Abstract
Red blood cell (RBC) transfusion is associated with alterations in systemic concentrations of IL-8/CXCL8 functional homologues in a murine model. Whether RBC transfusion alters systemic neutrophil chemokine concentrations in individuals sustaining traumatic injury is not known. We conducted a retrospective, single-center study of severely injured trauma patients presenting within 12 h of injury with a base deficit > 6 and hypotension in the field. Plasma concentrations of twenty-five chemokines, cytokines, and growth factors were obtained from both transfused (N=22) and non-transfused (N=33) groups in the first 48 h following admission. The transfused group (mean RBC units ± SD: 2.7 ±1.7) tended to be older (49.9 ±21.1 versus 40.4 ±19.9 years, p=0.10), with a higher percentage of females (40.9% versus 18.2%, p=0.06), and a higher injury severity score (ISS) (27.1 ±12.7 versus 21.4 ±10.2, p=0.07). In univariate and multivariate analyses, transfusion was associated with increased hospital and ICU length of stay but not ventilator-free days. Plasma CXCL8 concentrations were higher in the transfused (mean ±SD: 84 ±88 pg/mL) than the non-transfused group (31 ±21 pg/mL, p=0.003). Using a linear prediction model to calculate bioanalyte concentrations standardized for age, gender, ISS, and admission SBP, we observed that CXCL8 concentrations diverged within 12 hours following injury, with the transfused group showing persistently elevated CXCL8 concentrations by contrast to the decay observed in the non-transfused group. Other bioanalytes showed no differences across time. RBC transfusion is associated with persistently elevated neutrophil chemokine CXCL8 concentrations following traumatic injury.
Keywords: Chemokines, RBC transfusion, Inflammation, trauma
Introduction
Traumatic injury induces the systemic inflammatory response (SIRS) even in the absence of infection(1, 2), and risk factor analysis clearly demonstrates that allogeneic blood transfusion in the first 24 h following trauma is associated with increased SIRS and death(3, 4). Although red blood cell (RBC) transfusion is a primary therapy for hemorrhagic shock that accompanies severe traumatic injury, this treatment is also associated with many adverse outcomes, including multiple organ dysfunction (MODS)(5, 6), acute respiratory distress syndrome (ARDS)(7), increased infectious complications(8), and mortality(3).
While controversy exists regarding the implications of RBC transfusions and associated adverse outcomes(9), several mechanisms have been postulated by which RBC transfusion may impart harm in individuals with critical illness(10). RBC transfusion has been implicated as having immunomodulatory or suppressive effects(11–13), however components of RBC units early following transfusion may heighten inflammation(14),(15),(16–18). The release of endogenous damage-associated molecular patterns (DAMP) such as mitochondrial formyl peptides and mitochondrial DNA and subsequent neutrophil activation has been implicated in the SIRS accompanying traumatic injury(19, 20). Furthermore, plasma from aged, stored red cell units is capable of inducing CXCL8 release in addition to secretory phospholipase A2 from human neutrophils(21). Whether or not blood transfusion is capable of enhancing concentrations of neutrophil activating substances in trauma patients, however, remains unknown. In a murine model of systemic inflammation induced by Gram-negative bacterial endotoxin, we have shown that transfusion of stored RBC increased plasma concentrations of keratinocyte-derived cytokine (KC)/CXCL1 and macrophage inflammatory protein-2 (MIP-2)/CXCL2, functional homologues of human CXCL8(17). In this study, we translate these experimental observations into a clinical study to evaluate plasma bioanalyte concentrations of various chemokines, cytokines and growth factors available from subjects obtained from a severely injured cohort. We hypothesize that altered neutrophil chemokine concentrations, in particular CXCL8, occurs during the initial response to injury following RBC transfusion and may be mechanistically associated with a worse outcome.
Materials and Methods
Study design
We conducted an observational, retrospective cohort analysis of severely injured subjects recruited into the Mathematical Modeling of the Acute Inflammatory Response Following Injury at the University of Pittsburgh Medical Center for the period January 1, 2004, to September 30, 2008. The dates of hospital admission, age, sex, severity of illness, and detailed biochemical data were available.
Subjects and patient samples
Individuals were recruited into the Mathematical Modeling of the Acute Inflammatory Response Following Injury, an investigation that characterizes the temporal changes in immunity that occur in response to severe injury. The study was approved by the University of Pittsburgh Institutional Review Board, and informed consent was obtained from each patient or a designated surrogate prior to the use of any data in this study. Data from individual subjects were de-identified.
