Abstract
Family 1 glycosyltransferases catalyse the glycosylation of small molecules and play an important role in maintaining cell homeostasis and regulating plant growth and development. In this study, a putative glycosyltransferase gene of family 1, PtGT1, was cloned from poplar (Populus tomentosa Carr.). Sequence analysis showed that this gene encodes a protein of 481 amino acid residues with a conserved PSPG box at its C-terminal, suggesting that it is active in the glycosylation of plant secondary products. The PtGT1 gene was expressed in poplar stems and leaves, with a particularly high expression level in elongating stems. Transgenic tobacco plants ectopically over-expressing PtGT1 were obtained and phenotypes were analysed. Wiesner and Mäule staining showed that stem xylem of transgenic tobacco plants stained more strongly than controls. Measurement of the Klason lignins showed much higher lignin content in the transgenic lines than in control plants. Furthermore, the ectopic over-expression of PtGT1 in tobacco resulted in an early flowering phenotype. These findings offer a possible starting point towards better understanding of the function of poplar PtGT1, and provide a novel strategy for lignin engineering and flowering control in plants through the genetic manipulation of a poplar glycosyltransferase gene.
Keywords: Flowering, glycosyltransferase, lignin, poplar, tobacco
Introduction
Glycosylation occurs in a wide range of biological processes in plants and is thought to play an important role in the production of a range of plant compounds. Glycosyltransferases (GTs) are enzymes responsible for the glycosylation of plant compounds. Substrate recognition, sequence similarity, and phylogenetic analysis have shown that GTs can be divided into 94 distinct families. Family 1 contains the greatest number of GTs found in plants and most contain a carboxy-terminal consensus sequence termed the ‘plant secondary product glycosyltransferase box’ (PSPG box). Family 1 GTs usually recognize substrates of low-molecular-weight lipophilic compounds and use uridine 5'-diphospho sugars as the sugar donors, and are thus termed UDP-sugar glycosyltransferases (UGTs) (Jones and Vogt, 2001; Bowles et al., 2005; Wang and Hou, 2009). Completion of the genome sequence analysis of Arabidopsis thaliana has allowed comprehensive analysis of the multigene families of glycosyltransferases and the discovery of a large glycosyltransferase superfamily consisting of 119 putative UGT genes (Li et al., 2001).
Although glycosyltransferase activities and glycosylated products have long been known in a variety of plants, the enzymes and genes involved in glycosylation have only recently been isolated, and their roles in plant growth and development are now better understood. In recent years, dozens of glycosyltransferase genes have been identified, and many have been functionally characterized. It is now recognized that the glycosylation of low-molecular-weight compounds in plants, through the addition of a sugar moiety to the acceptors, usually changes acceptors in terms of their bioactivity, stability, solubility, subcellular localization, and binding properties to other molecules (Bowles et al., 2005). Therefore, glycosyltransferases might have an important role in maintaining cell homeostasis and regulating plant growth, development, and defence responses to stressful environments (Jones and Vogt, 2001; Lim and Bowles, 2004). For example, glycosyltransferases have been shown to play a role in processes such as the synthesis of various secondary metabolites, the modification of hormones, detoxification, and cell wall synthesis (Hou et al., 2004; Wang and Hou, 2009). The study of glycosyltransferases and glycosylation of plant molecules has attracted considerable research interest because understanding the catalytic mechanisms of these enzymes and their physiological roles would be of great significance for in vitro design and synthesis of valuable glycosides, and for in vivo metabolic engineering of crops for important agronomic traits (Kristensen et al., 2005; Lim, 2005; Weis et al., 2008).
Poplar (Populus spp.) is a woody plant of great commercial and ecological value. Its small genome size, ease of transformation, and rapid growth make it an ideal model for other forest trees. Publication of the Populus trichocarpa genome offers unique opportunities to characterize glycosyltransferase genes in at least one woody plant model system. Recently, Geisler-Lee et al. (2006) identified over 1 600 genes encoding carbohydrate-active enzymes (CAZymes), including glycosyltransferases, in P. trichocarpa and found some key differences in metabolism and gene expression of the CAZyme at the genomic scale between it and the herbaceous Arabidopsis. Approximately 840 GTs were identified in their study. Interestingly, the GT1 family of secondary metabolite-glycosylating enzymes was the largest and most diverse gene family identified, and much larger in poplar than in Arabidopsis, perhaps due to additional specialized functions in processes such as wood formation, dormancy, and longevity. The functions of several individual poplar glycosyltransferase genes have also been characterized. For example, Zhou et al. (2007) cloned two poplar glycosyltransferase genes, PoGT8D and PoGT43B (belonging to the GT8 and GT43 families, respectively) and found that they were associated with the secondary wall and involved in glucuronoxylan biosynthesis. Although the largest GT1 family of poplar has been identified at the genome scale, little is known so far about the impact of individual poplar GT1 family genes on plant growth and development. No association between glycosyltransferase and lignin biosynthesis has yet been reported.
In this study, the cloning of a putative GT1 family glycosyltransferase gene, PtGT1, from poplar is reported and its over-expression in tobacco analysed. It was found that the over-expression of PtGT1 in tobacco substantially increases lignin content. Moreover, the expression of PtGT1 also results in an early flowering phenotype. Our data suggest a possible starting point for deeper studies into the function of poplar PtGT1, and a novel approach to lignin engineering and flowering control in plants using a genetically modified poplar glycosyltransferase gene.
Materials and methods
Plant materials and growth conditions
Plant materials used in this study were Chinese white poplar (Populus tomentosa Carr.) and tobacco (Nicotiana tabacum Linn, Wisconsin 38). Their tissue cultures and regenerated plantlets were maintained in a culture room at 23±2 °C under a 14/10 h light/dark photoperiod, and a light intensity of 60 μmol·m−2 s−1. Plants grown in flowerpots in soil–Perlite mixtures were maintained in a greenhouse at 25±2 °C under a 16/8 h light/ dark photoperiod and a light intensity of 100 μmol·m−2 s−1.
