Abstract
The aspartyl protease BACE1 is the rate limiting enzyme in the synthesis of amyloid beta, which accumulation in the human brain is a hallmark of Alzheimer’s disease (AD). BACE1 has been proposed as a surrogate marker of AD; however, very few BACE1 immunoassays have been reported. In the present study we have screened ten BACE1 antibodies by Western blot and several antibody pairs to develop a new BACE1 sandwich ELISA procedure. We identified one pair that showed little background and good reproducibility. Several dilution buffers and sample denaturation methods were tried to partially unfold BACE1 before capture. We found that dilution in PBS followed by 10 min incubation at 50 °C critically improves the performance of the assay. Finally, we successfully measured BACE1 levels in a few human brain and platelet lysates as well as in plasma and AD CSF. We anticipate that this assay will lay the ground to accurately measure BACE1 levels in human tissues, which could facilitate the molecular diagnosis of AD in the near future.
Keywords: BACE1, ELISA, Human, Brain, Plasma, Platelets
1. Introduction
Beta-site APP-cleaving enzyme 1 (BACE1) is a 501 amino acid-long glycosylated type I transmembrane endoprotease (Vassar et al., 2009). It belongs to the aspartyl protease family (EC 3.4.23.46) which comprises BACE1 homolog BACE2, cathepsin D, cathepsin E, rennin, and pepsin (Gruninger-Leitch et al., 2002). BACE1 expression in the body is ubiquitous, though higher expression was noted in the brain (Hussain et al., 1999; Sinha et al., 1999; Vassar et al., 1999; Yan et al., 1999). BACE1 is synthesized as a pre-proprotein, and becomes fully active only after removal of the pro-region (Ermolieff et al., 2000). Several substrates have been identified for BACE1, which include the low-density lipoprotein receptor-related protein (von Arnim et al., 2005), P-selectin glyco-protein ligand-1 (Lichtenthaler et al., 2003), α2.6-sialyltransferase in the Golgi (Kitazume et al., 2001), voltage-gated sodium channel (Nav 1) β2 subunit (Wong et al., 2005), neuregulins 1 and 3 (Hu et al., 2008), enteropeptidase in the pancreas (Hoffmeister et al., 2009), and amyloid precursor protein (APP) and its homolog proteins APLP1 and APLP2 (Li and Sudhof, 2004), suggesting important physiological roles for BACE1 that have yet to be elucidated. Among these substrates, APP has been the most studied since its cleavage by BACE1 initiates the synthesis of amyloid beta peptides, which aggregation into dense-core senile plaques in the human brain is associated with Alzheimer’s disease (AD) (Vassar et al., 2009).
BACE1 is the rate-limiting enzyme in amyloidogenesis (Cole and Vassar, 2008), and measuring its levels and activity have been proposed as surrogate biomarkers for Alzheimer’s disease. Several groups have reported an increase in BACE1 levels and β-secretase activity in the post-mortem analysis of AD patient brains (Ahmed et al., 2010; Fukumoto et al., 2002; Holsinger et al., 2002; Yang et al., 2003). Recently, several investigations have suggested that CSF BACE1 levels and β-secretase activity may be increased in patients suffering mild-cognitive impairment, which is considered to be early AD (Holsinger et al., 2006; Verheijen et al., 2006; Zetterberg et al., 2008; Zhong et al., 2007). However, measurement of BACE1 levels and activity are currently limited by the lack of specific and sensitive tools (reviewed in Decourt and Sabbagh, 2011). Only a few BACE1 ELISA procedures have been reported in the literature and one assay is commercially available (supplemental Table S1). However, all these assays have tissue- and BACE1 isoform-limited applications. Because of its major role in and potential utilization as a biomarker for AD, we aimed at developing a specific BACE1 sandwich ELISA protocol. In the present study, we report the testing of a large number of antibodies and parameters to develop a novel procedure that is specific for BACE1 and shows excellent reproducibility and good linearity with several human tissues.
2. Materials and methods
2.1. Reagents
All reagents were from Sigma–Aldrich, unless stated otherwise. 96-Well microplates were from Nunc (model number 456537). All the antibodies tested are listed in Table 1. The standard used for the ELISA procedure was the human soluble recombinant BACE1 from Calbiochem/EMD (PF125). For specificity and reproducibility tests, the following proteins were also used: human soluble recombinant BACE1 from Enzo Life Sciences (BML-SE531) and R&D Systems (931-AS-050); mouse recombinant BACE1 (R&D Systems 2976-AS-050); human recombinant renin (Enzo life Sciences ALX-201-256); cathepsin D from human liver (Sigma–Aldrich C8596); human recombinant cathespin E (R&D Systems 1294-AS-10); and human soluble recombinant BACE2 (Enzo life Sciences BML-SE550). HRP-conjugated secondary antibodies were from Santa-Cruz biotechnologies: human adsorbed goat anti-mouse (SC-2055), mouse and human adsorbed goat anti-rabbit (SC-2301), and rabbit anti-goat (SC-2768). Odyssey blocking buffer was from Li-Cor Biosciences. 5% alkali-soluble casein was from Novagen. 3,3,5,5-tetramethylbenzidine (TMB) was from Thermo-Fisher. Sulfuric acid was from Mallinckrodt.
Table 1.
BACE1 antibodies screened by Western blot.
