Abstract
Soil sodium, while toxic to most plants at high concentrations, can be beneficial at low concentrations, particularly when potassium is limiting. However, little is known about Na+ uptake in this ‘high-affinity’ range. New information is provided here with an insight into the transport characteristics, mechanism, and ecological significance of this phenomenon. High-affinity Na+ and K+ fluxes were investigated using the short-lived radiotracers 24Na and 42K, under an extensive range of measuring conditions (variations in external sodium, and in nutritional and pharmacological agents). This work was supported by electrophysiological, compartmental, and growth analyses. Na+ uptake was extremely sensitive to all treatments, displaying properties of high-affinity K+ transporters, K+ channels, animal Na+ channels, and non-selective cation channels. K+, NH4+, and Ca2+ suppressed Na+ transport biphasically, yielding IC50 values of 30, 10, and <5 μM, respectively. Reciprocal experiments showed that K+ influx is neither inhibited nor stimulated by Na+. Sodium efflux constituted 65% of influx, indicating a futile cycle. The thermodynamic feasibility of passive channel mediation is supported by compartmentation and electrophysiological data. Our study complements recent advances in the molecular biology of high-affinity Na+ transport by uncovering new physiological foundations for this transport phenomenon, while questioning its ecological relevance.
Keywords: Barley, compartmental analysis, electrophysiology, high-affinity Na+ uptake, ion transport, pharmacology, potassium, radiotracers, salinity, sodium
Introduction
The kinetics and mechanism of sodium transport into plant roots were first investigated in detail by Rains and Epstein (1965, 1967a, b), who demonstrated that transport of Na+ into roots of barley (Hordeum vulgare L.) has a biphasic character. Sodium fluxes at low external Na+ concentrations (typically, [Na+]ext <1 mM) were shown to be mediated by a high-affinity transport system (HATS) that was suppressible by both Ca2+ and K+, influenced by the counterion (e.g. Cl−, F−, ), and affected by temperature and a small range of metabolic inhibitors. At higher [Na+]ext, including the range at which plants encounter sodium toxicity, a low-affinity system (LATS), was shown to operate. This type of biphasic flux pattern has been well documented in plants with respect to other important ions, including potassium (Szczerba et al., 2009), ammonium (Jackson et al., 2008), and nitrate (Jackson et al., 2008).
Largely because of the urgent agronomic importance of salinity stress, the high-affinity system received little attention relative to the LATS for three decades following these discoveries (Rodríguez-Navarro and Rubio, 2006). Nevertheless, at low to moderate concentrations, sodium is commonly found to be a benign and even beneficial element (Marschner, 1995; Subarrao et al., 2003), which can stimulate growth in many plant species, particularly when individuals are K-deprived (Hawker et al., 1974; Marschner, 1995; Subarrao et al., 2003; Horie et al., 2007). In addition, sodium is a required micronutrient for the growth of many plant species possessing the C4 photosynthetic pathway (Brownell and Bielig, 1996). Moreover, the understanding of high-affinity Na+ transport may provide important clues as to the nature of Na+ fluxes in the toxic range (Kronzucker and Britto, 2011).
Interest in the sodium HATS has been substantially revived in recent years with the cloning and expression of genes belonging to the HKT family. Initially believed to represent high-affinity K+ transporters (Schachtman and Schroeder, 1994), HKTs now appear to be more closely involved with Na+ transport in both high- and low-affinity ranges, and may function either via a Na+ uniport mechanism or by K+/Na+ symport (note: the high functional diversity of HKT transporters should be kept in mind; see reviews by Rodríguez-Navarro and Rubio, 2006; Huang et al., 2008; Munns and Tester, 2008; Corratge-Faillie et al., 2010; Kronzucker and Britto, 2011). Consistent with both the sodium–potassium inhibition studies of Rains and Epstein (1965, 1967b), and with the role proposed for Na+ as an ion that can partially substitute for K+, it has been shown that expression of HKT genes, and HKT mediation of Na+ transport into plant roots, are suppressed by the presence of K+ in solution (Wang et al., 1998; Garciadeblás et al., 2003; Horie et al., 2007; Haro et al., 2010). Conversely, in several plant species, the enhancement of HKT mRNA levels has been observed in response to K+ starvation (Wang et al., 1998; Laurie et al., 2002; Garciadeblás et al., 2003; Mian et al., 2011; also see Buschmann et al., 2000).
While ion channels of varying classes, especially non-selective cation channels (NSCCs) have been strongly implicated in the transport of Na+ into plant roots in the toxic range (Munns and Tester, 2008; Kronzucker and Britto, 2011), their contribution to high-affinity Na+ fluxes should not be ruled out. Although channels are generally considered to catalyse fluxes from millimolar concentrations, it has been demonstrated that the K+ channel AKT1 can transport K+ into root cells from external concentrations as low as 10 μM (Hirsch et al., 1998; Spalding et al., 1999). Conversely, HKT transporters have been reported to display channel-like properties (Lan et al., 2010; Xue et al., 2011), and to have evolved from bacterial K+ channels (Durell et al., 1999), which partially explains their ability to operate in the low-affinity range of Na+ transport, not just the high-affinity range (Munns and Tester, 2008).
Despite these and other recent advances, the physiological basis and significance of high-affinity Na+ transport system in higher plants is still not well understood (Rodríguez-Navarro and Rubio, 2006; Haro et al., 2010), and some fundamental questions about the system’s characteristics remain open. These questions fall into two major areas of interest: (i) the kinetics and mechanism of this transport system, and (ii) its nutritional and ecological significance. In the present work, existing knowledge of the physiological and ecophysiological foundations of high-affinity Na+ transport in these two areas has been extended, chiefly by use of tracer-flux, electrophysiological, and compartmental analyses in the model system barley.
Materials and methods
Plant culture
Barley seeds (Hordeum vulgare L. cv. ‘A.C. Metcalfe’) were surface-sterilized for 10 min in 1% sodium hypochlorite followed by a 3 h rinse with distilled water. Seeds were germinated for 3 d in acid-washed sand prior to placement for 4 d in 12 l aerated hydroponic vessels, containing either: simple solutions of NaCl (100 μM) or CaSO4 (200 μM, removed prior to influx measurement), or a modified, complete Hoagland’s solution: 5 mM KNO3, 0.25 mM KH2PO4, 2 mM MgSO4, 0.1 mM Ca(NO3)2, 0.2 μM Na2MoO4, 50 μM FeSO4, 70 μM H3BO3, 14 μM MnCl2, 1 μM ZnSO4, 0.5 μM CuSO4 , and 100 μM NaCl. The pH was adjusted to 6.3–6.4 by adding KOH or H2SO4. To prevent nutrient depletion, solutions were exchanged on days 3 and 5. Plants were cultured in climate-controlled walk-in growth chambers under fluorescent lights with an irradiation, at plant height, of 200 μmol photons m−2 s−1 (Philips Silhouette High Output F54T5/850HO; Philips Electronics Ltd., Markham, ON, Canada). A 16 h photoperiod with day and night temperatures of 20 °C and 15 °C, respectively, was used. The relative humidity was 60%. For flux experiments, plants were bundled in groups of 6–8 at the base of the shoot with a plastic collar, 0.5 cm in height, one day prior to measurement.
