Abstract
Knowledge about the root system structure and the uptake efficiency of root orders is critical to understand the adaptive plasticity of plants towards salt stress. Thus, this study describes the phenological and physiological plasticity of Citrus volkameriana rootstocks under severe NaCl stress on the level of root orders. Phenotypic root traits known to influence uptake processes, for example frequency of root orders, specific root area, cortical thickness, and xylem traits, did not change homogeneously throughout the root system, but changes after 6 months under 90 mM NaCl stress were root order specific. Chloride accumulation significantly increased with decreasing root order, and the Cl− concentration in lower root orders exceeded those in leaves. Water flux densities of first-order roots decreased to <20% under salinity and did not recover after stress release. The water flux densities of higher root orders changed marginally under salinity and increased 2- to 6-fold in second and third root orders after short-term stress release. Changes in root order frequency, morphology, and anatomy indicate rapid and major modification of C. volkameriana root systems under salt stress. Reduced water uptake under salinity was related to changes of water flux densities among root orders and to reduced root surface areas. The importance of root orders for water uptake changed under salinity from root tips towards higher root orders. The root order-specific changes reflect differences in vulnerability (indicated by the salt accumulation) and ontogenetic status, and point to functional differences among root orders under high salinity.
Keywords: Anatomy, biomass, Citrus volkameriana, miniature depletion chambers, NaCl accumulation, phenotypic plasticity, root architecture, root order, salt stress, water flux density
Introduction
Salinity is a major concern for agriculture worldwide; at least 20% of all irrigated lands are salt affected, with some estimates being as high as 50% (Pitman and Läuchli, 2004). Secondary salinization is particularly widespread in arid and semi-arid environments whose agricultural systems are often associated with cultivation of one of the various Citrus varieties. With global food production having to meet the demands of a growing world population, understanding plant responses to salinity is decisive to improve the salt tolerance of crops.
Phenotypical or physiological changes in response to environmental conditions often enhance the fitness of plants (Sultan, 2000). Root systems can exhibit enormous plasticity on the level of biomass, morphology, and/or physiology in response to different environmental parameters such as water and nutrient availability (e.g. Sorgonà et al., 2007; Wang et al., 2009; Gruber et al., 2011) or excess ions (Deak and Malamy, 2005; Zolla et al., 2010; Rewald et al., 2011b, c). Previous studies addressing salinity effects on tree crops such as Citrus spp. and Olea europaea found, for example, increased root:shoot ratios (Zekri and Parsons, 1989), reduced root branching (Gucci and Tattini, 1997), modified axial root conductivity (Rewald et al., 2011c), and a well-developed Casparian strip closer to the root apex (Walker et al., 1984). While salt exclusion, compartmentation, and osmoregulation are the mechanisms particularly considered to increase the salt tolerance of Citrus spp. and other woody glycophytes, adaptation to salinity is determined by the integrating effects of several mechanisms (Zekri and Parsons, 1992; Maas, 1993; Kozlowski, 1997; Munns, 2002). Thus, it is reasonable to speculate that root system modifications under salinity are a trade-off between the capacity to exclude excess ions and sustained water or nutrient uptake. However, studies on the (structural) differences among salt-stressed root systems that may partially underlie uptake capacities have received less attention (Vadez et al., 2007; Rewald et al., 2011b).
Because root system traits, such as water uptake rates per surface area, are defined by the properties of individual root segments (see Rewald et al., 2011a, and references within), detailed studies about the abundance, morphology, anatomy, and physiology of individual roots are needed. Due to the fact that traits often vary according to the position of individual root segments among the root branching hierarchy (i.e. ‘root order’; Pagès and Kervella, 1990; Pregitzer et al., 2002; Valenzuela-Estrada et al., 2008), analysis by root order is a powerful approach to understand complex woody root systems under stress. However, the morphological/anatomical properties and frequencies of the most distal root orders have been determined to date on <40 woody species world-wide (e.g. Pregitzer et al., 2002; Wang et al., 2006; Guo et al., 2008a); even fewer studies have quantified total number, biomass, and/or surface area of root orders (Valenzuela-Estrada et al., 2008; Rewald et al., 2011a).
Most previous studies have used indirect, specifically morphological and anatomical, analyses to estimate differences in root order functionalities (e.g. Valenzuela-Estrada et al., 2008; Huang et al., 2010). Direct hydraulic measurements on certain root orders were restricted for a long time to abscised (e.g. Schulte, 2006; Bramley et al., 2007) or distal (Zwieniecki and Boersma, 1997) root segments. However, Rewald et al. (2011a) have recently developed a method to determine water fluxes among root orders. It was shown that water flux densities under homogeneous, non-stressed conditions are determined by root order but not by root diameter or the position of a root segment within a root branch or the whole root system. Because water uptake is reduced under salinity and during periods of salt stress release (Cimato et al., 2010; Rewald et al., 2011b), detailed knowledge on water uptake capacities within the root branching system is key to understanding plant functioning under salinity.
To understand the adaptive response of Citrus volkameriana rootstocks under severe NaCl stress, the present work studies root traits and water uptake on the level of root orders. Two questions are addressed in detail. (i) Which architectural, morphological, and anatomical changes occur in salt-stressed C. volkameriana rootstocks? (2) What contribution do specific root orders make to water uptake under salinity and after a rapid release of salt stress? It is hypothesized that the type of plasticity (e.g. architectural, morphological, and anatomical) differs among root orders and that the water uptake by salt-stressed root systems is highly influenced by changes in abundance and water flux density among specific root orders.
Materials and methods
Plant material and growth conditions
Citrus volkameriana Ten. & Pasq. rootstocks are of economic importance because of their resistance to the Citrus tristeza virus and as medium salt excluders (Levy and Shalhevet, 1990; Ramin and Alirhezanezhad, 2005). In 2006, 1-year-old Citrus sinensis Osbeck var. Newhall shoots were grafted on adequately sized C. volkameriana rootstocks. The plants were grown in fertigated, soil-filled 10 litre pots in a greenhouse at ambient temperature until October 2009. As of this time, eight equal sized plants were selected, roots were rinsed, and plants were moved to constantly aerated hydroponics (Supplementary Fig. S1 available at JXB online). Plants were placed into opaque 20 litre pots filled with ∼17 litres of either 1.0 strength Long Ashton (LA; Ottow, 2005) solution (control treatment) or 1.0 strength LA plus 90 mM NaCl (salt treatment). The osmolalities of the solutions were 24±1 mmol kg−1 and 162±1 mmol kg−1, respectively (mean ±SE, n=10; Vapro 5520, Wescor, Logan, UT, USA). The pots, tightly covered to prevent light penetration and evaporation, were placed in a controlled growth room [air temperature ≤28 °C (day), 20 °C (night); relative humidity 30–40% (day), 70% (night); photosynthetic photon flux density (PPFD) 300–400 μmol m−2 s−1 (daylength 12.5 h)]. The temperature of the hydroponic system was kept at 20±0.1 °C (BL-30, MRC, Holon, Israel); transpired water was refilled every other day and the entire solution was exchanged every other week. After 5 months, leaf stomatal conductance between 12:00 h and 13:00 h was 81.6±7.4 mmol m−2 s−1 (control) and 35.5±3.5 mmol m−2 s−1 in plants under salinity (mean ±SE, n=22–24; SC-1 Porometer, Decagon, Pullmann, WA, USA).