| Antibody | Provider (Cat. #) | Epitope | Tissue tested | Western blot results |
|---|---|---|---|---|
| Goat anti-BACE1 ectodomain | R&D Systems (AF931) | 22–460 | Human & mouse brains | Low signals, bands at 55 and 70 kDa |
| Mouse anti-BACE1 C-term | Millipore (MAB5308) | C-term | Human & mouse brains | Low signals, bands at 55 and 70 kDa |
| Mouse anti-BACE1 ectodomain | R&D Systems (MAB 9311) | 22–460 | Human & mouse brains | No signal |
| Mouse anti-BACE1 ectodomain | R&D Systems (MAB931) | 22–460 | Human & mouse brains | Strong signal for bands at 55 and 70 kDa |
| Rabbit anti-BACE1 C-term | Calbiochem (195102) | 487–501 | Mouse brain | Several unspecific extra-bands |
| Rabbit anti-BACE1 C-term | Sigma (B0806) | 485–501 | Human brain | Low signals, bands at 55 and 70 kDa |
| Rabbit anti-BACE1 C-term | Thermo Fisher (PA1-757) | 485–501 | Human brain | Very low signals |
| Rabbit anti-BACE1 N-term | Calbiochem (195101) | 46–65 | Mouse brain | Several unspecific extra-bands |
| Rabbit anti-BACE1 N-term | IBL (28051) | 42–57 | Human & mouse brains | Very low signals |
| Rabbit anti-BACE1 N-term | Sigma (B0681) | 46–62 | Human & mouse brains | Strong signal for bands at 55 and 70 kDa |
2.2. Sample preparation
Human blood was collected in EDTA tubes by a licensed phlebotomist from three volunteers among the laboratory staff. Processing of blood specimens was adopted from the protocol by Bush et al. (1990). At the end, platelets were resuspended in an excess of ice-cold HBSS to wash the remaining plasma and spun again. The plasma and platelet fractions were aliquoted and stored at −80 °C until use. Platelets were thawed and homogenized in lysis buffer (135 mM NaCl; 2.7 mM KCl; 10 mM Na2HPO4; 1.8 mM KH2PO4; pH 7.4; 1%Triton X100; 0.1% SDS; 0.05% NP-40; protease cocktail inhibitor [Complete mini tablets, Roche]) and centrifuged for 20 min at 10,000 × g at 4 °C. Supernatants were isolated and used for ELISA experiments.
Cerebral spinal fluid (CSF) was collected from three locally-recruited subjects: two suffering mild to moderate Alzheimer’s disease per NINCDS-ADRDA criteria (McKhann et al., 1984) and one age-matched non-demented subject. Informed consents approved by the Banner Sun Health Research Institute (BSHRI) Institutional Review Board (IRB) were obtained for all the three subjects. Within 10 min after collection, the CSF was aliquoted and stored at −80 °C until use.
Human post-mortem brain tissues (n = 2) were obtained from the Banner Sun Health brain donation program (Beach et al., 2008). Samples were homogenized in lysis buffer and centrifuged for 30 min at 10,000 × g at 4 °C. Supernatants were isolated and used for ELISA and Western blot experiments.
C57BL/6 mice were purchased from Jackson laboratories and reproduced in house in compliance with a protocol approved by the BSHRI Institutional Animal Care and Use Committee (IACUC). Two twelve month-old mice were anaesthetized with CO2, decapitated, and the brains were quickly removed, snap frozen on dry ice and stored at −80 °C. Brains were thawed and homogenized in mouse brain lysis buffer (50 mM Tris HCl, pH 7.5; 150 mM NaCl; 0.5% NP-40, 0.5% Triton X100, 0.2% Sodium deoxycholate, 0.1% SDS and proteinase inhibitor cocktail [Complete mini tablets, Roche]), then centrifuged at 10,000 × g for 20 min at 4 °C. Supernatants were collected for ELISA and Western blot analyses.
2.3. Western blotting
All Western blot experiments were done in duplicate. Brain lysates (40 μg), platelet lysates (10 μg), plasma diluted to 10% in PBS, and 50 μL CSF were directly separated by SDS-PAGE using 9% gels. After transfer onto PVDF membranes and blocking with 5% dry milk in TBS (25 mM tris base, 137 mM NaCl, 2.7 mM KCl, pH 7.4), primary antibodies were incubated overnight at 4 °C. Membranes were washed several times with TBS and TBST (TBS plus 0.05% Tween 20), incubated for 1 h with corresponding HRP-conjugated secondary antibodies, washed again, incubated with ECL (Millipore), and signals detected with autoradiographic films. The films were scanned. The pictures were processed (levels adjustment, cropping, resizing, and adjustment of brightness and contrast), and the figures were assembled in Photoshop.
2.4. ELISA general procedure
For all the ELISAs performed, 100 μL of the diluted capture antibodies were loaded into RIA-grade 96-well plates, and incubated overnight at 4 °C. The plates were then washed four times with TBST, loaded with 200 μL of blocker (3% bovine serum albumin, except where indicated otherwise), incubated for 1–2 h at room temperature (RT), and the samples were loaded without wash and incubated overnight at 4 °C. After washes, the detection antibodies were loaded and incubated for 2 h at RT, followed by washes and incubation with HRP-conjugated secondary antibodies for 1 h at RT. Detection was carried out using TMB reagent and stopped with 2 M sulfuric acid. Colorimetric signals were measured with a microplate reader (Synergy HT; Biotek Instruments) set at 450 nm. For specificity tests, several aspartyl proteases were used for direct ELISA using the same protocol as above, except that the proteins tested were directly coated on the plates instead of the capture antibody. All ELISAs were carried out at least twice, with duplicate wells each time. Data shown in graphs are optical densities (OD) of samples minus OD of blank (dilution buffer only). Based on the initial standard curves obtained, we used standard dilutions in the dynamic range of the assay for all other tests, and all curve fittings were done by the linear regression method. Slopes, y-intercepts, and R2 values are indicated for each curve. R2 values showed high correlation between the corrected OD values and fitting curves. A patent has been filed for the new ELISA procedure (LMS #0432-12-25204).
3. Results
3.1. Antibody selection
The first step to develop our new sandwich ELISA protocol was to review the literature and select antibodies that had been used previously to detect BACE1 by Western blot and ELISA. This pre-screening led us to test a total of ten candidates by Western blot on human and mouse brain lysates to determine their specificity and signal intensity. Globally, antibodies against sequences located in the BACE1 N-terminus and catalytic domains produced stronger signals than antibodies against the C-terminus intracellular domain (Table 1). The MAB9311 antibody did not recognize BACE1 in denaturing and reducing Western blot procedures, however, the provider indicates that it has the potential to inhibit BACE1 activity in in vitro assays. Furthermore, it is commonly used to immunoprecipitate BACE1 from human and mouse tissues and cell lysates (Kume et al., 2009). All these observations suggest that MAB9311 recognizes a conformational epitope inside or in close proximity to the catalytic site of BACE1, and that it can capture the protein when covalently linked to a solid phase, which we tested (see paragraph below).