Flux analysis
Unidirectional influx was determined over a 5 min uptake period, as described in detail previously (Kronzucker et al., 1999; Szczerba et al., 2006). In brief, roots of intact, bundled seedlings were immersed in 240 ml labelling vessels containing growth solution and one of two γ-emitting radioisotopes, 24Na+ or 42K+ (McMaster University Nuclear Reactor, Hamilton, ON, Canada), followed by a 5 s dip and 5 min desorption in non-radioactive growth solution. Unless otherwise noted, flux experiments were conducted with plants grown in simple solutions of 100 μM NaCl. For the experiment presented in Table 2, inhibitors were applied 10 min prior to the uptake step, and over its duration, at the following concentrations: KCN+SHAM (10 mM each); KCl (5 mM); (NH4)2SO4 (5 mM); CaCl2 (10 mM); tetraethylammonium chloride (TEACl, 10 mM); BaCl2 (5 mM); CsCl (10 mM); LaCl3 (50 μM); GdCl3 (50 μM and 5 mM); quinine (1 mM), lidocaine (as lidocaine hydrochloride monohydrate, 10 mM), and dopamine (as dopamine hydrochloride, 5 mM). Chemicals were obtained from Sigma-Aldrich Co. (St Louis, MO, USA) and Alfa Aesar (Ward Hill, MA, USA). In the pH 4.2 treatment, H2SO4 was used to lower the pH, while the 4 °C condition was maintained by immersion of uptake vessels in an ice water bath. Concentration of inhibitors and exposure times were chosen according to published protocols (Demidchik et al., 2002; Essah et al., 2003).
Table 2.
Effects of pharmacological, physical, and nutritional treatments on high-affinity Na+ influx in intact barley seedlings grown at 100 μM, and measured at 10 and 100 μM Na+.Treatments were applied 10 min prior to, and during, the 5 min labelling step, dip (5 s), and desorption (5 min). Bottom of table: Na+ influx measurements at 10 μM Na+ external in plant grown at 0.2 mM Ca2+, where the Ca2+ is withdrawn prior to the uptake. Measurements significantly different from the control are designated with a *, P <0.05. Values represent mean ±SEM (n=4–19).
| Treatment (measured at 100 μM Na+) | Na+ influx (nmol g−1 fw h−1) | Suppression (%) |
| Control | 1124.7±18.3 | 0 |
| CN−+SHAM (10 mM) | 61.8*±2.2 | 94.5 |
| pH 4.2 | 238.7*±11.9 | 78.8 |
| 4 °C | 218.7*±9.0 | 80.6 |
| K+ (5 mM) | 87.4*±3.7 | 92.2 |
| NH4+ (5 mM) | 71.2*±4.7 | 93.7 |
| Ca2+ (5 mM) | 91.1*±4.4 | 91.9 |
| TEA+ (10 mM) | 150.3*±5.5 | 86.6 |
| Ba2+ (5 mM) | 118.1*±4.9 | 89.5 |
| Cs+ (10 mM) | 88.6*±3.9 | 92.1 |
| La3+ (50 μM) | 293.3*±17.1 | 73.9 |
| Gd3+ (50 μM) | 211.0*±6.3 | 81.2 |
| Gd3+ (5 mM) | 213.3*±12.6 | 81.0 |
| Zn2+ (10 mM) | 85.8*±3.9 | 92.4 |
| Quinine (1 mM) | 134.1*±14.4 | 88.1 |
| Lidocaine (10 mM) | 101.9*±3.2 | 90.9 |
| Dopamine (5 mM) | 158.1*±11.6 | 85.9 |
|
Treatment (measured at 10 μM Na+) | ||
| Control | 593.4±17.0 | 0 |
| Gd3+ (50 μM) | 334.0*±12.7 | 43.7 |
| TEA+ (10 mM) | 259.1*±8.6 | 56.3 |
| Cs+ (10 mM) | 102.4*±4.0 | 82.7 |
| Lidocaine (10 mM) | 90.5*±2.2 | 84.7 |
Compartmental analysis by tracer efflux was conducted as previously published (Siddiqi et al., 1991; Malagoli et al., 2008), with minor modifications. In brief, roots of intact, bundled seedlings were first immersed for 1 h in growth medium containing the radiotracer 24Na+. Seedlings were then attached to efflux funnels, in which roots were eluted of radioactivity by a series of 36 aliquots of non-labelled growth solution. The desorption series was timed as follows: 15 s (×4), 20 s (×3), 30 s (×2), 40 s (×2), and 60 s (×25). Solutions were continuously mixed and aerated by a fine stream of air bubbles. In efflux protocols involving amiloride treatment, the amiloride was applied 19.5 min into the desorption series, as amiloride hydrochloride (0.1 mM; Cuin et al., 2011).
In all flux protocols, plant roots were detached from shoots immediately after labelling and desorption, and spun in a low-speed centrifuge for 30 s to remove surface water prior to weighing. Radioactivity in all plant parts and eluates was measured by gamma counting using one of two γ-counters (Canberra-Packard Quantum Cobra Series II, model 5003, Packard Instrument Co., Meriden, CT, USA; PerkinElmer Wallac 1480 Wizard 3”, Turku, Finland), and corrected for isotopic decay.
Tissue Na+ content
To determine Na+ tissue content, roots of 7-d-old barley seedlings, grown in simple solutions of 0.01, 0.1, 1, 5, 10, 25, or 50 mM NaCl, were desorbed for 5 min in 10 mM CaSO4, to remove excess Na+ from the apoplast. Shoots and roots were separated, weighed, and oven-dried for a minimum of 3 d in a drying oven at 80–85 °C, then reweighed, pulverized, and acid-digested with 30% nitric acid for three more days. Na+ tissue content was measured with a single-channel flame photometer (Digital Flame Analyzer model 2655-00; Cole Parmer; Cole-Parmer, Anjou, QC, Canada).
Electrophysiological measurements
All electrical measurements were performed in a grounded Faraday cage, using intact roots of plants grown in a simple solution of 100 μM NaCl. A single seminal root of a seedling (aged 7–8 d) was isolated and positioned over platinum pins in a narrow Plexiglas chamber initially containing 100 μM NaCl. The seedling’s other roots were immersed in a sub-chamber adjacent to and continuous with the chamber containing the isolated root. This apparatus (total volume=125 ml) was installed on the stage of an inverted Leica DME microscope (Leica Microsystems Inc., Concord, Ontario, Canada). Impalements were made with a micromanipulator (SD instruments; MX310R, Siskiyou Corporation, Grants Pass, Oregon, USA) under 100× magnification. The root was secured by small foam-rubber plugs to avoid movement during impalement or solution exchange. Impalements were made with an electrode in a grounded, aerated bathing solution, 2–3 cm from the root tip. A reference electrode was placed adjacent to the impalement. Borosilicate glass pipettes (I.D.=0.75 mm; O.D.=1.0 mm) from World Precision Instruments Inc. (World Precision Instruments Inc., Sarasota, Florida, USA) were used to make microelectrodes (tip diameter <1 μm) with an electrode puller (Sutter Instrument Co.; P-30). The microelectrodes (R=14.6±0.5 MΩ) were filled with 3 M KCl solution (pH=2 to reduce tip potential). Both the impaling and the reference electrodes were made in this manner. A solenoid coil (World Precision Instruments Inc. Sarasota, Florida, USA) was positioned around the reference electrode to reduce electrical interference. Membrane potential measurements were made with an electrometer (World Precision Instruments Inc.; Duo 773) and recorded on an oscilloscope (Tektronix; TDS2002B, Tektronix Inc., Beaverton, Oregon, USA). Once a steady reading was obtained, treatment solution was perfused through 1/16-inch Tygon tubing via a peristaltic pump at a rate of approximately 7.5 ml min−1. Each treatment solution consisted of a single salt in a background of 0.1 mM NaCl. The salt concentrations of individual treatments were 30 μM KCl, 50 μM KCl, 5 μM (NH4)2SO4, 30 μM (NH4)2SO4, 5 μM CaCl2, or 30 μM CaCl2.
Statistics
For flux measurements, each plant bundle was treated as a single replicate. Direct influx measurements were compared by ANOVA followed by the Tukey post-hoc test. Traces of efflux runs following amiloride application were compared with control traces using a Student’s t test. Statistical values and Michaelis–Menten parameters were estimated by the use of GraphPad Prism statistical software (v. 5.01).