Inclusion criteria included a base deficit > 6, hypotension in the field and initial presentation within 12 h of injury. Clinical and plasma bioanalyte data were available for 88 individuals. Routine practice for transfusion in trauma patients at the University of Pittsburgh Presbyterian hospital is to provide non-leukoreduced RBC units, utilizing a first-in/first-out protocol. Thirty-three of the 88 individuals received massive transfusion (> 10 packed RBC units) and/or had ongoing transfusions on arrival to the emergency room. As these individuals represented a clinically distinct cohort with higher hospital mortality(22–24), we excluded them from further analysis. We restricted our temporal analysis to subjects with at least 3 plasma samples within the first 48 h of admission in order to ensure an appropriate sampling for longitudinal analysis and to minimize any bias stemming from missing data. Based upon these criteria, plasma samples from 33 non-transfused individuals and 22 transfused individuals were available for analysis.
Plasma bioanalyte measurements
Plasma bioanalytes (CCL2, CCL3, CCL4, CCL5, CCL11, CXCL8, CXCL9, CXCL10, GM-CSF, IL-1β, IL-1ra, IL-2, IL-2R, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p40, IL-13, IL-15, IL-17, IFN-α, IFN-γ, TNF-α) were measured using human-specific Luminex™ beadsets (Biosource-Invitrogen, Austin, TX) utilizing a Luminex™ 100 IS apparatus (MiraiBio, Austin, TX) as we have previously described(25).
Statistical analysis
Univariate analyses of continuous and categorical variables were performed with Student’s t test and Pearson’s χ2 test as appropriate. We determined the mean bioanalyte concentration for each patient who had at least 3 and up to 5 measurements during the first 48 hours of admission. The mean values of each subject were obtained and then averaged in the non-transfused (n=33) and transfused (n=22) populations. As the study was driven by the hypothesis that transfusions increase systemic neutrophil chemokine CXCL8 concentration similar to our murine model, a p value of <0.05 was considered appropriate. However, we incorporated some degree of conservatism by applying a modified Bonferroni correction where the analytes examined were separated into 3 categories (chemokines, cytokines, growth factors), and we focused upon our chemokine hypothesis. A formal Bonferroni correction for our chemokine hypothesis (0.05/8 chemokines = p<0.00625) was considered substantially conservative(26). Thus a ‘significance’ threshold was determined by the sequential methods of Sidak-Holm(27) in order to balance Type I and Type II errors. In circumstances in which this method was used, the adjusted p-value (padj) is presented in parentheses.
We also performed a random effects multivariate, longitudinal linear regression to assess the association between natural log mediator concentration over time and transfusion, accounting for the correlation within subjects, repeated measures over time, and covariates specified a priori as potential confounders. The primary outcome of interest was the longitudinal pattern of bioanalyte concentration. Sufficient plasma sampling occurred within each quartile of the entire 48 hour period: first quartile (n=41), second quartile (n=91), third quartile (n=54), and the fourth quartile (n=23). Thus, time was modeled categorically (0–1, 13–24, 25–36, 37–48 h) so as to avoid making any assumption about the relationship between time and bioanalyte concentration and also to avert any potential bias involved in pre-specifying the functional form. Modeling each quartile separately yielded a model sensitive to non-linear changes in concentrations over time, such as a rise and fall, which was commonly observed. As the distribution of bioanalyte concentrations deviated from normality, all values were natural log-transformed to better approximate a Gaussian distribution.
We addressed potential confounding due to variation in case mix by controlling for the severity of illness, as defined by Injury Severity Score (ISS), admission systolic blood pressure (SBP), gender, age, and time after injury. Ln(analyte) concentrations for each cohort were predicted, utilizing the raw data and the generated coefficients. A standardized linear prediction model generated predicted values of lnCXCL8 concentrations across time had the other covariates been identical between the two cohorts. The values for standardizing the age, ISS, and SBP were determined by the mean values of each variable for the entire population.
Because some samples obtained in the transfused cohort might precede transfusion or be obtained long after (>24 hours) transfusion is complete, we also modeled post- and pre-transfusion periods. Mean changes in bioanalyte concentration were estimated using linear regression to compare samples obtained during periods in which transfusion would have its effect (i.e. ‘exposed’) to those in which transfusion would not (i.e. ‘not exposed’), using the kinetics of transfusion effects observed in our prior murine studies to guide the assumptions(17). The exponentiated form of this coefficient is the mean absolute increase in bioanalyte concentration that is attributable to transfusion. Though the variability in mean bioanalyte concentration in each cohort is considerable in univariate analysis, in a multivariate model much of this variability is accounted for by the other covariates, thus enabling us to determine attributable change due to transfusion. For the multivariate analysis, a p<0.05 was considered significant. Statistical analyses were performed with Stata 11 (StataCorp LP, College Station, TX).
