Abstract
Background
Down syndrome appears to be associated with a virtually certain risk of fibrillar amyloid-β (Aβ) pathology by the age of 40 and a very high risk of dementia at older ages. The positron emission tomography (PET) ligand florbetapir F18 has been shown to characterize fibrillar Aβ in the living human brain and to provide a close correlation with subsequent Aβ neuropathology in individuals proximate to and after the end of life. The extent to which the most frequently used PET ligands can be used to detect fibrillar Aβ in patients with Down syndrome remains to be determined.
Objectives
To characterize PET estimates of fibrillar Aβ burden in a Down syndrome patient very close to the end of life and to compare them with neuropathologic assessment made after his death.
Design/Methods
With the family’s informed consent, florbetapir PET was used to study a 55-year-old Down syndrome patient with Alzheimer disease near the end of life; his brain was donated for neuropathologic assessment when he died 14 days later. Visual ratings of cerebral florbetapir uptake were performed by trained readers who were masked to the patient’s diagnosis as part of a larger study, and an automated algorithm was used to characterize regional-to-cerebellar standard uptake value ratios in 6 cerebral regions of interest. Neuropathologic assessments were performed masked to the patient’s diagnosis or PET measurements.
Results
Visual ratings and automated analyses of the PET image revealed a heavy fibrillar Aβ burden in cortical, striatal, and thalamic regions, similar to that reported for patients with late-onset Alzheimer disease. This matched neuropathologic findings of frequent neuritic and diffuse plaques, as well as frequent amyloid angiopathy, except for neuropathologically demonstrated frequent cerebellar diffuse plaques and amyloid angiopathy that were not detected by the PET scan.
Conclusions
Florbetapir PET can be used to detect increased cerebral-to-cerebellar fibrillar Aβ burden in a Down syndrome patient with Alzheimer disease, even in the presence of frequent amyloid angiopathy and diffuse plaques in the cerebellum. Additional studies are needed to determine the extent to which PET could be used to detect and to track fibrillar Aβ and to evaluate investigational Aβ-modifying treatments in the presymptomatic and symptomatic stages of Alzheimer disease.
alzheimer disease (AD) IS a common cause of dementia in geriatric populations and is characterized by the extracellular accumulation of amyloid-β (Aβ) peptide plaques and neurofibrillary tangles, as well as a decrease of cholinergic neurons, in the basal forebrain region.1 There are a variety of ways AD can be triggered in chromosomally typical patients, including genetic predisposition, environmental factors, and traumatic brain injury.1 Patients with Down syndrome (DS) have an extremely high incidence of early-onset AD,2,3 with virtually all DS patients developing the characteristic plaques and tangles by the age of 35 years, a gradual increase in the rate of AD between ages 40 and 60 years, and up to 50% prevalence.4 Patients with DS are subject to subtle preclinical changes that can be detected by early dementia ratings, such as those obtained by the Dementia Questionnaire for Persons with Mental Retardation (DMR),5 and are associated with neurologic findings including increased glucose metabolism in a number of brain regions6-8 and decreased volume in striatum and hippocampal gray matter.6 These neurologic changes before the onset of dementia could be used to predict the extent of potential decline in more severe stages of AD.
Because all DS patients demonstrate significant Aβ pathology but not all clearly develop the profound dementia characteristic of sporadic AD,7,8 this population provides an experimental model uniquelysuited to the exploration of the relationship between early neurologic changes, dementia, and Aβ plaques.9 A better understanding of the early markers of AD would allow for earlier and more efficient treatment of AD in individuals with DS as well as a potentially broader understanding of the early signs of idiopathic AD in general. Here, we describe a case involving a DS patient with clinically confirmed AD who underwent florbetapir imaging proximate to death and autopsy evaluation, as part of a phase 3, multisite autopsy comparison study conducted by Avid Radiopharmaceuticals.10
REPORT OF A CASE
The patient was a 55-year-old right-handed white man with DS and 8 years of formal education through special education. At the time of presentation at age 51 years, the patient was having difficulty maintaining occupational duties at his job at a gas station, difficulty multitasking, confusion with well-known tasks, word and naming difficulty, and a loss of pleasurable activities. He was having difficulty with doing his own cooking and maintaining his home environment. He also had become less fastidious regarding appearance. He had no change in his personality or mood, no weight loss, no reported hallucinations or delusions, and no change in gait or posture.
