Abstract
BACKGROUND AND PURPOSE
Inflammation and reactive oxygen species are associated with the promotion of various cancers. The use of non-steroidal anti-inflammatory drugs (NSAIDs) in cancer prevention treatments has been promising in numerous cancers. We report the evaluation of NSAIDs chemically modified by the addition of a redox-active nitroxide group. TEMPO-aspirin (TEMPO-ASA) and TEMPO-indomethacin (TEMPO-IND) were synthesized and evaluated in the lung cancer cell line A549.
EXPERIMENTAL APPROACHES
We evaluated physico-chemical properties of TEMPO-ASA and TEMPO-IND by electron paramagnetic resonance and cyclic voltammetry. Superoxide dismutase-like properties was assayed by measuring cytochrome c reduction and anti-inflammatory effects were assayed by measuring production of prostaglandin E2 (PGE2) and leukotriene B4 (LTB4). MTT proliferation assay and clonogenic assay were evaluated in the A549 lung carcinoma cell line. Maximum tolerated doses (MTD) and acute ulcerogenic index were also evaluated in in vivo.
KEY RESULTS
MTD were: TEMPO (140 mg·kg−1), ASA (100 mg·kg−1), indomethacin (5 mg·kg−1), TEMPO-ASA (100 mg·kg−1) and TEMPO-IND (40 mg·kg−1). While TEMPO-ASA was as well tolerated as ASA, TEMPO-IND showed an eightfold improvement over indomethacin. TEMPO-IND showed markedly less gastric toxicity than the parent NSAID. Both TEMPO-ASA and TEMPO-IND inhibited production of PGE2 and LTB4 in A549 cells with maximum effects at 100 µg·mL−1 or 10 µg·mL−1 respectively.
CONCLUSIONS AND IMPLICATIONS
The nitroxide-NSAIDs retained superoxide scavenging capacity of the parent nitroxide and anti-inflammatory effects, inhibiting cyclooxygenase and 5-lipoxygenase enzymes. These redox-modified NSAIDs might be potential drug candidates, as they exhibit the pharmacological properties of the parent NSAID with antioxidant activity decreasing NSAID-associated toxicity.
Keywords: NSAIDS, nitroxides, cancer, nitrosative stress, inflammation, antioxidants
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) have been used to treat numerous inflammatory conditions. Many conditions such as arthritis and cancer require chronic administration of NSAIDs for extended period of time. For example, aspirin (acetyl salicylic acid, ASA) and selective inhibitors of COX-2 such as celecoxib and rofecoxib have been shown to reduce some types of cancer by over 50% when taken for an extended period of time (Shishodia et al., 2004; Bastos-Pereira et al., 2010). Although there are beneficial effects, the long-term use even with COX-2 selective agents has problematic side effects such as thrombosis (Dajani and Islam, 2008).
The unexpected cardiovascular side effects observed with highly selective COX-2 inhibitors and the subsequent withdrawal of rofecoxib (as Vioxx) have lead to the investigation of other potential NSAIDs. Redox-modified NSAIDs have emerged as potential therapeutic compounds reducing both gut toxicity and thrombosis. Compounds such as the NCX series, which are COX-inhibitory NO donors, opened up the potential of NO-based NSAIDs (Del Soldato et al., 1999). Recently, analogous NSAID NO donors have been developed from the NONOate class of NO donors that exhibited beneficial cardiovascular effects as well as abating gastro-intestinal toxicity (Velázquez et al., 2005; 2007). Other NSAIDs have been synthesized to release H2S and these derivatives have also been shown to have lower gastric toxicity along with beneficial effects in the cardiovascular system (Li et al., 2007). Taken together, these compounds have potentially useful properties under different conditions.
In addition to the NSAIDs, other compounds can be used to treat inflammatory conditions. Antioxidants have been shown over several decades to have marked beneficial effects in numerous inflammatory diseases and particularly in oxidative stress (Halliwell, 1991; Soule et al., 2007; Nakao et al., 2009). The nitroxides have been shown to have such beneficial effects. One agent, 4-hydroxy-TEMPO (TEMPOL) is a superoxide dismutase (SOD) mimetic, as well as a powerful antioxidant (Samuni et al., 1990; 1991) protecting against oxidative stress from peroxides and radiation (Soule et al., 2007). The electrochemical properties of this molecule make it ideal for scavenging reactive oxygen species and abating oxidative stress (Samuni et al., 2001). In vivo studies with TEMPOL showed decreased ischaemia-reperfusion injury and radiation-induced tissue injury, a decreased weight gain and prevention of the induction of some cancers (DeGraff et al., 1992; Cotrim et al., 2005; Patel et al., 2006; Moens et al., 2008; Zhang et al., 2008; Samuni et al., 2010).
In this report, we have synthesized the nitroxide derivatives of the NSAIDs aspirin (ASA) and indomethacin (namely, 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO)-ASA and TEMPO-IND) to yield compounds exerting the beneficial effects of both the NSAID and the antioxidant. These TEMPO-modified NSAIDs retained inhibition of COX while the nitroxide portion of the compound scavenged superoxide anion. In addition, we found that TEMPO-ASA was as well tolerated as ASA while TEMPO-IND exhibited markedly lower gastric toxicity than indomethacin. The coupling of NSAIDs with the antioxidant nitroxide provides a new class of redox NSAIDs.
Methods
Synthesis of TEMPO-NSAIDs
General
4-hydroxy-TEMPO, indomethacin and O-acetylsalicyloyl chloride were purchased from Sigma Aldrich Chemical Company and used as received. Analytical TLC was performed on silica gel plates with QF-254 indicator. Visualization was accomplished with UV light, iodine and phosphomolybdic acid. Solvents for extraction and purification were technical grade and used as received. All reactions were performed in flame-dried glassware under an inert atmosphere of dry argon. 1H NMR and 13C NMR spectra were recorded in CDCl3 using a Bruker Avance 300 MHz NMR spectrometer. Chemical shifts are given in ppm (δ); multiplicities are indicated by s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet) and b (broadened). Mass spectra were obtained on an Agilent Technologies 1100 LC/MSD Trap instrument.
