Stem cells are clonogenic cells that have two remarkable features, the ability to differentiate into multiple mature cell types (multipotency) and to simultaneously replenish the stem cell pool (self-renewal), that allow them to sustain tissue development and maintenance1 (Box 1). The surge of stem cell research arose when it became clear that all blood cell components were derived from a rare subset of bone marrow (BM)-residing haematopoietic stem cells (HSCs), which can be isolated and assayed in vitro and in vivo. Currently, HSCs are the paradigmatic and best-characterized example of tissue-specific stem cells.
Preface.
Mesenchymal stem cells (MSCs) are a diverse subset of multipotent precursors present in the stromal fraction of many adult tissues, which have drawn intense interest from translational and basic investigators. MSCs have been operationally defined by their ability to differentiate into osteoblasts, adipocytes and chondrocytes after in vitro expansion. Nevertheless, their identity in vivo, heterogeneity, anatomical localization and their functional roles in adult tissue homeostasis have remained enigmatic and are only starting to be uncovered.
Soon after the discovery of HSCs, studies by Friedenstein and colleagues reported that the BM stroma can generate bone, fat cells, cartilage and reticular cells following heterotopic transplantation (that is, transplantation into a different tissue from that of origin) in mice. This suggested the existence of non-haematopoietic BM multipotent precursor cells with skeletal and adipose potential2, 3. It was later shown that these precursors were a subset of fibroblast-like cells (defined as colony-forming unit fibroblast (CFU-F)4 in analogy to their haematopoietic counterpart) that could be selected by adherence to plastic surfaces and expanded in vitro. Further studies substantiated the ability of cultured cells derived from single CFU-Fs to proliferate while preserving the ability to differentiate to osteoblasts, adipocytes and chondrocytes in vitro5. Multilineage capacity and proliferation in vitro were interpreted at the time as indicativeof in vivo multipotency and self-renewal, the hallmarks of `stemness'. Thus, the term mesenchymal stem cell (MSC) was coined and gained acceptance to refer to these newly identified precursor cells6, 7. Since their original description, stromal cells categorized as MSCs based on trilineage potential (osteoblast, adipocyte and chondrocyte) in vitro have been isolated from the adherent fraction of many adult and embryonic tissues in multiple species (Figure 1)8–11.
Figure 1. Mesencymal stem cells and multipotent mesenchymal stromal cells.
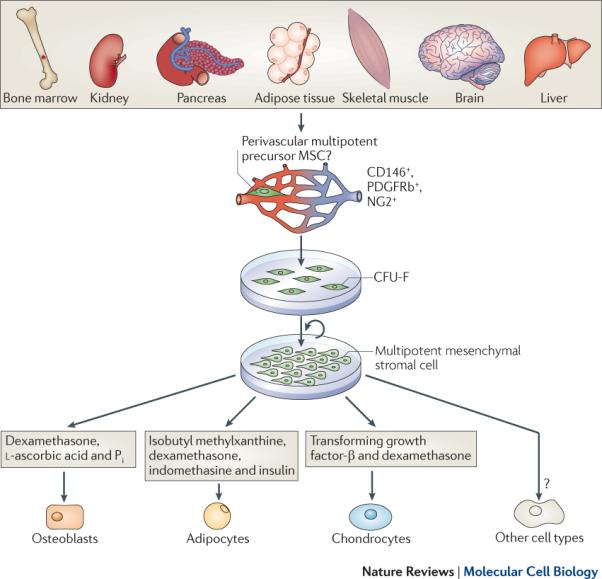
The plastic-adherent cellular fraction of many organs contains stromal progenitor cells that can give rise to colonies of fibroblastic morphology. This cellular subset, known as colony forming units-fibroblasts (CFU-F), totally or partially corresponds to a proposed multipotent progenitor cell population, most likely heterogeneous in nature and origin, which resides in the proximity of blood vessels in all tissues studied thus far and has been shown to express pericyte specific markers (CD146, NG2 and PDGFRβ). When cultured under the appropriate cell densities, colonies derived from single CFU-Fs can be isolated and expanded after multiple passages in vitro without losing their multipotent mesenchymal capacity. These cultured cells, classically referred to as mesenchymal stem cells, are now termed multipotent mesenchymal stromal cells. The hallmark, and unique criteria to that define mesenchymal stromal cells is their ability to differentiate into adipocytes, chondrocytes and osteoblasts when placed under inductive stimuli. Differentiation into multiple non-mesenchymal mature cell types has been reported but remains a matter of debate.
