Abstract
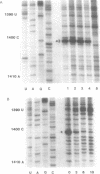
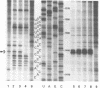
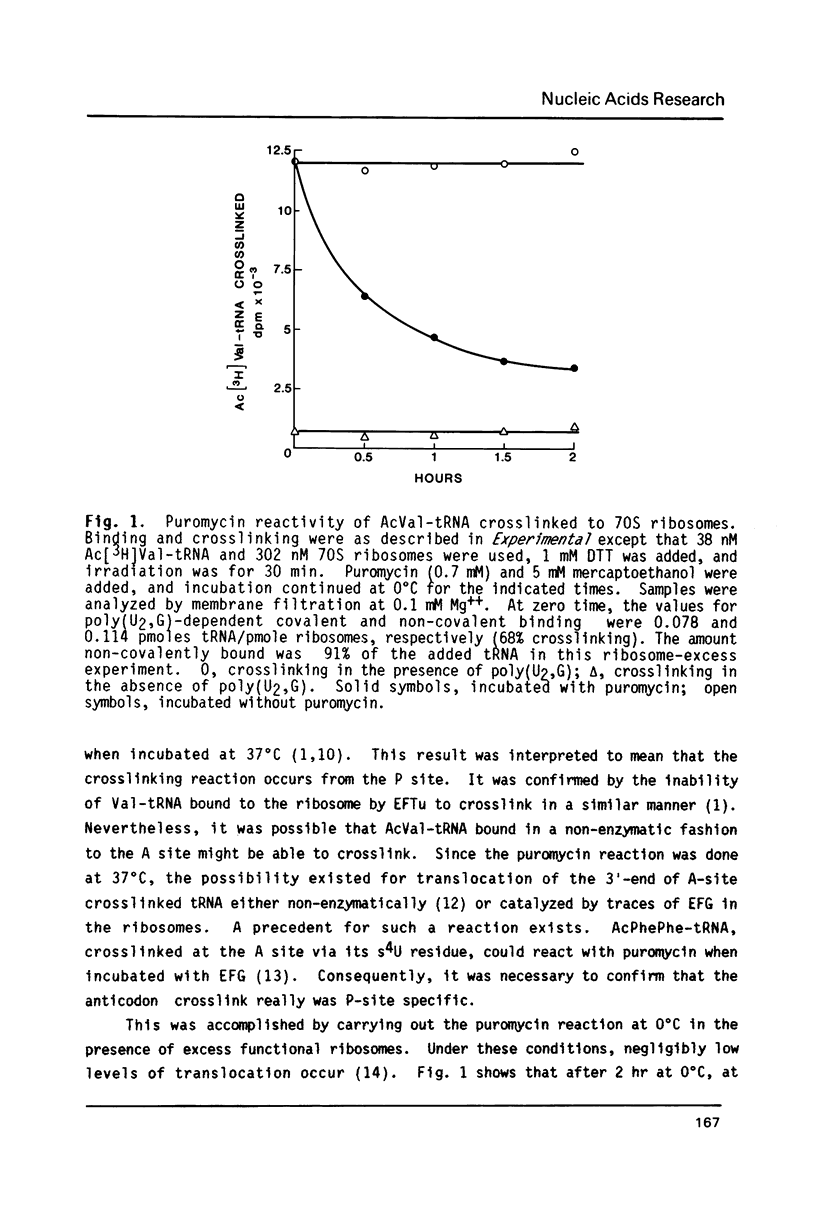
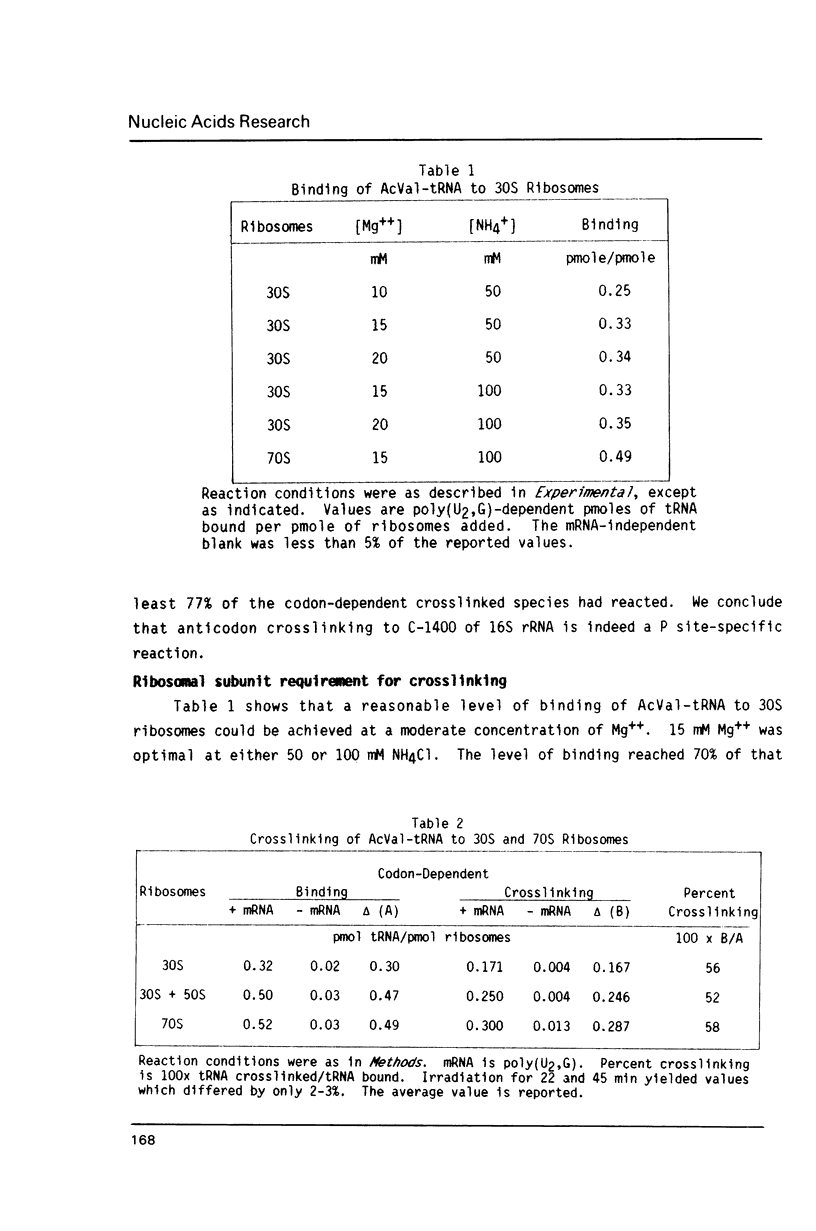
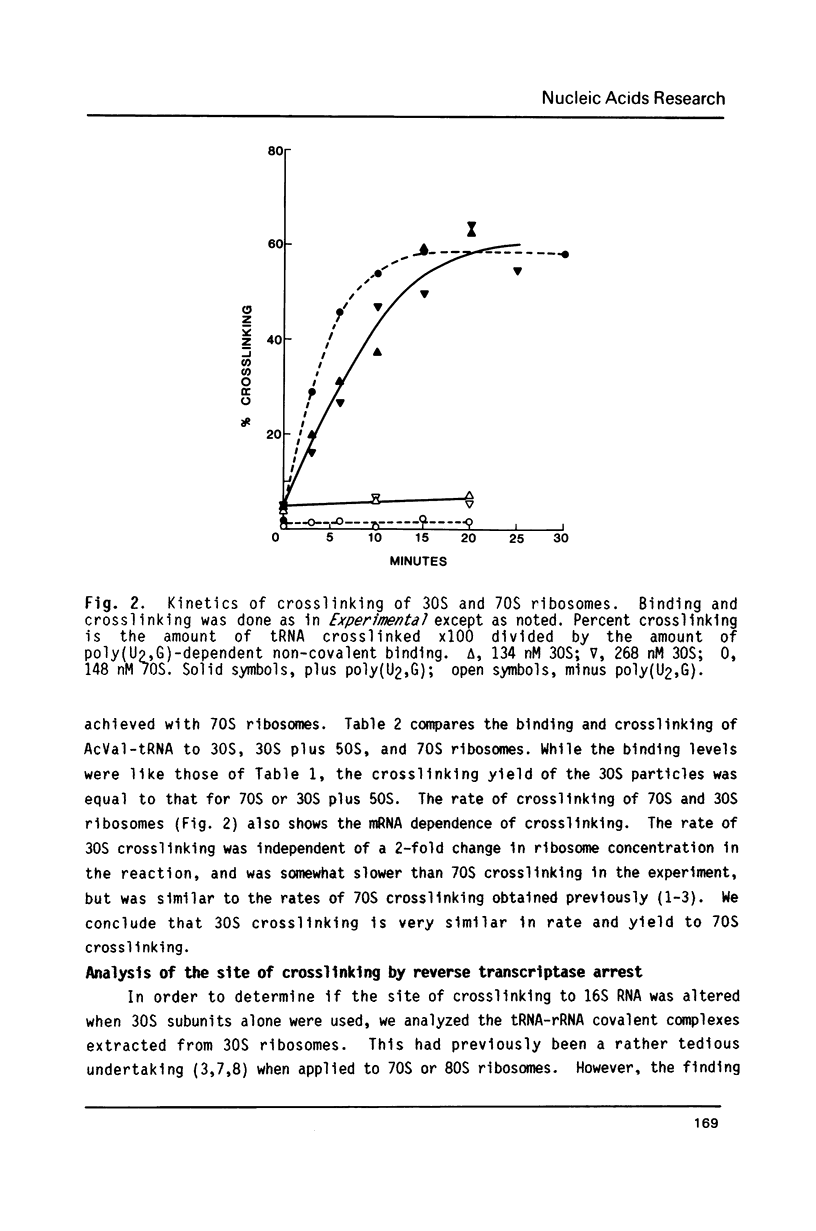
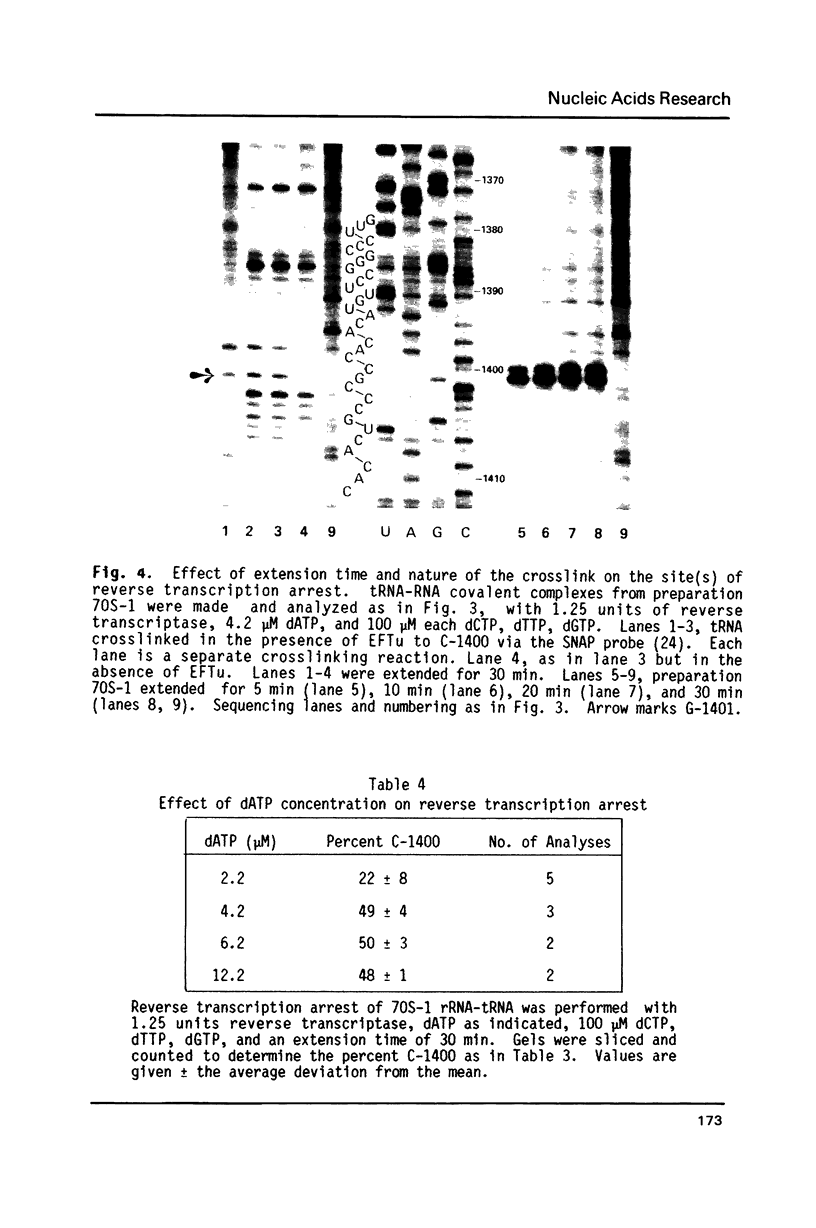
Crosslinking of the 5'-anticodon base of ribosomal P site bound AcVal-tRNA to residue C-1400 of 16S RNA or to its equivalent in 18S RNA has been shown to occur on 70S or 80S ribosomes of both prokaryotes and eukaryotes [Ciesiolka, J., Nurse, K., Klein, J. and Ofengand, J. (1985) Biochemistry 24, 3233-3239]. In the present work, we show that the crosslinking rate, crosslinking yield, and site of crosslinking are all unchanged when the 50S subunit is omitted. Therefore, all of the positional features of tRNA-ribosome complexes which allow crosslinking to occur are entirely contained in the 30S subunit. Blockage of reverse transcription by crosslink formation was used to determine the site of crosslinking. This analysis revealed that RNA modifications which do not directly block base-pairing ligands sometimes allow the modified base to be transcribed, leading to doublet band formation even when there is only a single crosslink site.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barta A., Steiner G., Brosius J., Noller H. F., Kuechler E. Identification of a site on 23S ribosomal RNA located at the peptidyl transferase center. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3607–3611. doi: 10.1073/pnas.81.12.