Isolation of poplar GT cDNA
Populus trichocarpa was used as a bridge to obtain the P. tomentosa PCR primers for the amplification of the full-length glycosyltransferase gene PtGT1 because its genome had already been sequenced. The P. trichocarpa genome database (http://genome.jgi-psf.org/Poptr1/Poptr1.home.html) was first searched for glycosyltransferase homologues of Arabidopsis thaliana UGT72E1-E3 cDNA, which were already known to glucosylate several phenylpropanoids (Lim et al., 2001) and were used as reference sequences in this study. Two primers were designed according to the P. trichocarpa homologues obtained: a forward primer 5′-ATAGGATCCATGCAAAACACAAAACCTCA-3′ with a BamHI cloning site at the 5′ end; and a reverse primer 5′-ATACCCGGGCTAGGCACCTTGAGCCTTTG-3′ with a SmaI cloning site at the 5′ end. Populus tomentosa total RNA was extracted from its young shoots using the Trizol method, and this RNA was used in an RT-PCR reaction using the primer pairs mentioned above. The amplified products of three separate RT-PCRs were cloned into appropriate intermediate vectors and sequenced. After comparing the sequence results to determine the consistent sequences, the clone containing the correct GT cDNA sequences of P. tomentosa was selected. PtGT1 cDNA sequences were deposited in GenBank under the accession number HM776516.
Sequence analysis of PtGT1
The conserved protein domain sequences of poplar PtGT1 and Arabidopsis UGT72E1-E3 were obtained from the Proteomics Server of the Expert Protein Analysis system (ExPASy) of the Swiss Institute of Bioinformatics (http://cn.expasy.org; (Gasteiger et al., 2003) using their cDNA sequences. The amino acid sequences of PtGT1 and UGT72E1-E3 were aligned using the ClustalX program and the online website (http://www.ch.embnet.org/software/BOX_form.html). Genetic distance matrices were obtained from the alignments using ClustalX 2 and Neighbor–Joining trees constructed with bootstrap sampling of 1000 replications using MEGA 4.0 programs (Zhou et al., 2006, 2007; An et al., 2010). The sequences of all the published Arabidopsis family 1 GTs used in the phylogenetic tree were obtained from the Carbohydrate-active enzymes database ( http://www.cazy.org/GT1_eukaryota.html) and the NCBI database (Aspeborg et al., 2005)
Expression pattern analysis of PtGT1 in poplar (Populus. tomentosa Carr.)
For the expression pattern analyses of PtGT1, 2-month-old seedlings regenerated from poplar tissue culture were cut into six parts: young leaves, mature leaves, top stems (rapidly elongating stems), middle stems (elongating stems near cessation), bottom stems (non-elongating stems), and roots. RNA was isolated from each of the six parts using the same method as described in the section relating to RT-PCR analysis of the transgenic tobacco plants. The RT-PCR reactions were repeated three times with identical results. The expression level of the 18S rRNA reference gene (primers: 5′-CTGCCCGTTGCTCTGATGATTCA-3′ and 5′-CCTTGGATGTGGTAGCCGTTTCT-3′) was used as an internal control.
Construction of the expression vector
The PtGT1 cDNA was cut out from the intermediate plasmid using the BamHI and SmaI restriction endonucleases. Plant expression plasmid pBI121 was cut with SacI, blunted and then cut again with BamHI. The PtGT1 cDNA was inserted into the pBI121 plasmid, behind the CaMV 35S promoter, to produce the recombinant expression vector pBI121-PtGT1. The recombinant plasmid was then confirmed by appropriate restriction endonuclease digestion and transferred to tobacco using the Agrobacterium-mediated method (Li et al., 1992).
Plant transformation and transgenic plant regeneration
Leaf discs from 6-week-old sterile tobacco seedlings were prepared for the transformation experiment. Leaf discs were immersed for 20 min in a suspension of recombinant A. tumefaciens LBA4404 containing plant expression vector pBI121-PtGT1, and were then dried on sterile filter paper.The leaf discs were then co-cultured with recombinant A. tumefaciens LBA4404 for 2 d on a solid MS medium (Murashige and Skoog, 1962) in the dark. The leaf discs were then transferred to a differentiation medium (a solid MS medium with 1 mg l−1 6-BA, 0.1 mg l−1 IAA, 500 mg l−1 cephalosporins, and 100 mg l−1 kanamycin). Once the buds of the T0 generation had grown sufficiently, they were transferred to a root-inducing medium (1/2 solid MS medium with 500 mg l−1 cephalosporins and 100 mg l−1 kanamycin). T0 plants with developed root systems were transferred to flowerpots and grown in a greenhouse until T1 seed set. T2 homozygous transgenic plants were used for the transgene expression analysis and further experiments.
Identification of the transgenic tobacco plants by PCR and RT-PCR
A total of 100 mg of fresh leaves from wild-type (WT) and T2 homozygous transgenic tobacco plants were used for DNA extraction using the method described by Yoshimura et al. (2004). About 100 ng of their genomic DNA was used to amplify the target gene PtGT1 using PCR. The PtGT1-F primer (5′-CGACAACCAACCCTACGAAT-3′) and the PtGT1-R primer (5′-GAGGAACCACCTTTACTGCT-3′) were used in the PCR with the following thermal regime: an initial 94 °C for 5 min; followed by 31 cycles of 94 °C for 50 s, 56 °C for 50 s, 72 °C for 1 min; with a final 72 °C for 10 min. Agarose electrophoresis (1% agarose) was performed and the amplified bands photographed under UV light.