Analysis of root morphology, surface area, and biomass
After ∼6 months in hydroponics, the rootstocks of three Citrus plants per treatment were severed from the stem above the highest root. Aboveground biomass was separated into leaves and branches, dried (70 °C, 48 h), and weighed. Subsequently 12 ‘large root branches’ (i.e. branches with 5–6 root orders) and four ‘small root branches’ (≤4 root orders present) attached to the tap root were randomly severed per individual. This ratio was chosen according to a visual pre-examination of the control rootstocks. The 16 root branches per plant were dissected into root orders and were kept moist constantly. The architectural classification follows the stream classification approach (Pregitzer et al., 2002), allowing most distal root segments (root tips) to be defined persistently as first-order roots even if the total number of orders is subject to change or unknown. Roots that possess only first-order side roots were named second-order roots, root segments bearing exclusively first- and second-order side roots were named third-order roots (starting at the most distal point along the root axis where two second-order roots met), and so on (see Supplementary Fig. S1A at JXB online).
The remaining roots (i.e. roots beside the 16 root branches analysed in detail) were separated into fourth root order and higher, while the biomass of the root orders 1–3 was later divided by the ratios calculated from the detailed dissection of root branches. Finally, ∼60–70% of the root systems were analysed in detail. Live roots were distinguished from dead roots (Rewald and Leuschner, 2009); dead roots and any adhering particles were discarded.
After dissection, root segments were stored in closed Petri dishes with small amounts of tap water to keep the roots hydrated (4 °C, <5 d), separated by treatment, plant, root branch, and order, until digital images capturing of root orders 1–6 took place on a flatbed scanner (grey scale, 400 dpi). To determine root diameters and surface areas, images were analysed with the software WinRhizo 2005c PRO (Régent, Quebec, Canada). Finally, root samples were dried (70 °C, 48 h) and weighed to a precision of ±0.1 mg using an analytical scale (CP225D, Sartorius, Göttingen, Germany). The specific root area [SRA, cm2 g dry weight (d.wt)−1) and the root diameter were calculated per root order using data of the 16 dissected root branches per plant. The relative biomass and surface area of root orders 1–6 within root branches was calculated, using the 12 ‘large root branches’ per plant. The root to leaf biomass ratio (‘root:leaf ratio’), the total plant biomass (DMT), and the rootstock biomass were calculated from dry weights per plant individual.
Analysis of root anatomy
Tissue sections from root order 1 were obtained close to the basal site of the root segments. Root orders 2–4 were sampled for tissue sections in the middle between two side branches. Eight randomly selected tissue sections of root orders 1–4 in each treatment were studied; root orders 5 and 6 were not analysed due to the difficult preparation of heterogeneously dense samples. The sections were fixed for 48 h by immersion [5% formaldehyde, 5% acetic acid, 90% ethanol (70%)]. Dehydration of the tissue sections was accomplished in a graded ethanol series (50, 70, 95, and 100%, 30 min each) followed by immersion in tert-butanol (8 h) and embedding in Paraplast Plus. After hardening, 12 μm thick cross-sections were cut with a rotation microtome (RM2235, Leica, Nussloch, Germany). Cross-sections were collected on glass slides and placed on a warming tray (40 °C, 3 h). The tissue sections were deparaffinized in xylene (3× 10 min) and rehydrated (ethanol 100, 95, 70, and 50%, 5 min each). The washed sections (H2O, 1 min) were successively stained with safranin (0.5%) and fast green (0.5%; Ruzin, 1999, and references within), cleared with 100% xylene (3× 10 min), and air dried. Digital images were taken (Zeiss AxioImager A1 microscope) and cross-sections were analysed (AxioVision 4.6, Carl Zeiss, Wetzlar, Germany). Measured parameters included the number of exodermal layers, and area and diameter of the root cross-section, the cortex, the stele, and the xylem. Relative proportions of the cortex diameter and the xylem area were calculated. The xylem was analysed in detail by quantifying the number, radii, and areas of xylem vessels; the hydraulically weighted average conduit diameter [HWCD, i.e. 2(Σr5 (Σr4)−1)] was calculated (Lewis and Boose, 1995). Furthermore the cross-sections were analysed for differences in the suberization of endodermis and peri-/exodermis after staining with aniline blue using fluorescence microscopy (Brundrett et al., 1988).
Plant chloride and sodium analysis
Dry materials of leaves and root orders 1–6, separated by plant individual, were ground to a powder and extracted overnight with distilled water (0.1 g of dry material in 10 ml of double-distilled water). Chloride (Cl−) concentration was determined by silver ion titration (Chloride Analyzer 926, Corning, MA, USA), while sodium (Na+) analyses were carried out using a Corning Flame Photometer 410 (Raveh, 2005).
Miniature depletion chamber set-up
Rewald et al. (2011a) constructed ‘miniature depletion chambers’ to measure the water fluxes of C. volkameriana root orders under fresh water supply (‘control’ treatment). The current study measured the water flux rates under salinity and after released salt stress. The measurements took place after the plants were growing under salinity for >4 months and in parallel to the measurements on fresh water-grown plants.
In brief, the chambers were manufactured from small plastic tubes (diameter=15 mm) which were shortened to 15 mm in length (Supplementary Fig. S1 at JXB online). Septa (IceBlue, Restek, Bellefonte, PA, USA) were glued in place (LocTite Super Glue-3, Henkel, Boulogne, France) on both ends. Both septa and plastic tubes were cut open on one side, allowing for root insertion by spreading the chamber open along the section. Septa were pre-drilled (diameter=0.3–3.5 mm) to enable sealing of inserted roots (diamter=0.5–4.1 mm) while preventing excessive squeezing. For measuring water fluxes of the first-root order, chambers with only one pre-drilled septum were used, with the root tip ending within the chamber.
For chamber placement, the root systems were lifted out of the hydroponics and fixed in mid-air for <10 min. A root segment was chosen by the following criteria: lack of side braches on a length of ≥17 mm, no signs of decay (e.g. dark-coloured, shrivelled), and undamaged epi-/peridermis. The segment was gently blotted dry using a paper towel, placed in the septa holes, and the chamber was closed by a clamp. Cuts were sealed with either hot glue (plastic tube) or superglue (septa); the root–septa interfaces were sealed by the pressure of the septa and a small amount of superglue.
Two different measurements were performed on four salt-stressed rootstocks and the first four root orders: (i) 0.5 strength LA plus 90 mM NaCl (osmolality: 156±2 mM kg−1; mean ±SE, n=10; Vapro 5520, Wescor) was inserted into the chamber to measure the water fluxes under salt stress (‘salt’ treatment) or (ii) 0.5 strength LA (osmolality: 15±1 mM kg−1) was used to measure the flux rates after rapid release of salt stress (‘stress release’ treatment). In each case, 1.3 ml of aerated (>18 h) solution was injected into the chamber at 9:00 h to allow for 2 h of equilibration before measurement started at 11:00 h.
Determination of water flux rates per root order
In brief, a thin plastic tube attached to a hollow needle was used to connect the ‘miniature depletion chambers’ to a storage container placed on an analytical scale (see above; Supplementary Fig. S1 at JXB online). Both the tube and the storage container were filled with the type of solution added to the chamber. To prevent bias by gravimetric force, solution levels in the storage container and the hydroponic pot were brought to the same height. The weight of the storage container was recorded every minute (Sartorius Connect 1.0; Sartorius, Göttingen, Germany). To induce high, measurable mass flux rates (Fm; g h−1) the period between 11:00 h and 14:00 h was chosen because transpiration maxima (related to temperature maxima and relative humidity minima) were expected during this time.
Five to 11 flux measurements were performed per solution type and per root order 1–4; higher replicate numbers were used if the first five measurements were very heterogeneous. Linear regressions (R2=0.43–0.99, P < 0.01) were performed to determine Fm from the 3 h measurement period and it was correlated with the surface area (cm2) of segments to calculate the water flux density (Js; g cm−2 h−1).
