ, Bolton JS, McKinnon WM, Farr GH, Jr, Champaign J, Ordoyne K, Chung K, Fuhrman GM. Appropriate management of atypical ductal hyperplasia diagnosed by stereotactic core needle breast biopsy. Ann Surg Oncol. 1997;4:283–286. doi: 10.1007/BF02303575. [DOI] [PubMed] [Google Scholar]
- 66.Meyer JE, Smith DN, Lester SC, DiPiro PJ, Denison CM, Harvey SC, Christian RL, Richardson A, Ko WD. Large-needle core biopsy: non-malignant breast abnormalities evaluated with surgical excision or repeat core biopsy. Radiology. 1998;206:717–720. doi: 10.1148/radiology.206.3.9494490. [DOI] [PubMed] [Google Scholar]
- 67.Lin PH, Clyde JC, Bates DM, Garcia JM, Matsumoto GH, Girvin GW. Accuracy of stereotactic core-needle breast biopsy in atypical ductal hyperplasia. Am J Surg. 1998;175:380–382. doi: 10.1016/s0002-9610(98)00047-6. [DOI] [PubMed] [Google Scholar]
- 68.Fuhrman GM, Cederbom GJ, Bolton JS, King TA, Duncan JL, Champaign JL, Smetherman DH, Farr GH, Kuske RR, McKinnon WM. Image-guided core-needle breast biopsy is an accurate technique to evaluate patients with nonpalpable imaging abnormalities. Ann Surg. 1998;227:932–939. doi: 10.1097/00000658-199806000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brem RF, Behrndt VS, Sanow L, Gatewood OM. Atypical ductal hyperplasia: histologie underestimation of carcinoma in tissue harvested from impalpable breast lesions using 11-gauge stereotacti-cally guided directional vacuum-assisted biopsy. AJR Am J Roentgenol. 1999;172:1405–1407. doi: 10.2214/ajr.172.5.10227526. [DOI] [PubMed] [Google Scholar]
- 70.Burak WE, Jr, Owens KE, Tighe MB, Kemp L, Dinges SA, Hitchcock CL, Olsen J. Vacuum-assisted stereotactic breast biopsy: histologie underestimation of malignant lesions. Arch Surg. 2000;135:700–703. doi: 10.1001/archsurg.135.6.700. [DOI] [PubMed] [Google Scholar]
- 71.Philpotts LE, Lee CH, Horvath LJ, Lange RC, Carter D, Tocino I. Underestimation of breast cancer with 11-gauge vacuum suction biopsy. AJR Am J Roentgenol. 2000;175:1047–1050. doi: 10.2214/ajr.175.4.1751047. [DOI] [PubMed] [Google Scholar]
- 72.O'Hea BJ, Tornos C. Mild ductal atypia after large-core needle biopsy of the breast: is surgical excision always necessary? Surgery. 2000;128:738–743. doi: 10.1067/msy.2000.108222. [DOI] [PubMed] [Google Scholar]
- 73.Adrales G, Turk P, Wallace T, Bird R, Norton HJ, Greene F. Is surgical excision necessary for atypical ductal hyperplasia of the breast diagnosed by mammotome? Am J Surg. 2000;180:313–315. doi: 10.1016/s0002-9610(00)00451-7. [DOI] [PubMed] [Google Scholar]
- 74.Darling ML, Smith DN, Lester SC, Kaelin C, Selland DL, Denison CM, DiPiro PJ, Rose DI, Rhei E, Meyer JE. Atypical ductal hyperplasia and ductal carcinoma in situ as revealed by large-core needle breast biopsy: results of surgical excision. AJR Am J Roentgenol. 2000;175:1341–1346. doi: 10.2214/ajr.175.5.1751341. [DOI] [PubMed] [Google Scholar]
- 75.Cangiarella J, Waisman J, Symmans WF, Gross J, Cohen JM, Wu H, Axelrod D. Mammotome core biopsy for mammary microcalcification: analysis of 160 biopsies from 142 women with surgical and radiologie followup. Cancer. 2001;91:173–177. doi: 10.1002/1097-0142(20010101)91:1<173::aid-cncr22>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 76.Lai JT, Burrowes P, MacGregor JH. Diagnostic accuracy of a stereotaxically guided vacuum-assisted large-core breast biopsy program in canada. Can Assoc Radiol J. 2001;52:223–227. [PubMed] [Google Scholar]
- 77.Jackman RJ, Birdwell RL, Ikeda DM. Atypical ductal hyperplasia: can some lesions be defined as probably benign after stereotactic 11-gauge vacuum-assisted biopsy, eliminating the recommendation for surgical excision? Radiology. 2002;224:548–554. doi: 10.1148/radiol.2242011528. [DOI] [PubMed] [Google Scholar]
- 78.Liberman L, Kaplan JB, Morris EA, Abramson AF, Menell JH, Dershaw DD. To excise or to sample the mammographie target: what is the goal of stereotactic 11-gauge vacuum-assisted breast biopsy? AJR Am J Roentgenol. 2002;179:679–683. doi: 10.2214/ajr.179.3.1790679. [DOI] [PubMed] [Google Scholar]
