Abstract
Identification of hypersensitive cell death (HCD) regulators is essential to dissect the molecular mechanisms underlying plant disease resistance. In this study, combined proteomic and RNA interfering (RNAi) analyses were employed to identify genes required for the HCD conferred by the tomato resistance gene Cf-4 and the Cladosporium fulvum avirulence gene Avr4. Forty-nine proteins differentially expressed in the tomato seedlings mounting and those not mounting Cf-4/Avr4-dependent HCD were identified through proteomic analysis. Among them were a variety of defence-related proteins including a cysteine protease, Pip1, an operative target of another C. fulvum effector, Avr2. Additionally, glutathione-mediated antioxidation is a major response to Cf-4/Avr4-dependent HCD. Functional analysis through tobacco rattle virus-induced gene silencing and transient RNAi assays of the chosen 16 differentially expressed proteins revealed that seven genes, which encode Pip1 homologue NbPip1, a SIPK type MAP kinase Nbf4, an asparagine synthetase NbAsn, a trypsin inhibitor LeMir-like protein NbMir, a small GTP-binding protein, a late embryogenesis-like protein, and an ASR4-like protein, were required for Cf-4/Avr4-dependent HCD. Furthermore, the former four genes were essential for Cf-9/Avr9-dependent HCD; NbPip1, NbAsn, and NbMir, but not Nbf4, affected a nonadaptive bacterial pathogen Xanthomonas oryzae pv. oryzae-induced HCD in Nicotiana benthamiana. These data demonstrate that Pip1 and LeMir may play a general role in HCD and plant immunity and that the application of combined proteomic and RNA interfering analyses is an efficient strategy to identify genes required for HCD, disease resistance, and probably other biological processes in plants.
Keywords: Cf, Cladosporium fulvum, defence, gene silencing, hypersensitive cell death, proteomics, regulation, resistance, RNAi
Introduction
Plants have evolved two lines of defence to counter-attack pathogens’ infection: pathogen-associated molecular pattern-triggered immunity and effector-triggered immunity (ETI) (Jones and Dangl, 2006; De Wit, 2007). ETI is initiated by recognition of pathogen effector proteins, including avirulence (Avr) proteins by plant resistance (R) proteins, and is hallmarked by development of hypersensitive cell death (HCD).
The pathosystem of tomato (Solanum lycopersicum Mill.) and its leaf mould fungal pathogen Cladosporium fulvum is a model system to study ETI conferred by receptor-like protein type R proteins (Joosten and De Wit, 1999; Rivas and Thomas, 2005; Wang et al., 2006b; Stergiopoulos and De Wit, 2009). A set of C. fulvum effector genes and tomato R genes (Cfs), including four complementary R/Avr gene pairs, have been cloned from this pathosystem. These are Cf-9/Avr9 (Van den Ackerveken et al., 1992; Jones et al., 1994), Cf-4/Avr4 (Joosten et al., 1994; Thomas et al., 1997), Cf-2/Avr2 (Dixon et al., 1996; Luderer et al., 2002), and Cf-4E/Avr4E (Takken et al., 1998; Westerink et al., 2004). All known Avr products are secreted to the extracellular space and carry an even number of cysteine residues, but show no other sequence similarity (Van den Ackerveken et al., 1992; Joosten et al., 1994; Luderer et al., 2002; Westerink et al., 2004). However, all Cfs are homologous, extracellular, membrane-anchored glycoproteins that mainly consist of leucine-rich repeats (LRR) (Jones et al., 1994; Dixon et al., 1996; Thomas et al., 1997; Takken et al., 1998). Although over 91% of the amino acids of the Cf-4 and Cf-9 proteins are identical (Jones et al., 1994; Thomas et al., 1997), the HCD resulting from Avr9 and Avr4 recognition by Cf-9 and Cf-4, respectively, is distinct in cell death pattern and intensity (Cai et al., 2001) and sensitivity to environmental conditions (Thomas et al., 2000; Van der Hoorn et al., 2000; Cai et al., 2001; De Jong et al., 2002; Wang et al., 2005). In comparison with the Avr9/Cf-9-dependent HCD, Avr4/Cf-4-dependent HCD is more rapid, with necrosis appearing primarily in the veins (Thomas et al., 2000; Van der Hoorn et al., 2000; Cai et al., 2001), and less sensitive to high temperature and high relative humidity (De Jong et al., 2002; Wang et al., 2005).
Significant progress has been made to understand the defence signal transduction initiated with Cf/Avr recognition. An oxidative burst, calcium-dependent kinases, MAP kinases, and a K+ efflux are involved in regulation of Cf-9/Avr9-initiated defence responses (Piedras et al., 1998; Blatt et al., 1999; Romeis et al., 1999, 2000, 2001; De Jong et al., 2000). A thioredoxin, CITRX, negatively regulates Cf-9-dependent cell death (Rivas et al., 2004). Transcript profiling reveals that expression of several hundreds of genes alters upon Cf/Avr recognition, which include 290 ACRE (Avr9/Cf-9 rapidly elicited) (Durrant et al., 2000), 442 ART (Avr4-responsive tomato) (Gabriëls et al., 2006), and 367 ACE (Avr/Cf elicited) (Hong et al., 2007; Zhu et al., 2008) genes. Recently, further studies employing virus-induced gene silencing (VIGS) identified several genes that are essential for Cf-dependent HCD and/or resistance, which include a CC-NB-LRR type resistance protein analogue gene NRC1 (Gabriëls et al., 2007), protein kinase genes ACIK1 (Rowland et al., 2005) and LeMPKs (Stulemeijer et al., 2007), two E3 ubiquitin ligase genes CMPG1 (Gonzalez-Lamothe et al., 2006) and PUB17 (Yang et al., 2006), and a F-box protein gene ACIF1 (Van den Burg et al., 2008), demonstrating that protein phosphorylation and the ubiquitin-proteasome pathway are pivotal in regulation of Cf-dependent HCD and resistance. Additionally, Avr2 inhibits cysteine protease Rcr3 and thereby initiates Cf-2-dependent defence signalling (Kruger et al., 2002; Rooney et al., 2005). Avr2 also inhibits another cysteine protease, Phytopathora-inhibited protease 1 (Pip1), which has been suggested to be an operative target of Avr2 in a recently developed ‘decoy’ model (Shabab et al., 2008; van der Hoorn and Kamoun, 2008; van Esse et al., 2008). However, the exact role of Pip1 in defence response to C. fulvum is still largely unknown. Additionally, 12 proteins were differentially phosphorylated in the tomato seedlings mounting Cf-4-dependent HCD and the control HCD− seedlings (Stulemeijer et al., 2009); however, role of these phosphoproteins in Cf-4-dependent HCD and resistance is still unclear.
To further understand the molecular mechanism underlying Cf-conferred HCD and resistance to C. fulvum, this study identified a set of novel genes required for Cf-4-dependent HCD employing combined proteomic and RNA interfering (RNAi) analyses.
Materials and methods
Plant growth and sampling
Tomato (Solanum lycopersicon) lines of Moneymaker (MM) carrying the C. fulvum resistance gene Cf-4 (MM-Cf-4) or carrying no known Cf gene but transformed with the C. fulvum avirulence gene Avr4 (MM-Avr4), and the F1 progeny of crossing between MM-Cf-4 and MM-Avr4 (MM-Avr4/Cf-4) (Cai et al., 2001) were used in this study. Seeds were surface sterilized, germinated, sown in trays, and grown in plant growth chambers (RXZ-450D, Saifu Instrument Manufacturer, Ningbo, China) with a light/dark regime of 16/8 h, 70% humidity at 22°C, as described previously (Wang et al., 2005). Cotyledons of the MM-Avr4/Cf-4 seedlings were collected when pin-point hypersensitive necrosis was just visible by the naked eye on the lower sides of the cotyledons. Meanwhile, cotyledons of the MM-Cf-4 seedlings were also collected as the control. The cotyledon samples were immediately frozen in liquid nitrogen and stored at –80 °C prior to protein extraction.
Preparation of protein samples for two-dimensional polyacrylamide gel electrophoresis
Protein was extracted from sampled cotyledons by trichloracetic acid (TCA) method as described by Damerval et al. (1986) with some modifications. One gram of tomato cotyledons were finely powdered with a pestle in a mortar under liquid nitrogen and suspended in cold acetone (−20 °C) containing 10% (w/v) TCA and 0.07%(v/v) β-mercaptoethanol. Proteins were precipitated overnight at −20°C and centrifuged at 40,000 g at 4 °C for 1 h. Then the pellets were washed three times with cold acetone containing 0.07% (v/v) β-mercaptoethanol and centrifuged at 40,000 g at 4 °C for 1 h. The precipitates were lyophilized and solubilized in lysis buffer (7 M urea, 2 M thiourea, 4% (w/v) CHAPS, 65 mM DTT, 0.4% (w/v) each carrier ampholyte pH 5–7 and pH 3–10). Insoluble debris was removed by centrifugation at 40,000 g at 4 °C for 1 h and supernatants were collected, separated into aliquots, and stored at −80 °C. Protein concentration was determined by Bradford assay using bovine serum albumin as a standard.