Water flux rates on the level of root branches
The 12 large root branches per plant were used to calculate ‘standardized’ Citrus branches under fresh water and salinity. The biomass and surface area proportions of root orders 1–4 were used to determine their absolute surface areas (SAs; cm2) in a root branch of 1 g dry weight. Root orders 5 and 6 were excluded from estimates of biomass and surface area proportions owing to their small surface area (<4% and <6% SA of large root branches under control treatment and salt stress, respectively) and because they were not measured for water flux.
To determine the mean water flux densities () of the standardized root branches, the water flux densities (Js) under fresh water, salinity, or stress release conditions were weighted by the surface area of the respective root order (A) under fresh water or salt stress, respectively. By setting the total flux rates per branch as 100% and dividing them by the flux rates of the four root orders, the relative proportion of different root orders on the total flux rates of the root branch was calculated.
Statistics
Statistical calculations were conducted with SAS version 9.2 (SAS Institute, Cary, NC, USA). Data sets were tested for Gaussian distribution with the Shapiro–Wilk test and for homogeneity of variances with the Levene test. Because of unbalanced data, a general linear model (PROC GLM) was used to test for significant influences of treatment, order, and interactive effect on root traits and for root order-specific changes in traits between treatments. For traits expressed as percentages, the Bliss angular transformation was applied. To test for salt effect on the root biomass and the root:leaf ratio, the DMT was used as a covariate in PROC GLM. Parametric Tukey test was used for examination of tissue NaCl concentrations. Analyses of variance comparing root order, treatment, and their interaction were performed by the PROC ANOVA procedure for Na+ and Cl− contents in roots; the interaction was removed later because of non-significance. Critical α for all tests was set at ≤0.05.
Results
Plant biomass, total root biomass, and root:leaf biomass ratio
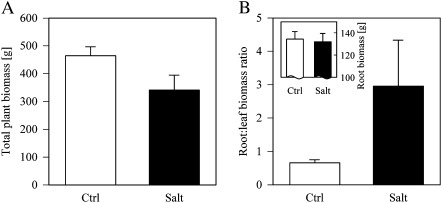
Total plant biomass was reduced by 27% under salinity (Fig. 1A), caused by a major reduction in leaf biomass, some dead branches, and a minor reduction in total root biomass (∼2%; Fig. 1B inset, Table 1). While the reduction in root biomass was marginally significant (P=0.09), the root:leaf biomass ratio increased significantly (P < 0.03) from 0.65 under fresh water to 2.96 after 6 months under high salinity (Fig. 1B, Table 1).
Fig. 1.
(A) Total plant biomass and (B) root:leaf biomass ratio of Citrus spp. after 6 months under fresh water (Ctrl, open bars) and salinity (Salt, filled bars). The inset in B shows the root biomass per treatment (mean ±SE, n=3). See Table 1 for statistics.
Table 1.
GLM results for the effect of salt stress on the root:leaf biomass ratio and root biomass (n=3)
| Parameter | Class | Covariate | |
| Salinity | DMT | ||
| Root:leaf biomass ratio | F | 16.24 | 8.62 |
| P | 0.026 | 0.061 | |
| Root biomass | F | 6.34 | 13.12 |
| P | 0.086 | 0.036 |
The total plant dry mass (DMT) was used as covariate for root:leaf ratio and root biomass.
Root architecture
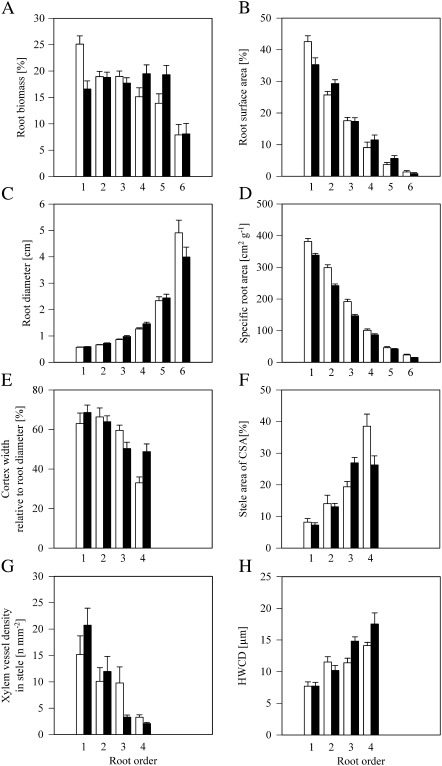
After 6 months in hydroponics, the C. volkameriana rootstocks had eight root orders in total under fresh water (control) and seven root orders when grown under salt stress (data not shown). In both treatments, the highest root order formed the tap root; the first root orders were clearly distinguishable as root tips. The architecture of C. volkameriana rootstocks; that is, the proportion of root orders within the branching root system, changed under salinity in respect to both biomass and surface area (Fig. 2A, B). Both biomass and surface area frequencies changed significantly among root orders 1–6 (P < 0.001) and as an interactive effect of root order and salinity (P < 0.5; Table 2).
Fig. 2.
Structural, morphological, and anatomical traits of Citrus volkameriana root orders 1–4/6 after 6 months under fresh water (ctrl, open bars) or salt stress (salt, filled bars). (A and B) Relative root biomass and root surface area of orders 1–6 in root branches (mean ±SE, n=36). (C and D) Root diameter and specific root area (mean ±SE, n=36–197). (E–H) Cortex diameter relative to root diameter, percentage of the stele on the root cross-section area (CSA), xylem vessel density related to the area of the stele, and hydraulically weighed conduit diameter (HWCD; mean ±SE, n=8). See Table 2 for statistics.
Table 2.
Frequency of root orders (in respect to biomass and surface area; n=36), root diameter, specific root area (n=36–197), cortex and stele dimensions, xylem density, and the hydraulically weighed conduit diameter (HWCD; n=8) were analysed by two-way GLM either pooled or separated by root order
| Parameter | Salinity effect | Root order effect | Salinity × root order | Salinity effect by root order |
||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |||||
| Frequency of root order (biomass)a | F | 0.76 | 135.65 | 10.31 | 14.08 | 0.01 | 0.68 | 2.29 | 3.16 | 0.00 |
| P | 0.385 | <0.001 | 0.001 | <0.001 | 0.925 | 0.412 | 0.134 | 0.079 | 0.954 | |
| Frequency of root order (surface area)a | F | 1.47 | 1780.67 | 4.47 | 6.16 | 4.58 | 0.02 | 0.90 | 3.49 | 0.64 |
| P | 0.226 | <0.001 | 0.035 | 0.015 | 0.035 | 0.089 | 0.345 | 0.065 | 0.424 | |
| Root diameter | F | 12.21 | 1194.64 | 0.20 | 2.01 | 6.39 | 6.34 | 6.45 | 0.25 | 2.33 |
| P | 0.001 | <0.001 | 0.652 | 0.157 | 0.012 | 0.012 | 0.012 | 0.621 | 0.125 | |
| Specific root area | F | 62.54 | 2247.65 | 10.76 | 13.63 | 28.75 | 26.55 | 4.62 | 0.41 | 1.31 |
| P | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | 0.032 | 0.524 | 0.260 | |
| Cortex diameter:root diameter ratio | F | 0.67 | 41.14 | 0.81 | 5.23 | 0.20 | 5.10 | 10.44 | n.d. | n.d. |
| P | 0.416 | <0.001 | 0.372 | 0.026 | 0.664 | 0.041 | 0.006 | |||
| Stele area : root area ratio | F | 0.89 | 115.36 | 2.63 | 0.44 | 0.12 | 9.78 | 6.49 | n.d. | n.d. |
| P | 0.349 | <0.001 | 0.110 | 0.519 | 0.731 | 0.007 | 0.023 | |||
| Xylem density | F | 0.23 | 33.03 | 5.23 | 1.34 | 0.23 | 4.38 | 11.70 | n.d. | n.d. |
| P | 0.636 | <0.001 | 0.026 | 0.267 | 0.636 | 0.055 | 0.004 | |||
| HWCD | F | 4.57 | 86.00 | 6.73 | 0.00 | 1.38 | 11.56 | 3.40 | n.d. | n.d. |
| P | 0.037 | <0.001 | 0.012 | 0.976 | 0.300 | 0.004 | 0.086 | |||
Bliss angular transformed. n.d., not determined.