The patient’s medical history at initial presentation included DS, cataracts, and gout, with no remarkable surgical history except for cataract extraction or medical allergies. The patient’s medications included allopurinol and mouthwash for stomatitis. His family history indicated risk for diabetes mellitus, hypertension, and coronary disease, as well as a mother who died with AD. The patient’s social history was unremarkable. He resided in a group home. The patient was not reported to have incontinence. The review of systems was not significant for notable change in appetite, weight, or sleep and was not indicative of anxiety or depression.
The patient’s physical examination at initial evaluation revealed normal vital signs, including regular cardiovascular rate and rhythm as well as normotensive blood pressure. He exhibited a rash on his scalp and morphologic features consistent with a diagnosis of DS.
His neurologic examination revealed intact cranial nerves. His motor examination indicated normality in all muscle groups. The patient exhibited intact sensation peripherally and intact coordination and stood toe to heel in tandem. The patient’s deep tendon reflexes were symmetric and brisk throughout.
The patient’s initial cognitive examination indicated that his mentation was alert and his speech fluent. His premorbid IQ was difficult to gauge, but his medical records indicated that neuropsychologic testing performed 11 years before presentation put his full-scale IQ at 55, his verbal IQ at 53, and his performance IQ at 63, which are all consistent with mild retardation. His Mini-Mental State Examination11 score at the time of the initial neurologic evaluation for cognitive decline was derived and ascertained to be approximately 11/23. During the next few years, he declined in terms of cognitive ability and developed agitation and aggressive behavior. He was treated with Aricept (donepezil hydrochloride; Pfizer Inc), Namenda (memantine hydrochloride; Forest), and antidepressant and antipsychotic medications. In 2006, he had a seizure (not described in detail). Beginning in September 2007, he was noted to have a slow shuffling gait, and later his posture also became stooped. In the last year of life, he had visual hallucinations and multiple falls, as well as myoclonic jerking and tremors. He was using a wheelchair. Carbidopa-levodopa was added to his treatment regimen. A magnetic resonance imaging scan performed in March 2009 showed enlarged lateral ventricles and dystrophic calcifications of the head of the caudate nucleus and globus pallidus. The patient progressively declined in a manner typical of AD during the 4 years ensuing from the low initial assessment baseline until he was nontestable, with his last Mini-Mental State Examination score recorded as 0. The patient was enrolled in a florbetapir histopathology study.
FLORBETAPIR IMAGING METHODS
The patient received a 10-minute florbetapir positron emission tomography (PET) scan, beginning 50 minutes after a single intravenous bolus of 370 MBq (10 mCi) F-18 florbetapir. Images were acquired with a 128×128 matrix (zoom 2) and were reconstructed with an iterative reconstruction algorithm. Images were assessed using a semiquantitative visual image score ranging from 0 (no cortical amyloid) to 4 (high levels of amyloid).10 Three independent readers, masked to all clinical, demographic, and neuropathologic information, performed the visual rating. The median rating of these 3 readers served as the primary outcome variable. Independently, the images were first normalized to a standard template in Talairach space (SPM2, http://www.fil.ion.ucl.ac.uk/spm/), and then a semiautomated algorithm was used to calculate standard uptake value ratios (SUVRs) in predefined anatomically relevant cortical regions (frontal, temporal, parietal, anterior cingulate, posterior cingulate, and precuneus), with the whole cerebellum as the reference region.
To explore the Aβ deposition pattern, voxel-wise SUVRs (parametric imaging) were generated using the same reference region. The patient died 14 days after imaging. The brain was collected at autopsy for neuropathologic analysis. Neuropathologic studies were performed without knowledge of the clinical and PET data.
NEUROPATHOLOGIC EVALUATION
At the time of death, the brain was removed and processed according to the standard autopsy protocol for the Avid A07 clinical trial (NCT00857415). The brain was placed in fixative for 2 weeks before dissection at Banner Sun Health Research Institute, Sun City, Arizona.