Results
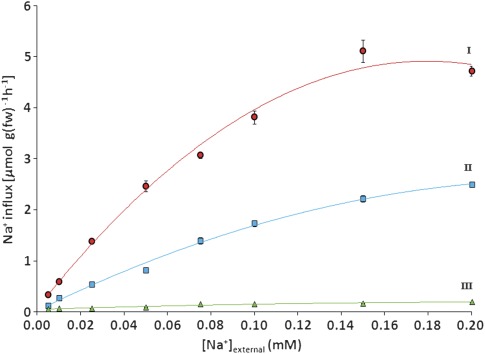
The sodium-concentration dependence of unidirectional Na+ influx was measured in plants grown under three conditions, using a 5 min 24Na uptake protocol (Fig. 1). Maximal influxes were observed in plants that were grown only with CaSO4 (200 μM), which was withdrawn 10 min prior to tracer application. By contrast, the fluxes were suppressed by more than 90% when Ca2+ was not withdrawn (not shown). The suppressed fluxes were considerably lower than those observed by Rains and Epstein (1967a) in the presence of higher (500 μM) Ca2+, which only reduced Na+ fluxes by about half compared to Ca2+-free conditions, at a representative [Na+]ext of 100 μM. Plants grown and measured with full nutrient solution showed the lowest fluxes, which were barely detectable, while plants grown at 100 μM NaCl transported sodium at an intermediate rate. For the three growth/measurement conditions, influx showed saturating patterns with increasing [Na+]ext, and Michaelis–Menten constants were determined (Table 1). In general, Na+ fluxes shown in the upper two curves of Fig. 1 (‘I’ and ‘II’) were of a magnitude similar to those measured by other workers (Rains and Epstein, 1967a; Jeschke, 1982; Wrona and Epstein, 1985), and also to those of high-affinity K+ fluxes into barley roots (Szczerba et al., 2006). In addition, the half-maximal saturation (Km) values in Table 1 were in good general agreement with those provided in Haro et al. (2010) for multiple species, including barley.
Fig. 1.
Concentration dependence of unidirectional, high-affinity Na+ influx, measured in 7-d-old barley seedlings grown with 200 μM Ca2+ (I, circles), 100 μM Na+ (II, squares), or full nutrient medium (III, triangles). Values represent means ±SEM (n=4–6). (This figure is available in colour at JXB online.)
Table 1.
Michaelis–Menten parameters for high-affinity Na+ influx, derived from concentration-dependence curves in Fig. 1: values represent mean ±SEM (n=4–6)
| Concentration-dependence curve | Growth condition | Vmax (μmol g−1 fw h−1) | Km (μM) |
| I | 200 μM Ca2+ | 7.91 | 110 |
| II | 100 μM Na+ | 5.34 | 219 |
| III | Full medium | 0.22 | 36 |
More detailed experiments were conducted with plants grown at 100 μM NaCl (curve ‘II’), in the absence of inhibitory calcium. Table 2 illustrates the high malleability of unidirectional Na+ influx in these plants, when exposed to 16 pharmacological, nutritional, and physicochemical treatments. In all cases, the flux of Na+ was profoundly reduced, with inhibition ranging from 74% to 95%. The strongest suppression was seen when both respiratory electron transport pathways were blocked using cyanide and SHAM. The presence of Ca2+ in solution also abolished almost the entire flux, as did the presence of K+, confirming the observations of others (Rains and Epstein, 1967a, b; Garciadeblás et al., 2003; Horie et al., 2007; Haro et al., 2010), but, in addition, it was found that another common nutrient, ammonium (), brought Na+ influx to very low values, for which no precedent could be found in the literature. Na+ influx responded negatively to the application of a wide range of channel inhibitors: both the NSCC blockers Gd3+, La3+, Zn2+, and quinine, and the K+ channel blockers TEA+, Ba2+, and Cs+, powerfully suppressed Na+ influx (Demidchik and Tester, 2002; Kader and Lindberg, 2005; Volkov and Amtmann, 2006). In addition, it was found that the anaesthetic lidocaine and the neurotransmitter dopamine, two compounds known to block Na+-specific channels in animal systems (Ragsdale et al., 1996; Cantrell and Catterall, 2001), both greatly reduced Na+ influx, a novel finding with respect to plant Na+ transport.
Effects of selected inhibitors were further investigated in plants measured at 10 μM [Na+]ext. Fluxes in these plants were also suppressed by the channel blockers Gd3+, TEA+, Cs+, and lidocaine, although to a slightly lesser extent than plants measured at 100 μM (Table 2).
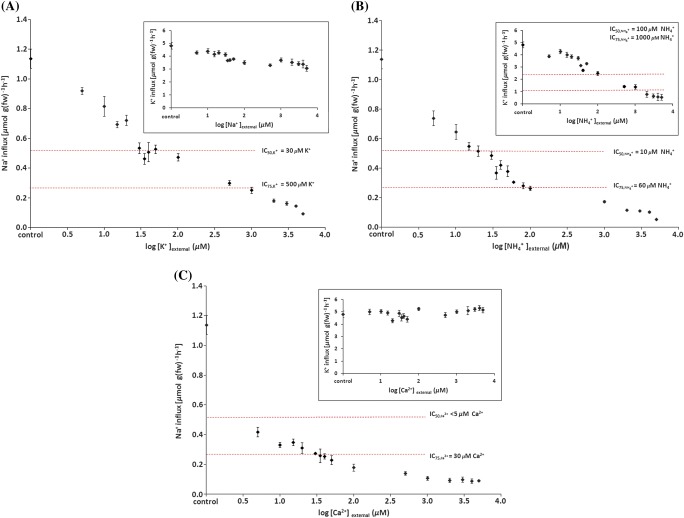
Of the inhibitors given in Table 2, the effects on Na+ influx of those that are commonly regarded as plant nutrients (i.e. K+, , and Ca2+) were investigated more thoroughly. Figure 2A shows how strongly the presence of K+ reduces Na+ influx: half-maximal inhibition of the control flux occurs at only 30 μM [K+]ext (=), and is suppressed by another 50% at 500 μM [K+]ext (=). By contrast, the reciprocal effect, i.e. suppression of K+ influx (as measured using 42K) by Na+ was much less pronounced; an IC50 was not found, even with Na+ application of up to 5 mM (Fig. 2A, inset). A somewhat different picture emerged when was present during uptake (Fig. 2B). Both Na+ and K+ influxes were greatly reduced by , particularly in the case of Na+, which was suppressed with IC50 and IC75 values of 10 and 60 μM, respectively, while those for K+ influx were 100 and 1000 μM (inset). By contrast, Na+ influx was highly sensitive to extremely small amounts of Ca2+, ( <5 μM and =30 μM; Fig. 2C), whereas K+ influx showed no signs of suppression by Ca2+ supply (Fig. 2C, inset).
Fig. 2.
Influences of (A) K+, (B) NH4+, and (C) Ca2+ on high-affinity Na+ influx (and of Na+, NH4+, and Ca2+ on high-affinity K+ influx, respectively; insets). Fluxes were measured at 100 μM Na+ or K+. Dashed lines represent IC50 and IC75 values for the inhibition of control fluxes. Values represent means ±SEM (n=3–14). (This figure is available in colour at JXB online.)