First-order roots (root tips) provided 25±2% of the biomass under fresh water; under salinity this amount was significantly (P < 0.001) reduced to 17±2% (mean ±SE; Fig. 2A, Table 2). The abundance of the root order-specific biomass declined markedly with increasing root order under fresh water supply; biomass was more homogeneously distributed among root orders 1–5 under salinity. No significant changes were found in biomass frequencies of root orders 2–6 under salt stress (Table 2).
The surface area shares of root orders (SA%) varied from the biomass distribution due to differences in SRAs (see below). Root orders 1 and 2 showed contrasting changes under salinity; the relative SA provided by the first-root order (root tips) decreased significantly (P < 0.05) from 42±2% (Ctrl) to 35±2% (Salt), while the second-order roots accounted for a significantly (P < 0.05) higher percentage (3%) of root branch SA under salinity (29±1%; mean ±SE; Fig. 2B, Table 2). The SA% of root orders 3–6 did not change significantly between treatments.
Morphology of root orders
Root diameter increased significantly under salinity (P < 0.01) and with increasing root order (P < 0.001; Fig. 2C, Table 2). However, when separately analysed by root order, only the diameter increases in root orders 2, 3, and 4 were significant (P < 0.05). For example, the root diameter of third order roots increased from 0.86±0.02 mm (control) to 0.98±0.04 mm under salinity (mean ±SE; Fig. 2C).
The SRA decreased significantly (P < 0.001) with increasing root order under both treatments and was significantly lower (P < 0.001) under salinity (Table 2). The interaction effect between treatment and root order was found to be significant (P < 0.01). Analysed by order, the SRAs of root orders 1–4 were significantly (P < 0.05) reduced under salinity while the SRA of root orders 5 and 6 did not change significantly (Fig. 2D). For example, the SRAs of root orders 1 and 4 were 382±10 cm2 g−1 and 101±4 cm2 g−1 under fresh water and 338±6 cm2 g−1 and 87±4 cm2 g−1 under salt stress. respectively (mean ±SE).
Anatomy of root orders
The cortex thickness increased significantly (P < 0.01) in higher root orders but showed no homogeneous change under salinity (Fig. 2E, Table 2; Supplementary Fig. S2 at JXB online). Split up into root orders, the relative cortex thickness decreased significantly in root order 3 and increased significantly in root order 1 (root tips) and 4 under salinity (P < 0.05).
The proportion of the stele increased significantly (P < 0.001) in higher root orders (Table 2). However, the direction of change differed among orders under salinity: stele proportions were unchanged in first-order roots, increased significantly in third-order roots (∼39%; P < 0.01), and decreased significantly in fourth-order roots (∼32%; P < 0.05; Fig. 2F, Table 2).
The xylem density was significantly lower in higher root orders (P < 0.001) and a significant interaction effect between salinity and root order was found (P < 0.05; Table 2). Fourth-order roots of salt-stressed plants had a significantly (P < 0.01) lower xylem vessel density (2.1±0.2 n mm−2) than control plants (3.3±0.5 n mm−2; mean ±SE, Fig. 2G). No significant changes in xylem density associated with salinity were found for root orders 1–3 (Table 2); however, the xylem vessel density tended to decrease (P=0.06) with salinity in third-order roots.
HWCD increased significantly (P < 0.001) in higher root orders (Fig. 2H; Table 2). Changes in HWCD were also significant (P < 0.05) between treatments and a significant (P < 0.05) interaction effect between salt stress and root order was found. Analysed per root order, the HWCD of root order 3 increased significantly (P < 0.01) from 11.4±0.7 μm under control treatment to 14.8±0.7 μm (mean ±SE) under salt stress, and the HWCD of root order 4 tended to increase (P=0.09) by the same magnitude (Table 2).
No differences in the number of peri- and exodermal layers and the suberinization of the endodermis and the peri-/exodermis were found between treatments (data not shown). However, in higher root orders, larger areas of both endo- and exodermis were suberized compared with low root orders (data not shown).
Na and Cl ion accumulation in leaves and roots
Sodium and chloride ion concentrations increased significantly (P < 0.01) in salt-stressed leaves (7- and 4-fold, respectively; Table 3). In roots, the concentration of both ions differed significantly (P < 0.001) between root orders, with lower concentrations in higher root orders (Table 3, Supplementary Table S1 at JXB online). For example, the Cl− concentration in salt-stressed root tips (root order 1) was 13.65±4.08 mg g d.wt−1, while sixth-order roots had a Cl− concentration of 3.68±0.27 mg g d.wt−1 (mean ±SE). Ion concentrations in low root orders were often significantly (P < 0.05) higher than in leaves (Table 3). Between treatments, concentrations of both ions increased in roots under salinity; while the increase was marginally significant (P = 0.07) for sodium, possibly due to low Na+ concentrations in higher root orders, the increase in chloride concentrations was highly significant (P <0.001; Supplementary Table S1).
Table 3.
Sodium (Na+) and chloride (Cl−) ion concentration in Citrus spp. leaf and root (root orders 1–6) tissues under fresh water (Control) and salinity (90 mM NaCl)
| Organ | Ion concentration in tissues (mg g d.wt−1) |
|||
| Control |
Salt |
|||
| Na+ | Cl− | Na+ | Cl− | |
| Leaves | 1.02±0.10 a | 1.82±0.10 a | 7.49±0.09 a,b | 6.04±0.03 b,c |
| First-order roots | 7.95±1.35 b | 6.84±2.07 b | 10.86±2.98 a | 13.65±4.08 a |
| Second-order roots | 8.19±1.64 b | 7.14±1.22 b | 9.39±1.92 a | 12.84±2.48 a,b |
| Third-order roots | 5.79±1.31 b | 6.21±0.38 b | 6.08±1.48 a,b | 10.38±1.70 a,b,c |
| Fouth-order roots | 2.12±0.39 a | 3.86±0.36 ab | 3.36±0.22 b | 6.45±0.28 a,b |
| Fifth-order roots | 1.08±0.32 a | 2.19±0.22 a | 2.51±0.04 b | 4.62±0.31 c |
| Sixth-order roots | 0.99±0.18 a | 2.00±0.16 a | 2.12±0.03 b | 3.68±0.27 c |
Different lower letters denote differences within columns (mean ±SE; Tukey, P < 0.05, n=3–10); see Supplementary Table S1 at JXB online for ANOVA results on the influence of treatment and root order on the ion concentrations.