The second step was to test several antibody pairs in a common ELISA procedure (see Section 2). The antibodies with poor specificity in Western blot experiments were not used. We utilized the human soluble recombinant BACE1 protein from Calbiochem/EMD as the detection target, and analyzed which antibody pair produced the best signal-to-noise ratio. This recombinant BACE1 was chosen because it has been used in numerous activity assays, as well as in other biochemical analyses of BACE1 (Kalvodova et al., 2005; Kienlen-Campard et al., 2006). Out of the 10 combinations investigated, most produced either high background or very low signals (Table 2). However, we found that capture with mouse anti-BACE1 ectodomain MAB9311 (R&D Systems) and detection with rabbit anti-BACE1 N-terminus B0681 (Sigma–Aldrich) antibodies produced the most specific and intense signals (Table 2). Therefore, we decided to optimize our new assay using this antibody pair.
Table 2.
Summary of the antibody pairs tested during the development of the new BACE1 ELISA.
| Coating antibody (concentration) | Detection antibody (concentration) | Samples used | Results |
|---|---|---|---|
| IBL 28051 (1.0, 2.5, and 5 μg/mL) | R&D Systems MAB9311 (500 and 250 ng/mL) | Calbiochem/EMD BACE1 | Low signal/noise ratio |
| IBL 28051 (1.0, 2.5, and 5 μg/mL) | R&D Systems MAB931 (500 and 250 ng/mL) | Calbiochem/EMD BACE1 | Low signal/noise ratio |
| Sigma B0681 (1:2000) | R&D System AF931 (100 ng/mL) | Calbiochem/EMD BACE1; plasma and serum diluted 50% and 25% | Low signal and high inter-assay variation |
| R&D System AF931 (100 ng/mL) | Sigma B0681 (1:2000) | Calbiochem/EMD BACE1; plasma and serum diluted 50% and 25% | Plasma and serum have higher readings than the standards, indicating high background with biological samples |
| R&D Systems MAB9311 (500 ng/mL) | R&D System AF931 (100 ng/mL) | Calbiochem/EMD BACE1; plasma diluted 50% and 25% | No signal above background |
| Sigma B0681 (1:500, 1:1000, 1:2000 dilutions) | R&D Systems MAB9311 (500 ng/mL) | Calbiochem/EMD BACE1 | Low background. Plasma signals very low |
| Sigma B0681 (1:500, 1:1000, 1:2000 dilutions) | R&D Systems MAB931 (500 ng/mL) | Calbiochem/EMD BACE1 | Low signal/noise ratio |
| Sigma B0681 (1:2000) | R&D Systems MAB9311 (500 ng/mL) | Calbiochem/EMD BACE1; plasma diluted 50%, 25%, 12.5%, and 6.2% | Low background. Plasma signals very low |
| Sigma B0681 (1:2000) | R&D Systems MAB931 (500 ng/mL) | Calbiochem/EMD BACE1; plasma diluted 50%, 25%, 12.5%, and 6.2% | High background. Plasma signals very low |
| R&D Systems MAB9311 (500 ng/mL) | Sigma B0681 (1:2000) | Calbiochem/EMD BACE1; plasma and serum diluted 50%, 25%, 12.5%, and 6.2% | Strong plasma signals. Low background. Good reproducibility |
3.2. Assay specificity and performance characteristics
We determined whether the antibody pair we selected was specific for BACE1 when used for ELISA. For this, two tests were conducted. In the first set of experiments, we examined the cross-reactivity with several aspartyl proteases by direct ELISA. Human soluble recombinant BACE1 (Calbiochem/EMD and Enzo Life Sciences), human recombinant renin, human cathepsin D, human recombinant cathespin E and human soluble recombinant BACE2 diluted to 3.5–250 ng/mL (two-fold serial dilutions) were directly coated on 96-well plates. Rabbit anti-BACE1 N-terminus B0681 (Sigma–Aldrich) was then used for detection. This resulted in signals above background only in the presence of BACE1 proteins, but not with any other aspartyl protease (data not shown). In the second set of experiments, we explored eventual cross-reactivity with the same aspartyl proteases by sandwich ELISA. In this aim, microplates were coated with mouse anti-BACE1 ectodomain MAB9311 (R&D Systems), then the aspartyl proteases diluted in PBS and denatured for 10 min at 50 °C (see Section 3.3) were added into the wells. The detection procedure was the same as for the direct ELISA. Again, no signal above background was detected for any aspartyl protease other than BACE1 (supplemental Table S2).
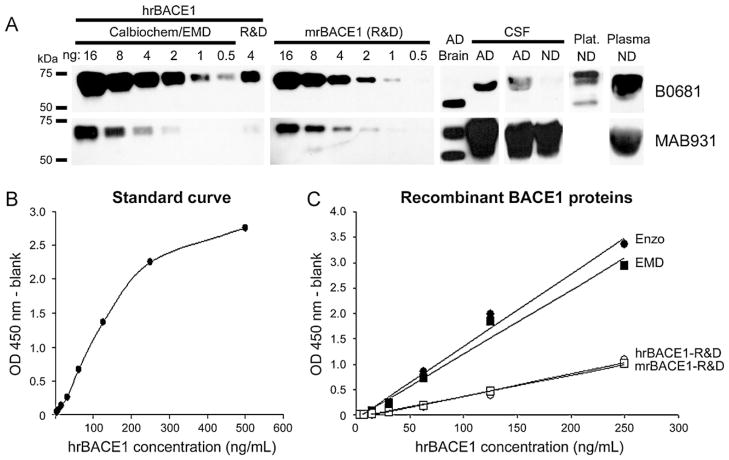
Following the specificity analysis, we investigated the performance characteristics of our new BACE1 ELISA. For this, we carried out both Western blot and ELISA tests on two-fold serial dilutions of three human and one mouse soluble recombinant BACE1 proteins (see Section 2 for details). Western blots were carried out with rabbit anti-BACE1 N-terminus B0681 (Sigma–Aldrich) on recombinant BACE1 quantities ranging from 5 pg to 160 ng. We found a lower limit of detection of 0.5 ng with the recombinant BACE1 protein from Calbiochem/EMD (Fig. 1A), though we occasionally observed weaker signals in some human tissues (e.g. CSF). The human soluble recombinant BACE1 protein from Enzo Life Sciences generated similar results as the Calbiochem/EMD recombinant BACE1 (data not shown). However, both the human and mouse recombinant BACE1 proteins from R&D showed lower signals (Fig. 1A). In addition, stripping and reprobing of the membranes with mouse anti-BACE1 ectodomain MAB931 (R&D Systems), which showed strong signal during the antibody screening (Table 1), also resulted in lower signals than the antibody B0681 with all recombinant BACE1 proteins (Fig. 1A).