Three independent methods were used to identify and to quantify cerebral amyloid burden. The first method used immunohistochemistry with Aβ antibody 4G8 (1:2000 dilution; antibody localization was amplified with an avidinbiotin peroxidase [VECTASTAIN Elite ABC System; Vector Laboratories, Inc, Burlingame, CA] and visualized with 3,3′-diaminobenzidine enhanced with a mixture of nickel and ammonium sulfate as horseradish peroxidase substrate [Signet, Dedham, Massachusetts; provided by Covance, Princeton, New Jersey]) at Biospective Inc, Montreal, Canada. The stained slides were digitized using a Zeiss MIRAX high-resolution automated slide scanner (Carl Zeiss Canada, Toronto, Ontario). Image quantification was performed using the PERMITS image processing/analysis software (Biospective Inc). This automated quantification method segments chromogen-positive pixels based on red-green-blue intensity and generates a parametric map of Aβ aggregation over the entire tissue section. The Aβ burden (percentage of gray matter containing Aβ aggregates) was calculated for each tissue section and averaged across multiple slides for each anatomical region.
The second method used a modified Bielchowsky silver stain applied to 6-μm sections cut from each region of interest and cerebellum. Two sections, separated by 300 μm, were evaluated from each region, and the average was used to represent the plaque density. Density of both neuritic and diffuse plaques was assessed by 2 independent experienced neuropathology raters using the Consortium to Establish a Registry for Alzheimer’s Disease templates. The results were reviewed by a senior neuropathologist (T.G.B.); all were masked to the clinical information and imaging results.
The third method of estimating plaque burden was that used as a standard protocol at the Banner Sun Health Research Institute Brain and Body Donation Program. Standardized fixed and cryoprotected 4×5-cm tissue blocks were sectioned at 40μm on a sliding freezing micro tome, and sections were stained using the Campbell-Switzer Gallyas silver stains. 12 Average total (neuritic and diffuse) plaque densities as well as average neuro fibrillary tangle densities were estimated using the Consortium to Establish a Registry for Alzheimer’s Disease templates as zero, sparse, moderate, or frequent in the following regions: frontal lobe cortex at the coronal level of the genu of the corpus callosum, temporal lobe cortex at the coronal level of the amygdala and at the level of the lateral geniculate nucleus, and parietal lobe cortex at a level 1-cm caudal to the splenium of the corpus callosum. Density descriptive terms were converted to a 0-to-4 scale for statistical purposes. Braak neuro fibrillary tangle stage was established according to the original publication.10
FLORBETAPIR FINDINGS
The DS patient’s florbetapir PET image (analyzed both in terms of raw counts and cerebral-to-whole cerebellar SUVRs) revealed a pattern of cortical fibrillar amyloid burden similar to that in patients with late-onset AD (Figure 1). All 6 cortical regions evaluated demonstrated significant cortical florbetapir uptake in both visual reads and SUVR analysis (Figure 2). The image also revealed fibrillar amyloid burden in the striatum and thalamus. On visual inspection, there was also more tracer uptake in the cerebellum than typically seen.13 However, whole cerebellar uptake quantitated within the normal range, resulting in cortical-to-whole cerebellar SUVRs in the expected range for florbetapir.13
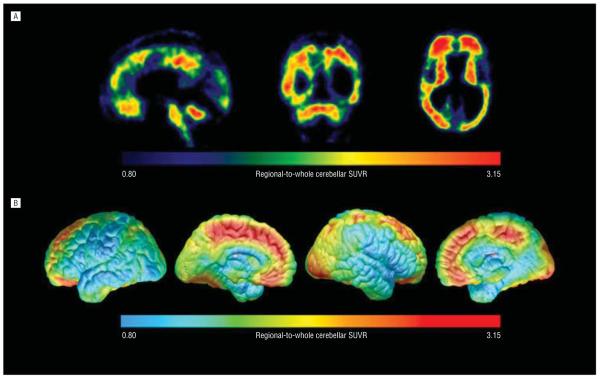
Figure 1.