Surface areas of root orders within root branches
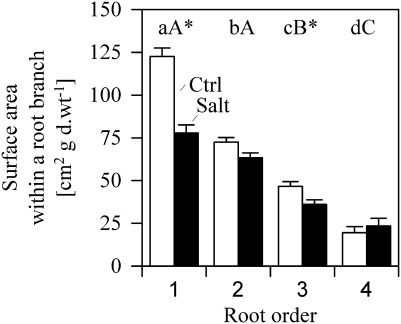
When root systems were analysed on the level of root orders 1–4 (which were measured for water flux density, see below), 1 g of root biomass (d.wt) built 261 cm2 root SA under control conditions and 201 cm2 under salt stress. Approximateloy 39–50% of the SA was provided by root order 1 (root tips); that is, a SA of 122±5 cm2 under fresh water in contrast to 78±5 cm2 under salinity (mean±SE; Fig. 3). Root order 2 accounted for 28–32%, root order 3 for 18%, and the fourth root order for 7–12% of root branch SA. While the SA generally decreased with increasing root order under both control and salinity, no significant difference was found between SA of first and second root orders under salinity. Significant differences were found between the root SA built by root orders 1 and 3 between the fresh water and saline treatment, with significantly lower SA in first- and third-order roots under salinity (P < 0.05).
Fig. 3.
Surface area of root orders 1–4 under fresh water (Ctrl, open bars) and salinity (Salt, filled bars) in a standardized root branch of 1 g d.wt. Different lower/upper case letters denote significant differences between root orders within control and saline treatments respectively; Asterisks denote significant differences between treatments (mean ±SE; Mann–Whitney U-test, P < 0.05, n=189–214).
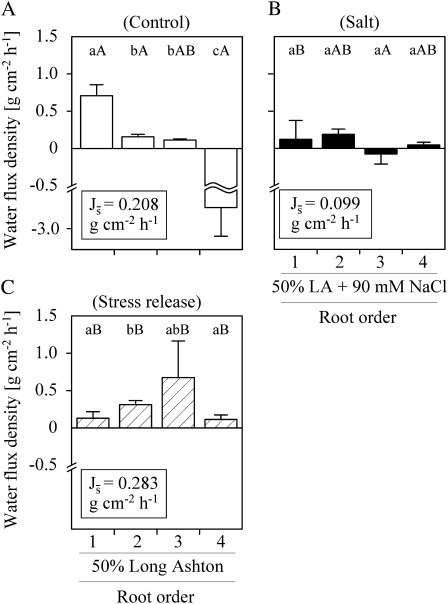
Water flux density
Water flux densities (Js) differed significantly between C. volkameriana root orders and treatments (Fig. 4). Water uptake under fresh water (control) declined significantly (P < 0.05) from root order 1 (root tips) to root orders 2 and 3 (0.71±0.15, 0.16±0.04, and 0.11±0.02 g cm−2 h−1, respectively; mean ±SE); fourth root orders possessed water excess (–2.49±0.69 g cm−2 h−1; Fig. 4A). Under salinity and after stress release, Js of first-order roots was significantly reduced by >80% to 0.12±0.25 g cm−2 h−1 and 0.13±0.09 g cm−2 h−1, respectively (Fig. 4B, C). Mean water flux densities () in root orders 1–4 were generally low under salinity (–0.07 g cm−2 h−1 to 0.18 g cm−2 h−1) while the variability in water flux densities increased significantly as compared with the control (data not shown). Under stress release conditions (i.e. 0.5 LA solution in the miniature depletion chamber, root system placed in 1.0 LA + 90 mM NaCl) all root orders took up water (Fig. 4C). The highest Js values under stress release conditions were found in second- (0.31±0.06 g cm−2 h−1) and third-order roots (0.67±0.49 g cm−2 h−1); second-order roots had significantly (P < 0.05) higher uptake rates of fresh water after stress release than under continuous fresh water treatment (control). The fluxes of stress-released third-order roots were significantly higher than under salinity (P < 0.05; Fig. 4B, C).
Fig. 4.
Water flux density (Js) of Citrus volkameriana root orders 1–4 under (A) fresh water (control), (B) salinity, and (C) stress release conditions. Plants were placed either in 1.0 strength LA (A) or 1.0 strength LA + 90 mM NaCl (B, C). The miniature depletion chambers were filled with either 0.5 strength LA (A, C) or 0.5 LA + 90 mM NaCl (B), respectively. Different lower case letters denote significant differences between root order-specific flux densities; different upper case letters denote significant differences between treatments (mean ±SE; Mann–Whitney U-test, P < 0.05, n=5–11). Mean water flux densities () of root branches of 1 g d.wt. are given.
Up-scaled, using the surface area and Js of root orders, a root branch with four root orders and 1 g d.wt (Fig. 3) had mean water flux densities () of 0.208, 0.099, and 0.283 g cm−2 h−1 under control conditions, salinity, and stress release, respectively (Fig. 4, insets).
The relative contribution of root orders to the branch water flux density differed among treatments (see Supplementary Fig. S3 at JXB online). For example, 57% of the water fluxes were mediated by first-order roots under fresh water (control), in contrast to 38% under salinity and 18% after stress release conditions. Second-order roots mediated 48% of the water fluxes under salinity and 35% after salt release in contrast to 7% under fresh water supply (Supplementary Fig. S3).
Discussion
Changes in phenotype in response to salinity are often adaptive by enhancing the fitness of plants. For example, increased root:shoot ratios are thought to improve the ‘source:sink ratio’ for water and nutrients under salinity (Zekri and Parsons, 1989). In this study, the root:leaf ratio of Citrus increased significantly while the rootstock biomass was marginally reduced under salinity (Fig. 1, Table 1). However, because root functions, such as water uptake, are strongly related to root tissue differentiation (Doussan et al., 1998) and root order (Pregitzer et al., 2002; Comas and Eissenstat, 2009; Rewald et al., 2011a), total root mass and root:shoot ratios cannot effectively determine the functionality of woody root systems under stress. Thus, to predict uptake, knowledge of the active root surface area and the flux density is needed (Hinsinger et al., 2011)
Structural, morphological, and anatomical changes under salinity
Several previous studies on woody species found reduced numbers of lateral roots under salinity (e.g. Reinhard and Rost, 1995; Eshel and Waisel, 1996; Croser et al., 2001); similarly, this study provides evidence that severe NaCl stress reduces the number of Citrus root orders from eight to seven. However, more importantly, the current study shows that the frequency of root orders and morphological and anatomical traits known to influence uptake processes, for example root system branching (Dunabin et al., 2004), SRA (Trubat et al., 2006), cortex thickness (Rieger and Litvin, 1999), and xylem traits (Rodríguez-Gamir et al., 2010), do not change homogenously throughout C. volkameriana root systems under salinity but that changes are often root order specific (Table 2).
Changes among order frequencies of Citrus roots were previously reported under altered nitrate supply (Sorgonà et al., 2007, 2011). Similarly, in this study, lower root orders, especially root tips, were most plastic in frequency, expressed as both relative biomass and surface area per root branch (Fig. 2A, B, Table 2). Because low root orders have a high SRA, the 3% loss in total root system biomass under salinity caused a major reduction of active root surface area by ∼23% (Figs 1–3). Because uptake is strongly coupled to root SA (e.g. Korn, 2004), the reduced SA provided by root tips under salinity is indicative of a decrease in functionality, in terms of uptake, of this order. In contrast, the relative importance of root orders ≥2 for water and/or nutrient uptake should increase accordingly. The high plasticity of first (second) root orders in respect of biomass (SA) frequency and morphology was anticipated as lower root orders have relatively high turnover rates (Guo et al., 2008b, and references within) and are considered most vulnerable to environmental stresses. However, in addition to results from Citrus spp., seedlings under varied nitrate supply, in which the morphology of second and third (i.e. tap roots of these seedlings) root orders were considerably less plastic than those of root tips (Sorgonà et al., 2007, 2011), the present result showed that even intermediate, third and fourth, root orders of more mature plants are able to undergo significant morphological changes within 6 months (Table 2).