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauclerk A. A., Cundliffe E. Sites of action of two ribosomal RNA methylases responsible for resistance to aminoglycosides. J Mol Biol. 1987 Feb 20;193(4):661–671. doi: 10.1016/0022-2836(87)90349-4. [DOI] [PubMed] [Google Scholar]
- Bergemann K., Nierhaus K. H. Spontaneous, elongation factor G independent translocation of Escherichia coli ribosomes. J Biol Chem. 1983 Dec 25;258(24):15105–15113. [PubMed] [Google Scholar]
- Ciesiolka J., Gornicki P., Ofengand J. Identification of the site of cross-linking in 16S rRNA of an aromatic azide photoaffinity probe attached to the 5'-anticodon base of A site bound tRNA. Biochemistry. 1985 Aug 27;24(18):4931–4938. doi: 10.1021/bi00339a031. [DOI] [PubMed] [Google Scholar]
- Ciesiolka J., Nurse K., Klein J., Ofengand J. Conservation of RNA sequence and cross-linking ability in ribosomes from a higher eukaryote: photochemical cross-linking of the anticodon of P site bound tRNA to the penultimate cytidine of the UACACACG sequence in Artemia salina 18S rRNA. Biochemistry. 1985 Jun 18;24(13):3233–3239. doi: 10.1021/bi00334a024. [DOI] [PubMed] [Google Scholar]
- Ehresmann C., Ehresmann B., Millon R., Ebel J. P., Nurse K., Ofengand J. Cross-linking of the anticodon of Escherichia coli and Bacillus subtilis acetylvalyl-tRNA to the ribosomal P site. Characterization of a unique site in both E. coli 16S and yeast 18S ribosomal RNA. Biochemistry. 1984 Jan 31;23(3):429–437. doi: 10.1021/bi00298a006. [DOI] [PubMed] [Google Scholar]