TEMPO-ASA
Pyridine (0.45 mL, 5.50 mmol) was added to a solution of 4-hydroxy-TEMPO (0.86 g, 5.00 mmol), and acetylsalicyloyl chloride (1.09 g, 5.50 mmol) in CH2Cl2 (6 mL) at 0°C. The solution was stirred at room temperature overnight and the precipitate was removed by filtration. The filtrate was washed with water (6 mL), 10% aq. NaHCO3 (10 mL), 2 M aq. HCl (10 mL) and water (6 mL). The CH2Cl2 layer was dried, filtered, concentrated and purified by silica gel chromatography (7:3 hexanes : EtOAc, Rf= 0.39) to give an orange oil (0.8 g, 48%). 1H NMR (CDCl3 with 2 drops of TFA-d) 7.96 (d, 1H, J= 7.0 Hz), 7.60 (t, 1H, J= 7.1 Hz), 7.33 (d, 1H, J= 7.5), 7.19 (d, 1H, J= 7.5 Hz), 5.44 (bt, 1H, J= 11.7 Hz), 2.37 (s, 3H), 2.42–2.00 (m, 4H), 1.54 (s, 6H), 1.46 (s, 6H); 13C NMR 169.8, 163.36, 150.96, 134.59, 131.66, 126.25, 123.98, 122.68, 67.37, 63.94, 41.74, 27.77, 20.7; MS: m/z, 335 [M+1]; Analysis calculated for C18H24NO5: C, 64.65; H, 7.23, N, 4.19; Found: C, 64.73, H, 7.35, N, 4.20.
TEMPO-IND
Dicyclohexyl carbodiimide (DCC, 1.32 g, 6.4 mmol) was added to a solution of indomethacin (2.08 g, 5.8 mmol), 4-hydroxy-TEMPO (1 g, 5.8 mmol) and 4-dimethylaminopyridine (DMAP, 0.16 g, 5.8 mmol) in CH2Cl2 (45 mL) at 0°C. The mixture was allowed to warm to rt and stirred overnight. The solvent was evaporated in vacuum and the residue was purified by silica gel chromatography (hexanes : EtOAc 7:3, Rf= 0.36) to give an orange solid (2.3 g, 84%). 1H NMR (CDCl3 with 2 drops of TFA-d) 7.69 (d, 2H, J= 8.7 Hz), 7.55 (d, 2H, J= 8.4 Hz), 6.8–7.4 (m, 3H), 5.32 (bt, 1H, J= 11.4), 3.95 (s, 3H), 3.82 (s, 2H), 2.38 (s, 3H), 2.35–2.1 (m, 4H), 1.56 (s, 6H), 1.49 (s, 6H); 13C NMR 174.08, 172.13, 155.83, 141.34, 137.83, 132.81, 132.06, 130.18, 120.74, 116.97, 116.20, 113.41, 113.03, 109.42, 69.81, 66.18, 57.29, 41.65, 30.56, 28.14, 20.47, 13.60; MS: m/z 512 [M+]; Analysis calculated for C28H32ClN2O5: C, 65.68; H, 6.30, N, 5.47; Found: C, 66.28, H, 6.52, N, 5.38.
Electrochemical analysis: cyclic voltammetry
Cyclic voltammetry measurements of the nitroxide-NSAIDs were taken with an EG Potensiostat/Galvanostat Model 273A, from AMETEK Princeton Applied Research (Oak Ridge, TN). Measurements were made at room temperature in PBS or 50% organic solvents, using the platinum auxiliary electrode and Ag/AgCl (saturated KCl) reference electrode.
Electron paramagnetic resonance
Electron paramagnetic resonance (EPR) measurements were made in a gas-permeable Teflon capillary tube of 0.81 mm inner diameter, 0.38 mm wall thickness and 15 cm length (Samuni et al., 2004). The capillary tube was folded twice, and inserted in a narrow quartz tube, then placed in the cavity of a Varian E-109 X-band spectrometer. The measurements were done under aerobic conditions and at room temperature. EPR parameters were: Field Set at 3355 G, Scan Range 10 × 101 G, time constant: 0.128, Mod. Ampl. 0.5 × 101 H, RG: 8.0 × 102, 9.36 GHz microwave frequency, 100 kHz modulation frequency and 10 mW microwave power.
Dismutation of superoxide anions (superoxide dismutase (SOD) mimetic actions) by nitroxide-NSAIDs
Superoxide anions were generated from the reaction of hypoxanthine (500 µM) with xanthine oxidase (10 mU) in PBS (pH 7.4, 25°C), containing the metal chelator diethylenetriaminepentaacetic acid (DTPA, 50 µM). The rate of superoxide formation was assessed by reduction of ferricytochrome c (cyt c) (60 µM), measured at 550 nm (ε= 21 000 M−1·cm−1), as previously described (Wasserman et al., 1980). A range of concentrations (5–100 µM) of the nitroxide-NSAIDs were used to scavenge superoxide.
Cell line and cell culture
The A549 cells (purchased from American Type Culture Collection (ATCC), Manassas, VA) were grown in DMEM also supplemented with 10% heat inactivated fetal bovine serum and penicillin-streptomycin. The cells were maintained at 37°C in an atmosphere of 95% room air and 5% CO2. The media was changed twice a week.
Proliferation assay
Cell viability was determined by using a standard colorimetric assay with 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT). The cells were grown for 24 h in a 96-well cultured plate with cell density 5.0 × 103 cells per well with 100 µL of media. The cells were treated with different concentrations of individual compounds (0–300 µM) along with the respective controls for 24 h. After that 10 µL of MTT (Sigma-Aldrich, St. Louis, MO, USA) was added to the culture media (5 mg·mL−1, PBS) and incubated at 37°C for 3 h. The culture medium was decanted and 100 µL of DMSO was added to each well to dissolve the formazan crystals. After 5 min of agitation at 37°C, the absorbance was measured at 590 nm using a Perkin Elmer HTS 7000 Bio Assay Reader.
Clonogenic survival assay
A standard clonogenic assay was performed to assess cell survival. A549 cells were trypsinized and plated (1 × 106 cells per dish) in 60 cm2 culture dishes. The cells were incubated for 24 h, and then treated with 30, 100 and 300 µM of TEMPO-ASA or TEMPO-IND, and appropriate controls for 24 h. After treatment, the cells were washed with PBS, trypsinized, counted and then plated for colony formation. For each drug dose, the cells were plated in triplicates and incubated at 37°C for 14 days. The colonies were stained with Crystal violet and counted.
PGE2 and leukotriene (LT)B4elisa assays
PGE2 was measured by enzyme elisa (Cayman Chemical, Ann Arbor, MI). TEMPO-ASA and TEMPO-IND were dissolved in DMSO at a concentration of 10 mg·mL−1 for the in vitro experiments. A549 cells in 24-well plates were treated with the compounds for 30 min then arachidonic acid (20 µg·mL−1) was added and the mixture incubated at 37°C for 5 min. The samples were frozen at −80°C, until assayed for PGE2. A volume of 100 µL sample was added to EIA buffer (900 µL). Routinely 25 µL of the diluted sample was then added to a 96-well plate. The samples were incubated overnight at 4°C. The plate was then washed five times (200 µL each) with wash buffer and then Edman's reagent (200 µL per well) was added, according to the assay protocol. The absorbance at 450 nm was determined in an elisa reader. Similarly, production of LTB4 from A549 cells (treatment described above) was measured using a elisa assay kit (Cat no. 520111). The assay was performed as described in the assay kit booklet.