Two-dimensional polyacrylamide gel electrophoresis and image analysis
IPG strips (Immobiline DryStrip pH 3–7 NL, 24 cm, GE Healthcare) were rehydrated at 25 °C for 12 h with 450 μl rehydration buffer (7 M urea, 2 M thiourea, 2% (w/v) CHAPS, 65 mM DTT, 0.2% (w/v) each carrier ampholyte pH 5–7 and pH 3–10), 0.002% (w/v) bromophenol blue) containing 300 μg protein for analytical gels or 1 mg protein for preparative gels. The rehydrated strips were electrofocused with Ettan IPGphor II IEF unit following the protocol provided by the manufacturer (Amersham Biosciences, GE Healthcare) with the addition of the following two steps at the beginning to make the salt removal more efficient: 100 V step and hold for 1 h; 250 V step and hold for 1 h. After focusing, the IPG strips were incubated first in equilibration buffer (6 M urea, 30% (w/v) glycerol, 2% (w/v) SDS, 75 mM TRIS-HCl, pH 8.8) containing 1% (w/v) DTT and then in the same equilibration buffer but containing 2.5% (w/v) iodoacetamide. The second-dimension separation was performed using the Ettan Daltsix electrophoresis system (Amersham Biosciences) with 12% polyacrylamide gels at 1 W/gel for 1 h followed by 13 W/gel until the dye front reached the bottom of the gels. Six gels (three each for samples of MM-Cf-4 and MM-Cf-4/Avr4) were run simultaneously each time as three technical replicates and the two-dimensional polyacrylamide gel electrophoresis (2-D PAGE) analysis was repeated three times for three independent sets of biological samples as three biological replicates. Analysis gels were visualized by silver staining compatible with MS analysis (Yan et al., 2000) and scanned with the Imagescanner UMAX with Labscan v. 5.0 at 300 dots/inch (Amersham Biosciences). Spot detection and nomalization, gel matching, and statistical data analysis (P-value <0.05) were conducted with ImageMaster 2D Platinum v. 6.0 (Amersham Biosciences) following the manufacturer’s instructions. Proteins that displayed two-fold or greater changes in the spot’s relative volume (spot volume/total spot volume × 100) were collected as differentially expressed proteins.
In-gel tryptic digestion, MS analysis, and database search
Approximately 1 mg protein from each sample (MM-Cf-4/Avr4 and MM-Cf-4) was separated by 2-D PAGE and the gels were stained using Coomassie Brilliant Blue (CBB). Protein spots that showed different abundance in gels loaded with the MM-Cf-4/Avr4 (HCD+) and MM-Cf-4 (HCD−) samples were excised from CBB-stained gels and destained with 100 mM NH4HCO3 in 30% acetonitrile (ACN). Subsequently, the destaining buffer was removed and the gel pieces were lyophilized and rehydrated in 30 μl of 50 mM NH4HCO3 containing 50 ng trypsin (Promega, USA). After overnight digestion at 37 °C, the peptides were extracted three times with 0.1% trifluoroacetic acid (TFA) in 60% ACN. Extracts were pooled together and lyophilized. The resulting peptide mixtures were kept at –80 °C for MS analysis. A protein-free gel piece was treated similarly and used for a control to identify the proteolysis products.
MS analysis of protein spots was performed at the Research Centre for Proteome Analysis, Chinese Academy of Sciences, Shanghai, China. The peptide mixtures were redissolved with an equal volume of cyano-4-hydroxycinnamic acid (10 mg/ml, Sigma), saturated with 50% acetonitrile in 0.05% TFA and analysed with an AutoFlex MALDI-TOF/TOF mass spectrometer (Bruker Daltonics, Bremen, Germany). Proteins were first analysed by MALDI-TOF MS. The samples that were unable to be identified by MALDI-TOF MS analysis were subjected to MALDI-TOF/TOF MS analysis with ‘LIFT’ technology (Suckau et al., 2003). The lists of peaks for both the MALDI-TOF and the MALDI-TOF/TOF mass spectra were generated by flexAnalysis 2.4. Peaks with intensity >500 and S/N >6 were automatically picked up. The known contaminant ions (keratin and tryptic autodigest peptides) were excluded. The peptide calibration standard (Bruker) was used for external calibration, and the matrix and autolytic peaks of trypsin were used for internal calibration to ensure the accuracy of protein identification.
MS and MS/MS spectra were directly analysed by database searching using MASCOT 2.1 (MatrixScience, London, UK) with BioTools 3.0 (Bruker Daltonics, Bremen, Germany). Mass spectra were searched against the NCBI nonredundant protein (NCBInr) database within the taxonomy Viridiplantae (green plants). Protein-matching searches that resulted in a score of over 68 were considered significant (P < 0.05). Individual ion scores of over 40 indicated identity or extensive homology (P < 0.05) existing in the compared proteins. A retrieved protein with the highest score in each Mascot search was accepted as the target protein for the spot subjected to identification. If peptides matched to multiple members of a protein family with the same or very similar scores, the S. lycopersicum protein with the highest score was the first choice; otherwise, a protein with pI and molecular weight mostly coinciding with the value predicted from 2-D PAGE profiles was considered as the identified protein. The search parameters were set as follows: enzyme, trypsin; fixed modifications, carbamidomethyl cysteine; variable modifications, methionine oxidation; mass values, monoisotopic; protein mass, unrestricted; peptide charge state, 1+; maximum missed cleavages, 1; peptide mass tolerance, ±100 ppm; fragment mass tolerance, ±0.8 Da.
Cloning of Nicotiana benthamiana and tomato cDNA fragments corresponded to the differentially expressed proteins
cDNAs corresponding to the differentially expressed proteins were cloned from Nicotiana benthamiana and tomato through reverse-transcription (RT) PCR. The tomato cDNAs and their N. benthamiana homologues corresponding to the differentially expressed proteins were obtained through tBLASTn search against the DFCI The Gene Indices database (http://compbio.dfci.harvard.edu/tgi/) and the SOL Tobacco Gene Indices database (http://www.pngg.org/tgi/) using amino acid sequences of the differentially expressed proteins as queries. Primers were designed according to the retrieved cDNA sequences for the parts that had the highest identity to the tomato target proteins and had restriction enzyme sites added to the 5′-end (Supplementary Table S1, available at JXB online). Total RNA was isolated from normal tomato and N. benthamiana plants using TRIZOL reagent. cDNA generation from total RNA and subsequent PCR were performed using PrimeScript RT-PCR kit (TaKaRa Biotechnology, China). Products of RT-PCR were ligated into pUC-mT vector (Sangon, China) and sequenced. The obtained sequences were analysed for homology to the tomato differentially expressed proteins using DNAstar software (DNASTAR, USA) and deposited in the NCBI database.
Tobacco rattle virus-induced gene silencing analysis
The target cDNA fragments were ligated into the tobacco rattle virus (TRV) VIGS vector pYL156 (provided by Dr SP Dinesh-Kumar, Yale University) using the appropriate enzymes (Supplementary Table S1). The recombinant vector as well as pTRV1 was electroporated into cells of Agrobacterium tumefaciens strain GV3101. VIGS in N. benthamiana was performed as described previously (Wang et al., 2006a; Xu et al., 2007). Three weeks after agroinfiltration, the plants were subjected to HCD analysis. For each gene, over 10 plants were used for each VIGS analysis experiment and the VIGS experiments were conducted in duplicate.
HCD analysis
For Cf-4/Avr4- and Cf-9/Avr9-dependent HCD assessment, agrobacteria expressing an Avr and a Cf, respectively, were cultured as described (Wang et al., 2006a). The pellets were collected by centrifugation and suspended to give an OD600 of 4.0. After recovery, suspensions of agrobacteria expressing an Avr and its complementary Cf were mixed in a 1:1 ratio to obtain the final agro-inocula for infiltration. The agro-inocula were infiltrated into the top three completely developed leaves of each N. benthamiana, Nicotiana tabacum, and tomato plants with a sterilized syringe without a needle. The agro-inoculated plants were grown in plant growth chambers at 25 °C with a light/dark 16/8 h regime. Three days later, the HCD in infiltrated area was investigated.
Phenotypes of HCD were briefly grouped into three grades based on the intensity and emergence percentage of tissue death in the agroinfiltrated area: full and nearly full HCD; partial HCD; and no HCD (Supplementary Fig. S1). A full HCD meant that tissue over the whole agroinfiltrated area completely died and collapsed (Supplementary Fig. S1A and B); a nearly full HCD signified that most tissue of the agroinfiltrated area (over 75%) coherently died and collapsed (Supplementary Fig. S1C); a partial HCD indicated that only partial tissue of the agroinfiltrated area (less than 75%) died and the dead tissues often scattered into several patches frequently with yellowish margins (Supplementary Fig. S1D–F); and no HCD referred to none of the tissue of the agroinfiltrated area dying (Supplementary Fig. S1G–I).
To analyse Xanthomonas oryzae pv. oryzae (Xoo)-induced HCD, Xoo was cultured in Wakimoto’s medium plates at 28 °C for 2 days. Colonies were collected with sterilized water and suspended to give an OD600 of 0.1. Leaf infiltration was performed as described above.
Reverse-transcription PCR analysis
Transcript abundance of the genes for VIGS and transient RNAi analysis in N. benthamiana and N. tabacum plants was examined by semi-quantitative RT-PCR analysis as described (Cai et al., 2007). Total RNA was extracted with TRIZOL reagents (Invitrogen, USA). cDNA generation from total RNA and subsequent PCR were performed with the primers listed in Supplementary Table S2 using a PrimeScript RT-PCR kit (TaKaRa Biotechnology).
Transient RNAi analysis
A 300bp fragment of Pip1 was amplified with primers PIP1-F3 (CAGAGCTC(SacI)GGTACC(KpnI)GACGAGTCATCGTTGTTGAAA) and PIP1-R3 (CAGGATCC(BamHI)CTCGAG(XhoI)AGCAGTAGGGAACGACGCAAC) using as template the plasmids carrying Pip1 cloned earlier in this study. This fragment was cloned into pBS-IN flanking the intron sequence of Phaseolus vulgaris nitrite reductase gene (U10419) first in the reverse direction with XhoI/KpnI and then in the forward direction with SacI/BamHI. The released hairpin sequence cassette was then cloned into binary vector pC1305-35S after the CaMV 35S promoter with KpnI to obtain the Pip1 RNAi construct pC1305-35S::PIP1-RNAi. Agrobacteria transformed with this construct were infiltrated into all sectors of left halves of leaves of Sumsun NN tobacco plants while agrobacteria transformed with empty vector, as control, were infiltrated into the right half leaves. The plants were grown at 25 °C in plant growth chambers for Pip1 gene silencing. Two days later, HCD in each sector of the RNAi-treated leaves was evaluated as described above.