Similar to earlier reports, the cortex diameter decreased and the proportion of the stele increased in higher root orders under fresh water supply (Fig. 2E, F; Guo et al., 2008a). The increase in root diameter under salinity was expected to be caused by increasing cortex dimensions as reported, for example, for cotton roots (Casenave et al., 1999). However, under salinity, significant changes of cortex and stele dimensions were found in third and fourth root orders only, and reaction norms differed in direction (Fig. 2E, F, Table 2). It is hypothesized that the contrasting changes are related to the different functions of these two root orders for water uptake under fresh water; in brief, third-order roots of C. volkameriana were found to perform water uptake under fresh water supply, while fourth-order roots showed water excess (Fig. 4A). The outflow of water in fourth-order roots was related to changes in chamber solute osmolalities, possible caused by exudation (for details, see Rewald et al., 2011a).
The lack of significant changes in gross root anatomy (i.e. cortex and stele dimension) and xylem traits in first and second root orders was surprising because these root orders have a larger number of passage cells (Eissenstat and Achor, 1999), and are the preferred sites of water and nutrient uptake under fresh water (Fig. 4; Supplementary S2 at JXB online; Peterson and Enstone, 1996; Rewald et al., 2011a). Previous studies on cotton radicles and roots of herbaceous plants found smaller xylem vessels at higher frequencies under salinity, probably caused by a repression in the development of metaxylem vessels and altered cambial activity (Reinhardt and Rost, 1995; Casenave et al., 1999; Boughalleb et al., 2009). However, wider and fewer xylem vessels have been found in both stems and some coarse roots after exposure to salinity (e.g. Eckstein et al., 1978, Rewald et al., 2011c), thus the present results might indicate a different reaction norm in ephemeral roots (such as root order 1 and 2) and more persistent woody roots (such as root order 3 and 4) under salinity. While the underlying molecular mechanisms and the functional significance of these changes in the xylem structure remain open, the differences between root orders under salt stress are probably related to the varied accumulation of sodium and chloride ions (Table 3). Lower salt concentrations are suggested to impair the cambial activity and metaxylem differentiation in higher root orders to a lesser extent. Suberization of the endo- and exodermis increased in older (higher) Citrus root orders (data not shown) as found elsewhere (e.g. Eissenstat and Volder, 2005) and is likely to be one factor underlying the significantly lower Na+ and Cl− accumulation in higher root orders of C. volkameriana (Table 3, Supplementary Table S1 at JXB online; Krishnamurthy et al., 2009). In contrast to findings in herbaceous plants, which often have lower Na+ and Cl− concentrations in roots than the external solution (Munns, 2002), salt accumulation in Citrus root orders 1–5 was explicitly higher than those of the surrounding solution and partially higher than those in leaves (see also Arbona et al., 2005). Because physiological damage in Citrus spp. is associated with tissue chloride build-up rather than with sodium accumulation (Romero-Aranda et al., 1998), the high Cl− concentration in lower root orders backs up the hypothesis that many structural, morphological, and anatomical changes were driven by accumulating salt.
Effect of salinity on root water uptake
It was hypothesized that changes in water flux rates in salt-stressed Citrus rootstocks are related to changes in both root surface area and root anatomy/physiology, as originally suggested by Storey and Walker (1999). As mentioned above, changes were observed in abundance and SRA of root orders 1–4 that resulted in a 23% reduction of root branch surface area under salinity compared with fresh water (Fig. 3). Changes among anatomical (see above) and physiological parameters (e.g. membrane properties) are also known to modify root hydraulic conductivity (Lpr; Peterson and Enstone, 1996; Steudle, 2000; Vandeleur et al., 2009). In the present study we did not find major changes in gross root anatomy; thus, besides the lower water potential of the salt solution, fine-scale anatomical or physiological changes and damage caused by high salt accumulation (see above) have probably contributed to the 50% reduction of root branch mean water flux densities () in this study (Fig. 4A, B). This is supported by the finding that Js did not increase in first-order roots after release of the salt stress (Fig. 4C) as expected if only temporarily caused by the lower water potential of the salt solution. Because water flow rates among Citrus root systems follow the same trend as root conductivities (Zekri and Parsons, 1989), it is valid to hypothesize that the reduction of in salt-stressed Citrus rootstocks is related to changes in both root surface area and root physiology.
Interestingly, the water flow densities (Js) of root orders changed differently under salinity (Fig. 4A, B). The decrease in under salinity was mainly caused by a significant reduction (>80%) of the water uptake by first-order roots. The degree of Js reduction is in accordance with measurements on apical segments of corn roots, among which Lpr was reduced by 80% under salinity (Evlagon et al., 1990). The water flux densities of the other three Citrus root orders did not change significantly under salinity (Fig. 4B). Together with the different reduction in surface areas (see above), the varying water flux densities are changing the contribution of root orders to the overall water flux density of the root system (Fig. 4, Supplementary Fig. S3A, B at JXB online). For example, second-order roots contributed only 7% to the water fluxes under fresh water but nearly 50% under salinity. The increased importance of higher root orders for water uptake under salinity may help to explain previous results of Zekri and Parsons (1989) who found the highest reductions of root length under salinity in Citrus species which were most tolerant to salinity in terms of water flow rate or root conductivity. This has been thought to be in contrast to studies which found that Citrus rootstocks with high specific root lengths tend to exhibit high hydraulic conductivities under fresh water supply (Graham and Syvertsen, 1985; Eissenstat, 1997). However, the overall root length density might cause an overestimation of the water uptake capacity under salt stress if thin, first-order roots contribute less to water uptake (as seen in the current study). This might be true as well if parts of the root system are temporarily released from salt stress; in this study, second-order and third-orders roots contributed more to water uptake under stress relief than under both control and salt treatments (Fig. 4, Supplementary Fig. S3C). A temporal and spatial release of roots from salinity might occur in situ, for example in saline water-irrigated orchards (during ‘salt leaching’) or after rainfall events (Cimato et al., 2010).
While Sorgonà et al. (2007) stated that more distal root orders have a prominent adaptive significance in Citrus, possibly due to their high number of passage cells (Eissenstat and Achor, 1999), this study demonstrated that the importance of specific root orders for water uptake and tolerance of the whole root system is subject to changes in response to environmental conditions. The underlying anatomical and physiological factors still remain open but root order-specific changes in the development of Casparian bands, suberin lamellae, passage cells (Peterson et al., 1993; Peterson and Enstone, 1996), aquaporin expression (Vandeleur et al., 2009), or a different susceptibility to reactive oxygen species (Li et al., 2009) or salt accumulation (this study) might have resulted in the observed differences. Further investigation is needed to determine the parameters underlying the (i) different susceptibility of root tissues to salt stress and ion accumulation and (ii) the different water flux densities among root orders, and should seek to compare the function of different salt-tolerant rootstocks on the level of root orders.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Schematic side view of a ‘miniature depletion chamber’ (A), attached to a second-order root, and a drawing of the experimental set-up (B).
Figure S2. Photographs of Citrus volkameriana root orders 1–4 after 6 months under fresh water (A–D) and salinity (E–H).
Figure S3. Relative contribution of root orders 1–4 to the total water flux (100%) under (A) fresh water (control), (B) salt stress, and (C) after stress release.
Table S1. Influence of treatment and root order on the Na+ and Cl− concentrations in Citrus volkameriana roots.
Acknowledgments
The authors wish to thank L. Summerfield and O. Shelef for their help regarding root dissection and image analyses. L. Rose and two anonymous reviewers provided helpful comments on earlier drafts of the manuscript. BR was partially supported by a post-doctoral fellowship awarded by the Jacob Blaustein Center for Scientific Cooperation (BCSC), Israel.
