Abstract
Matrix metalloproteinases (MMPs) have been implicated in the modulation of synaptic plasticity, glial activation and long-term potentiation in the CNS. Here we demonstrate for the first time a mechanism for the regulation of nociceptive processing by spinal MMP-3 during peripheral inflammation. We first determined by western blotting that the catalytic (active) form of MMP-3 (cMMP-3) is increased in lumbar spinal cord following peripheral inflammation in rats. The peripheral inflammation induced thermal hyperalgesia and tactile hypersensitivity was transiently (2–3 hours) attenuated by intrathecal (IT) pretreatment with either an MMP-3 inhibitor (NNGH), or a broad spectrum MMP inhibitor (GM6001). In addition, IT delivery of cMMP-3 evoked hypersensitivity whereas the pro (enzymatically inactive) form of MMP-3 did not. This suggests a pro-algesic effect of spinal MMP-3 mediated by an enzymatic mechanism. This cMMP-3 induced hypersensitivity is concurrent with increased TNF in the spinal cord. The hypersensitivity behavior is prevented intrathecal etanercept (TNF blockade). Treatment with cMMP-3 resulted in an increase in TNF release from spinal primary microglial, but not astrocyte cultures. These findings thus present direct evidence implicating MMP-3 in the coordination of spinal nociceptive processing via a spinal TNF dependent mechanism.
Keywords: matrix metalloproteinase 3, tumor necrosis factor, pain, inflammation, hyperalgesia, allodynia
1
Matrix metalloproteinases (MMPs) are zinc dependent endopeptidases, which have been widely investigated regarding their dynamic role in the processing of extra cellular matrix (ECM) protein (Ganea et al., 2007). Activation of matrix metalloproteinases during inflammation and injury have been implicated (Agrawal et al., 2008b) in a variety of neurologic diseases including multiple sclerosis (Gijbels et al., 1993), spinal cord injury (Noble et al., 2002), cerebral ischemia (Rosenberg et al., 1996), and neuropathic pain (Shubayev and Myers, 2000). Pain-like behavior and hypersensitivity result, in part, from the release of inflammatory mediators, which activate and sensitize peripheral and central nociceptive components (McMahon et al., 2005). Here, we demonstrate for the first time the role of MMP-3 in mediating spinal nociceptive processing through a TNF dependent pathway activated by peripheral inflammation.
Constitutive RNA expression of MMP-2, 3, 7, 9, and 13 have been demonstrated in the spinal cord (Clements et al., 1997). The expression of particular MMPs and related regulatory enzymes such as tissue inhibitors of metalloproteinase (TIMPs) in microglia, astrocytes or neurons are differentially regulated during the initiation of neurological pathologies (Yong et al., 2001). Such differential regulation correlates with enhanced spinal secretion of cytokines including TNF, IL-1β, and IL-10. These cytokines have been implicated in spinal sensitization (Nuttall et al., 2007). MMP-3 is of particular interest as it lies upstream in the pro to catalytically active MMP cascade. MMP-3 will activate, for example, proMMP-9 (Ogata et al., 1992), which in turn has been demonstrated to activate microglia and regulate onset of neuropathic pain in an IL-1β dependent manner (Shubayev and Myers, 2004; Kawasaki et al., 2008). In addition, MMP-3 activates other key nociceptive linkages including nerve growth factor and brain derived neurotrophic factor (Lee et al., 2001), osteopontin (Agnihotri et al., 2001), and IL-1β (Schonbeck et al., 1998), which can regulate neuronal and non-neuronal cell excitability (Cauwe et al., 2007).
Recent work from our laboratory and that of others indicates that generation and maintenance of enhanced pain states after peripheral injury often involves activation of spinal non-neuronal cells such as microglia and astrocytes (Milligan and Watkins, 2009). Intrathecal minocycline pretreatment will attenuate glial activation and pain hypersensitivity arising from tissue injury (Hua et al., 2005) and reduce MMP activity (Duivenvoorden et al., 1997; Vidal et al., 2007). Given the enzymatic activation by MMP-3 of known pro-nociceptive mediators, the aim of this study was to investigate spinal catalytic MMP-3 activity via pharmacological blockade during peripheral inflammation induced hypersensitivity and explore catalytic MMP-3 regulation of TNF release during non-neuronal cell signaling.
2. Experimental Procedures
2.1 Animals
All experiments were carried out according to protocols approved by the Institutional Animal Care and Use Committee of the University of California, San Diego. Male rats (250–350 g; Harlan, Holtzman Sprague Dawley) were housed in individual cages at room temperature and maintained on a 12 hour light/dark cycle. Testing was performed during the light cycle. Food and water were available ad libitum.
2.2 Intrathecal catheter implantation
For intrathecal (IT) bolus drug delivery, lumbar catheters were surgically implanted into the IT space using a modification of a previously described protocol (Malkmus and Yaksh, 2004). Briefly, animals were anesthetized, secured in a stereotaxic head holder, the cisternal membrane exposed and an incision made through which a single lumen 8.5 cm polyethylene catheter (PE-5) was inserted ending at the L3–L4 spinal segments. The other end of the catheter was fused to a piece of PE-10 tubing and externalized. Rats were allowed to recover for five to six days before experimentation.
2.3 Behavioral tests
To assess the thermally evoked paw withdrawal response, a Hargreaves-type testing device was used (UARDG, Department of Anesthesiology, La Jolla, CA). Rats are placed individually in plexiglass cubicles on a glass surface (maintained at 25 °C) and a thermal stimulus below the glass surface regulates a timer. Latency was defined as the time required for the paw briskly withdrawal as detected by motion sensors controlling the timer and terminating the stimulus (Dirig et al., 1997).
Mechanical sensitivity was assessed using von Frey hairs with hair values ranging from 3.61 g to 15 g and the up–down method was applied as previously described (Chaplan et al., 1994). The 50% withdrawal threshold (force of the von Frey hair to which an animal reacts to 50% of the presentations) was recorded.
Data were also presented as a hyperalgesic index, which represents the area under the time effect curve after stimulation. The formula is [(baseline latency − post-drug latency) × 100 (baseline latency)−1]×min, where latency is expressed in seconds. Decreased values indicate analgesic efficacy. For post-treatments, hyperalgesic index values were calculated using the value at the time point before drug delivery as the baseline value.
2.4 Drugs and drug delivery
Local inflammation was induced with 100 µl of 2% carrageenan in saline (lambda, Sigma-Aldrich) injected subcutaneously into the plantar surface of the hind paw. Intrathecally delivered drugs were prepared in a final volume of 10 µL and followed by a 10 µL saline flush. The following drugs and their doses were employed in vivo: GM6001, (N-[(2R)-2-(Hydroxamidocarbonylmethyl)-4-methylpentanoyl]-L-tryptophan Methylamide) (Calbiochem, 0.01 µg, 0.1 µg or 1 µg); NNGH (N-Isobutyl-N-(4-methoxyphenylsulfonyl)-glycylhydroxamic Acid), (Calbiochem, 2.5 µg or 7.5 µg); PY-2 (synthesized in the laboratory of S.M. Cohen, Dept of Chemistry and Biochemistry, UCSD (Agrawal et al., 2008a), 0.3 µg, 1 µg, or 3 µg); Etanercept (Amgen, 100 µg); cMMP-3 and proMMP-3 (EMD biosciences, 1 pmol). GM6001, NNGH, and PY-2 were prepared in 3% DMSO, which was also used as the respective vehicle. The vehicle for the inhibitors is consistent; therefore the 3% DMSO control groups were combined so as to reduce the number of animals used. Etanercept, cMMP-3, and proMMP-3 were prepared in saline, which was also used as the vehicle. Etanercept was delivered as a 1 hour pretreatment. GM6001, NNGH, and PY-2 were delivered as 30 minute pretreatments in all experiments, except where delivered as a post-treatment at 105 minutes after carrageenan. In vitro the following drugs and their doses were employed: GM6001 (7.5 µg/mL), NNGH (7.5 µg/mL), LPS (Sigma, 100 ng/mL), cMMP-3 (400 ng/mL). The experimenter was blinded to drug treatments during all behavioral testing.
2.5 Western blotting
Rats were anesthetized in 4% isoflurane, decapitated, and spinal cords hydroextruded. 1.5 cm of the spinal cord lumbar enlargement was collected in 3% SDS lysis buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA, 0.5% Triton X-100, 3% SDS pH 7.4) containing protease and phosphatase inhibitors and homogenized by sonication. 30 µg of protein were loaded with 0.1 M DTT and 1xLDS loading buffer (Invitrogen) and separated on a 4–12% Bis-Tris gel (Invitrogen). Proteins were transferred to a 0.45µm nitrocellulose membrane (Invitrogen), and blocked with 5% non-fat milk. Blots were incubated in anti-MMP-3 (Sigma; 1:500) or β-actin (Sigma; 1:100,000) followed by anti-rabbit/ anti-mouse secondary HRP conjugated antibody (Cell Signaling), for 1 hour at room temperature in 5% non-fat milk. Blots were developed using femto (anti-MMP-3) or pico (anti-β-actin) sensitive enhanced chemiluminescent detection system (SuperSignal Pierce). Western blots were scanned and quantified by densitometry using ImageQuant (Molecular Dynamics).
2.6 Tissue Preparation and Immunohistochemistry
Naïve rats were anesthetized with 0.5 mL Euthasol (Virbac), perfused intracardially with 0.9% NaCl followed by 4% paraformaldehyde (PFA) in 0.1 M sodium phosphate buffer (PBS), and spinal cords post fixed in 4% PFA overnight and cryoprotected in sucrose.
Floating sections (30 µm) were blocked (5% normal serum, 0.2% Triton X-100 in PBS) for 1 hour at room temperature before incubating with anti-MMP-3 (1:50; Sigma), and anti Iba-1 (1:500; Abcam), or anti GFAP (1:1000; Sigma) for 72 hours at 4 °C. MMP-3 was amplified with a biotinylated anti-rabbit secondary (1:250; Vector) and an Alexa 488 avidin conjugate. All other primaries were visualized using the appropriate secondary linked to Alexa 555 (1: 5000; Molecular Probes). The anti-MMP-3 antibody has been characterized and deposited in the protein atlas database (Ponten et al., 2008). Sections were mounted and cover slipped (prolong gold antifade medium; Invitrogen).
