Summary
New neurons and glial cells are generated in an extensive germinal niche adjacent to the walls of the lateral ventricles in the adult brain. The primary progenitors (B1 cells) have astroglial characteristics, but retain important neuroepithelial properties. Recent work shows how B1 cells contact all major compartments of this niche. They share the “shoreline” on the ventricles with ependymal cells, forming a unique adult ventricular zone (VZ). In the subventricular zone (SVZ), B1 cells contact transit amplifying (Type C) cells, chains of young neurons (A cells), and blood vessels. How signals from these compartments influence the behavior of B1 or C cells remains largely unknown, but recent work highlights growth factors, neurotransmitters, morphogens, and the extracellular matrix as key regulators of this niche. The integration of emerging molecular and anatomical clues forecasts an exciting new understanding of how the germ of youth is actively maintained in the adult brain.
Keywords: Subventricular zone, Adult Neurogenesis, Subependymal Zone, Stem Cell Niche, Neural Stem Cells
The phenomenon of adult neurogenesis raises fundamental questions about its biology, including the identity of primary neuronal precursors, the regulation of cell birth and long-range migration, and the function of neuronal replacement. Elucidating the properties of adult neural progenitors may also provide directions for their use in the treatment of brain injuries and neurological disorders. In particular, better understanding the regulation of adult neurogenesis raises new possibilities for effecting brain repair. Additionally, greater knowledge of the mechanisms of neurogenesis may improve our knowledge of the etiology of brain tumors by identifying pathways that affect potential cells of origin for these malignancies. Here we will focus on the birth of new neurons in the adult brain; specifically, the most extensive niche where neurogenesis occurs.
Two discrete regions of the adult brain continue to generate new neurons – the walls of the lateral ventricles and the subgranular zone in the dentate gyrus of the hippocampus (Ming and Song, 2005; Zhao et al., 2008; Kriegstein and Alvarez-Buylla, 2009). Large numbers of immature neurons are generated by primary progenitors in the walls of the lateral ventricles. These newly born neuroblasts migrate long distances to the olfactory bulb (Lois and Alvarez-Buylla, 1994; Carleton et al., 2003). This extensive adult neurogenic niche is heterogeneous, such that NSCs in different locations generate distinct types of neurons. Recent work has also shown that NSCs have a stereotypic architecture that allows them to simultaneously contact the cerebrospinal fluid (CSF) and blood vessels. Therefore this extensive adult germinal niche not only includes a subventricular compartment, but also a ventricular zone: we will refer to this region as the adult VZ-SVZ.
Here, we review how the unique spatial location and molecular properties of stem cells and their neighbors affect signaling and consequently neurogenesis in the adult VZ-SVZ. Contacts with neighboring cells, the CSF, and the vasculature provide three major routes for molecular signals to affect neural stem cell self-renewal and proliferation and the identity of VZ-SVZ-derived progeny. Many pathways have been shown to alter the composition of this niche, either by altering the patterns of progenitor proliferation and division or by directly impacting the migration of progenitors. We briefly discuss the use of specific gene products as stem cell markers, and the effects of epigenetic and transcriptional events downstream of niche-derived factors. A major challenge for the field going forward will be to understand how the many signals that have been shown to affect the VZ-SVZ are integrated to maintain this important germinal niche throughout life.
Adult Stem Cells and Their Neighbors
The adult VZ-SVZ exhibits a high degree of organization, with stem cells themselves as well as other cell types contributing important features to the niche (Figure 1). The proliferative unit of the adult VZ-SVZ contains both slowly dividing primary progenitors (type B cells) and rapidly dividing progeny (type C cells). Non-dividing ependymal cells lining the ventricle are multiciliated, and their motile cilia contribute to the flow of cerebrospinal fluid (Spassky et al., 2005; Sawamoto et al., 2006; Carlen et al., 2009; Mirzadeh et al., 2010a). Astrocyte-like type B cells can be subdivided into two types based on differences in their location and morphology (Doetsch et al., 1997). Type B1 cells are generally closely associated with ependymal cells, and frequently extend a small apical process to contact the ventricle between their cell bodies (Figure 1)(Doetsch et al., 1999b; Mirzadeh et al., 2008; Shen et al., 2008). This apical process contains a non-motile primary cilium, which extends into the cerebrospinal fluid (CSF). Type B2 cells, in contrast, are more frequently located close to the underlying striatal parenchyma. In the apical compartment of the VZ-SVZ, these cells form homotypic connections with each other, as well as heterotypic connections with the ependyma, through gap and adherens junctions (illustrated in Figure 1). Type B1 cells also contact the basal lamina and extensive vascular network that underlie the SVZ.
Figure 1. The Periventricular Adult Stem Cell Niche.
This illustration summarizes recent advances on our understanding of the adult VZ-SVZ niche. The apical ventricular zone is shown at top. Ependymal cells (E, in gray) are multiciliated, and the basal bodies of these cilia are oriented in the direction of cerebrospinal fluid (CSF) flow. Ependymal cells form pinwheel-like structures around the apical processes of type B1 cells (shown in blue). Type B1 cells extend a short, non-motile primary cilium into the ventricle. These cells maintain contact with the ventricle, but disassemble the primary cilium, while dividing. Type B1 cells also frequently extend a basal process with an endfoot that contacts blood vessels (Bv). Type B2 cells, in contrast, have astrocytic characteristics but do not contact the ventricle. Transit-amplifying type C cells (in green) are found close to type B cells. Dividing C cells are also often found in close proximity to blood vessels (shown at left). Type B1 cells also contact their more differentiated progeny, the chains of migrating type A neuroblasts (shown in red). Type A cells migrate tangentially in chains (shown towards right of figure) that ultimately coalesce to form the rostral migratory stream taking these young neurons to the olfactory bulb for terminal differentiation. The VZ-SVZ also includes extracellular matrix (shaded) that contacts all the cell types in this region, including blood vessels and microglia (in purple).
Type C cells, the immediate progeny of type B1 astrocytes, are also referred to as transit amplifying cells or intermediate precursor cells (IPCs) (Kriegstein and Alvarez-Buylla, 2009). Proliferating type C cells are located close to their progenitors and are also often in close proximity to blood vessels (Doetsch et al., 1999a; Shen et al., 2008; Tavazoie et al., 2008). Type B cells also directly contact type A neuroblasts and form a glial sheath around chains of these young neurons as they migrate anteriorly towards the olfactory bulb (Lois and Alvarez-Buylla, 1994; Jankovski and Sotelo, 1996; Doetsch et al., 1997; Jankovski et al., 1998; Kaneko et al., 2010). The adult VZ-SVZ also generates oligodendrocyte precursors labeled by the chondroitin sulfate proteoglycan NG2 and oligodendrocytes, although in much lower numbers than the type A neuroblasts (Hack et al., 2005; Menn et al., 2006; Komitova et al., 2009). This niche also includes microglia, the primary immune cell type present in the brain (Walton et al., 2006; Ekdahl et al., 2009; Thored et al., 2009). The VZ-SVZ – type B1 cells in particular – is therefore poised to receive informational inputs via cell-cell contacts and extracellular signals from ependymal cells, more committed progenitors, the extracellular matrix, the SVZ vasculature, the cerebrospinal fluid, and surrounding neural tissue. Below, we review current evidence on the extensive array of signals that maintain the delicate balance between self-renewal and proliferation within this long-lived germinal region.
The Elusive Nature of Cell Type Markers in the VZ-SVZ
Type B1 cells have been reported to express several cell-surface and cytoskeletal proteins that distinguish them from other cells within the niche. In addition to their astrocytic morphology and ultrastructure, type B cells express markers that have been associated with astroglia or neural progenitors, including astrocyte-specific glutamate transporter (GLAST), brain-lipid-binding protein (BLBP), connexin 30, and the cytoskeletal proteins glial fibrillary acidic protein (GFAP), vimentin, and nestin (Doetsch et al., 1999a; Hartfuss et al., 2001; Platel et al., 2008a; Pastrana et al., 2009; Nomura et al., 2011). Ependymal cells express CD24 and S100β, while type B1 cells do not (Raponi et al., 2007; Mirzadeh et al., 2008; Pastrana et al., 2009; Beckervordersandforth et al., 2010). Both the ependyma and Type B1 cells have been reported to express prominin/CD133 (Coskun et al., 2008; Mirzadeh et al., 2008; Beckervordersandforth et al., 2010; Nomura et al., 2011), a protein often employed as a stem cell marker (Shmelkov et al., 2005; Shmelkov et al., 2008). However, the CD133 staining of apical endings of type B1 cells is variable and may be dynamically regulated (Mirzadeh et al., 2008). The cell surface carbohydrate Lewis X (LeX)/CD15/SSEA-1 has also been proposed to label the stem cell population in the VZ-SVZ (Capela and Temple, 2002; Imura et al., 2006; Shen et al., 2008).
The epidermal growth factor receptor (EGFR) and the transcription factors Ascl1 (Mash1) and Dlx2 are generally used as C cell markers (Doetsch et al., 2002; Parras et al., 2004), while doublecortin (Dcx) and polysialylated neural cell adhesion molecule (PSA-NCAM) distinguish Dlx2-positive young neuroblasts from C cells (Seki and Arai, 1993; Francis et al., 1999; Yang et al., 2004; Couillard-Despres et al., 2006). Flow cytometric sorting of dissected adult VZ-SVZ cells suggests that many of the proteins employed as stem or progenitor markers are expressed at particular points along a continuous stem-progenitor-neuroblast cell lineage. While GFAP, for example, labels type B cells within the VZ-SVZ, GLAST is also present in a limited number of C cell progeny (Pastrana et al., 2009), possibly due to perdurance after proliferation of the primary progenitors. Similarly, the orphan nuclear receptor Tlx, which was initially thought to be expressed only in nestin-positive type B cells (Shi et al., 2004), is also transcribed at high levels in C cells. Mash1 and EGFR are present in a limited number of B cells, and are now suggested to possibly distinguish a population of “activated” B cells in addition to the GFAP-negative C cells (Doetsch et al., 2002; Pastrana et al., 2009; Kim et al., 2011). In addition, subsequent studies of markers that were suggested to be exclusive to the progenitor cell compartment, such as nestin, have found that these proteins are more broadly expressed within the brain (Hendrickson et al., 2011). These results argue that the large number of putative stem and progenitor cell markers are likely to identify overlapping but not identical subsets of adult VZ-SVZ cells, highlighting the need for caution and accompanying functional studies when assigning biological characteristics to the stem cell population (for further discussion of this topic, see (Chojnacki et al., 2009)). Given that Type B1 cells have many astroglial characteristics, finding potential markers to distinguish Type B1 cells from other non-germinal astrocytes within the SVZ and in the brain parenchyma would be extremely useful in future studies of this region.