Toxicity studies
All animal care and experimental procedures complied with the Guide for the care and use of laboratory animal resources (National Research Council, 1996) and were approved by the National Cancer Institute Animal Care and Use Committee. Female athymic nude mice were supplied at 6 weeks of age by the Frederick Cancer Research Center, housed five per cage in a climate-controlled environment. TEMPO-ASA, TEMPO-IND, ASA and indomethacin were prepared in DMSO while TEMPO was prepared in 5% DMSO/PBS pH 7.4. Each animal was weighed individually on the day of the i.p. injection. A range of doses were used to determine the maximum dose tolerated (MTD) by the animals. Each animal was monitored for up to 24 h; death was the end point of the experiment.
Anti-inflammatory assay and acute ulcerogenesis assay
The in vivo anti-inflammatory activity was evaluated using the carrageenan-induced foot paw oedema model (Winter et al., 1962). TEMPO-IND and the reference drug indomethacin were tested at three different doses: 1, 5, 10 mg·kg−1, suspended in 1% methylcellulose solution and administered orally (gavage) 60 minutes before carrageenan injection (50 µL of 1% carrageenan). The paw volume was measured (with a plethysmometer) before and 3 h after injection of carrageenan. The percentage inhibition of inflammation (control inflammation was oedema after treatment with vehicle (1% methylcellulose) alone) was determined at the tested doses and ED50 was calculated graphically from the dose-response curve (R2 > 0.8).
For the ulcerogenesis assay (Winter et al., 1962; Velázquez et al., 2005), TEMPO-IND (30 mg·kg−1) and the reference drug indomethacin (30 mg·kg−1) were suspended in 1.2 mL of 1% methylcellulose solution and administered p.o. The animals were fasted for 24 h prior to drug administration. 6 h after giving the drugs, the rats were killed, and their stomachs were removed and kept on ice. A magnifier lens was used to determine the number and length of the ulcers observed in each stomach. The severity of each gastric lesion was measured by its greatest length in mm. The ‘ulcer index’ (UI) for each compound was calculated by adding the total length in mm of individual ulcers in each stomach, divided by the number of animals in each treatment group.
Data analysis
Data are presented as means ± SD or SE, as shown in the text. Data were analysed by Student's t-test or, where necessary, by one-way ANOVA followed by Dunnett's test for multiple comparisons, using Prism (GraphPad Software, USA).
Materials
Dimethyl sulphoxide (DMSO), 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT), acetyl salicylic acid (aspirin), indomethacin, TEMPO and TEMPOL were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Results
Synthesis
Figure 1 illustrates the chemical synthesis of TEMPO-ASA and TEMPO-IND. Condensation of 4-hydroxy-TEMPO with acetylsalicyloyl chloride gave TEMPO-ASA in 48% yield and DCC-mediated coupling of 4-hydroxy-TEMPO with indomethacin gave TEMPO-IND in 84% yield. NMR spectroscopy, mass spectrometry and elemental analysis support the structure of both products, as shown.
Figure 1.
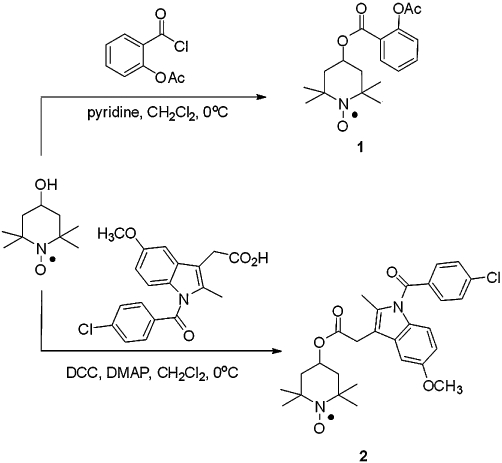
Synthesis of ASA (TEMPO-ASA, 1) and indomethacin (TEMPO-IND, 2) nitroxide conjugates.
Physical properties
Beyond the biological properties, nitroxide-NSAIDs lend themselves to characterization via different physical techniques such as EPR and electrochemistry. Nitroxides, such as TEMPO, have paramagnetic characteristics that can be examined using EPR (Monti et al., 1996). In Figure 2A, the EPR spectra showed a triplet characteristic of this type of nitroxide in both the ASA and indomethacin derivatives. These spectra demonstrate that the integrity of the nitroxide was preserved with the modification of the NSAID. Nitroxides have been shown to be reduced by cells indicated by loss of the triplet. Using this parameter, a single peak can be monitored to obtain kinetic information of cellular and biochemical reduction rates (Swartz et al., 1986). In the presence of A549 cells (Figure 2B) a rapid loss of the triplet was observed (monitored at 3360 G) indicating reduction of these compounds, on incubation with cells, with a half life (t1/2) for TEMPO-IND of ∼24 min and for TEMPO-ASA of ∼2.4 min. The comparable value for TEMPO was ∼13 min. The different rates of reduction for these compounds are indicative of their rates of cellular uptake and intracellular reduction.
Figure 2.
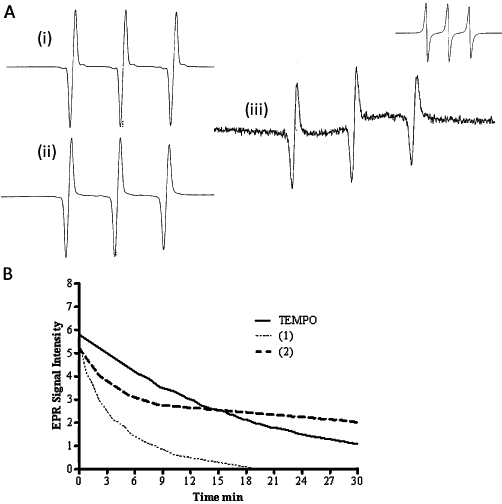
EPR and reduction rate of nitroxide-NSAIDs in A549 cells. (A) EPR of TEMPO-ASA and TEMPO-IND: X-band EPR signal of TEMPO 200 µM in buffer saline solution. Scan Range 8 × 10 G, time constant: 0.128, Mod. Ampl: 1.25 × 101 H, Field Set: 3360 G; Receiving Gain (i) = TEMPO (1.25 × 103) in PBS, (ii) = TEMPO-ASA (RG: 1.25 × 103) in PBS, (iii) = TEMPO-IND (RG: 1.25 × 103) in PBS/10% DMF (upper right: TEMPO-IND in 100% DMF, RG 8.0 × 102), microwave power: 10 mV, microwave frequency: 9.36 GHz, modulation frequency: 100 Hz, at room temperature. (B) This graph shows the rapid uptake of the nitroxides by A549 cells. The decay curves shows the reduction of TEMPO to TEMPO-H and similar reduction of TEMPO-ASA (1) and TEMPO-IND (2). X-band EPR: Scan Range 10 × 101 G, time constant: 0.128, Mod. Ampl: 0.5 × 101 H, Field Set: 3360 G; cell density 2.2 × 107 cells·mL−1, 37°C.