Staining and visualization procedures were performed as described for floating sections. All images were captured with a Leica TCS SP5 confocal imaging system and processed using Adobe Photoshop CS2 software.
2.7 TNF ELISA
Rats were deeply anesthetized with 4% isoflurane, decapitated, and spinal cords hydroextruded. 1.5 cm of the spinal cord lumbar enlargement (approximately L2–L7) was collected in a modified RIPA buffer containing protease and phosphatase inhibitors and homogenized by sonication, and centrifuged for 12,000×g for 15 minutes at 4 °C. The supernatant was collected and total protein was established using the Bradford method. 1000 µg of total protein in a volume of 100 µl was added to each well and TNF levels were quantified according to manufacturer’s instructions using an ELISA kit (BD Biosciences). TNF content in the spinal cord samples was expressed as pg/mL of spinal cord extract.
2.8 Cell culture
Purified cultures of rat spinal microglia and astrocytes were prepared using a method described previously with some modifications (Hua et al., 2005). One- to three-day-old Holtzman Sprague-Dawley rat pups were anaesthetized, the spinal cords ejected, mechanically triturated, centrifuged at 215 g for 5 minutes, re-suspended in DMEM containing 10% fetal bovine serum (FBS; Gibco), 1% penicillin/streptomycin (P/S; Gibco), plated in a flask previously coated with poly l-lysine (Sigma) and maintained at 37 °C in a humidified 5% CO2 incubator. On day 14, microglia were removed by shaking for 2 hours at 37 °C, centrifuged at 215 g for 5 minutes and plated onto 96-well plates at 40,000 cells/well and allowed to adhere for 24 hours.
Mother cultures were shook a second time and cells were determined to be astrocytes, trypsanized, centrifuged at 215×g for 5 minutes, re-suspended in DMEM with 10% FBS and 1% P/S, and plated until they reached 70–80% confluence.
2.9 Statistics
All the data are presented as mean ± S.E.M. Statistics were performed using Student's t-tests or one-way ANOVA with multiple post hoc comparisons made using the Bonferroni tests (Prism Statistical Software, San Diego, CA, USA) except for TNF ELISAs, which were evaluated by Dunnett’s multiple comparison test using the vehicle as the control value. Critical values corresponding to p < 0.05 were deemed statistically significant.
3. Results
3.1 Inhibition of spinal MMP-3 reduces inflammation-induced thermal and tactile hypersensitivity
3.1.1 Carregeenan thermal hyperalgesia and tactile allodynia
Carrageenan injected into the ipsilateral hind paw of male Holtzman rats elicited a time-dependent increase in the thickness of the injected paw (not shown) and decrease in the thermal latency of the inflamed paw with no significant changes in the response latency of the non-injected contralateral paw. Thus, in animals receiving intrathecal (IT) injections of vehicle (3% DMSO), the baseline thermal escape latency of the ipsilateral paw decreased from 11.4 seconds ± 0.7 to 3.2 seconds ± 0.6 between baseline and 2 hours, (p < 0.05 vs. baseline; Figure 1A, B, C). In a separate group of animals, we showed that in addition to decreased thermal latencies, intraplantar carrageenan also produced a time-dependent decrease in the tactile threshold of the inflamed paw. Thus, the tactile threshold of the ipsilateral paw in IT 3% DMSO vehicle treated rats decreased from the baseline 15 g to 2.1 g ± 0.5 at 2 hours (p < 0.05, Figure 2A).
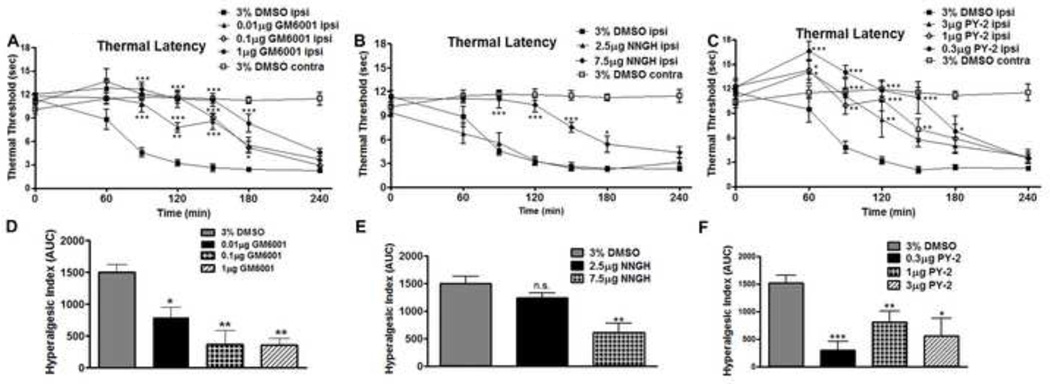
Figure 1.
Spinal MMP inhibition delays onset of thermal hyperalgesia following peripheral inflammation in a dose dependent manner. A 30 minute IT pretreatment delayed thermal hyperalgesia onset compared to a 3% DMSO vehicle treatment for (A) 3 hours with GM6001 (1 µg; p < 0.05), which are graphed (D) according to the hyperalgesic index; for (B) 2.5 hours with NNGH (7.5 µg; p < 0.05), which are graphed (E) according to the hyperalgesic index; and for (C) 2 hours with PY-2 (1 µg; p < 0.05), which are graphed (F) according to the hyperalgesic index. 3% DMSO control groups were combined for all inhibitors tested and plotted accordingly. n = 6 rats for all groups. (* = p < 0.05, ** = p < 0.01, *** = p < 0.001)
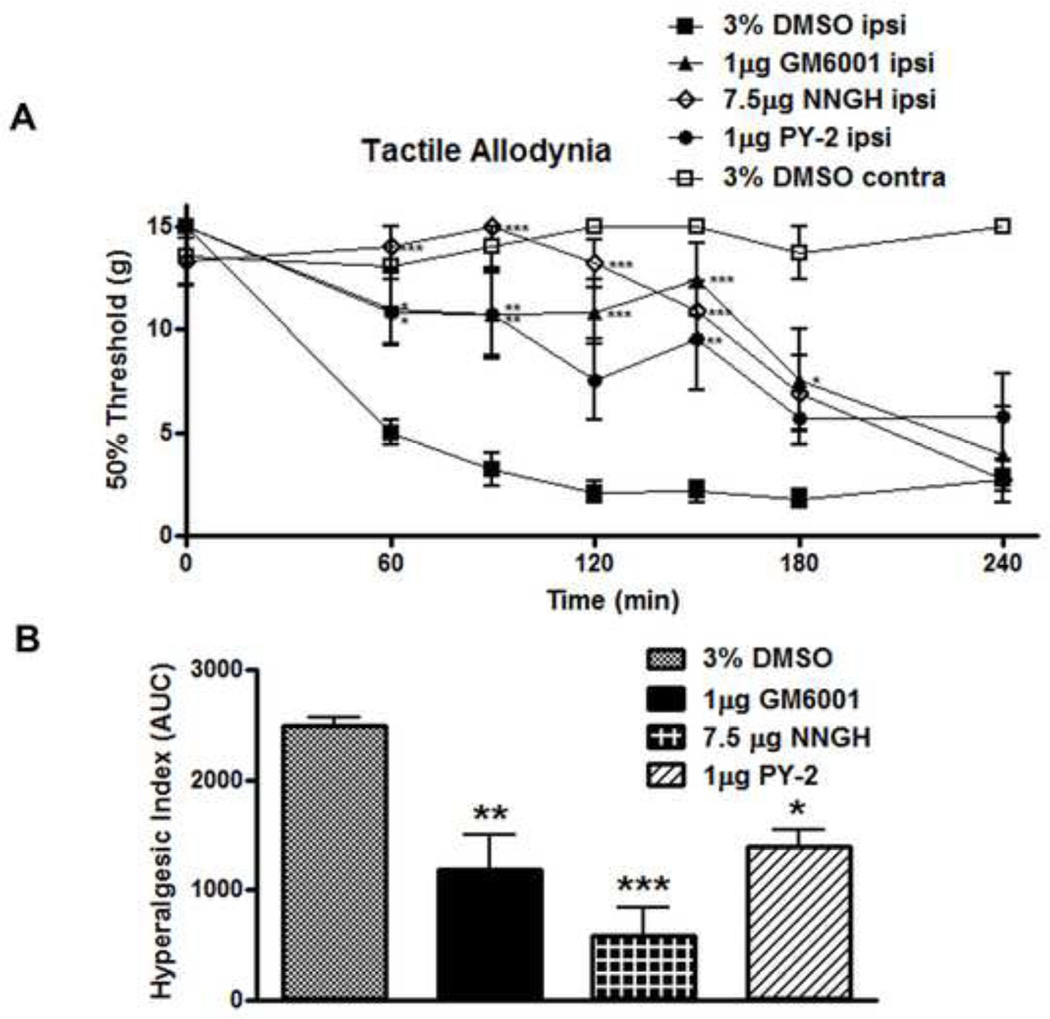
Figure 2.
Spinal MMP inhibition delays onset of tactile allodynia following peripheral inflammation. (A) A 30 minute IT pretreatment with GM6001 (1 µg), NNGH (7.5 µg), or PY-2 (1 µg) significantly delayed the onset of tactile allodynia through 150 minutes (p < 0.05) vs. 3% DMSO vehicle, which is graphed (B) according to the hyperalgesic index. n = 6 rats for all groups. (* = p < 0.05, ** = p < 0.01, *** = p < 0.001)
3.1.2 IT MMP-3 inhibition and pretreatment
To define the role of endogenous spinal MMP-3 during inflammation evoked thermal hyperalgesia, rats received an IT pretreatment (30 minutes in advance of intraplantar carrageenan) of several agents known to inhibit MMP-3 enzymatic activity. Here, IT pretreatment delivery of GM6001, a broad spectrum MMP inhibitor (Figure 1A), NNGH, a semi-selective MMP-3 inhibitor (Figure 1B), or PY-2, a semi-selective MMP-3 inhibitor (Figure 1C) resulted in an attenuation of the onset of intraplantar carrageenan evoked thermal hyperalgesia. When the data for the ipsilateral, inflamed paw, are presented according to the hyperalgesic index, a dose dependent anti-nociceptive effect as compared to a 3% DMSO vehicle is observed with: GM6001 (Figure 1D: 0.01 µg; p < 0.05, 0.1 µg; p < 0.01, 1 µg; p < 0.001) and NNGH (Figure 1E: 2.5 µg; not significant, 7.5 µg; p < 0.001). For PY-2 all doses display a significant reduction as compared to vehicle control (0.3 µg; p < 0.001, 1 µg; p < 0.01, 3 µg; p < 0.05).