References
- Arbona V, Marco AJ, Iglesias DJ, López-Climent MF, Talon M, Gómez-Cadenas A. Carbohydrate depletion in roots and leaves of salt-stressed potted Citrus clementina L. Plant Growth Regulation. 2005;46:153–160. [Google Scholar]
- Boughalleb F, Denden M, Tiba B. Anatomical changes induced by increasing NaCl salinity in three fodder shrubs, Nitraria retusa, Atriplex halimus and Medicago arborea. Acta Physiologiae Plantarum. 2009;31:947–960. [Google Scholar]
- Bramley H, Turner NC, Turner DW, Tyerman SD. Comparison between gradient-dependent hydraulic conductivities of roots using the root pressure probe: the role of pressure propagations and implications for the relative roles of parallel radial pathways. Plant, Cell and Environment. 2007;30:861–874. doi: 10.1111/j.1365-3040.2007.01678.x. [DOI] [PubMed] [Google Scholar]
- Brundrett MC, Enstone DE, Peterson CA. A berberine–aniline blue fluorescent staining procedure for suberin, lignin, and callose in plant tissue. Protoplasma. 1988;146:133–142. [Google Scholar]
- Casenave EC, Degano CAM, Toselli ME, Catan EA. Statistical studies on anatomical modifications in the radicle and hypocotyl of cotton induced by NaCl. Biological Research. 1999;32:289–295. [Google Scholar]
- Cimato A, Castelli S, Tattini M, Traversi ML. An ecophysiological analysis of salinity tolerance in olive. Environmental and Experimental Botany. 2010;68:214–221. [Google Scholar]
- Comas LH, Eissenstat DM. Patterns in root trait variation among 25 co-existing North American forest species. New Phytologist. 2009;182:919–928. doi: 10.1111/j.1469-8137.2009.02799.x. [DOI] [PubMed] [Google Scholar]
- Croser C, Renault S, Franklin J, Zwiazek J. The effect of salinity on the emergence and seedling growth of Picea mariana, Picea glauca, and Pinus banksiana. Environmental Pollution. 2001;115:9–16. doi: 10.1016/s0269-7491(01)00097-5. [DOI] [PubMed] [Google Scholar]
- Deak KI, Malamy J. Osmotic regulation of root system architecture. The Plant Journal. 2005;43:17–28. doi: 10.1111/j.1365-313X.2005.02425.x. [DOI] [PubMed] [Google Scholar]
- Doussan C, Vercambre G, Pagès L. Modelling of the hydraulic architecture of root systems: an integrated approach to water absorption – distribution of axial and radial conductances in maize. Annals of Botany. 1998;81:225–232. [Google Scholar]
- Dunbabin VM, Rengel Z, Diggle AJ. Simulating form and function of root systems: efficiency of nitrate uptake is dependent on root system architecture and the spatial and temporal variability of nitrate supply. Functional Ecology. 2004;18:204–211. [Google Scholar]
- Eckstein D, Liese W, Plossl P. Histometrische Untersuchungen zur unterschiedlichen Streusalztoleranz von Weiden (Salix spp.) Forstwissenschaftliches Centralblatt. 1978;97:335–341. [Google Scholar]
- Eissenstat DM. Trade-offs in root form and function. In: Jackson LE, editor. Ecology in agriculture. San Diego, CA: Academic Press; 1997. pp. 173–199. [Google Scholar]
- Eissenstat DM, Achor DS. Anatomical characteristics of roots of citrus rootstocks that vary in specific root length. New Phytologist. 1999;141:309–321. doi: 10.1046/j.1469-8137.1999.00342.x. [DOI] [PubMed] [Google Scholar]
- Eissenstat DM, Volder A. The efficiency of nutrient acquisition over the life of a root. In: BassiriRad H, editor. Nutrient acquisition by plants—an ecological perspective. Berlin: Springer; 2005. pp. 185–220. [Google Scholar]
- Eshel A, Waisel Y. Multiform and multifunction of various constituents of one root system. In: Waisel Y, Eshel A, Kafkafi U, editors. Plant roots: the hidden half. New York: Marcel Dekker; 1996. pp. 175–192. [Google Scholar]
- Evlagon D, Ravina I, Neumann P. Interactive effects of salinity and calcium on hydraulic conductivity, osmotic adjustment, and growth in primary roots of maize seedlings. Israel Journal of Botany. 1990;39:239–247. [Google Scholar]
- Graham JH, Syvertsen JP. Host determinants of mycorrhizal dependency of citrus rootstock seedlings. New Phytologist. 1985;101:667–676. [Google Scholar]
- Gruber V, Zahaf O, Diet A, Zélicourt A, Lorenzo L, Crespi M. Impact of the environment on root architecture in dicotyledoneous plants. In: Oliveira ACd, Varshney RK., editors. Root genomics. Heidelberg: Springer; 2011. pp. 113–132. [Google Scholar]
- Gucci R, Tattini M. Salinity tolerance in olive. Horticultural Reviews. 1997;21:177–214. [Google Scholar]
- Guo DL, Li H, Mitchell RJ, Han W, Hendricks JJ, Fahey TJ, Hendrick RL. Fine root heterogeneity by branch order: exploring the discrepancy in root turnover estimates between minirhizotron and carbon isotopic methods. New Phytologist. 2008b;177:443–456. doi: 10.1111/j.1469-8137.2007.02242.x. [DOI] [PubMed] [Google Scholar]
- Guo DL, Xia MX, Wei X, Chang WJ, Liu Y, Wang ZQ. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytologist. 2008a;180:673–683. doi: 10.1111/j.1469-8137.2008.02573.x. [DOI] [PubMed] [Google Scholar]
- Hinsinger P, Brauman A, Devau N, Gérard F, Jourdan C, Laclau JP, Le Cadre E, Jaillard B, Plassard C. Acquisition of phosphorus and other poorly mobile nutrients by roots. Where do plant nutrition models fail? Plant and Soil. 2011;348:29–61. [Google Scholar]
- Huang G, Zhao XY, Zhao HL, Huang YX, Zuo XA. Linking root morphology, longevity and function to root branch order: a case study in three shrubs. Plant and Soil. 2010;336:197–208. [Google Scholar]
- Korn S. Experimental investigation of water uptake and hydraulic properties of the root system of six European tree species (in German) 2004 PhD thesis, University of Göttingen, Germany. [Google Scholar]
- Kozlowski TT. Responses of woody plants to flooding and salinity. Tree Physiology Monograph. 1997;1:1–29. [Google Scholar]
- Krishnamurthy P, Ranathunge K, Franke R, Prakash H, Schreiber L, Mathew M. The role of root apoplastic transport barriers in salt tolerance of rice (Oryza sativa L.) Planta. 2009;230:119–134. doi: 10.1007/s00425-009-0930-6. [DOI] [PubMed] [Google Scholar]
- Levy Y, Shalhevet J. Ranking the salt tolerance of citrus rootstocks by juice analysis. Scientia Horticulturae. 1990;45:89–98. [Google Scholar]
- Lewis AM, Boose ER. Estimating volume flow-rates through xylem conduits. American Journal of Botany. 1995;82:1112–1116. [Google Scholar]
- Li HY, Wang YC, Jiang J, Liu GF, Gao CQ, Yang CP. Identification of genes responsive to salt stress on Tamarix hispida roots. Gene. 2009;433:65–71. doi: 10.1016/j.gene.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Maas EV. Salinity and citriculture. Tree Physiology. 1993;12:195–216. doi: 10.1093/treephys/12.2.195. [DOI] [PubMed] [Google Scholar]
- Munns R. Comparative physiology of salt and water stress. Plant, Cell and Environment. 2002;25:239–250. doi: 10.1046/j.0016-8025.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- Ottow EA. Molecular and ecophysiological responses of Populus euphratica (Oliv.) and Arabidopsis thaliana (L.) to salt stress. 2005 PhD thesis, University of Göttingen, Germany. [Google Scholar]
