Abstract
Objectives
To define the pre-operative risk factors, outcomes and adverse events related to the motor subtypes of post-operative delirium.
Design
Prospective cohort study.
Setting
Referral medical center.
Patients
Subjects 50 years and older with planned post-operative intensive care unit admission following an elective operation were recruited.
Main Outcome Measures
Pre-operatively, a standardized frailty assessment was performed. Post-operatively, delirium and its motor subtypes were measured using the validated tools of the Confusion Assessment Method-ICU and the Richmond Agitation Sedation Scale. Statistical analysis included univariate (t test and chi-square) and ANOVA with post-hoc analysis.
Results
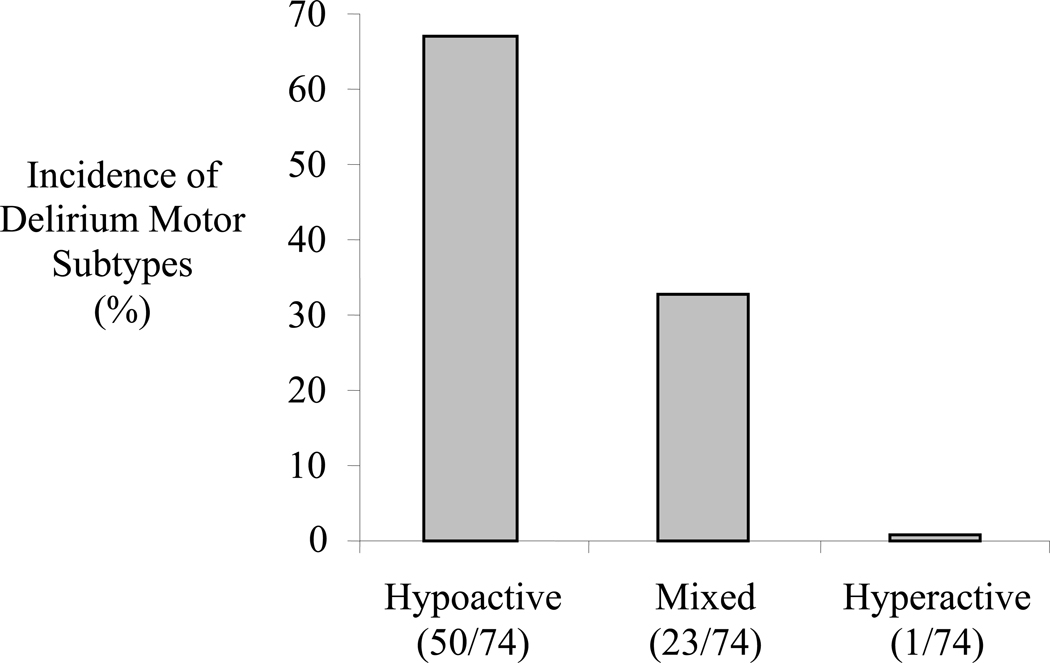
Delirium occurred in 43% (74/172); including the motor subtypes of hypoactive 68% (50/74), mixed 31% (23/74) and hyperactive 1% (1/74). Subjects with hypoactive delirium were found to be older (71±9 vs. 65±9 years; p=0.002) and more anemic (36±8% vs. 41±6%, *p=0.002). Subjects with hypoactive delirium were found to have higher six-month mortality (32% (16/50) vs. 9% (2/23); p=0.041). Delirium related adverse events occurred in 24% (18/74) of delirious subjects; pulled lines/tubes occurred more frequently in the mixed group (p=0.006) and decubitus ulcers were more common in the hypoactive group (p=0.002).
Conclusion
Delirium motor subtypes alert the clinician to differing prognosis and adverse event profiles in the post-operative geriatric patient. Hypoactive delirium is the most common motor subtype and is associated with worse prognosis (one in three six-month mortality). Delirium motor subtypes are related to differing adverse event profiles; this knowledge can modify the management of the delirious elderly patient.