Results
Two-dimensional polyacrylamide gel electrophoresis protein profiles of Cf-4/Avr4 tomato seedlings mounting a HCD
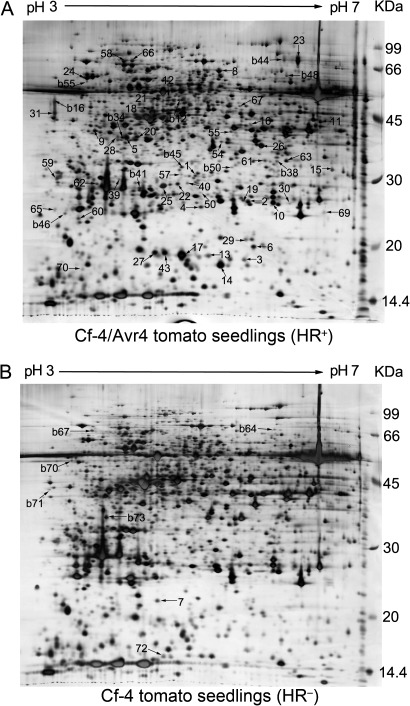
To investigate the change on protein expression during development of Cf-4/Avr4-dependent HCD, the proteomes of the cotyledons of tomato seedlings carrying the gene pair Cf-4/Avr4 and thus mounting a HCD (HCD+ seedlings) and those carrying only the Cf-4 gene and thus developing no HCD (HCD− seedlings) were comparatively analysed. Typical protein profiles for the two type seedlings are shown in Fig. 1. According to the statistical data of the 2-D PAGE experiments, 2345 ± 75 protein spots for the HCD− seedlings and 2387 ± 68 for the HCD+ seedlings were detected in silver-stained 2-D gels loaded with 300 μg protein per gel. In total, 66 protein spots were expressed differentially at least two-fold between the HCD+ and HCD− seedlings (Fig. 1). Among them, 59 were up-regulated (Fig. 1A), while seven were down-regulated (Fig. 1B) in the HCD+ seedlings. As expected, this result indicates that expression of proteins significantly and globally altered during development of Cf-4/Avr4-dependent HCD.
Fig. 1.
Two-dimensional polyacrylamide gel electrophoresis profiles of proteins from Cf-4/Avr4 tomato seedlings mounting a hypersensitive cell death (HCD). (A) Profile from Cf-4/Avr4 tomato seedlings (HCD+). (B) Profile from Cf-4 tomato seedlings (HCD−). A total of 300 μg protein per gel was loaded. After 2-D PAGE using IPG strips (24 cm, pH 3–7 NL), the gels were silver stained. Protein spots that expressed differentially over two-fold in the cotyledons of the HCD+ and HCD− seedlings are numbered and indicated with arrows in the gels in which the spots displayed with higher abundance. Spot numbers are as given in Supplementary Table S3.
MS identification of proteins differentially expressed between the HCD+ and HCD− seedlings
To identify the differentially expressed proteins, preparative 2-D PAGE followed by CBB gel staining instead of silver staining was performed so that the loading volume could be increased from 300 μg to 1 mg per gel and thus the protein spots could be more easily identified successfully. The differentially expressed protein spots were excised from the gels and then subjected to in-gel trypsin digestion and subsequent MS identification analysis. Forty-nine proteins were successfully identified (Supplementary Table S3). The MS spectra of these identified proteins are listed in Supplementary Fig. S2. The close-up spot-to-spot comparison of differential expression of the identified 49 proteins in the HCD+ and HCD− seedlings are shown in Fig. 2.
Fig. 2.
Close-up view of the protein spots differentially expressed between Cf-4/Avr4 (hypersensitive cell death; HCD+) and Cf-4 (HCD−) tomato seedlings. The differentially expressed protein spots are circled and grouped according to their functions.
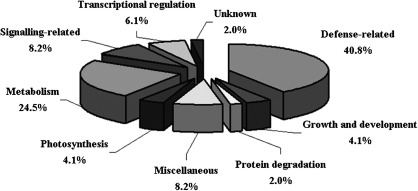
Among the identified protein spots, one was a predicted protein with unknown function, while the other 48 were annotated to be involved in defence response (40.8%), signal transduction (8.2%), metabolism (24.5%), transcriptional regulation (6.1%), protein degradation (2.0%), growth and development (4.1%), miscellaneous functions (8.2%), and photosynthesis (4.1%) (Fig. 3, Supplementary Table S3). The majority of the identified proteins (46/49) were up-regulated in the HCD+ seedlings.
Fig. 3.
Functional classification of the identified proteins differentially expressed in Cf-4/Avr4 (hypersensitive cell death; HCD+) and Cf-4 (HCD−) tomato seedlings.
Nearly half of the 46 up-regulated proteins were defence-related proteins, among which were four glutathione S-transferases belonging to different classes, such as phi and tau, and two phospholipid hydroperoxide glutathione peroxidases (PHGPx). Glutathione S-transferases are involved in oxidative-stress metabolism and detoxification and PHGPx are antioxidant selenoenzymes that directly reduce membrane-bound lipid hydroperoxides (Dixon et al., 2009 and references therein). This indicates that glutathione-mediated antioxidation is one of the major responses upon the occurrence of Cf-4/Avr4-dependent HCD and/or that glutathione-mediated metabolism and maintenance of reductive state of membrane-bound lipids is important in regulation of Cf-4/Avr4-dependent HCD. The importance of glutathione in disease resistance has been shown with a pad2 mutant (Parisy et al., 2007). The cysteine protease Phytopathora-inhibited protease 1 (Pip1, spot 59) and a trypsin-inhibitor family protein LeMir (Lycopersicon Esculentum Miraculin, spot b60), were not detected in the HCD− seedlings but significantly accumulated in seedlings mounting Cf-4/Avr4-dependent HCD (Fig. 2, Supplementary Table S3). Additional up-regulated defence-related proteins were a set of well-known pathogenesis-related (PR) proteins including four PR10, three PR7 (P69), a PR5 and a PR9, and two chitinases, as well as a β-1,3-glucanase. Some of them were up-regulated nearly or over 10 times in the HCD+ seedlings over the HCD− seedlings. For instance, a subtilisin-like protease P69 (spot 23), a peroxidase (spot 31), a PR10 protein STH-2 (spot 14), and a basic 30 kDa endochitinase (spot 15) were up-regulated 39, 18.6, 9.8, and 9.4 times, respectively, in the HCD+ seedlings compared with the HCD− seedlings (Fig. 2, Supplementary Table S3).
There were four signalling-related proteins that were up-regulated in the HCD+ seedlings. These included a SIPK type MAP kinase (LeMPK1), a Ras-related GTP-binding protein, and two ACC oxidases. Other up-regulated proteins in the HCD+ seedlings included an APF1-like transcription factor, a S-adenosylmethionine-dependent methyltransferase, several molecular chaperons such as a protein disulfide isomerase and two luminal-binding proteins, and a functionally unknown protein.
Three down-regulated spots corresponded to a chloroplast thiazole biosynthetic protein, a thioredoxin, and an ASR (Abscissic acid, Stress, Ripening) protein (ASR4). A thioredoxin, CITRX, has been reported to specifically negatively regulate Cf-9-dependent HCD (Rivas et al., 2004). It will be intriguing to investigate the role of the thioredoxin identified in this study in Cf-dependent HCD and resistance.
Taken together, the proteomic data clearly show that Cf-4/Avr4 plants are reprogrammed at the translational level to activate defence responses.
Comparison of expression profiles for Cf-4/Avr4-dependent HCD in protein and transcript levels
Previously, the present study laboratory and others have identified the transcript profiles for Cf-4/Avr4-dependent HCD through cDNA-amplified fragment length polymorphism (AFLP) analysis and have cloned 278 differentially expressed ACE fragments that correspond to 128 type proteins (Hong et al., 2007; Zhu et al., 2008) and 343 ART (Avr4-responsive tomato) fragments (Gabriëls et al., 2006). However, as far as is known, this is the first report on the proteomic analysis of expression profiles for Cf/Avr-dependent HCD. To better understand regulation of Cf-4/Avr4-dependent HCD at different levels and to validate the proteomic analysis in this study, the expression profiles for HCD at the protein level (this study) and the transcript level (previous studies: Gabriëls et al., 2006; Hong et al., 2007; Zhu et al., 2008) were compared. As shown in Table 1, genes encoding 22 out of the 49 differentially expressed spots identified in this study, which corresponded to 13 type proteins, were detected to be differentially expressed at the transcript level as well. These proteins were involved in defence response, signal transduction, metabolism, protein synthesis, miscellaneous functions, and photosynthesis (Table 1). This partial overlap between the two expression profiles validates the proteomic analysis in this study and demonstrates that Cf-4/Avr4-dependent HCD is regulated at both the transcriptional and the translational levels. Proteomic analysis could thus provide additional information related to the regulation of Cf-4/Avr4-dependent HCD and resistance.
Table 1.