Nitroxides undergo one electron reduction and oxidation, which is at the heart of their antioxidant characteristics. In Figure 3A, the cyclic voltammogram in PBS showed an oxidation potential for TEMPO-ASA (Eox= 0.72 V vs. Ag/AgCl or 0.83 vs. NHE), similar to that for TEMPO (Eox= 0.622 V vs. NHE, E1/2= 197 mV) or TEMPOL (Eox= 0.810 V vs. NHE) (Krishna et al., 1992). Cyclic voltammetry for TEMPO-IND was performed in acetonitrile. Figure 3B shows the cyclic voltammogram of this compound, the presence of 100 mM tetrabutylammonium chloride (Eox= 1.312 V vs. Ag/AgCl) compared with TEMPO under the same conditions (Eox= 1.0 V vs. NHE). The cyclic voltammograph for both TEMPO-ASA and TEMPO-IND was reversible indicating stable one electron oxidation, comparable to that of the parent nitroxides. These results of the EPR and electrochemistry suggest that both TEMPO-ASA and TEMPO-IND exhibited physico-chemical properties characteristic of nitroxides.
Figure 3.
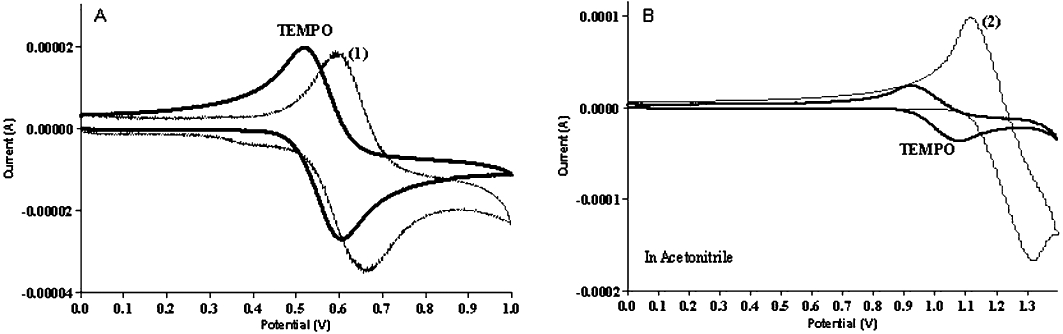
Measurement of physical properties. Cyclic voltagram of nitroxide-NSAIDs. (A) Measurements were done under the following conditions: TEMPO and TEMPO-ASA (1) in PBS in the presence of 10 mM tetrabutyl ammonium chloride. (B) TEMPO and TEMPO-IND (2) in acetonitrile in the presence of 10 mM tetrabutyl ammonium chloride, under aerobic conditions at room temperature. Reference: (Ag/AgCl), working electrode: glass carbon, auxiliary electrode: platinum, sweep scan 100 mV.
SOD mimetic actions
Nitroxides have the capability to mimic superoxide dismutase (SOD) thereby catalytically dismutate superoxide (Krishna et al., 1996). The similarity of the electrochemical behavior between compounds (1) and (2) and TEMPO suggest that these might be capable of detoxifying O2-. As described above, superoxide was generated by HX/XO and ferricytochrome c reduction was monitored at 550 nm. Increasing concentration of TEMPO, compound (1), and (2) decreased the reduction rate of ferricytochrome c characteristic of the competition for superoxide. The solubility of these compounds limited the use of higher doses (>200 mM) in aqueous solutions for this test. The rate constant of these compounds were calculated from the typical plot shown on Figure 4. We obtained a rate of 1.00 × 104 ± 0.06 M−1 s−1 and 1.04 × 104 ± 0.08 M−1 s−1 for compound (1) and (2) respectively. Previous reports at pH 7.4 show a rate of 1.2 × 105 M−1 s−1 for TEMPO and 6.5 × 104 M−1 s−1 for TEMPOL (Samuni et al., 1990; Krishna et al., 1992). These findings show that nitroxide-NSAIDs can scavenge superoxide like other nitroxides retaining the redox properties of nitroxides.
Figure 4.
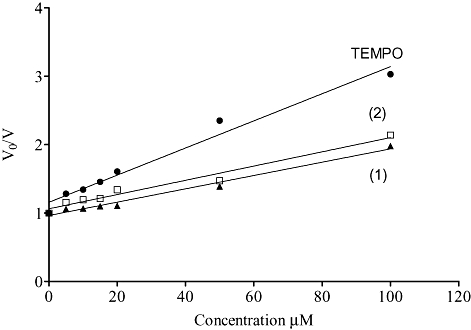
SOD mimetic effects of TEMPO-NSAIDs. Superoxide generation was assessed by Hypoxanthine/Xanthine Oxidase system, 500 µM and 10 mU respectively and 60 µM of ferricytochrome c in PBS (pH 7.4, 25°C /DTPA, 50 µM). The initial rates of cyt c III generation were monitored spectrophotometrically at 550 nm determined in, the presence (V) and absence (V0) of nitroxide in varying concentrations (5–100 µM); (•) TEMPO, (▴) (TEMPO-ASA, 1), and (□) (TEMPO-IND, 2). The data were plotted according to the equation: V0/V = 1 + (knitroxide/scavenger × [nitroxide])/ (kcyt c × [cyt c]) as described in Samuni et al., 1990. The data points were fitted using a linear regression analysis, which gave correlation coefficients of 0.97, 0.98, and 0.97 for TEMPO and compounds TEMPO-ASA and TEMPO-IND respectively.