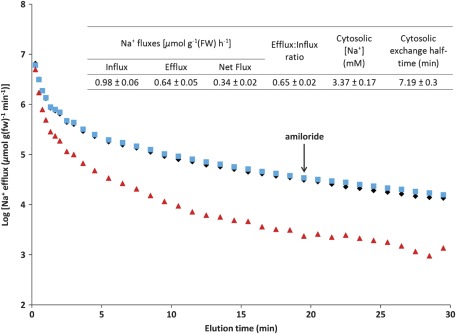
Figure 3 shows the results of 24Na+-efflux experiments with plants grown and measured at 100 μM Na+ (i.e. steady-state conditions). It was found that applying amiloride, a well-known blocker of Na+/H+ antiporters (Canessa et al., 1994; Cuin et al., 2011), midway through the elution period, did not affect the efflux of 24Na+ (Fig. 3). By contrast, application of high (5 mM) K+ in the same manner did produce a small but detectable increase in Na+ efflux (not shown), a phenomenon previously observed by Jeschke (1970). Compartmental analysis of 24Na+-efflux and -retention data yielded key flux and pool-size parameters (Fig. 3, tabular inset). The value of Na+ influx was similar to that given in Fig. 1, but a surprising result was that 65% of the absorbed 24Na+ was subsequently released by root cells, in a futile-cycling scenario not usually observed at such low substrate concentrations (Britto and Kronzucker, 2006; Coskun et al., 2010), but very commonly at high concentrations, especially for Na+ (Essah et al., 2003; Britto and Kronzucker, 2006; Malagoli et al., 2008; Britto and Kronzucker, 2009). The other parameter of high interest in this analysis was the Na+ pool size estimated for the cytosolic compartment (see below for discussion about phase identification). Its value was found to be 3.37 mM, within the range of [Na+]cytosol previously determined, under saline conditions, by Carden et al. (2003) in two cultivars of barley by use of Na-selective microelectrodes (2–28 mM), but well below normal cytosolic [K+] values near 100 mM (Kronzucker et al., 2003b).
Fig. 3.
Traces of 24Na+ efflux from roots of intact barley seedlings grown and measured at 100 μM Na+. Diamonds, steady-state conditions with no inhibitors; squares, sudden application of 0.1 mM amiloride as shown by the arrow (at 19.5 min); triangles, 5 mM K+ present in the labelling medium and during subsequent elution. Each point on the efflux traces represents the mean of nine replicates for control and amiloride treatments, and the mean of four replicates for the K+ treatment. Tabular inset: flux parameters derived from compartmental analysis by tracer efflux, under steady-state conditions (mean ±SEM; n=9). (This figure is available in colour at JXB online.)
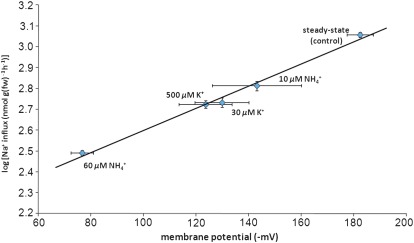
Figure 4 shows the strong (R2=0.99) semi-logarithmic correlation between Na+ influx and the corresponding depolarization of the electrical potential (ΔΨ) across the plasma membrane of barley roots cells. As with the influx of sodium, membrane potentials responded immediately to the addition of IC50 and IC75 concentrations of K+ and NH4+ (but not Ca2+, not shown), in the presence of 0.1 mM Na+. Membrane depolarizations brought about by K+ and are well known to occur in plant root cells, even at such low concentrations (Cheeseman and Hanson, 1979; Ayling, 1993; Wang et al., 1994).
Fig. 4.
Relationship between membrane electrical potential and unidirectional Na+ influx, and in response to NH4+ and K+. NH4+ and K+ concentrations represent IC50 and IC75 values for the suppression of Na+ influx by these ions (see Fig. 2). Na+ fluxes were measured at 100 μM [Na+]ext. The data represent means of 4–23 replicates ±SEM. (This figure is available in colour at JXB online.)
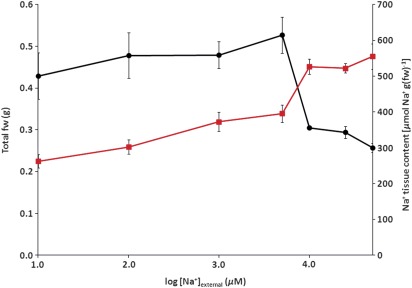
Figure 5 depicts Na+ shoot fresh weight and total Na+ content of 7-d-old barley seedlings grown under seven sodium regimes, ranging from 0.01 to 50 mM [Na+]ext. Initially, rising tissue Na+ was positively associated with an increase in fresh weight. However, once [Na+]ext reached or exceeded 10 mM, fresh weight declined rapidly, while Na+ content continued to rise. This decline in biomass was more severe than previously observed for barley plants growing in complete solution, probably due to a lack of essential nutrients (Kronzucker et al., 2006). Interestingly, even under steady-state growth on 0.01 mM Na+, barley seedlings accumulated almost half of what was accumulated by seedlings growing at 5000 times the Na+ supply.
Fig. 5.
Tissue Na+ content (squares) and fresh weight (circles) of barley seedlings was determined in plants grown at 0.01, 0.1, 1, 5, 10, 25, and 50 mM Na+ steady state. Values represent means ±SEM (n=6–10). (This figure is available in colour at JXB online.)
Discussion
This study investigates high-affinity Na+ transport in plant roots from a variety of perspectives. Sodium influx values were determined by use of a 5 min 24Na-absorption protocol, which agreed well with those determined by the alternative method of compartmental analysis by 24Na efflux. While these procedures resulted in basal values consistent with those reported in the literature, the inhibitory profiles, electrophysiological data, compartmental analyses, and growth and tissue data presented here provide new information about this transport phenomenon, and the possible mechanisms by which it occurs, as well as its ecological significance.
Interactions between Na+ uptake and nutrient provision
One of the most striking findings here is the degree to which three nutrients commonly found in soils can inhibit the influx of sodium under high-affinity conditions (Table 2; Fig. 2): external concentrations of K+, , and Ca2+ as low as 500, 60, and 30 μM, respectively, were sufficient to reduce Na+ influx by 75% (Fig. 2). While other studies have shown K+ and Ca2+ to significantly reduce Na+ uptake (Rains and Epstein, 1967a, b; Garciadeblás et al., 2003; Haro et al., 2010), none has shown that can also accomplish this, despite the well-known inhibition by of high-affinity K+ transport (Santa-Maria et al., 2000, Kronzucker et al., 2003b). Moreover, it is shown just how strong these effects can be, by application of a wide concentration range of inhibitory nutrients and using a short-term labelling procedure that yields unidirectional influx values of high precision. Most studies in this area have used depletion or longer-term tracer-uptake protocols, which do not provide unidirectional influx measurements; for this reason, they may have underestimated the Na+ influx capacity and detail (Britto and Kronzucker, 2001), particularly if the high ratio (0.65) of efflux to influx seen here is a general feature of Na+ HATS in higher plants, as appears to be the case with low-affinity Na+ influx (Essah et al., 2003; Malagoli et al., 2008; Britto and Kronzucker, 2009).
Ammonium, while typically not the major source of N for plants, is often found in soils well above the IC75 of 60 μM (Glass et al., 2002; Kronzucker et al., 2003a, and references therein). In addition, 500 μM K+ is not uncommon in soils (let alone the IC50 of 30 μM; Marschner, 1995; Szczerba et al., 2006), and soil [Ca2+] values, almost universally, far exceed the IC75 of 30 μM reported here (Zidan et al., 1991; Hirschi, 2004). This suggests that influx of Na+ in the high-affinity range is appreciable only when measured under rather unrealistic conditions, even when considering that soils can have considerable chemical heterogeneity over small spatial scales (Jackson and Caldwell, 1993). The nutritionally suppressed influx of Na+, which is far lower than normal uptake rates of K+ (Kronzucker et al., 2003b), calls into question the significance of sodium uptake as a K-replacement strategy under K limitation (see Introduction), at least in this range of external [Na+] for this cultivar of barley. It is also, however, at odds with Horie et al. (2007), who found, in rice seedlings, that both OsHKT2;1-mediated high-affinity Na+ uptake and Na+-stimulated growth under K+ deprivation, occurred in the presence of 1 mM Ca2+. These issues are at least partially related to the expression and functioning of Na+ HATS being highly species- (and cultivar-) dependent, as is the degree to which Na+ may substitute for K+. In another contrasting finding, net Na+ fluxes in the HATS range were not suppressed by K+ in the case of sunflower (Garciadeblás et al., 2003), which is regarded as a salt-tolerant sodium accumulator (Bhatt and Indirakutty, 1973). Thus, in future work, it will be interesting to investigate nutritional sensitivities (or lack thereof) of the sodium HATS in plant species, such as sugar beet, that are able beneficially to accumulate large amounts of Na+, even in complete growth media, and also in C4 plants that require Na+ as a micronutrient.