Introduction
Post-operative delirium is the most common complication in elderly surgical patients.1 More than half of all operations performed in the United States are on patients older than 65 years.2 Risk factors for post-operative delirium include cognitive dysfunction, functional impairment, multiple co-morbidities, malnutrition and older age.3,4 Outcomes associated with post-operative delirium include worse outcomes including prolonged length of stay, increased incidence of discharge institutionalization and higher mortality.3 With an aging population, understanding the clinical presentation and outcomes of post-operative delirium will become increasingly relevant.
A potential means of increasing understanding about post-operative delirium is recognition of delirium motor subtypes. The motor subtypes of delirium include hyperactive (pure agitation), hypoactive (pure lethargy), and mixed (fluctuation between lethargy and agitation).5 While a close relationship between hospitalized delirium and poor outcomes is well established; outcomes following the development of the individual motor subtypes of delirium are less well defined. Conflicting reports describe improved outcomes in both hyperactive delirium6 and hypoactive delirium.7 Adverse event profiles have also been shown to differ for delirium presenting with agitation (hyperactive or mixed) in comparison to lethargic (hypoactive) delirium.
Increased knowledge regarding delirium motor subtypes may aid the clinician in the management of geriatric post-operative patients. The specific aims of this study were to: (1) determine the incidence and natural history of the motor subtypes of post-operative delirium; (2) describe the pre-operative risk factors for developing the motor subtypes of post-operative delirium; (3) determine the outcomes related to the motor subtypes of delirium; and (4) describe the adverse events related to the development of the different motor subtypes of delirium.
Methods
The study was performed at the Denver Veteran Affairs Medical Center. Regulatory approval was obtained through the Colorado Multi-Institutional Review Board (COMIRB 05-0281) prior to initiating the study. Written informed consent was obtained from all participants. Risk factors and outcomes of developing post-operative delirium were previously reported in 144 of the 172 subjects included in this study.3
Inclusion criteria were subjects older than 50 years and undergoing an operation with a planned post-operative intensive care unit admission. Exclusion criteria were: (1) subjects undergoing intracranial surgery, (2) subjects who were vision or hearing impaired (making the delirium assessment tool unreliable), (3) subjects who could not speak English (making the delirium assessment tool unreliable) and (4) subjects who could not undergo informed consent.
Delirium was determined using the Confusion Assessment Method-ICU (CAM-ICU) which was administered daily at 7AM for all participants throughout their ICU stay. The CAM-ICU has established reliability and validity in the diagnosis of delirium.8 The CAM-ICU defines delirium by assessing four features: (1) fluctuation in mental status, (2) inattention, (3) disorganized thinking and (4) altered levels of consciousness.8 Delirium is diagnosed when features one, two and either three or four are present. The CAM-ICU utilizes the Richmond Agitation Sedation Score (RASS) to determine the level of consciousness.9 The RASS is a 10-point scale ranging from (−5) unarousable, (0) calm to (+4) combative.9 The RASS has established reliability and validity in rating the level of consciousness of ICU patients.9 If RASS scores of −4 or −5 were obtained (revealing deep sedation or un-arousable), the CAM-ICU was not completed (as previously described10) to define the presence or absence of delirium because delirium cannot be assessed in subjects with this level of sedation (for example, delirium cannot be assessed on a subject who is unarousable because they are paralyzed). Because delirium is transient and fluctuates over time, previous delirium researchers have validated a chart review process which defines the presence or absence of delirium which occurs at times other than during the administration of the CAM-ICU.11 The previously described methods identify delirium by searching the chart for keywords related to delirium by asking “Is there any evidence from the chart of acute confusional state (e.g., delirium, mental status change, inattention, disorientation, hallucinations, agitation, inappropriate behavior, etc.)?”11 The importance of including the chart review is that it provides a validated, reproducible dynamic picture of delirium (a process which is transitory and fluctuates throughout a 24 hour day) over an entire day which is not possible by only performing the CAM-ICU assessment. In this study, delirium was defined as present if either the CAM-ICU or the validated chart review were positive. Our methods for detected delirium have been described previously.3,12
The delirium motor subtypes were defined using the daily RASS scores as previously described.13,14 Hyperactive delirium was defined as present in subjects with all positive daily RASS scores (ranging from +1 to +4) associated with every positive CAM-ICU assessment. Hypoactive delirium was defined as present in subjects with all neutral or negative daily RASS scores (ranging from 0 to −3) associated with every CAM-ICU positive assessment. Mixed type delirium was defined as present when daily RASS scores included both positive values (ranging from +1 to +4) and neutral or negative values (ranging from 0 to −3) associated with every positive CAM-ICU assessment. The validated chart review previously described to determine the presence/absence of delirium in conjunction with the CAM-ICU was not used to define which motor subtype was present because this chart review methodology is not validated to determine motor subtypes.11 Only the daily RASS scores were used to define which motor subtype was present.