Florbetapir F18 positron emission tomography (PET) images of fibrillar amyloid-β (Aβ) burden in an end-of-life, neuropathologically verified patient with Down syndrome and Alzheimer disease. A, Sagittal, coronal, and horizontal brain sections from the patient’s florbetapir F18 PET image. B, The patient’s PET data projected onto lateral and medial surfaces of a spatially standardized magnetic resonance imaging template from the laboratory of Paul Thompson, UCLA (University of California, Los Angeles). Each figure is shown in terms of a regional-to-whole cerebellar standard uptake value ratio color scale. The patient had PET evidence of fibrillar Aβ burden in the cortical regions previously reported in other early- and late-onset AD patients, as well as in striatal and cerebellar regions previously reported in early-onset AD patients—evidence subsequently confirmed by postmortem neuropathologic assessment.
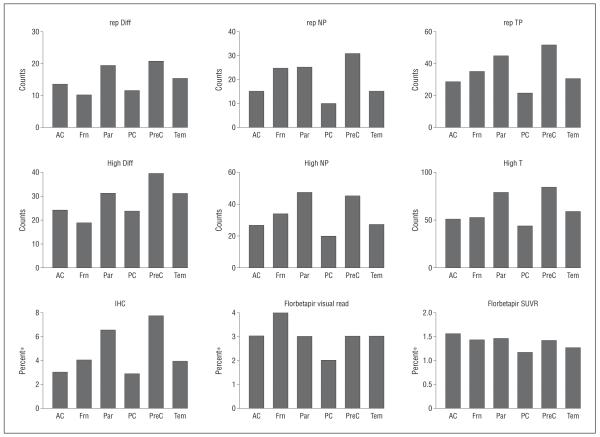
Figure 2.
Diffuse amyloid plaques, neuritic plaques, and percentage of cortical amyloid on immunohistochemistry on pathology were elevated in all 6 regions assessed. Amyloid pathology was consistent with regional quantitative florbetapir standard uptake value ratios and visual reads. AC indicates anterior cingulate; Frn, frontal; High, highest counts; IHC, amyloid-β immunohistochemistry; Par, parietal; PC, posterior cingulate; PreC, precuneus; rep Diff, representative diffuse plaque counts; rep NP, representative neuritic plaque counts; rep TP, representative total plaque counts; SUVR, cerebral-to-whole-cerebellum standard uptake value ratio; and Tem, temporal. Asterisk indicates percentage of gray matter containing amyloid.
AUTOPSY FINDINGS
On gross examination, the anterior part of the temporall obes showed gyral atrophy with no herniation, as well as marked atrophy of the amygdala, head and body of the hippocampus, and parahippocampal gyrus.4 The cerebellum and brainstem were externally unremarkable. Cerebral slices revealed no cortical lesions but moderate to marked dilation of the lateral and third ventricles. Histology with hematoxylin-eosin showed substantial gliosis of the upper neocortical layers, with many Aβ plaques and neurofibrillary tangles.2 Mild depigmentation of the substantia nigra, without Lewy bodies, and calcification of the basal ganglia were observed; the latter is common in DS. Otherwise, sections of the basal ganglia, thalamus, subthalamic regions, cerebellum, brainstem, spinal cord, and paraspinal sympathetic ganglia were unremarkable. Amyloid staining revealed frequent diffuse, neuritic, and cored Aβ plaques and frequent neurofibrillary tangles in neocortex and limbic areas (Figure 3). The Braak stage was VI. Diffuse Aβ plaques were observed in the cerebellum, striatum, and thalamus, with frequent amyloidotic blood vessels in all cerebral lobes, thalamus, striatum, and cerebellum. Immunohistochemical staining for phosphorylated α-synuclein showed no evidence of neuronal cytoplasmic inclusions or neurites in multiple sections of the olfactory bulb, brainstem, amygdala, cerebral cortex, spinal cord, or paraspinal sympathetic ganglia. The final neuropathologic diagnosis, based on established National Institutes of Health Alzheimer Disease Center criteria,14 was AD with trisomy 21.