Results
Subjects
Subjects who received RBC transfusion were older (49.9 ±21.1 versus 40.4 ±19.9, p=0.10), more likely to be female (40.9% versus 18.2%, p=0.06), and more severely injured with higher ISS (27.1 ±12.7 versus 21.4 ±10.2, p=0.07) and lower SBP (102 ±6 versus 116 ±4, p=0.06), than the non-transfused cohort (Table 1). These patients were transfused a mean 2.7 ±1.7 units of packed RBC (pRBC).
Table 1.
Baseline characteristics of patients with traumatic injury
| No Transfusion (n=33) |
Transfusion (n=22) |
p value | |
|---|---|---|---|
| Age, mean years (sd)a | 40.4 (19.9) | 49.9 (21.1) | 0.10b |
| Female gender (%) | 6 (18.2%) | 9 (40.9%) | 0.06c |
| ISS (sd)a | 21.4 (10.2) | 27.1 (12.7) | 0.07b |
| SBP, mean mmHg (sd) | 116 (4) | 102 (6) | 0.06 |
| RBC units transfused (sd)a | 0 (0) | 2.7 (1.7) | NA |
Data expressed as mean (sd)
Comparisons were made using non-parametric Mann-Whitney test for continuous variables.
Comparisons were made using chi-square analysis for dichotomous predictor variables.
Bioanalytes
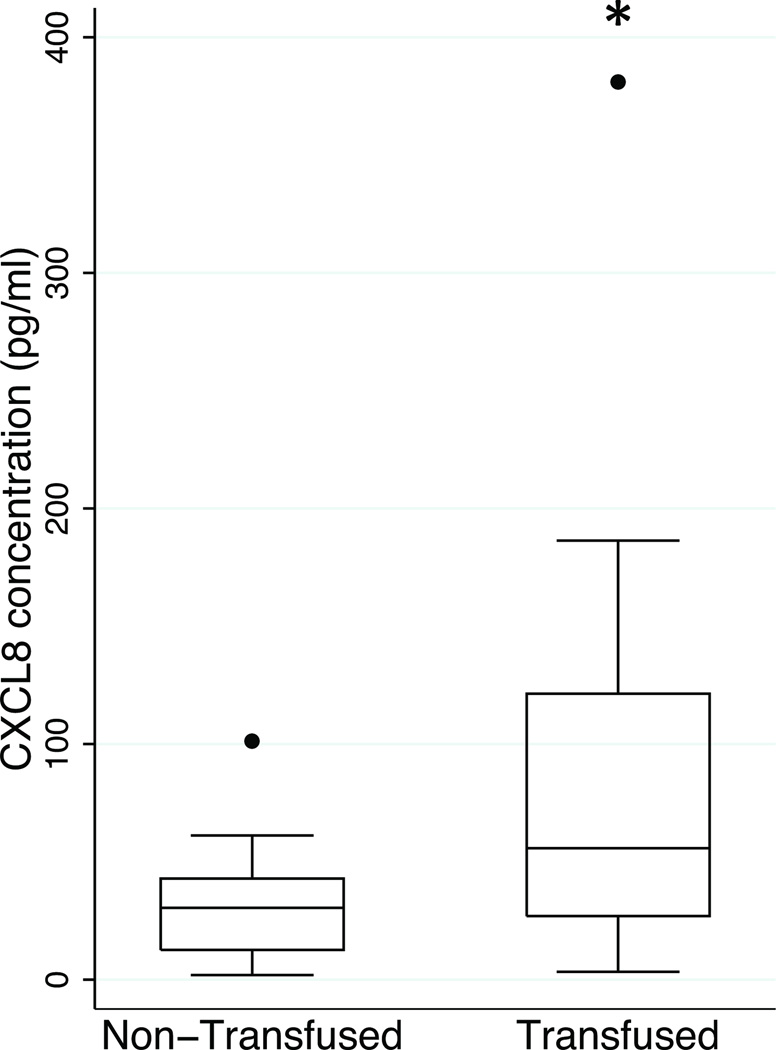
Twenty-five bioanalytes were assayed, and grouped as a chemokine, growth factor or cytokine. The mean plasma bioanalyte concentrations in the first 48 h were examined (Table 2). Plasma CXCL8 concentrations were higher in the transfused group than the non-transfused group (mean ±SD: 84 ±88 pg/mL vs. 31 ±21 pg/mL, p=0.003 (padj=0.02)) (Figure 1). All other bioanalyte concentrations were not significantly different between the two groups (Table 2).