Possible mechanisms of high-affinity Na+ uptake
It is worth noting that, even while the flux of Na+ may be highly suppressed under normal nutritional conditions, a very high substrate concentration, as is found in the toxic range, may yet drive Na+ into the plant via this same mechanism, at rates sufficient to cause detrimental Na+ accumulation (Kronzucker and Britto, 2011). This may be particularly important given that HKT transporters have characteristics of both carriers and ion channels (see Introduction). In this vein, Laurie et al. (2002) showed that TaHKT2;1-mediated Na+ influx reduces growth in wheat roots, when exposed to high (200 mM) Na+, and deprived of K+. Interestingly, the authors concluded that Na+ transport to the stele was a major control point at which HKT appeared to operate, consistent with the foliar accumulation of Na+ as a significant aspect of Na+ stress (Munns and Tester, 2008; cf. Kronzucker and Britto, 2011). Additional support for the possibility that high-affinity Na+ transporters catalyse fluxes in the toxic range can be seen in the electrophysiological work of Buschmann et al. (2000), who showed that Na+ currents were enhanced in response to K+ deprivation (also see Mian et al., 2011).
The inhibitor data presented in Table 2 provide additional clues about the mechanism underlying high-affinity Na+ influx. Numerous treatments known to block several types of ion channels (NSCCs, K+-specific channels, and Na+-specific channels in animals) were able to diminish the Na+ flux profoundly in the present study. Seven of these inhibitory treatments (Ca2+, Gd3+, La3+, Zn2+, Ba2+, low pH, and quinine) are known to affect NSCCs (Kronzucker and Britto, 2011), which have been strongly implicated in Na+ uptake in the toxic range (Munns and Tester, 2008). However, Cs+ and tetraethylammonium (TEA+), two agents to which most NSCCs have been reported to be insensitive (Kronzucker and Britto, 2011), also strongly blocked Na+ transport. Nevertheless, it has been shown that NSCCs can transport both Cs+ and TEA+ (Davenport and Tester, 2000; Demidchik and Tester, 2002), which could cause an electrical depolarization of the plasma membrane and, therefore, a reduction in the driving force for channel-mediated Na+ uptake (see below); this may in itself explain the inhibition of the flux observed here. Similarly, low temperature (4 °C) and cyanide/SHAM applications can also cause depolarizations (Lew, 1991) and thus reduce the flux, if it is driven by the membrane potential.
Interestingly, HKT-type transporters are thought to have evolved from prokaryotic K+ channels (Shafrir et al., 2008). They have, in addition, been suggested to be a sub-class of NSCCs, for which no genetic identities have yet been unequivocally ascribed (Horie et al., 2009) and, indeed, rice OsHKT2;4 has been shown to be sensitive to both Gd3+ and La3+ (Lan et al., 2010). Catalysis via HKT-type transporters of the high-affinity fluxes presented here is suggested by these studies, as well as by demonstrations that HKT-mediated Na+ transport is highly sensitive to external K+ (Horie et al., 2007; Haro et al., 2010; also see Introduction). Curiously, the Arabidopsis genome encodes only one HKT-type transporter, AtHKT1.1, which had been suggested to play a role in low-affinity Na+ transport in the toxic range (Rus et al., 2001), but more recent evidence indicates that its major role is in the internal redistribution of Na+, rather than its primary acquisition (Berthomieu et al., 2003; Møller et al., 2009).
Thermodynamics of high-affinity Na+ transport
If it is indeed correct that ion channels (or channel-like HKTs) mediate high-affinity Na+ transport, possibly in a manner similar to that proposed for high-affinity K+ transport by Hirsch et al. (1998), a thermodynamic scenario would have to exist that permits a ‘passive’ influx of Na+. Thermodynamic evaluation necessitates information about the concentration gradient of Na+ across the membrane, as well as its state of electrical polarization. To determine the internal (cytosolic) Na+ pool, by use of efflux trials as shown in Fig. 3, the intracellular origin of the released tracer was first verified (Britto and Kronzucker, 2003; Coskun et al., 2010); this was done in several ways. First, the influx values determined from tracer-efflux and -retention data were very close to those determined by the ‘direct-influx’ procedure at 100 μM [Na+]ext (Table 2; Fig. 3, tabular inset). Second, the presence of 5 mM K+ in the external medium during the 1 h labelling period resulted in a dramatic reduction in tracer release during elution (Fig. 3, lower trace); this is readily explained by the blockage of 24Na+ influx during labelling by K+ (Fig. 2); such a powerful effect would not be expected had the origin of the 24Na+ trace been extracellular spaces of the root. A third line of evidence comes from comparing the most suppressed influx in Table 2 (i.e. in response to the cyanide+SHAM treatment) to the efflux determined in Fig. 3. The small influx that remains after this powerful metabolic treatment (0.06 μmol g−1 fw h−1) represents the maximum contribution to influx that the apoplastic component can make. Because the measured efflux of 0.65 μmol g−1 fw h−1 far exceeds this quantity, it can be concluded that >90% of released tracer originated intracellularly. Finally, it was of interest to determine which intracellular compartment(s) was/were the source of released tracer. Prior work indicates that half-times for vacuolar Na+ exchange in barley seedlings can be 77–231 h (Jeschke, 1982), far longer than the 1 h labelling period employed in the present study. In addition, the quantity of Na+ found in the traced pool (0.169 μmol g−1 fw) was far less than can account for the tissue accumulation of Na+ (300 μmol g−1 fw; Fig. 5), which is likely to be mostly vacuolar. Thus, very little of the released tracer is likely to have originated in the vacuole, suggesting that, instead, its origin was the cytosolic compartment of root cells.
This information, in conjunction with the finding that root cell membranes of plants grown at 100 μM Na+ are highly electrically polarized, with a steady-state potential of –182 mV (Fig. 4), a condition not uncommon in K-deprived plants (Cheeseman and Hanson, 1979), allows the Nernst equation to be used to indicate the direction of passive flux. Given the small Na+ pool and the highly polarized membrane, this direction is clearly inward, even at 100 μM [Na+]ext (i.e. ΔΨ is negative of the calculated Nernst potential of –92 mV), supporting the idea of a passive, possibly channel-mediated influx of Na+. This possibility is further supported by the strong correlation between ΔΨ and Na+ influx (Fig. 4). Interestingly, this relationship is not linear but log-linear, indicating that the behaviour of the transport system is not Ohmic but may involve voltage gating, or be influenced by asymmetries in Na+ concentrations or channel structure (Hille, 2001).
‘Can channels do it all?’
The finding that high-affinity Na+ transport shares many characteristics with ion-channel behaviour provides new evidence in the ongoing debate summarized in the title of an essay by Kochian and Lucas (1993), ‘Can K+ channels do it all?’ Although that paper was specifically about K+, its conclusion that K+ channels cannot catalyse active, high-affinity K+ uptake is relevant here. The term ‘high affinity’ refers to the substrate binding characteristics of the enzyme or transporter (Marschner, 1995), as identified by the Michaelis constant (Km). Here, Na+ transport from low external concentrations does display saturable Michaelis–Menten properties with low Km values, characteristic of high-affinity systems (Table 1), while at the same time showing pharmacological sensitivities typical of ion channels, even at external [Na+] of only 10 μM (Table 2). However, thermodynamic analysis indicates that high-affinity Na+ transport is not an example of ‘active transport’, but appears to be driven by the membrane electrical potential, as is the case with channels (Fig. 4; Britto and Kronzucker, 2006). In addition, the strong inhibition of the flux by low temperature (4 °C) and cyanide/SHAM application is not necessarily a hallmark of active transport, but these treatments can act on channels via alterations in membrane potential (Lew, 1991). Thus, our study suggests that either channels, or channel-like transporters (such as HKTs) may indeed ‘do it all’, with respect to sodium uptake. However, this scenario differs from the proposed channel-mediated uptake of K+ from low external concentrations (Hirsch et al., 1998; Spalding et al., 1999), in that many K+-specific, high-affinity carrier-type transporters are known, which are likely to operate in concert with channel mediation; this is not so with Na+. A remaining question is whether ion channels, which are known to be saturable in some instances (Hille, 2001), can have Km values as low as those presented here.
