Abstract
Background
Viral-associated trichodysplasia of immunosuppression is a rare cutaneous eruption that is characterized by follicularly based shiny papules and alopecia with characteristic histopathologic findings of abnormally anagen follicules with excessive inner root sheath differentiation. Prior reports have described the histopathologic characteristics on vertical sections; however, to our knowledge, immunohistochemical analysis of polyomavirus proteins has not been previously performed.
Observations
We discuss the thorough diagnostic evaluation and therapy of an unusual case of viral-associated trichodysplasia due to a newly described human polyomavirus that occurred in a patient with post-treatment chronic lymphocytic leukemia and an abnormal white blood cell count. Unique to our study is the immunohistochemical staining for the polyomavirus middle T antigen, which demonstrated positive staining of cellular inclusions within keratinocytes that compose the inner root sheath. Further evaluation with scanning electron microscopy and polymerase chain reaction analysis of viral DNA confirmed the presence of the virus. Treatment with topical cidofovir resulted in dramatic clinical improvement and hair regrowth.
Conclusions
Several tools, including immunohistochemical staining for the polyomavirus middle T antigen, can be used to identify the pathogenic virus associated with viral-associated trichodysplasia. This case highlights the utility of multiple diagnostic modalities and a robust response to a topical therapeutic agent, cidofovir.
Viral-associated trichodysplasia (VAT) of immunosuppression (also known as trichodysplasia spinulosa, pilomatrix dysplasia, cyclosporine-induced folliculodystrophy, and trichodysplasia of immunosuppression) is a rare cutaneous eruption that is characterized by erythematous to skin-colored follicularly based spiny papules that predominantly affect the central face as well as by the histopathologic findings of abnormally maturing anagen follicles with excessive inner root sheath differentiation. Variable amounts of alopecia also have been reported, especially involving the eyebrows, eyelashes, and other hair-bearing parts of the face.
We report an exceptional case of VAT that was confirmed by the results of immunohistologic analysis, histopathologic examination, electron microscopy, and polymerase chain reaction (PCR) assay. We also describe the patient’s dramatic response to topical cidofovir therapy.
REPORT OF A CASE
A 57-year-old woman with a history of chronic lymphocytic leukemia presented with a new rash that started 6 months after she completed chemotherapy with rituximab, cyclophosphamide, and cytarabine. She remained on maintenance therapy with monthly intravenous immunoglobulin therapy. Dry skin on her nose and forehead developed into more distinctive skin-colored and erythematous papules that in turn coalesced into plaques. She had a rough texture to her skin as well as alopecia of her eyebrows and eyelashes and the frontal aspect of her scalp. The skin-colored papules subsequently spread to her chest, arms, and legs. She was initially treated with cimetidine, topical imiquimod, salicylic acid, and hydrocortisone for presumed verruca vulgaris at an outside institution, with limited benefit. She was taking no other medications.
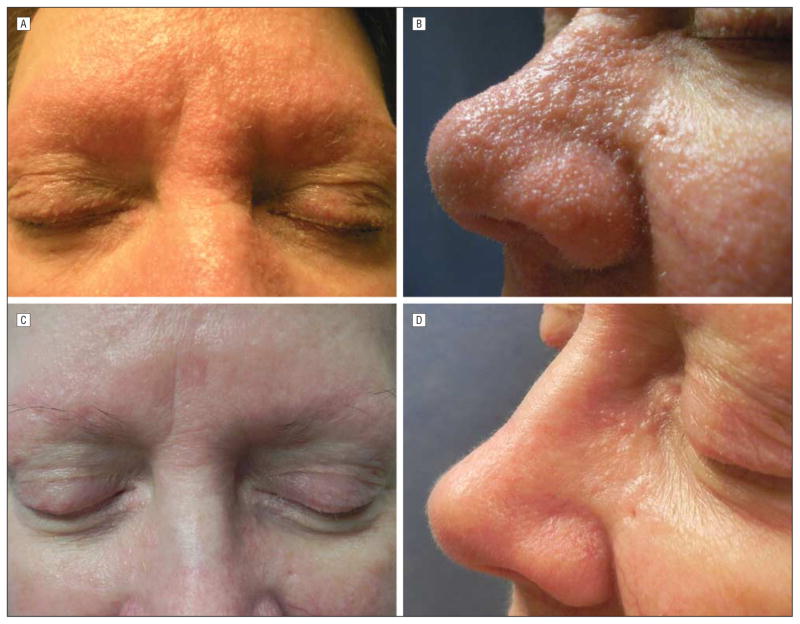
Physical examination revealed skin-colored papules that were nearly confluent on her nose, forehead, cheeks, and chin, with background erythema and nonscarring alopecia. She also had small white spicules protruding from some papules on her nose, madarosis of the eyebrows (Figure 1A and B), and small skin-colored papules on her arms, thighs, chest, neck, and ears as well as alopecia within the affected areas. Laboratory tests were remarkable for a white blood cell count of 3600 μ/L (to convert to ×109/L, multiply by 0.001) (reference range, 4000–10 900 μ/L), with an otherwise normal complete blood cell count and comprehensive metabolic panel.