For the RT-PCR analysis, total RNA was isolated using Trizol (Invitrogen, Auckland, New Zealand) according to the manufacturer’s instructions. Approximately 30 μg of the total RNA was treated with RNase-free DNase. RNA concentration and quality were assessed photometrically (Biophotometer; Eppendorf, Hamburg, Germany) and by gel electrophoresis. Approximately 3 μg of total RNA was utilized to synthesize single-strand cDNA using reverse transcriptase (Superscript III, Invitrogen) and oligo-DT18 primers, according to the manufacturer’s instructions. The first strand cDNA was diluted 1:10 ddH2O, and 2 μl of the diluted cDNA was used as a template for RT-PCR analysis. The RT-PCR system was performed in a total volume of 20 μl, with 2 μl of 10× PCR buffer, 1 μl of 2.5 mM dNTPs, and 1 μl of 10 μM PtGT1-F/R primer mentioned above, and 1 unit Taq DNA polymerase. PCR conditions were the same as used previously for PCR analysis.
Histochemical staining
In order to examine the lignified cell walls in stems, the transgenic and WT tobacco plants were grown at the same conditions for ∼2 months. The second internodes of stems (from ground level) were excised, the bark removed, and the internodes hand-cut into 20–30 μm thick slices, and subjected to histochemical analysis. Wiesner staining was performed by incubating sections in 1% phloroglucinol (w/v) in 6 mol l−1 HCl for 5 min, and the sections observed under a dissecting microscope (Pomar et al., 2002; Weng et al., 2010). For Mäule staining, hand-cut stem sections were soaked in 1% KMnO4 for 5 min, then rinsed with water, destained in 30% HCl, washed with water, mounted in concentrated NH4OH, and examined under a dissecting microscope (Atanassova et al., 1995; Weng et al., 2010).
Assay of Klason lignin content
The second internodes of stems (from ground level) of transgenic and WT tobacco plants grown in the same conditions for approximately 2 months, were excised, the bark removed, and the internodes then cut into thin sections and put into an 80 °C oven. The dried stem materials were ground into a fine powder, extracted four times in methanol and dried. Then 200 mg of the extract was mixed with 5 ml of 72% (w/w) sulphuric acid at 30 °C and hydrolysed for 1 h. The hydrolysate was diluted to 4% sulphur by the addition of water and then cooked for 1 h in boiling water. The solid residue was filtered through a glass filter. Finally, the sample was washed, dried at 80 °C overnight and then weighed. The lignin content was measured and expressed as a percentage of the original weight of cell wall residue (Dence, 1992).
Phenotype analysis of transgenic tobacco plants
Fifteen transgenic plants from each line were grown alongside WT plants in the same conditions in a greenhouse. The date and number of leaves on each plant were recorded when each one flowered.
Data collection and statistical analysis
Data presented are the means ±SE of at least three independent experiments, with at least 10 plant samples per genotype. The experimental data were analysed by one-way ANOVA; the comparisons between the mean values of transgenics and WT were evaluated by the least-significant difference test at P <0.01 (indicated with double stars above the bars in the figures). Statistical analyses were performed using SPSS/PC ver. 14.0 software.
Results
The cloning and sequence analysis of the PtGT1 gene
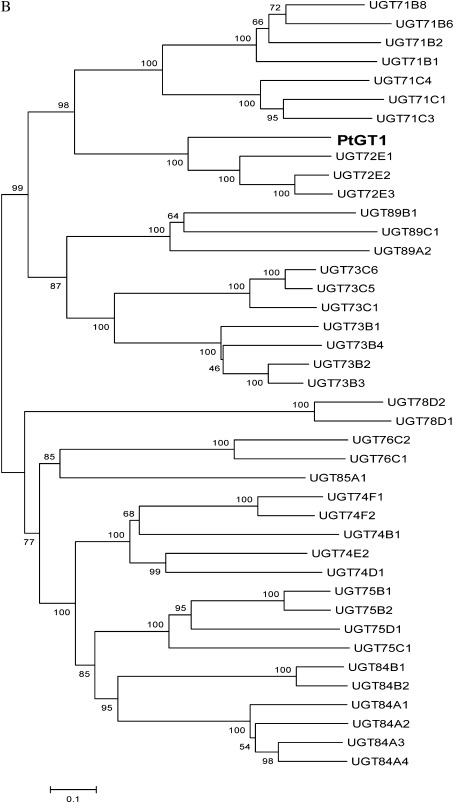
In order to clone the glucosyltransferase from P. tomentosa, P. trichocarpa was used as a bridge, because of the availability of its genome sequences, to obtain the homologues of Arabidopsis thaliana UGT72E1-E3 cDNA. According to the P. trichocarpa homologues, the glucosyltransferase gene, PtGT1, was isolated from P. tomentosa (Fig. 1). After sequencing, the open reading frame of PtGT1 was found to be 1 446 bp long, and encoded a putative protein of 481 amino acid residues. Arabidopsis UGT72E1-E3 is a small cluster of three closely related genes encoding glucosyltransferases shown to glucosylate several phenylpropanoids in vitro, including monolignols, hydroxycinnamic acids, and hydroxycinnamic aldehydes. In this study, the deduced amino acid sequences of PtGT1 were aligned with UGT72E1-E3 and it was found that PtGT1 exhibits 56%, 52%, and 51% identity and 75%, 73%, and 73% similarity to the Arabidopsis UGT72E2, UGT72E3, and UGT72E1 proteins, respectively. The conserved PSPG box for secondary metabolite glycosyltransferases was found in the carboxy-terminal domain of the PtGT1 protein (marked by asterisks in Fig. 2A), suggesting that PtGT1 is a putative secondary metabolite glycosyltransferase. PtGT1 sequences were deposited in GenBank under the accession number HM776516. A phylogenetic comparison was made between PtGT1 and all 40 published Arabidopsis family 1 GTs, and the poplar PtGT1 was found to be located on a unique branch with Arabidopsis UGT72E1-E3 (Fig. 2B). This suggests that the poplar PtGT1 might recognize the same substrates as Arabidopsis UGT72E1-E3. However, when the PtGT1 enzymatic activity was examined in vitro, PtGT1 did not show the same activity towards monolignols and other phenylpropanoid compounds as Arabidopsis UGT72E1-E3 (data not shown), suggesting that the substrate specificities can not be predicted based on sequence similarities only.