To define the role of endogenous spinal MMP-3 during inflammation evoked tactile allodynia, the most efficacious doses of GM6001, NNGH, and PY-2, as determined during the dose response thermal hypersensitivity experiments, were delivered as 30 minute IT pretreatments. These doses delayed the onset of carrageenan induced tactile allodynia for GM6001 (1 µg; p < 0.001), NNGH (7.5 µg; p < 0.001) and PY-2 (1 µg; p < 0.01) as compared to the 3% DMSO vehicle (Figure 2A and 2B). None of the IT inhibitors or the vehicle at the doses employed in this pretreatment series caused any sedation or signs of motor dysfunction (e.g. rats displayed normal spontaneous symmetrical ambulation, hind paw placing and stepping, pinnae and blink reflexes).
3.2 IT MMP-3 inhibition and post-treatment
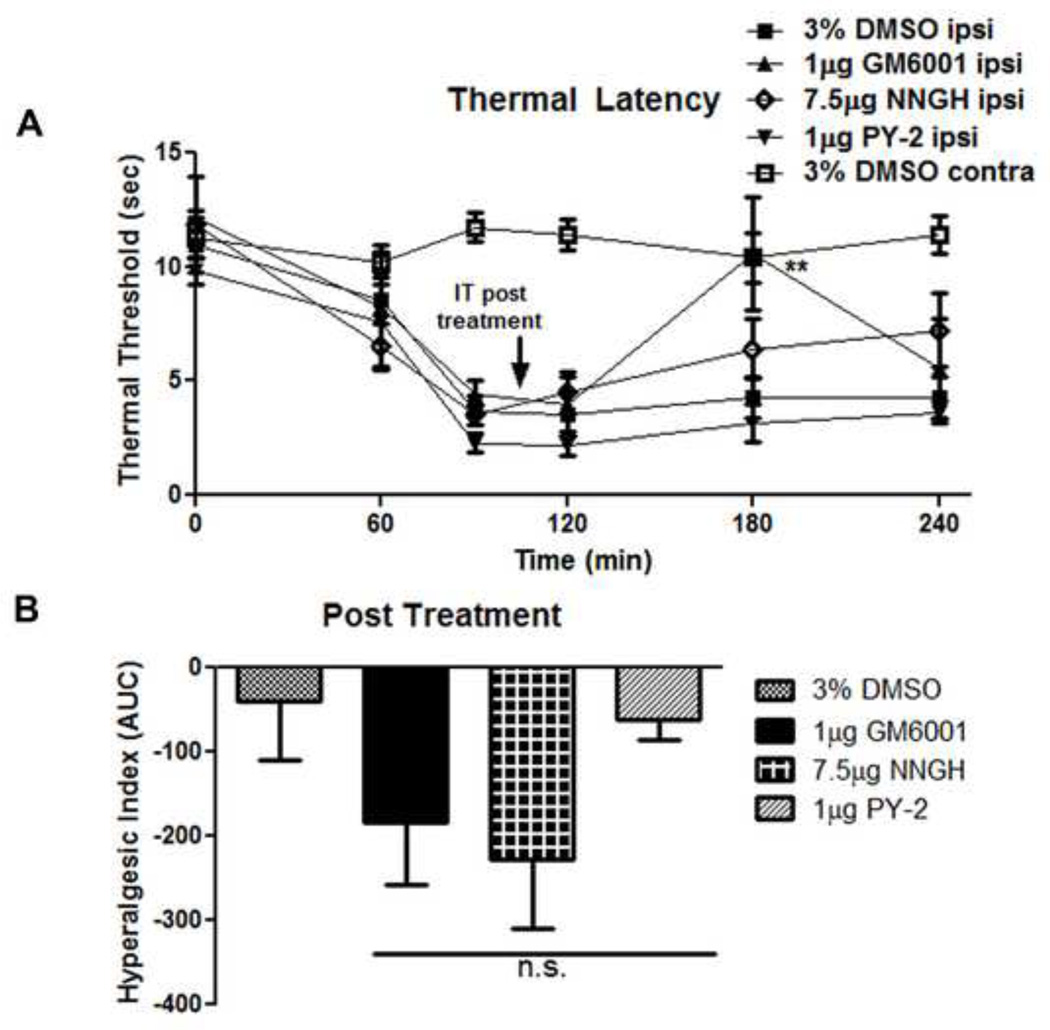
At 105 min after intraplantar carrageenan injection we delivered IT the efficacious doses of GM6001 (1 µg), NNGH (7.5 µg), and PY-2 (1 µg) and recorded the changes in thermal thresholds in rats from 120–240 minutes (Figure 3A) to determine if MMP inhibition altered hypersensitivity after initiation of peripheral inflammation. To determine the effect of post-treatments upon the hyperalgesic index, baseline values were set according to 90 minute values and the area under the curve from 120–240 minutes was calculated. Post-treatment with the MMP-3 specific inhibitors NNGH and PY-2 indicate no significant improvements in thermal thresholds as compared to 3% DMSO vehicle. IT GM6001 post-treatment demonstrated a transient increase in thermal thresholds at 180min following carrageenan (Figure 3A; p < 0.05 vs 3% DMSO), however, calculation of the hyperalgesic index showed no significant anti-hyperalgesic effects in thermal thresholds as compared to 3% DMSO vehicle. Given that GM6001 also inhibits MMP-1(Grobelny et al., 1992), MMP-2, MMP-8, and MMP-9 (Knight et al., 1992); the post-treatment efficacy of GM6001 but not NNGH or PY-2 is likely the result of broad spectrum MMP inhibition, rather than MMP-3 specific inhibition.
Figure 3.
A post treatment with NNGH (7.5 µg) or PY-2 (1 µg) at 105 minutes after carrageenan administration had no significant effect upon thermal hyperalgesia. (A) A post treatment with GM6001 (1 µg) increased thermal thresholds 180 minutes after carrageenan injection (p < 0.01). Graphed (B) according to the hyperalgesic index IT post treatment of GM6001, NNGH, and PY-2 had no significant effect upon the hyperalgesic index on the inflamed paw. n = 5–7 rats for all groups. (* = p < 0.05, ** = p < 0.01).
3.3 Peripheral inflammation increases spinal MMP-3 protein expression
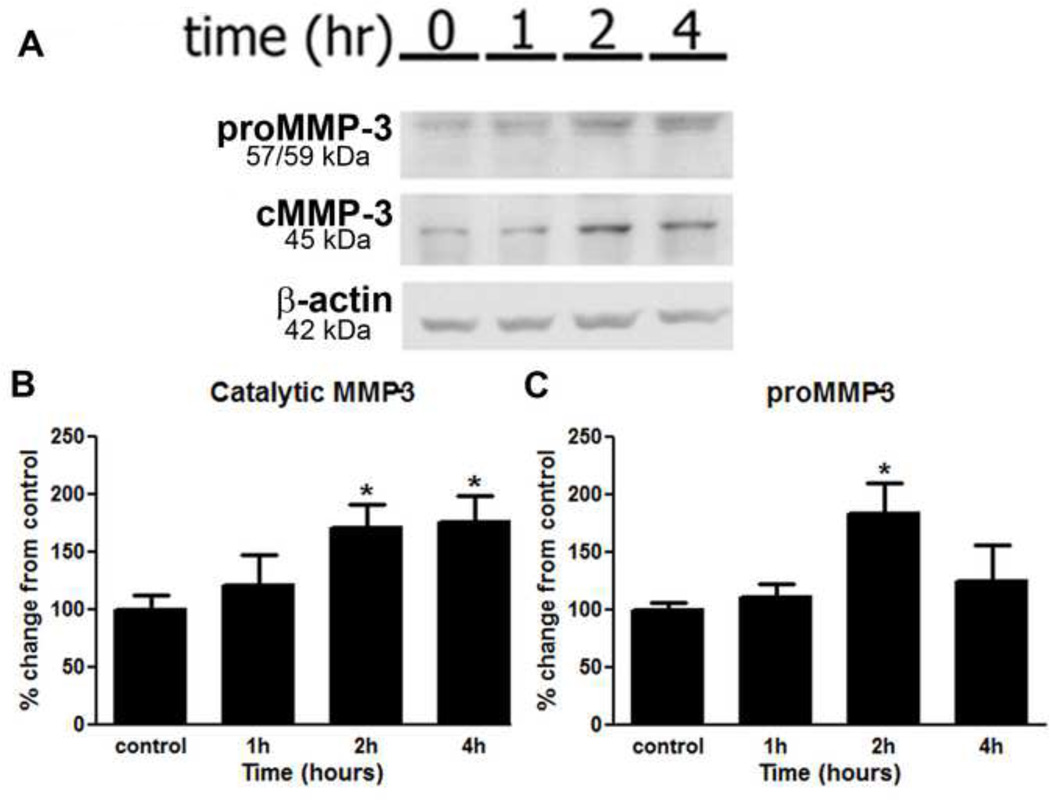
The pharmacological behavioral data suggested that catalytically active spinal MMP-3 was mediating the inflammation evoked hyperalgesia. To characterize spinal MMP-3 activation, protein levels were measured by western blotting for the pro form and the catalytically active forms of MMP-3 in the lumbar spinal cord (L4–L6) following intraplantar carrageenan (Figure 4A). There was indeed a significant increase in catalytic MMP-3, 2 and 4 hours post-injection (p < 0.05, Figure 5B) and in proMMP-3, 2 hours after the intraplantar carrageenan (p < 0.05; Figure 5C) as compared to naïve animals. This increase in the catalytically active MMP-3 cleavage product coincides with the time at which the carrageenan induced hyperalgesia and allodynia, attenuated by spinal MMP-3 inhibition, were observed.