- Ehresmann C., Ofengand J. Two-dimensional gel electrophoresis technique for determination of the cross-linked nucleotides in cleavable covalent RNA-RNA complexes. Application to Escherichia coli and Bacillus subtilis acetylvalyl-tRNA covalently linked to E. coli 16S and yeast 18S ribosomal RNA. Biochemistry. 1984 Jan 31;23(3):438–445. doi: 10.1021/bi00298a007. [DOI] [PubMed] [Google Scholar]
- Geigenmüller U., Hausner T. P., Nierhaus K. H. Analysis of the puromycin reaction. The ribosomal exclusion principle for AcPhe-tRNA binding re-examined. Eur J Biochem. 1986 Dec 15;161(3):715–721. doi: 10.1111/j.1432-1033.1986.tb10498.x. [DOI] [PubMed] [Google Scholar]
- Gnirke A., Nierhaus K. H. tRNA binding sites on the subunits of Escherichia coli ribosomes. J Biol Chem. 1986 Nov 5;261(31):14506–14514. [PubMed] [Google Scholar]
- Hagenbüchle O., Santer M., Steitz J. A., Mans R. J. Conservation of the primary structure at the 3' end of 18S rRNA from eucaryotic cells. Cell. 1978 Mar;13(3):551–563. doi: 10.1016/0092-8674(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Henderson R. E., Kirkegaard L. H., Leonard N. J. Reaction of diethyl pyrocarbonate with nucleic acid components. Adenosine-containing nucleotides and dinucleoside phosphates. Biochim Biophys Acta. 1973 Feb 4;294(1):356–364. doi: 10.1016/0005-2787(73)90090-7. [DOI] [PubMed] [Google Scholar]