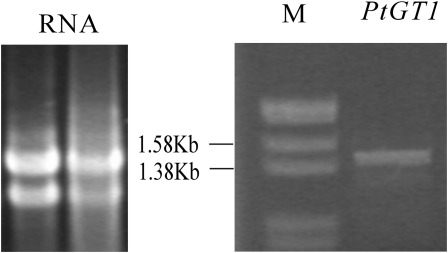
Fig. 1.
The cloning of PtGT1. The RNA was isolated from poplar (Populus tomentosa Carr.) (Left) and the PtGT1 cDNA was obtained (Right). M: DNA molecular weight marker.
Fig. 2.
The sequence analysis of PtGT1 cDNA. (A) Amino acid sequence alignment of poplar PtGT1 and Arabidopsis UGT72E1-E3. The numbers shown on the left of each sequence are the positions of amino acid residues in the corresponding proteins. Gaps (marked with dashes) were introduced to maximize the sequence alignment. Identical and similar amino acid residues are shaded with black and grey, respectively. The PSPG box is marked by asterisks. (B) Neighbor–Joining tree representing the relationships of PtGT1 and all 40 published Arabidopsis family 1 GTs. Bootstrap values are shown in percentages at the nodes. The GT sequences were retrieved from the Carbohydrate-active enzymes database ( http://www.cazy.org/GT1_eukaryota.html) and the NCBI database. The GenBank accession numbers for each sequence are: UGT71B1 (AEE76548.1); UGT71B2 (AAK50110.1); UGT71B6 (AEE76551.1); UGT71B8 (AEE76553.1); UGT71C1 (AAC35226.1); UGT71C3 (AAF82195.1); UGT71C4 (AAG18592.1); UGT72E1 (AAK83619.1); UGT72E2 (AAL32714.1); UGT72E3 (AAC26233.1); UGT73B1 (AAL57652.1); UGT73B2 (AAK59668.1); UGT73B3 (AAL32831.1); UGT73B4 (AAP37678.1); UGT73C1 (AAD20151.1); UGT73C5 (AAD20156.1); UGT73C6 (AAD20155.1); UGT74B1 (AAC00570.1); UGT74D1 (AAD32297.1); UGT74E2 (AAD30627.1); UGT74F1 (AAB64022.1); UGT74F2 (AAB64024.1); UGT75B1 (AAF79730.1); UGT75B2 (AAF79732.1); UGT75C1 (AAL32667.1); UGT75D1 (AAB58497.1); UGT76C1 (AAP21281.1); UGT76C2 (AAK73975.1); UGT78D1 (AAF19756.1); UGT78D2 (AAL61932.1); UGT84A1 (AAN72025.1); UGT84A2 (AAM13998.1); UGT84A3 (AAL15277.1); UGT84A4 (AAS99717.1); UGT84B1 (AAB87119.1); UGT84B2 (AAB87106.1); UGT85A1 (AAF18537.1); UGT89A2 (ABH04465.1); UGT89B1 (AEE35520.1); UGT89C1 (AAF80123.1).
The expression pattern of the poplar PtGT1 gene
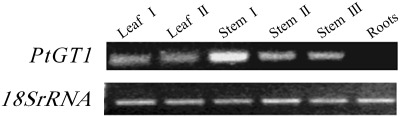
To examine the expression pattern of the poplar PtGT1 gene, RNA was isolated from younger leaves, older leaves, upper stems (elongating stems), middle stems, lower stems (non-elongating stems), and roots from 2-month-old poplar and the relative expression levels assessed using RT-PCR (Fig. 3). Experimental results showed that upper stems contained higher levels of PtGT1 transcripts than the other tissues, indicating that PtGT1 is highly expressed where stems are elongating and secondary cell walls are being laid down. PtGT1 was expressed at lower levels in leaves and other parts of the stems, and was undetectable in roots.
Fig. 3.
RT-PCR analysis of the expression of PtGT1 gene in different poplar tissues. The expression level of the 18S rRNA gene was used as an internal control. Leaf I was young and at the expanding stage, whereas leaf II was the older leaves. Stem I and stem III were from the top (elongating) and bottom (non-elongating) parts of the stems, respectively. Stem II was from the middle part between stem I and stem III.
Over-expression of the poplar PtGT1 gene in tobacco plants
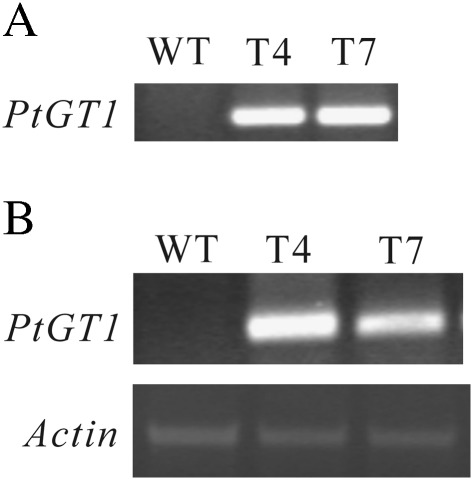
In order to obtain information on the effects of constitutive over-expression of PtGT1 on plant growth and development, the PtGT1 gene was first integrated into tobacco plants. The T2 homozygous transgenic tobacco plants were identified using PCR of their genomic DNA. The expected fragment was amplified in transgenic lines but not in the non-transformed plants (Fig. 4A).
Fig. 4.
The analysis results of PCR and RT-PCR for PtGT1 cDNA in transgenic tobacco plants and the wild type. (A) DNA PCR in wild type (WT) and transgenic T4 and T7 tobacco plants. (B) RT-PCR in wild type and transgenic T4 and T7 tobacco plants. The expression level of the Actin gene was used as an internal control. Data are identical from two independent biological replicates.