Multipotentiality in vitro, as well as ease of isolation and expansion, rapidly positioned MSCs as promising therapeutic agents in regenerative medicine and made them the subject of intensive clinical research12 (Box 2). Nevertheless, many important aspects regarding MSC biology are still unclear. In this Opinion article we discuss the well-established properties of MSCs cultured in vitro, and focus on how these properties relate to recent studies that are beginning to uncover their localization and function in vivo.
Box 1. Criteria used to define stem cells as they apply to haematopoietic stem cells and Bone Marrow Mesenchymal Stem Cells.
1. Multipotency
The first criterion used to define stem cells is their ability to differentiate into multiple types of functionally mature specialized cells. Haematopoietic stem cells can give rise to all blood cell components, including neutrophils, lymphocytes, natural killer cells, dendritic cells, macrophages and monocytes. Bone marrow mesenchymal stem cells can give rise to osteoblasts, chondrocytes, adipocytes and reticular stroma.
2. Self-renewal
Stem cells also possess the capacity of self-renewal; that is, the ability to undergo numerous cell divisions while retaining their stem cell identity. HSCs have been shown to self-renew: HSCs transplanted into irradiated mice can reconstitute the haematopoietic system, including giving rise to a population of HSCs that can be serially retransplanted. BM-resident MSCs have also been shown to possess this ability, as transplantation gives rise to `ossicles' composed of bone, cartilage and reticular stroma, as well as to a population of serially retransplatable MSCs.
Studying MSCs in vitro
Defining MSCs
Stem cells are classically defined by their multipotency and self-renewal (Box 1). Based on these criteria, the central and most disputed issue is the use of the term mesenchymal stem cell. This term was first used to refer to a hypothetical postnatal, multipotent and self-renewing precursor derived from an original embryonic MSC, the function of which was to maintain the turnover of skeletal tissues in homeostasis or tissue repair during adulthood. As described for HSCs, MSCs would lie at the top of the mesenchymal cell hierarchy and progress through discrete stages of differentiation in an orderly manner to give rise to functionally and phenotypically mature tissues, including bone, smooth muscle, tendons and cartilage6.
This theoretical model provided an attractive conceptual framework in which the stromal multipotent precursor cell described by Friedenstein was rapidly regarded as the prototypical MSC, despite the fact that such a cell had not been shown to strictly fulfill the attributes conveyed in the term MSC. First, the biological properties of multipotent progenitors had been mostly inferred from the analysis of clonal and non-clonal in vitro expanded populations owing to the inability to isolate and assay them directly from tissues. Until recently, the multipotency and self-renewal of uncultured progenitors had not been fully probed using stringent in vitro and in vivo assays. Furthermore, the existence of a common post-natal `mesenchymal' progenitor has been questioned, as bone and muscle derive from different progenitors during embryonic development, and because whether MSCs give rise to muscle cells in vivo has not been convincingly demonstrated to date. For this reason, alternative names such as osteogenic or skeletal stem cells have been suggested. Regardless of its inaccuracy13, 14, the term MSC has remained prevalent to date to designate stromal precursors with trilineage potential isolated from the BM, and by extension, from any other mammalian tissue. Of note, the common use of the name MSC to indistinctively refer to both in vivo precursors as well as their in vitro expanded progeny, has frequently lead to misconceptions in the field. The International Society for Cellular Therapy has recommended the use of the name multipotent mesenchymal stromal cell (also abbreviated to MSCs but not used in this Opinion article) for the in vitro cultured cells, restricting the term stem cell to designate the proposed in vivo precursors/stem cells15, 16.
Characterization of mesenchymal stromal cells
Beyond their ability to generate osteoblasts, adipocytes and chondrocytes in vitro5, mesenchymal stromal cells give rise to bone and cartilage after ectopic implantation in vivo17, 18 and have been documented to contribute to bone regeneration in animal models of genetic bone disorders19. Many studies have further reported mesenchymal stromal cell differentiation into multiple other cell types of mesodermal and non-mesodermal origin, including endothelial cells20, cardiomyocytes21, hepatocytes22 and neural cells23, 24. Nevertheless, such multipotential capabilities of mesenchymal stromal cells are not universally accepted. There are concerns because of the lack of globally standardized methods for their isolation, expansion and identification, as well as the range in assays used to define terminally differentiated, functionally mature populations. Claims for in vivo differentiation into other cell types are equally controversial, as BM-derived mesenchymal stromal cell cultures have been shown to contribute to many tissues upon transplantation through fusion with endogenous cells and not through differentiation into mature cell types25. How multipotent mesenchymal stromal cells really are remains unclear.