The Origins of the Niche: Development from Radial Glia
Neural stem cells are present transiently at many locations along the developing neuraxis, as this complex tissue generates the many cell types required in the mature CNS (Alvarez-Buylla et al., 2001; Noctor et al., 2007). At birth, the walls of the lateral ventricles still bear many similarities to the ventricular zone present in the immature neuroepithelium. They are comprised mainly of radial glia, progenitors with cell bodies close to the ventricles and a long radial process that contacts the pial surface of the brain (Hartfuss et al., 2001; Merkle et al., 2004). Radial glia, which function as neural stem cells (NSCs) in the embryonic and fetal brain, generate an immense diversity of neurons and glial cells within a short period of time – days in the mouse and weeks in the human – to assemble the central nervous system (CNS). A select group of radial glia then transform into unique subpopulations of astrocytes that continue to function as primary neural progenitors during juvenile and adult life. Viral targeting of these cells via their radial processes, as well as anatomical studies, have demonstrated that during the next several days of postnatal development, the radial glia located on the walls of the lateral ventricles retract their long RC2-positive distal process, lose RC2 expression, and give rise to the type B1 astrocytes that become the slow-cycling stem cells of the VZ-SVZ (Merkle et al., 2004; Spassky et al., 2005; Merkle et al., 2007). Lineage tracing of radial glia by neonatal viral infection of their basal also shows that they give rise to multiciliated ependymal cells, striatal astrocytes, and oligodendrocytes, indicating that radial glia are the developmental predecessors of the adult VZ-SVZ. Type B1 cells have several characteristics that are reminiscent of their radial glial progenitors – a thin apical process with a primary cilium extending into the ventricular lumen, a basal process that extends to reach blood vessels, and behavior that is reminiscent of the interkinetic nuclear migration observed in the embryo (Doetsch et al., 1997; Mirzadeh et al., 2008; Shen et al., 2008; Tavazoie et al., 2008). Therefore, this periventricular germinal niche, previously referred to as the SVZ, also includes a compartment that directly contacts the ventricle, reminiscent of the ventricular zone (VZ) in the embryo.
Ependyma, the Cerebrospinal Fluid, and the Primary Cilium
Multiple lines of evidence suggest that signals arising from the ciliated ependymal cells and the cerebrospinal fluid (CSF) in the ventricle may influence the activity of cells in the adult VZ-SVZ. The walls of the lateral ventricles exhibit a specific planar organization: the small apical processes of one or more type B1 cells are surrounded by a rosette of ependymal cells, forming pinwheel structures on this surface (Figure 1)(Mirzadeh et al., 2008). This organization is unique to regions of the ventricular wall where neurogenesis continues throughout life. Interestingly, B1 cells establish symmetric adherens junctions with other adjoining B1 cells in the center of pinwheels and asymmetric contacts with surrounding ependymal cells, highlighting a possible mechanism for affecting stem cell state via direct adhesive contacts. Mapping of the numbers of ventricle-contacting type B1 cells along the ventricular surface reveals “hotspots” where large numbers of these cells are observed, suggesting a possible correlation with sites of stem cell activation or increased division (Mirzadeh et al., 2008). While it is clear from lineage tracing experiments that ventricle-contacting astrocytes are neurogenic, it is not yet known whether ventricular contact, or specialized contact with the ependyma, is a requirement for neurogenesis. In fact, neurogenic stem cells are present all along the RMS, where there is no apparent open ventricle (Vicario-Abejon et al., 2003; Merkle et al., 2007; Alonso et al., 2008). Specifically, the role of CSF components in the regulation of the proliferation and differentiation of B1 cells remains unknown.
The unique location of the type B1 cell primary cilium contacting the CSF raises several intriguing possibilities for the regulation of stem cell activity. The primary cilium can have a mechanosensory function, suggesting that the force of CSF flow itself may exert an influence on the proliferative state of the stem cell (Singla and Reiter, 2006). The primary cilium also functions as an antenna for specific signaling pathways – this structure has been implicated in Hedgehog (Hh), Wnt, and PDGF signaling, which are thought to occur in this region (Corbit et al., 2005; Jackson et al., 2006; Eggenschwiler and Anderson, 2007; Rohatgi et al., 2007; Corbit et al., 2008), and is essential for the formation of postnatal NSCs in the hippocampus (Han et al., 2008). Mice carrying reporter alleles for Hh or Wnt activity indicate that both pathways are active in the adult VZ-SVZ, and that stem cells respond to these pathways (Ahn and Joyner, 2005; Adachi et al., 2007). Wnt signaling is upregulated under conditions that promote asymmetrical neural stem cell division, as in reconstitution of the VZ-SVZ after antimitotic treatment, and has therefore been suggested to function in maintaining the stem cell pool (Piccin and Morshead, 2011). A similar role has also been suggested for Wnt signaling in stem-like cells within brain tumors, with inhibition of Wnt signaling enhancing a differentiated phenotype in cultured tumor cells (Zheng et al., 2010). Wnt signaling may also occur in type C cells: elevated levels of β-catenin, a downstream mediator of Wnt signaling, result in increased progenitor proliferation within the VZ-SVZ and an increase in neurons in the olfactory bulb (Adachi et al., 2007). Similar to Wnt signaling, Hh pathway activity has been identified in the slow-dividing stem cell compartment, although it may also occur in other VZ-SVZ cell types (Ahn and Joyner, 2005). Hh signaling affects stem cell self-renewal in the maturing VZ-SVZ, and the Hh pathway component Smoothened has also been shown to play an important role in transit-amplifying cell divisions and neuroblast migration (Machold et al., 2003; Palma et al., 2005; Balordi and Fishell, 2007a, b). Type B1 cells also express the PDGF receptor alpha (PDGFRα), and exhibit hyperproliferation when exogenous PDGF ligand is infused into the lateral ventricle (Jackson et al., 2006). In all three cases, the source of activating ligands for these pathways has not been determined, raising the question of whether these proteins are generated locally, released into the CSF from other ventricle-contacting cells in the brain, or delivered to type B1 cells through other mechanisms.
The motile cilia of ependymal cells, which contribute to CSF flow, also affect the migrating young neurons. Ependymal cells are required to create gradients of Slit chemorepellents that guide anterior neuroblast migration from the adult VZ-SVZ towards the olfactory bulb (Nguyen-Ba-Charvet et al., 2004; Sawamoto et al., 2006). Ependymal cells themselves, as well as their radial glia progenitors, also exhibit polarization corresponding to the direction of flow (Mirzadeh et al., 2010b). Primary cilia in progenitor radial glia, motile cilia in immature ependymal cells, and CSF flow all seem to contribute to the organization of planar cell polarity in development (Guirao et al., 2010; Mirzadeh et al., 2010b). Interestingly, Slit1 expression within the migrating neuroblasts themselves is also involved in opening a migratory path by inhibiting astrocytic processes from invading the chains of neuroblasts (Kaneko et al., 2010).
Ependymal cells further contribute to the neurogenic environment by affecting the activity of bone morphogenetic proteins (BMPs). BMP2 and BMP4 are present in the adult brain and type B and/or C cells in the adult VZ-SVZ express both these ligands and their cognate receptors (Lim et al., 2000; Peretto et al., 2004). Type B cells can be induced to differentiate both in vitro and in vivo after treatment with BMPs. BMPs appear to have distinct roles in the different cell types of the adult VZ-SVZ. One explanation for these distinct effects may be the localized regulation of BMP signaling via ependymal cell contact with type B1 cells. Ependymal cells express the BMP inhibitor Noggin, potentially modulating BMP signaling in stem cells and creating an environment that is permissive for neurogenesis. Ectopic Noggin expression appears to enhance neurogenesis in grafted SVZ cells (Lim et al., 2000). Treatment of stem-like cells in brain tumor isolates with BMP4 promotes differentiation of these cells at the expense of self-renewal and neurosphere formation (Piccirillo et al., 2006). However, additional studies have found that neurogenesis is dependent on Smad4, a downstream effector of BMP signaling, and that infusion of exogenous Noggin decreases neuroblast production in the adult VZ-SVZ and increases oligodendrogenesis (Colak et al., 2008). It has also been suggested that type B cells themselves express Noggin (Peretto et al., 2004). Taken together, these results suggest that the levels of BMP signaling within the adult VZ-SVZ are likely tightly regulated to allow production of differentiated progeny while preserving the stem cell population, and that localized signaling via cell-cell contacts in the niche may allow BMP/Noggin interactions of different types in different cells. The studies of the apical surface of type B1 cells and the ventricular face as a whole highlight the importance of ependymal cells in maintaining the neurogenic function of stem cells as well as influencing the migration of more differentiated progenitors. Cerebrospinal fluid influences both the large-scale organization of the subventricular zone, through its effects on chain migration, and the subcellular organization of basal bodies and cilia in ependymal cells and stem cells.
The Underside of the Adult VZ-SVZ: Extracellular Matrix and Vascular Contacts
In addition to the emerging role of apical contacts with the CSF, the basal face of the adult VZ-SVZ also presents a potential source of signals regulating progenitor cell behavior. The adult VZ-SVZ contains an extensive basal lamina, and early studies of the proteins expressed in this lamina identified some which are rare in the adult brain. For example, the extracellular matrix protein tenascin-C, which is expressed more broadly during neural development, is most prominent in the adult VZ-SVZ in the adult brain (Gates et al., 1995). Studies of the anatomy of this region found that it contains extensive basement membrane and extracellular matrix (ECM) components, including laminin and heparan sulfate proteoglycans (HSPGs). These matrix components appear to contact all the cell types in the adult VZ-SVZ (Mercier et al., 2002; Shen et al., 2008). The high degree of basement membrane organization in the adult VZ-SVZ is absent in other areas of the brain. HSPGs can directly control local availability of growth factors such as fibroblast growth factor (FGF), and have since been suggested to affect proliferative signaling and progenitor activity (Kerever et al., 2007).