A pilot study of 100 daily encounters was completed prior to starting the main study to assess inter-rater reliability for delirium assessment using the CAM-ICU. Researchers evaluating for delirium (T.N.R and C.D.R.) had a high inter-rater reliability with a concordance rate of 98% and a kappa statistic of 0.96 (95% confidence interval, 0.91 to 1.00).
Routine pre-operative clinical variables were recorded. In addition, pre-operative assessment of (1) burden of co-morbidities (Charlson’s Index – a sum of co-morbidities which assigns weighted values to co-morbidities based on their one year risk of mortality)15, (2) cognitive function (Mini-Cog Test – a combination of three item recall with a clock drawing task which accurately predicts cognitive dysfunction in the elderly)16 and (3) functional status (Barthel Index – evaluates independence in activities of daily living).17 Routine post-operative outcome measures were recorded. Mortality at 30 days and six-months was determined by both chart review and phone contact. Institutionalization was defined as discharge to another facility (rehabilitation, nursing home, long term care facility). A subject was not considered to be newly institutionalized post-discharge if they resided in a care facility immediately pre-operatively.
Statistical analysis performed included univariate analysis (t test and chi-square) and analysis of variance (ANOVA). Post-hoc multiple comparisons of the ANOVA results were performed using Tukey’s HSD test.18 Results were reported as mean ± standard deviation, and statistical significance was set at p<0.05.
Results
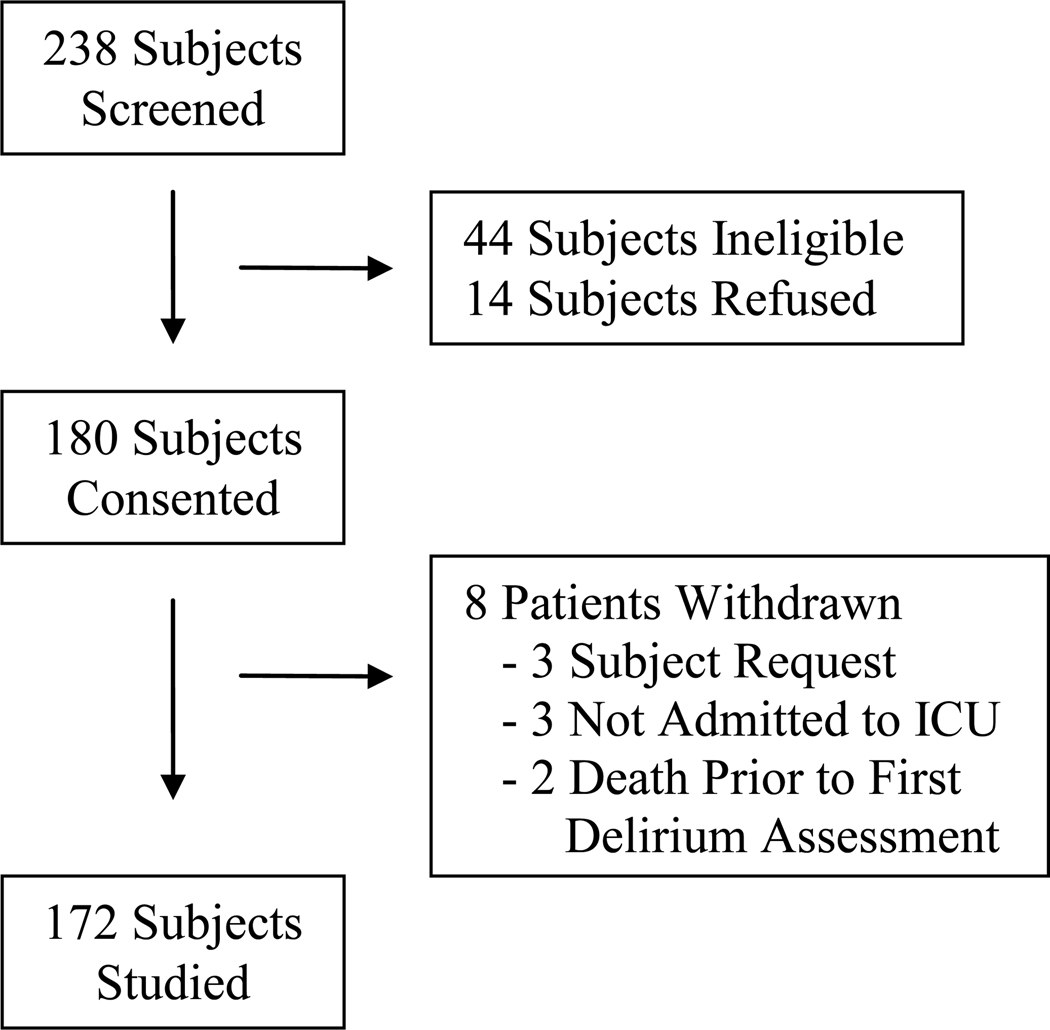
Between September 2006 and October 2007, 172 subjects were studied. Study enrollment is reviewed in Figure 1. Delirium occurred in 43% (74/172); including the motor subtypes of hypoactive 68% (50/74), mixed 31% (23/74) and hyperactive 1% (1/74). The one subject in whom purely hyperactive delirium occurred was excluded from statistical comparisons because of the small group size (i.e., n=1). The average age was 64±8 years and 97% (166/172) were male. The average time to the initial presentation of delirium in all delirious subjects was 2.3±1.8 days. The time to initial onset of delirium was not different in the hypoactive (2.4±1.7 days) and mixed (2.2±1.8 days; p=0.655) delirium groups. The average duration of delirium in all hypoactive and mixed delirious subjects was 3.5±4.5 days. The duration of delirium was not different in the hypoactive (2.8±1.4 days) and mixed (3.9±5.4 days; p=0.341) delirium groups.