Discrepancies in the reported properties of MSCs might be partially explained by the presence in tissues of diverse precursor types, heterogeneous in nature and origin that seem similar on the basis of their in vitro characteristics. However, heterogeneity is obvious at the level of mesenchymal stromal cell cultures (reviewed in26), with the presence of clones of different morphologies8, 27, 28, proliferative capacities29 multidifferentiation capacity in vitro and ability to generate bone in ectopic implants in vivo27, 30, 31. Single-cell derived clones of mesenchymal stromal cells from human umbilical cord progenitors that display varying degrees of multipotency and extensive self-renewal in vitro have been shown to generate daughter clones that gradually lose their multilineage differentiation capacity32. Together, these observations suggest that conventional mesenchymal stromal cell cultures arise from and contain, a heterogeneous pool of mesenchymal progenitors/stem cells that can be organized in a hierarchical manner, analogous to that of other well-described stem cell systems. Beyond MSCs, more primitive multipotent cell subsets with the potential to give rise to cells of all three germ layers have been proposed to be present within the tissue-resident pool of cells and co-purify with mesenchymal stromal progenitors. Nevertheless, it should be emphasized that the existence of stem cell populations of such nature, which include multipotent adult progenitor cells (MAPCS)33 or Muse cells (for Multilineage differentiating stress enduring cells)34 is highly controversial, as a detailed characterization of their biological properties and in vivo identity is lacking to date.
An additional important consideration at this point is that mesenchymal stromal cells derived from various postnatal or embryonic tissues using identical culture conditions display significant differences in colony morphology, differentiation potential and gene expression8, 35–37. This raises the question of whether MSCs from different anatomical locations, selected by classic adherence and in vitro culture methods, are biologically equivalent. Collectively, these results suggest that mesenchymal stromal cell cultures may originate from an array of tissue-specific multipotent precursor cells that are present in native tissues and have diverse degrees of plasticity and self-renewal.
Studying MSCs in vivo
After years of investigating MSCs out of their native context, little has been learned regarding the identity and function of their precursors in vivo. It is important to note that the fundamental biological properties of mesenchymal stromal cells are likely to be altered by culture conditions and thus should not be directly ascribed to their presumed in vivo counterpart as has often been done in the published literature. Progress in our understanding of bona fide MSCs largely relies in having the capacity to recognize progenitor cells in situ, prospectively isolate them and finally assay their multipotency and self-renewal capacity in vivo.
Prospective isolation, multipotency and self-renewal
The unequivocal identification of MSCs in vivo has been hindered by their extremely low frequency in tissues38 and the lack of a distinct MSC-specific immunophenotype to enable their isolation. Indeed, cultured human mesenchymal stromal cells express a panel of cell surface markers such as CD73, CD105, CD90 and lack endothelial or haematopoietic cell markers (CD34, CD31 and CD45)16. Nevertheless, these are not homogeneously expressed throughout stromal cultures, vary with isolation protocols and passage, and therefore are not necessarily representative of MSCs in vivo. Several labeling strategies have been used to successfully enrich for CFU-Fs in human and mouse BM; these include the use of combinations of markers such as Stro-1 and CD10639, 40, SSEA-4 (also known as FUT4)41, CD271, CD56, MSCA-1 and D7-FIB (a fibroblast orepithelial cell marker)42–44.
Recent studies have provided valuable insight into the identity and physiology of BM-resident MSCs using new markers to track and purify MSC-enriched populations and assay them in vivo. Of note, unlike the haematopoietic system, in which haematopoietic reconstitution of myeloablated recipients is the gold-standard to assess HSC self-renewal and differentiation at a single-cell level, no universally accepted assay has been established to date to probe for the activity of MSCs in vivo.
Using the capacity to form bone and assemble a functional BM stroma at heterotopic sites as an indication of MSC potential in vivo, one group identified a population of CD146+ perivascular self-renewing osteoprogenitors that were present in the outermost connective tissue layer covering BM microvessels (known as adventitia)45. Furthermore, in mice, combined expression of surface cell antigen 1 (Sca1) and platelet-derived growth factor receptor-α (PDGFRα) specifies a subset of non-haematopoietic cells that resides close to arteries and gives to rise to osteoblasts, reticular cells and adipocytes in vivo upon transplantation into an irradiated recipient46. Finally, the neural stem cell marker Nestin was recently reported to label BM-resident MSCs in a selective manner. This study showed for the first time that MSCs are the progenitors of osteochondral mature cell types in the BM under physiologic conditions. Nestin+ BM-derived MSCs could be cultured under non-adherent conditions and could be serially transplanted, therefore demonstrating a robust self-renewal capacity47.