Fig. 1.
Dynamic range and specificity analysis of BACE1 antibodies and recombinant proteins. (A) Two antibodies that showed strong signal during initial screening were further tested for their lower limit of detection and specificity by Western blot. Human recombinant BACE1 from Calbiochem/EMD and R&D Systems (R&D) (left), and mouse recombinant BACE1 from R&D Systems (center) were separated by SDS-PAGE and blotted with antibody B0681 (Sigma; top). After stripping the membranes were reprobed with MAB931 (R&D Systems; bottom). B0681 showed higher sensitivity than MAB931 with all recombinant BACE1 proteins. In addition, the recombinant BACE1 protein from Calbiochem/EMD showed more intense signal than the R&D Systems proteins. All proteins were migrated in the same gel and blotted on the same membrane, allowing qualitative comparison. No band was observed for 0.5 ng of recombinant BACE1 when MAB931 was exposed longer times or when MAB931 was detected before B0681 (data not shown). The same antibodies were used on human AD brain, AD and non-demented (ND) CSF, ND platelets (Plat.; only B0681 used), and ND plasma diluted to 10% (right). Bands at 55 and 70 kDa were observed in all tissues with B0681 antibody (top), though the strong band observed for plasma suggests some unspecific signal exists. MAB931 showed specific bands for the brain sample, but very strong, likely unspecific, signal for all CSF and plasma samples tested (bottom). (B) A standard curve was constructed for the assay using the Calbiochem/EMD BACE1 protein. This curve revealed a dynamic range 5–250 ng/mL of recombinant protein. (C) Human recombinant BACE1 from Calbiochem/EMD (EMD; black squares; y = 0.0126x − 0.0638; R2 = 0.982), Enzo Life Sciences (Enzo; black dots; y = 0.0143x − 0.0856; R2 = 0.988), and R&D Systems (hrBACE1-R&D; open circles; y = 0.0044x − 0.0689; R2 = 0.982), as well as mouse recombinant BACE1 from R&D Systems (mrBACE1-R&D; open squares; y = 0.0041x − 0.0503; R2 = 0.996), were tested on our newly developed ELISA (with thermal denaturation). Both proteins from R&D Systems showed lower slopes compared to Calbiochem/EMD and Enzo Life Sciences proteins.
In parallel to the Western blot analysis, we carried out sandwich ELISA experiments with the same above-mentioned recombinant BACE1 proteins using concentrations in the range 3.9–250 ng/mL (0.39–25 ng BACE1 protein since 100 μL were loaded per well). This range was chosen because standard curves built from concentration ranging 3.9–500 ng/mL of recombinant BACE1 (Calbiochem/EMD) showed a lower limit of detection of 3 ng/mL and a dynamic range 5–250 ng/mL (Fig. 1B). Very similar to the Western blot, we observed that the human soluble recombinant BACE1 proteins from Calbiochem/EMD and Enzo Life Sciences showed higher performance than human and mouse recombinant BACE1 proteins from R&D Systems (Fig. 1C). The signals measured for the R&D Systems proteins were 50–75% lower than the signals measured for Calbiochem/EMD and Enzo Life Sciences BACE1 proteins (supplemental Table S2). Altogether, these results indicate that our new assay is highly specific for BACE1 and has a dynamic range of 5–250 ng/mL (0.5–25 ng of BACE1 protein).
3.3. Parameters optimization
In the model proposed by Ermolieff et al. (2000), the BACE1 catalytic domain is partially auto-inhibited by the pro-peptide until the latter is cleaved, likely by furin-like enzymes (Creemers et al., 2001). Alternatively, one may suggest that denaturing the protein would release the pro-peptide, as well as endogenous substrates and inhibitors, from the catalytic site to free the access for antibodies binding the BACE1 active site (e.g. MAB9311). This is commonly achieved either by thermal denaturation, or changing the pH, or adding denaturing agents like detergents to the buffer used to dilute the samples. We explored all three possibilities.
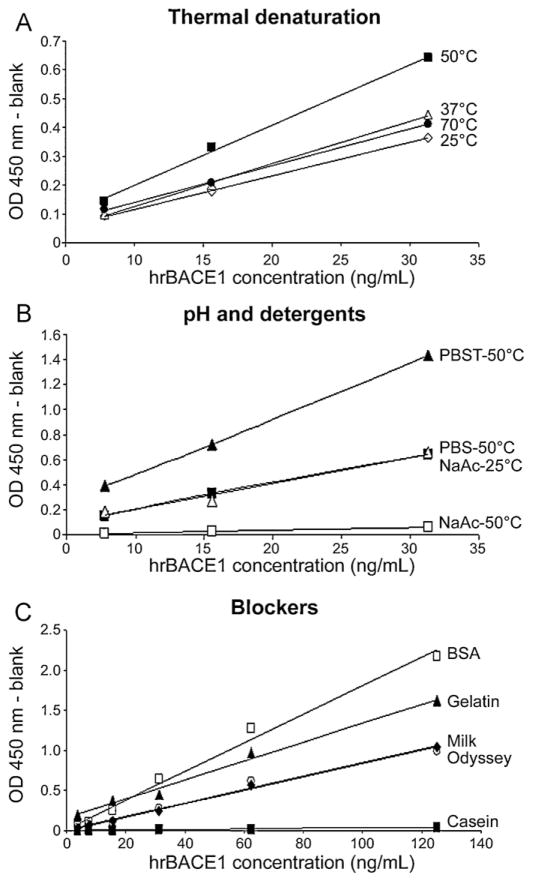
Study of the thermal unfolding profile has shown that human recombinant BACE1 diluted in a neutral pH buffer conserves most of its natural conformation when heated up to 55 °C, but linearizes very fast beyond this temperature (Hayley et al., 2009). Thus, we have investigated the effect of temperature on the performance of our ELISA protocol. Three dilutions (7.8, 15.6, and 31.2 ng/mL) of human recombinant BACE1 (Calbiochem/EMD) protein were prepared in PBS and incubated at room temperature, 37 °C, 50 °C, and 70 °C for 10 min before loading into microplates coated with MAB9311 antibody. At the end, optical densities were measured. We observed that denaturing BACE1 at 50 °C produced the strongest signal for each dilution tested, while the other temperatures generated signals 40% lower in average (Fig. 2A). Later, we noticed that this temperature also produces the best results with biological specimens (see Section 3.4), demonstrating that adding this denaturation step greatly improves the performance of our BACE1 ELISA. To note, although bovine serum albumin (BSA) is usually added to sample dilution buffers in ELISA protocols, in our new assay the denaturation at 50 °C results in BSA agglomeration; therefore BSA was removed from the dilution buffer.