Table 2.
Bioanalyte concentrations in the first 48 h
| Bioanalyte (pg/mL)a | Non-transfused (n=33) |
Transfused (n=22) |
p-valueb |
|---|---|---|---|
| CCL2 | 532 (289) | 549 (262) | 0.834 |
| CCL3 | 132 (71) | 134 (77) | 0.904 |
| CCL4 | 468 (675) | 224 (159) | 0.130 |
| CCL5 | 21851 (21762) | 52165 (139348) | 0.270 |
| CCL11 | 65 (39) | 56 (23) | 0.376 |
| CXCL8 | 31 (21) | 84 (88) | 0.003 |
| CXCL9 | 135 (113) | 135 (111) | 0.996 |
| CXCL10 | 75 (70) | 62 (40) | 0.468 |
| GM-CSF | 86 (191) | 87 (146) | 0.991 |
| IL-1β | 65 (84) | 62 (87) | 0.893 |
| IL1-RA | 1360 (2656) | 2219 (2063) | 0.237 |
| IL-2 | 22 (24) | 25 (27) | 0.668 |
| IL-2R | 813 (393) | 1042 (700) | 0.148 |
| IL-4 | 139 (164) | 139 (58) | 0.995 |
| IL-5 | 68 (143) | 83 (129) | 0.703 |
| IL-6 | 201 (190) | 231 (186) | 0.596 |
| IL-7 | 187 (221) | 377 (465) | 0.060 |
| IL-10 | 24 (30) | 32 (36) | 0.402 |
| IL12p40 | 506 (273) | 467 (250) | 0.619 |
| IL13 | 51 (26) | 47 (26) | 0.591 |
| IL-15 | 72 (107) | 84 (100) | 0.705 |
| IL-17 | 132 (126) | 134 (92) | 0.904 |
| IFN-α | 93 (82) | 102 (78) | 0.692 |
| IFN-γ | 101 (59) | 125 (96) | 0.277 |
| TNF-α | 23 (24) | 14 (9) | 0.132 |
Plasma bioanalyte concentrations are reported as mean pg/mL (sd).
Comparisons were made between the non-transfused and transfused groups using two-sample t test with equal variances.
Figure 1.
Box-and-whiskers plot of plasma CXCL8 concentrations in the non-transfused and transfused groups in the first 48 h of injury. (bar = median, box = 25–75 percentile; whiskers extend to 1.5 × interquartile range (IQR) distance from the 25th and 75th percentile and, by convention, is “rolled back” to an actual data point; black circle = values greater than 1.5 times IQR). *, p=0.003; padj=0.02.
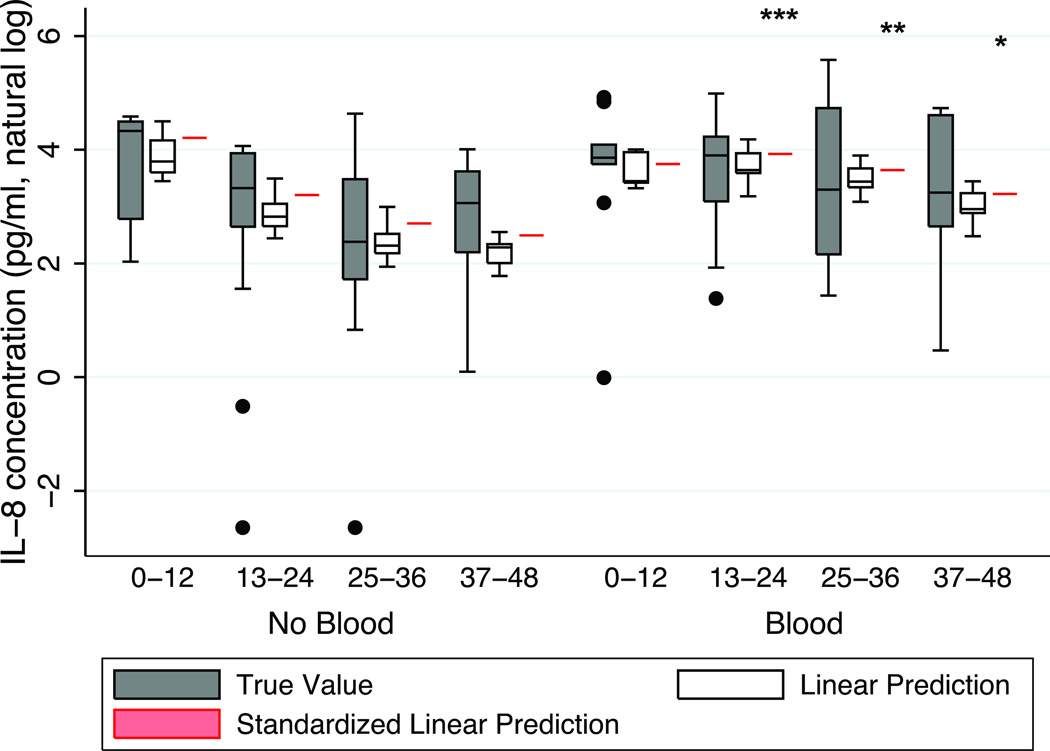
We next performed a multivariate analysis to compare the kinetics of plasma bioanalyte concentrations during the first 48 h following injury in the transfused and non-transfused group. Figure 2 displays the kinetics of natural log transformed plasma CXCL8 (lnCXCL8) concentrations dividing the 48 h studied into quartiles of time (0–12, 13–24, 25–36, 37–48 h). The lnCXCL8 concentrations decreased over time in the non-transfused cohort, whereas they remained stably elevated in the transfused cohort. In multivariate analysis, lnCXCL8 concentrations increased over time in individuals receiving blood relative to those who were not transfused: hours 13–24 (β-coefficient 1.18, p=0.001), hours 25–36 (β-coefficient 1.40, p=0.004), and hours 37–48 (β-coefficient 1.19, p=0.027). We conducted a standardized linear prediction model, standardizing each cohort to the mean age (41 years), ISS (21), SBP (108 mmHg), and male gender, and thus predicted lnCXCL8 concentrations in each cohort had these covariates been identical. As shown in Figure 2 (red), the transfused cohort had persistently higher lnCXCL8 concentrations, by comparison to the non-transfused cohort.