Together, these studies have convincingly shown the self-renewing and differentiation potential of a specific population of MSCs in the BM. It remains to be determined whether and to what extent the specificity of these markers and the functional characteristics of these BM-resident MSCs can be used to describe MSC populations from different adult tissues.
Perivascular localization in vivo
A key aspect for assessing MSC function in vivo is to define their microanatomical localization in situ in diverse organs. Efforts to track the identity of tissue-resident MSCs have consistently suggested that these cells lie adjacent to blood vessels48. Evidence for such association, came from initial observations that pericytes (also known as Rouget cells or mural cells), which are defined by their perivascular location and morphology, display MSC-like features49. Pericyte-derived cultures are similar to mesenchymal stromal cell cultures in terms of morphology and cell-surface antigen expression, and can be induced to differentiate into osteoblasts, chondrocytes, adipocytes, but also smooth muscle cells and myocytes under appropriate conditions50–52. Cells expressing some mesenchymal stromal cell markers were found to localize to blood vessel walls in human bone marrow and dental pulp53. Conversely, MSC-like cultures were generated from cells enriched directly from tissues based on expression of pericyte-specific markers54. However, evidence that pericytes and MSCs are biologically equivalent has remained indirect for a long time. A recent study identified a combination of markers, such as NG2(also known as CSPG4), CD146, and PDGFRβ, that seemed to specifically label pericytes in a range of human organs, including fetal and adult skin, pancreas, heart, brain, lungs, bone marrow and placenta. Long-term cultures derived from prospectively isolated pericytes directly from these organs based on specific expression of those marker, displayed similar morphological features to those of cultured mesenchymal stromal cells, as well as trilineage potential in vitro and osteogenic potential in vivo55.
Collectively, these results strongly suggest that the precursors of cultured mesenchymal stromal cells preferentially reside close to blood vessels in vivo, a trait that is not unique to MSCs but pertinent to other multipotent stem or progenitor cells present in adult tissues. In this respect, HSCs (discussed below), as well as other ill-defined tissue progenitors such as white fat progenitor cells and skeletal muscle stem cells, have been reported to reside in perivascular spaces of bone marrow, adipose tissue and skeletal muscle microvessels, respectively56–59. Nevertheless, it is important to note that the terms pericyte and MSC are not equivalent or interchangeable. Although widely used to refer to cells surrounding blood vessels, the word pericyte strictly refers to cells adjacent to capillaries and post-capillary venules49; however, multipotent MSC-like precursors have been isolated from the walls of other vascular types, including arteries and veins60, 61. Furthermore, because pericytes show an extensive tissue distribution along diverse microvascular beds and have many proposed functions (including vessel stabilization, phagocytosis and regulation of vascular integrity and tone49) it is likely that functionally heterogeneous, non-equivalent cell subsets are included under the vague term pericyte. Thus, despite being perivascular, not all MSCs can be referred to as pericytes, and not all pericytes exhibit MSC-specific properties.
MSCs and haematopoiesis
The stromal compartment of BM was the first biological material from which MSCs were isolated. Since then, BM-derived MSCs have been the most widely studied and are the best characterized and are thought to be key regulators of BM physiology (Figure 2).
Figure 2. Proposed biological functions of BM-resident MSCs in vivo.
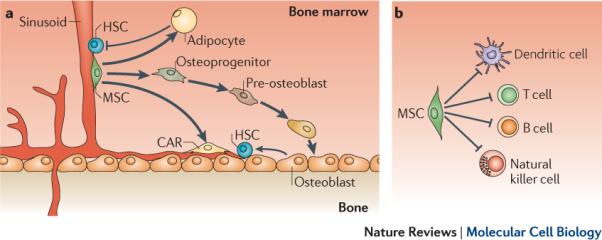
a. Bone marrow (BM)-derived mesenchymal stem cells (MSCs) differentiate into osteoblasts, adipocytes and reticular cells, which provide the supportive environment for haematopoietic development, and are thought to be responsible for the natural turnover of these mesenchymal cell types in the bone marrow. Osteoblasts are key components of haematopoietic stem cell (HSC) niches and have been proposed to directly interact and positively regulate quiescence of some HSCs in the BM, whereas adipocytes negatively regulate HSC activity. HSCs have also been shown to lie adjacent to CXCL12-abundant reticular cells (CAR cells), which are poorly characterized cells with adipogenic and osteogenic potential and may correspond to, or originate from, BM-resident MSCs. In addition to giving rise to haemosupportive environment, BM-resident MSCs expressing the neural stem cell marker Nestin have been shown to physically associate with HSCs in perivascular BM 'dual stem cell niches` and regulate HSC homeostasis. b. BM-resident MSCs are found in perivascular areas of BM microenvironments, where they may associate with cells of the immune system, including B cells, T cells and dendritic cells (DCs). Furthermore, mesenchymal stromal cells, which are though to directly derive from MSCs in vivo, are known to regulate the function of lymphocytes (B cells and T cells), DCs and natural killer (NK) cells. It is therefore hypothesized that BM-resident MSCs may regulate immune responses occurring in the BM in vivo.