Figure 1.
Photographs of the patient before (A and B) and after (C and D) treatment. A and B, The patient presented with skin-colored papules that were nearly confluent on her nose, forehead, cheeks, and chin, with background erythema, nonscarring alopecia, and small, white spicules. C and D, Dramatic improvement is seen after 4 months of treatment with topical cidofovir ointment, 1%, once daily. Note the flattening of the papules, absence of white spicules, and improvement in erythema.
Skin biopsy specimens from her eyebrow and arm were examined in both vertical and horizontal cross sections. Merkel cell carcinoma samples and scar tissue from re-excisions served as controls. Formalin-fixed, paraffin-embedded human skin sections, 8 to 10 μm thick, were obtained from the Penn Skin Disease Research Center Tissue Bank according to protocols approved by the institutional review board of the University of Pennsylvania, Philadelphia.
Routine hematoxylin-eosin staining and immunohistochemical analysis for the polyomavirus middle T antigen were performed on sections (Lifespan Biosciences Inc). Immunohistochemical analysis was performed using a mouse anti–SV-40 antibody (EMD Chemicals), which recognized the NH2 terminus within amino acids 83–128 of a large tumor antigen of SV-40 and JCV.1 Unstained slides were deparaffinized, rehydrated, quenched with endogenous peroxidase, and immunostained using the biotin-peroxidase technique (Spring Bioscience). Antigen retrieval was done with 10mM citrate buffer at sub-boiling temperature. Negative and positive controls were run at the same time.
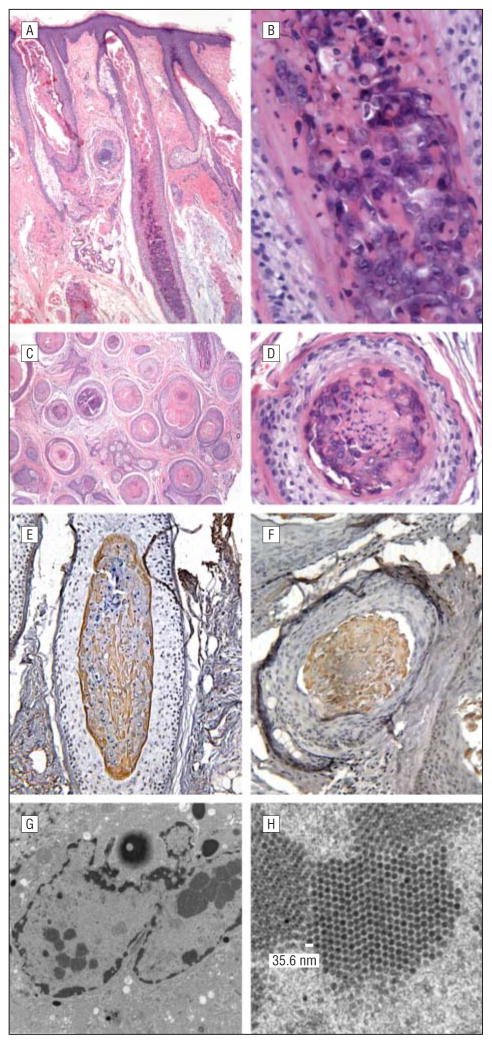
Histopathologically, aberrant keratinization of the inner root sheath hair shaft cells was observed, with numerous enlarged, bulbous anagen hairs and a thin layer of basophilic, germinative cells transitioning to inner root sheath–type cells containing several enlarged bluish gray inclusions. There also were vacuolated keratinocytes with pyknotic nuclei and coarse keratohyaline granules. (Figure 2A and D) Immunohistochemical analysis for the polyomavirus middle T antigen demonstrated positive staining of cellular inclusions within keratinocytes composing the inner root sheath in all of the follicles examined in the patient’s samples (Figure 2E and F). Follicles of control samples (Merkel cell carcinoma, representing another polyomavirus cutaneous infection, and normal skin) demonstrated negative staining. An additional skin biopsy specimen was obtained for electron microscopy and revealed small (35.6-nm), icosahedral, regularly spaced, intracellular viral particles within these inclusions, consistent with polyomavirus (Figure 2G and H).