The question of Na+-K+ symport
Another controversy surrounding high-affinity Na+ uptake concerns the possibility that it is linked to symport with K+ (Rubio et al., 1995; Maathuis et al., 1996; Corratgé-Faillie et al., 2010; Kronzucker and Britto, 2011). No evidence that this occurs was found; on the contrary, Na+ uptake was profoundly reduced by low K+, while Na+ (up to 5 mM) suppressed K+ uptake only moderately (Fig. 2A). This is in agreement with Maathuis et al. (1996), who found no in planta evidence for Na+/K+ symport. In this context, it is also worthwhile to ask whether the system that transports Na+ also transports other ions. At 100 μM substrate concentrations, K+ influx was not affected by either Ca2+ (Fig. 2C, inset), Gd3+ or Ba2+ (not shown), nor was NH4+ influx altered by Ba2+ or Cs+ (not shown); the contrast of this finding with the inhibitory profile of Na+ influx (Table 2) indicates that neither K+ nor is a substrate for this transport system.
Electrophysiological analysis (Fig. 4) suggests that K+ and inhibit high-affinity Na+ influx, in the short term, by depolarizing root cell membranes (Cheeseman and Hanson, 1979; Ayling, 1993; Wang et al., 1994). By contrast, Ca2+ did not depolarize (not shown); thus, its suppression of the Na+ flux appears to occur via a different means, presumably a direct action upon the transporter.
An unknown mechanism of Na+ efflux?
In our thermodynamic analysis, an inwardly passive mechanism would imply an active extrusion of Na+. Various mechanisms of outward Na+ pumping from plant roots have been proposed, including a sodium ATPase (Lunde et al., 2007; Jacobs et al., 2011) and a Na+/H+ antiport (Mennen et al., 1990). The former has been identified in algae and bryophytes, but not in higher plants, while the latter appears to be catalysed by the SOS1 protein, and is strongly tied to salt tolerance in many higher plants (Shi et al., 2000; Cuin et al., 2011; see Kronzucker and Britto, 2011, for a review). However, SOS1 has been reported to be directly or indirectly sensitive to the Na+/H+ antiport-inhibitor amiloride (Cuin et al., 2011), which yielded no change to the tracer-efflux line in Fig. 3. Thus, it appears that the transporter mediating Na+ efflux under high-affinity conditions is a previously unidentified component of this cellular sodium cycle.
Na+ accumulation and compartmentation
Another significant outcome of the compartmental analysis shown here is that the cytosolic pool of Na+ was estimated at only 3.37 mM, at an extracellular concentration of 100 μM, and in the absence of K+ (Fig. 3, tabular inset). This value is within the range measured by Carden et al. (2003) using Na+-selective intracellular electrodes, albeit under salinity stress (to our knowledge, our values are the first cytosolic [Na+] estimates to be determined in the high-affinity range). Nevertheless, this value is far lower than typical values of cytosolic [K+], which are about 100 mM (Kronzucker et al., 2003b). On the other hand, the tissue accumulation of Na+ under these conditions is substantial, at 300 μmol g−1 fw (Fig. 5), and is similar in magnitude to that of K+ accumulation at 100 μM [K+]ext (Szczerba et al., 2006). This suggests that, under conditions that optimize Na+ uptake in the high-affinity range, and under growth conditions where nutrients inhibitory to its flux are not present, Na+ can substitute for the osmolytic function of K+ on a total-tissue basis, probably accumulating in the vacuoles, but cannot replace the critical requirement of high [K+] in the cytosol. It is likely that this osmolytic function is responsible for the positive relationship between Na+ accumulation and growth between 0.01 and 5 mM [Na+]ext, depicted in Fig. 5.
Concluding remarks
It was found that high-affinity, unidirectional influx of Na+ exhibits values comparable to those of high-affinity K+ uptake, but can only do so under special conditions; i.e. in the near absence of the major soil nutrients K+, Ca2+, and NH4+. Ecologically, therefore, the relevance of high-affinity Na+ transport should be viewed with caution. However, the study of this phenomenon, even under ecologically unrealistic conditions, reveals surprising results which may have implications for Na+ acquisition in the toxic range. Chief amongst these is the dual display of both channel and carrier characteristics, in terms of inhibitory profiles, saturability with low Km, and thermodynamic conditions. The possible involvement of HKT-type transporters, for which channel-like behaviour (and descent from bacterial channels) has recently been shown, is supported for high-affinity Na+ influx. No evidence, however, was seen for the coupling of K+ influx to that of Na+. In addition, it is shown that Na+ influx is part of a futile cycle even at low [Na+]ext, and results in only small cytosolic accumulation (<5 mM), but total tissue accumulation that can rival that of K+.
Acknowledgments
We thank M Butler and R Pasuta at McMaster University (Hamilton, Ontario, Canada) for supplying us with the radiotracers 24Na+ and 42K+. We also thank J Bakshi and D Fiorini for assistance with experiments. Funding for this study was generously provided by the Natural Sciences and Engineering Council of Canada (NSERC), the Canadian Foundation for Innovation (CFI), and the Canada Research Chairs (CRC) program.