Figure 4.
cMMP-3 increased in the lumbar spinal cord following peripheral inflammation. (A) The L4–L6 region of the spinal cord was blotted against MMP-3 following peripheral inflammation (B) cMMP-3 increased significantly in the spinal cord of rats 2 and 4 hours and (C) proMMP-3 increased significantly in the spinal cord of rats 2 hours following induction of peripheral inflammation as determined by densitometry (p < 0.05) and normalized to β-actin. Densitometric results shown are the average of three independent experiments. * = p < 0.05.
Figure 5.
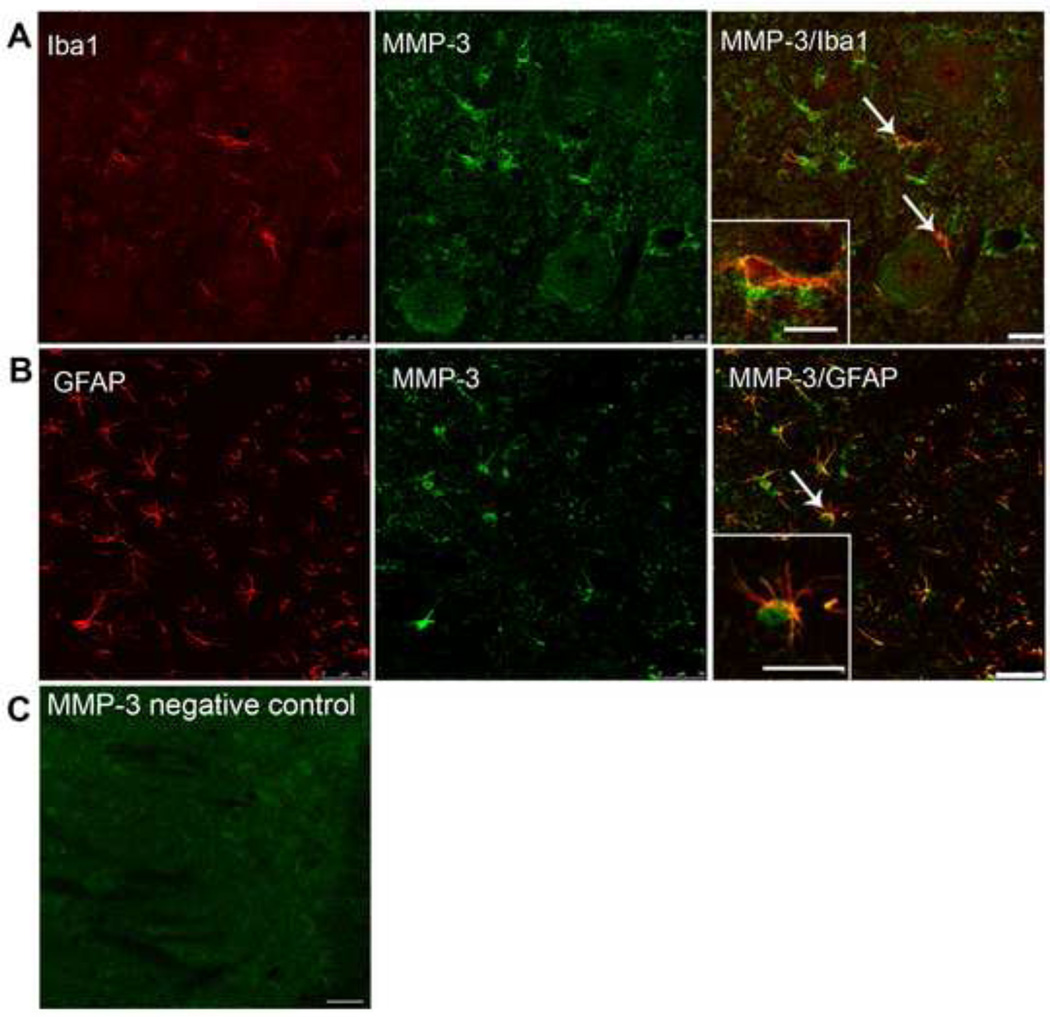
MMP-3 co-labeled with the markers for astrocytes, microglia, and neurons in the spinal cord. MMP-3 (green) co-stained in (A) with the microglia marker Iba-1 (red) and in (B) with the astrocyte marker GFAP (red). (C) Is a negative control slide for staining. All slides are representative slices from the L4–L6 lumbar section of the superficial dorsal horn in naïve rats. Scale bars are 25 and 50 µm respectively. Inset scale bars are 10 and 15 µm respectively.
3.4 MMP-3 is expressed in spinal astrocytes and microglia
We next investigated the distribution and cellular location of MMP-3 in the rat spinal cord by immunohistochemistry. MMP-3 immunoreactivity was seen in all laminae in the dorsal and ventral horn. As shown in Figure 5, cells co-labeled with antibodies against MMP-3 and with antibodies against protein markers for astrocytes (GFAP), and microglia (Iba1).
3.5 Spinal administration of catalytic MMP-3 (cMMP-3) causes thermal hyperalgesia and tactile allodynia
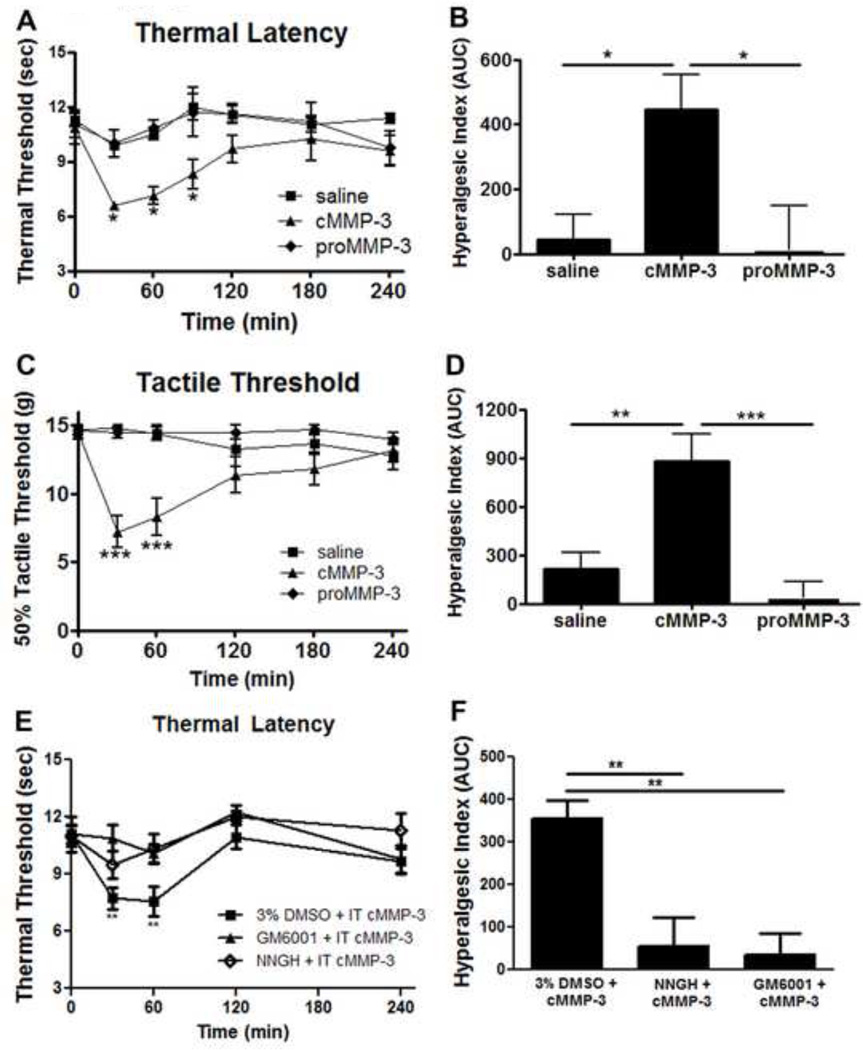
Given the delay in peripheral inflammation induced hypersensitivity onset following spinal MMP-3 inhibition and the appearance of increased spinal cMMP-3, we next sought to determine if exogenous cMMP-3 or proMMP-3 delivered intrathecally would mimic the pro-algesic actions of the peripheral inflammation. Rats were injected IT with cMMP-3 (1 pmol), proMMP-3 (1 pmol) or saline and evaluated for changes in thermal and tactile thresholds. 30 minutes after IT injection of cMMP-3, the thermal escape latency dropped from baseline 10.8 seconds ± 0.9 to 6.6 seconds ± 0.2 (p < 0.05; Figure 6A) and remained significantly decreased for 90 minutes, whereas neither the pro form of MMP-3 (1 pmol) nor the saline control had any effect. Changes are presented according to the hyperalgesic index in Figure 6B, indicating a significant increase in pain-like behavior following catalytic but not proMMP-3 or saline intrathecal delivery (p < 0.05). Similarly, 30 minutes after IT cMMP-3 the tactile threshold dropped from baseline 14.5 g ±0.5 to 7.2 g ±1.2 (p < 0.001; Figure 6C) and remained decreased for 60 minutes, with no changes in tactile threshold following IT saline or proMMP-3. Changes presented according to the hyperalgesic index in Figure 6D show a significant increase in pain-like behavior following catalytic but not after IT proMMP-3 or saline (p < 0.01). An increase in nociceptive behavior by the spinal application of catalytic MMP-3 but not the unprocessed pro form indicates an enzymatic role for this protein in the spinal nociceptive processing.
Figure 6.
IT cMMP-3 reduced thermal and tactile thresholds. (A) IT cMMP-3 (1 pmol), but not proMMP-3 (1 pmol) induced thermal hyperalgesia 30, 60, and 90 minutes after delivery (p < 0.05) vs. saline vehicle, which is graphed (B) according to the hyperalgesic index. (C) IT cMMP-3 (1 pmol), but not proMMP-3 (1 pmol) induced tactile allodynia 30 and 60 minutes after delivery (p <0.05) vs. saline vehicle, which is graphed (D) according to the hyperalgesic index. (E) IT cMMP-3 induced thermal hyperalgesia is attenuated by IT GM6001 or NNGH pretreatment, which is graphed (F) according to the hyperalgesic index. n = 4–8 rats. * = p < 0.05, ** = p < 0.01, *** = p < 0.001.