- Pagès L, Kervella J. Growth and development of root systems: geometrical and structural aspects. Acta Biotheoretica. 1990;38:289–302. [Google Scholar]
- Peterson CA, Enstone DE. Functions of passage cells in the endodermis and exodermis of roots. Physiologia Plantarum. 1996;97:592–598. [Google Scholar]
- Peterson CA, Murrmann M, Steudle E. Location of the major barriers to water and ion movement in young roots of Zea mays L. Planta. 1993;190:127–136. [Google Scholar]
- Pitman M, Läuchli A. Global impact of salinity and agricultural ecosystems. In: Läuchli A, Lüttge U, editors. Salinity: environment—plants—molecules. Dordrecht, The Netherlands: Springer; 2004. pp. 3–20. [Google Scholar]
- Pregitzer KS, DeForest JL, Burton AJ, Allen MF, Ruess RW, Hendrick RL. Fine root architecture of nine North American trees. Ecological Monographs. 2002;72:293–309. [Google Scholar]
- Ramin AA, Alirezanezhad A. Effects of citrus rootstocks on fruit yield and quality of Ruby red and Marsh grapefruit. Fruits. 2005;60:311–317. [Google Scholar]
- Raveh E. Methods to assess potential chloride stress in citrus: analysis of leaves, fruit, stem-xylem sap and roots. Horttechnology. 2005;15:17–21. [Google Scholar]
- Reinhardt DH, Rost TL. On the correlation of primary root growth and tracheary element size and distance from the tip in cotton seedlings grown under salinity. Environmental and Experimental Botany. 1995;35:575–588. [Google Scholar]
- Rewald B, Ephrath JE, Rachmilevitch S. A root is a root is a root? Water uptake rates of Citrus root orders. Plant, Cell and Environment. 2011a;34:33–42. doi: 10.1111/j.1365-3040.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- Rewald B, Leuschner C. Belowground competition in a broad-leaved temperate mixed forest: pattern analysis and experiments in a four-species stand. European Journal of Forest Research. 2009;128:387–398. [Google Scholar]
- Rewald B, Leuschner C, Wiesman Z, Ephrath JE. Influence of salinity on root hydraulic properties of three olive varieties. Plant Biosystems. 2011c;145:12–22. [Google Scholar]
- Rewald B, Rachmilevitch S, McCue MD, Ephrath JE. Influence of saline drip-irrigation on fine root and sap-flow densities of two mature olive varieties. Environmental and Experimental Botany. 2011b;72:107–114. [Google Scholar]
- Rieger M, Litvin P. Root system hydraulic conductivity in species with contrasting root anatomy. Journal of Experimental Botany. 1999;50:201–209. [Google Scholar]
- Rodríguez-Gamir J, Intrigliolo DS, Primo-Millo E, Forner-Giner MA. Relationships between xylem anatomy, root hydraulic conductivity, leaf/root ratio and transpiration in citrus trees on different rootstocks. Physiologia Plantarum. 2010;139:159–169. doi: 10.1111/j.1399-3054.2010.01351.x. [DOI] [PubMed] [Google Scholar]
- Romero-Aranda R, Moya JL, Tadeo FR, Legaz F, Primo-Millo E, Talon M. Physiological and anatomical disturbances induced by chloride salts in sensitive and tolerant citrus: beneficial and detrimental effects of cations. Plant, Cell and Environment. 1998;21:1243–1253. [Google Scholar]
- Ruzin SE. Plant microtechnique and microscopy. New York: Oxford University Press; 1999. [Google Scholar]
- Schulte PJ. Water flow through junctions in Douglas fir roots. Plant, Cell and Environment. 2006;29:70–76. doi: 10.1111/j.1365-3040.2005.01401.x. [DOI] [PubMed] [Google Scholar]
- Sorgonà A, Abenavoli MR, Gringeri PG, Cacco G. Comparing morphological plasticity of root orders in slow- and fast-growing Citrus rootstocks supplied with different nitrate levels. Annals of Botany. 2007;100:1287–1296. doi: 10.1093/aob/mcm207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgonà A, Lupini A, Abenavoli MR. Nitrate use-efficiency: a morphological analysis of the above- and below-ground functional traits in two Citrus rootstocks. Global Journal of Plant Ecophysiology. 2011;1:26–37. [Google Scholar]
- Steudle E. Water uptake by plant roots: an integration of views. Plant and Soil. 2000;226:45–56. [Google Scholar]
- Storey R, Walker RR. Citrus and salinity. Scientia Horticulturae. 1999;78:39–81. [Google Scholar]
- Sultan SE. Phenotypic plasticity for plant development, function and life history. Trends in Plant Science. 2000;5:537–542. doi: 10.1016/s1360-1385(00)01797-0. [DOI] [PubMed] [Google Scholar]
- Trubat R, Cortina J, Vilagrosa A. Plant morphology and root hydraulics are altered by nutrient deficiency in Pistacia lentiscus (L.) Trees-Structure and Function. 2006;20:334–339. [Google Scholar]
- Vadez V, Krishnamurthy L, Kashiwagi J, et al. Exploiting the functionality of root systems for dry, saline, and nutrient deficient environments in a changing climate. SAT eJournal. 2007;4:1–61. [Google Scholar]
- Valenzuela-Estrada LR, Vera-Caraballo V, Ruth LE, Eissenstat DM. Root anatomy, morphology, and longevity among root orders in Vaccinium corymbosum (Ericaceae) American Journal of Botany. 2008;95:1506–1514. doi: 10.3732/ajb.0800092. [DOI] [PubMed] [Google Scholar]
- Vandeleur RK, Mayo G, Shelden MC, Gilliham M, Kaiser BN, Tyerman SD. The role of plasma membrane intrinsic protein aquaporins in water transport through roots: diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiology. 2009;149:445–460. doi: 10.1104/pp.108.128645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RR, Sedgley M, Blesing MA, Douglas TJ. Anatomy, ultrastructure and assimilate concentrations of roots of Citrus genotypes differing in ability for salt exclusion. Journal of Experimental Botany. 1984;35:1481–1494. [Google Scholar]
- Wang H, Siopongco J, Wade LJ, Yamauchi A. Fractal analysis on root systems of rice plants in response to drought stress. Environmental and Experimental Botany. 2009;65:338–344. [Google Scholar]
- Wang ZQ, Guo DL, Wang XR, Gu JC, Mei L. Fine root architecture, morphology, and biomass of different branch orders of two Chinese temperate tree species. Plant and Soil. 2006;288:155–171. [Google Scholar]
- Zekri M, Parsons LR. Growth and root hydraulic conductivity of several citrus rootstocks under salt and polyethylene glycol stresses. Physiologia Plantarum. 1989;77:99–106. [Google Scholar]
- Zekri M, Parsons LR. Salinity tolerance of Citrus rootstocks: effects of salt on root and leaf mineral concentrations. Plant and Soil. 1992;147:171–181. [Google Scholar]
- Zolla G, Heimer YM, Barak S. Mild salinity stimulates a stress-induced morphogenic response in Arabidopsis thaliana roots. Journal of Experimental Botany. 2010;61:211–224. doi: 10.1093/jxb/erp290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwieniecki MA, Boersma L. A technique to measure root tip hydraulic conductivity and root water potential simultaneously. Journal of Experimental Botany. 1997;48:333–336. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.