Figure 1. Study Enrollment Summary.
Ineligible 44 subjects included: operations on the brain (39), hearing impaired (3) and vision impaired (2).
An overview of all operations performed included: abdominal 45% (77/172), cardiac 38% (65/172), non-cardiac thoracic 9% (16/172), and vascular 8% (14/172). There was no difference in regard to the types of operations performed in subjects with hypoactive and mixed motor subtypes of delirium. (see Table 1)
TABLE 1.
The motor subtypes of post-operative delirium
| No Delirium | DELIRIUM | |||
|---|---|---|---|---|
| Hypoactive | Mixed | |||
| n=98 | n=50 | n=23 | p value | |
| PRE-OPERATIVE VARIABLES | ||||
| Clinical | ||||
| Age (years) | 61±6 | 71±9 | 65±9 | p=0.002 |
| Co-Morbidities(Charlson Indexa) | 1.9±1.5 | 4.2±2.3 | 5.0±2.1 | p=0.146 |
| Cognitive Function (Mini-Cogb) | 4.6±0.7 | 2.7±1.6 | 2.7±1.5 | p=0.971 |
| Functional Status (Barthel Indexc) | 99±3 | 90±11 | 93±11 | p=0.173 |
| Laboratory | ||||
| Albumin (g/dL) | 3.9±0.5 | 3.3±0.7 | 3.4±0.7 | p=0.753 |
| Hematocrit (%) | 44±4 | 36±8 | 41±6 | p=0.002 |
| Sodium (mEq/L) | 139±3 | 137±3 | 139±3 | p=0.422 |
| Potassium (mEq/L) | 4.3±0.5 | 4.1±0.5 | 4.2±0.5 | p=0.580 |
| Creatinine (mg/dL) | 1.1±0.5 | 1.2±0.7 | 1.2±0.7 | p=1.000 |
| Glucose (mg/dL) | 115±41 | 119±44 | 112±34 | p=0.756 |
| OPERATIONS | ||||
| Abdominal | 38% (37/98) | 58% (29/50) | 48% (11/23) | p=0.457 |
| Cardiac | 46% (45/98) | 26% (13/50) | 26% (6/23) | p=1.000 |
| Non-Cardiac Thoracic | 8% (8/98) | 10% (5/50) | 13% (3/23) | p=0.701 |
| Vascular | 8% (8/98) | 6% (3/50) | 13% (3/23) | p=0.372 |
| OUTCOME MEASURES | ||||
| ICU Stay (days) | 4.6±2.6 | 8.8±8.7 | 9.2±5.5 | p=0.970 |
| Hospital Stay (days) | 8.0±4.6 | 14.4±11.2 | 14.5±9.6 | p=0.998 |
| Institutionalization | 3% (3/98) | 42% (21/48)e | 26% (6/22)e | p=0.296 |
| 30-Day Mortality | 1% (1/98) | 14% (7/50) | 4% (1/23) | p=0.421 |
| 6-Month Mortality | 2% (2/96)d | 32% (16/50) | 9% (2/23) | p=0.041 |
The p value describes comparison of mixed and hypoactive motor subtypes of delirium.