Inhibition of PGE2 and LTB4 production by nitroxide-NSAIDs
The classical target for the NSAIDs is the COX–catalysed production of PGs (Vane 1971; Vane et al., 1998). Here we evaluated the nitroxide-NSAIDs as inhibitors of the synthesis of PGE2. When the A549 cells were treated with TEMPO-ASA or TEMPO-IND, the IC50 of PGE2 production was 30 µg·mL−1 (90 µM) for TEMPO-ASA and 10 µg·mL−1 (19 µM) for TEMPO-IND (Table 1A). Corresponding data with ASA, indomethacin and TEMPO are shown in Table 1B.
Table 1A.
Inhibition of PGE2 production by A549 cells by TEMPO-NSAIDs
| Dose (µg·mL−1) | TEMPO-ASA | TEMPO-IND | ||||
|---|---|---|---|---|---|---|
| % | (PGE2 pg·mL−1) | µM | % | (PGE2 pg·mL−1) | µM | |
| 0 | 100 | (282 ± 32) | 0 | 100 | (282 ± 32) | 0 |
| 1 | 82 | (230 ± 10) | 3 | 78 | (220 ± 30) | 2 |
| 3 | 81 | (227 ± 2) | 9 | 66 | (187 ± 37)** | 6 |
| 10 | 71 | (200 ± 10)** | 30 | 49 | (140 ± 40)** | 20 |
| 30 | 50 | (142 ± 32)** | 90 | 36 | (102 ± 22)** | 60 |
| 100 | 29 | (82 ± 8)** | 300 | 27 | (77 ± 2)** | 200 |
Table 1B.
Inhibition of PGE2 production by A549 cells by TEMPO, ASA and indomethacin
| PGE-2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Concentration (µg·mL−1) | ASA | Indomethacin | TEMPO | ||||||
| % | (PGE2 pg·mL−1) | µM | % | (PGE2 pg·mL−1) | µM | % | (PGE2 pg·mL−1) | µM | |
| 0 | 100 | (42 ± 3) | 0 | 100 | (42 ± 3) | 0 | 100 | (42 ± 3) | 0 |
| 10 | 107 | (45 ± 7) | 55 | 50 | (21 ± 3) | 28 | 133 | (56 ± 10) | 64 |
| 100 | 23 | (10 ± 2)** | 555 | 12 | (5 ± 1)** | 280 | 123 | (52 ± 8) | 640 |
The values shown in the Tables are the means ± SD of three determinations. PGE2 was measured in supernatant from A549 cells incubated with 20 µM arachidonic acid for 5 min. % represents the %:control production of PGE2 in pg·mL−1
P < 0.001, ANOVA.
Several modified NSAIDs target both PG and LT synthesis (Martel-Pelletier et al., 2003). As nitroxides inhibit lipoxygenase (Rachmilewitz et al., 1994), we evaluated the effects of these nitroxide-NSAIDs on the production of LTB4 by A549 cells. As seen in Table 2A, both TEMPO-ASA and TEMPO-IND blocked LTB4 formation, with IC50 values of ∼30 µM for TEMPO-ASA and of ∼20 µM for TEMPO-IND. However, no inhibition was observed for any of the parent compounds (ASA indomethacin TEMPO) at concentrations up to 250–600 µM (Table 2B). These results indicated that the nitroxide-NSAIDs inhibited both COX and 5-LO in A549 cells.
Table 2A.
Inhibition of LTB4 production by A549 cells by TEMPO-NSAIDs
| Dose (µg·mL−1) | TEMPO-ASA | TEMPO-IND | ||||
|---|---|---|---|---|---|---|
| % | (pg·mL−1) | µM | % | (pg·mL−1) | µM | |
| 0 | 100 | (46.6 ± 0.3) | 0 | 100 | (46.6 ± 0.3) | 0 |
| 0.01 | 98 | (45.9 ± 0.3) | 0.03 | 96 | (44.5 ± 0.3) | 0.02 |
| 0.1 | 95 | (44.2 ± 0.3) | 0.30 | 76 | (35.4 ± 0.3)** | 0.20 |
| 1 | 91 | (42.5 ± 0.3) | 3.00 | 66.5 | (31.0 ± 0.3)** | 2.00 |
| 10 | 51 | (23.6 ± 0.3)** | 30.00 | 62.8 | (29.3 ± 0.3)** | 20.00 |
Table 2B.
Inhibition of LTB4 production by A549 cells by TEMPO, ASA and indomethacin
| Dose (µg·mL−1) | ASA | Indomethacin | TEMPO | ||||||
|---|---|---|---|---|---|---|---|---|---|
| % | (LTB4 pg·mL−1) | µM | % | (LTB4 pg·mL−1) | µM | % | (LTB4 pg·mL−1) | µM | |
| 0 | 100 | (100 ± 1) | 0 | 100 | (100 ± 1) | 0 | 100 | (100 ± 1) | 0 |
| 10 | 64 | (64 ± 13) | 55 | 100 | (100 ± 10) | 28 | 100 | (100 ± 2) | 64 |
| 100 | 62 | (62 ± 5)** | 555 | 82 | (82 ± 9) | 250 | 100 | (100 ± 1) | 640 |
The values shown in the Tables are the means ± SD of three determinations. PGE2 was measured in supernatant from A549 cells incubated with 20 µM arachidonic acid for 5 min. % represents the %:control production of PGE2 in pg·mL−1
P < 0.001. ANOVA.
Cell viability and proliferation assays
NSAIDs have been used to induce cytotoxicity and reduce proliferation in lung tumour cells (Choy and Milas, 2003; Liao et al., 2005). To assess the effectiveness and toxicity of the nitroxide-NSAIDs in cancer cells, we used two assays (i) proliferation assay with MTT and (ii) a clonogenic assay. As shown in Figure 5, A549 cells were incubated with the compounds for 48 h. There was inhibition of proliferation by both TEMPO-ASA and TEMPO-IND but not with the parent compounds (TEMPO, ASA and indomethacin) alone. Similar results were obtained in the clonogenic assay where the cells were exposed for 24 h to the compounds and showed increased cell death with TEMPO-ASA and TEMPO-IND, compared with the parent compounds (Figure 5B). Both assays suggested that the nitroxide-NSAIDs were anti-proliferative for lung adenoma cells.
Figure 5.