Figure 2.
Plasma CXCL8 concentrations in non-transfused and transfused groups as a function of time. Gray box-and-whiskers plot depicts the natural log transformed absolute CXCL8 concentrations. White box-and whiskers plot depicts the linear prediction of log transformed absolute CXCL8 concentrations. Red line depicts the standardized linear prediction of log transformed absolute CXCL8 concentration standardized to the mean age (41 years), mean ISS (21), mean SBP (108 mmHg), and male gender. (bar = median; box = 25–75 percentile; whiskers extend to 1.5 × interquartile range (IQR) distance from the 25th and 75th percentile and, by convention, “rolled back” to an actual data point; black circle = greater than 1.5 times IQR). ***, p=0.001; **, p=0.004; *, p=0.027.
We then modeled whether a bioanalyte sample was ‘exposed’ or ‘not exposed’ to transfusion, guided by our prior data indicating that the effects of transfusion on CXCL8 occur within 2 hours and decay to baseline within 24 hours(17). Transfusion was associated with a mean 1.72 pg/mL (95% CI 1.06–2.92; p=0.01) increase in systemic CXCL8 concentration. Transfusion was also associated with a significant mean increase in IL-6 concentration of 1.50 pg/mL (95% CI 1.16 – 1.94; p=0.002) after pRBC transfusion (data not shown).
The transfused group also trended toward higher plasma chemokine CCL2 concentrations at hours 37–48 (β-coefficient 0.620, p=0.047) vs. the non-transfused group. Plasma IL-5 concentrations were higher in the transfused group at hours 13–24 (β-coefficient 0.894, p=0.009) vs. the non-transfused group. All other bioanalytes showed no significant differences in changes over time between the transfused and non-transfused group.
Clinical outcomes
Transfused subjects demonstrated longer ICU (11.9 versus 5.4 days, p=0.005) and hospital lengths of stay (18.2 vs. 10.2 days, p=0.003) as compared to non-transfused individuals; there was no significant difference in either ventilator-free days (21.1 versus 22.7 days, p=0.54) or mortality (9 vs. 5%; p=0.53) (Table 3). In multivariate analysis, those patients who were transfused had an increased ICU length of stay (RR 1.8; p=0.001) and hospital length of stay (RR 1.5; p=0.001). There was no difference in mortality (p=0.21).
Table 3.