Fig. 2.
Representative data for the optimization of the new BACE1 assay. (A) Human BACE1 recombinant protein (Calbiochem/EMD) diluted at 7.8, 15.6, and 31.2 ng/mL in PBS was incubated for 10 min at room temperature (25 °C, open diamonds; y = 0.0116x − 0.0002; R2 = 0.999), 37 °C (open triangles; y = 0.0148x − 0.0225; R2 = 0.999), 50 °C (black squares; y = 0.0209x − 0.0089; R2 = 0.997), and 70 °C (black dots; y = 0.0127x + 0.0141; R2 = 0.999) before capture. Corrected optical densities (OD) were plotted on one graph, which showed that denaturation at 50 °C produces the best performance for the assay. (B) Human BACE1 recombinant protein (Calbiochem/EMD) was diluted at 7.8, 15.6, and 31.2 ng/mL in PBS (black squares; y = 0.0209x − 0.0089; R2 = 0.997), PBST (black triangles; y = 0.0445x + 0.0362; R2 = 0.999) (both pH 7.4), and 50 mM sodium acetate (NaAc; pH 4.5; open triangles [y = 0.0209x − 0.0091; R2 = 0.974] and squares [y = 0.0022x − 0.0074; R2 = 0.995]). Standards were incubated for 10 min at room temperature (25 °C), or 50 °C before capture. Standards diluted in sodium acetate at room temperature generated signals comparable to standards diluted in PBS and heat-denatured. Standards diluted in PBST and heat-denatured showed stronger signals than PBS. Standards diluted in sodium acetate and heat-denatured did not have signal above background. (C) After coating of the microplates with the capture antibody, several blockers were used: 3% BSA (open squares; y = 0.018x + 0.0123; R2 = 0.989), 3% gelatin (black triangles; y = 0.0119x + 0.1528; R2 = 0.991), 3% milk (open circles; y = 0.0083x + 0.0038; R2 = 0.984), Odyssey blocking buffer (black diamonds; y = 0.0086x − 0.0089; R2 = 0.997), and 5% casein (black squares; y = 0.0003x − 0.0015; R2 = 0.995). Two-fold serial dilutions of human BACE1 recombinant protein in the range 3.9–125 ng/mL were loaded. BSA showed the best performance among all blockers used.
Next, we examined the effect of low pH on BACE1 ELISA signals. Most β-secretase activity assays use a buffer at pH 4.5, which is the optimum pH for BACE1 (Vassar et al., 1999). Moreover, the thermal unfolding of BACE1 at pH 4.5 occurs at lower temperature than at physiological pH (Hayley et al., 2009). We diluted recombinant human BACE1 (7.8, 15.6, and 31.2 ng/mL) in 50 mM sodium acetate, pH 4.5, and incubated the samples at room temperature and 50 °C for 10 min. The results were compared to recombinant BACE1 diluted in PBS, pH 7.4, and incubated at 50 °C. In agreement with the thermal unfolding data (Hayley et al., 2009), BACE1 diluted in sodium acetate pH 4.5 and incubated at room temperature produced signals identical to BACE1 diluted in PBS and incubated at 50 °C (Fig. 2B). However, heating the standards diluted in sodium acetate at 50 °C resulted in signals close to background (Fig. 2B). But, when biological specimens were diluted in sodium acetate (±BSA), pH 4.5, and incubated at room temperature we found that the ELISA signals were lower than dilution in PBS plus denaturation at 50 °C (supplemental Table S3). Thus, we concluded that sodium acetate, pH 4.5, is not an appropriate sample dilution buffer for our BACE1 assay.
To assess whether detergents could increase BACE1 ELISA signals, we added several detergents into the sample dilution buffer (PBS) and incubated the standards and samples at 50 °C for 10 min. Data showed that adding 0.05% Tween 20 to PBS (PBST) increased the signals detected for standards (Fig. 2B). However, signals were lower than PBS alone when PBST was used to dilute biological specimens (data not shown), thus we eliminated Tween 20 in later experiments. We also added 0.005% SDS, 0.05% Triton X-100, 0.05% NP-40, and 0.01% sodium deoxycholate, but all resulted in ELISA signals lower than PBS alone (data not shown).
Two additional parameters we tested were the salt composition of the sample dilution buffer and the blocking agent applied before loading the samples into microplates. As indicated above, PBS pH 7.4 produced better results than sodium acetate pH 4.5 for biological specimens in our new BACE1 assay. Other buffers commonly used in ELISA are TBS and TBST, pH 7.4. Intriguingly, when TBS and TBST were used as dilution buffers for recombinant BACE1 we noted a 10–30% decrease in optical densities compared to PBS (supplemental Table S4) and did not pursue further with these buffers. Next, we examined the effect of several blockers. In these experiments, 3% non-fat dry milk, 3% gelatin, and ready-to-use 5% casein and Odyssey blocker (see Section 2) were compared to 3% BSA. The data indicated that 3% BSA was the best of all blockers tested (Fig. 2C). Blocking with 3% gelatin resulted in signals 25–30% lower than BSA, but was better than 3% milk and Odyssey blocking buffer that both generated corrected OD values 40–60% lower than BSA. Casein seemed to prevent the binding of BACE1 to the antibodies as no signal above background was detected at any concentration of standard loaded (Fig. 2C). Altogether, these experiments indicated that blocking the microplates with 3% BSA, and dilution in PBS (without BSA or detergent), pH 7.4, plus denaturation at 50 °C for 10 min were the best parameters to analyze biological specimens with our new BACE1 ELISA.