The extensive vasculature underlying the adult VZ-SVZ also provides a route for interactions between endothelial cells, blood-borne factors, and neural progenitors. Type B1 cells, in addition to their apical contacts with the CSF, extend a long process terminating in an endfoot that directly contacts blood vessels (Figure 1)(Mirzadeh et al., 2008; Tavazoie et al., 2008). Type C cells, and in particular clusters of proliferating C cells, are also closely associated with blood vessels, suggesting that the perivascular environment contains signals that allow transit-amplifying cell generation or proliferation (Shen et al., 2008; Tavazoie et al., 2008). Studies using injected tracer molecules indicate that the vasculature in this region is more “leaky” or permissive than the blood-brain barrier in other regions, possibly allowing signals from the bloodstream to diffuse into this region and impact the niche (Tavazoie et al., 2008). Endothelial cells themselves may secrete factors that contribute to stem cell self-renewal or proliferation, and coculture of endothelial cells has been reported to enhance in vitro neurosphere generation from embryonic progenitors (Shen et al., 2004). Blood vessels have also been shown to serve as a scaffold for neuroblast migration, potentially through the release of neurotrophic factors (Snapyan et al., 2009; Whitman et al., 2009). Recent studies have identified SDF-1/CXCR4-mediated signaling as one pathway by which endothelial cells appear to promote progenitor activation, alter the binding of progenitor cells to laminin in the ECM, and affect neuroblast migration in the adult VZ-SVZ (Kokovay et al., 2010).
Vascular endothelial growth factor (VEGF) signaling has been implicated in NSC and progenitor survival, proliferation within the VZ-SVZ, and neuroblast migration and maturation, highlighting a pathway that may be able to act on both VZ-SVZ progenitors and the vasculature in this region (Zhang et al., 2003; Schanzer et al., 2004; Gotts and Chesselet, 2005c; Meng et al., 2006; Wada et al., 2006; Mani et al., 2009; Wittko et al., 2009; Licht et al., 2010). Angiogenesis elsewhere in the brain, after tumor growth or injury, has also been reported to induce proliferation and migration of neural progenitors (Schmidt et al., 2009; Harms et al., 2010). After focal ischemia, in addition to angiogenesis at the site of damage, adult VZ-SVZ-derived progenitors have been proposed to proliferate and migrate to the site of injury (Arvidsson et al., 2002; Gotts and Chesselet, 2005a, b; Yamashita et al., 2006; Kojima et al., 2010). The close contact between adult VZ-SVZ progenitors and endothelial cells is again reminiscent of the embryonic and neonatal brain, in which neuroepithelial and radial glial progenitors also maintain basal contacts with the vasculature (Noctor et al., 2001). This contact also underscores the glial nature of adult neural stem cells, as astrocytes often maintain close contacts with blood vessels in the adult brain (Tavazoie et al 2008). The close contact between type B1 cells and blood vessels suggests that vasculature-derived signals are important in regulation of neural stem cells, offering the promising prospect that identification of these signals could allow therapeutic reprogramming of other non-germinal areas and subsequent neuronal repopulation in the injured brain.
Neurotransmitter Regulation of Adult VZ-SVZ Progenitor Development
In addition to being the birthplace of thousands of young neurons that likely have tonic secretion of neurotransmitters, the adult VZ-SVZ could also receive input via projections from neurons in adjoining or distant regions. A limited number of studies have focused on neurotransmitters and neural input in the regulation of adult VZ-SVZ neurogenesis, and have found roles for localized signaling via neurotransmitter production (reviewed in (Young et al., 2011)). The inhibitory neurotransmitter γ-aminobutyric acid (GABA) is produced by migrating type A cells. Type B cells express GABAA receptor and GABA transporters (Wang et al., 2003; Bolteus and Bordey, 2004; Liu et al., 2005). GABA signaling appears to have two roles: it inhibits the proliferation of type B1 cells, and slows the migration of type A neuroblasts. The production of GABA by immature neuroblasts has been proposed to act as a negative feedback mechanism to control proliferation of primary progenitors, which are closely associated with chains of migrating neuroblasts and are therefore optimally positioned to respond to changes in local GABA concentration (Liu et al., 2005). In addition to GABA, the excitatory neurotransmitter glutamate has also been suggested to positively regulate neurogenesis, potentially by increasing transit-amplifying (type C) cells. Immunostaining within the adult VZ-SVZ has suggested that type B cells are a source of glutamate within this region (Platel et al., 2010). The precise populations of cells that are responsive to glutamate have not been identified, but cells within the RMS and olfactory bulb express functional AMPA/kainaite, NMDA, and glutamate receptors, implying that neuroblasts may also respond to glutamate and upregulate these receptors as they move towards the OB (Carleton et al., 2003; Platel et al., 2007; Platel et al., 2008b; Platel et al., 2010).
Dopamine signaling has also been reported to affect neurogenesis in the adult VZ-SVZ. The striatum and nucleus accumbens adjoining the region are immunopositive for tyrosine hydroxylase, indicating the presence of dopaminergic fibers possibly projecting from the substantia nigra (Baker et al., 2004). TH-positive dopaminergic neurites have also been reported to contact VZ-SVZ progenitors directly, with a high percentage of these neurites appearing in association with EGFR-positive type C cells (Hoglinger et al., 2004). Although existing data argue most convincingly for an effect of dopamine on type C cells, the precise identification of the VZ-SVZ cells that could respond to dopamine remains unclear. This is due in part to the five distinct dopamine receptors, which have been variously reported to be expressed in type B, C, or A cells (Coronas et al., 2004; Hoglinger et al., 2004; Kippin et al., 2005a; Kim et al., 2010). However, loss of dopamine within the VZ-SVZ clearly impacts the region: ablation of dopamine-producing neuronal populations or treatment with dopamine receptor antagonist results in decreased proliferation within the VZ-SVZ (Hoglinger et al., 2004; Kim et al., 2010). Other brain regions may also have fibers that reach the VZ-SVZ, allowing signals from neural circuitry to affect the production of new neurons and therefore the tuning of the olfactory circuit. The recent identification of neurons in the ventral forebrain that produce the Shh ligand and extend processes toward the VZ-SVZ highlights one example of VZ-SVZ patterning that may rely on more distant neuronal signals (Ihrie et al, in press). If innervation from more distant sources does occur, it would be of great interest to understand how contacts between neuronal terminals and adult VZ-SVZ cells are structured, and what signaling molecules are involved in these interactions. The presence of these terminals from mature neurons in an adult germinal region hints at possible neural mechanisms for regulation of progenitors. The logic of how these neural signals may modify the behavior of progenitors or neuronal output from the SVZ remains unknown.
Growth Factor Signaling in the Adult VZ-SVZ
External signaling pathways, including receptor tyrosine kinase signaling and morphogens in addition to those discussed above, have been implicated in the control of postnatal neurogenesis in the adult VZ-SVZ. Stem cells were first isolated in vitro and stimulated to grow as “neurospheres” from cells isolated from tissue containing the walls of the lateral ventricle (Reynolds and Weiss, 1992; Morshead et al., 1994). Neurosphere assays, as well monolayer culture systems, employ epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), or a combination of both (Reynolds and Weiss, 1992; Vescovi et al., 1993; Scheffler et al., 2005). Early analyses indicated that FGF- and EGF-responsive cells corresponded to distinct populations within the VZ-SVZ, and subsequent immunostaining for FGFR and EGFR has supported this conclusion (Jackson et al., 2006). Infusion of FGF-2 increased proliferation in the adult VZ-SVZ, but also resulted in decreased numbers of newly born neurons, suggesting that FGF-2 might function to maintain the self-renewing VZ-SVZ population (Kuhn et al., 1997). FGF-2 loss also resulted in a decrease in the slow-dividing stem cell pool and less neurogenesis (Zheng et al., 2004). EGFR is primarily expressed on type C cells and a limited number of type B1 cells, and studies of the EGFR-expressing population have indicated that most neurospheres arise from the C cell population (Vescovi et al., 1993; Doetsch et al., 2002). Exogenous stimulation of the EGFR by ventricular infusion of EGF has striking effects within the adult VZ-SVZ. First, an increased number of type B1 cells contacting the ventricle are visible by electron microscopy (Doetsch et al., 2002). Second, VZ-SVZ cells exhibit increased proliferation, and generate progeny that invade the surrounding parenchyma (Craig et al., 1996; Doetsch et al., 2002; Aguirre et al., 2005; Aguirre et al., 2007; Gonzalez-Perez et al., 2009). Elevated EGF signaling biases VZ-SVZ cells towards the oligodendrocytic lineage – rather than giving rise to neurons, labeled EGF-stimulated progenitors largely differentiate into oligodendrocytes or oligodendrocyte precursor cells (Gonzalez-Perez et al., 2009). The most likely endogenous ligand for this pathway is transforming growth factor-alpha (TGF-α). TGF-α deficient mice exhibit decreased proliferation within the adult VZ-SVZ, and these proliferation defects can be rescued in vitro by administration of EGF (Tropepe et al., 1997). More recently, TGF-α treatment has been suggested to decrease the percentage of highly motile neuroblasts within the RMS (Kim et al., 2009), but EGFR overexpression in NG2-positive progenitors has been reported to increase migration, suggesting that this pathway may have different functions in distinct cell types (Aguirre et al., 2005). Intriguingly, the related receptor ErbB4 and its ligands, neuregulin 1 and 2, are also expressed in the adult VZ-SVZ and have been implicated in progenitor proliferation and the initiation of neuroblast migration (Ghashghaei et al., 2006).