The Differentially expressed proteins which were consistently detected in both this proteomic analysis and the previous cDNA-amplified fragment length polymorphism (AFLP) transcript profiling analysis (Hong et al., 2007; Zhu et al., 2008)
| Differentially expressed proteins | Corresponding protein spot number in this proteomic analysis | Corresponding transcript number in previous cDNA-AFLP transcript profiling analysis |
| Glutathione S-transferase | 2, 10, 22, 40 | 95, 148, 198 |
| Pathogenesis-related protein STH-2 | 3, 14 | 140 |
| Pathogenesis-related protein 10 | 13, 17 | 78, 79, 114 |
| Subtilisin-like protease | 23, b44 | 150, 189 |
| Acidic 26 kDa endochitinase | 15, 25 | 152, 153, 158, 192, 237, 283, 285a |
| Ribulose-1,5-bisphosphate carboxylase | 63, b50 | 237, 238, 259, 328, 330, 357, 358a |
| 1-Aminocyclopropane-1-carboxylate oxidase 1 (ACC oxidase 1) | 5, b34 | 162, 164 |
| Asparagine synthetase | b48 | 180 |
| Quinone-oxidoreductase | 4 | 10 |
| Glucan endo-1,3-beta-glucosidase A | 26 | 116, 186 |
| Peroxidase | 31 | 203 |
| Inorganic pyrophosphatase | 39 | 4 |
| Esterase | 20 | 117, 208 |
Identification of genes required for Cf-4/Avr4-dependent HCD through VIGS analysis
To evaluate the function of the differentially expressed proteins and thereby to identify the genes required for Cf-4/Avr4-dependent HCD, VIGS analysis, a rapid reverse-genetics gene function analysis technique, was conducted in N. benthamiana for genes corresponding to the differentially expressed proteins. N. benthamiana instead of the host plant tomato was chosen for VIGS analysis because the former is the model plant for VIGS analysis, with the highest and most reproducible silencing efficiency, and the agroinfiltration-based Cf-4/Avr4-dependent HCD detection analysis is easier and more repeatable in this plant, as reported earlier (Gabriëls et al., 2006). From the total of 49 differentially expressed proteins, 16, most possibly related to HCD and defence regulation according to sequence annotation, were selected for further functional analysis (Supplementary Table S1). Fragments of N. benthamiana homologues of the genes encoding the tomato differentially expressed proteins were cloned employing RT-PCR with primers designed according to the N. benthamiana homologue cDNAs retrieved from tBLASTn search against either the DFCI The Gene Indices database or the Tobacco Gene Indices database (Supplementary Table S1). Bioinformatic analysis of the sequencing data revealed that most of the cloned N. benthamiana fragments had sequence identity higher than 70% at the amino acid level compared with the counterparts of the tomato differentially expressed proteins (Supplementary Table S1, Supplementary Fig. S3). These 16 N. benthamiana fragments were subcloned into VIGS vector pYL156 for TRV-induced gene silencing analysis.
For most of the selected genes, VIGS-treated plants grew and developed normally, similarly to the control plants treated with agrobacteria transformed with the empty silencing vector (EV). However, VIGS-induction treatment (agroinfiltration) for six out of the 16 genes resulted in abnormal growth and development of the treated plants (Fig. 4). These genes encoded a proteasome 20S beta 1.1 subunit (NbPb, 19), a luminal-binding protein (NbBiP, 58), a SIPK type MAP kinase (Nbf4, b12), an asparagine synthetase (NbAsn, b48), an ASR protein (NbASR, b67), and a chloroplast thiazole biosynthetic protein (NbTHI, b73) (Table 2). VIGS-induction treatment for both NbPb and NbBiP caused severe necrosis and finally death of the whole plant, although the dynamics of plant death were different. In NbPb-silencing-treated plants, the newly developed leaves were crinkly and showed clear necrotic symptoms 12 days after VIGS treatment (dat) (Fig. 4A, B). One week later, the whole plant died (Fig. 4C). VIGS-induction treatment for NbBiP led to a more rapid and severe necrosis in comparison with NbPb. Necrosis occurred in the infiltrated areas and the top newly developed leaves as early as 7 dat in NbBiP-silencing-treated plants. The whole plants died at 14 dat (Fig. 4D). This result demonstrates that the proteasome 20S beta 1.1 subunit and BiP genes are somehow associated with plant cell death. Silencing induction treatment for both Nbf4 and NbAsn led to leaf roll and inflorescence malformation. However, phenotype development resulting from the Nbf4-VIGS treatment was much weaker and slower. No obvious phenotype was observed within 4 weeks after VIGS treatment (wat). Around 5 wat, leaf edges of the VIGS-treated plants rolled to the lower sides and twisted (Fig. 4E), while calyxes and corolla crinkled (Fig. 4F). For NbAsn, however, the growth of the VIGS-treated plants was significantly retarded at 3 wat: the sizes of newly developed leaves were much smaller and the development of shoots and inflorescences was significantly delayed (Fig. 4G). Furthermore, interestingly, unlike Nbf4-silencing-treated plants, the top newly developed leaves of the NbAsn-silencing-treated plants rolled to the upper sides tightly (Fig. 4G). At 6 wat, inflorescence was distorted, with corolla twisted and broken (Fig. 4H). Additionally, NbASR-silencing-treated plants were dwarfish with leaves turning in thicker (Fig. 4I), while NbTHI-silencing-treated plants showed leaf chlorosis before 3 wat and later bleaching (Fig. 4J). These data reveal that the SIPK type MAP kinase Nbf4, ASN, and ASR might be related with plant growth and development.
Fig. 4.
Influence of virus-induced gene silencing (VIGS)-inducing treatment for six genes on growth and development of N. benthamiana plants. Genes subjected to VIGS analyses encoded (A–C) a proteasome 20S beta 1.1 subunit (NbPb, 19), (D) a luminal-binding protein (NbBiP, 58), (E, F) a SIPK type MAP kinase (Nbf4, b12), (G, H) an asparagine synthetase (NbAsn, b48), (I) an ASR (Abscissic acid, Stress, Ripening) protein (NbASR, b67), and (J) a chloroplast thiazole biosynthetic protein (NbTHI, b73). Plants were infiltrated with cell suspensions of Agrobacterium transformed with TRV VIGS vector carrying a fragment of the target gene or just empty vector (EV; K, L). The photographs were taken 12 days (A, B), 14 days (D, K), 19 days (C), 21 days (G, J), 30 days (I, L), and 42 days (E, F, H) after agroinfiltration. Spot numbers at bottom right are as given in Supplementary Table S3.
Table 2.
List of genes affecting plant growth and development in the virus-induced gene silencing (VIGS) assay
| dat, days after VIGS-inducing treatment; wat, weeks after VIGS-inducing treatment. | ||
| Target protein spot | Target gene product | Phenotype of the VIGS-treated plant |
| 19 | Proteasome 20S beta1.1 subunit | Severe necrosis, resulting in death of whole plant about 20 dat |
| 58 | Luminal-binding protein 5 (BiP 5) | Severe necrosis, finally resulting in death of whole plant at 14 dat |
| b12 | SIPK type MAP kinase similar to NbNTF4 and LeMPK1/2 | Leaves rolled to lower side and twisted, and calyxes and corolla crinkled around 5 wat |
| b48 | Asparagine synthetase | Leaves rolled to upper side tightly, plant growth and development retarded 3 wat, inflorescence distorted 6 wat |
| b67 | ASR4-like protein | Dwarf plants and thicker leaves |
| b73 | Chloroplast thiazole biosynthetic protein | Leaf chlorosis and bleaching |
With exclusion of the two lethal genes NbPb and NbBiP, the remaining 14 genes were subjected to evaluation of their role in Cf-4/Avr4-dependent HCD by comparison of HCD in the silenced plants and nonsilenced EV plants at 3 wat. In EV plants, HCD strongly formed in the agroinfiltrated areas, most belonging to the full HCD category. In VIGS-treated plants, however, severity of HCD varied depending on the gene subjected to silencing analysis. As shown in Table 3 and Fig. 5, Cf-4/Avr4-dependent HCD in VIGS-treated plants for four genes, which encode a SIPK type MAP kinase (Nbf4, b12), a Pip1-like protein (NbPip1, 59), an ASN (NbAsn, b48), and a LeMir-like protein (NbMir, b60), was completely abolished (no HCD) in over 50% of the agroinfiltrated leaves and was much weaker and in a smaller area (partial HCD) in the remaining agroinfiltrated leaves than in the EV plants, demonstrating that these four genes are essential to Cf-4/Avr4-dependent HCD. HCD in plants treated by VIGS for three genes, which encode a small GTP-binding protein (NbRas, b16), a late embryogenesis (Lea)-like protein (NbLea, b46), and an ASR4-like protein (NbASR, b67), was also significantly compromised but to a relatively weaker extent compared with the above four genes. HCD in these plants was abolished (no HCD) in over 30% of the agroinfiltrated leaves, indicating that these genes were also required for Cf-4/Avr4-dependent full HCD.
Table 3.
Effect of virus-induced gene silencing (VIGS)-inducing treatment for 14 genes on Cf-4/Avr4-dependent hypersensitive cell death (HCD)
| Only leaves producing full and nearly full HCD (refer to text and Supplementary Fig. S1) were calculated. | ||||
| Target protein spot | Target gene product | Experiment | Leaves producing HCD/total investigated agroinfiltrated leaves | Average % leaves producing HCD |
| CK | − | 1 | 42/45 | 92.2 |
| 2 | 41/45 | |||
| 5 | 1-Aminocyclopropane-1-carboxylate oxidase 1 | 1 | 20/27 | 88.9 |
| 2 | 36/36 | |||
| 6 | Phospholipid hydroperoxide glutathione peroxidase (PHGPx) | 1 | 15/21 | 89.5 |
| 2 | 36/36 | |||
| 16 | Transcription factor APFI-like | 1 | 16/21 | 80.7 |
| 2 | 30/36 | |||
| 24 | Protein disulfide isomerase | 1 | 24/24 | 83.3 |
| 2 | 31/42 | |||
| 59 | Phytophthora-inhibited protease 1 (Pip1)-like protein | 1 | 9/24 | 35.0 |
| 2 | 12/36 | |||
| 72 | Thioredoxin | 1 | 24/30 | 83.3 |
| 2 | 26/30 | |||
| b60 | LeMir-like protein | 1 | 16/30 | 48.3 |
| 2 | 13/30 | |||
| b12 | SIPK type MAP kinase similar to NbNTF4 and LeMPK1/2 | 1 | 15/42 | 42.3 |
| 2 | 18/36 | |||
| b16 | Rab1-like small GTP-binding protein | 1 | 21/30 | 60.9 |
| 2 | 21/39 | |||
| b44 | Subtilisin-like protease | 1 | 27/30 | 91.7 |
| 2 | 39/42 | |||
| b46 | Late embryogenesis (Lea)-like protein | 1 | 18/30 | 63.3 |
| 2 | 20/30 | |||
| b48 | Asparagine synthetase | 1 | 12/30 | 38.1 |
| 2 | 12/33 | |||
| b67 | ASR4-like protein | 1 | 25/36 | 69.7 |
| 2 | 21/30 | |||
| b73 | Chloroplast thiazole biosynthetic protein | 1 | 27/27 | 94.7 |
| 2 | 27/30 | |||
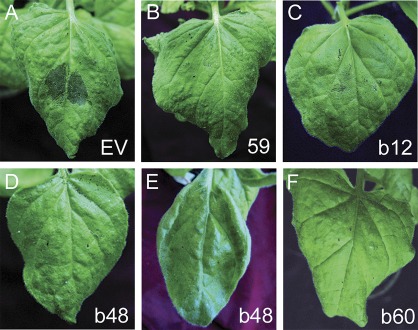
Fig. 5.