References
- Ayling SM. The effect of ammonium ions on membrane potential and anion flux in roots of barley and tomato. Plant, Cell and Environment. 1993;16:297–303. [Google Scholar]
- Berthomieu P, Conéjéro G, Nublat A, et al. Functional analysis of AtHKT1 in Arabidopsis shows that Na+ recirculation by the phloem is crucial for salt tolerance. EMBO Journal. 2003;22:2004–2014. doi: 10.1093/emboj/cdg207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt J, Indirakutti K. Salt uptake and salt tolerance by sunflower. Plant and Soil. 1973;39:457–460. [Google Scholar]
- Britto DT, Kronzucker HJ. Cytosolic ion exchange dynamics: insights into the mechanisms of component ion fluxes and their measurement. Functional Plant Biology. 2003;30:355–363. doi: 10.1071/FP02119. [DOI] [PubMed] [Google Scholar]
- Britto DT, Kronzucker HJ. Futile cycling at the plasma membrane: a hallmark of low-affinity nutrient transport. Trends in Plant Science. 2006;11:529–534. doi: 10.1016/j.tplants.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Britto DT, Kronzucker HJ. Ussing's conundrum and the search for transport mechanisms in plants. New Phytologist. 2009;183:243–246. doi: 10.1111/j.1469-8137.2009.02872.x. [DOI] [PubMed] [Google Scholar]
- Britto DT, Kronzucker HJ. Can unidirectional influx be measured in higher plants? A mathematical approach using parameters from efflux analysis. New Phytologist. 2001;150:37–47. [Google Scholar]
- Brownell PF, Bielig LM. The role of sodium in the conversion of pyruvate to phosphoenolpyruvate in mesophyll chloroplasts of C4 plants. Australian Journal of Plant Physiology. 1996;23:171–177. [Google Scholar]
- Buschmann PH, Vaidyanathan R, Gassmann W, Schroeder JI. Enhancement of Na+ uptake currents, time-dependent inward-rectifying K+ channel currents, and K+ channel transcripts by K+ starvation in wheat root cells. Plant Physiology. 2000;122:1387–1397. doi: 10.1104/pp.122.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of 3 homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- Cantrell AR, Catterall WA. Neuromodulation of Na+ channels: aAn unexpected form of cellular plasticity. Nature Reviews Neuroscience. 2001;2:397–407. doi: 10.1038/35077553. [DOI] [PubMed] [Google Scholar]
- Carden DE, Walker DJ, Flowers TJ, Miller AJ. Single-cell measurements of the contributions of cytosolic Na+ and K+ to salt tolerance. Plant Physiology. 2003;131:676–683. doi: 10.1104/pp.011445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman JM, Hanson JB. Mathematical analysis of the dependence of cell potential on external potassium in corn roots. Plant Physiology. 1979;63:1–4. doi: 10.1104/pp.63.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corratgé-Faillie C, Jabnoune M, Zimmermann S, Véry AA, Fizames C, Sentenac H. Potassium and sodium transport in non-animal cells: the Trk/Ktr/HKT transporter family. Cellular and Molecular Life Sciences. 2010;67:2511–2532. doi: 10.1007/s00018-010-0317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun D, Britto DT, Kronzucker HJ. Regulation and mechanism of potassium release from barley roots: an in planta 42K+ analysis. New Phytologist. 2010;188:1028–1038. doi: 10.1111/j.1469-8137.2010.03436.x. [DOI] [PubMed] [Google Scholar]
- Cuin TA, Bose J, Stefano G, Jha D, Tester M, Mancuso S, Shabala S. Assessing the role of root plasma membrane and tonoplast Na+/H+ exchangers in salinity tolerance in wheat: in planta quantification methods. Plant, Cell and Environment. 2011;34:947–961. doi: 10.1111/j.1365-3040.2011.02296.x. [DOI] [PubMed] [Google Scholar]
- Davenport RJ, Tester M. A weakly voltage-dependent, nonselective cation channel mediates toxic sodium influx in wheat. Plant Physiology. 2000;122:823–834. doi: 10.1104/pp.122.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Davenport RJ, Tester M. Non-selective cation channels in plants. Annual Review of Plant Biology. 2002;53:67–107. doi: 10.1146/annurev.arplant.53.091901.161540. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Tester M. Sodium fluxes through non-selective cation channels in the plasma membrane of protoplasts from Arabidopsis roots. Plant Physiology. 2002;128:379–387. doi: 10.1104/pp.010524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durell SR, Hao YL, Nakamura T, Bakker EP, Guy HR. Evolutionary relationship between K+ channels and symporters. Biophysical Journal. 1999;77:775–788. doi: 10.1016/S0006-3495(99)76931-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essah PA, Davenport R, Tester M. Sodium influx and accumulation in Arabidopsis. Plant Physiology. 2003;133:307–318. doi: 10.1104/pp.103.022178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garciadeblás B, Senn ME, Bañuelos MA, Rodríguez-Navarro A. Sodium transport and HKT transporters: the rice model. The Plant Journal. 2003;34:788–801. doi: 10.1046/j.1365-313x.2003.01764.x. [DOI] [PubMed] [Google Scholar]
- Glass ADM, Britto DT, Kaiser BN, et al. The regulation of nitrate and ammonium transport systems in plants. Journal of Experimental Botany. 2002;53:855–864. doi: 10.1093/jexbot/53.370.855. [DOI] [PubMed] [Google Scholar]
- Haro R, Bañuelos MA, Rodríguez -Navarro A. High-affinity sodium uptake in land plants. Plant and Cell Physiology. 2010;51:68–79. doi: 10.1093/pcp/pcp168. [DOI] [PubMed] [Google Scholar]
- Hawker JS, Marschner H, Downton WJS. Effect of sodium and potassium on starch synthesis in leaves. Australian Journal of Plant Physiology. 1974;1:491–501. [Google Scholar]
- Hille B. Ionic channels of excitable membranes. 3rd edn. Sunderland, MA: Sinauer Associates, Inc; 2001. [Google Scholar]
- Hirsch RE, Lewis BD, Spalding EP, Sussman MR. A role for the AKT1 potassium channel in plant nutrition. Science. 1998;280:918–921. doi: 10.1126/science.280.5365.918. [DOI] [PubMed] [Google Scholar]
- Hirschi KD. The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiology. 2004;136:2438–2442. doi: 10.1104/pp.104.046490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Costa A, Kim TH, Han MJ, Horie R, Leung HY, Miyao A, Hirochika H, An G, Schroeder JI. Rice OsHKT2;1 transporter mediates large Na+ influx component into K+-starved roots for growth. EMBO Journal. 2007;26:3003–3014. doi: 10.1038/sj.emboj.7601732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Hauser F, Schroeder JI. HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends in Plant Science. 2009;14:660–668. doi: 10.1016/j.tplants.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Spielmeyer W, Lagudah ES, Munns R. Comparative mapping of HKT genes in wheat, barley, and rice, key determinants of Na+ transport, and salt tolerance. Journal of Experimental Botany. 2008;59:927–937. doi: 10.1093/jxb/ern033. [DOI] [PubMed] [Google Scholar]
- Jackson LE, Martin B, Cavagnaro TR. Roots, nitrogen transformations, and ecosystem services. Annual Review of Plant Biology. 2008;59:341–363. doi: 10.1146/annurev.arplant.59.032607.092932. [DOI] [PubMed] [Google Scholar]
- Jackson RB, Caldwell MM. Geostatistical patterns of soil heterogeneity around individual perennial plants. Journal of Ecology. 1993;81:683–692. [Google Scholar]
- Jacobs A, Ford K, Kretschmer J, Tester M. Rice plants expressing the moss sodium pumping ATPase PpENA1 maintain greater biomass production under salt stress. Plant Biotechnology Journal. 2011;9:838–847. doi: 10.1111/j.1467-7652.2011.00594.x. [DOI] [PubMed] [Google Scholar]
- Jeschke WD. Evidence for a K+-stimulated Na+ efflux at the plasmalemma of barley root cells. Planta. 1970;94:240–245. doi: 10.1007/BF00386136. [DOI] [PubMed] [Google Scholar]
- Jeschke WD. Shoot-dependent regulation of sodium and potassium fluxes in roots of whole barley seedlings. Journal of Experimental Botany. 1982;33:601–618. [Google Scholar]
- Kader MA, Lindberg S. Uptake of sodium in protoplasts of salt-sensitive and salt-tolerant cultivars of rice, Oryza sativa L. determined by the fluorescent dye SBFI. Journal of Experimental Botany. 2005;56:3149–3158. doi: 10.1093/jxb/eri312. [DOI] [PubMed] [Google Scholar]
- Kochian LV, Lucas WJ. Can K+ channels do it all? The Plant Cell. 1993;5:720–721. doi: 10.1105/tpc.5.7.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Britto DT. Sodium transport in plants: a critical review. New Phytologist. 2011;189:54–81. doi: 10.1111/j.1469-8137.2010.03540.x. [DOI] [PubMed] [Google Scholar]