The specificity of the IT cMMP-3 action was further defined by pretreatment with MMP and MMP-3 inhibitors. Pretreatment (30 minutes) with IT GM6001 (1 µg) or IT NNGH (7.5 µg) abrogated the IT cMMP-3 induced thermal hypersensitivity (Figure 6E). At no time point was there a significant difference between baseline thresholds and thresholds 30 or 60 minutes after IT cMMP-3 when rats were pretreated with GM6001 or NNGH. Changes presented according to the hyperalgesic index in Figure 6F show a significant increase in pain-like behavior in the four hours after IT cMMP-3, which is attenuated by NNGH or GM6001 pretreatment (p < 0.01).
3.6 IT cMMP-3 but not pro-MMP-3 increases spinal TNF concentrations
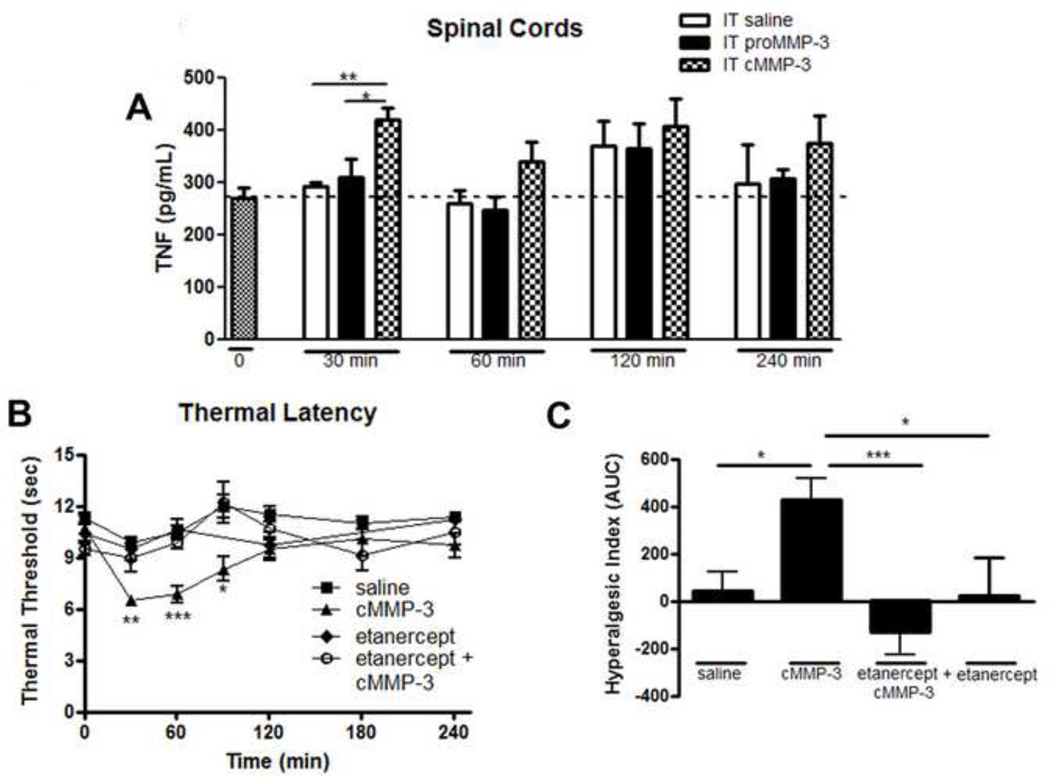
MMP-3 application increases TNF levels in vitro in microglial cell cultures (Kim et al., 2005). Given that TNF is a potent pro-inflammatory cytokine and produces enhanced nociception after IT delivery (Junger and Sorkin, 2000; Svensson et al., 2005), we investigated if MMP-3 could induce TNF levels in vivo in the spinal cord following intrathecal delivery. Following IT saline or IT proMMP-3, TNF levels were not different 30 minutes after injection 295 pg/mL +/− 7 vs. 311 pg/mL +/− 35. In contrast, at 30 minutes after injection, IT cMMP-3 significantly increased spinal cord TNF concentration 423 pg/mL +/− 22 (Figure 7A; p < 0.01 vs. saline, p < 0.05 vs. proMMP-3). At later times, measured out to 4 hour, TNF levels were numerically greater than either the saline or proMMP-3 groups, but these differences did not reach statistical significance.
Figure 7.
IT cMMP-3 reduced thermal and tactile thresholds is TNF dependent. (A) IT cMMP-3 will significantly increase spinal TNF levels as compared to saline and proMMP-3 injections 30 minutes but not 60 minutes, 120 minutes, or 240 minutes after delivery. (B) A 60 minute IT pretreatment with etanercept (100 µg) prevented IT cMMP-3 induced thermal hyperalgesia at 30, 60, and 90 minutes vs. control, which is graphed (C) according to the hyperalgesic index. n = 5–6 rats for all behavior groups. n = 4 rats for TNF ELISA groups. * = p < 0.05, ** = p < 0.01, *** = p < 0.001.
3.7 Thermal hyperalgesia and tactile allodynia evoked by IT cMMP-3 is mediated by TNF
To functionally assess the role of TNF in cMMP-3 mediated nociception, we examined the spinal effect of etanercept, a TNF sequestering molecule, on IT cMMP-3 induced hyperalgesia. As before, IT cMMP-3 produced a significant reduction in thermal threshold at 30 minutes and the effect persisted for 90 minutes, which was prevented by a 1 hour pretreatment with IT etanercept (Figure 7B). This data is also presented according to the hyperalgesic index, indicating a pretreatment (1 hour) with IT etanercept effectively prevented an IT cMMP-3 induced thermal hyperalgesia (p < 0.001, Figure 7C). Rats pretreated with etanercept and subsequently received cMMP-3 showed no difference from control (saline) animals (p > 0.05 at any time point).
3.8 Peripheral inflammation induces spinal TNF, which is regulated by MMPs
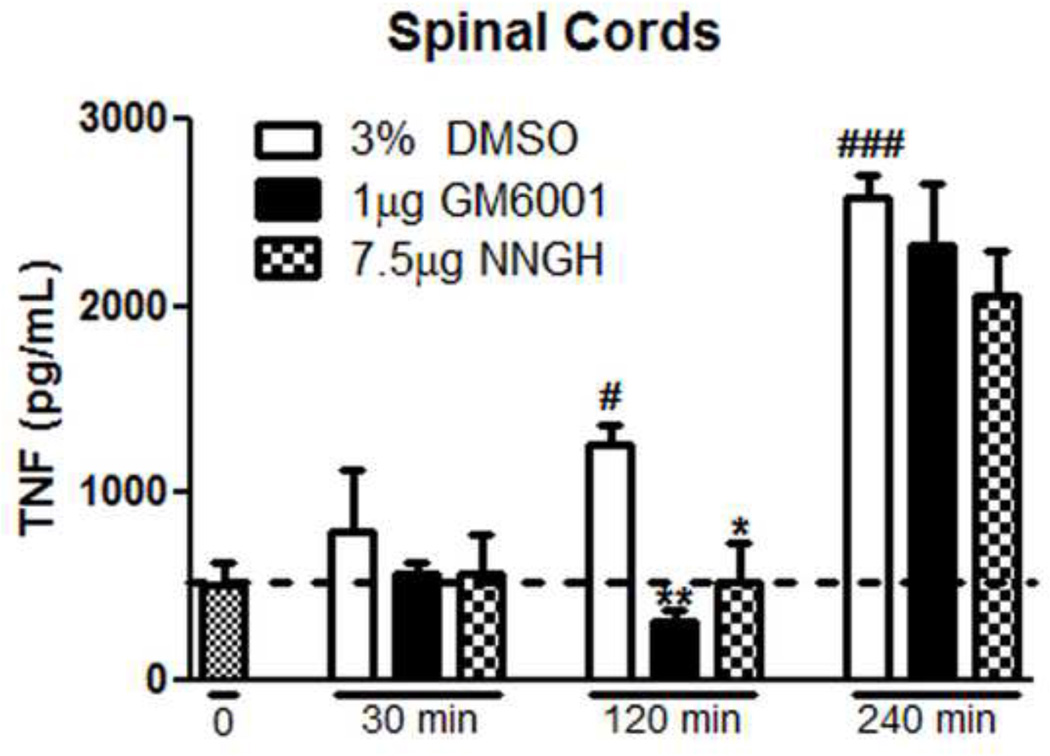
To further establish the relationship between spinal MMP-3 activity and spinal TNF production we measured by TNF ELISA the concentrations of TNF in the lumbar spinal cord from groups of rats receiving IT pretreatments (30 minutes in advance of the intraplantar carrageenan) with: IT 3% DMSO (vehicle control); IT 1 µg GM6001, or IT 7.5 µg NNGH. Animals were then randomly assigned to undergo tissue harvest at 30 minutes, 120 minutes, or 240 minutes after intraplantar carrageenan injection. Spinal TNF concentrations in control animals 512 pg/mL +/− 127 were increased in a time dependent fashion after intraplantar carrageenan, reaching statistical significance, 1062 pg/mL +/− 208 at 120 minutes after injection (Figure 8; p < 0.05) and continuing to rise through 240 minutes to 2579 pg/mL +/− 117 (Figure 8; p < 0.001). These intraplantar inflammation induced increases in spinal TNF concentrations were significantly reduced at the 120 minute time point by pretreatment with IT GM6001 (316 pg/mL +/− 61; p < 0.01) and by IT NNGH (532 pg/mL +/− 209; p < 0.05). By 4.5 hours after drug delivery, the inhibitory effects of IT GM6001 (2320 pg/mL +/− 342) or NNGH (206 pg/mL +/− 241) were lost. These spinal TNF concentrations did not differ from those observed in the vehicle control animals at this time (2579 pg/mL +/− 117). This attenuation of the time dependent increase in spinal TNF levels corresponds with the IT GM6001, NNGH, and PY-2 regulated behavioral attenuation of thermal and tactile hypersensitivity demonstrated in the intraplantar carrageenan treated animal.