Effect of virus-induced gene silencing (VIGS)-inducing treatment for five genes on Cf-4/Avr4-dependent hypersensitive cell death in N. benthamiana plants. (A) Empty vector control (EV). Genes subjected to VIGS analyses encode (B) a Phytophthora-inhibited protease 1 (Pip1)-like protein (59), (C) a SIPK type of MAP kinase (b12), (D) an asparagine synthetase (b48), (E) a LeMir-like protein (b60), and (F) a subtilisin-like protease (b44). Plants were infiltrated with cell suspensions of Agrobacterium transformed with TRV VIGS vector carrying a fragment of the target gene or just empty vector (EV). The photographs were taken 3 days after agroinfiltration. Spot numbers at bottom right are as given in Supplementary Table S3.
RT-PCR analysis to verify gene silencing
To verify gene silencing in the VIGS-treated plants, RT-PCR analysis was conducted with the primers listed in Supplementary Table S2. Transcripts of the target genes Nbf4, NbPip1, NbAsn, and NbMir accumulated to much lower levels in the VIGS-treated plants compared with the EV plants (Fig. 6), indicating that the observed phenotypes in these plants are caused by the targeted gene silencing.
Fig. 6.
RT-PCR analysis for confirmation of the gene silencing in virus-induced gene silencing (VIGS)-treated plants. Mesophyll tissues of leaf areas corresponding to the place for agroinfiltration for induction of Cf-4/Avr4-dependent hypersensitive cell death in either a target gene-VIGS-treated N. benthamiana plants (VIGS) or an empty vector-VIGS-treated plants (CK1) were sampled for total RNA isolation 3 weeks after VIGS-inducing agroinfiltration. Products from 30 cycles of RT-PCR (CK2: without RTase) were loaded. Actin was used as an inner standard gene for loading check. Spot numbers are as given in Supplementary Table S3.
Functional validation of Pip1, asparagine synthetase, miraculin-like proteinase inhibitor, and MAP kinase in tomato
To validate the HCD-regulating function of Pip1, asparagine synthetase, and miraculin-like proteinase inhibitor in tomato, 450 bp fragments of the tomato genes homologous to NbPip1, NbAsn and NbMir, which corresponded to the identified differentially expressed tomato protein and have been functionally analysed by VIGS in N. benthamiana, were cloned and checked by sequencing (Supplementary Table S1). VIGS analysis was executed for these genes in tomato. As observed earlier for N. benthamiana genes, silencing of the tomato homologues resulted in complete loss of Cf-4/Avr4-dependent HCD in tomato plants (Fig. 7). Tomato MAP kinases LeMAP2 and LeMAP3 have been reported to play important role in Cf-4/Avr4-dependent HCD (Stulemeijer et al., 2007). To further support the similar role of the MAP kinase gene Nbf4, VIGS for this gene was conducted in tomato. The Cf-4/Avr4-dependent HCD did not occur in Nbf4 homologue-silenced tomato leaves, indicating a role of the Nbf4 tomato homologue(s) in Cf-4/Avr4-dependent HCD.
Fig. 7.
Virus-induced gene silencing analysis for function of four genes in Cf-4/Avr4-dependent hypersensitive cell death in tomato plants: (A) empty vector control (EV), (B, C) a Phytophthora-inhibited protease 1 (Pip1)-like protein (59), (D) a SIPK type of MAP kinase (b12), (E) an asparagine synthetase (b48), and (F) a LeMir-like protein (b60). The analysis is similar to that for Fig. 5 except that tomato plants were used. Spot numbers at bottom right are as given in Supplementary Table S3.
VIGS functional analysis of NbPip1, Nbf4, NbAsn, and NbMir in Cf-9-dependent and X. oryzae pv. oryzae-induced HCD in N. benthamiana
For the sake of understanding function of NbPip1, Nbf4, NbAsn, and NbMir in other Cf-dependent HCD, effect of these genes on Cf-9-dependent HCD was examined by VIGS in N. benthamiana. Silencing of these four genes blocked Cf-9-dependent HCD (Fig. 8), indicating that these genes are required for both Cf-4- and Cf-9-dependent HCD.
Fig. 8.
Virus-induced gene silencing analysis for function of four genes in Cf-9/Avr9-dependent hypersensitive cell death (HCD) in N. benthamiana plants: (A) empty vector control (EV), (B) a Phytophthora-inhibited protease 1 (Pip1)-like protein (59), (C) a SIPK type of MAP kinase (b12), (D, E) an asparagine synthetase (b48), and (F) a LeMir-like protein (b60). The analysis is similar to that for Fig. 5 except that Cf-9/Avr9-dependent HCD instead of Cf-4/Avr4-dependent HCD was checked. Spot numbers at bottom right are as given in Supplementary Table S3.
To assess the roles of NbPip1, Nbf4, NbAsn, and NbMir in HCD dependent on genes other than Cf, effect of these genes on X. oryzae pv. oryzae (Xoo)-induced HCD was investigated. Xoo is a nonadaptive pathogen to N. benthamiana and induces HCD in this nonhost plant. Strong HCD occurred at the infiltration area in leaves of normal plants and Nbf4-silenced plants within 24 hours post infiltration with Xoo suspension. However, no significant HCD was observed with leaves of NbPip1-, NbAsn-, and NbMir-silenced plants (Fig. 9). HCD in these plants was delayed at least 1–2 days. This result demonstrates that silencing of NbPip1, NbAsn, and NbMir, but not Nbf4, attenuated Xoo-induced HCD.
Fig. 9.
Virus-induced gene silencing analysis for function of four genes in X. oryzae pv. oryzae (Xoo)-induced hypersensitive cell death (HCD) in N. benthamiana plants: (A) empty vector control (EV), (B) a Phytophthora-inhibited protease 1 (Pip1)-like protein (59), (C) a SIPK type of MAP kinase (b12), (D, E) an asparagine synthetase (b48), and (F) a LeMir-like protein (b60). The analysis is similar to that for Fig. 5 except that Xoo-induced HCD instead of Cf-4/Avr4-dependent HCD was checked. Spot numbers at bottom right are as given in Supplementary Table S3.
Transient RNAi analysis for function of Pip1 in Cf-4-, Cf-9-dependent, and Xoo-induced HCD in tobacco
To confirm the VIGS results, transient RNAi analysis was further executed to investigate the function of HCD-affecting genes represented by Pip1 in Cf-4-, Cf-9-dependent, and Xoo-induced HCD in tobacco. A Pip1 RNAi construct was made for this transient RNAi analysis, which carried a hairpin sequence cassette that comprised of two copies of a 300 bp fragment of Pip1 flanking the intron sequence of the P. vulgaris nitrite reductase gene in the opposite direction. On the control halves of the leaves, all three types of HCD occurred strongly at 3 days post infiltration, while on the Pip1-RNAi-treated halves, no obvious HCD developed (Fig. 10). This confirmed what was observed earlier in VIGS experiments and once more demonstrated that Pip1 is required for Cf-4-, Cf-9-dependent, and Xoo-induced HCD.
Fig. 10.
RNAi analysis for function of Pip1 in Cf-4/Avr4- and Cf-9/Avr9-dependent hypersensitive cell death (HCD) and X. oryzae pv. oryzae-induced HCD in tobacco plants. A Pip1 RNAi construct pC1305-35S::PIP1-RNAi was made, which carried a hairpin sequence cassette that comprised of two copies of a 300bp fragment of Pip1 flanking the intron sequence of P. vulgaris nitrite reductase gene (U10419) in the opposite direction. Agrobacterium transformed with this construct was infiltrated into all sectors of left half leaves while Agrobacterium transformed with empty vector (EV) as control, into right half leaves of Sumsun NN tobacco plants. Two days after agroinfiltration, HCD in each sector of the RNAi-treated leaves was evaluated as described above.
Discussion
Genes required for Cf-4/Avr4-dependent HCD and disease resistance
The tomato−C. fulvum pathosystem is one of the model systems for studying gene-for-gene resistance (Joosten and De Wit, 1999; Rivas and Thomas, 2005; Wang et al., 2006b; Stergiopoulos and De Wit, 2009). Great effort has been made in several laboratories over the world to understand the molecular mechanisms of the Cf-dependent defence response. Several important regulators of Cf-dependent HCD and resistance have been identified. However, the signal transduction pathways leading to Cf-dependent HCD and resistance are still far from clear.
The present study employed a combined proteomic and RNAi analysis system and successfully identified a set of genes required for Cf-4/Avr4-dependent HCD, among which are a Pip1-like protein (59), a SIPK type MAP kinase (b12), an ASN (b48), and a LeMir-like protein (b60).