Clinical outcomes of patients with traumatic injury
| No Transfusion (n=33) |
Transfusion (n=22) |
p value | |
|---|---|---|---|
| Hospital LOS, days (sd)a | 10.2 (6.4) | 18.2 (12.9) | 0.001 |
| ICU LOS, days (sd)a | 5.4 (6.0) | 11.9 (10.3) | 0.001b |
| Ventilator free days (sd)a | 22.7 (9.2) | 21.1 (9.3) | 0.54 |
| Mortality, n(%) | 3 (9.1%) | 1 (4.6) | 0.53 |
Data expressed as mean (sd)
Comparisons were made using chi-square analysis for dichotomous predictor variables.
Discussion
The main finding of this study is that RBC transfusion is associated with increased plasma CXCL8 concentrations in individuals presenting with traumatic injury. The strength of our study is that in every analysis, the bioanalyte CXCL8 concentration remained significant: univariate comparison of cohorts, the multivariate time-series analysis examining the kinetics of CXCL8 concentrations over time in the two cohorts, as well as comparisons of post- to pre-transfusion samples. After adjusting for differences in age, gender, injury severity score, and temporal trends over time after injury, the significant difference in CXCL8 concentration persisted. In a standardized linear prediction model, the transfused group showed sustained and higher plasma CXCL8 concentrations following injury, whereas the non-transfused group showed a gradual decay in concentrations over the initial 48 hours studied.
These findings are consistent with results in our pre-clinical mouse model. In those studies, plasma concentrations of murine keratinocyte-derived cytokine (KC)/CXCL1 and macrophage inflammatory protein-2 (MIP-2)/CXCL2, the functional homologs of CXCL8, increased following RBC transfusion in endotoxemic mice(17). While the current human study cannot show causality, RBC transfusion is associated with maintenance of persistently elevated CXCL8 concentrations in those individuals with traumatic injury. Collectively, our murine and now human data support the hypothesis that transfusion of stored RBC can enhance the effects of a pre-existing inflammatory response, specifically CXCL8 concentration, in a susceptible host.
CXCL8 is a key innate immune activation signal functioning as a potent neutrophil chemoattractant and can induce the respiratory burst(28). CXCL8 is a biomarker of inflammation that is consistently elevated in studies of acute lung injury and ARDS(29, 30), which are common complications in individuals sustaining severe traumatic injury and resuscitation. Ware and colleagues have shown that CXCL8 is one of the few biomarkers with the ability to discriminate survivors from non-survivors of ARDS(29, 30). In our cohort, there were no differences in ventilator-free days that might suggest an association between RBC transfusion and risk of acute respiratory failure between the transfused and non-transfused group. Due to the small sample size, our study may be underpowered to identify differences in the clinical outcomes of ventilator-free days and mortality. Nonetheless, RBC transfusion was associated with increased hospital and ICU lengths of stay.
Our study is limited by the retrospective nature that precludes the ability to determine precisely the age of each RBC unit as well as the exact time of at which any given transfusion was administered in relation to the blood draw. Thus, our study does not attempt to address whether or not the age of the transfused RBC contributes to enhanced plasma bioanalyte concentrations in the transfused group. Moreover, we took a conservative approach in analyzing the kinetics of plasma bioanalyte concentrations in the two groups, and therefore made no assumptions as to the exact timing of the RBC transfusion and blood draw. Finally, the small sample size may be underpowered to show potential differences in plasma concentrations of other analytes in the different quartiles of time examined. Early, elevated plasma IL-6 concentrations have been shown to occur in individuals sustaining severe traumatic injury(13) and have value in predicting the development of organ failure(31, 32). Others have shown that intraoperative RBC transfusions were associated with elevated plasma IL-6 concentrations for up to 18 h following reperfusion in cardiac surgical patients undergoing aortic cross-clamping(14). We too observed elevated IL-6 concentrations with transfusion. A larger cohort may uncover additional bioanalytes that are associated with RBC transfusion.
In summary, this is the first study to show that RBC transfusion is associated with persistently elevated CXCL8 concentrations in individuals with traumatic injury. The kinetics of CXCL8 concentrations in the non-transfused and transfused group diverge following the initial 12 h following presentation, with the transfused group showing persistently elevated CXCL8 concentrations lasting 48 h in contrast to the decay in CXCL8 concentrations observed in the non-transfused group. Our findings are in agreement with our pre-clinical model of systemic inflammation in which RBC transfusion enhanced plasma neutrophil chemokine concentrations. We conclude that RBC transfusion contributes to ongoing inflammatory responses during the early phase of traumatic injury.