Figure 8.
Peripheral inflammation increases spinal TNF levels, which are reduced by 30 minute pretreatment with MMP inhibitors. Carrageenan injection significantly increases TNF in the spinal cord 120 and 240 minutes after injection (p < 0.05 vs. t = 0). This increase in TNF is blocked by IT pretreatment with GM6001 (1 µg; p < 0.01) or NNGH (7.5µg; p < 0.05) at 120 minutes but not 240 minutes after carrageenan. n = 4 rats; * = p < 0.05 vs. 3% DMSO, ** = p < 0.01 vs. 3% DMSO, # = p < 0.05 vs. t = 0, ### = p < 0.001 vs. t = 0.
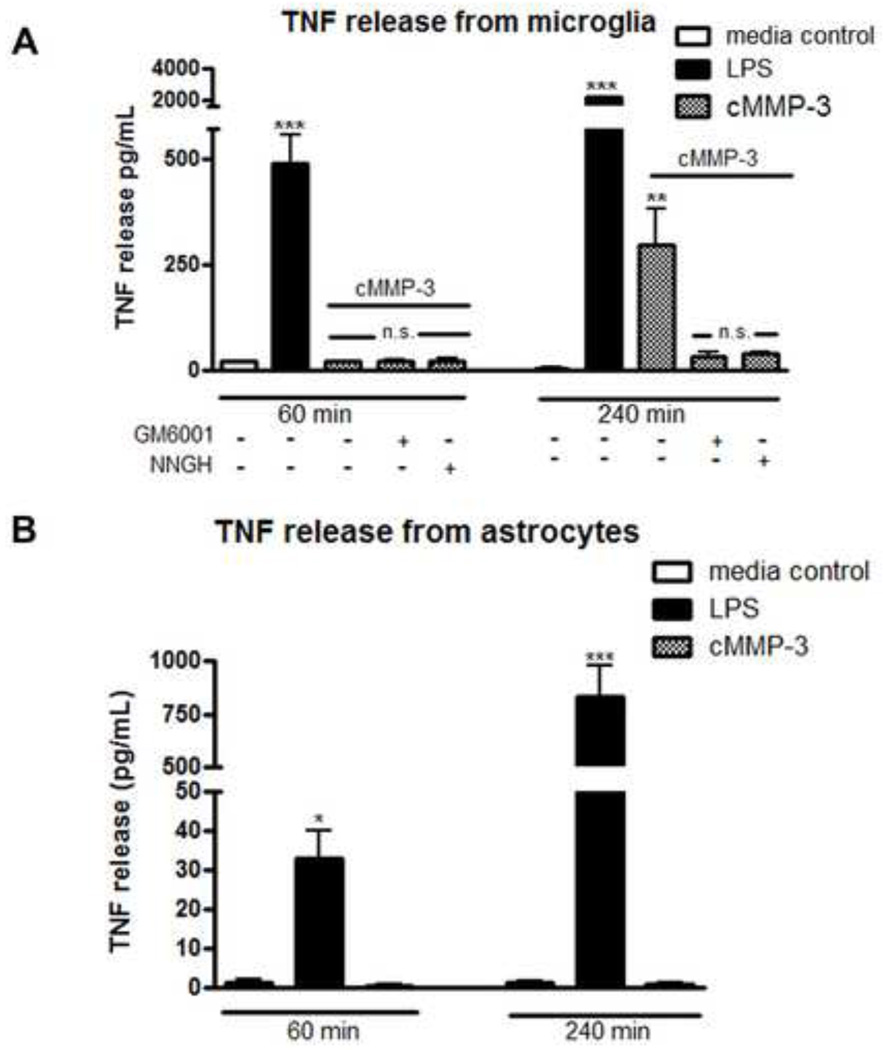
3.9 cMMP-3 evoked TNF release from microglia, but not astrocytes
Considerable work has demonstrated that stimulated astrocytes and microglia display increased extracellular concentrations of TNF (Sawada et al., 1989). Given the role of TNF in the spinal MMP-3 actions, we examined whether cMMP-3 would initiate TNF release from primary spinal microglia and astrocyte cultures. The addition of cMMP-3 to microglia cultures resulted in a prominent increase in TNF as compared to media controls 4.5 pg/mL ± 2.6 vs. cMMP-3 application 296.5 pg/mL ± 84.2 at four hours (Figure 9A; p < 0.01). In contrast, application of cMMP-3 did not, at any time point, induce significant release of TNF from astrocytes as compared to media control values (Figure 9B; p > 0.05). This lack of effect in astrocytes stands in contrast to the effect of the TLR4 agonist, LPS (100 ng/mL), which induced a significant increase in TNF released from microglia (p < 0.01) and from astrocytes (p < 0.05) at all time points (Figure 9A and 9B). This increase in TNF release from microglia is blocked by pretreatment with GM6001 (31.6 pg/mL ± 12.4, p > 0.05 vs. media control) or with NNGH (40.3 pg/mL ± 2.8, p > 0.05 vs. media control).
Figure 9.
cMMP-3 induced TNF release from microglia but not astrocytes. (A) Primary spinal microglia were treated for 1 or 4 hours with cMMP-3 (400 ng/mL) in both the presence and absence of either GM6001 (7.5 µg/mL) or NNGH (7.5 µg/mL). cMMP-3 induced significant TNF release at 4 hours (p < 0.01) vs. media and was prevented by pretreatment with GM001 and NNGH. (B) Treatment of primary spinal astrocytes with cMMP-3 did not induce an increase in TNF release (p > 0.05) at any time. LPS (10 ng/mL), induced TNF released in microglia and astrocytes at all time points (p < 0.05) vs. media control. Results are the average of 3–4 independent experiments.
4. Discussion
Matrix metalloproteinases are a diverse class of zinc-dependent enzymes, which are recognized to play an important regulatory role in the organization and remodeling of extracellular matrix proteins. The present studies emphasize a novel role for catalytically active MMP-3, showing it to be a key component in a spinal cascade initiated by peripheral inflammation and resulting in thermal hyperalgesia and tactile allodynia. We hypothesize that the peripheral inflammation leads to an increase in spinal cMMP-3 resulting in release of TNF, likely from non-neuronal cells to evoke a facilitated state of dorsal horn processing. Convergent data supporting this hypothesis will be considered below.
4.1 The facilitated state is initiated by the catalytically active form of MMP-3
In the present study, IT MMP-3 resulted in a potent and reversible tactile allodynia and thermal hyperalgesia. Thus, i) IT delivery of exogenous cMMP-3, but not proMMP-3 initiated a short latencied and reversible thermal hyperalgesia and tactile allodynia; ii) the cMMP-3, but not proMMP-3 resulted in increased spinal TNF in vivo and iii) the effects of the cMMP-3 on TNF concentrations were blocked by MMP and MMP-3 inhibitors GM6001 and NNGH, respectively.
These MMP-3 specific inhibitors exert their actions by binding at the enzymatically active site (Grobelny et al., 1992; MacPherson et al., 1997; Romero-Perez et al., 2009), thus providing further confirmation that it is the catalytically active form that is responsible for the endogenous and exogenous actions.
4.2 Peripheral inflammation leads to a significant elevation of spinal cMMP-3
In agreement with other reports, western blotting and imunohistochemistry indicate that MMP-3 is constitutively expressed in spinal cord (Pagenstecher et al., 1998; Nuttall et al., 2007; Gawlak et al., 2009) and differentially regulated during inflammatory CNS diseases including multiple sclerosis, stroke, and neuropathic pain (as reviewed in (Agrawal et al., 2008b). In the present work there was a significant increase in MMP-3 protein expression in the spinal cord at 2 hours following peripheral inflammation. This observation is consistent with previous work that demonstrated MMP-3 up-regulation by signaling pathways including MAPK and PI3K/AKT (Ito et al., 2007). Inhibition of these pathways will diminish expression of MMPs (Qiu et al., 2004; Prast et al., 2008) and attenuate post-inflammation hyperalgesia (Choi et al., 2010; Svensson et al., 2003).
Conversion of proMMP-3 to its active form (cMMP-3) can occur through cleavage by a variety of extracellular serine protease systems including those for plasmin/plasminogen (Chakraborti et al., 2003). Tissue type plasminogen is induced in vivo in spinal astrocytes after injury and its blockade is associated with a loss of hyperalgesia (Kozai et al., 2007). Future investigation of the upstream regulatory mechanisms for MMP-3 cleavage during acute peripheral inflammation would be of great scientific interest.
4.3 Endogenous spinal cMMP-3 mediates inflammation evoked hyperalgesia
Intrathecal delivery of a broad spectrum MMP and two MMP-3 preferring inhibitors resulted in an attenuation in the onset of the tactile and thermal hypersensitivity otherwise observed following peripheral inflammation. While reports indicate that GM6001 has off target effects including inactivation of TNF-alpha converting enzyme (TACE) (Jacobsen et al., 2008), which would itself lead to decreased TNF, the consistent results obtained by two structurally diverse inhibitors : NNGH and PY-2, provides strong evidence for the contributions of MMP-3 in this series of experiments. NNGH shows little inhibition of TACE but does have modest inhibitory effects against MMP-12 (Bertini et al., 2005) and MMP-10 (Bertini et al., 2004). PY-2 is a semi-selective MMP-3 inhibitor, which also inhibits MMP-8 and -12, but has little efficacy against MMP-1, 2, 7, 9, and 13 (Agrawal et al., 2008a). In vitro PY-2 has greater specificity against MMP-3 (0.13 µM NNGH vs. 0.077 µM PY-2 Ki) (Agrawal et al., 2008a) with no inhibitory effects against TACE. The block of the thermal and tactile hyperalgesia onset afforded by an IT pretreatment with either MMP-3 inhibitors suggests MMP-3 specificity. The post-treatment efficacy of GM6001 but not NNGH or PY-2 is likely the result of broad spectrum MMP inhibition, not MMP-3 specific inhibition. While there has been little work regarding the role of MMPs in maintenance of inflammatory pain, current post-treatment data in neuropathic pain models utilizing an IT MMP-2 specific inhibitor and an IT endogenous inhibitor of MMP-2 (TIMP2) suggests an MMP-2 specific maintenance mechanism is more likely (Kawasaki et al., 2008). The ineffectiveness of post-treatment MMP-3 specific inhibition suggests a prominent role for MMP-3 during the initiation of carrageenan-induced hyperalgesia and allodynia, but not during the maintenance.