- Inoue T., Cech T. R. Secondary structure of the circular form of the Tetrahymena rRNA intervening sequence: a technique for RNA structure analysis using chemical probes and reverse transcriptase. Proc Natl Acad Sci U S A. 1985 Feb;82(3):648–652. doi: 10.1073/pnas.82.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzosiak W., Denman R., Nurse K., Hellmann W., Boublik M., Gehrke C. W., Agris P. F., Ofengand J. In vitro synthesis of 16S ribosomal RNA containing single base changes and assembly into a functional 30S ribosome. Biochemistry. 1987 Apr 21;26(8):2353–2364. doi: 10.1021/bi00382a042. [DOI] [PubMed] [Google Scholar]
- Kyogoku Y., Lord R. C., Rich A. The effect of substituents on the hydrogen bonding of adenine and uracil derivatives. Proc Natl Acad Sci U S A. 1967 Feb;57(2):250–257. doi: 10.1073/pnas.57.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manderschied U., Bertram S., Gassen H. G. Initiator-tRNA recognizes a tetranucleotide codon during the 30 S initiation complex formation. FEBS Lett. 1978 Jun 1;90(1):162–166. doi: 10.1016/0014-5793(78)80321-4. [DOI] [PubMed] [Google Scholar]
- Moazed D., Stern S., Noller H. F. Rapid chemical probing of conformation in 16 S ribosomal RNA and 30 S ribosomal subunits using primer extension. J Mol Biol. 1986 Feb 5;187(3):399–416. doi: 10.1016/0022-2836(86)90441-9. [DOI] [PubMed] [Google Scholar]
- Moazed D., Van Stolk B. J., Douthwaite S., Noller H. F. Interconversion of active and inactive 30 S ribosomal subunits is accompanied by a conformational change in the decoding region of 16 S rRNA. J Mol Biol. 1986 Oct 5;191(3):483–493. doi: 10.1016/0022-2836(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Noller H. F. Structure of ribosomal RNA. Annu Rev Biochem. 1984;53:119–162. doi: 10.1146/annurev.bi.53.070184.001003. [DOI] [PubMed] [Google Scholar]