Abbreviations – ICU (intensive care unit)
Charlson Index is a measure of burden of co-morbidities [range of 0=no co-morbidities, 19=severe co-morbidities]
Mini-Cog test assesses cognitive function [range of 5=no cognitive dysfunction to 0=severe cognitive dysfunction]
Barthel Index measures independence performing activities of daily living [range from 100=no functional impairment to 0=severe functional dependence]
n=96 because two subjects were lost to six month follow-up
Two subjects with hypoactive delirium and one subject with mixed delirium were institutionalized prior to their operation and were not considered to be newly institutionalized post-discharge.
Pre-operative variables were compared in the groups of participants with hypoactive and mixed types of delirium. (see Table 1) Subjects with hypoactive delirium were older (71±9 years versus 65±9 years; p=0.002) and more anemic (36±8% versus 41±6%, p=0.002) in comparison to subjects with mixed type delirium.
Intra-operative variables of blood transfusions, length of operation and type of anesthesia were compared in the groups with hypoactive (n=50) and mixed (n=23) delirium motor subtypes. Type of anesthesia is described as the percent of subjects who received general endotracheal anesthesia (all other subject received general endotracheal anesthesia with an epidural). Blood transfusions were similar in the two groups (3.0±3.2 units in the hypoactive group versus 3.6±3.8 units in the mixed group; p=0.644), operating room time was similar in the two groups (268±117 minutes in the hypoactive group versus 325±155 minutes in the mixed group; p=0.118) and the use of general endotracheal anesthesia was similar in the two groups (80% in the hypoactive group and 69% in the mixed group; p=0.350).
Outcomes were compared in the groups of subjects with hypoactive and mixed types of delirium. Hypoactive delirium subjects had higher six-month mortality compared to mixed (32% (16/50) versus 9% (2/23); p=0.041). (see Table 1) Outcomes were compared in subjects who did not develop delirium (n=98) to subjects who developed delirium of any motor subtype (n=74). Subjects who developed delirium of any motor subtype had longer ICU stays (9.1±7.7 versus 4.6±2.6 days; p<0.001), longer hospital stays (14.6±10.7 versus 8.0±4.6 days; p<0.001), a higher rate of discharge institutionalization (39% versus 3%; p<0.001), a higher 30-day morality (11% versus 1%; p=0.005) and a higher rate of six-month mortality (24% versus 2%; p<0.001) in comparison to subjects who did not develop delirium.
Of the 73 subjects with hypoactive or mixed post-operative delirium, 17 (23%) had one or more delirium related adverse events. Two subjects had 2 adverse events. A total of 19 adverse events occurred during the study in subjects with hypoactive or mixed subtypes of delirium. Adverse events included: 58% (11/19) tube/line inadvertent removals, 32% (6/19) sacral skin breakdown and 11% (2/19) falls. (see Table 2) In subjects with delirium related adverse events, sacral skin breakdown occurred in 67% (6/9) subjects with hypoactive delirium versus none (0/10) in the mixed motor type group (p=0.002), and pulled lines/tubes occurred in 90% (9/10) of mixed subjects in comparison to 22% (2/9) of hypoactive (p=0.006). The one subject who developed purely hyperactive post-operative delirium had three delirium related adverse events (2 peripheral IV pulls and 1 self-extubation).