Acknowledgments
Funding Support: American Association for the Surgery of Trauma (AAST)/Novo Nordisk Research Scholarship Award in Hemostasis and Resuscitation (MRR); NIH HL086884, Vascular Medicine Institute Hemostasis and Vascular Biology Grant from the Institute for Transfusion Medicine (JSL), NIH P50-GM-53789 (DB, RM, YV); NIH GM065914 (JBO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Janet S. Lee, Email: leejs3@upmc.edu.
Jason L. Sperry, Email: sperryjl@upmc.edu.
Juan B. Ochoa, Email: ochoajb@upmc.edu, Juan.Ochoa@US.nestle.com.
Derek Barclay, Email: barclayd@upmc.edu.
Rami Namas, Email: namasra2@upmc.edu.
Yoram Vodovotz, Email: vodovotzy@upmc.edu.
Matthew R Rosengart, Email: rosengartmr@upmc.edu.
References
- 1.Malone DL, Kuhls D, Napolitano LM, McCarter R, Scalea T. Back to basics: validation of the admission systemic inflammatory response syndrome score in predicting outcome in trauma. J Trauma. 2001;51(3):458–463. doi: 10.1097/00005373-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Napolitano LM, Ferrer T, McCarter RJ, Jr, Scalea TM. Systemic inflammatory response syndrome score at admission independently predicts mortality and length of stay in trauma patients. J Trauma. 2000;49(4):647–652. doi: 10.1097/00005373-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Dunne JR, Malone DL, Tracy JK, Napolitano LM. Allogenic blood transfusion in the first 24 hours after trauma is associated with increased systemic inflammatory response syndrome (SIRS) and death. Surg Infect (Larchmt) 2004;5(4):395–404. doi: 10.1089/sur.2004.5.395. [DOI] [PubMed] [Google Scholar]
- 4.Bochicchio GV, Napolitano L, Joshi M, Bochicchio K, Meyer W, Scalea TM. Outcome analysis of blood product transfusion in trauma patients: a prospective, risk-adjusted study. World J Surg. 2008;32(10):2185–2189. doi: 10.1007/s00268-008-9655-0. [DOI] [PubMed] [Google Scholar]
- 5.Cryer HG, Leong K, McArthur DL, Demetriades D, Bongard FS, Fleming AW, et al. Multiple organ failure: by the time you predict it it's already there. J Trauma. 1999;46(4):597–604. doi: 10.1097/00005373-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Sauaia A, Moore FA, Moore EE, Norris JM, Lezotte DC, Hamman RF. Multiple organ failure can be predicted as early as 12 hours after injury. J Trauma. 1998;45(2):291–301. doi: 10.1097/00005373-199808000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Chaiwat O, Lang JD, Vavilala MS, Wang J, MacKenzie EJ, Jurkovich GJ, et al. Early packed red blood cell transfusion and acute respiratory distress syndrome after trauma. Anesthesiology. 2009;110(2):351–360. doi: 10.1097/ALN.0b013e3181948a97. [DOI] [PubMed] [Google Scholar]
- 8.Napolitano LM, Kurek S, Luchette FA, Corwin HL, Barie PS, Tisherman SA, et al. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit Care Med. 2009;37(12):3124–3157. doi: 10.1097/CCM.0b013e3181b39f1b. [DOI] [PubMed] [Google Scholar]
- 9.Ruttinger D, Wolf H, Kuchenhoff H, Jauch KW, Hartl WH. Red cell transfusion: an essential factor for patient prognosis in surgical critical illness? Shock. 2007;28(2):165–171. doi: 10.1097/shk.0b013e31803df84d. [DOI] [PubMed] [Google Scholar]
- 10.Silliman CC, Moore EE, Johnson JL, Gonzalez RJ, Biffl WL. Transfusion of the injured patient: proceed with caution. Shock. 2004;21(4):291–299. doi: 10.1097/00024382-200404000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Prins HA, Houdijk AP, Nijveldt RJ, Teerlink T, Huygens P, Thijs LG, et al. Arginase release from red blood cells: possible link in transfusion induced immune suppression? Shock. 2001;16(2):113–115. doi: 10.1097/00024382-200116020-00005. [DOI] [PubMed] [Google Scholar]
- 12.Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2007;21(6):327–348. doi: 10.1016/j.blre.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Hensler T, Heinemann B, Sauerland S, Lefering R, Bouillon B, Andermahr J, et al. Immunologic alterations associated with high blood transfusion volume after multiple injury: effects on plasmatic cytokine and cytokine receptor concentrations. Shock. 2003;20(6):497–502. doi: 10.1097/01.shk.0000095058.62263.1f. [DOI] [PubMed] [Google Scholar]