Precursors of the haematopoietic microenvironment
During adulthood, the sustained production of blood cells occurs primarily in the BM. MSCs have long been proposed to be the in vivo precursors of some of the non-haematopoietic components of the BM that regulate hematopoiesis, such as osteoblasts, adipocytes and fibroblastic reticular cells2. Consequently, MSCs are likely to contribute to the homeostasis of the haematopoietic compartment in vivo through the regulatory properties of their mature progeny (Figure 2).
Inside the BM microenvironment, HSCs are thought to reside in confined niches, which are created by surrounding cells, soluble factors and extracellular matrix proteins that ultimately promote HSC maintenance. Osteoblasts have been postulated to crucially contribute to HSC niches and regulate HSC homeostasis through direct cell-to-cell interactions62, 63. Although the existence of an osteoblastic HSC niche is controversial64, it seems clear that either directly or through the secretion of soluble factors, osteoblasts are essential constituents of the BM microenvironment and have regulatory roles at many stages of haematopoietic development (reviewed in65). The BM stroma is also composed of MSC-derived adipocytes, which function as negative regulators of early haematopoietic progenitors through unknown molecular mechanisms66.
Hence, MSCs are the source of two coexisting mature cell types with apparently antagonistic properties on HSCs. Many open questions remain concerning the precise developmental stages that MSCs undergo during differentiation in situ, the pathways governing lineage commitment decisions in vivo and the global impact of the balance of osteoblast and adipocyte production in haematopoietic environments (Figure 2).
HSC niche components
Multipotent immature BM-resident MSCs have long been proposed to provide modulatory signals to haematopoietic progenitors based on the fact that mixed cultures derived from the adherent fraction of BM stroma promote survival and proliferation of HSCs ex vivo67. In addition, MSCs are isolated from all fetal hematopoietic sites even before the HSC colonization of those tissues10, 68. It is therefore thought that MSCs have a key role in the organization of HSC niches, either through direct interaction or, as proposed13, through their reported ability to organize vascular networks, which are key structural and functional components of haematopoietic sites45, 69. Indeed, the existence of a `dual stem cell niche', in which MSCs and HSCs directly interact in perivascular spaces of the BM, is conceptually attractive and has been proposed in two recent publications. HSCs have been shown to co-localize with a subset of poorly characterized reticular cells that are defined by high expression of the chemokine CXCL12 and are known as CXCL12-abundant reticular (CAR cells)70. These cells hold osteogenic and adipogenic potential and might therefore correspond to or immediately derive from MSCs71. Moreover, an association between HSCs and BM-resident MSCs has been visualized using mice expressing nestin tagged with green fluorescent protein (nestin-GFP)47. Selective depletion of CAR cells or nestin-GFP+ MSCs, both of which express high levels of HSC-regulatory factors, had a direct impact in HSC numbers and homeostasis, further indicating a role for BM-resident MSCs in HSC biology47, 71. Notably, nestin-GFP+ BM-resident MSCs are directly innervated and respond to signals from the sympathetic nervous system (SNS)47, thus providing a link by which HSC homeostasis is regulated by the SNS as previously demonstrated72, 73. Further studies are needed to fully understand where and how HSCs, MSCs and SNS interact.
Immunomodulatory agents
One of the most remarkable and unforeseen aspects of mesenchymal stromal cells pertains to their immunomodulatory activity (reviewed in74, 75). In vitro, mesenchymal stromal cells inhibit T cell activation, dendritic cell differentiation, B cell proliferation and impair the cytolytic potential of natural killer cells. Immunosuppression after MSC infusion in vivo has also been documented in diverse animal models of disease12, 74. These effects are partially explained by the ability of mesenchymal stromal cells to secrete a vast array of soluble mediators, some of which have immunomodulatory properties, such as interleukin-10 (IL-10), prostaglandin E2, nitric oxide or transforming growth factor (TGFβ)12.