TABLE 2.
Adverse events and delirium motor subtypes
| HYPOACTIVE | MIXED | ||
|---|---|---|---|
| 8 subjects 9 adverse events |
9 subjects 10 adverse events |
p value | |
| Incidence - Adverse Events | 16% (8/50) | 39% (9/23) | |
| ADVERSE EVENT | |||
|
Pulled lines/tubes Peripheral IV Naso-Gastric Tube Central Venous Catheter Urinary Catheter |
22% (2/9) (1) (1) |
90% (9/10) (3) (3) (2) (1) |
p=0.006 |
| Sacral Skin Breakdown | 67% (6/9) | 0 (0/10) | p=0.002 |
| Falls | 11% (1/9) | 10% (1/10) | p=0.999 |
Of the 73 subjects with hypoactive or mixed post-operative delirium, 17 (23%) had a delirium related adverse events.
Discussion
Hypoactive delirium occurred in two thirds and mixed delirium in one third of elderly patients who developed post-operative delirium in the surgical intensive care unit. Purely hyperactive delirium rarely occurred. Those with hypoactive delirium were older and more frequently presented with pre-operative anemia. Subjects with hypoactive delirium had higher six-month mortality in comparison to mixed type delirium. Delirium related adverse event profiles were different for the mixed and hypoactive groups. Pulled lines/tubes occurred more frequently in the mixed group and sacral skin breakdown occurred more frequently in the hypoactive delirium group. A major limitation of this study is that the majority of subjects were male; a fact which does not allow the findings to be generalized to both males and females because gender differences in the motor subtypes of delirium were not able to be detected.
The incidence of the motor subtypes of delirium reported has varied depending on the patient population studied. Two previous studies have evaluated the motor subtypes of delirium in post-operative patients.14,7 In patients who required mechanical ventilation in the surgical ICU with an average age of 55 years, the overall incidence of post-operative delirium was 73%, hypoactive delirium occurred in 88% of all cases of delirium, mixed type delirium occurred in 12% of all cases of delirium and no patient developed purely hyperactive delirium.14 In patients following hip fracture repair with an average age of 79 years, 71% of all delirium was purely hypoactive.7 Elderly patients in the ICU, whether surgical or medical, rarely develop purely hyperactive delirium.13,14 This study’s results confirm previous findings which suggest that hypoactive delirium is most common delirium motor subtype following an operation in geriatric patients.
It is important to recognize that different patient populations will present with different incidences of the delirium motor subtypes. Younger patient populations are more likely to present with more purely hyperactive delirium.12,19 We previously reported the incidence of the hyperactive motor subtype as 15% of all delirium in trauma ICU patients (average age 44 years) for whom substance withdrawal was a significant issue.12 Purely hyperactive delirium has been reported as 30% of all delirium in psychiatric inpatients.19 The prevalence of the motor subtypes of delirium have not previously been recognized to differ in male and female patients.6, 7, 13, 19–21
Our study found that the pre-operative risk factors for developing hypoactive delirium were older age and greater anemia. Older age as a risk for hypoactive delirium has been reported in medical intensive care unit patients.13 Anemia has not been previously related to the occurrence of hypoactive delirium. So, why is older age and anemia associated with the development of hypoactive delirium? The answer is likely that older age and anemia are both markers of frailty. Frailty is defined as a state of reduced physiologic reserve which increases susceptibility to disability and creates limited reserve to withstand stressors.22,23 Our previous work related the accumulation of four or more markers of frailty in elderly individuals to the occurrence of higher six-month post-operative mortality.24 Given that both frail patients and patients who develop hypoactive delirium both have high six-month mortality, it seems reasonable that pre-operative markers of frailty would be related to the occurrence of post-operative hypoactive delirium. However, frailty is not the only possible explanation for relating older age and greater anemia to developing hypoactive delirium; increased burden of chronic disease may result from advanced age and cause anemia.