3.4. Detection of endogenous human BACE1
After optimizing the assay, we conducted tests on several human tissues. Since this new BACE1 ELISA was developed to study AD, we have focused our attention on tissues that are of interest for this pathology. These included post-mortem human brain tissues, CSF, and plasma and platelets freshly isolated from blood draws. The specificity of two antibodies for BACE1 in these tissues was first confirmed by Western blot. We observed that rabbit anti-BACE1 N-terminus B0681 (Sigma–Aldrich) had good specificity for brain, platelets and CSF, but we noticed very strong signals in plasma suggesting some unspecific binding in this tissue (Fig. 1A). Mouse anti-BACE1 ectodomain MAB931 (R&D Systems), which generated strong signal in our initial antibody screening (Table 1), produced similar results with brain samples (Fig. 1A), but showed very strong, likely unspecific, signals with CSF and plasma (Fig. 1A). MAB931 antibody was not tested on platelet lysates. These data indicated that the B0681 antibody had greater specificity for BACE1 in human tissues than the other antibodies tested here.
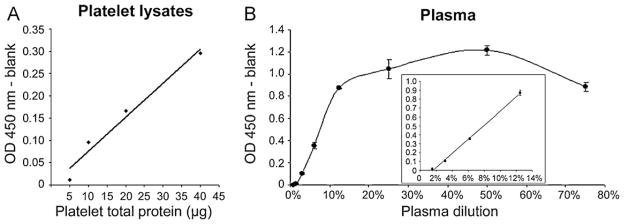
Following Western blots, we tested all tissues in our novel BACE1 assay. A few brain and platelets lysates, as well as plasma, were diluted in PBS and denatured at 50 °C for 10 min before loading into microplates coated with MAB9311 antibody (R&D Systems). CSF samples were used raw (not diluted in PBS) and heat-denatured. Brains from AD subjects (n = 2) had levels ranging 10–70 pg BACE1/μg total proteins. Platelets samples from non-demented subjects (n = 3) had levels in the range of 4–25 pg BACE1/μg total proteins. Furthermore, platelets showed good signal linearity when measuring optical densities of two-fold serial dilutions of the lysates (Fig. 3A). We also detected BACE1 in plasma and CSF. Plasma showed signal linearity only for dilutions up to 15% (Fig. 3B, inset). For dilutions between 15% and 75% signals were saturated (Fig. 3B), likely indicative of increasing matrix effects. Plasma measurements have been difficult to confirm between the few subjects used in this study. Therefore, we are currently collecting data from a larger sample population to try determining quantitative values that we expect to report in a close future. However, we believe that 10% dilution will provide accurate results since the signals will be less affected by matrix effects. The few CSF samples tested revealed that BACE1 levels in cognitively normal subjects may be very low (here the corrected OD values were below the lower limit of quantification, i.e. <5 ng/mL; n = 1), confirming the Western blot data (Fig. 1A). CSF BACE1 levels in AD subjects were slightly higher but remained low (<10 ng/mL; n = 2). Finally, it was difficult to analyse mouse brain lysates using this new BACE1 ELISA protocol since the mouse standard used showed poor performance compared to human recombinant BACE1 (Fig. 1C). Further optimization will, therefore, be required before working with tissues from rodent origin. Nonetheless, based on these preliminary data, we concluded that 10–100 μg of total protein from human tissue lysates, 10–15% dilutions of plasma, and 200 μL of crude CSF should be used to measure BACE1 levels by this ELISA protocol.
Fig. 3.
Linearity tests on human samples. (A) Representative data for linearity test of BACE1 ELISA on platelet lysates. Two-fold serial dilutions of platelet lysate (5, 10, 20, and 40 μg total proteins) were prepared in PBS and heat-denatured at 50 °C before loading. We observed a good linearity of the signal with the corrected OD values doubling as the quantity of total protein increased (y = 0.0076x − 0.0006; R2 = 0.967). (B) Human plasma was diluted to 0.8–75% in PBS and heat-denatured before loading. We noticed good linearity for dilution up to 15% (inset; y = 7.9188x − 0.1252; R2 = 0.998). Above 15%, there had saturation of the signal, likely due to matrix effects.
4. Discussion
In the present study, we screened ten BACE1 antibodies by Western blot and several antibody pairs to develop a new BACE1 sandwich ELISA procedure. We found that capture with mouse anti-BACE1 MAB9311 (R&D Systems) and detection with rabbit anti-BACE1 B0681 (Sigma) antibodies generated the most specific and intense signals. Optimization of the sample preparation showed that dilution in PBS and denaturation at 50 °C for 10 min increases the performance of the assay. Testing of human tissues with this immunoassay indicated that BACE1 levels can be measured in brain and platelet lysates, as well as plasma and CSF.
Most BACE1 ELISA protocols published previously employed antibody pairs that detect the full-length protein containing the intracellular C-terminus portion (supplement Table S1). However, recent evidence suggests that BACE1 may be shed from the plasma membrane resulting in the release of its large catalytic ectodomain into the extracellular milieu (Murayama et al., 2005; Verheijen et al., 2006). This implies that full-length BACE1 may represent only a fraction of total BACE1 present in tissues, and likely very little full-length BACE1 is found in biological fluids, limiting the usage of ELISA protocols detecting the full-length isoform of BACE1 protein. The new assay we describe here uses two antibodies raised against the BACE1 extracellular region, thus has the potential to detect both full-length and membrane-shed BACE1, which likely provides more accurate measurement of endogenous BACE1 levels.