Nevertheless, these immunomodulatory effects require, at least in part, direct cell-to-cell contact. Notably, immunomodulation in vivo and in vitro has been reported exclusively for mesenchymal stromal cells and no evidence exists to date to suggest that such regulatory properties can be ascribed to MSCs in vivo. However, given that the BM is one of the sites where adaptive immune responses are generated, and that BM-resident MSCs share perisinusoidal locations with dendritic cells and circulating B cells76, 77, it seems plausible that MSC-immune cell interactions may be of physiological relevance, which merits further investigation (Figure 2).
Concluding remarks
The discovery of a subset of adult multipotent cells, which could be readily purified by adherence from multiple tissues and rapidly expanded ex vivo, was enthusiastically received in the hope that these would become an alternative to embryonic stem cells and free of the ethical implications associated with their therapeutic application in humans. As a consequence, investigations oriented towards characterizing mesenchymal stromal cells and harnessing their therapeutic potential (Box 2) rapidly proliferated, whereas fundamental biological questions regarding their in vivo counterpart populations remained largely unanswered. In our view, the term MSC is misleading in that it has been widely used to refer to a heterogeneous pool of tissue-specific multipotent perivascular progenitors, which likely possess diverse in vivo functions and differentiation potential, but have similar features after in vitro culture. Among these, the only well characterized in terms of biological properties and in vivo stem cell features are BM-resident MSCs, which sustain the homeostatic turnover of skeletal cell types in the BM in vivo.
Major challenges at hand are to define the biological equivalence and hierarchical relationships between progenitors in diverse anatomical locations, understand their developmental origin, characterize their multipotential capabilities and elucidate their in vivo roles during homeostasis and tissue repair. Resolving these questions will require comprehensive experimental approaches including the use of stringent in vivo assays to define the multipotentiality of MSC populations, advanced in vivo microscopy techniques to track their distribution and dynamics in diverse tissues, and the use of inducible genetic MSC-specific animal models. Ultimately, a more refined insight into the biological attributes of MSCs is expected to result in a more rational exploitation of their therapeutic use.
Box 2. Therapeutic exploitation of mesenchymal stromal cells.
Although clinical interest in cultured mesenchymal stem cells (known as mesenchymal stromal cells) initially focused on the potential of their stem cell-like properties for tissue regeneration and repair, the discovery of their paracrine properties markedly increased the range of therapeutic applications for which they are currently studied. Systemic infusion of mesenchymal stromal cells has proved beneficial in different preclinical models of acute lung injury, myocardial infarction, diabetes, multiple sclerosis, as well as renal and hepatic failure74, 78. Although the mechanisms underlying the therapeutic effects of mesenchymal stromal cells in these disease models are not well characterized, they are thought to partially arise from the release of a combination of multiple bioactive molecules with anti-inflammatory, antiproliferative, antiapoptotic and angiogenic properties (reviewed in 12). The current hypothesis is that paracrine factors secreted by mesenchymal stromal cells provide protective microenvironmental cues and promote repair by local tissue-resident progenitor populations, thereby explaining the detection of favourable effects even in the absence of prolonged mesenchymal stromal cell engraftment in sites of injury12, 74, 75.
These findings have prompted clinical studies on the therapeutic potential of mesenchymal stromal cells. For instance, the osteogenic properties of mesenchymal stromal cells have been used to treat children with osteogenesis imperfecta and have shown promising outcomes79, 80. On the basis of their immunoregulatory and tissue protective properties, mesenchymal stromal cells are also being tested for the treatment and prevention of graft-versus-host disease, Crohn's disease and certain haematologic malignancies78, 81, 82.
Nevertheless, in most cases, these studies are preliminary, and treatment efficacy has not been conclusively established. Some of the major questions that still need to be resolved concern the standardization of protocols for the isolation of mesenchymal stem cells and their expansion into mesenchymal stromal cells in vitro, the safety of such cell-based therapies and the homing and engraftment of mesenchymal stromal cells to their target tissues.
Acknowledgements
L.E.S. is supported by grants P01 HL095489, R01 HL093139, and contract HHSN268201000009C from the National Heart Lung and Blood Institute. J.R. is supported by grant P01 CA78378 from the National Cancer Institute, and grant P01 CA142106 and contract HHSN268201000009C from the National Heart Lung and Blood Institute. C.N.A is a recipient of a Human Frontiers in Science Program (HFSP) Long Term fellowship 00194/2008-L.