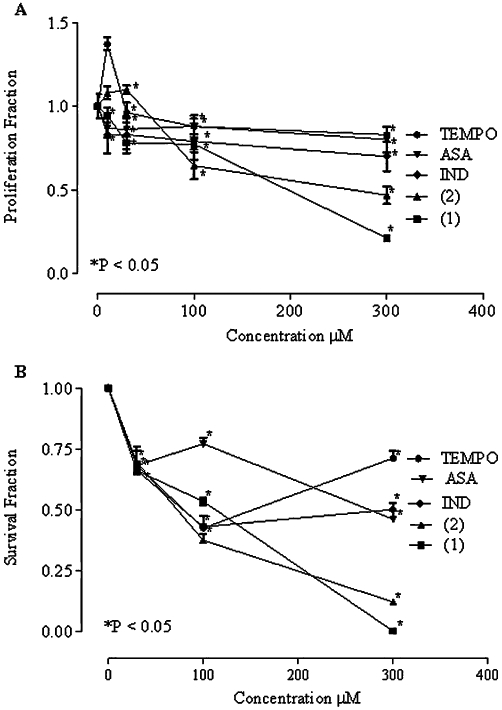
Cell proliferation and clonogenic assays. (A) Proliferation assay of A549 cells after 48 h treatment with nitroxide-NSAID. The mean values of 18 determinations for each individual culture. Absorbance measured at 590 nm, cell density of 5 × 103 cell per well. Values (proliferation fraction) were obtained by dividing the average of the measurements of each treatment (TEMPO, ASA, indomethacin (IND) TEMPO-ASA (1), TEMPO-IND (2)) by the average of control cultures. (B) Clonogenic survival assay of A549 after 24 h treatment with TEMPO, ASA, indomethacin (IND) TEMPO-ASA (1), TEMPO-IND (2). Colonies were counted after 2 weeks of seeding the cells. The mean values of experiments in triplicate are shown, P < 0.05, t-test.
Toxicity studies, anti-inflammatory assays and ulcerogenic index
In Table 3 is the summary of the maximum tolerable dose (MTD) for these drugs when injected i.p. in nude mice. TEMPO showed a MTD of 140 mg·kg−1, which was similar to that of other nitroxides such as TEMPOL (275 mg·kg−1) (Hahn et al., 1992). The MTD of TEMPO-ASA was similar to that of ASA.; However, TEMPO-IND had an eightfold higher MTD (decrease in toxicity), compared with its parent compound indomethacin. The addition of the nitroxide abates the toxicity of these new modified NSAIDs.
Table 3.
Determination of maximum tolerated dose of TEMPO-NSAIDs and parent compounds
| Compound | Molecular weight (g·mol−1) | Maximum tolerated dose (mg·kg−1) |
|---|---|---|
| TEMPO | 156.25 | 140 |
| ASA | 180.15 | 100 |
| TEMPO-ASA | 334.39 | 50–100 |
| Indomethacin | 357.30 | 5 |
| TEMPO-IND | 512.02 | 40 |
The maximum tolerated dose was determined in female athymic nude mice (n= 5 per group). Different dose ranges for each drug (given i.p.) were used: TEMPO: 140, 150, and 160 mg·kg−1, ASA: 50, 75, and 100 mg·kg−1, indomethacin: 5, 7.5, and 10 mg·kg−1, TEMPO-ASA: 50, 75, and 100 mg·kg−1, and TEMPO-IND: 20, 40, and 50 mg·kg−1. The animal was monitored for 24 h after injection, death was the end point of the experiment.
Subsequently, anti-inflammatory and ulcerogenesis assays were performed with TEMPO-IND compared with indomethacin and are summarized in Table 4. Results from the anti-inflammatory assay showed that TEMPO-IND was about 15% more potent (ED50= 3.2 mg·kg−1), compared with indomethacin (ED50= 4.2 mg·kg−1). Based on this, we also evaluated the ulcerogenic side effects of TEMPO-IND using an acute model in rats. The chemical modification of indomethacin with the nitroxide antioxidant moiety (TEMPO) significantly reduced the UI for TEMPO-IND almost ten-fold, compared with that of the parent drug indomethacin (Table 4). These results demonstrate that the addition of the antioxidant moiety TEMPO to the parent indomethacin may have improved the anti-inflammatory activity of TEMPO-IND but clearly decreased the gastric toxicity associated with the parent compound.
Table 4.
Anti-inflammatory activity and ulcerogenic index (UI)
| Compound | Anti-inflammatory ED50 mg·kg−1 | UI |
|---|---|---|
| Indomethacin | 4.2 | 34.1 ± 2.3 |
| TEMPO-IND | 3.2 | 3.6 ± 1.3* |
The anti-inflammatory ED50 values were calculated graphically from dose-response curves of evaluated drugs (TEMPO-IND and indomethacin) at doses of 1, 5, 10 mg·kg−1, in groups of 4 rats, (R2 > 0.8).
The UI was determined at a single dose (30 mg·kg−1) of either indomethacin or TEMPO-IND, given orally 6h before assessment of gastric damage, in groups of 4 rats. Values shown are means ± SEM.
P < 0.05, significantly different from indomethacin.
Discussion and conclusions
Numerous diseases associated with inflammation have increased oxidative stress and inflammatory mediators such as PGs and LTs. Diseases such as arthritis showed an improvement in the health of the patient when NSAIDs are used for prolonged periods of time at high doses and, more recently, NSAIDs have been in clinical trials for cancer (Ulrich et al., 2006; Liao et al., 2007). However, chronic use of these drugs can lead to a significant risk of thrombosis, increasing the likelihood of stroke and heart attack in addition to the risk of developing gastric and small intestine ulcers, sometimes fatal (Dajani and Islam, 2008; Martínez-González and Badimon, 2007). A classic example is indomethacin. It has been shown to be an effective and a potent anti-inflammatory drug; however, the side effects such as gastric ulceration have limited its use (Suleyman et al., 2010). In our data, the gastric toxicity of indomethacin was much reduced by the nitroxide compound TEMPO-IND. These results are analogous to those of Rachmilewitz et al., (1994) who found TEMPOL to abolish gastric toxicity induced by indomethacin or ASA. In our experiments (see Table 2B), it was surprising that the TEMPO-NSAIDs were able to inhibit LTB4 production from arachidonic acid, whereas neither TEMPO nor the parent NSAIDs were inhibitors at much higher concentrations. This additional finding is a potentially important property of this type of NSAID in that both COX and 5-LO pathways of arachidonic acid metabolism were inhibited.
Studies of the chemical properties of these nitroxide-NSAIDs showed that the reduction potential of these compounds was similar to the parent TEMPO compound and they exhibited similar EPR spectra. However, in the presence of cells the nitroxide moiety was readily reduced indicating that these compounds could penetrate into cells and behaved similarly to nitroxide. Additionally, the nitroxide-NSAIDs scavenged superoxide anion, comparably to TEMPO and TEMPOL (rate constants ∼105 M−1·s−1) (Samuni et al., 1990;Krishna et al., 1992). The addition of the NSAID moiety did not alter the chemical properties of the nitroxide group, which is important for further biophysical studies.