- 79.Zhao L, Freimanis R, Bergman S, Shen P, Perrier ND, Lesko N, Pulaski T, Pulaski S, Carr JJ, Levine EA. Biopsy needle technique and the accuracy of diagnosis of atypical ductal hyperplasia for mammographie abnormalities. Am Surg. 2003;69:757–762. discussion 762. [PubMed] [Google Scholar]
- 80.Lourenco AP, Mainiero MB, Lazarus E, Giri D, Schepps B. Stereotactic breast biopsy: comparison of histologie underestimation rates with 11- and 9-gauge vacuum-assisted breast biopsy. AJR Am J Roentgenol. 2007;189:W275–279. doi: 10.2214/AJR.07.2165. [DOI] [PubMed] [Google Scholar]
- 81.Doren E, Hulvat M, Norton J, Rajan P, Sarker S, Aranha G, Yao K. Predicting cancer on excision of atypical ductal hyperplasia. Am J Surg. 2008;195:358–361. doi: 10.1016/j.amjsurg.2007.11.008. discussion 361-352. [DOI] [PubMed] [Google Scholar]
- 82.Wagoner MJ, Laronga C, Acs G. Extent and histologie pattern of atypical ductal hyperplasia present on core needle biopsy specimens of the breast can predict ductal carcinoma in situ in subsequent excision. Am J Clin Pathol. 2009;131:112–121. doi: 10.1309/AJCPGHEJ2R8UYFGP. [DOI] [PubMed] [Google Scholar]
- 83.Arora S, Moezzi M, Kim U, Menés TS. Is surgical excision necessary for atypical ductal hyperplasia diagnosed with 8 gauge stereotactic biopsy? Breast J. 2009;15:673–674. doi: 10.1111/j.1524-4741.2009.00821.x. [DOI] [PubMed] [Google Scholar]
- 84.Chae BJ, Lee A, Song BJ, Jung SS. Predictive factors for breast cancer in patients diagnosed atypical ductal hyperplasia at core needle biopsy. World J Surg Oncol. 2009;7:77. doi: 10.1186/1477-7819-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eby PR, Ochsner JE, DeMartini WB, Allison KH, Peacock S, Lehman CD. Frequency and upgrade rates of atypical ductal hyperplasia diagnosed at stereotactic vacuum-assisted breast biopsy: 9-versus 11-gauge. AJR Am J Roentgenol. 2009;192:229–234. doi: 10.2214/AJR.08.1342. [DOI] [PubMed] [Google Scholar]
- 86.Youk JH, Kim EK, Kim MJ. Atypical ductal hyperplasia diagnosed at sonographically guided 14-gauge core needle biopsy of breast mass. AJR Am J Roentgenol. 2009;192:1135–1141. doi: 10.2214/AJR.08.1144. [DOI] [PubMed] [Google Scholar]
- 87.Guerra-Wallace MM, Christensen WN, White RL., Jr A retrospective study of columnar alteration with prominent apical snouts and secretions and the association with cancer. Am J Surg. 2004;188:395–398. doi: 10.1016/j.amjsurg.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 88.Piubello Q, Parisi A, Eccher A, Barbazeni G, Franchini Z, Iannucci A. Rat epithelial atypia on core needle biopsy: which is the right management? Am J Surg Pathol. 2009;33:1078–1084. doi: 10.1097/PAS.0b013e31819d0a4d. [DOI] [PubMed] [Google Scholar]
- 89.Hayes BD, O'Doherty A, Quinn CM. Correlation of needle core biopsy with excision histology in screen-detected b3 lesions: the Merrion Breast Screening Unit experience. J din Pathol. 2009;62:1136–1140. doi: 10.1136/jcp.2009.067280. [DOI] [PubMed] [Google Scholar]
- 90.Noel JC, Fayt I, Fernandez-Aguilar S, Buxant F, Boutemy R. Proliferating activity in columnar cell lesions of the breast. Virchows Arch. 2006;449:617–621. doi: 10.1007/s00428-006-0296-0. [DOI] [PubMed] [Google Scholar]
- 91.Noske A, Pahl S, Fallenberg E, Richter-Ehrenstein C, Buckendahl AC, Weichert W, Schneider A, Dietel M, Denkert C. Hat epithelial atypia is a common subtype of b3 breast lesions and is associated with noninvasive cancer but not with invasive cancer in final excision histology. Hum Pathol. 2010;41:522–527. doi: 10.1016/j.humpath.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 92.Ingegnoli A, d'Aloia C, Frattaruolo A, Pallavera L, Martella E, Crisi G, Zompatori M. Rat epithelial atypia and atypical ductal hyperplasia: carcinoma underestimation rate. Breast J. 2010;16:55–59. doi: 10.1111/j.1524-4741.2009.00850.x. [DOI] [PubMed] [Google Scholar]