References
- 1.Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–68. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 2.Friedenstein AJ, Piatetzky S, II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–90. [PubMed] [Google Scholar]
- 3.Tavassoli M, Crosby WH. Transplantation of marrow to extramedullary sites. Science. 1968;161:54–6. doi: 10.1126/science.161.3836.54. [DOI] [PubMed] [Google Scholar]
- 4.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 5.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 6.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–50. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 7.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–4. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 8.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–13. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 9.Kuznetsov SA, et al. Circulating skeletal stem cells. J Cell Biol. 2001;153:1133–40. doi: 10.1083/jcb.153.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendes SC, Robin C, Dzierzak E. Mesenchymal progenitor cells localize within hematopoietic sites throughout ontogeny. Development. 2005;132:1127–36. doi: 10.1242/dev.01615. [DOI] [PubMed] [Google Scholar]
- 11.Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004;32:414–25. doi: 10.1016/j.exphem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–27. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Bianco P, Robey PG, Saggio I, Riminucci M. “Mesenchymal” stem cells in human bone marrow (skeletal stem cells): a critical discussion of their nature, identity, and significance in incurable skeletal disease. Hum Gene Ther. 21:1057–66. doi: 10.1089/hum.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–9. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horwitz EM, et al. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–5. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 16.Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 17.Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13:81–8. doi: 10.1016/8756-3282(92)90364-3. [DOI] [PubMed] [Google Scholar]
- 18.Ashton BA, et al. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin Orthop Relat Res. 1980:294–307. [PubMed] [Google Scholar]
- 19.Li F, Wang X, Niyibizi C. Bone marrow stromal cells contribute to bone formation following infusion into femoral cavities of a mouse model of osteogenesis imperfecta. Bone. 47:546–55. doi: 10.1016/j.bone.2010.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oswald J, et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377–84. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 21.Makino S, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snykers S, De Kock J, Rogiers V, Vanhaecke T. In vitro differentiation of embryonic and adult stem cells into hepatocytes: state of the art. Stem Cells. 2009;27:577–605. doi: 10.1634/stemcells.2008-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26:1787–95. doi: 10.1634/stemcells.2007-0979. [DOI] [PubMed] [Google Scholar]
- 24.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez-Dolado M, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–73. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 26.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–92. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 27.Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113(Pt 7):1161–6. doi: 10.1242/jcs.113.7.1161. [DOI] [PubMed] [Google Scholar]
- 28.Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A. 2000;97:3213–8. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci U S A. 2001;98:7841–5. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Digirolamo CM, et al. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999;107:275–81. doi: 10.1046/j.1365-2141.1999.01715.x. [DOI] [PubMed] [Google Scholar]
- 31.Kuznetsov SA, et al. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res. 1997;12:1335–47. doi: 10.1359/jbmr.1997.12.9.1335. [DOI] [PubMed] [Google Scholar]
- 32.Sarugaser R, Hanoun L, Keating A, Stanford WL, Davies JE. Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy. PLoS One. 2009;4:e6498. doi: 10.1371/journal.pone.0006498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang Y, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 34.Kuroda Y, et al. Unique multipotent cells in adult human mesenchymal cell populations. Proc Natl Acad Sci U S A. 107:8639–43. doi: 10.1073/pnas.0911647107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panepucci RA, et al. Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells. 2004;22:1263–78. doi: 10.1634/stemcells.2004-0024. [DOI] [PubMed] [Google Scholar]
- 36.Lee RH, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14:311–24. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- 37.Kaltz N, et al. Novel markers of mesenchymal stem cells defined by genome-wide gene expression analysis of stromal cells from different sources. Exp Cell Res. doi: 10.1016/j.yexcr.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–7. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 39.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 40.Gronthos S, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116:1827–35. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 41.Gang EJ, Bosnakovski D, Figueiredo CA, Visser JW, Perlingeiro RC. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109:1743–51. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- 42.Jones EA, et al. Optimization of a flow cytometry-based protocol for detection and phenotypic characterization of multipotent mesenchymal stromal cells from human bone marrow. Cytometry B Clin Cytom. 2006;70:391–9. doi: 10.1002/cyto.b.20118. [DOI] [PubMed] [Google Scholar]