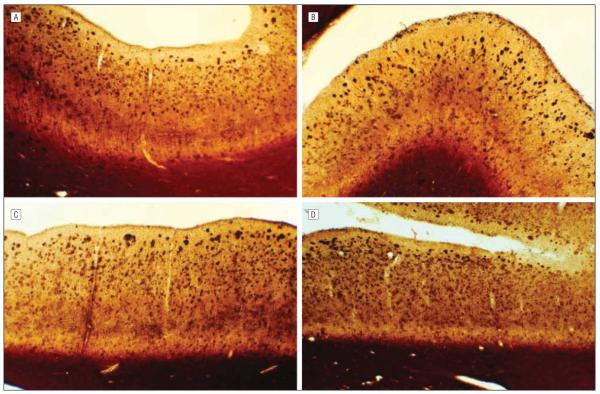
Figure 3.
Sections of cerebral cortex regions stained for amyloid with the Campbell-Switzer Gallyas stain, revealing frequent diffuse, neuritic, and cored amyloid plaques throughout the cerebral cortex. A, Middle frontal gyrus. B, Anterior cingulate gyrus. C, Middle temporal gyrus. D, Posterior cingulate gyrus.
COMPARISON OF FLORBETAPIR AND AUTOPSY FINDINGS
Diffuse amyloid plaques, neuritic plaques, and percentage of cortical amyloid on immunohistochemistry on pathology were elevated in all 6 regions assessed (Figure 2). Amyloid pathologic features were consistent with regional quantitative florbetapir SUVRs and visual reads (Figure 2).
COMMENT
To our knowledge, this is the first report of florbetapir imaging in a DS patient with AD. Because the images were gathered proximate to death, it was also possible to correlate imaging findings with pathologic features at autopsy. This case reveals an association between antemortem amyloid PET findings and postmortem neuropathologic findings in a DS patient with clinical and neuropathologic evidence of AD. Florbetapir PET revealed a characteristic pattern of fibrillar amyloid burden in the cerebral cortex in a DS patient with AD. Both visual reads and quantitative binding measures were consistent with amyloid seen on pathology.10,15 Quantification of florbetapir PET was useful even when using a cerebellar reference region found to have elevated florbetapir uptake and neuropathologic evidence of diffuse plaques and vascular amyloid compared with what is typically found in late onset.16,17 Although additional studies are needed, our findings suggest the promise of florbetapir PET to detect and to track AD-related fibrillar amyloid pathology and to evaluate amyloidmodifying treatments in DS patients.
Although this is a single case report, florbetapir has recently been shown to be highly accurate in identifying amyloid on pathology.10 An ongoing phase 3 study of 152 terminally ill participants and 74 healthy young controls is comparing florbetapir imaging and postmortem amyloid immunohistochemistry. This study recently demonstrated high levels of correlation between florbetapir imaging and postmortem amyloid pathology in the first 35 cases to come to autopsy,10 with high levels of correlation between visual reads of raw PET images (r=0.78) and quantitative measures with immunohistochemistry (r=0.76). Overall, florbetapir showed 99% accuracy, 98% positive predictive value, and 100% negative predictive value for determining underlying amyloid pathology. In the image-to-pathology study, there was a comparable correlation between image (SUVR) and either diffuse or neuritic plaques, with the best correlation for total plaque.10 The DS case gives a similar result. In addition, florbetapir binds to vascular amyloid in vitro. This supports the use of florbetapir as a true biomarker of underlying amyloid pathology and provides secondary validation for the results from our single case report.
Molecular imaging with florbetapir in DS subjects could be important for several reasons. First, life expectancy of the DS population has increased dramatically during the past few decades,8 and this population is at extremely high risk for AD even in middle age.4 As such, if early detection is critical in late-onset AD subjects, it may be as much or more so in DS. Second, the virtual universality of Aβ deposition in the DS population may make DS subjects optimal for tracking Aβ pathology over time. Whereas one would have to recruit and to scan hundreds of normal elderly to arrive at the few who develop Aβ pathology in a longitudinal study, all DS subjects will do so by middle age or younger.2-4 Third, despite the ubiquity of Aβ deposition in DS, many DS individuals reportedly do not develop overt AD.8 Thus, the DS population may also be optimal for research into the salience of Aβ pathology in AD. For example, differential distributions of Aβ over the various cortical areas might be found to account for discrepancies in the development of AD, although many more cases will be necessary to confirm the unusual Aβ distribution observed in the present study.