The platelet-derived growth factor (PDGF) signaling pathway also alters stem cell properties and lineage decisions, although the endogenous source of ligand for this pathway is unknown. The PDGFRα is expressed by most GFAP-positive cells within the adult VZ-SVZ, and PDGF enhances in vitro neurosphere generation in cooperation with bFGF (Jackson et al., 2006). Infusion of PDGF, like EGF, induces elevated proliferation in VZ-SVZ cells, and many of these progenitors give rise to oligodendrocytes after ligand infusion has ended. However, PDGFRα staining and EGFR staining label separate populations of cells within the adult VZ-SVZ, suggesting that they affect stem and transit-amplifying populations respectively. Strikingly, PDGF stimulation results in local hyperplastic growths within the adult VZ-SVZ, rather than increased invasion of these progenitors into the parenchyma. As both PDGF and EGF pathways are frequently affected in human brain tumors, dissecting the differing effects of these two stimuli on the properties of immature cells may offer insight into the invasive and proliferative properties of distinct classes of gliomas. In fact recent work suggests that deregulation of proliferation or oncogenic mutations within progenitor populations of the VZ-SVZ could lead to brain tumor formation (Persson et al., 2002; Zhu et al., 2005; Zheng et al., 2008; Alcantara Llaguno et al., 2009; Jacques et al., 2009)
Another subset of the receptor tyrosine kinase family, ephrin receptors, is also active in the adult VZ-SVZ. Eph receptors and their transmembrane ephrin ligands function in guidance of migratory cells and establishment of boundaries in developing tissues. Within the adult VZ-SVZ, both EphB and EphA receptors are expressed, and ephrin signaling appears to impact both type B cell proliferation and type A cell migration (Conover et al., 2000; Liebl et al., 2003; Ricard et al., 2006). Infusion of EphB2 ligand results in disrupted and aberrant chains of migratory neuroblasts, and also in increased BrdU incorporation by type B1 cells. Intriguingly, infusion also appears to increase the number of stem cells contacting the ventricle, possibly indicating increased type B1 cell activation. More recently, EphB2 signaling has also been suggested to act downstream of Notch signaling to maintain ependymal cell identity and regulate the conversion of ependymal cells to astrocytes after injury to the ventricular face (Nomura et al., 2011). EphA4 signaling has been proposed to act as an anti-apoptotic signal within the adult VZ-SVZ, as removal of ephrinB3 in the adult results in increased apoptosis (Furne et al., 2009).
EGFR signaling has also been proposed to modulate Notch signaling – a fundamental pathway in nervous system development (Aguirre et al., 2010). Notch is essential for maintaining asymmetric division and stem cell pools in multiple tissues (Maillard et al., 2003; Mizutani et al., 2007). In the adult VZ-SVZ, loss of Notch signaling compromises stem cell self-renewal, while activation of this pathway enhances neurosphere formation (Hitoshi et al., 2002; Alexson et al., 2006). Postnatal deletion of Numb/Numblike, which inhibit Notch signaling by mediating degradation of the Notch protein, has also been shown to affect the VZ-SVZ niche. Acute deletion of Numb/Numbl in nestin-positive VZ-SVZ cells resulted in extensive defects in maturation of the neonatal VZ/SVZ, alterations in ependymal cell maturation and adhesion, and decreased neuroblast survival, likely due to excess Notch activity (Kuo et al., 2006). Intriguingly, animals with elevated EGFR signaling in type C cells also exhibited non-cell-autonomous defects in Notch signaling and elevated Numb levels in stem cells, suggesting that signaling from the type C cells may regulate the self-renewal and proliferation of the type B1 cells through this pathway (Aguirre et al., 2010).
Wnt and Hh morphogens both play essential and sometimes opposing roles in development of the central nervous system, and can act to affect both proliferation and cell fate (reviewed in (Rowitch et al., 1997; Fuccillo et al., 2006; Ulloa and Briscoe, 2007)). Both pathways are also active in the adult VZ-SVZ and affect self-renewal, proliferation, and migration, as discussed above. To date, it is unknown whether these two pathways interact functionally in this context. It is possible that Wnt may act in concert with FGF signaling and/or in opposition to Shh signaling, as is the case in early nervous system development (Ulloa et al., 2007; Alvarez-Medina et al., 2008). Going forward, it will be fascinating to understand how the many growth factor and morphogen-driven pathways active in the SVZ are functionally integrated to affect progenitor proliferation and differentiation. With some exceptions noted above, most of these pathways have been examined in isolation, and determining how these pathways interact will be essential to understanding the normal regulation of neurogenesis.
Transcription Factors and Epigenetic Regulation
In addition to the wealth of extracellular signaling pathways that are thought to act within the adult VZ-SVZ, intracellular actors, including transcription factors, nuclear receptors, chromatin-modifying complexes, and microRNAs, have been reported to affect the neural stem cell lineage. The transcription factors Dlx2, Mash1, and NeuroD1 are all associated with a neurogenic fate, while Olig2 is primarily gliogenic (Parras et al., 2004; Hack et al., 2005; Marshall et al., 2005; Menn et al., 2006; Petryniak et al., 2007; Gao et al., 2009). However, it is still unclear how niche-provided signals and subsequent intracellular signaling cascades ultimately result in the expression of specific neurogenic or gliogenic transcription factors.
Recent work has highlighted the essential role of epigenetic regulators such as the chromatin-modifying protein Mll1 and the microRNA miR-124 in the control of neurogenesis (Lim et al., 2006; Cheng et al., 2009; Lim et al., 2009) (reviewed in Hwang, Alvarez-Buylla, and Lim, in press). The orphan nuclear receptor Tlx is also required for neural stem cell self-renewal and may mediate the repression of cell cycle inhibitory factors through the recruitment of Bmi-1 (Sun et al., 2007; Liu et al., 2008; Liu et al., 2010). The expression of epigenetic regulators like Bmi-1 is altered as the organism ages and stem cell function declines (Molofsky et al., 2003; Molofsky et al., 2006; Fasano et al., 2009). Although Mll1 and Bmi-1 are broadly expressed within the VZ-SVZ lineage, both proteins appear to function at specific points in this lineage to permit division or neurogenesis. These studies highlight the impact of epigenetic regulators on neurogenesis: the epigenetic state of the cell affects its ability to respond to the extracellular stimuli present in the niche and initiate programs of maintenance or differentiation. With respect to both proliferation and fate determination, chromatin modification therefore represents an important mechanism for maintaining the adult VZ-SVZ stem cell pool during the lifetime of the organism. Determining how signaling by niche-provided factors ultimately drives transcriptional activity will help to develop a unified understanding of how neurogenesis and stem cell persistence is maintained.
Regional Heterogeneity of the Adult Periventricular Germinal Niche
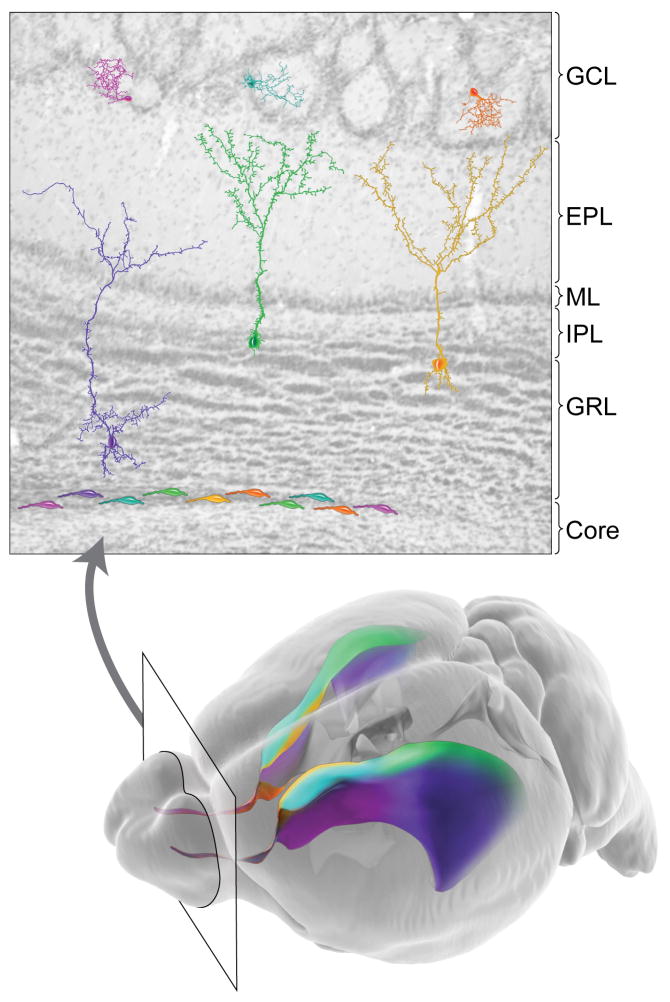
Neurogenesis in the adult VZ-SVZ extends over a large area – approximately six square millimeters in mice. It was unclear why this large region evolved to support postnatal neuronal generation, and why newly generated neurons had to migrate so far to integrate into the olfactory circuitry. Clues to the biological heterogeneity of the adult VZ-SVZ came from examining the expression of transcription factors such as Pax6, which is present in specific subpopulations of migrating neuroblasts and olfactory bulb interneurons (Hack et al., 2005; Kohwi et al., 2005). Subsequent experiments using viral targeting or genetic lineage tracing in neonatal and adult mice revealed that specific subtypes of interneurons are made within specific adult VZ-SVZ locations (Figure 2)(Kelsch et al., 2007; Kohwi et al., 2007; Merkle et al., 2007; Ventura and Goldman, 2007; Young et al., 2007). While superficial granule interneurons are largely generated by stem cells in the dorsal VZ-SVZ, deep granule interneurons are primarely derived from the ventral VZ-SVZ. Distinct populations of periglomerular cells (PGCs) also arise from specific locations within the anterior and medial adult VZ-SVZ, and a population of glutamatergic olfactory bulb neurons is derived from the dorsal SVZ (Merkle et al., 2007; Brill et al., 2009). Intriguingly, stem cells continued to generate specific types of progeny even after transplantation or multiple passages in culture, suggesting that the differentiation program for neuronal progeny is encoded at least in part by cell-intrinsic factors (Merkle et al., 2007). Although this patterning is present at birth, it is not yet known at what stage in embryonic development regional specification in the adult VZ-SVZ is organized, and whether there is a window of time during development when the fate of stem cell progeny has not yet become restricted. Subregions of the adult VZ-SVZ express transcription factors that are involved in regional specification of the developing brain, suggesting that some of the same coding at play in development may continue to be active in the adult. However, the mechanisms by which this specification is generated and maintained are unknown. Additionally, the production of particular olfactory interneuron types appears to decline after birth, indicating that the repertoire of neuronal types derived from the VZ-SVZ may change over time (De Marchis et al., 2007; Kohwi et al., 2007). New studies on Hh pathway activity within the VZ-SVZ highlight one potential pathway involved in regional fate specification. The transcription factor gli1, which indicates high levels of pathway activation, is expressed predominantly in the ventral half of the VZ-SVZ (Machold et al., 2003; Palma et al., 2005), a region that is associated with the generation of deep granule interneurons and calbindin-positive PGCs (Ihrie et al, in press). It is likely that additional signaling pathways are activated in the other subregions of the adult VZ-SVZ and maintain the heterogeneous patterning of neural progenitors.