Defining the long term prognosis associated with the different motor subtypes of delirium is the first important finding of our study. Higher six-month mortality was found in subjects who developed hypoactive delirium in comparison to mixed delirium. In fact, one third of all patients who developed hypoactive delirium died within six months of their operation. These findings are in line with previous work which has recognized a worse prognosis in medically hospitalized patients who develop hypoactive delirium.6,20 However, contradictory findings reporting improved outcomes following the development of hypoactive delirium after surgery for hip fractures exist;7 which might reflect differing study patient populations and/or our incomplete understanding regarding of the etiology of the motor subtypes of delirium. The clinician should not make false assumptions that delirium equates to mortality. The cause of mortality is likely multi-factorial. Baseline frailty of elderly individuals both predisposes to delirium and mortality. There is no evidence that by eliminating delirium, pharmacologically or otherwise, will improve outcomes. Recognizing the poor prognosis associated with hypoactive delirium may provide a better understanding of the prognosis of elderly post-operative patients.
The description of the distinct inpatient adverse event profiles of the different motor subtypes of delirium is the second important finding of our study. Individuals with the hypoactive motor subtype of delirium had more frequent sacral skin breakdown. Similar findings have been previously described in medically hospitalized patients.20 Those with delirium which clinically presented with agitation, either mixed or purely hyperactive, more often pulled lines and tubes. This finding appears unique to post-operative surgery patients and has not been previously recognized by delirium studies evaluating medical hospitalized patients. In such work agitation was associated with falls20 and urinary incontinence.25
Limitations of this study include: (1) The small sample of purely hyperactive delirium did not allow for statistical comparison of this motor subtype of delirium with the other groups. The fact that our study found such a small group of hyperactive post-operative delirium likely reflects the elderly population studied and not all post-operative patients. (2) The majority of our subjects were male. As a result, this study would not be able to detect potential gender differences in the motor subtypes of delirium. Our study population’s gender distribution reflects the patient population at the Denver Veterans Affairs Medical Center and not a selection bias. (3) The fact that delirium is fluctuating and transient in nature makes delirium research methodology challenging. The delirium research community has developed validated tools which quantitatively define the presence / absence of delirium (i.e., the CAM-ICU8 and the validated chart review11). There is no single methodology that is universally accepted to detect delirium.10 Changes in research methods will change the incidence of delirium detected; a fact which partially accounts for the wide variability of the incidences of delirium reported in the literature. Our methodology of daily CAM-ICU and chart review assessments has been validated as reasonable methods for detecting delirium.11 (4) The final major limitation is that the relationship of delirium and post-operative complications and narcotics/sedatives was not accounted for in our study design. Benzodiazepines have been found to promote the transition into delirium26 and future work might focus on examining the relationship of post-operative factors and the development of the motor subtypes of delirium.
Our study found that hypoactive delirium occurred in two thirds of post-operative elderly patients with delirium. Those with hypoactive delirium were older and had higher six-month mortality. Future research is warranted to understand the underlying pathophysiology of the delirium motor subtypes and the possibility that differing post-operative treatment regimens may be tailored to optimally manage the individual motor subtypes of delirium.
Figure 2. The Incidence of the Motor Subtypes of Post-Operative Delirium.
Acknowledgments
This work was supported by the American Geriatric Society’s Jahnigen Scholars Award (TNR) and NIH K24-HL-089223 (MM).
Footnotes
Manuscript Presentation: Western Surgical Association, San Antonio, TX (Nov. 11, ‘09).