To assess the expression of the PtGT1 gene in transgenic tobacco plants, 7-week-old transgenic seedlings were subjected to RT-PCR analysis. Two lines, T4 and T7, showed relatively high levels of PtGT1 transcripts (Fig. 4B) showing that poplar PtGT1 had been transferred into the tobacco genome and was highly expressed in the transgenic tobacco seedlings. TheT4 and T7 lines were therefore chosen for further investigation.
Lignin staining and Klason lignin content analysis of transgenic tobacco plants
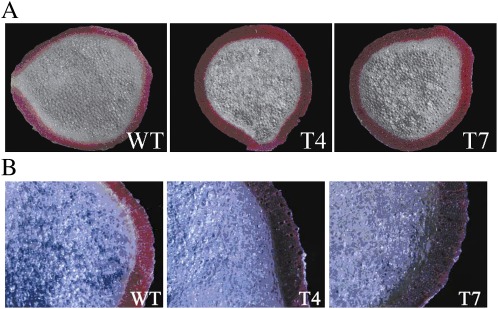
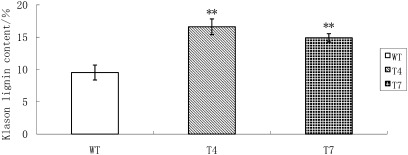
Because the PtGT1 gene is more highly expressed in elongating stems than in other poplar tissues, there was a need to examine the possible impacts of the PtGT1 gene on stem growth and development in transgenic plants. Although the stems of transgenic plants showed no obvious phenotypic characters, lignin deposition was higher in the transgenic tobacco plants. Wiesner and Mäule staining of stem cross-sections showed that transgenic tobacco plants produced more intensive xylem tissue staining than WT (Fig. 5A, B), suggesting that the transgenic tobacco plants have higher total lignin than WT. To verify this finding further, the total lignin contents of transgenic and WT tobacco stem tissues were quantified by Klason analysis (Fig. 6). Two transgenic lines (T4 and T7) had significantly higher Klason lignin contents than WT, consistent with the histochemical staining results and showing that PtGT1 over-expression impacts on lignin content in tobacco. The total cellulose contents of stems were also measured in this study, but no substantial differences were found between the total cellulose contents of transgenic and WT tobacco plants (data not shown).
Fig. 5.
Wiesner staining (A) and Mäule staining (B) of 2-month-old tobacco stems. WT, wild type; T4, transgenic line T4; T7, transgenic line T7.
Fig. 6.
Klason lignin content analysis of 2-month-old tobacco stems. WT, wild type; T4, transgenic line T4; T7, transgenic line T7. Error bars represent standard deviations based on assays of biological duplicates. Double stars above the bars represent a very significant difference compared with the wild type.
Flowering phenotype of transgenic tobacco plants
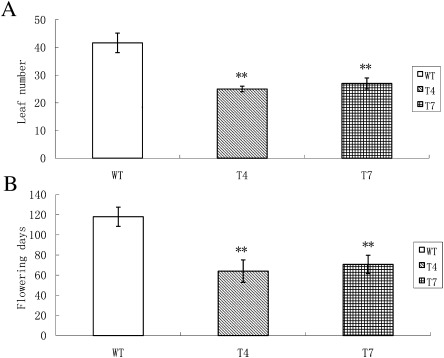
Transgenic tobacco lines and WT plants were grown in a greenhouse to observe their phenotypes. Surprisingly, it was found that the transgenic tobacco lines bolted earlier than WT plants (Fig. 7). The leaf numbers and days to flowering of the transgenic tobacco plants and WT were recorded. Leaf numbers of the WT plants were approximately 1.5 times those of the transgenic plants at bolting (Fig. 8A), and the number of days to WT flowering were nearly twice those of the transgenic plants (Fig. 8B). The transgenic plants showed almost no differences in leaf shape or size.
Fig. 7.
The flowering phenotype of transgenic tobacco plants and the wild type. The transgenic tobacco plants bolted earlier than the wild type. WT, wild-type tobacco plants; T4, transgenic tobacco plant T4; T7, transgenic tobacco plant T7.
Fig. 8.
The leaf numbers (A) and days (B) up to flowering of tobacco plants. WT, wild-type tobacco plants; T4, transgenic tobacco plant T4; T7, transgenic tobacco plant T7. Error bars represent standard deviations based on assays of biological duplicates. Double stars above the bars represent very significant difference compared with wild type.
Discussion
In previous research, three glycosyltransferase genes UGT72E1-E3 were identified as involved in sinapate and monolignol metabolism in Arabidopsis (Lim et al., 2001; Lanot et al., 2006, 2008). To obtain their homologues from P. tomentosa, P. trichocarpa genome sequences were used as a bridge to design the PCR primers for amplifying the full-length of glycosyltransferase gene PtGT1 of P. tomentosa (because of the availability of its genome sequence). The PtGT1 amino acid sequences were deduced according to the obtained cDNA sequences. In its carboxy-teminal domain, a 44-amino acid consensus was found, called the PSPG box, a characteristic of family 1 GTs, indicating that the PtGT1 gene is most probably a glycosyltransferase gene active in the glycosylation of plant secondary metabolites (Paquette et al., 2003). Because no data on poplar family 1 glycosyltransferases have been available up to now, a phylogenetic tree was constructed using only PtGT1 and the 40 family 1 glycosyltransferases of Arabidopsis so far identified. PtGT1 was located on the phylogenetic tree in a unique clade with UGT72E1-E3. This may indicate that PtGT1 is involved in the glycosylation of phenylpropanoids (including monolignols) similar to UGT72E1-E3. However, our in vitro biochemical assays indicated that the PtGT1 recombinant enzyme does not recognize phenylpropanoids as substrates. It is therefore likely that PtGT1 participates in a metabolite pathway in poplar distinct from its Arabidopsis homologues, UGT72E1-E3.