Figure 2. The Adult VZ-SVZ Niche is Patterned and Heterogeneous.
A coronal section of the mouse olfactory bulb is shown at top. Neuroblasts derived from the VZ-SVZ enter at the core of the olfactory bulb (at bottom of image), then migrate radially to populate the granular layer (GRL) and glomerular layer (GCL) of the olfactory bulb. Color gradients in the mouse brain depicted at bottom indicate the sites of origin of the differently colored olfactory neurons. The ventral VZ-SVZ principally generates deep granule cells (purple) and calbindin-positive periglomerular cells (magenta). Deep granule cells typically have cell bodies close to the core of the olfactory bulb and dendritic projections that contact the inner half of the external plexiform layer (EPL), close to the mitral cell layer (ML). The dorsal SVZ, by contrast, produces superficial granule cells (green) and tyrosine hydroxylase-positive periglomerular cells (teal). Finally, the medial face of the VZ-SVZ generates calretinin-positive superficial granule cells (yellow) and periglomerular cells (orange). Note that periglomerular cells tend to be produced in more anterior regions of the VZ-SVZ, as indicated by the gradients shown in the lower image.
Cell Division, Specification, and Long-Term Survival: Open Questions for the Future
Our knowledge of the anatomy and molecular properties of the adult VZ-SVZ stem cell niche has advanced significantly in the past decade. A number of studies have implicated various growth factors, neurotransmitters, morphogens, epigenetic regulators and transcription factors in the maintenance of the stem cell pool, activation of progenitors, and neuroblast migration. We also know that the precise mosaic patterning of the adult VZ-SVZ is present at birth, even before this germinal region has reached a fully mature state. How the effects of the multiple signaling pathways we describe here are integrated within the progenitors of the VZ-SVZ is a particular important challenge for future research. Higher resolution subcellular localization of specific receptors coupled to dynamic studies of lineage progression may provide important clues of when and where the different extracellular molecules have their effects. However, many other fundamental questions remain unanswered. First, the lineage of an individual stem cell in vivo has not been traced – we do not know how many times a single stem cell divides, how many of these divisions are self-renewing, or whether this varies depending on location. Recent in vitro studies have begun to suggest possible lineages and patterns of cell division in intermediate progenitor cells that generate young neurons (Costa et al., 2011). Neurogenesis also decreases significantly with age (Kippin et al., 2005b; Luo et al., 2006; Molofsky et al., 2006). Little is known about how the pattern of stem cell division might change with age, and whether the pool of quiescent stem cells is depleted with time or if these cells are prevented from proliferating. The effect of niche-specific factors on the long-term retention of stem cells is largely unknown.
Second, although anatomical studies have highlighted the organization of the apical surface of the adult VZ-SVZ, we do not know whether ventricular contact by type B cells is essential for their activation or specification. Although multiple candidate pathways may signal through the primary cilia of type B1 cells, the role of primary cilia in adult VZ-SVZ neural stem cells remains unknown. It is unclear whether signals such as Shh are provided through the cerebrospinal fluid, through more specialized contacts on the ventricular surface, or even through interactions in the basal compartment distant from the primary cilium.
Third, the collection of molecular markers that distinguish astrocyte-like stem cells in the adult VZ-SVZ niche from astrocytes elsewhere in the brain has not been elucidated. It has been suggested that a wounding stimulus elsewhere in the brain may create a temporarily permissive environment that resembles the stem cell niche, but the signals involved in this environment are unknown (Buffo et al., 2008). Equally interesting is the suggestion that reactive astrocytes located outside the adult VZ-SVZ may be induced to behave as neural progenitors (Robel et al., 2011). Fourth, the finding that the adult VZ-SVZ is patterned as a spatial mosaic raises questions about the initiation and maintenance of this pattern – principally, at what time in development do individual progenitors become committed to a particular neuronal fate, and what pathways are associated with the generation of specific types of neurons?
Finally, the field awaits the application of the many discoveries in model organisms to our knowledge of the human VZ-SVZ during development, in the mature brain, and after disease or injury. The majority of detailed studies of the adult VZ-SVZ niche have been completed in rodents, but therapeutic application of our understanding of neural stem cells will require knowledge of how this germinal niche is structured in the human brain. Comparative studies in mammals have highlighted differences in anatomy and proliferative activity between species (Pérez-Martín et al., 2000; Kornack and Rakic, 2001; Luzzati et al., 2003; Sawamoto et al., 2011). Although neurospheres can be isolated from adult human VZ-SVZ (Kukekov et al., 1999; Sanai et al., 2004), proliferation levels in this region are significantly lower than the rates observed in mouse, and there has been extensive debate over whether chains of migrating neurons are present in the adult human brain (Sanai et al., 2004; Quinones-Hinojosa et al., 2006; Curtis et al., 2007; Sanai et al., 2007). Recent studies of the developing fetal brain suggest that more robust neuronal production and migration may occur earlier in the development of this region (Guerrero-Cazares et al., 2011). Determining the capacity of human VZ-SVZ cells to proliferate and generate immature neurons as the brain develops, matures, and ages will be essential to harnessing the potential of these cells for therapeutic ends. In model organisms and humans, understanding how the many structural and molecular elements within this region interact to maintain stem cell self-renewal and neurogenesis will be a fascinating challenge as the field advances. Already, our maturing knowledge of how stem cells and neurogenesis are maintained begins to point the way towards expanding and reprogramming these progenitors for neuronal and glial replacement.
Acknowledgments
We apologize to those authors whose work was not cited due to space constraints. The authors thank the members of the Alvarez-Buylla, Lim, Kriegstein, and Rowitch laboratories at UCSF for thought-provoking and informative discussions, and Kenneth X. Probst for preparation of the illustrations. R.A.I. was supported by postdoctoral fellowships from the Damon Runyon Cancer Research Foundation (DRG1935-07) and the American Association for Cancer Research/National Brain Tumor Society, in memory of Bonnie Brooks. Work in the Alvarez-Buylla laboratory is funded by the NIH (HD32116, NS28478), the Goldhirsh Foundation, the John G. Bowes Research Fund, and the Sandler Foundation. A. A.-B. is the Heather and Melanie Muss Endowed Chair of Neurological Surgery at UCSF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, Peltz G, Gong L, Kawase T, Alvarez-Buylla A, et al. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007:990–1002. doi: 10.1038/nn1938. [DOI] [PubMed] [Google Scholar]
- Aguirre A, Rizvi TA, Ratner N, Gallo V. Overexpression of the epidermal growth factor receptor confers migratory properties to nonmigratory postnatal neural progenitors. J Neurosci. 2005;25:11092–11106. doi: 10.1523/JNEUROSCI.2981-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre A, Rubio ME, Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature. 2010;467:323–327. doi: 10.1038/nature09347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- Alcantara Llaguno S, Chen J, Kwon CH, Jackson EL, Li Y, Burns DK, Alvarez-Buylla A, Parada LF. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. 2009;15:45–56. doi: 10.1016/j.ccr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexson TO, Hitoshi S, Coles BL, Bernstein A, van der Kooy D. Notch signaling is required to maintain all neural stem cell populations--irrespective of spatial or temporal niche. Dev Neurosci. 2006;28:34–48. doi: 10.1159/000090751. [DOI] [PubMed] [Google Scholar]
- Alonso M, Ortega-Pérez I, Grubb MS, Bourgeois JP, Charneau P, Lledo P. Turning astrocytes from the rostral migratory stream into neurons: a role for the olfactory sensory organ. J Neurosci. 2008:11089–11102. doi: 10.1523/JNEUROSCI.3713-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2.2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- Alvarez-Medina R, Cayuso J, Okubo T, Takada S, Marti E. Wnt canonical pathway restricts graded Shh/Gli patterning activity through the regulation of Gli3 expression. Development. 2008;135:237–247. doi: 10.1242/dev.012054. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Baker SA, Baker KA, Hagg T. Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur J Neurosci. 2004;20:575. doi: 10.1111/j.1460-9568.2004.03486.x. [DOI] [PubMed] [Google Scholar]
- Balordi F, Fishell G. Hedgehog signaling in the subventricular zone is required for both the maintenance of stem cells and the migration of newborn neurons. J Neurosci. 2007a;27:5936–5947. doi: 10.1523/JNEUROSCI.1040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balordi F, Fishell G. Mosaic removal of hedgehog signaling in the adult SVZ reveals that the residual wild-type stem cells have a limited capacity for self-renewal. J Neurosci. 2007b;27:14248–14259. doi: 10.1523/JNEUROSCI.4531-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckervordersandforth R, Tripathi P, Ninkovic J, Bayam E, Lepier A, Stempfhuber B, Kirchhoff F, Hirrlinger J, Haslinger A, Lie DC, et al. In vivo fate mapping and expression analysis reveals molecular hallmarks of prospectively isolated adult neural stem cells. Cell Stem Cell. 2010;7:744–758. doi: 10.1016/j.stem.2010.11.017. [DOI] [PubMed] [Google Scholar]
- Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24:7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill MS, Ninkovic J, Winpenny E, Hodge RD, Ozen I, Yang R, Lepier A, Gascon S, Erdelyi F, Szabo G, et al. Adult generation of glutamatergic olfactory bulb interneurons. Nat Neurosci. 2009;12:1524–1533. doi: 10.1038/nn.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Gotz M. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc Natl Acad Sci U S A. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Carlen M, Meletis K, Goritz C, Darsalia V, Evergren E, Tanigaki K, Amendola M, Barnabe-Heider F, Yeung MS, Naldini L, et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci. 2009;12:259–267. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojnacki AK, Mak GK, Weiss S. Identity crisis for adult periventricular neural stem cells: subventricular zone astrocytes, ependymal cells or both? Nat Rev Neurosci. 2009;10:153–163. doi: 10.1038/nrn2571. [DOI] [PubMed] [Google Scholar]
- Colak D, Mori T, Brill MS, Pfeifer A, Falk S, Deng C, Monteiro R, Mummery C, Sommer L, Götz M. Adult neurogenesis requires Smad4-mediated bone morphogenic protein signaling in stem cells. J Neurosci. 2008:434–446. doi: 10.1523/JNEUROSCI.4374-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover JC, Doetsch F, García-Verdugo JM, Gale NW, Yancopoulos GD, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and cell proliferation in the subventricular zone of the adult mouse brain. Nature Neuroscience. 2000;3:1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Reiter JF. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- Coronas V, Bantubungi K, Fombonne J, Krantic S, Schiffmann SN, Roger M. Dopamine D3 receptor stimulation promotes the proliferation of cells derived from the post-natal subventricular zone. J Neurochem. 2004;91:1292–1301. doi: 10.1111/j.1471-4159.2004.02823.x. [DOI] [PubMed] [Google Scholar]
- Coskun V, Wu H, Blanchi B, Tsao S, Kim K, Zhao J, Biancotti JC, Hutnick L, Krueger RC, Jr, Fan G, et al. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc Natl Acad Sci U S A. 2008;105:1026–1031. doi: 10.1073/pnas.0710000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa MR, Ortega F, Brill MS, Beckervordersandforth R, Petrone C, Schroeder T, Gotz M, Berninger B. Continuous live imaging of adult neural stem cell division and lineage progression in vitro. Development. 2011;138:1057–1068. doi: 10.1242/dev.061663. [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Karl C, Lindemann G, Schmid P, Aigner R, Laemke J, Bogdahn U, Winkler J, Bischofberger J, et al. Targeted transgene expression in neuronal precursors: watching young neurons in the old brain. Eur J Neurosci. 2006;24:1535–1545. doi: 10.1111/j.1460-9568.2006.05039.x. [DOI] [PubMed] [Google Scholar]
- Craig CG, Tropepe V, Morshead CM, Reynolds BA, Weiss S, Van der Kooy D. In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J Neurosci. 1996;16:2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtas S, van Roon-Mom WM, Bjork-Eriksson T, Nordborg C, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- De Marchis S, Bovetti S, Carletti B, Hsieh YC, Garzotto D, Peretto P, Fasolo A, Puche AC, Rossi F. Generation of distinct types of periglomerular olfactory bulb interneurons during development and in adult mice: implication for intrinsic properties of the subventricular zone progenitor population. J Neurosci. 2007;27:657–664. doi: 10.1523/JNEUROSCI.2870-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999a;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci U S A. 1999b;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol. 2007;23:345–373. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- Fasano CA, Phoenix TN, Kokovay E, Lowry N, Elkabetz Y, Dimos JT, Lemischka IR, Studer L, Temple S. Bmi-1 cooperates with Foxg1 to maintain neural stem cell self-renewal in the forebrain. Genes Dev. 2009;23:561–574. doi: 10.1101/gad.1743709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet M-CG, McDonnell N, Reiner O, Kahn A, McConnell SK, et al. Doublecortin Is a Developmentally Regulated, Microtubule-Associated Protein Expressed in Migrating and Differentiating Neurons. Neuron. 1999;23:247–256. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- Fuccillo M, Joyner AL, Fishell G. Morphogen to mitogen: the multiple roles of hedgehog signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:772–783. doi: 10.1038/nrn1990. [DOI] [PubMed] [Google Scholar]
- Furne C, Ricard J, Cabrera JR, Pays L, Bethea JR, Mehlen P, Liebl DJ. EphrinB3 is an anti-apoptotic ligand that inhibits the dependence receptor functions of EphA4 receptors during adult neurogenesis. Biochim Biophys Acta. 2009;1793:231–238. doi: 10.1016/j.bbamcr.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, Goebbels S, Eisch AJ, Hsieh J. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci. 2009;12:1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates MA, Thomas LB, Howard EM, Laywell ED, Sajin B, Faissner A, Gotz B, Silver J, Steindler DA. Cell and molecular analysis of the developing and adult mouse subventricular zone of the cerebral hemispheres. J Comp Neurol. 1995;361:249–266. doi: 10.1002/cne.903610205. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Weber J, Pevny L, Schmid R, Schwab MH, Lloyd KC, Eisenstat DD, Lai C, Anton ES. The role of neuregulin-ErbB4 interactions on the proliferation and organization of cells in the subventricular zone. Proc Natl Acad Sci U S A. 2006;103:1930–1935. doi: 10.1073/pnas.0510410103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perez O, Romero-Rodriguez R, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Epidermal growth factor induces the progeny of subventricular zone type B cells to migrate and differentiate into oligodendrocytes. Stem Cells. 2009;27:2032–2043. doi: 10.1002/stem.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts JE, Chesselet MF. Mechanisms of subventricular zone expansion after focal cortical ischemic injury. J Comp Neurol. 2005a;488:201–214. doi: 10.1002/cne.20609. [DOI] [PubMed] [Google Scholar]
- Gotts JE, Chesselet MF. Migration and fate of newly born cells after focal cortical ischemia in adult rats. J Neurosci Res. 2005b;80:160–171. doi: 10.1002/jnr.20434. [DOI] [PubMed] [Google Scholar]
- Gotts JE, Chesselet MF. Vascular changes in the subventricular zone after distal cortical lesions. Exp Neurol. 2005c;194:139–150. doi: 10.1016/j.expneurol.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Guerrero-Cazares H, Gonzalez-Perez O, Soriano-Navarro M, Zamora-Berridi G, Garcia-Verdugo JM, Quinones-Hinojosa A. Cytoarchitecture of the lateral ganglionic eminence and rostral extension of the lateral ventricle in the human fetal brain. J Comp Neurol. 2011;519:1165–1180. doi: 10.1002/cne.22566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirao B, Meunier A, Mortaud S, Aguilar A, Corsi JM, Strehl L, Hirota Y, Desoeuvre A, Boutin C, Han YG, et al. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat Cell Biol. 2010;12:341–350. doi: 10.1038/ncb2040. [DOI] [PubMed] [Google Scholar]
- Hack MA, Saghatelyan A, de Chevigny A, Pfeifer A, Ashery-Padan R, Lledo PM, Gotz M. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci. 2005 doi: 10.1038/nn1479. [DOI] [PubMed] [Google Scholar]
- Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- Harms KM, Li L, Cunningham LA. Murine neural stem/progenitor cells protect neurons against ischemia by HIF-1alpha-regulated VEGF signaling. PLoS One. 2010;5:e9767. doi: 10.1371/journal.pone.0009767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartfuss E, Galli R, Heins N, Gotz M. Characterization of CNS precursor subtypes and radial glia. Dev Biol. 2001;229:15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- Hendrickson ML, Rao AJ, Demerdash ON, Kalil RE. Expression of nestin by neural cells in the adult rat and human brain. PLoS One. 2011;6:e18535. doi: 10.1371/journal.pone.0018535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, Conlon RA, Mak TW, Bernstein A, van der Kooy D. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16:846–858. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- Hwang WW, Alvarez-Buylla A, Lim D. The glial nature of adult neural stem cells: Neurogenic competence at the end of the radial glial developmental continuum. In: Rao MS, editor. Neural Development and Stem Cells. Totowa, NJ: Humana Press; In press. [Google Scholar]
- Ihrie RA, Shah JK, Harwell CC, Levine JH, Guinto CD, Lezameta M, Kriegstein AR, Alvarez-Buylla A. Sonic hedgehog regulates the positional identity of neural stem cells in the adult brain. Neuron. 2011 doi: 10.1016/j.neuron.2011.05.018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura T, Nakano I, Kornblum HI, Sofroniew MV. Phenotypic and functional heterogeneity of GFAP-expressing cells in vitro: differential expression of LeX/CD15 by GFAP-expressing multipotent neural stem cells and non-neurogenic astrocytes. Glia. 2006;53:277–293. doi: 10.1002/glia.20281. [DOI] [PubMed] [Google Scholar]
- Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, Roy M, Quinones-Hinojosa A, Vandenberg S, Alvarez-Buylla A. PDGFRalpha-Positive B Cells Are Neural Stem Cells in the Adult SVZ that Form Glioma-like Growths in Response to Increased PDGF Signaling. Neuron. 2006;51:187–199. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Jacques TS, Swales A, Brzozowski MJ, Henriquez NV, Linehan JM, Mirzadeh Z, COM, Naumann H, Alvarez-Buylla A, Brandner S. Combinations of genetic mutations in the adult neural stem cell compartment determine brain tumour phenotypes. EMBO J. 2009;29:222–235. doi: 10.1038/emboj.2009.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovski A, Garcia C, Soriano E, Sotelo C. Proliferation, migration and differentiation of neuronal progenitor cells in the adult mouse subventricular zone surgically separated from its olfactory bulb. European Journal of Neuroscience. 1998;10:3853–3868. doi: 10.1046/j.1460-9568.1998.00397.x. [DOI] [PubMed] [Google Scholar]