References
- 1.Marcantonio ER, Juarez G, Goldman L, et al. The relationship of postoperative delirium with psychoactive medications. JAMA. 1994;272(19):1518–1522. [PubMed] [Google Scholar]
- 2.Geriatric Review Syllabus - A Core Curriculum in Geriatric Medicine. Sixth Edition ed. New York: American Geriatrics Society; 2006. [Google Scholar]
- 3.Robinson TN, Raeburn CD, Tran ZV, et al. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249(1):173–178. doi: 10.1097/SLA.0b013e31818e4776. [DOI] [PubMed] [Google Scholar]
- 4.Dasgupta M, Dumbrell AC. Preoperative risk assessment for delirium after noncardiac surgery: a systematic review. J Am Geriatr Soc. 2006;54(10):1578–1589. doi: 10.1111/j.1532-5415.2006.00893.x. [DOI] [PubMed] [Google Scholar]
- 5.Lipowski ZJ. Transient cognitive disorders (delirium, acute confusional states) in the elderly. Am J Psychiatry. 1983;140(11):1426–1436. doi: 10.1176/ajp.140.11.1426. [DOI] [PubMed] [Google Scholar]
- 6.Liptzin B, Levkoff SE. An empirical study of delirium subtypes. Br J Psychiatry. 1992;161:843–845. doi: 10.1192/bjp.161.6.843. [DOI] [PubMed] [Google Scholar]
- 7.Marcantonio E, Ta T, Duthie E, Resnick NM. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc. 2002;50(5):850–857. doi: 10.1046/j.1532-5415.2002.50210.x. [DOI] [PubMed] [Google Scholar]
- 8.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 9.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 10.Moraga AV, Rodriguez-Pascual C. Acurate diagnosis of delirium in elderly patients. Curr Opin Psychiatry. 2007;20(3):262–267. doi: 10.1097/YCO.0b013e3280ec52e5. [DOI] [PubMed] [Google Scholar]
- 11.Pisani MA, Araujo KL, Van Ness PH, et al. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10(4):R121. doi: 10.1186/cc5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angles EM, Robinson TN, Biffl WL, et al. Risk factors for delirium after major trauma. Am J Surg. 2008;196(6):864–869. doi: 10.1016/j.amjsurg.2008.07.037. discussion 9–70. [DOI] [PubMed] [Google Scholar]
- 13.Peterson JF, Pun BT, Dittus RS, et al. Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc. 2006;54(3):479–484. doi: 10.1111/j.1532-5415.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 14.Pandharipande P, Cotton BA, Shintani A, et al. Motoric subtypes of delirium in mechanically ventilated surgical and trauma intensive care unit patients. Intensive Care Med. 2007;33(10):1726–1731. doi: 10.1007/s00134-007-0687-y. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Borson S, Scanlan JM, Chen P, Ganguli M. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51(10):1451–1454. doi: 10.1046/j.1532-5415.2003.51465.x. [DOI] [PubMed] [Google Scholar]
- 17.Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud. 1988;10(2):61–63. doi: 10.3109/09638288809164103. [DOI] [PubMed] [Google Scholar]
- 18.Rosner B. Fundamentals of biostatistics. 6th ed ed. Belmont, CA: Duxbury Press; 2006. [Google Scholar]
- 19.Meagher DJ, O'Hanlon D, O'Mahony E, Casey PR, Trzepacz PT. Relationship between symptoms and motoric subtype of delirium. J Neuropsychiatry Clin Neurosci. 2000;12(1):51–56. doi: 10.1176/jnp.12.1.51. [DOI] [PubMed] [Google Scholar]
- 20.O'Keeffe ST, Lavan JN. Clinical significance of delirium subtypes in older people. Age Ageing. 1999;28(2):115–119. doi: 10.1093/ageing/28.2.115. [DOI] [PubMed] [Google Scholar]
- 21.de Rooij SE, Schuurmans MJ, van der Mast RC, Levi M. Clinical subtypes of delirium and their relevance for daily clinical practice: a systematic review. Int J Geriatr Psychiatry. 2005;20(7):609–615. doi: 10.1002/gps.1343. [DOI] [PubMed] [Google Scholar]
- 22.Buchner DM, Wagner EH. Preventing frail health. Clin Geriatr Med. 1992;8(1):1–17. [PubMed] [Google Scholar]
- 23.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 24.Robinson TN, Eiseman B, Wallace JI, et al. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg. 2009;250(3):449–455. doi: 10.1097/SLA.0b013e3181b45598. [DOI] [PubMed] [Google Scholar]
- 25.O'Keeffe S, Lavan J. The prognostic significance of delirium in older hospital patients. J Am Geriatr Soc. 1997;45(2):174–178. doi: 10.1111/j.1532-5415.1997.tb04503.x. [DOI] [PubMed] [Google Scholar]
- 26.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]