Pip1 is a papain-like cysteine protease. Expression of Pip1 is induced by butylhydroxytoluene (BTH) treatment and infection of pathogens (Tian et al., 2007; Shabab et al., 2008). Pip1 was found to map at the same genetic locus with another cysteine protease gene Rcr3, which mediates the recognition of Avr2 by Cf-2, initiating Cf-2-dependent defence signalling (Kruger et al., 2002; Rooney et al., 2005; Shabab et al., 2008; van Esse et al., 2008). Avr2 can inhibit both Pip1 and Rcr3, and Pip1 predominates over Rcr3, in apoplasts of BTH-treated tomato leaves (Shabab et al., 2008). Thus, it has been suggested that Rcr3 may act as a decoy to trap the fungus into a recognition event of Avr2 by Cf-2, but rather Pip1 is the operative target of Avr2 (Shabab et al., 2008; van der Hoorn and Kamoun, 2008). However, the other role of Pip1 independent of Avr2, such as in other Cf/Avr-dependent, or even Cf/Avr-independent, HCD and defence, is unclear. This study found that Pip1 protein (spot 59) is not detected in seedlings that do not develop Cf-4/Avr4-dependent HCD, but accumulate abundantly in seedlings mounting the HCD (Figs. 1 and 2). Employing VIGS analysis, the N. benthamiana homologue of the tomato Pip1 (NbPip1) was found to be required not only for Cf-4/Avr4- and Cf-9/Avr9-dependent host plant defence to fungal pathogen C. fulvum but also for Cf/Avr-independent nonhost plant defence to the bacterial pathogen Xoo (Figs. 5–10, Table 3). This phenomenon that a protease may mediate defence to distinct types of pathogens has been reported for two papain-like cysteine proteases Rcr3 and C14. Rcr3 is the target of two types of protease inhibitors, EPICs of the oomycete pathogen Phytophthora infestans and Avr2 of fungal pathogen C. fulvum, and plays a role in defence to both pathogens (Song et al., 2009). C14 is a target of even more protease inhibitors from fungi, oomycetes, and bacteria. Among them are EPICs and a RXLR effector, AvrBlb2, of P. infestans, Avr2 of C. fulvum, and RIP1 of Pseudomonas syringae (discussion in Kaschani et al., 2010, and references therein). Thus C14 has been suggested to play a general role in immunity to fungal, oomycete, and bacterial diseases (Kaschani et al., 2010). Taken together, Pip1, like C14, may play a role in plant defence against a wide range of pathogens. Plant and pathogen interactors of Pip1 remain to be further identified.
Previously, several MAP kinases have been found to be involved in regulation of Cf-9/Avr9-initiated defence responses. Two MAP kinases, WIPK (wounding-induced protein kinase) and SIPK (salicylic acid-induced protein kinase), are rapidly and transiently activated after elicitation in Cf-9 transgenic tobacco cell suspensions by Avr9 elicitor (Romeis et al., 1999). Recently, studies employing VIGS revealed that tomato MAP kinases LeMPK1/2/3 have different but also overlapping roles in Cf-4/Avr4-dependent HCD and resistance. LeMPK3, a tomato paralogue of WIPK, is essential to both Cf-4/Avr4-dependent HCD and resistance, while LeMPK1 and LeMPK2, two tomato paralogues of SIPK, are only required for Cf-4/Avr4-dependent resistance and HCD, respectively (Stulemeijer et al., 2007). This study identified a differentially expressed protein spot, b12, as being the MAP kinase LeMPK1, which accumulates to a very high level in seedlings mounting Cf-4/Avr4-dependent HCD compared with those not showing HCD (Figs. 1 and 2). To further understand the role of b12, this study cloned a cDNA fragment from N. benthamiana. This 557 bp fragment has over 97% amino acid sequence identity to the counterparts of a set of plant SIPKs, including NbNTF4, NbSIPK, Ntf4, Ntf4-1, Ntf4-2, NtSIPK, and LeMPK1/2 (Supplementary Fig. S3A). Silencing of the gene(s) corresponding to this fragment abolished not only Cf-4/Avr4- but also Cf-9/Avr9-dependent HCD in N. benthamiana (Figs. 5–8; Table 3). This result, together with the previously reported data, clearly demonstrates that SIPKs are pivotal in regulation of Cf-dependent HCD and resistance. However, unlike NbPip1, silencing of Nbf4 (b12) did not alter Xoo-induced HCD (Fig. 9), implying that the role of Nbf4 (b12) is not so wide as NbPip1.
LeMir is named after its sequence similarity (54% amino acid identity) to miraculin, a protein converting a sour into a sweet taste by altering human taste perception (Brenner et al., 1998). However, according to the BLAST database similarity search, LeMir is most homologous to a tobacco tumour-related protein (mRNA, U66263; protein, AAC49969) with 82% identity at the amino acid level. Both LeMir and its two homologues belong to the soybean trypsin-inhibitor family on the basis of the sequences; however, their proteinase-inhibitory activity remains to be confirmed (Brenner et al., 1998). LeMir is accumulated in and secreted from roots in response to nematode infection, suggesting a role of LeMir in plant defence against nematode infection (Brenner et al., 1998). Furthermore, over-expression of the tobacco homologue of LeMir results in formation of HCD-like lesions (Karrer et al., 1998), which is in accordance with the current results. This study found that LeMir is translationally up-regulated in HCD+ tomato seedlings (Figs. 1 and 2) and that silencing of a LeMir homologue (Supplementary Table S1, Supplementary Fig. S3C) significantly compromised Cf-4/Avr4- and Cf-9/Avr9-dependent HCD and Xoo-induced HCD in N. benthamiana (Figs. 5–9; Table 3). Collectively, these data show that LeMir plays an important role in the regulation of a variety of types of HCD. However, whether this function is related to its possible proteinase-inhibitory activity requires further confirmation.
Asparagine synthetase (Asn) catalyses the ATP-dependent conversion of aspartate into asparagine, which is a central intermediate in nitrogen metabolism and contributes to nitrogen transport and storage in many higher plants. Thus Asn is a key regulator of nitrogen metabolism and flow. Role of Asn in plant disease resistance is unknown except that there is a clue that an Asn gene is transcriptionally up-regulated in tomato leaves infected by the bacterial pathogen Pseudomonas syringae pv. tomato (Olea et al., 2004). The current study found that Asn protein increasingly accumulates in HCD+ tomato seedlings compared with HCD− seedlings (Figs. 1 and 2) and that silencing of a LeAsn homologue in N. benthamiana, which has 79.3% amino acid sequence identity to LeAsn (Supplementary Table S1, Supplementary Fig. S3D), significantly compromised Cf-4/Avr4- and Cf-9/Avr9-dependent HCD and Xoo-induced HCD (Figs. 5–9, Table 3), indicating that maintenance of Asn-mediated nitrogen metabolism and transport pathway is important for the establishment of these types of HCD. Additionally, the growth of NbAsn-silenced plants was significantly retarded, with smaller and narrower leaves, and later the plants developed shoots and inflorescences (Fig. 4). These results are in agreement with those obtained from plants over-expressing an Asn gene, which shows more numerous and wider leaves and precocious bolting and flowering compared with control plants (Giannino et al., 2008). Therefore, the Asn genes merit exploitation in the breeding of crops with simultaneously high disease resistance and other good agronomic traits such as high yields and a short vegetative stage.
Other proteins possibly required for Cf-4/Avr4-dependent HCD included a small GTP-binding protein (b16), a late embryogenesis (Lea)-like protein (b46), and an ASR4-like protein (b67).
Combined differential expression profiling and RNAi analysis system is an efficient strategy to identify genes required for HCD and resistance and probably other biological processes
This study employed a strategy involving combined proteomic and RNAi analyses to identify genes required for HCD and disease resistance in plants. This strategy is one example of combining differential expression profiling and RNAi assays to identify important genes involved in biological processes. RNAi assays include a common technique that requires the construction of transgenic plants with a binary vector harbouring a hairpin-resulting sequence cassette and another more rapid and straightforward technique, VIGS, which avoids plant transformation (Burch-Smith et al., 2004; Xu et al., 2008).
The combined differential expression profiling and RNAi analysis system has been used to identify factors required for Cf/Avr-dependent HCD and disease resistance. Using cDNA-AFLP as a differential gene expression profiling tool and VIGS as a RNAi tool, a set of essential regulators of Cf-9/Avr9- and Cf-4/Avr4-dependent HCD and disease resistance were successfully identified, the former identifying the protein kinase gene ACIK1 (Durrant et al., 2000; Rowland et al., 2005), two E3 ubiquitin ligase genes CMPG1 (Gonzalez-Lamothe et al., 2006) and PUB17 (Yang et al., 2006), and a F-box protein gene ACIF1 (Van den Burg et al., 2008) and the latter identifying a CC-NB-LRR type resistance protein analogue gene NRC1 (Gabriëls et al., 2006, 2007). This study executed differential expression profiling at the translational level. Finally seven regulators of Cf-4/Avr4-dependent HCD were screened out (Table 3, Figs. 5–10).
The expression profiles for Cf-4/Avr4-dependent HCD at the protein level (this study) and the transcript level (Gabriëls et al., 2006; Hong et al., 2007; Zhu et al., 2008) are not identical. This could be because some genes are highly transcribed but are somehow translated less or not at all. In addition, this difference could be due to the experimental design: i.e., for some differentially expressed protein spots detected by 2-D PAGE analysis, the corresponding transcripts might be not identified by the previous cDNA-AFLP analysis because of the restriction enzymes (only one) used for this analysis (Hong et al., 2007; Zhu et al., 2008). Similarly, for some differentially expressed transcripts detected by cDNA-AFLP analysis, the corresponding proteins might not have been identified by 2-D PAGE analysis in this study because of the pH range of IPG (pH 3−7) and staining method (CBB) used for this analysis. Taken together, the combination of proteomic analysis and VIGS assay to identify genes required for Cf/Avr-dependent HCD in this study is still valuable, has been proved to be efficient, and is complementary to the previously employed combined cDNA-AFLP analysis and VIGS assay to identify new important components of Cf/Avr-dependent HCD.
Collectively, the current and others’ data demonstrate that differential expression profiling and RNAi analysis system is an efficient strategy to identify novel essential HCD and disease resistance regulators. Additionally, the major techniques included in the strategy are differential expression profiling analysis and RNAi (including VIGS) assay, which have been more and more extensively employed to dissect many other plant biological processes such as insect resistance, stress, development, and metabolism. In fact, this study found two genes, which encode a proteasome 20S beta 1.1 subunit and a BiP, that might be involved in regulation of plant cell death, and three genes, which encode a SIPK type MAP kinase, an ASN, and an ASR-like protein, that might be related with plant growth and development (Fig. 4, Table 2). Therefore, the combined differential expression profiling. The RNAi analysis system is potentially a versatile strategy to dissect a variety of plant biological processes.