Figure 2.
Histologic features. A–D, Hematoxylin-eosin staining of tissues in vertical (A, low power; B, high power) and horizontal (C, low power; D, high power) sections demonstrates aberrant keratinization of the inner root sheath hair shaft cells, with numerous enlarged, bulbous anagen hairs and a thin layer of basophilic, germinative cells transitioning to inner root sheath–type cells containing several enlarged bluish gray inclusions. Vacuolated keratinocytes with pyknotic nuclei and coarse keratohyaline granules also are seen. E and F, Immunohistochemical staining for the polyomavirus middle T antigen on both vertical (E) and horizontal (F) sections demonstrates strongly positive staining of cellular inclusions within keratinocytes composing the inner root sheath, confirming the presence of polyomavirus. G and H, Scanning electron microscopy reveals small (35.6-nm), icosahedral, regularly spaced, intracellular viral particles within these inclusions, consistent with polyomavirus.
Detection of the polyomavirus genome was performed by PCR on genomic DNA isolated from paraffin sections (Qiagen). The PCR assay was performed with primers TSV288-F 5′-TATGTTTGCAGTGGGTGG-3′1775–1792 andTSV295-RCTTTGAACTTGGATGTGC-3′3096–3113, multiple primer pairs that are specific for the human polyomavirus that is associated with trichodysplasia spinulosa.2 The PCR conditions were as follows: 95°C for 5 minutes, 95°C for 1 minute, 48°C for 1 minute, and 72°C for 1½ minutes for 40 cycles; elongation at 72°C for 10 minutes; and then incubation at 4°C. The PCR products were run on 0.8% agarose gel at 100 V for 1 hour. Sequencing of PCR products confirmed the presence of TSV.2,3
All of the clinicohistopathologic findings supported a diagnosis of VAT. We treated our patient with topical cidofovir ointment, 1%, once daily based on prior reports of successful use.2,4 She had dramatic improvement in the papules and white spicules, with slow improvement in overall alopecia over a period of 6 months. Her renal function remained normal, and her white blood cell count remained stable throughout her course. To spread more diffusely, topical cidofovir was compounded into a lotion with increased ability to expand over large surfaces, with continued clinical response. Attempts to wean therapy in clinically improved areas resulted in a recurrence of the papules, and untreated areas remained affected, strongly supporting the efficacy of the topical treatment (Figure 1C and D).
COMMENT
Viral-associated trichodysplasia was originally described in 1999 as a folliculocentric viral infection in a patient who was receiving cyclosporine after kidney and pancreas transplantation.5 Since that time, it has been reported under a variety of names (eg, cyclosporine-induced folliculodystrophy, trichodysplasia spinulosa, pilomatrix dysplasia) and in association with other immunosuppressant agents that are used in cases of organ transplantation or as part of chemotherapy regimens, including tacrolimus, azathioprine, prednisone, mycophenolate mofetil, cyclophosphamide, methotrexate, rituximab, fludarabine, intravenous immunoglobulin, and vincristine. Both children and adults have been affected by VAT, and it has been reported to occur after lung, heart, and kidney transplantations and in the setting of acute lymphocytic leukemia.2,3,6–15 In 1 case, the diagnosis of VAT preceded a relapse of non-Hodgkin lymphoma in a 68-year-old man.4 Similar to our case, there is 1 report of VAT that occurred 2 months after completion of chemotherapy in a 70-year-old man with chronic lymphocytic leukemia.16 Screening for this virus by PCR assay in unaffected immunosuppressed patients demonstrated a 4% prevalence, suggesting that active viral infection may be underreported or that the virus may lead only to overt clinical disease in a subset of immunocompromised patients.2
Our case highlights all of the previously reported findings, including the classic histopathologic appearance of large granules and the electron microscopic findings of intranuclear, icosahedral viral particles between 35 and 36 nm in diameter (reported virion diameters range from 28 to 46 nm).3–5,8,9,11,17 Also, sequencing of PCR products confirmed the presence of the human polyomavirus TSV, as reported previously.2,3 Unique to this report is the immunohistochemical identification of the human polyomavirus middle T antigen corresponding to large eosinophilic cellular inclusions in keratinocytes of the inner root sheath, which were visualized on both vertical and horizontal sections. In the initial description of VAT, immunohistochemical stains demonstrated increased Ki-67 protein expression and negative staining for both papillomavirus and the large T antigen of the BK polyomavirus.5 At that time, the initial virus was assumed to be part of the Papovaviridae family, which has subsequently been split into the Papillomaviridae and Polyomaviridae families.18 The PCR and sequencing confirmation of the human polyomvirus DNA, as well as the results of immunohistochemical staining for specific polyomavirus middle T antigen, supports this as a polyomavirus and not a papillomavirus. In our study, the positive staining for the polyomavirus middle T antigen in the trichohyaline granules suggests that this target may be a useful tool for the identification of this disease process and further supports the existing data that the human polyomavirus is responsible for the characteristic findings of VAT.