An effective path toward treatment of AD depends on the successful development of noninvasive methods for early diagnosis, including measurements of Aβ load in the brain.18 Current measures of Aβ load, such as Aβ levels in cerebrospinal fluid, may not be direct enough to accurately assess brain Aβ pathology.18 In typical late-onset AD, as well as in DS patients, florbetapir may offer a unique opportunity for presymptomatic identification of patients in the early stages of AD. Its half-life of 110 minutes makes it easier to store and to use than other radioactive materials used in PET scans, and it appears to have high selectivity and optimum kinetics for Aβ plaques.19 Trials using florbetapir have shown that healthy control patients exhibited minimal accumulation of florbetapir in white matter areas, whereas patients with AD exhibited high tracer uptake in areas of the brain where Aβ plaques are commonly observed at autopsy.
Acknowledgments
Funding/Support: This study was supported by grant P30 AG 019610 from the National Institute on Aging; the Banner Sun Health Research Institute; the Banner Alzheimer’s Institute; and Avid Radiopharmaceuticals, Inc.
Additional Information: We thank Paul Thompson, PhD, for allowing us to use the human brain template and cortical brain surface display technique from the Laboratory of Neuro Imaging (LONI) in our case report.
Footnotes
Author Contributions:Study concept and design: Sabbagh, Fleisher, Rogers, Pontecorvo, Skovronsky, Jacobson, and Cole. Acquisition of data: Sabbagh, Pontecorvo, Sue, Liebsack, Charney, Belden, and Beach. Analysis and interpretation of data: Sabbagh, Fleisher, Chen, Reiman, Pontecorvo, Mintun, Jacobson, and Charney. Drafting of the manuscript: Sabbagh, Fleisher, Rogers, Berk, Jacobson, Cole, and Beach. Critical revision of the manuscript for important intellectual content: Sabbagh, Fleisher, Chen, Reiman, Pontecorvo, Mintun, Skovronsky, Jacobson, Sue, Liebsack, Charney, Belden, and Beach. Statistical analysis: Chen. Obtained funding: Sabbagh, Rogers, Pontecorvo, Skovronsky, and Beach. Administrative, technical, and material support: Sabbagh, Pontecorvo, Mintun, Skovronsky, Sue, Liebsack, Charney, Cole, Belden, and Beach. Study supervision: Sabbagh, Reiman, and Skovronsky.
Financial Disclosure: Dr Sabbagh has worked in an advisory capacity for Eisai Co, Ltd; Pfizer Inc; Amerisciences, LP; and GlaxoSmithKline plc; has received royalties from John Wiley & Sons, Inc and Amerisciences, LP; and has grants and contracts with Eli Lilly and Company; Baxter Healthcare Corporation; Bayer AG; General Electric Company; Bristol-Myers Squibb; Eisai Co, Ltd; Janssen Pharmaceuticals, Inc; Wyeth Pharmaceuticals/Elan Corporation, plc; Avid Radiopharmaceuticals, Inc; and Medivation, Inc. Dr Rogers is a consultant for Eisai Co, Ltd. Drs Pontecorvo and Skovronsky are employees and Dr Mintun is chief medical officer of Avid Radiopharmaceuticals, Inc, the maker of florbetapir. Dr Jacobson receives royalties from American Psychiatric Publishing and research support for clinical trials from Bristol-Myers Squibb; Avid Radiopharmaceuticals, Inc; Forest; General Electric Company; Bayer AG; Baxter Healthcare Corporation; Wyeth Pharmaceuticals; Janssen Pharmaceuticals, Inc; Eli Lilly and Company; and Medivation, Inc. Dr Beach has received scientific support from Avid Radiopharmaceuticals, Inc and Bayer Schering Pharma AG.