Supplementary material
Supplementary data are available at JXB online.
Supplementary Table S1. Cloned tomato and N. benthamiana cDNA fragments corresponding to the differentially expressed proteins selected for VIGS functional analysis.
Supplementary Table S2. PCR primers used for amplification of the VIGS target gene fragments.
Supplementary Table S3. List of the protein spots differentially expressed between Cf-4/Avr4 (HCD+) and Cf-4 (HCD−) tomato seedlings and identified by MALDI-TOF or MALDI-TOF/TOF MS analyses.
Supplementary Fig. S1. HCD phenotypes.
Supplementary Fig. S2. The MS spectra data for the differentially expressed protein spots.
Supplementary Fig. S3. Alignments of amino acid sequences predicted from the cloned N. benthamiana fragments with the counterparts of the reported homologous sequences from tomato, N. tabacum and N. benthamiana.
Acknowledgments
The authors are grateful to Prof Pierre JGM de Wit (Wageningen University, The Netherlands) for providing seeds of tomato lines MM-Cf-4 and MM-Cf-9 and Dr SP Dinesh-Kumar (Yale University, USA) for providing the TRV silencing vector. They also thank the 985-Institute of Agrobiology and Environmental Sciences of Zhejiang University for providing the experimental equipment. This work was financially supported by grants from the National Basic Research Program of China (no. 2009CB119000), the genetically modified organisms breeding major projects (no. 2009ZX08009-044B), the National Natural Science Foundation of China (no. 30871608 and 30671352), the PCSIRT project (no. IRT0943), the NCET project (no. NCET-08-0485), and the Fundamental Research Funds for the Central Universities (no. 2011XZZX006).
References
- Blatt MR, Grabov A, Brearley J, Hammond-Kosack KE, Jones JDG. K+ channels of Cf-9 transgenic tobacco guard cells as targets for Cladosporium fulvum Avr9 elicitor-dependent signal transduction. The Plant Journal. 1999;19:453–462. doi: 10.1046/j.1365-313x.1999.00534.x. [DOI] [PubMed] [Google Scholar]
- Brenner ED, Lambert KN, Kaloshian I, Williamson VM. Characterization of LeMir, a root-knot nematode-induced gene in tomato with an encoded product secreted from the root. Plant Physiology. 1998;118:237–247. doi: 10.1104/pp.118.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch-Smith TM, Anderson JC, Martin GB, Dinesh-Kumar SP. Applications and advantages of virus-induced gene silencing for gene function studies in plants. The Plant Journal. 2004;39:734–746. doi: 10.1111/j.1365-313X.2004.02158.x. [DOI] [PubMed] [Google Scholar]
- Cai X, Takken FLW, Joosten MHAJ, De Wit PJGM. Specific recognition of AVR4 and AVR9 results in distinct patterns of hypersensitive cell death in tomato, but similar patterns of defence-related gene expression. Molecular Plant Pathology. 2001;2:77–86. doi: 10.1046/j.1364-3703.2001.00053.x. [DOI] [PubMed] [Google Scholar]
- Cai XZ, Wang CC, Xu YP, Xu QF, Zheng Z, Zhou XP. Efficient gene silencing induction in tomato by a viral satellite DNA vector. Virus Research. 2007;125:169–175. doi: 10.1016/j.virusres.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Damerval C, de Vienne D, Zivy M, Thiellement H. The technical improvements in two-dimensional electrophoresis increase the level of genetic variation detected in wheat-seedling proteins. Electrophoresis. 1986;7:52–54. [Google Scholar]
- De Jong CF, Honée G, Joosten MHAJ, De Wit PJGM. Early defence responses induced by AVR9 and mutant analogues in tobacco cell suspensions expressing the Cf-9 resistance gene. Physiological and Molecular Plant Pathology. 2000;56:169–177. [Google Scholar]
- De Jong CF, Takken FLW, Cai X, De Wit PJGM, Joosten MHAJ. Attenuation of Cf-mediated defence responses at elevated temperatures correlates with a decrease in elicitor-binding sites. Molecular Plant–Microbe Interactions. 2002;15:1040–1049. doi: 10.1094/MPMI.2002.15.10.1040. [DOI] [PubMed] [Google Scholar]
- De Wit PJGM. How plants recognize pathogens and defend themselves. Cellular and Molecular Life Sciences. 2007;64:2726–2732. doi: 10.1007/s00018-007-7284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DP, Hawkins T, Hussey PJ, Edwards R. Enzyme activities and subcellular localization of members of the Arabidopsis glutathione transferase superfamily. Journal of Experimental Botany. 2009;60:1207–1218. doi: 10.1093/jxb/ern365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon MS, Jones DA, Keddie JS, Thomas CM, Harrison K, Jones JDG. The tomato Cf-2 disease resistance locus comprises two functional genes encoding leucine-rich repeat proteins. Cell. 1996;84:451–459. doi: 10.1016/s0092-8674(00)81290-8. [DOI] [PubMed] [Google Scholar]
- Durrant WE, Rowland O, Piedras P, Hammond-Kosack KE, Jones JDG. cDNA-AFLP reveals a striking overlap in race specific resistance and wound response gene expression profiles. The Plant Cell. 2000;12:963–977. doi: 10.1105/tpc.12.6.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriëls SHEJ, Takken FLW, Vossen JH, et al. cDNA-AFLP combined with functional analysis reveals novel genes involved in the hypersensitive response. Molecular Plant–Microbe Interactions. 2006;19:567–576. doi: 10.1094/MPMI-19-0567. [DOI] [PubMed] [Google Scholar]
- Gabriëls SHEJ, Vossen JH, Ekengren SK, et al. An NB-LRR protein required for HR signalling mediated by both extra- and intracellular resistance proteins. The Plant Journal. 2007;50:14–28. doi: 10.1111/j.1365-313X.2007.03027.x. [DOI] [PubMed] [Google Scholar]
- Giannino D, Nicolodi C, Testone G, Frugis G, Pace E, Santamaria P, Guardasole M, Mariotti D. The overexpression of asparagine synthetase A from E. coli affects the nitrogen status in leaves of lettuce (Lactuca sativa L.) and enhances vegetative growth. Euphytica. 2008;162:11–22. [Google Scholar]
- Gonzalez-Lamothe R, Tsitsigiannis DI, Ludwig AA, Panicot M, Shirasu K, Jones JDG. The U-Box protein CMPG1 is required for efficient activation of defence mechanisms triggered by multiple resistance genes in tobacco and tomato. The Plant Cell. 2006;18:1067–1083. doi: 10.1105/tpc.106.040998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W, Xu YP, Zheng Z, Cao JS, Cai XZ. Comparative transcript profiling by cDNA-AFLP reveals similar patterns of Avr4/Cf-4- and Avr9/Cf-9-dependent defence gene expression. Molecular Plant Pathology. 2007;8:515–527. doi: 10.1111/j.1364-3703.2007.00412.x. [DOI] [PubMed] [Google Scholar]
- Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Jones DA, Thomas CM, Hammond-Kosack KE, Balint-Kurti PJ, Jones JDG. Isolation of the tomato Cf-9 gene for resistance to Cladosporium fulvum by transposon tagging. Science. 1994;266:789–793. doi: 10.1126/science.7973631. [DOI] [PubMed] [Google Scholar]
- Joosten MHAJ, Cozijnsen TJ, De Wit PJGM. Host resistance to a fungal tomato pathogen lost by a single base-pair change in an avirulence gene. Nature. 1994;367:384–386. doi: 10.1038/367384a0. [DOI] [PubMed] [Google Scholar]
- Joosten MHAJ, De Wit PJGM. The tomato–Cladosporium fulvum interaction: a versatile experimental system to study plant–pathogen interactions. Annual Review of Phytopathology. 1999;37:335–367. doi: 10.1146/annurev.phyto.37.1.335. [DOI] [PubMed] [Google Scholar]
- Karrer EE, Beachy RN, Holt CA. Cloning of tobacco genes that elicit the hypersensitive response. Plant Molecular Biology. 1998;36:681–690. doi: 10.1023/a:1005949304445. [DOI] [PubMed] [Google Scholar]
- Kaschani F, Shabab M, Bozkurt T, Shindo T, Schornack S, Gu C, Ilyas M, Win J, Kamoun S, van der Hoorn RAL. An effector-targeted protease contributes to defence against Phytophthora infestans and is under diversifying selection in natural hosts. Plant Physiology. 2010;154:1794–1804. doi: 10.1104/pp.110.158030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger J, Thomas CM, Golstein C, Dixon MS, Smoker M, Tang S, Mulder L, Jones JDG. A tomato cysteine protease required for Cf-2-dependent disease resistance and suppression of autonecrosis. Science. 2002;296:744–747. doi: 10.1126/science.1069288. [DOI] [PubMed] [Google Scholar]
- Luderer R, Takken FLW, De Wit PJGM, Joosten MHAJ. Cladosporium fulvum overcomes Cf-2-mediated resistance by producing truncated AVR2 elicitor proteins. Molecular Microbiology. 2002;45:875–884. doi: 10.1046/j.1365-2958.2002.03060.x. [DOI] [PubMed] [Google Scholar]
- Olea F, Perez-Garcia A, Canton FR, Rivera ME, Canas R, Avila C, Cazorla FM, Canovas FM, de Vicente A. Up-regulation and localization of asparagine synthetase in tomato leaves infected by the bacterial pathogen Pseudomonas syringae. Plant and Cell Physiology. 2004;45:770–780. doi: 10.1093/pcp/pch092. [DOI] [PubMed] [Google Scholar]
- Parisy V, Poinssot1 B, Owsianowski L, Buchala A, Glazebrook J, Mauch F. Identification of PAD2 as a c-glutamylcysteine synthetase highlights the importance of glutathione in disease resistance of Arabidopsis. The Plant Journal. 2007;49:159–172. doi: 10.1111/j.1365-313X.2006.02938.x. [DOI] [PubMed] [Google Scholar]