- Kronzucker HJ, Glass ADM, Siddiqi MY. Inhibition of nitrate uptake by ammonium in barley: Analysis of component fluxes. Plant Physiology. 1999;120:283–292. doi: 10.1104/pp.120.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM, Britto DT. Root ammonium transport efficiency as a determinant in forest colonization patterns: an hypothesis. Physiologia Plantarum. 2003a;117:164–170. [Google Scholar]
- Kronzucker HJ, Szczerba MW, Britto DT. Cytosolic potassium homeostasis revisited: 42K-tracer analysis in Hordeum vulgare L. reveals set-point variations in [K+] Planta. 2003b;217:540–546. doi: 10.1007/s00425-003-1032-5. [DOI] [PubMed] [Google Scholar]
- Kronzucker HJ, Szczerba MW, Moazami-Goudarzi M, Britto DT. The cytosolic Na+:K+ ratio does not explain salinity-induced growth impairment in barley: a dual-tracer study using 42K+ and 24Na+ Plant, Cell and Environment. 2006;29:2228–2237. doi: 10.1111/j.1365-3040.2006.01597.x. [DOI] [PubMed] [Google Scholar]
- Lan W-Z, Wang W, Wang S-M, Li L-G, Buchanan BB, Lin H-X, Gao J-P, Luan S. A rice high-affinity potassium transporter (HKT) conceals a calcium-permeable cation channel. Proceedings of the National Academy of Sciences, USA. 2010;107:708–7094. doi: 10.1073/pnas.1000698107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie S, Feeney KA, Maathuis FJM, Heard PJ, Brown SJ, Leigh RA. A role for HKT1 in sodium uptake by wheat roots. The Plant Journal. 2002;32:139–149. doi: 10.1046/j.1365-313x.2002.01410.x. [DOI] [PubMed] [Google Scholar]
- Lew RR. Electrogenic transport properties of growing Arabidopsis root hairs. Plant Physiology. 1991;97:1527–1534. doi: 10.1104/pp.97.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde C, Drew DP, Jacobs AK, Tester M. Exclusion of Na+ via sodium ATPase (PpENA1) ensures normal growth of Physcomitrella patens under moderate salt stress. Plant Physiology. 2007;144:1786–1796. doi: 10.1104/pp.106.094946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis FJM, Verlin D, Smith FA, Sanders D, Fernandez JA, Walker NA. The physiological relevance of Na+-coupled K+-transport. Plant Physiology. 1996;112:1609–1616. doi: 10.1104/pp.112.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagoli P, Britto DT, Schulze LM, Kronzucker HJ. Futile Na+ cycling at the root plasma membrane in rice (Oryza sativa L.): kinetics, energetics, and relationship to salinity tolerance. Journal of Experimental Botany. 2008;59:4109–4117. doi: 10.1093/jxb/ern249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H. Mineral nutrition of higher plants. 2nd edn. San Diego, CA: Academic Press; 1995. [Google Scholar]
- Mennen H, Jacoby B, Marschner H. Is sodium-proton antiport ubiquitous in plant cells? Journal of Plant Physiology. 1990;137:180–183. [Google Scholar]
- Mian A, Oomen RJFJ, Isayenkov S, Sentenac H, Maathuis FJM, Véry A-A. Over-expression of an Na+- and K+-permeable HKT transporter in barley improves salt tolerance. The Plant Journal. 2011;68:468–479. doi: 10.1111/j.1365-313X.2011.04701.x. [DOI] [PubMed] [Google Scholar]
- Møller IS, Gilliham M, Jha D, Mayo GM, Roy SJ, Coates JC, Haseloff J, Tester M. Shoot Na+ exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na+ transport in Arabidopsis. The Plant Cell. 2009;21:2163–2178. doi: 10.1105/tpc.108.064568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annual Review of Plant Biology. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proceedings of the National Academy of Sciences, USA. 1996;93:9270–9275. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rains DW, Epstein E. Transport of sodium in plant tissue. Science. 1965;148:1611. doi: 10.1126/science.148.3677.1611. [DOI] [PubMed] [Google Scholar]
- Rains DW, Epstein E. Sodium absorption by barley roots: role of dual mechanisms of alkali cation transport. Plant Physiology. 1967a;42:314–318. doi: 10.1104/pp.42.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rains DW, Epstein E. Sodium absorption by barley roots: its mediation by mechanism 2 of alkali cation transport. Plant Physiology. 1967b;42:319–323. doi: 10.1104/pp.42.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Navarro A, Rubio F. High-affinity potassium and sodium transport systems in plants. Journal of Experimental Botany. 2006;57:1149–1160. doi: 10.1093/jxb/erj068. [DOI] [PubMed] [Google Scholar]
- Rubio F, Gassmann W, Schroeder JI. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science. 1995;270:1660–1663. doi: 10.1126/science.270.5242.1660. [DOI] [PubMed] [Google Scholar]
- Rus A, Yokoi S, Sharkhuu A, Reddy M, Lee BH, Matsumoto TK, Koiwa H, Zhu JK, Bressan RA, Hasegawa PM. AtHKT1 is a salt tolerance determinant that controls Na+ entry into plant roots. Proceedings of the National Academy of Sciences, USA. 2001;98:14150–14155. doi: 10.1073/pnas.241501798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa-Maria GE, Danna CH, Czibener C. High-affinity potassium transport in barley roots. Ammonium-sensitive and -insensitive pathways. Plant Physiology. 2000;123:297–306. doi: 10.1104/pp.123.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Schroeder JI. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher-plants. Nature. 1994;370:655–658. doi: 10.1038/370655a0. [DOI] [PubMed] [Google Scholar]
- Shafrir Y, Durell SR, Guy HR. Models of the structure and gating mechanisms of the pore domain of the NaChBac ion channel. Biophysical Journal. 2008;95:3650–3662. doi: 10.1529/biophysj.108.135327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi HZ, Ishitani M, Kim CS, Zhu JK. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proceedings of the National Academy of Sciences, USA. 2000;97:6896–6901. doi: 10.1073/pnas.120170197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi MY, Glass ADM, Ruth TJ. Studies of the uptake of nitrate in barley. 3. Compartmentation of . Journal of Experimental Botany. 1991;42:1455–1463. [Google Scholar]
- Spalding EP, Hirsch RE, Lewis DR, Qi Z, Sussman MR, Lewis BD. Potassium uptake supporting plant growth in the absence of AKT1 channel activity: inhibition by ammonium and stimulation by sodium. Journal of General Physiology. 1999;113:909–918. doi: 10.1085/jgp.113.6.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao GV, Ito O, Berry WL, Wheeler RM. Sodium: a functional plant nutrient. Critical Reviews in Plant Sciences. 2003;22:391–416. [Google Scholar]
- Szczerba MW, Britto DT, Kronzucker HJ. Rapid, futile K+ cycling and pool-size dynamics define low-affinity potassium transport in barley. Plant Physiology. 2006;141:1494–1507. doi: 10.1104/pp.106.082701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczerba MW, Britto DT, Kronzucker HJ. K+ transport in plants: physiology and molecular biology. Journal of Plant Physiology. 2009;166:447–466. doi: 10.1016/j.jplph.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Volkov V, Amtmann A. Thellungiella halophila, a salt-tolerant relative of Arabidopsis thaliana, has specific root ion-channel features supporting K+/Na+ homeostasis under salinity stress. The Plant Journal. 2006;48:342–353. doi: 10.1111/j.1365-313X.2006.02876.x. [DOI] [PubMed] [Google Scholar]
- Wang MY, Glass ADM, Shaff JE, Kochian LV. Ammonium uptake by rice roots. III. Electrophysiology. Plant Physiology. 1994;104:899–906. doi: 10.1104/pp.104.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TB, Gassmann W, Rubio F, Schroeder JI, Glass ADM. Rapid up-regulation of HKT1, a high-affinity potassium transporter gene, in roots of barley and wheat following withdrawal of potassium. Plant Physiology. 1998;118:651–659. doi: 10.1104/pp.118.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrona AF, Epstein E. Potassium and sodium-absorption kinetics in roots of two tomato species. Lycopersicon esculentum and Lycopersicon cheesmanii. Plant Physiology. 1985;79:1064–1067. doi: 10.1104/pp.79.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue SW, Yao X, Luo W, Jha D, Tester M, Horie T, Schroeder JI. AtHKT1;1 mediates Nernstian sodium channel transport properties in Arabidopsis root stelar cells. PLoS ONE. 2011;6:1–9. doi: 10.1371/journal.pone.0024725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidan I, Jacoby B, Ravina I, Neumann PM. Sodium does not compete with calcium in saturating plasma-membrane sites regulating 22Na influx in salinized maize roots. Plant Physiology. 1991;96:331–334. doi: 10.1104/pp.96.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]