All these findings clearly suggest that the modifications of these NSAIDs with the nitroxide moiety provide several advantages, including a decreased gastric toxicity, with no loss of anti-inflammatory potency, relative to indomethacin. The NSAID and nitroxide moeities in these compounds are linked through an ester bond but they could be linked with other bonds (R-groups) to lower toxicity and increase anti-inflammatory activity. These ester prodrugs have activity either as the intact compound or after cleavage by esterases to their respective individual components. Other ester prodrugs require cleavage by esterases of one or both the components. For example, the NO-NSAIDs require NO release through cleavage of the compound to the NSAID and the NO donor component to abate gut toxicity (Kaza et al., 2002; Rigas et al., 2003). Other bifunctional nitroxides have been developed which act as SOD mimetics and NO donors (Haj-Yehia and Nassar, 2002; Dhanasekaran et al., 2005; Jiang et al., 2009). The nitroxide-NSAIDs described here exert a double action, abating both oxidative stress and inflammatory processes making them potentially suitable for treatment of cancer as well as other diseases.
Acknowledgments
This project has been funded through the Intramural Research Program of the NIH, National Cancer Institute.
Glossary
- 5-LO
lipoxygenase 5
- ASA
acetyl salicylic acid
- DMSO
dimethyl sulphoxide
- EPR
electron paramagnetic resonance
- LTB4
leukotriene B4
- MTT
1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan
- NHE
normal hydrogen electrode
- NSAID
non-steroidal anti-inflammatory drug
- SOD
superoxide dismutase
- TEMPO
2,2,6,6-tetramethyl-1-piperidinyloxy
- TEMPOL
4-hydroxy-TEMPO
Conflict of interest
The authors state no conflict of interests.
References
- Bastos-Pereira AL, Lugarini D, de Oliveira-Christoff A, Avila TV, Teixeira S, Pires AD, et al. Celecoxib prevents tumor growth in an animal model by a COX-2 independent mechanism. Cancer Chemother Pharmacol. 2010;65:267–276. doi: 10.1007/s00280-009-1031-8. [DOI] [PubMed] [Google Scholar]
- Choy H, Milas L. Enhancing radiotherapy with cyclooxygenase-2 enzyme inhibitors: a rational advance? J Natl Cancer Inst. 2003;95:1440–1452. doi: 10.1093/jnci/djg058. [DOI] [PubMed] [Google Scholar]
- Cotrim AP, Sowers AL, Lodde BM, Vitolo JM, Kingman A, Russo A, et al. Kinetics of tempol for prevention of xerostomia following head and neck irradiation in a mouse model. Clin Cancer Res. 2005;11:7564–7568. doi: 10.1158/1078-0432.CCR-05-0958. [DOI] [PubMed] [Google Scholar]
- Dajani EZ, Islam K. Cardiovascular and gastrointestinal toxicity of selective cyclo-oxygenase-2 inhibitors in man. J Physiol Pharmacol. 2008;59(Suppl. 2):117–133. [PubMed] [Google Scholar]
- DeGraff WG, Krishna MC, Russo A, Mitchell JB. Antimutagenicity of a low molecular weight superoxide dismutase mimic against oxidative mutagens. Environ Mol Mutagen. 1992;19:21–26. doi: 10.1002/em.2850190105. [DOI] [PubMed] [Google Scholar]
- Del Soldato P, Sorrentino R, Pinto A. NO-aspirins: a class of new anti-inflammatory and antithrombotic agents. Trends Pharmacol Sci. 1999;20:319–323. doi: 10.1016/s0165-6147(99)01353-x. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran A, Kotamraju S, Karunakaran C, Kalivendi SV, Thomas S, Joseph J, et al. Mitochondria superoxide dismutase mimetic inhibits peroxide-induced oxidative damage and apoptosis: role of mitochondrial superoxide. Free Radic Bio Med. 2005;39:567–583. doi: 10.1016/j.freeradbiomed.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Hahn SM, Tochner Z, Krishna CM, Glass J, Wilson L, Samuni A, et al. Tempol, a stable free radical, is a novel murine radiation protector. Cancer Res. 1992;52:1750–1753. [PubMed] [Google Scholar]
- Haj-Yehia A, Nassar T. Beneficial effects of tempoyl nitrate, a bifunctional SOD-mimic NO-donor, on diabetes induced endothelial dysfunction in rat. J Mol Cell Cardiol. 2002;34:A27–A27. [Google Scholar]
- Halliwell B. Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am J Med. 1991;91:14S–22S. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- Jiang JF, Stoyanovsky DA, Belikova NA, Tyurina YY, Zhao Q, Tungekar MA, et al. A mitochondria-targeted triphenylphosphonium-conjugated nitroxide functions as a radioprotector/mitigator. Radiat Res. 2009;172:706–717. doi: 10.1667/RR1729.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaza CS, Kashfi K, Rigas B. Colon cancer prevention with NO-releasing NSAIDs. Prostaglandins Other Lipid Mediat. 2002;67:107–120. doi: 10.1016/s0090-6980(02)00003-5. [DOI] [PubMed] [Google Scholar]
- Krishna MC, Grahame DA, Samuni A, Mitchell JB, Russo A. Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation of superoxide. Proc Natl Acad Sci USA. 1992;89:5537–5541. doi: 10.1073/pnas.89.12.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna MC, Russo A, Mitchell JB, Goldstein S, Dafni H, Samuni A. Do nitroxide antioxidants act as scavengers of O2-. or as SOD mimics? J Biol Chem. 1996;271:26026–26031. doi: 10.1074/jbc.271.42.26026. [DOI] [PubMed] [Google Scholar]
- Li L, Rossoni G, Sparatore A, Lee LC, Del Soldato P, Moore PK. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radic Biol Med. 2007;42:706–719. doi: 10.1016/j.freeradbiomed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Liao Z, Komaki R, Milas L, Yuan C, Kies M, Chang JY, et al. A phase I clinical trial of thoracic radiotherapy and concurrent celecoxib for patients with unfavorable performance status inoperable/unresectable non-small cell lung cancer. Clin Cancer Res. 2005;11:3342–3348. doi: 10.1158/1078-0432.CCR-04-1741. [DOI] [PubMed] [Google Scholar]