4.4 Extracellular cMMP-3 initiates a facilitatory state though the release of TNF
In the spinal cord, IT cMMP-3 resulted in a prominent increase in spinal TNF concentrations. The time course of TNF release paralleled the temporal profile of the pain-like behavior observed with the same doses of IT cMMP-3. The functional significance of this increase is evidenced by the block of the IT cMMP-3 evoked hyperalgesia by the spinal pretreatment of etanercept (a TNF sequestering protein). These observations are consistent with previous reports that intrathecal TNF evokes a significant hyperalgesic state mediated by TNF receptor 1 (TNFR1) (Reeve et al., 2000; Schafers et al., 2004; Youn et al., 2008). Activation of these spinal TNF receptors will activate kinases, which, through phosphorylation of voltage sensitive channels (Czeschik et al., 2008), NMDA ionophores (Chao et al., 1995) and AMPA ionophore trafficking to the neuronal membrane (Stellwagen et al., 2005) will lead to an enhanced excitability of dorsal horn neurons. This enhanced excitability is believed to underlie the hyperalgesic and allodynic states (Zhang et al., 2010). These results emphasize the likely role of downstream TNF release in mediating the cascade initiated by both exogenous and endogenous spinal cMMP-3. Moreover, these early effects upon spinal TNF concentrations were blocked by MMP-3 inhibitors at spinal doses that were shown to prevent the ITcMMP-3 induced hyperalgesia.
4.5 Non-Neuronal Cells
Spinal immunohistochemistry demonstrates the presence of MMP-3 immunoreactivity in both astrocytes and microglia in accordance with previous reports (Pagenstecher et al., 1998; Nuttall et al., 2007; Gawlak et al., 2009). Manipulation of spinal microglial and astrocyte function will influence development and maintenance of acute and chronic pain conditions (Watkins and Maier, 2003; Marchand et al., 2005; Tsuda et al., 2005). To further consider this mechanism, we undertook work with primary spinal cell cultures to determine the effects of cMMP-3 on these subpopulations. The primary spinal cell culture work provided two unexpected observations. The first was that cMMP-3 served to release TNF from microglia, but not astrocytes. Release of TNF by cMMP-3 from myeloid derived macrophages (Steenport et al., 2009) and microglia (Kim et al., 2005) has been previously reported, but this is the first description of a microglia but not astrocyte specific effect. Consistent with the in vivo work, TNF release from microglia was produced by the catalytic form and was blocked by cMMP-3 inhibitors previously shown to be active in vivo. Secondly, the cMMP-3 effects in microglia were evident only after four hours, a finding in contrast to the robust and early onset in increased spinal TNF observed after IT cMMP-3 in vivo. Although the interpretation of these results must be tempered by the fact that the work involves primary cell cultures from neonatal rats, these two results stand in contrast to the parallel observations in the present studies that the TLR4 agonist LPS resulted in an immediate increase in TNF release from both astrocytes and microglia, indicating that these actions of the cMMP-3 were peculiar to this exogenous agent and not the culture system. Our unexpected results show that cMMP-3 has a distinct effect upon microglia vs. astrocytes and suggests that, while both astrocytes and microglia express MMP-3 and both are capable of releasing TNF, the two cell types have a distinct response to exogenous cMMP-3. These results raise the speculative hypothesis that cMMP-3 exerts its actions in a cell specific regulated manner. It further suggests that the net effects of the exogenous agent are not the result of a simple cleavage of TNF from the cell membrane, in accordance with previous biochemical data (Mohan et al., 2002). The observed delay in TNF release within the cell culture system reflects upon the distinction between homogenous cell types vs. the intact organ system where complex interactions invariably occur. Therefore, caution must be employed when attributing these finding to the animal system.
4.6 Concluding Overview
These results indicate the constitutive presence of MMP-3 and a functional role of exogenous cMMP-3 and endogenous cMMP-3 in initiating a facilitated state of nociceptive processing in the spinal cord. Our novel observations regarding cMMP-3-induced TNF release in the spinal lumbar region, prompts the hypothesis that cMMP-3 could be released and serve in a paracrine capacity in the activation of non-neuronal cells and may function in a capacity to initiate and sustain a facilitated pain state similar to other enzyme agonists including secretory PLA2 (Farooqui and Horrocks, 2004) or proteases acting though the proteinase activated receptors (PARs) (Vergnolle et al., 2001; Bunnett, 2006) to regulate at the spinal level the response following tissue injury and inflammation. cMMP-3 may also directly cleave TNF in a manner similar to TACE, though this mechanism would suggest that cMMP-3 would have an effect in all TNF-expressing cell systems, a characteristic that is not supported by the astrocyte cell culture data. While the specific target for the enzymatic activity of cMMP-3 remains to be elucidated, this novel role for cMMP-3 as a factor in the initiation of pro-inflammatory cytokine release and in the facilitatory cascades in the dorsal horn relevant to spinal pain processing are clearly delineated by these present studies.
Highlights.
IT MMP antagonism attenuates peripheral inflammation induced pain-like behavior
IT MMP-3 induces pain-like behavior
Spinal MMP-3 action requires TNF activity
Acknowledgements
This work was supported by grants from the National Institutes of Health (NS 16541, DA02110 and F31 NS064787).
Abbreviations used
- IT
intrathecal
- MMP-3
matrix metalloproteinase 3
- cMMP-3
catalytic MMP-3
- NNGH
N-Isobutyl-N-(4-methoxyphenylsulfonyl)glycyl hydroxamic acid
- GM6001
N-[(2R)-2-(Hydroxamidocarbonylmethyl)-4-methylpentanoyl]-L tryptophanmethylamide
- TNF
tumor necrosis factor
- LPS
lipopolysaccharide
- DMSO
dimethyl sulfoxide
- APMA
p-aminophenylmercuric acetate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agnihotri R, Crawford HC, Haro H, Matrisian LM, Havrda MC, Liaw L. Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin) J Biol Chem. 2001;276:28261–28267. doi: 10.1074/jbc.M103608200. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Romero-Perez D, Jacobsen JA, Villarreal FJ, Cohen SM. Zinc-binding groups modulate selective inhibition of MMPs. ChemMedChem. 2008a;3:812–820. doi: 10.1002/cmdc.200700290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal SM, Lau L, Yong VW. MMPs in the central nervous system: where the good guys go bad. Semin Cell Dev Biol. 2008b;19:42–51. doi: 10.1016/j.semcdb.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Bertini I, Calderone V, Cosenza M, Fragai M, Lee YM, Luchinat C, Mangani S, Terni B, Turano P. Conformational variability of matrix metalloproteinases: beyond a single 3D structure. Proc Natl Acad Sci U S A. 2005;102:5334–5339. doi: 10.1073/pnas.0407106102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertini I, Calderone V, Fragai M, Luchinat C, Mangani S, Terni B. Crystal structure of the catalytic domain of human matrix metalloproteinase 10. J Mol Biol. 2004;336:707–716. doi: 10.1016/j.jmb.2003.12.033. [DOI] [PubMed] [Google Scholar]
- Bunnett NW. Protease-activated receptors: how proteases signal to cells to cause inflammation and pain. Semin Thromb Hemost. 2006;32(Suppl 1):39–48. doi: 10.1055/s-2006-939553. [DOI] [PubMed] [Google Scholar]
- Cauwe B, Van den Steen PE, Opdenakker G. The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit Rev Biochem Mol Biol. 2007;42:113–185. doi: 10.1080/10409230701340019. [DOI] [PubMed] [Google Scholar]
- Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu S, Ehrlich L, Peterson PK. Interleukin-1 and tumor necrosis factor-alpha synergistically mediate neurotoxicity: involvement of nitric oxide and of N-methyl-D-aspartate receptors. Brain Behav Immun. 1995;9:355–365. doi: 10.1006/brbi.1995.1033. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Choi JI, Svensson CI, Koehrn FJ, Bhuskute A, Sorkin LS. Peripheral inflammation induces tumor necrosis factor dependent AMPA receptor trafficking and Akt phosphorylation in spinal cord in addition to pain behavior. Pain. 2010;149:243–253. doi: 10.1016/j.pain.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JM, Cossins JA, Wells GM, Corkill DJ, Helfrich K, Wood LM, Pigott R, Stabler G, Ward GA, Gearing AJ, Miller KM. Matrix metalloproteinase expression during experimental autoimmune encephalomyelitis and effects of a combined matrix metalloproteinase and tumour necrosis factor-alpha inhibitor. J Neuroimmunol. 1997;74:85–94. doi: 10.1016/s0165-5728(96)00210-x. [DOI] [PubMed] [Google Scholar]
- Czeschik JC, Hagenacker T, Schafers M, Busselberg D. TNF-alpha differentially modulates ion channels of nociceptive neurons. Neurosci Lett. 2008;434:293–298. doi: 10.1016/j.neulet.2008.01.070. [DOI] [PubMed] [Google Scholar]
- Dirig DM, Salami A, Rathbun ML, Ozaki GT, Yaksh TL. Characterization of variables defining hindpaw withdrawal latency evoked by radiant thermal stimuli. J Neurosci Methods. 1997;76:183–191. doi: 10.1016/s0165-0270(97)00097-6. [DOI] [PubMed] [Google Scholar]
- Duivenvoorden WC, Hirte HW, Singh G. Use of tetracycline as an inhibitor of matrix metalloproteinase activity secreted by human bone-metastasizing cancer cells. Invasion Metastasis. 1997;17:312–322. [PubMed] [Google Scholar]