During the optimization process, we found that partially unfolding BACE1 improved significantly the performance of the assay. To our knowledge, such approach has not been attempted for other BACE1 ELISA procedures, but may be key to develop more sensitive BACE1 immunoassays. This original finding is in agreement with the model proposed by Ermolieff et al. (2000) in which BACE1 catalytic domain is partially auto-inhibited by the pro-peptide until it is cleaved. Whether the BACE1 protein detected by our assay contains a high proportion of pro-BACE1 is unknown. Alternatively, one may speculate that the catalytic site is occupied not only by the pro-peptide, but also by endogenous BACE1 substrates and inhibitors which may be removed during the denaturation step. Cross-linking experiments coupled with mass spectrometry analysis could be used to verify this hypothesis. In addition, with the antibody pair we used, thermal denaturation of the biological specimen diluted in PBS, pH 7.4, at 50 °C resulted in higher signals than dilution in an acidic buffer (sodium acetate pH 4.5), though no difference was observed with pure recombinant BACE1. This suggests that MAB9311 antibody binds BACE1 at pH as low as 4.5. However, such a low pH may alter the biochemical properties of other components present in biological samples, which could result in unspecific interactions with BACE1 and prevent recognition by the capture antibody. Moreover, heat-denaturation combined with low pH resulted in low signals. Altogether, these data are in agreement with the thermal unfolding properties reported for BACE1, which unfolds faster when diluted in an acidic buffer (Hayley et al., 2009). Whether our observations are applicable to other BACE1 antibody pairs remains to be elucidated.
Despite our best efforts to optimize this novel BACE1 immunoassay and increase its performance, the lower limit of quantification we observed is 5 ng/mL (0.5 ng) of BACE1 protein. Our Western blot analysis suggests that the limit of quantification derives from the detection antibody (Fig. 1A), though the performance of the capture antibody was not assessed in this study. This limit of quantification may be due to the poor immunogenicity of BACE1, as advanced by Dr. Vassar’s group (Zhao et al., 2007). This idea is emphasized by the low signal intensity of antibodies raised against BACE1 C-terminus intracellular domain, which usually require longer exposure times of autoradiographic films in Western blot experiments (Table 1 and unpublished observations). However, we anticipate that the development of new and more sensitive BACE1 antibodies will allow decreasing the limit of detection of our assay in the future. In addition, we noticed that recombinant BACE1 protein from different providers affects the performance of the assay, with the protein from R&D Systems displaying lower sensitivity than the protein from Calbiochem/EMD. Why these two proteins elicit different results remains unclear since both lack the transmembrane and intracellular domains, are expressed in NSO cells, harbor a His-tag on their C-terminus and are provided as a mixture of immature and mature isoforms. On the other hand, the recombinant BACE1 protein from Enzo Life Sciences, which has the same performance as the Calbiochem/EMD one, is expressed in bacteria (hence is not glycosylated) and is shorter in length. Testing of additional recombinant BACE1 proteins might provide clues towards the understanding of these initial observations.
In conclusion, we have successfully developed a new sandwich ELISA for the quantitative detection of BACE1 in human tissues. The major innovation in our procedure is the thermal denaturation of samples before capture which improves the performance of the assay. Further experimentation will determine whether values obtained with this assay correlate AD diagnosis on known samples and, later, whether it has prognostics potential for AD.
Supplementary Material
Acknowledgments
This study was supported by National Institute on Aging grants R01AG034155 and P30AG019610-09.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jneumeth.2011.08.030.
Contributor Information
Amanda Gonzales, Email: Amanda.Gonzales@bannerhealth.com.
Boris Decourt, Email: Boris.Decourt@bannerhealth.com.
Aaron Walker, Email: Aaron.Walker@bannerhealth.com.
Hikmet Nural, Email: Hikmet.Nural@bannerhealth.com.
Marwan N. Sabbagh, Email: Marwan.Sabbagh@bannerhealth.com.
References
- Ahmed RR, Holler CJ, Webb RL, Li F, Beckett TL, Murphy MP. BACE1 and BACE2 enzymatic activities in Alzheimer’s disease. J Neurochem. 2010;112:1045–53. doi: 10.1111/j.1471-4159.2009.06528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Sue LI, Walker DG, Roher AE, Lue L, Vedders L, et al. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Bank. 2008;9:229–45. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush AI, Martins RN, Rumble B, Moir R, Fuller S, Milward E, et al. The amyloid precursor protein of Alzheimer’s disease is released by human platelets. J Biol Chem. 1990;265:15977–83. [PubMed] [Google Scholar]
- Cole SL, Vassar R. BACE1 structure and function in health and Alzheimer’s disease. Curr Alzheimer Res. 2008;5:100–20. doi: 10.2174/156720508783954758. [DOI] [PubMed] [Google Scholar]
- Creemers JW, Ines Dominguez D, Plets E, Serneels L, Taylor NA, Multhaup G, et al. Processing of beta-secretase by furin and other members of the proprotein convertase family. J Biol Chem. 2001;276:4211–7. doi: 10.1074/jbc.M006947200. [DOI] [PubMed] [Google Scholar]
- Decourt B, Sabbagh MN. BACE1 as a potential biomarker for Alzheimer’s Disease. J Alzheimers Dis. 2011;2(Suppl):53–9. doi: 10.3233/JAD-2011-110017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermolieff J, Loy JA, Koelsch G, Tang J. Proteolytic activation of recombinant pro-memapsin 2 (pro-beta-secretase) studied with new fluorogenic substrates. Biochemistry. 2000;39:12450–6. doi: 10.1021/bi001494f. [DOI] [PubMed] [Google Scholar]