- Ofengand J., Gornicki P., Chakraburtty K., Nurse K. Functional conservation near the 3' end of eukaryotic small subunit RNA: photochemical crosslinking of P site-bound acetylvalyl-tRNA to 18S RNA of yeast ribosomes. Proc Natl Acad Sci U S A. 1982 May;79(9):2817–2821. doi: 10.1073/pnas.79.9.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofengand J., Liou R. Correct codon--anticodon base pairing at the 5'-anticodon position blocks covalent cross-linking between transfer ribonucleic acid and 16S RNA at the ribosomal P site. Biochemistry. 1981 Feb 3;20(3):552–559. doi: 10.1021/bi00506a017. [DOI] [PubMed] [Google Scholar]
- Ofengand J., Liou R. Evidence for pyrimidine-pyrimidine cyclobutane dimer formation in the covalent cross-linking between transfer ribonucleic acid and 16S ribonucleic acid at the ribosomal P site. Biochemistry. 1980 Oct 14;19(21):4814–4822. doi: 10.1021/bi00562a016. [DOI] [PubMed] [Google Scholar]
- Ofengand J., Liou R., Kohut J., 3rd, Schwartz I., Zimmermann R. A. Covalent cross-linking of transfer ribonucleic acid to the ribosomal P site. Mechanism and site of reaction in transfer ribonucleic acid. Biochemistry. 1979 Oct 2;18(20):4322–4332. doi: 10.1021/bi00587a010. [DOI] [PubMed] [Google Scholar]