- Piedras P, Hammond-Kosack KE, Harrison K, Jones JDG. Rapid, Cf-9- and Avr9-dependent production of active oxygen species in tobacco suspension cultures. The Plant Cell. 1998;11:1155–1166. [Google Scholar]
- Rivas S, Rougon-Cardoso A, Smoker M, Schauser L, Yoshioka H, Jones JDG. CITRX thioredoxin interacts with the tomato Cf-9 resistance protein and negatively regulates defence. The EMBO Journal. 2004;23:2156–2165. doi: 10.1038/sj.emboj.7600224. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rivas S, Thomas CM. Molecular interactions between tomato and the leaf mold pathogen Cladosporium fulvum. Annual Review of Phytopathology. 2005;43:395–436. doi: 10.1146/annurev.phyto.43.040204.140224. [DOI] [PubMed] [Google Scholar]
- Romeis T, Ludwig AA, Martin R, Jones JDG. Calcium-dependent protein kinases play an essential role in a plant defence response. The EMBO Journal. 2001;20:5556–5567. doi: 10.1093/emboj/20.20.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis T, Piedras P, Jones JDG. Resistance gene-dependent activation of a calcium-dependent protein kinase in the plant defence response. The Plant Cell. 2000;12:803–815. doi: 10.1105/tpc.12.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis T, Piedras P, Zhang S, Klessig DF, Hirt H, Jones JDG. Rapid Avr9- and Cf-9-dependent activation of MAP kinases in tobacco cell cultures and leaves: convergence of resistance gene, elicitor, wound, and salicylate responses. The Plant Cell. 1999;11:273–287. doi: 10.1105/tpc.11.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney HCE, van ‘t Klooster JW, van der Hoorn RAL, Joosten MHAJ, Jones JDG, de Wit PJGM. Cladosporium Avr2 inhibits tomato Rcr3 protease required for Cf-2-dependent disease resistance. Science. 2005;308:1783–1786. doi: 10.1126/science.1111404. [DOI] [PubMed] [Google Scholar]
- Rowland O, Ludwig AA, Merrick CJ, et al. Functional analysis of Avr9/Cf-9 rapidly elicited genes identifies a protein kinase, ACIK1, that is essential for full Cf-9-dependent disease resistance in tomato. The Plant Cell. 2005;17:295–310. doi: 10.1105/tpc.104.026013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabab M, Shindo T, Gu C, Kaschani F, Pansuriya T, Chintha R, Harzen A, Colby T, Kamoun S, van der Hoorn RAL. Fungal effector protein AVR2 targets diversifying defence-related Cys proteases of tomato. The Plant Cell. 2008;20:1169–1183. doi: 10.1105/tpc.107.056325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Win J, Tian M, Schornack S, Kaschani F, Ilyas M, van der Hoorn RA, Kamoun S. Apoplastic effectors secreted by two unrelated eukaryotic plant pathogens target the tomato defence protease Rcr3. Proceedings of the National Academy of Sciences USA. 2009;106:1654–1659. doi: 10.1073/pnas.0809201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiopoulos I, de Wit PJGM. Fungal effector proteins. Annual Review of Phytopathology. 2009;47:233–263. doi: 10.1146/annurev.phyto.112408.132637. [DOI] [PubMed] [Google Scholar]
- Stulemeijer IJE, Joosten MHAJ, Jensen ON. Quantitative phosphoproteomics of tomato mounting a hypersensitive response reveals a swift suppression of photosynthetic activity and a differential role for Hsp90 isoforms. Journal of Proteome Research. 2009;8:1168–1182. doi: 10.1021/pr800619h. [DOI] [PubMed] [Google Scholar]
- Stulemeijer IJE, Stratmann JW, Joosten MHAJ. Tomato mitogen-activated protein kinases LeMPK1, LeMPK2, and LeMPK3 are activated during the Cf-4/Avr4-induced hypersensitive response and have distinct phosphorylation specificities. Plant Physiology. 2007;144:1481–1494. doi: 10.1104/pp.107.101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suckau D, Resemann A, Schuerenberg M, Hufnagel P, Franzen J, Holle A. A novel MALDI LIFT-TOF/TOF mass spectrometer for proteomics. Analytical and Bioanalytical Chemistry. 2003;376:952–965. doi: 10.1007/s00216-003-2057-0. [DOI] [PubMed] [Google Scholar]
- Takken FLW, Schipper D, Nijkamp HJJ, Hille J. Identification and Ds-tagged isolation of a new gene at the Cf-4 locus of tomato involved in disease resistance to Cladosporium fulvum race 5. The Plant Journal. 1998;14:401–411. doi: 10.1046/j.1365-313x.1998.00135.x. [DOI] [PubMed] [Google Scholar]
- Thomas CM, Jones DA, Parniske M, Harrison K, Balint-Kurti PJ, Hatzixanthis K, Jones JDG. Characterization of the tomato Cf-4 gene for resistance to Cladosporium fulvum identifies sequences that determine recognitional specificity in Cf-4 and Cf-9. The Plant Cell. 1997;9:2209–2224. doi: 10.1105/tpc.9.12.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CM, Tang S, Hammond-Kosack K, Jones JDG. Comparison of the hypersensitive response induced by the Cf-4 and Cf-9 genes in Nicotiana spp. Molecular Plant–Microbe Interactions. 2000;13:465–469. doi: 10.1094/MPMI.2000.13.4.465. [DOI] [PubMed] [Google Scholar]
- Tian M, Win J, Song J, van der Hoorn RAL, van der Knaap E, Kamoun S. A Phytophthora infestans cystatin-like protein targets a novel tomato papain-like apoplastic protease. Plant Physiology. 2007;143:364–377. doi: 10.1104/pp.106.090050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Ackerveken GFJM, Van Kan JAL, De Wit PJGM. Molecular analysis of the avirulence gene avr9 of the fungal tomato pathogen Cladosporium fulvum fully supports the gene-for-gene hypothesis. The Plant Journal. 1992;2:359–366. doi: 10.1111/j.1365-313x.1992.00359.x. [DOI] [PubMed] [Google Scholar]
- Van den Burg HA, Tsitsigiannis DI, Rowland O, Lo J, Rallapalli G, MacLean D, Takken FLW, Jones JDG. The F-box protein ACRE189/ACIF1 regulates cell death and defence responses activated during pathogen recognition in tobacco and tomato. The Plant Cell. 2008;20:697–719. doi: 10.1105/tpc.107.056978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoorn RA, Kamoun S. From guard to decoy: a new model for perception of plant pathogen effectors. The Plant Cell. 2008;20:2009–2017. doi: 10.1105/tpc.108.060194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Hoorn RA, Laurent F, Roth R, De Wit PJGM. Agroinfiltration is a versatile tool that facilitates comparative analyses of Avr9/Cf-9-induced and Avr4/Cf-4-induced necrosis. Molecular Plant–Microbe Interactions. 2000;13:439–446. doi: 10.1094/MPMI.2000.13.4.439. [DOI] [PubMed] [Google Scholar]
- van Esse HP, van’t Klooster JW, Bolton MD, Yadeta KA, Van Baarlen P, Boeren S, Vervoort J, de Wit PJGM, Thomma BPHJ. The Cladosporium fulvum virulence protein Avr2 inhibits host proteases required for basal defence. The Plant Cell. 2008;20:1948–1963. doi: 10.1105/tpc.108.059394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Cai X, Wang X, Zheng Z. Optimisation of tobacco rattle virus-induced gene silencing in Arabidopsis. Functional Plant Biology. 2006a;33:347–355. doi: 10.1071/FP05096. [DOI] [PubMed] [Google Scholar]
- Wang CC, Cai XZ, Xu YP. Molecular mechanism of interaction between tomato and leaf mold pathogen Cladosporium fulvum. Acta Phytopathologica Sinica. 2006b;36:385–391. [Google Scholar]
- Wang C, Cai X, Zheng Z. High humidity represses Cf-4/Avr4- and Cf-9/Avr9-dependent hypersensitive cell death and defence gene expression. Planta. 2005;222:947–956. doi: 10.1007/s00425-005-0036-8. [DOI] [PubMed] [Google Scholar]
- Westerink N, Brandwagt BF, de Wit PJGM, Joosten MHAJ. Cladosporium fulvum circumvents the second functional resistance gene homologue at the Cf-4 locus (Hcr9–4E) by secretion of a stable avr4E isoform. Molecular Microbiology. 2004;54:533–545. doi: 10.1111/j.1365-2958.2004.04288.x. [DOI] [PubMed] [Google Scholar]
- Xu YP, Xu QF, Song XY, Zhang ZX, Cai XZ. Virus-induced gene silencing. Journal of Zhejiang University (Agric and Life Sci) 2008;34:119–131. [Google Scholar]
- Xu YP, Zheng LP, Xu QF, Wang CC, Zhou XP, Wu ZJ, Cai XZ. Efficiency for gene silencing induction in Nicotiana species by a viral satellite DNA vector. Journal of Integrative Plant Biology. 2007;49:1726–1733. [Google Scholar]
- Yan JX, Wait R, Berkelman T, Harry RA, Westbrook JA, Wheeler CH, Dunn MJ. A modified silver staining protocol for visualization of proteins compatible with matrix-assisted laser desorption/ionization and elecctrospray ionizationmass spectrometry. Electrophoresis. 2000;21:3666–3672. doi: 10.1002/1522-2683(200011)21:17<3666::AID-ELPS3666>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Yang CW, Gonzalez-Lamothe R, Ewan RA, Rowland O, Yoshioka H, Shenton M, Ye H, O’Donnell E, Jones JDG, Sadanandom A. The E3 ubiquitin ligase activity of Arabidopsis PLANT U-BOX17 and its functional tobacco homolog ACRE276 are required for cell death and defence. The Plant Cell. 2006;18:1084–1098. doi: 10.1105/tpc.105.039198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JW, Xu YP, Zhang ZX, Cao WY, Cai XZ. Transcript profiling for Avr4/Cf-4- and Avr9/Cf-9-dependent defence gene expression. European Journal of Plant Pathology. 2008;122:307–314. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.