REFERENCES
- 1.Yaari R, Corey-Bloom J. Alzheimer’s disease. Semin Neurol. 2007;27(1):32–41. doi: 10.1055/s-2006-956753. [DOI] [PubMed] [Google Scholar]
- 2.Mann DM, Esiri MM. The pattern of acquisition of plaques and tangles in the brains of patients under 50 years of age with Down’s syndrome. J Neurol Sci. 1989;89(2-3):169–179. doi: 10.1016/0022-510x(89)90019-1. [DOI] [PubMed] [Google Scholar]
- 3.Wisniewski KE, Dalton AJ, McLachlan C, Wen GY, Wisniewski HM. Alzheimer’s disease in Down’s syndrome: clinicopathologic studies. Neurology. 1985;35(7):957–961. doi: 10.1212/wnl.35.7.957. [DOI] [PubMed] [Google Scholar]
- 4.Leverenz JB, Raskind MA. Early amyloid deposition in the medial temporal lobe of young Down syndrome patients: a regional quantitative analysis. Exp Neurol. 1998;150(2):296–304. doi: 10.1006/exnr.1997.6777. [DOI] [PubMed] [Google Scholar]
- 5.Evenhuis HM. Further evaluation of the Dementia Questionnaire for Persons with Mental Retardation (DMR) J Intellect Disabil Res. 1996;40(pt 4):369–373. doi: 10.1046/j.1365-2788.1996.786786.x. [DOI] [PubMed] [Google Scholar]
- 6.Beacher F, Daly E, Simmons A, et al. Alzheimer’s disease and Down’s syndrome: an in vivo MRI study. Psychol Med. 2009;39(4):675–684. doi: 10.1017/S0033291708004054. [DOI] [PubMed] [Google Scholar]
- 7.Devenny DA, Hill AL, Patxot O, Silverman WP, Wisniewski KE. Ageing in higher functioning adults with Down’s syndrome: an interim report in a longitudinal study. J Intellect Disabil Res. 1992;36(pt 3):241–250. doi: 10.1111/j.1365-2788.1992.tb00511.x. [DOI] [PubMed] [Google Scholar]
- 8.Zigman WB, Schupf N, Urv T, Zigman A, Silverman W. Incidence and temporal patterns of adaptive behavior change in adults with mental retardation. Am J Ment Retard. 2002;107(3):161–174. doi: 10.1352/0895-8017(2002)107<0161:IATPOA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Moncaster JA, Pineda R, Moir RD, et al. Alzheimer’s disease amyloid-β links lens and brain pathology in Down syndrome. PLoS One. 2010;5(5):e10659. doi: 10.1371/journal.pone.0010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark CM, Schneider JA, Bedell BJ, et al. AV45-A07 Study Group Use of florbetapir-PET for imaging β-amyloid pathology. JAMA. 2011;305(3):275–283. doi: 10.1001/jama.2010.2008. published correction appears in JAMA. 2011;305(11):1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 12.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 13.Lengyel Z, Balogh E, Emri M, et al. Pattern of increased cerebral FDG uptake in Down syndrome patients. Pediatr Neurol. 2006;34(4):270–275. doi: 10.1016/j.pediatrneurol.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 14.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56(10):1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131(pt 6):1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haier RJ, Head K, Head E, Lott IT. Neuroimaging of individuals with Down’s syndrome at-risk for dementia: evidence for possible compensatory events. Neuroimage. 2008;39(3):1324–1332. doi: 10.1016/j.neuroimage.2007.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Head E, Lott IT, Patterson D, Doran E, Haier RJ. Possible compensatory events in adult Down syndrome brain prior to the development of Alzheimer disease neuropathology: targets for nonpharmacological intervention. J Alzheimers Dis. 2007;11(1):61–76. doi: 10.3233/jad-2007-11110. [DOI] [PubMed] [Google Scholar]
- 18.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 19.Wong DF, Rosenberg PB, Zhou Y, et al. In vivo imaging of amyloid deposition in Alzheimer’s disease using the radioligand 18F-AV-45 (florbetapir F 18) [published correction appears. J Nucl Med. 2010;51(8):1327. doi: 10.2967/jnumed.109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]; J Nucl Med. 2010;51(6):913–920. doi: 10.2967/jnumed.109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]