- 14.Fransen E, Maessen J, Dentener M, Senden N, Buurman W. Impact of blood transfusions on inflammatory mediator release in patients undergoing cardiac surgery. Chest. 1999;116(5):1233–1239. doi: 10.1378/chest.116.5.1233. [DOI] [PubMed] [Google Scholar]
- 15.McFaul SJ, Corley JB, Mester CW, Nath J. Packed blood cells stored in AS-5 become proinflammatory during storage. Transfusion. 2009;49(7):1451–1460. doi: 10.1111/j.1537-2995.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- 16.Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115(21):4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mangalmurti NS, Xiong Z, Hulver M, Ranganathan M, Liu XH, Oriss T, et al. Loss of red cell chemokine scavenging promotes transfusion-related lung inflammation. Blood. 2009;113(5):1158–1166. doi: 10.1182/blood-2008-07-166264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong Z, Cavaretta J, Qu L, Stolz DB, Triulzi D, Lee JS. Red blood cell microparticles show altered inflammatory chemokine binding and release ligand upon interaction with platelets. Transfusion. 2011;51(3):610–621. doi: 10.1111/j.1537-2995.2010.02861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raoof M, Zhang Q, Itagaki K, Hauser CJ. Mitochondrial peptides are potent immune activators that activate human neutrophils via FPR-1. J Trauma. 2010;68(6):1328–1332. doi: 10.1097/TA.0b013e3181dcd28d. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zallen G, Moore EE, Ciesla DJ, Brown M, Biffl WL, Silliman CC. Stored red blood cells selectively activate human neutrophils to release IL-8 and secretory PLA2. Shock. 2000;13(1):29–33. doi: 10.1097/00024382-200013010-00006. [DOI] [PubMed] [Google Scholar]
- 22.Ball CG, Salomone JP, Shaz B, Dente CJ, Tallah C, Anderson K, et al. Uncrossmatched blood transfusions for trauma patients in the emergency department: incidence, outcomes and recommendations. Can J Surg. 2011;54(2):111–115. doi: 10.1503/cjs.032009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inaba K, Teixeira PG, Shulman I, Nelson J, Lee J, Salim A, et al. The impact of uncross-matched blood transfusion on the need for massive transfusion and mortality: analysis of 5,166 uncross-matched units. J Trauma. 2008;65(6):1222–1226. doi: 10.1097/TA.0b013e31818e8ff3. [DOI] [PubMed] [Google Scholar]
- 24.Mahambrey TD, Fowler RA, Pinto R, Smith TS, Callum JL, Pisani NS, et al. Early massive transfusion in trauma patients: Canadian single-centre retrospective cohort study. Can J Anaesth. 2009;56(10):740–750. doi: 10.1007/s12630-009-9151-5. [DOI] [PubMed] [Google Scholar]
- 25.Namas R, Ghuma A, Torres A, Polanco P, Gomez H, Barclay D, et al. An adequately robust early TNF-alpha response is a hallmark of survival following trauma/hemorrhage. PLoS ONE. 2009;4(12):e8406. doi: 10.1371/journal.pone.0008406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Streiner DL, Norman GR. Correction for multiple testing: is there a resolution? Chest. 2011;140(1):16–18. doi: 10.1378/chest.11-0523. [DOI] [PubMed] [Google Scholar]
- 27.Brown BW, Russell K. Methods of correcting for multiple testing: operating characteristics. Stat Med. 1997;16(22):2511–2528. doi: 10.1002/(sici)1097-0258(19971130)16:22<2511::aid-sim693>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 28.Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClintock D, Zhuo H, Wickersham N, Matthay MA, Ware LB. Biomarkers of inflammation, coagulation and fibrinolysis predict mortality in acute lung injury. Crit Care. 2008;12(2):R41. doi: 10.1186/cc6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ware LB, Koyama T, Billheimer DD, Wu W, Bernard GR, Thompson BT, et al. Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest. 2010;137(2):288–296. doi: 10.1378/chest.09-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuschieri J, Bulger E, Schaeffer V, Sakr S, Nathens AB, Hennessy L, et al. Early elevation in random plasma IL-6 after severe injury is associated with development of organ failure. Shock. 2010;34(4):346–351. doi: 10.1097/SHK.0b013e3181d8e687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sperry JL, Friese RS, Frankel HL, West MA, Cuschieri J, Moore EE, et al. Male gender is associated with excessive IL-6 expression following severe injury. J Trauma. 2008;64(3):572–578. doi: 10.1097/TA.0b013e3181650fdf. [DOI] [PubMed] [Google Scholar]