- Farooqui AA, Horrocks LA. Brain phospholipases A2: a perspective on the history. Prostaglandins Leukot Essent Fatty Acids. 2004;71:161–169. doi: 10.1016/j.plefa.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Ganea E, Trifan M, Laslo AC, Putina G, Cristescu C. Matrix metalloproteinases: useful and deleterious. Biochem Soc Trans. 2007;35:689–691. doi: 10.1042/BST0350689. [DOI] [PubMed] [Google Scholar]
- Gawlak M, Gorkiewicz T, Gorlewicz A, Konopacki FA, Kaczmarek L, Wilczynski GM. High resolution in situ zymography reveals matrix metalloproteinase activity at glutamatergic synapses. Neuroscience. 2009;158:167–176. doi: 10.1016/j.neuroscience.2008.05.045. [DOI] [PubMed] [Google Scholar]
- Gijbels K, Proost P, Masure S, Carton H, Billiau A, Opdenakker G. Gelatinase B is present in the cerebrospinal fluid during experimental autoimmune encephalomyelitis and cleaves myelin basic protein. J Neurosci Res. 1993;36:432–440. doi: 10.1002/jnr.490360409. [DOI] [PubMed] [Google Scholar]
- Grobelny D, Poncz L, Galardy RE. Inhibition of human skin fibroblast collagenase, thermolysin, and Pseudomonas aeruginosa elastase by peptide hydroxamic acids. Biochemistry. 1992;31:7152–7154. doi: 10.1021/bi00146a017. [DOI] [PubMed] [Google Scholar]
- Hua XY, Svensson CI, Matsui T, Fitzsimmons B, Yaksh TL, Webb M. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur J Neurosci. 2005;22:2431–2440. doi: 10.1111/j.1460-9568.2005.04451.x. [DOI] [PubMed] [Google Scholar]
- Ito S, Kimura K, Haneda M, Ishida Y, Sawada M, Isobe K. Induction of matrix metalloproteinases (MMP3, MMP12 and MMP13) expression in the microglia by amyloid-beta stimulation via the PI3K/Akt pathway. Exp Gerontol. 2007;42:532–537. doi: 10.1016/j.exger.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Jacobsen FE, Buczynski MW, Dennis EA, Cohen SM. A macrophage cell model for selective metalloproteinase inhibitor design. Chembiochem. 2008;9:2087–2095. doi: 10.1002/cbic.200800148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junger H, Sorkin LS. Nociceptive and inflammatory effects of subcutaneous TNFalpha. Pain. 2000;85:145–151. doi: 10.1016/s0304-3959(99)00262-6. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, Gao YJ, Roy K, Corfas G, Lo EH, Ji RR. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14:331–336. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Kim SS, Cho JJ, Choi DH, Hwang O, Shin DH, Chun HS, Beal MF, Joh TH. Matrix metalloproteinase-3: a novel signaling proteinase from apoptotic neuronal cells that activates microglia. J Neurosci. 2005;25:3701–3711. doi: 10.1523/JNEUROSCI.4346-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight CG, Willenbrock F, Murphy G. A novel coumarin-labelled peptide for sensitive continuous assays of the matrix metalloproteinases. FEBS Lett. 1992;296:263–266. doi: 10.1016/0014-5793(92)80300-6. [DOI] [PubMed] [Google Scholar]
- Kozai T, Yamanaka H, Dai Y, Obata K, Kobayashi K, Mashimo T, Noguchi K. Tissue type plasminogen activator induced in rat dorsal horn astrocytes contributes to mechanical hypersensitivity following dorsal root injury. Glia. 2007;55:595–603. doi: 10.1002/glia.20483. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- MacPherson LJ, Bayburt EK, Capparelli MP, Carroll BJ, Goldstein R, Justice MR, Zhu L, Hu S, Melton RA, Fryer L, Goldberg RL, Doughty JR, Spirito S, Blancuzzi V, Wilson D, O'Byrne EM, Ganu V, Parker DT. Discovery of CGS 27023A, a non-peptidic, potent, and orally active stromelysin inhibitor that blocks cartilage degradation in rabbits. J Med Chem. 1997;40:2525–2532. doi: 10.1021/jm960871c. [DOI] [PubMed] [Google Scholar]
- Malkmus SA, Yaksh TL. Intrathecal catheterization and drug delivery in the rat. Methods Mol Med. 2004;99:109–121. doi: 10.1385/1-59259-770-X:011. [DOI] [PubMed] [Google Scholar]
- Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6:521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Cafferty WB, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp Neurol. 2005;192:444–462. doi: 10.1016/j.expneurol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan MJ, Seaton T, Mitchell J, Howe A, Blackburn K, Burkhart W, Moyer M, Patel I, Waitt GM, Becherer JD, Moss ML, Milla ME. The tumor necrosis factor-alpha converting enzyme (TACE): a unique metalloproteinase with highly defined substrate selectivity. Biochemistry. 2002;41:9462–9469. doi: 10.1021/bi0260132. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci. 2002;22:7526–7535. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttall RK, Silva C, Hader W, Bar-Or A, Patel KD, Edwards DR, Yong VW. Metalloproteinases are enriched in microglia compared with leukocytes and they regulate cytokine levels in activated microglia. Glia. 2007;55:516–526. doi: 10.1002/glia.20478. [DOI] [PubMed] [Google Scholar]
- Ogata Y, Enghild JJ, Nagase H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem. 1992;267:3581–3584. [PubMed] [Google Scholar]
- Pagenstecher A, Stalder AK, Kincaid CL, Shapiro SD, Campbell IL. Differential expression of matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase genes in the mouse central nervous system in normal and inflammatory states. Am J Pathol. 1998;152:729–741. [PMC free article] [PubMed] [Google Scholar]
- Ponten F, Jirstrom K, Uhlen M. The Human Protein Atlas--a tool for pathology. J Pathol. 2008;216:387–393. doi: 10.1002/path.2440. [DOI] [PubMed] [Google Scholar]
- Prast J, Saleh L, Husslein H, Sonderegger S, Helmer H, Knofler M. Human chorionic gonadotropin stimulates trophoblast invasion through extracellularly regulated kinase and AKT signaling. Endocrinology. 2008;149:979–987. doi: 10.1210/en.2007-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Q, Yang M, Tsang BK, Gruslin A. EGF-induced trophoblast secretion of MMP-9 and TIMP-1 involves activation of both PI3K and MAPK signalling pathways. Reproduction. 2004;128:355–363. doi: 10.1530/rep.1.00234. [DOI] [PubMed] [Google Scholar]
- Reeve AJ, Patel S, Fox A, Walker K, Urban L. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur J Pain. 2000;4:247–257. doi: 10.1053/eujp.2000.0177. [DOI] [PubMed] [Google Scholar]
- Romero-Perez D, Agrawal A, Jacobsen J, Yan Y, Thomas R, Cohen S, Villarreal F. Effects of novel semiselective matrix metalloproteinase inhibitors on ex vivo cardiac structure-function. J Cardiovasc Pharmacol. 2009;53:452–461. doi: 10.1097/FJC.0b013e3181a6aa83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg GA, Navratil M, Barone F, Feuerstein G. Proteolytic cascade enzymes increase in focal cerebral ischemia in rat. J Cereb Blood Flow Metab. 1996;16:360–366. doi: 10.1097/00004647-199605000-00002. [DOI] [PubMed] [Google Scholar]
- Sawada M, Kondo N, Suzumura A, Marunouchi T. Production of tumor necrosis factor-alpha by microglia and astrocytes in culture. Brain Res. 1989;491:394–397. doi: 10.1016/0006-8993(89)90078-4. [DOI] [PubMed] [Google Scholar]
- Schafers M, Marziniak M, Sorkin LS, Yaksh TL, Sommer C. Cyclooxygenase inhibition in nerve-injury- and TNF-induced hyperalgesia in the rat. Exp Neurol. 2004;185:160–168. doi: 10.1016/j.expneurol.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Schonbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol. 1998;161:3340–3346. [PubMed] [Google Scholar]
- Shubayev VI, Myers RR. Upregulation and interaction of TNFalpha and gelatinases A and B in painful peripheral nerve injury. Brain Res. 2000;855:83–89. doi: 10.1016/s0006-8993(99)02321-5. [DOI] [PubMed] [Google Scholar]
- Shubayev VI, Myers RR. Matrix metalloproteinase-9 promotes nerve growth factor-induced neurite elongation but not new sprout formation in vitro. J Neurosci Res. 2004;77:229–239. doi: 10.1002/jnr.20160. [DOI] [PubMed] [Google Scholar]
- Steenport M, Khan KM, Du B, Barnhard SE, Dannenberg AJ, Falcone DJ. Matrix metalloproteinase (MMP)-1 and MMP-3 induce macrophage MMP-9: evidence for the role of TNF-alpha and cyclooxygenase-2. J Immunol. 2009;183:8119–8127. doi: 10.4049/jimmunol.0901925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003;86:1534–1544. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Schafers M, Jones TL, Powell H, Sorkin LS. Spinal blockade of TNF blocks spinal nerve ligation-induced increases in spinal P-p38. Neurosci Lett. 2005;379:209–213. doi: 10.1016/j.neulet.2004.12.064. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in "small" glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, Bunnett NW, Sharkey KA, Brussee V, Compton SJ, Grady EF, Cirino G, Gerard N, Basbaum AI, Andrade-Gordon P, Hollenberg MD, Wallace JL. Proteinase-activated receptor-2 and hyperalgesia: A novel pain pathway. Nat Med. 2001;7:821–826. doi: 10.1038/89945. [DOI] [PubMed] [Google Scholar]
- Vidal A, Sabatini M, Rolland-Valognes G, Renard P, Madelmont JC, Mounetou E. Synthesis and in vitro evaluation of targeted tetracycline derivatives: effects on inhibition of matrix metalloproteinases. Bioorg Med Chem. 2007;15:2368–2374. doi: 10.1016/j.bmc.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Discov. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2:502–511. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn DH, Wang H, Jeong SJ. Exogenous tumor necrosis factor-alpha rapidly alters synaptic and sensory transmission in the adult rat spinal cord dorsal horn. J Neurosci Res. 2008;86:2867–2875. doi: 10.1002/jnr.21726. [DOI] [PubMed] [Google Scholar]
- Zhang H, Nei H, Dougherty PM. A p38 mitogen-activated protein kinase-dependent mechanism of disinhibition in spinal synaptic transmission induced by tumor necrosis factor-alpha. J Neurosci. 2010;30:12844–12855. doi: 10.1523/JNEUROSCI.2437-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]