Lignin is mainly present in secondary thickened plant cell walls and is composed of aromatic polymers resulting from the oxidative coupling of three major monolignols: coniferyl alcohol, sinapyl alcohol, and coumaryl alcohol (Boerjan et al., 2003; Ralph et al., 2006; Vanholme et al., 2010). The main biosynthetic pathways of the monolignols and lignin have been well described (Baucher et al., 2003; Boerjan et al., 2003; Chen and Dixon, 2007; Vanholme et al., 2008, 2010; Grabber et al., 2010; Novaes et al., 2010; Rahantamalala et al., 2010). Monolignol and lignin contents can be altered using metabolic engineering based on the genetic modification of lignin biosynthetic pathways. For example, many genes in the monolignol biosynthetic pathway, such as PAL, C4H, 4CL, HCT, C3H, CCoAOMT, CCR, CAD, influence lignin contents when their expression levels are altered (Baucher et al., 2003; Boerjan et al., 2003; Hoffmann et al., 2004; Sibout et al., 2005; Wagner et al., 2007; Mir Derikvand et al., 2008). Glycosylation is a key mechanism in the metabolic homeostasis of plant cells (Bowles et al., 2005). However, the impact on lignin biosynthesis of a glycosyltransferase, responsible for the glycosylation of plant secondary metabolites, has not been reported to date. In this study, it is shown that a putative glycosyltransferase, PtGT1, increased the lignin content when constitutively over-expressed in tobacco plants, suggesting the significance of glycosyltransferase PtGT1 in increasing lignin contents in angiosperms. Our findings may provide a novel strategy for lignin engineering in woody, and other, plants through the genetic manipulation of glycosyltransferase(s) such as PtGT1, increasing their value in industry and agriculture. Interestingly, the PtGT1 recombinant enzyme does not recognize the precursors of lignin, including monolignols, as substrates, and the transgenic tobacco plants which over-express the corresponding cDNA do not contain higher levels of monolignol glycosides (data not shown). It is, therefore, suggested that the impact of PtGT1 on lignin biosynthesis is not directly through the glycosylation of monolignols, and further research is needed to understand these molecular mechanisms better.
In addition to its effect on lignin content, the PtGT1 gene appears to influence flowering time when ectopically expressed in tobacco. Time of flowering is crucial for plants to ensure successful reproduction. The molecular mechanisms of flowering in A. thaliana, a model organism, have been intensively studied, and four pathways have been shown to control the development of flowers: the photoperiod pathway, vernalization pathway, autonomous pathway, and gibberellin pathway (Simpson, 2002). By contrast, very few genetic studies have been carried out on the flowering of poplar. Recently, An et al. (2010) reported that an inverted repeat PtLFY fragment (PtLFY-IR) of P. tomentosa effectively prevented flowering in transgenic tobacco plants. The ectopic expression of a poplar APETALA3-like gene in tobacco caused early flowering and fast growth (An et al., 2011). In Arabidopsis, several studies have reported that altered UGT levels can impact on hormone homeostasis and compromise the control of flowering. For example, increasing the expression of UGT84B1, an Arabidopsis glucosyltransferase of indole-3-acetic acid, in transgenic lines increased the number of bolts from the rosette and the degree of branching (Jackson et al., 2002). Mutants unable to express the UDP-glucose:thiohydroximate S-glucosyltransferase, UGT74B1, accumulated more indole-3-acetic acid, flowered later, and were partially sterile (Grubb et al., 2004). UGT73C5 is another Arabidopsis glycosyltransferase responsible for the glucosylation of brassinosteroids, and its over-expression resulted in dramatic changes in flowering time and flower development (Poppenberger et al., 2005). In poplar plants, however, the impact of glycosyltransferases on flowering time has not been studied. In this study, it was found that ectopic expression of a putative poplar glycosyltransferase gene, PtGT1, in tobacco caused early flowering, and provides a starting point for the further study of the function of the PtGT1 gene in the control of flowering time. The observed changes in lignin content are likely to be associated with changes in flowering time. Shadle et al. (2007) reported that down-regulation of hydroxycinnamoyl CoA:shikimate hydroxycinnamoyl transferase in transgenic alfalfa resulted in greatly reduced lignin content and a delay in flowering. The early flowering phenotype observed in this study, and the delayed flowering found in previous work (Shadle et al., 2007) provides the possibility that engineered changes in lignin quantity may allow beneficial changes in reproductive development.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No.90917006 and Grant No. 30971543). We thank Dr Zanmin Hu (The Institute of Genetics and Developmental Biology, Chinese Academy of China) for providing the poplar plants. We also thank Dr Lushan Wang for his assistance with the bioinformatics and in designing the phylogenetic tree.