- Liao Z, Mason KA, Milas L. Cyclo-oxygenase-2 and its inhibition in cancer: is there a role? Drugs. 2007;67:821–845. doi: 10.2165/00003495-200767060-00001. [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J, Lajeunesse D, Reboul P, Pelletier JP. Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective non-steroidal anti-inflammatory drugs. Ann Rheum Dis. 2003;62:501–509. doi: 10.1136/ard.62.6.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-González J, Badimon L. Mechanisms underlying the cardiovascular effects of COX-inhibition: benefits and risks. Curr Pharm Des. 2007;13:2215–2227. doi: 10.2174/138161207781368774. [DOI] [PubMed] [Google Scholar]
- Moens AL, Takimoto E, Tocchetti CG, Chakir K, Bedja D, Cormaci G, et al. Reversal of cardiac hypertrophy and fibrosis from pressure overload by tetrahydrobiopterin: efficacy of recoupling nitric oxide synthase as a therapeutic strategy. Circulation. 2008;117:2626–2636. [Google Scholar]
- Monti E, Cova D, Guido E, Morelli R, Oliva C. Protective effect of the nitroxide tempol against the cardiotoxicity of adriamycin. Free Radic Biol Med. 1996;21:463–470. doi: 10.1016/0891-5849(96)00124-4. [DOI] [PubMed] [Google Scholar]
- Nakao A, Sugimoto R, Billiar TR, McCurry KR. Therapeutic antioxidant medical gas. J Clin Biochem Nutr. 2009;44:1–13. doi: 10.3164/jcbn.08-193R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals (ISBN 0309053773). Institute of Laboratory Animal Research, Commission on Life Sciences. Washington DC: National Academy Press; 1996. [Google Scholar]
- Patel K, Chen Y, Dennehy K, Blau J, Connors S, Mendonca M, et al. Acute antihypertensive action of nitroxides in the spontaneously hypertensive rat. Am J Physiol Regul Integr Comp Physiol. 2006;290:R37–R43. doi: 10.1152/ajpregu.00469.2005. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D, Karmeli F, Okon E, Samuni A. A novel antiulcerogenic stable radical prevents gastric mucosal lesions in rats. Gut. 1994;35:1181–1188. doi: 10.1136/gut.35.9.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas B, Kalofonos H, Lebovics E, Vagenakis AG. NO-NSAIDs and cancer: promising novel agents. Dig Liver Dis. 2003;35(Suppl. 2):S27–S34. doi: 10.1016/s1590-8658(03)00049-5. [DOI] [PubMed] [Google Scholar]
- Samuni A, Krishna CM, Mitchell JB, Collins CR, Russo A. Superoxide reaction with nitroxides. Free Radic Res Commun. 1990;9:241–249. doi: 10.3109/10715769009145682. [DOI] [PubMed] [Google Scholar]
- Samuni A, Mitchell JB, DeGraff W, Krishna CM, Samuni U, Russo A. Nitroxide SOD-mimics: modes of action. Free Radic Res Commun. 1991;12–13(Pt 1):187–194. doi: 10.3109/10715769109145785. [DOI] [PubMed] [Google Scholar]
- Samuni AM, DeGraff W, Krishna MC, Mitchell JB. Cellular sites of H2O2-induced damage and their protection by nitroxides. Biochim Biophys Acta. 2001;1525:70–76. doi: 10.1016/s0304-4165(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Samuni Y, Gamson J, Samuni A, Yamada K, Russo A, Krishna MC, et al. Factors influencing nitroxide reduction and cytotoxicity in vitro. Antioxid Redox Signal. 2004;6:587–595. doi: 10.1089/152308604773934341. [DOI] [PubMed] [Google Scholar]
- Samuni Y, Cook JA, Choudhuri R, Degraff W, Sowers AL, Krishna MC, et al. Inhibition of adipogenesis by Tempol in 3T3-L1 cells. Free Radic Biol Med. 2010;49:667–673. doi: 10.1016/j.freeradbiomed.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishodia S, Koul D, Aggarwal BB. Cyclooxygenase (COX)-2 inhibitor celecoxib abrogates TNF-induced NF-kappa B activation through inhibition of activation of I kappa B alpha kinase and Akt in human non-small cell lung carcinoma: correlation with suppression of COX-2 synthesis. J Immunol. 2004;173:2011–2022. doi: 10.4049/jimmunol.173.3.2011. [DOI] [PubMed] [Google Scholar]
- Soule BP, Hyodo F, Matsumoto K, Simone NL, Cook JA, Krishna MC, et al. The chemistry and biology of nitroxide compounds. Free Radic Biol Med. 2007;42:1632–1650. doi: 10.1016/j.freeradbiomed.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suleyman H, Albayrak A, Bilici M, Cadirci E, Halici Z. Different mechanisms in formation and prevention of indomethacin-induced gastric ulcers. Inflammation. 2010;33:224–234. doi: 10.1007/s10753-009-9176-5. [DOI] [PubMed] [Google Scholar]
- Swartz HM, Sentjurc M, Morse PD., II Cellular metabolism of water-soluble nitroxides: effect on rate of reduction of cell/nitroxide ratio, oxygen concentrations and permeability of nitroxides. Biochim Biophys Acta. 1986;888:82–90. doi: 10.1016/0167-4889(86)90073-x. [DOI] [PubMed] [Google Scholar]
- Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer. 2006;6:130–140. doi: 10.1038/nrc1801. [DOI] [PubMed] [Google Scholar]
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for the aspirin-like drugs. Nature. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Vane JR, Bakhle YS, Botting RM. Cyclooxlygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- Velázquez C, Praveen Rao PN, Knaus EE. Novel nonsteroidal antiinflammatory drugs possessing a nitric oxide donor diazen-1-ium-1,2-diolate moiety: design, synthesis, biological evaluation, and nitric oxide release studies. J Med Chem. 2005;48:4061–4067. doi: 10.1021/jm050211k. [DOI] [PubMed] [Google Scholar]
- Velázquez CA, Praveen Rao PN, Citro ML, Keefer LK, Knaus EE. O2-acetoxymethyl-protected diazeniumdiolate-based NSAIDs (NONO-NSAIDs): synthesis, nitric oxide release, and biological evaluation studies. Bioorg Med Chem. 2007;15:4767–4774. doi: 10.1016/j.bmc.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GF, Nix PT, Koul AK, Warme PK. Semi-synthetic analogs of cytochrome c. Substitutions for methionine at position 80. Biochim Biophys Acta. 1980;623:457–460. doi: 10.1016/0005-2795(80)90275-5. [DOI] [PubMed] [Google Scholar]
- Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- Zhang QS, Eaton L, Snyder ER, Houghtaling S, Mitchell JB, Finegold M, et al. Tempol protects against oxidative damage and delays epithelial tumor onset in Fanconi anemia mice. Cancer Res. 2008;68:1601–1608. doi: 10.1158/0008-5472.CAN-07-5186. [DOI] [PubMed] [Google Scholar]