- Jankovski A, Sotelo C. Subventricular zone-olfactory bulb migratory pathway in the adult mouse: Cellular composition and specificity as determined by heterochronic and heterotopic transplantation. J Comp Neurol. 1996;371:376–396. doi: 10.1002/(SICI)1096-9861(19960729)371:3<376::AID-CNE3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Kaneko N, Marin O, Koike M, Hirota Y, Uchiyama Y, Wu JY, Lu Q, Tessier-Lavigne M, Alvarez-Buylla A, Okano H, et al. New neurons clear the path of astrocytic processes for their rapid migration in the adult brain. Neuron. 2010;67:213–223. doi: 10.1016/j.neuron.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsch W, Mosley CP, Lin CW, Lois C. Distinct mammalian precursors are committed to generate neurons with defined dendritic projection patterns. PLoS Biol. 2007;5:e300. doi: 10.1371/journal.pbio.0050300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, Efird JT, Mercier F. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells. 2007;25:2146–2157. doi: 10.1634/stemcells.2007-0082. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Ables JL, Dickel LK, Eisch AJ, Johnson JE. Ascl1 (mash1) defines cells with long-term neurogenic potential in subgranular and subventricular zones in adult mouse brain. PLoS One. 2011;6:e18472. doi: 10.1371/journal.pone.0018472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Comte I, Szabo G, Hockberger P, Szele FG. Adult mouse subventricular zone stem and progenitor cells are sessile and epidermal growth factor receptor negatively regulates neuroblast migration. PLoS One. 2009;4:e8122. doi: 10.1371/journal.pone.0008122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Wang WZ, Comte I, Pastrana E, Tran PB, Brown J, Miller RJ, Doetsch F, Molnar Z, Szele FG. Dopamine stimulation of postnatal murine subventricular zone neurogenesis via the D3 receptor. J Neurochem. 2010;114:750–760. doi: 10.1111/j.1471-4159.2010.06799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin TE, Kapur S, van der Kooy D. Dopamine specifically inhibits forebrain neural stem cell proliferation, suggesting a novel effect of antipsychotic drugs. J Neurosci. 2005a;25:5815–5823. doi: 10.1523/JNEUROSCI.1120-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin TE, Martens DJ, van der Kooy D. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev. 2005b;19:756–767. doi: 10.1101/gad.1272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi M, Osumi N, Rubenstein JL, Alvarez-Buylla A. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J Neurosci. 2005;25:6997–7003. doi: 10.1523/JNEUROSCI.1435-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi M, Petryniak MA, Long JE, Ekker M, Obata K, Yanagawa Y, Rubenstein JL, Alvarez-Buylla A. A subpopulation of olfactory bulb GABAergic interneurons is derived from Emx1- and Dlx5/6-expressing progenitors. J Neurosci. 2007;27:6878–6891. doi: 10.1523/JNEUROSCI.0254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T, Hirota Y, Ema M, Takahashi S, Miyoshi I, Okano H, Sawamoto K. Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells. 2010;28:545–554. doi: 10.1002/stem.306. [DOI] [PubMed] [Google Scholar]
- Kokovay E, Goderie S, Wang Y, Lotz S, Lin G, Sun Y, Roysam B, Shen Q, Temple S. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell. 2010;7:163–173. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komitova M, Zhu X, Serwanski DR, Nishiyama A. NG2 cells are distinct from neurogenic cells in the postnatal mouse subventricular zone. J Comp Neurol. 2009;512:702–716. doi: 10.1002/cne.21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proc Natl Acad Sci U S A. 2001;98:4752–4757. doi: 10.1073/pnas.081074998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukekov VG, Laywell ED, Suslov O, Davies K, Scheffler B. Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol. 1999;156:333. doi: 10.1006/exnr.1999.7028. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Mirzadeh Z, Soriano-Navarro M, Rasin M, Wang D, Shen J, Sestan N, Garcia-Verdugo J, Alvarez-Buylla A, Jan LY, et al. Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell. 2006;127:1253–1264. doi: 10.1016/j.cell.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht T, Eavri R, Goshen I, Shlomai Y, Mizrahi A, Keshet E. VEGF is required for dendritogenesis of newly born olfactory bulb interneurons. Development. 2010;137:261–271. doi: 10.1242/dev.039636. [DOI] [PubMed] [Google Scholar]
- Liebl DJ, Morris CJ, Henkemeyer M, Parada LF. mRNA expression of ephrins and Eph receptor tyrosine kinases in the neonatal and adult mouse central nervous system. J Neurosci Res. 2003;71:7–22. doi: 10.1002/jnr.10457. [DOI] [PubMed] [Google Scholar]
- Lim DA, DTA, Trevejo JM, Herrera DG, García-Verdugo JM, Alvarez-Buylla A. Noggin Antagonizes BMP Signaling to Create a Niche for Adult Neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Lim DA, Huang YC, Swigut T, Mirick AL, Garcia-Verdugo JM, Wysocka J, Ernst P, Alvarez-Buylla A. Chromatin remodelling factor Mll1 is essential for neurogenesis from postnatal neural stem cells. Nature. 2009;458:529–533. doi: 10.1038/nature07726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Suarez-Farinas M, Naef F, Hacker CR, Menn B, Takebayashi H, Magnasco M, Patil N, Alvarez-Buylla A. In vivo transcriptional profile analysis reveals RNA splicing and chromatin remodeling as prominent processes for adult neurogenesis. Mol Cell Neurosci. 2006;31:131–148. doi: 10.1016/j.mcn.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Liu H, Belz T, Bock D, Takacs A, Wu H, Lichter P, Chai M, Schütz G. The nuclear receptor tailless is required for neurogenesis in the adult subventricular zone. Genes & Development. 2008:2473–2478. doi: 10.1101/gad.479308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HK, Wang Y, Belz T, Bock D, Takacs A, Radlwimmer B, Barbus S, Reifenberger G, Lichter P, Schutz G. The nuclear receptor tailless induces long-term neural stem cell expansion and brain tumor initiation. Genes Dev. 2010;24:683–695. doi: 10.1101/gad.560310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8:1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Luo J, Daniels SB, Lennington JB, Notti RQ, Conover JC. The aging neurogenic subventricular zone. Aging Cell. 2006;5:139–152. doi: 10.1111/j.1474-9726.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- Luzzati F, Peretto P, Aimar P, Ponti G, Fasolo A, Bonfanti L. Glia-independent chains of neuroblasts through the subcortical parenchyma of the adult rabbit brain. Proc Natl Acad Sci U S A. 2003;100:13036–13041. doi: 10.1073/pnas.1735482100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- Maillard I, Adler SH, Pear WS. Notch and the immune system. Immunity. 2003;19:781–791. doi: 10.1016/s1074-7613(03)00325-x. [DOI] [PubMed] [Google Scholar]
- Mani N, Khaibullina A, Krum JM, Rosenstein JM. Vascular endothelial growth factor enhances migration of astroglial cells in subventricular zone neurosphere cultures. J Neurosci Res. 2009;88:248–257. doi: 10.1002/jnr.22197. [DOI] [PubMed] [Google Scholar]
- Marshall CA, Novitch BG, Goldman JE. Olig2 directs astrocyte and oligodendrocyte formation in postnatal subventricular zone cells. J Neurosci. 2005;25:7289–7298. doi: 10.1523/JNEUROSCI.1924-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Zhang Z, Zhang R, Liu X, Wang L, Robin AM, Chopp M. Biphasic effects of exogenous VEGF on VEGF expression of adult neural progenitors. Neurosci Lett. 2006;393:97–101. doi: 10.1016/j.neulet.2005.09.044. [DOI] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451:170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A. 2004;101:17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Mirzadeh Z, Doetsch F, Sawamoto K, Wichterle H, Alvarez-Buylla A. The subventricular zone en-face: wholemount staining and ependymal flow. J Vis Exp. 2010a doi: 10.3791/1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z, Han YG, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Cilia organize ependymal planar polarity. J Neurosci. 2010b;30:2600–2610. doi: 10.1523/JNEUROSCI.3744-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–355. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, Van der Kooy D. Neural stem cells in the adult mammalian forebrain: A relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Nguyen-Ba-Charvet KT, Picard-Riera N, Tessier-Lavigne M, Baron-Van Evercooren A, Sotelo C, Chedotal A. Multiple roles for slits in the control of cell migration in the rostral migratory stream. J Neurosci. 2004;24:1497–1506. doi: 10.1523/JNEUROSCI.4729-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Kriegstein AR. Neural stem and progenitor cells in cortical development. Novartis Found Symp. 2007;288:59–78. [PubMed] [Google Scholar]
- Nomura T, Goritz C, Catchpole T, Henkemeyer M, Frisen J. EphB signaling controls lineage plasticity of adult neural stem cell niche cells. Cell Stem Cell. 2011;7:730–743. doi: 10.1016/j.stem.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma V, Lim DA, Dahmane N, Sanchez P, Brionne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Buylla A, Ruiz i Altaba A. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–344. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parras CM, Galli R, Britz O, Soares S, Galichet C, Battiste J, Johnson JE, Nakafuku M, Vescovi A, Guillemot F. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. Embo J. 2004;23:4495–4505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrana E, Cheng LC, Doetsch F. Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. Proc Natl Acad Sci U S A. 2009;106:6387–6392. doi: 10.1073/pnas.0810407106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretto P, Dati C, De Marchis S, Kim HH, Ukhanova M, Fasolo A, Margolis FL. Expression of the secreted factors noggin and bone morphogenetic proteins in the subependymal layer and olfactory bulb of the adult mouse brain. Neuroscience. 2004;128:685–696. doi: 10.1016/j.neuroscience.2004.06.053. [DOI] [PubMed] [Google Scholar]
- Pérez-Martín M, Grondona JM, Cifuentes M, Pérez-Fígares JM, Jiménez JA, Fernández-LLebrez P. Ependymal explants from the lateral ventricle of the adult bovine brain: a model system for morphological and functional studies of the ependyma. Cell Tissue Res. 2000;300:11–19. doi: 10.1007/s004410000190. [DOI] [PubMed] [Google Scholar]
- Persson M, Stamataki D, te Welscher P, Andersson E, Bose J, Ruther U, Ericson J, Briscoe J. Dorsal-ventral patterning of the spinal cord requires Gli3 transcriptional repressor activity. Genes Dev. 2002;16:2865–2878. doi: 10.1101/gad.243402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryniak MA, Potter GB, Rowitch DH, Rubenstein JL. Dlx1 and Dlx2 control neuronal versus oligodendroglial cell fate acquisition in the developing forebrain. Neuron. 2007;55:417–433. doi: 10.1016/j.neuron.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccin D, Morshead CM. Wnt signaling regulates symmetry of division of neural stem cells in the adult brain and in response to injury. Stem Cells. 2011;29:528–538. doi: 10.1002/stem.589. [DOI] [PubMed] [Google Scholar]
- Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F, Vescovi AL. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- Platel JC, Dave KA, Gordon V, Lacar B, Rubio ME, Bordey A. NMDA receptors activated by subventricular zone astrocytic glutamate are critical for neuroblast survival prior to entering a synaptic network. Neuron. 2010;65:859–872. doi: 10.1016/j.neuron.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel JC, Gordon V, Heintz T, Bordey A. GFAP-GFP neural progenitors are antigenically homogeneous and anchored in their enclosed mosaic niche. Glia. 2008a doi: 10.1002/glia.20735. [DOI] [PubMed] [Google Scholar]
- Platel JC, Heintz T, Young S, Gordon V, Bordey A. Tonic activation of GLUK5 kainate receptors decreases neuroblast migration in whole-mounts of the subventricular zone. J Physiol. 2008b;586:3783–3793. doi: 10.1113/jphysiol.2008.155879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel JC, Lacar B, Bordey A. GABA and glutamate signaling: homeostatic control of adult forebrain neurogenesis. J Mol Histol. 2007;38:303–311. doi: 10.1007/s10735-007-9103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, Romero-Rodriguez R, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494:415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- Raponi E, Agenes F, Delphin C, Assard N, Baudier J, Legraverend C, Deloulme JC. S100B expression defines a state in which GFAP-expressing cells lose their neural stem cell potential and acquire a more mature developmental stage. Glia. 2007;55:165–177. doi: 10.1002/glia.20445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Ricard J, Salinas J, Garcia L, Liebl DJ. EphrinB3 regulates cell proliferation and survival in adult neurogenesis. Mol Cell Neurosci. 2006;31:713–722. doi: 10.1016/j.mcn.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Robel S, Berninger B, Gotz M. The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci. 2011;12:88–104. doi: 10.1038/nrn2978. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- Rowitch DH, Danielian PS, Lee SM, Echelard Y, McMahon AP. Cell interactions in patterning the mammalian midbrain. Cold Spring Harb Symp Quant Biol. 1997;62:535–544. [PubMed] [Google Scholar]
- Sanai N, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A. Comment on “Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension”. Science. 2007;318:393. doi: 10.1126/science.318.5849.393a. author reply 393. [DOI] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel-Garcia Verdugo J, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Sawamoto K, Hirota Y, Alfaro-Cervello C, Soriano-Navarro M, He X, Hayakawa-Yano Y, Yamada M, Hikishima K, Tabata H, Iwanami A, et al. Cellular composition and organization of the subventricular zone and rostral migratory stream in the adult and neonatal common marmoset brain. J Comp Neurol. 2011;519:690–713. doi: 10.1002/cne.22543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, Garcia-Verdugo JM, Marin O, Rubenstein JL, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- Schanzer A, Wachs FP, Wilhelm D, Acker T, Cooper-Kuhn C, Beck H, Winkler J, Aigner L, Plate KH, Kuhn HG. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol. 2004;14:237–248. doi: 10.1111/j.1750-3639.2004.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffler B, Walton NM, Lin DD, Goetz AK, Enikolopov G, Roper SN, Steindler DA. Phenotypic and functional characterization of adult brain neuropoiesis. Proc Natl Acad Sci U S A. 2005;102:9353–9358. doi: 10.1073/pnas.0503965102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt NO, Koeder D, Messing M, Mueller FJ, Aboody KS, Kim SU, Black PM, Carroll RS, Westphal M, Lamszus K. Vascular endothelial growth factor-stimulated cerebral microvascular endothelial cells mediate the recruitment of neural stem cells to the neurovascular niche. Brain Res. 2009;1268:24–37. doi: 10.1016/j.brainres.2009.02.065. [DOI] [PubMed] [Google Scholar]
- Seki T, Arai Y. Distribution and possible roles of the highly polysialylated neural cell adhesion molecule (NCAM-H) in the developing and adult central nervous system. Neurosci Res. 1993;17:265–290. doi: 10.1016/0168-0102(93)90111-3. [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ Stem Cells Lie in a Vascular Niche: A Quantitative Analysis of Niche Cell-Cell Interactions. Cell Stem Cell. 2008:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Chichung Lie D, Taupin P, Nakashima K, Ray J, Yu RT, Gage FH, Evans RM. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature. 2004;427:78–83. doi: 10.1038/nature02211. [DOI] [PubMed] [Google Scholar]
- Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, St Clair R, Baljevic M, White I, Jin DK, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133- metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmelkov SV, St Clair R, Lyden D, Rafii S. AC133/CD133/Prominin-1. Int J Biochem Cell Biol. 2005;37:715–719. doi: 10.1016/j.biocel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- Snapyan M, Lemasson M, Brill MS, Blais M, Massouh M, Ninkovic J, Gravel C, Berthod F, Gotz M, Barker PA, et al. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci. 2009;29:4172–4188. doi: 10.1523/JNEUROSCI.4956-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N, Merkle FT, Flames N, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Yu RT, Evans RM, Shi Y. Orphan nuclear receptor TLX recruits histone deacetylases to repress transcription and regulate neural stem cell proliferation. Proc Natl Acad Sci U S A. 2007;104:15282–15287. doi: 10.1073/pnas.0704089104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A Specialized Vascular Niche for Adult Neural Stem Cells. Cell Stem Cell. 2008:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thored P, Heldmann U, Gomes-Leal W, Gisler R, Darsalia V, Taneera J, Nygren JM, Jacobsen SE, Ekdahl CT, Kokaia Z, et al. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57:835–849. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Craig CG, Morshead CM, Van der Kooy D. Transforming growth factor-a null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. J Neurosci. 1997;17:7850–7859. doi: 10.1523/JNEUROSCI.17-20-07850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa F, Briscoe J. Morphogens and the control of cell proliferation and patterning in the spinal cord. Cell Cycle. 2007;6:2640–2649. doi: 10.4161/cc.6.21.4822. [DOI] [PubMed] [Google Scholar]
- Ulloa F, Itasaki N, Briscoe J. Inhibitory Gli3 activity negatively regulates Wnt/beta-catenin signaling. Curr Biol. 2007;17:545–550. doi: 10.1016/j.cub.2007.01.062. [DOI] [PubMed] [Google Scholar]
- Ventura RE, Goldman JE. Dorsal radial glia generate olfactory bulb interneurons in the postnatal murine brain. J Neurosci. 2007;27:4297–4302. doi: 10.1523/JNEUROSCI.0399-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovi AL, Reynolds BA, Fraser DD, Weiss S. bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal-astroglial) EGF-generated CNS progenitor cells. Neuron. 1993;11:951–966. doi: 10.1016/0896-6273(93)90124-a. [DOI] [PubMed] [Google Scholar]
- Vicario-Abejon C, Yusta-Boyo MJ, Fernandez-Moreno C, de Pablo F. Locally born olfactory bulb stem cells proliferate in response to insulin-related factors and require endogenous insulin-like growth factor-I for differentiation into neurons and glia. J Neurosci. 2003;23:895–906. doi: 10.1523/JNEUROSCI.23-03-00895.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Haigh JJ, Ema M, Hitoshi S, Chaddah R, Rossant J, Nagy A, van der Kooy D. Vascular endothelial growth factor directly inhibits primitive neural stem cell survival but promotes definitive neural stem cell survival. J Neurosci. 2006;26:6803–6812. doi: 10.1523/JNEUROSCI.0526-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP, 2nd, Scheffler B, Steindler DA. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54:815–825. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- Wang DD, Krueger DD, Bordey A. GABA depolarizes neuronal progenitors of the postnatal subventricular zone via GABAA receptor activation. J Physiol. 2003;550:785–800. doi: 10.1113/jphysiol.2003.042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman MC, Fan W, Rela L, Rodriguez-Gil DJ, Greer CA. Blood vessels form a migratory scaffold in the rostral migratory stream. J Comp Neurol. 2009;516:94–104. doi: 10.1002/cne.22093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittko IM, Schanzer A, Kuzmichev A, Schneider FT, Shibuya M, Raab S, Plate KH. VEGFR-1 regulates adult olfactory bulb neurogenesis and migration of neural progenitors in the rostral migratory stream in vivo. J Neurosci. 2009;29:8704–8714. doi: 10.1523/JNEUROSCI.5527-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, et al. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HK, Sundholm-Peters NL, Goings GE, Walker AS, Hyland K, Szele FG. Distribution of doublecortin expressing cells near the lateral ventricles in the adult mouse brain. J Neurosci Res. 2004;76:282–295. doi: 10.1002/jnr.20071. [DOI] [PubMed] [Google Scholar]
- Young KM, Fogarty M, Kessaris N, Richardson WD. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci. 2007;27:8286–8296. doi: 10.1523/JNEUROSCI.0476-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SZ, Taylor MM, Bordey A. Neurotransmitters couple brain activity to subventricular zone neurogenesis. Eur J Neurosci. 2011;33:1123–1132. doi: 10.1111/j.1460-9568.2011.07611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Vutskits L, Pepper MS, Kiss JZ. VEGF is a chemoattractant for FGF-2-stimulated neural progenitors. J Cell Biol. 2003;163:1375–1384. doi: 10.1083/jcb.200308040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zheng H, Ying H, Wiedemeyer R, Yan H, Quayle SN, Ivanova EV, Paik JH, Zhang H, Xiao Y, Perry SR, et al. PLAGL2 regulates Wnt signaling to impede differentiation in neural stem cells and gliomas. Cancer Cell. 2010;17:497–509. doi: 10.1016/j.ccr.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen AJ, Perry SR, Tonon G, Chu GC, Ding Z, et al. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 2008;455:1129–1133. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Nowakowski RS, Vaccarino FM. Fibroblast growth factor 2 is required for maintaining the neural stem cell pool in the mouse brain subventricular zone. Dev Neurosci. 2004;26:181–196. doi: 10.1159/000082136. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Guignard F, Zhao D, Liu L, Burns DK, Mason RP, Messing A, Parada LF. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell. 2005;8:119–130. doi: 10.1016/j.ccr.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]