- Fukumoto H, Cheung BS, Hyman BT, Irizarry MC. Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol. 2002;59:1381–9. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- Gruninger-Leitch F, Schlatter D, Kung E, Nelbock P, Dobeli H. Substrate and inhibitor profile of BACE (beta-secretase) and comparison with other mammalian aspartic proteases. J Biol Chem. 2002;277:4687–93. doi: 10.1074/jbc.M109266200. [DOI] [PubMed] [Google Scholar]
- Hayley M, Perspicace S, Schulthess T, Seelig J. Calcium enhances the proteolytic activity of BACE1: an in vitro biophysical and biochemical characterization of the BACE1-calcium interaction. Biochim Biophys Acta. 2009;1788:1933–8. doi: 10.1016/j.bbamem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Hoffmeister A, Dietz G, Zeitschel U, Mossner J, Rossner S, Stahl T. BACE1 is a newly discovered protein secreted by the pancreas which cleaves enteropeptidase in vitro. JOP. 2009;10:501–6. [PubMed] [Google Scholar]
- Holsinger RM, Lee JS, Boyd A, Masters CL, Collins SJ. CSF BACE1 activity is increased in CJD and Alzheimer disease versus [corrected] other dementias. Neurology. 2006;67:710–2. doi: 10.1212/01.wnl.0000229925.52203.4c. [DOI] [PubMed] [Google Scholar]
- Holsinger RM, McLean CA, Beyreuther K, Masters CL, Evin G. Increased expression of the amyloid precursor beta-secretase in Alzheimer’s disease. Ann Neurol. 2002;51:783–6. doi: 10.1002/ana.10208. [DOI] [PubMed] [Google Scholar]
- Hu X, He W, Diaconu C, Tang X, Kidd GJ, Macklin WB, et al. Genetic deletion of BACE1 in mice affects remyelination of sciatic nerves. FASEB J. 2008;22:2970–80. doi: 10.1096/fj.08-106666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain I, Powell D, Howlett DR, Tew DG, Meek TD, Chapman C, et al. Identification of a novel aspartic protease (Asp 2) as beta-secretase. Mol Cell Neurosci. 1999;14:419–27. doi: 10.1006/mcne.1999.0811. [DOI] [PubMed] [Google Scholar]
- Kalvodova L, Kahya N, Schwille P, Ehehalt R, Verkade P, Drechsel D, et al. Lipids as modulators of proteolytic activity of BACE: involvement of cholesterol, glycosphingolipids, and anionic phospholipids in vitro. J Biol Chem. 2005;280:36815–23. doi: 10.1074/jbc.M504484200. [DOI] [PubMed] [Google Scholar]
- Kienlen-Campard P, Feyt C, Huysseune S, de Diesbach P, N’Kuli F, Courtoy PJ, et al. Lactacystin decreases amyloid-beta peptide production by inhibiting beta-secretase activity. J Neurosci Res. 2006;84:1311–22. doi: 10.1002/jnr.21025. [DOI] [PubMed] [Google Scholar]
- Kitazume S, Tachida Y, Oka R, Shirotani K, Saido TC, Hashimoto Y. Alzheimer’s beta-secretase, beta-site amyloid precursor protein-cleaving enzyme, is responsible for cleavage secretion of a Golgi-resident sialyltransferase. Proc Natl Acad Sci USA. 2001;98:13554–9. doi: 10.1073/pnas.241509198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume H, Konishi Y, Murayama KS, Kametani F, Araki W. Expression of reticulon 3 in Alzheimer’s disease brain. Neuropathol Appl Neurobiol. 2009;35:178–88. doi: 10.1111/j.1365-2990.2008.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Sudhof TC. Cleavage of amyloid-beta precursor protein and amyloid-beta precursor-like protein by BACE 1. J Biol Chem. 2004;279:10542–50. doi: 10.1074/jbc.M310001200. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler SF, Dominguez DI, Westmeyer GG, Reiss K, Haass C, Saftig P, et al. The cell adhesion protein P-selectin glycoprotein ligand-1 is a substrate for the aspartyl protease BACE1. J Biol Chem. 2003;278:48713–9. doi: 10.1074/jbc.M303861200. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Murayama KS, Kametani F, Araki W. Extracellular release of BACE1 holoproteins from human neuronal cells. Biochem Biophys Res Commun. 2005;338:800–7. doi: 10.1016/j.bbrc.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, et al. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–40. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–41. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Vassar R, Kovacs DM, Yan R, Wong PC. The beta-secretase enzyme BACE in health and Alzheimer’s disease: regulation, cell biology, function, and therapeutic potential. J Neurosci. 2009;29:12787–94. doi: 10.1523/JNEUROSCI.3657-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheijen JH, Huisman LG, van Lent N, Neumann U, Paganetti P, Hack CE, et al. Detection of a soluble form of BACE-1 in human cerebrospinal fluid by a sensitive activity assay. Clin Chem. 2006;52:1168–74. doi: 10.1373/clinchem.2006.066720. [DOI] [PubMed] [Google Scholar]
- von Arnim CA, Kinoshita A, Peltan ID, Tangredi MM, Herl L, Lee BM, et al. The low density lipoprotein receptor-related protein (LRP) is a novel beta-secretase (BACE1) substrate. J Biol Chem. 2005;280:17777–85. doi: 10.1074/jbc.M414248200. [DOI] [PubMed] [Google Scholar]
- Wong HK, Sakurai T, Oyama F, Kaneko K, Wada K, Miyazaki H, et al. Beta Subunits of voltage-gated sodium channels are novel substrates of beta-site amyloid precursor protein-cleaving enzyme (BACE1) and gamma-secretase. J Biol Chem. 2005;280:23009–17. doi: 10.1074/jbc.M414648200. [DOI] [PubMed] [Google Scholar]
- Yan R, Bienkowski MJ, Shuck ME, Miao H, Tory MC, Pauley AM, et al. Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature. 1999;402:533–7. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- Yang LB, Lindholm K, Yan R, Citron M, Xia W, Yang XL, et al. Elevated beta-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med. 2003;9:3–4. doi: 10.1038/nm0103-3. [DOI] [PubMed] [Google Scholar]
- Zetterberg H, Andreasson U, Hansson O, Wu G, Sankaranarayanan S, Andersson ME, et al. Elevated cerebrospinal fluid BACE1 activity in incipient Alzheimer disease. Arch Neurol. 2008;65:1102–7. doi: 10.1001/archneur.65.8.1102. [DOI] [PubMed] [Google Scholar]
- Zhao J, Fu Y, Yasvoina M, Shao P, Hitt B, O’Connor T, et al. Beta-site amyloid precursor protein cleaving enzyme 1 levels become elevated in neurons around amyloid plaques: implications for Alzheimer’s disease pathogenesis. J Neurosci. 2007;27:3639–49. doi: 10.1523/JNEUROSCI.4396-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Ewers M, Teipel S, Burger K, Wallin A, Blennow K, et al. Levels of beta-secretase (BACE1) in cerebrospinal fluid as a predictor of risk in mild cognitive impairment. Arch Gen Psychiatry. 2007;64:718–26. doi: 10.1001/archpsyc.64.6.718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.