Although there are limited data available regarding the treatment of VAT, clinical improvement has been reported with various antiviral therapies and with reductions in immunosuppression. Our case demonstrates an impressive, persistent response to topical cidofovir therapy without any adverse effects (Figure 1A and C), a finding that is consistent with prior reports that suggested improvement in 3 patients who used topical cidofovir cream or ointment (1%–3%)2,4,8 and 1 report in which there was no response to topical cidofovir therapy but there was improvement with systemic valganciclovir therapy.13 Also, other patients experienced improvement with oral valacyclovir or valganciclovir therapy when being treated for herpes zoster17 or cytomegalovirus prophylaxis.12 A mixture of topical acyclovir, 2-deoxy-D-glucose, and epigallocatechin as a cost-effective alternative to topical cidofovir also has been demonstrated to be a moderately effective topical therapy in a single report.15 Other treatment modalities, including topical steroids, tacrolimus, antibiotics, imiquimod, topical retinoids, and oral minocycline and retinoids, have been tried, with limited success.7,8,10,11,16,19 Improvement in immune status also has led to resolution of symptoms, and there is a report of spontaneous remission.7,19 Notably, our patient had a recurrence of her lesions with cessation of topical cidofovir therapy and experienced improvement only in areas in which the topical cidofovir was directly applied, and while she has not been treated with immunosuppressive medications for more than 6 months, it is reasonable to presume that she may have some lingering immunodeficiency.
Our case of VAT is important because it provides a comprehensive evaluation of the disease, including the immunohistochemical staining for the middle T antigen or other viral proteins as a potentially more useful and practical way to establish the diagnosis compared with PCR assay or electron microscopy. Routine testing with PCR assay, electron microscopy, or immunhistochemical stains is not necessary in all cases, as routine histologic staining can be sufficient to make the diagnosis in cases of classic clinical presentations. Because the true incidence and clinical spectrum of this recently recognized disease is not known, these tools may be valuable in confirming the diagnosis in cases involving more subtle or atypical presentations. It is important that clinicians are aware of all of the potential evaluations that can be performed and that they recognize the newly described human polyomavirus as the infectious agent that is responsible for VAT, which may become significant if new viruses emerge with similar clinical findings. Further cases are required to verify the utility of immunohistochemical staining, because a negative result would not exclude the diagnosis. Also, we report a dramatic response to topical cidofovir therapy and confirm its efficacy as an important treatment for this cosmetically disfiguring disease.
Acknowledgments
Funding/Support: This work was supported in part by the Penn Skin Disease Research Center (NIAMS P30-AR057217) and by a National Hair Research Society mentorship grant.
Footnotes
Financial Disclosure: None reported.
Author Contributions: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Wanat, Holler, and Rosenbach. Acquisition of data: Wanat, Holler, Dentchev, Simbiri, Robertson, and Rosenbach. Analysis and interpretation of data: Wanat, Holler, Simbiri, Robertson, Seykora, and Rosenbach. Drafting of the manuscript: Wanat, Holler, Simbiri, and Rosenbach. Critical revision of the manuscript for important intellectual content: Wanat, Holler, Dentchev, Robertson, Seykora, and Rosenbach. Obtained funding: Wanat, Holler, Seykora, and Rosenbach. Administrative, technical, or material support: Wanat, Dentchev, Simbiri, Robertson, and Rosenbach. Study supervision: Robertson, Seykora, and Rosenbach.