- 43.Buhring HJ, et al. Novel markers for the prospective isolation of human MSC. Ann N Y Acad Sci. 2007;1106:262–71. doi: 10.1196/annals.1392.000. [DOI] [PubMed] [Google Scholar]
- 44.Battula VL, et al. Isolation of functionally distinct mesenchymal stem cell subsets using antibodies against CD56, CD271, and mesenchymal stem cell antigen-1. Haematologica. 2009;94:173–84. doi: 10.3324/haematol.13740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sacchetti B, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–36. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 46.Morikawa S, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206:2483–96. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mendez-Ferrer S, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 466:829–34. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corselli M, Chen CW, Crisan M, Lazzari L, Peault B. Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol. 30:1104–9. doi: 10.1161/ATVBAHA.109.191643. [DOI] [PubMed] [Google Scholar]
- 49.Hirschi KK, D'Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–98. [PubMed] [Google Scholar]
- 50.Farrington-Rock C, et al. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–32. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- 51.Doherty MJ, et al. Vascular pericytes express osteogenic potential in vitro and in vivo. J Bone Miner Res. 1998;13:828–38. doi: 10.1359/jbmr.1998.13.5.828. [DOI] [PubMed] [Google Scholar]
- 52.Collett GD, Canfield AE. Angiogenesis and pericytes in the initiation of ectopic calcification. Circ Res. 2005;96:930–8. doi: 10.1161/01.RES.0000163634.51301.0d. [DOI] [PubMed] [Google Scholar]
- 53.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 54.Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22:2903–11. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- 55.Crisan M, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 56.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 57.Dellavalle A, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–67. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 58.Tang W, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–6. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Traktuev DO, et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 60.Tintut Y, et al. Multilineage potential of cells from the artery wall. Circulation. 2003;108:2505–10. doi: 10.1161/01.CIR.0000096485.64373.C5. [DOI] [PubMed] [Google Scholar]
- 61.Hoshino A, Chiba H, Nagai K, Ishii G, Ochiai A. Human vascular adventitial fibroblasts contain mesenchymal stem/progenitor cells. Biochem Biophys Res Commun. 2008;368:305–10. doi: 10.1016/j.bbrc.2008.01.090. [DOI] [PubMed] [Google Scholar]
- 62.Lo Celso C, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–6. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xie Y, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457:97–101. doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- 64.Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- 65.Garrett RW, Emerson SG. Bone and blood vessels: the hard and the soft of hematopoietic stem cell niches. Cell Stem Cell. 2009;4:503–6. doi: 10.1016/j.stem.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 66.Naveiras O, et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–63. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dexter TM, Allen TD, Lajtha LG. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977;91:335–44. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- 68.Campagnoli C, et al. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 69.Melero-Martin JM, et al. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103:194–202. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–88. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 71.Omatsu Y, et al. The Essential Functions of Adipo-osteogenic Progenitors as the Hematopoietic Stem and Progenitor Cell Niche. Immunity. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 72.Katayama Y, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–21. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 73.Mendez-Ferrer S, Chow A, Merad M, Frenette PS. Circadian rhythms influence hematopoietic stem cells. Curr Opin Hematol. 2009;16:235–42. doi: 10.1097/MOH.0b013e32832bd0f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008 doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 75.Bernardo ME, Locatelli F, Fibbe WE. Mesenchymal stromal cells. Ann N Y Acad Sci. 2009;1176:101–17. doi: 10.1111/j.1749-6632.2009.04607.x. [DOI] [PubMed] [Google Scholar]
- 76.Pillai S, Cariappa A. The bone marrow perisinusoidal niche for recirculating B cells and the positive selection of bone marrow-derived B lymphocytes. Immunol Cell Biol. 2009;87:16–9. doi: 10.1038/icb.2008.89. [DOI] [PubMed] [Google Scholar]
- 77.Sapoznikov A, et al. Perivascular clusters of dendritic cells provide critical survival signals to B cells in bone marrow niches. Nat Immunol. 2008;9:388–95. doi: 10.1038/ni1571. [DOI] [PubMed] [Google Scholar]
- 78.Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 28:585–96. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Horwitz EM, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99:8932–7. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Horwitz EM, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–13. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 81.Tolar J, Le Blanc K, Keating A, Blazar BR. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells. 28:1446–55. doi: 10.1002/stem.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Auletta JJ, Cooke KR, Solchaga LA, Deans RJ, van't Hof W. Regenerative stromal cell therapy in allogeneic hematopoietic stem cell transplantation: current impact and future directions. Biol Blood Marrow Transplant. 16:891–906. doi: 10.1016/j.bbmt.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