- Piette J. G., Hearst J. E. Termination sites of the in vitro nick-translation reaction on DNA that had photoreacted with psoralen. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5540–5544. doi: 10.1073/pnas.80.18.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince J. B., Taylor B. H., Thurlow D. L., Ofengand J., Zimmermann R. A. Covalent crosslinking of tRNA1Val to 16S RNA at the ribosomal P site: identification of crosslinked residues. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5450–5454. doi: 10.1073/pnas.79.18.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaralingam M., Rao S. T., Abola J. Molecular conformation of dihydrouridine: puckered base nucleoside of transfer RNA. Science. 1971 May 14;172(3984):725–727. doi: 10.1126/science.172.3984.725. [DOI] [PubMed] [Google Scholar]
- Youvan D. C., Hearst J. E. A sequence from Drosophila melanogaster 18S rRNA bearing the conserved hypermodified nucleoside am psi: analysis by reverse transcription and high-performance liquid chromatography. Nucleic Acids Res. 1981 Apr 10;9(7):1723–1741. doi: 10.1093/nar/9.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youvan D. C., Hearst J. E. Reverse transcriptase pauses at N2-methylguanine during in vitro transcription of Escherichia coli 16S ribosomal RNA. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3751–3754. doi: 10.1073/pnas.76.8.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youvan D. C., Hearst J. E. Sequencing psoralen photochemically reactive sites in Escherichia coli 16 S rRNA. Anal Biochem. 1982 Jan 1;119(1):86–89. doi: 10.1016/0003-2697(82)90669-8. [DOI] [PubMed] [Google Scholar]