References
- 1.Bollag B, Prins C, Snyder EL, Frisque RJ. Purified JC virus T and T′ proteins differentially interact with the retinoblastoma family of tumor suppressor proteins. Virology. 2000;274(1):165–178. doi: 10.1006/viro.2000.0451. [DOI] [PubMed] [Google Scholar]
- 2.van der Meijden E, Janssens RW, Lauber C, Bouwes Bavinck JN, Gorbalenya AE, Feltkamp MC. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromized patient. PLoS Pathog. 2010;6(7):e1001024. doi: 10.1371/journal.ppat.1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthews MR, Wang RC, Reddick RL, Saldivar VA, Browning JC. Viral-associated trichodysplasia spinulosa: a case with electron microscopic and molecular detection of the trichodysplasia spinulosa-associated human polyomavirus. J Cutan Pathol. 2011;38(5):420–431. doi: 10.1111/j.1600-0560.2010.01664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osswald SS, Kulick KB, Tomaszewski MM, Sperling LC. Viral-associated trichodysplasia in a patient with lymphoma: a case report and review. J Cutan Pathol. 2007;34(9):721–725. doi: 10.1111/j.1600-0560.2006.00693.x. [DOI] [PubMed] [Google Scholar]
- 5.Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa—a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4(3):268–271. doi: 10.1038/sj.jidsp.5640227. [DOI] [PubMed] [Google Scholar]
- 6.Chastain MA, Millikan LE. Pilomatrix dysplasia in an immunosuppressed patient. J Am Acad Dermatol. 2000;43(1 pt 1):118–122. doi: 10.1067/mjd.2000.100967. [DOI] [PubMed] [Google Scholar]
- 7.Heaphy MR, Jr, Shamma HN, Hickmann M, White MJ. Cyclosporine-induced folliculodystrophy. J Am Acad Dermatol. 2004;50(2):310–315. doi: 10.1016/s0190-9622(03)00774-6. [DOI] [PubMed] [Google Scholar]
- 8.Sperling LC, Tomaszewski MM, Thomas DA. Viral-associated trichodysplasia in patients who are immunocompromised. J Am Acad Dermatol. 2004;50(2):318–322. doi: 10.1016/s0190-9622(03)01490-7. [DOI] [PubMed] [Google Scholar]
- 9.Wyatt AJ, Sachs DL, Shia J, Delgado R, Busam KJ. Virus-associated trichodysplasia spinulosa. Am J Surg Pathol. 2005;29(2):241–246. doi: 10.1097/01.pas.0000149691.83086.dc. [DOI] [PubMed] [Google Scholar]
- 10.Campbell RM, Ney A, Gohh R, Robinson-Bostom L. Spiny hyperkeratotic projections on the face and extremities of a kidney transplant recipient. Arch Dermatol. 2006;142(12):1643–1648. doi: 10.1001/archderm.142.12.1643-d. [DOI] [PubMed] [Google Scholar]
- 11.Sadler GM, Halbert AR, Smith N, Rogers M. Trichodysplasia spinulosa associated with chemotherapy for acute lymphocytic leukaemia. Australas J Dermatol. 2007;48(2):110–114. doi: 10.1111/j.1440-0960.2007.00348.x. [DOI] [PubMed] [Google Scholar]
- 12.Holzer AM, Hughey LC. Trichodysplasia of immunosuppression treated with oral valganciclovir. J Am Acad Dermatol. 2009;60(1):169–172. doi: 10.1016/j.jaad.2008.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benoit T, Bacelieri R, Morrell DS, Metcalf J. Viral-associated trichodysplasia of immunosuppression: report of a pediatric patient with response to oral valganciclovir. Arch Dermatol. 2010;146(8):871–874. doi: 10.1001/archdermatol.2010.175. [DOI] [PubMed] [Google Scholar]
- 14.Schwieger-Briel A, Balma-Mena A, Ngan B, Dipchand A, Pope E. Trichodysplasia spinulosa—a rare complication in immunosuppressed patients. Pediatr Dermatol. 2010;27(5):509–513. doi: 10.1111/j.1525-1470.2010.01278.x. [DOI] [PubMed] [Google Scholar]
- 15.Blake BP, Marathe KS, Mohr MR, Jones N, Novosel T. Viral-associated trichodysplasia of immunosuppression in a renal transplant patient. J Drugs Dermatol. 2011;10(4):422–424. [PubMed] [Google Scholar]
- 16.Lee JS, Frederiksen P, Kossard S. Progressive trichodysplasia spinulosa in a patient with chronic lymphocytic leukaemia in remission. Australas J Dermatol. 2008;49(1):57–60. doi: 10.1111/j.1440-0960.2007.00422.x. [DOI] [PubMed] [Google Scholar]
- 17.Elaba ZHS, Andrea A. Trichyodysplasia spinulosa in a lung transplant patient. Poster presented at: American Society of Dermatopathology 47th Annual Meeting; October 7–10, 2010; Atlanta, Georgia. [Google Scholar]
- 18.Büchen-Osmond C. Taxonomy and Classification of Viruses. 8. Vol. 2. Washington, DC: ASM Press; 2003. [Google Scholar]
- 19.Izakovic JBS, Büchner SA, Düggelin M, Guggenheim R, Itin PH. Hair-like hyper-keratoses in patients with kidney transplants: a new cyclosporin side-effect. Hautarzt. 1995;46(12):841–846. doi: 10.1007/s001050050350. [DOI] [PubMed] [Google Scholar]