References
- An XM, Wang DM, Wang ZL, Li B, Bo WH, Cao GL, Zhang ZY. Isolation of a LEAFY homolog from Populus tomentosa: expression of PtLFY in P. tomentosa floral buds and PtLFY-IR-mediated gene silencing in tobacco (Nicotiana tabacum) Plant Cell Reports. 2010;30:89–100. doi: 10.1007/s00299-010-0947-0. [DOI] [PubMed] [Google Scholar]
- An XM, Ye M, Wang DM, Wang ZL, Cao GL, Zheng H, Zhang ZY. Ectopic expression of a poplar APETALA3-like gene in tobacco causes early flowering and fast growth. Biotechnology Letter. 2011 doi: 10.1007/s10529-011-0545-4. doi: 10.1007/s10529-011-0545-4. [DOI] [PubMed] [Google Scholar]
- Aspeborg H, Schrader J, Coutinho PM, et al. Carbohydrate-active enzymes involved in the secondary cell wall biogenesis in hybrid aspen. Plant Physiology. 2005;137:983–997. doi: 10.1104/pp.104.055087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanassova R, Favet N, Martz F, Chabbert B, Tollier M-T, Monties B, Fritig B, Legrand M. Altered lignin composition in transgenic tobacco expressing O-methyltransferase sequence in sense and antisense orientation. The Plant Journal. 1995;8:465–477. [Google Scholar]
- Baucher M, Halpin C, Petit-Conil M, Boerjan W. Lignin: genetic engineering and impact on pulping. Critical Reviews in Biochemistry and Molecular Biology. 2003;38:305–350. doi: 10.1080/10409230391036757. [DOI] [PubMed] [Google Scholar]
- Boerjan W, Ralph J, Baucher M. Lignin biosynthesis. Annual Review of Plant Biology. 2003;54:519–546. doi: 10.1146/annurev.arplant.54.031902.134938. [DOI] [PubMed] [Google Scholar]
- Bowles D, Isayenkova J, Lim EK, Poppenberger B. Glycosyltransferases: managers of small molecules. Current Opinion in Plant Biology. 2005;8:254–263. doi: 10.1016/j.pbi.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Chen F, Dixon RA. Lignin modification improves fermentable sugar yields for biofuel production. Nature Biotechnology. 2007;25:759–761. doi: 10.1038/nbt1316. [DOI] [PubMed] [Google Scholar]
- Dence C. Lignin determination. In: Lin S, editor. Methods in lignin chemistry. Berlin: Springer-Verlag; 1992. pp. 33–61. [Google Scholar]
- Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Research. 2003;31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler-Lee J, Geisler M, Coutinho PM, et al. Poplar carbohydrate-active enzymes. Gene identification and expression analyses. Plant Physiology. 2006;140:946–962. doi: 10.1104/pp.105.072652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabber JH, Schatz PF, Kim H, Lu F, Ralph J. Identifying new lignin bioengineering targets. 1. Monolignol-substitute impacts on lignin formation and cell wall fermentability. BMC Plant Biology. 2010;10:114. doi: 10.1186/1471-2229-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb CD, Zipp BJ, Ludwig-Müller J, Masuno MN, Molinski TF, Abel S. Arabidopsis glucosyltransferase UGT74B1 functions in glucosinolate biosynthesis and auxin homeostasis. The Plant Journal. 2004;40:893–908. doi: 10.1111/j.1365-313X.2004.02261.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann L, Besseau S, Geoffroy P, Ritzenthaler C, Meyer D, Lapierre C, Pollet B, Legrand M. Silencing of hydroxycinnamoyl-coenzyme A shikimate/quinate hydroxycinnamoyltransferase affects phenylpropanoid biosynthesis. The Plant Cell. 2004;16:1446–1465. doi: 10.1105/tpc.020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou B, Lim EK, Higgins GS, Bowles DJ. N-glucosylation of cytokinins by glycosyltransferases of Arabidopsis thaliana. Journal of Biological Chemistry. 2004;279:47822–47832. doi: 10.1074/jbc.M409569200. [DOI] [PubMed] [Google Scholar]
- Jackson RG, Kowalczyk M, Li Y, Higgins G, Ross J, Sandberg G, Bowles DJ. Over-expression of an Arabidopsis gene encoding a glucosyltransferase of indole-3-acetic acid: phenotypic characterization of transgenic lines. The Plant Journal. 2002;32:573–583. doi: 10.1046/j.1365-313x.2002.01445.x. [DOI] [PubMed] [Google Scholar]
- Jones P, Vogt T. Glycosyltransferases in secondary plant metabolism: tranquilizers and stimulant controllers. Planta. 2001;213:164–174. doi: 10.1007/s004250000492. [DOI] [PubMed] [Google Scholar]
- Kristensen C, Morant M, Olsen CE, Ekstrom CT, Galbraith DW, Moller BL, Bak S. Metabolic engineering of dhurrin in transgenic Arabidopsis plants with marginal inadvertent effects on the metabolome and transcriptome. Proceedings of the National Academy of Sciences, USA. 2005;102:1779–1784. doi: 10.1073/pnas.0409233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanot A, Hodge D, Jackson RG, George GL, Elias L, Lim E-K, Vaistij FE, Bowles DJ. The glucosyltransferase UGT72E2 is responsible for monolignol 4-O-glucoside production in Arabidopsis thaliana. The Plant Journal. 2006;48:286–295. doi: 10.1111/j.1365-313X.2006.02872.x. [DOI] [PubMed] [Google Scholar]
- Lanot A, Hodge D, Lim EK, Vaistij FE, Bowles DJ. Redirection of flux through the phenylpropanoid pathway by increased glucosylation of soluble intermediates. Planta. 2008;228:609–616. doi: 10.1007/s00425-008-0763-8. [DOI] [PubMed] [Google Scholar]
- Li Y, Baldauf S, Lim EK, Bowles DJ. Phylogenetic analysis of the UDP-glycosyltransferase multigene family of Arabidopsis thaliana. Journal of Biological Chemistry. 2001;276:4338–4343. doi: 10.1074/jbc.M007447200. [DOI] [PubMed] [Google Scholar]
- Li Y, Hagen G, Guilfoyle TJ. Altered morphology in transgenic tobacco plants that overproduce cytokinins in specific tissues and organs. Developmental Biology. 1992;153:386–395. doi: 10.1016/0012-1606(92)90123-x. [DOI] [PubMed] [Google Scholar]
- Lim EK. Plant glycosyltransferases: their potential as novel biocatalysts. Chemistry—A European Journal. 2005;11:5486–5494. doi: 10.1002/chem.200500115. [DOI] [PubMed] [Google Scholar]