- 93.Liberman L, Sama M, Susnik B, Rosen PP, LaTrenta LR, Morris EA, Abramson AF, Dershaw DD. Lobular carcinoma in situ at percutaneous breast biopsy: surgical biopsy findings. AJR Am J Roentgenol. 1999;173:291–299. doi: 10.2214/ajr.173.2.10430122. [DOI] [PubMed] [Google Scholar]
- 94.Berg WA, Mrose HE, Ioffe OB. Atypical lobular hyperplasia or lobular carcinoma in situ at core-needle breast biopsy. Radiology. 2001;218:503–509. doi: 10.1148/radiology.218.2.r01fe32503. [DOI] [PubMed] [Google Scholar]
- 95.O'Driscoll D, Britton P, Bobrow L, Wishart GC, Sinnatamby R, Warren R. Lobular carcinoma in situ on core biopsy – what is the clinical significance? din Radiol. 2001;56:216–220. doi: 10.1053/crad.2000.0615. [DOI] [PubMed] [Google Scholar]
- 96.Renshaw AA, Cartagena N, Derhagopian RP, Gould EW. Lobular neoplasia in breast core needle biopsy specimens is not associated with an increased risk of ductal carcinoma in situ or invasive carcinoma. Am J Clin Pathol. 2002;117:797–799. doi: 10.1309/T4XF-C61J-C95Y-VR4Q. [DOI] [PubMed] [Google Scholar]
- 97.Man K, Brem RF. Surgical and mammographie follow-up of papillary lesions and atypical lobular hyperplasia diagnosed with stereotactic vacuum-assisted biopsy. Breast J. 2002;8:230–233. doi: 10.1046/j.1524-4741.2002.08408.x. [DOI] [PubMed] [Google Scholar]
- 98.Bauer VP, Ditkoff BA, Schnabel F, Brenin D, El-Tamer M, Smith S. The management of lobular neoplasia identified on percutaneous core breast biopsy. Breast J. 2003;9:4–9. doi: 10.1046/j.1524-4741.2003.09102.x. [DOI] [PubMed] [Google Scholar]
- 99.Bonnett M, Wallis T, Rossmann M, Pernick NL, Bouwman D, Carolin KA, Visscher D. Histopathologic analysis of atypical lesions in image-guided core breast biopsies. Mod Pathol. 2003;16:154–160. doi: 10.1097/01.MP.0000052375.72841.E2. [DOI] [PubMed] [Google Scholar]
- 100.Crisi GM, Mandavilli S, Cronin E, Ricci A., Jr Invasive mammary carcinoma after immediate and short-term follow-up for lobular neoplasia on core biopsy. Am J Surg Pathol. 2003;27:325–333. doi: 10.1097/00000478-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 101.Dmytrasz K, Tartter PI, Mizrachy H, Chinitz L. Rosenbaum Smith S, Estabrook A: The significance of atypical lobular hyperplasia at percutaneous breast biopsy. Breast J. 2003;9:10–12. doi: 10.1046/j.1524-4741.2003.09103.x. [DOI] [PubMed] [Google Scholar]
- 102.Middleton LP, Grant S, Stephens T, Stelling CB, Sneige N, Sahin AA. Lobular carcinoma in situ diagnosed by core needle biopsy: when should it be excised? Mod Pathol. 2003;16:120–129. doi: 10.1097/01.MP.0000051930.68104.92. [DOI] [PubMed] [Google Scholar]
- 103.Arpino G, Allred DC, Mohsin SK, Weiss HL, Conrow D, Elledge RM. Lobular neoplasia on core-needle biopsy – clinical significance. Cancer. 2004;101:242–250. doi: 10.1002/cncr.20318. [DOI] [PubMed] [Google Scholar]
- 104.Karabakhtsian RG, Johnson R, Sumkin J, Dabbs DJ. The clinical significance of lobular neoplasia on breast core biopsy. Am J Surg Pathol. 2007;31:717–723. doi: 10.1097/01.pas.0000213408.41182.1f. [DOI] [PubMed] [Google Scholar]
- 105.Hwang H, Barke LD, Mendelson EB, Susnik B. Atypical lobular hyperplasia and classic lobular carcinoma in situ in core biopsy specimens: routine excision is not necessary. Mod Pathol. 2008;21:1208–1216. doi: 10.1038/modpathol.2008.134. [DOI] [PubMed] [Google Scholar]
- 106.Menon S, Porter GJ, Evans AJ, Ellis IO, Elston CW, Hodi Z, Lee AH. The significance of lobular neoplasia on needle core biopsy of the breast. Virchows Arch. 2008;452:473–479. doi: 10.1007/s00428-008-0607-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gao F, Carter G, Tseng G, Chivukula M. Clinical importance of histologie grading of lobular carcinoma in situ in breast core needle biopsy specimens: current issues and controversies. Am J din Pathol. 2010;133:767–771. doi: 10.1309/AJCP04ZJQTJHQYVY. [DOI] [PubMed] [Google Scholar]