- Lim EK, Bowles DJ. A class of plant glycosyltransferases involved in cellular homeostasis. The EMBO Journal. 2004;23:2915–2922. doi: 10.1038/sj.emboj.7600295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim EK, Li Y, Parr A, Jackson R, Ashford DA, Bowles DJ. Identification of glucosyltransferase genes involved in sinapate metabolism and lignin synthesis in Arabidopsis. Journal of Biological Chemistry. 2001;276:4344–4349. doi: 10.1074/jbc.M007263200. [DOI] [PubMed] [Google Scholar]
- Mir Derikvand M, Sierra JB, Ruel K, Pollet B, Do CT, Thevenin J, Buffard D, Jouanin L, Lapierre C. Redirection of the phenylpropanoid pathway to feruloyl malate in Arabidopsis mutants deficient for cinnamoyl-CoA reductase 1. Planta. 2008;227:943–956. doi: 10.1007/s00425-007-0669-x. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum. 1962;15:473–497. [Google Scholar]
- Novaes E, Kirst M, Chiang V, Winter-Sederoff H, Sederoff R. Lignin and biomass: a negative correlation for wood formation and lignin content in trees. Plant Physiology. 2010;154:555–561. doi: 10.1104/pp.110.161281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette S, Moller BL, Bak S. On the origin of family 1 plant glycosyltransferases. Phytochemistry. 2003;62:399–413. doi: 10.1016/s0031-9422(02)00558-7. [DOI] [PubMed] [Google Scholar]
- Pomar F, Merino F, Barcel Ros A. O-4-Linked coniferyl and sinapyl aldehydes in lignifying cell walls are the main targets of the Wiesner (phloroglucinol–HCl) reaction. Protoplasma. 2002;220:17–28. doi: 10.1007/s00709-002-0030-y. [DOI] [PubMed] [Google Scholar]
- Poppenberger B, Fujioka S, Soeno K, et al. The UGT73C5 of Arabidopsis thaliana glucosylates brassinosteroids. Proceedings of the National Academy of Sciences, USA. 2005;102:15253–15258. doi: 10.1073/pnas.0504279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahantamalala A, Rech P, Martinez Y, Chaubet-Gigot N, Grima-Pettenati J, Pacquit V. Coordinated transcriptional regulation of two key genes in the lignin branch pathway—CAD and CCR—is mediated through MYB- binding sites. BMC Plant Biology. 2010;10:130–138. doi: 10.1186/1471-2229-10-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph J, Akiyama T, Kim H, Lu F, Schatz PF, Marita JM, Ralph SA, Reddy MS, Chen F, Dixon RA. Effects of coumarate 3-hydroxylase down-regulation on lignin structure. Journal of Biological Chemistry. 2006;281:8843–8853. doi: 10.1074/jbc.M511598200. [DOI] [PubMed] [Google Scholar]
- Shadle G, Chen F, Srinivasa Reddy MS, Jackson L, Nakashima J, Dixon RA. Down-regulation of hydroxycinnamoyl CoA:shikimate hydroxycinnamoyl transferase in transgenic alfalfa affects lignification, development and forage quality. Phytochemistry. 2007;68:1521–1529. doi: 10.1016/j.phytochem.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Sibout R, Eudes A, Mouille G, Pollet B, Lapierre C, Jouanin L, Seguin A. CINNAMYL ALCOHOL DEHYDROGENASE-C and -D are the primary genes involved in lignin biosynthesis in the floral stem of Arabidopsis. The Plant Cell. 2005;17:2059–2076. doi: 10.1105/tpc.105.030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GG. Arabidopsis, the Rosetta Stone of flowering time? Science. 2002;296:285–289. doi: 10.1126/science.296.5566.285. [DOI] [PubMed] [Google Scholar]
- Vanholme R, Demedts B, Morreel K, Ralph J, Boerjan W. Lignin biosynthesis and structure. Plant Physiology. 2010;153:895–905. doi: 10.1104/pp.110.155119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanholme R, Morreel K, Ralph J, Boerjan W. Lignin engineering. Current Opinion in Plant Biology. 2008;11:278–285. doi: 10.1016/j.pbi.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Wagner A, Ralph J, Akiyama T, Flint H, Phillips L, Torr K, Nanayakkara B, TeKiri L. Exploring lignification in conifers by silencing hydroxycinnamoyl-CoA:shikimate hydroxycinnamoyltransferase in Pinus radiata. Proceedings of the National Academy of Sciences, USA. 2007;104:11856–11861. doi: 10.1073/pnas.0701428104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hou BK. Glycosyltransferases: key players involved in the modification of plant secondary metabolites. Frontiers of Biology in China. 2009;4:39–46. [Google Scholar]
- Weis M, Lim EK, Bruce NC, Bowles DJ. Engineering and kinetic characterisation of two glucosyltransferases from Arabidopsis thaliana. Biochimie. 2008;90:830–834. doi: 10.1016/j.biochi.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Weng JK, Akiyama T, Bonawitz ND, Li X, Ralph J, Chapple C. Convergent evolution of syringyl lignin biosynthesis via distinct pathways in the lycophyte Selaginella and flowering plants. The Plant Cell. 2010;22:1033–1045. doi: 10.1105/tpc.109.073528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K, Miyao K, Gaber A, Takeda T, Kanaboshi H, Miyasaka H, Shigeoka S. Enhancement of stress tolerance in transgenic tobacco plants overexpressing Chlamydomonas glutathione peroxidase in chloroplasts or cytosol. The Plant Journal. 2004;37:21–33. doi: 10.1046/j.1365-313x.2003.01930.x. [DOI] [PubMed] [Google Scholar]
- Zhou GK, Zhong R, Himmelsbach DS, McPhail BT, Ye ZH. Molecular characterization of PoGT8D and PoGT43B, two secondary wall-associated glycosyltransferases in poplar. Plant and Cell Physiology. 2007;48:689–699. doi: 10.1093/pcp/pcm037. [DOI] [PubMed] [Google Scholar]
- Zhou GK, Zhong R, Richardson EA, WH Morrison, III, Wood-Jones A, Ye ZH. The poplar glycosyltransferase GT47C is functionally conserved with Arabidopsis Fragile fiber8. Plant and Cell Physiology. 2006;47:1229–1240. doi: 10.1093/pcp/pcj093. [DOI] [PubMed] [Google Scholar]